Open Access Article
Open Access ArticleSelective synthesis and structural transformation between a molecular ring-in-ring architecture and an abnormal trefoil knot†
Li-Long
Dang
a,
Xiang
Gao
a,
Yue-Jian
Lin
a and
Guo-Xin
Jin
 *ab
*ab
aDepartment of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200438, P. R. China
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, P. R. China. E-mail: gxjin@fudan.edu.cn
First published on 15th July 2020
Abstract
The synthesis of complicated supramolecular architectures and the study of their reversible structural transformations remains a fascinating challenge in the field of supramolecular chemistry. Herein, two types of novel coordination compounds, a non-intertwined ring-in-ring assembly and an abnormal trefoil knot were constructed from a strategically selected Cp*Rh building block and a semi-rigid N,N′-bis(4-pyridylmethyl)diphthalic diimide ligand via coordination-driven self-assembly. Remarkably, the reversible transformation between the abnormal trefoil knot and the ring-in-ring assembly or the corresponding tetranuclear macrocycle could be achieved by the synergistic effects of Ag+ ion coordination and alteration of the solvent. Single-crystal X-ray crystallographic data and NMR spectroscopic experiments support the structural assignments.
Introduction
In the field of supramolecular chemistry, the aesthetic features and potential applications of interlocked or intertwined compounds continue to motivate scientists to design and construct new compounds with intricate topologies.1–3 Among the many synthetic strategies applied, coordination-driven self-assembly has proven to be a particularly fruitful approach. Based on this, a wide range of topologies, such as catenanes,4–7 rotaxanes,8–13 knots,14–22 Solomon links,23–27 Borromean rings,28–32 and interlocked cages,33–35 have been constructed. Recent reports of the synthesis and structural transformations of molecular Borromean rings and Solomon links have marked considerable progress in the research field, in particular the development of unlinking transformations from Borromean rings to the corresponding component monomers by taking advantage of the inverse electron-demand Diels–Alder (IEDDA) reaction,36 and the successful realization of reversible transformation of a Solomon link to an unusual trefoil knot by utilizing the coordination ability of silver(I) ions.37,38Additionally, the successful synthesis of complicated molecular knots,18,19,39,40 and [n]catenanes (n = 3, 4, 6)41–46 are tremendous developments in the study of topologically complex molecules. Nevertheless, structural interconversion between intricate supramolecular architectures is seldom realized due to the difficulties inherent in their design and controllability.38,47
Molecular ring-in-ring motif is rarely observed in supramolecular host–guest chemistry, the structures of which are non-intertwined but consist of two chemically independent rings in which one ring encapsulates a second.48–53 Although an example of a metallarectangle-based molecular ring-in-ring structure has been reported by the group of Chi by using an arene-Ru acceptor and the rigid ligand 1,4-di(pyridin-4-yl)buta-1,3-diyne through π–π interactions,53 constructing a novel organometallic ring-in-ring complex with a nonrigid ligand and achieving its transformation to other intricate supramolecular structures remains an unfulfilled goal.
In recent works, a series of intricate complexes including molecular trefoil, figure-eight knots and Solomon links have been achieved using the building blocks [Cp2*M2(BiBzIm)](OTf)2 (M = Rh/Ir, BiBzIm = 2,2′-bisbenzimidazole).21,38 This success was based on the appropriate width and electron deficient characteristics of the BiBzIm plane, which provides the possibility of forming π–π stacking and C–H⋯π interactions.
In the ligand N,N′-bis(4-pyridylmethyl)-diphthalic diimide (L), the two pyridyl moieties are separated by diimide spacers, and the sp3-hybridized methylene carbon atoms in L allow the two pyridyl arms to rotate freely, thus making ligand L an example of a semi-rigid ligand. Moreover, the phthalic diimide (PDM) cores not only bear different angular geometries, but endow the ligand with rich hydrogen-bonding possibilities.54,55 Additionally, its π-conjugated phthalic diimide and pyridyl moieties can engender favorable aromatic π–π stacking and CH–π interactions. These unique advantages of [Cp2*M2(BiBzIm)](OTf)2 and ligand L provide the possibility of synthesizing novel ring-in-ring assemblies and other supramolecular complex architectures.
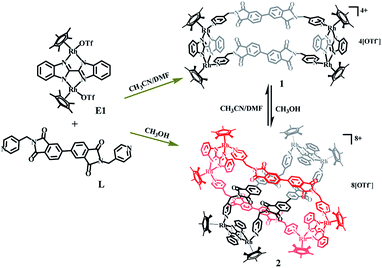
Herein, we report the reversible structural transformation between a molecular ring-in-ring complex and an abnormal trefoil knot for the first time, which can be achieved by the addition or removal of Ag+ ions. And these two structures can also transform to the corresponding tetranuclear macrocycle based on solvent effects and/or the presence of Ag+ ions. These three compounds are synthesised by coordination-driven self-assembly of the [Cp2*Rh2(BiBzIm)](OTf)2 building block and a semi-rigid diimide ligand (L) under mild conditions. π–π and C–H⋯π interactions may play synergetic roles in the formation of the ring-in-ring assembly and abnormal trefoil knot. In addition, these structures and their reversible transformation are unambiguously characterized by NMR spectroscopy, ESI-MS, and single-crystal X-ray diffraction analysis.
Results and discussion
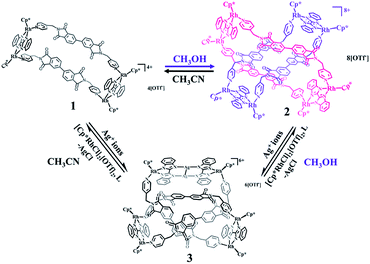
Self-assembly of tetranuclear macrocycle 1 and ring-in-ring complex 2
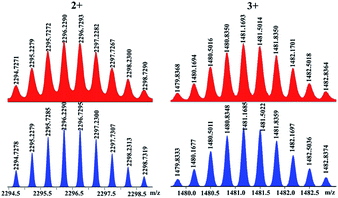
The reaction of [Cp2*Rh2(BiBzIm)](OTf)2 (E1) with AgOTf (2.0 equiv.) in CH3CN, followed by the addition of L, produced a tetranuclear macrocycle complex 1 (yield: 88%; Scheme 1). The structure of 1 was confirmed by NMR spectroscopy (Fig. S6–S9, ESI†) and X-ray crystallographic analysis (Fig. 1).The 1H NMR spectrum of 1 in CD3CN showed doublets at δ 8.26, 7.67 and 7.07 ppm and two singlets at δ 7.86 and 4.33 ppm corresponding to the protons of L, while the protons of BiBzIm appeared as two multiplet peaks at δ 7.93–7.94 and 7.42–7.44 ppm (Fig. S6, ESI†). The 1H DOSY NMR spectrum of 1 showed a single diffusion coefficient (D = 5.04 × 10−10 m2 s−1), suggesting that only one stoichiometry of assembly was formed (Fig. S8, ESI†). The structure of 1 in solution was also supported by ESI-MS. The prominent signal at m/z = 838.16 ([1–3OTf−]3+) is in good agreement with the theoretical isotopic distribution (Fig. 2A). In addition, only a single complex was detected in solution in a wide range of concentrations, suggesting the significant stability of 1 in CD3CN (Fig. S10, ESI†).
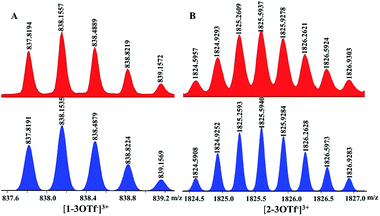 | ||
| Fig. 2 Calculated (bottom, blue) and experimental (top, red) ESI-MS spectra (3+) of (A) tetranuclear 1 and (B) ring-in-ring complex 2. | ||
To gain further information regarding complex 1, NMR spectroscopic experiments were carried out in [D7]-DMF solution (30.0 mM, with respect to Cp*Rh). The 1H NMR spectrum of the solution presented similar signals to those observed in CD3CN solution, indicating that 1 is similarly stable in [D7]-DMF solution (Fig. S11, ESI†).
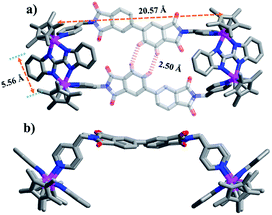
The Cp*Ir-based complex 1′ was prepared analogously to 1 by combination of E1′ and L in a yield of 83% and was crystallised by diffusion of diethyl ether into its acetonitrile solution. 1′ was found to be a boat-shaped tetranuclear macrocycle by single-crystal X-ray diffraction analysis (Fig. 1). The two binuclear (IrIII) molecular clips (E1′) are linked by two dipyridyl ligands, adopting U-conformations with dimensions of 5.56 Å (short Ir⋯Ir nonbonding distances) and 20.57 Å (long Ir⋯Ir nonbonding distances). The shortest H–H distance between two adjacent PDM of different L units is ca. 2.50 Å. This small internal space of the tetranuclear macrocycle 1 presumably hampers the formation of interlocked metallarectangles or other more complicated species.
In an effort to perturb the synthesis of 1 through solvent alteration, ligand L was added to a CH3OH solution of E1, and the mixture was stirred for 8 h, resulting in a yellow solution. Then, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of methanol and isopropyl ether as transition layer solution was gradually added to the above solution until the transition layer became colorless. After that, an equivalent volume of isopropyl ether was added. Single crystals 2 suitable for X-ray diffraction were obtained from this mixture in 82% yield after 3 days (Fig. 3).
1 mixture of methanol and isopropyl ether as transition layer solution was gradually added to the above solution until the transition layer became colorless. After that, an equivalent volume of isopropyl ether was added. Single crystals 2 suitable for X-ray diffraction were obtained from this mixture in 82% yield after 3 days (Fig. 3).
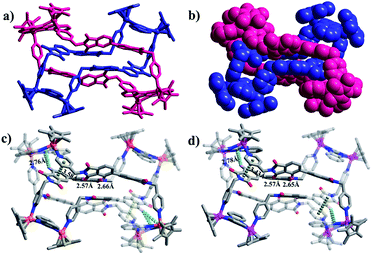
The solid-state structure of 2 shows that complex 2 has a non-catenane interlocked structure in which one macrocyclic unit threads another (chemical) identical macrocycle, thus forming a ring-in-ring complex. Close-contact analysis of the structure shows that the individual macrocycle forms a chair-shaped structure composed of two binuclear molecular clips E1 linked by two pyridyl ligands with Z-conformations. However, the phthalic diimide moieties in each macrocycle reveal different conformations (horizontal and parallel). The horizontal arrangement of adjacent phthalic diimide moieties in the outer macrocycle results in a distance that is wide enough to accommodate another macrocycle (minimum distance (r(H⋯O)): 5.1 Å), while the parallel arrangement of the inner macrocycle makes this unit narrower. The ring-in-ring structure is stabilized by parallel-displaced π–π interactions (of interlayer distance 3.59 Å) between the pyridyl moieties and phthalic diimide moieties of two macrocycles, as well as edge-to-face-type CH–π interactions (2.76 Å) between the BiBzIm moieties and the phenyl groups of the phthalic diimide ligands. In addition, the presence of four weak C–H⋯O hydrogen bonds56,57 (distances r(H⋯O) = 2.48–2.69 Å and angles ∠COH = 136.21–155.87°) suggest that inter-macrocycle interactions between the phthalic diimide moieties might also play a pivotal role in stabilizing the ring-in-ring structure (Fig. 2). Moreover, the analogous Cp*Ir-based ring-in-ring complex 2′ was separately constructed in a yield of 87%.
After determination of the solid-state molecular structure of ring-in-ring complex 2, extensive attempts were made to explore its behavior in solution by NMR spectroscopy, however, these were significantly hampered by the very poor solubility of complex 2 in CD3OD. However, the ESI-MS spectrum of 2 indicated that the ring-in-ring dimeric structure of the complex is retained in solution: [2–3OTf]3+ (m/z = 1825.59) (Fig. 2B). Additionally, the poor solubility of 2 might reflect the compactness of its ring-in-ring architecture.
A recent series of works have revealed that pyrene can induce the reversible conversion of some interlocked metallacycles to non-catenane rectangles through the inclusion of the former as a guest molecule.6,58 To investigate the inductive effect of pyrene on the dimeric ring-in-ring structure, a similar self-assembly reaction of E1 and L in the presence of pyrene was performed, resulting in the new complex 2-pyrene. The single-crystal X-ray analysis of 2-pyrene shows that the pyrene molecules are distributed around the ring-in-ring structure by forming π–π stacking interactions with the Cp* groups or as free guest molecules, clearly indicating that the pyrene molecules are unable to separate the macrocycles of the ring-in-ring structure (Fig. 4).
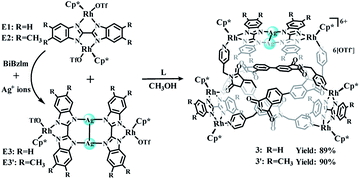
Self-assembly of abnormal trefoil knots 3 and 3′
As reported previously, the building block [Cp2*Ir2(BiBzIm)](OTf)2 (E1′) can react with two Ag+ ions and a BiBzIm ligand to form the Ag+-ions-bridged complex E3 based on the formation of N–Ag–N bonds,38 and E1′ can also be re-formed by removal of Ag+ ions by sunlight or Cl− ions, thus providing a reliable method for adjusting the size of the building block. This strategy encouraged us to explore whether the introduction of Ag+ ions into the ring-in-ring system could result in its supramolecular transformation. Thereby, a mixture of silver triflate and BiBzIm was added during the self-assembly process of complex 2. The resulting mixture was stirred for 6 h in the dark at room temperature, at which point evaporation of solvent and recrystallization provided compound 3 as a yellow solid (yield: 89%). In addition, the analogous E3-based complex 3′ was separately constructed in a yield of 90% (Scheme 2). The structures of 3 and 3′ were confirmed by NMR spectroscopy, ESI-MS, and single-crystal X-ray diffraction analysis.Complex 3 exhibited a far greater solubility in CH3OH than complex 2. Therefore, the relevant NMR spectroscopic experiments in CD3OD were performed to explore its solution behavior. The 1H NMR spectrum (Fig. S17, ESI†) of 3 shows very complicated peaks which can be analyzed by combination with 1H–1H COSY NMR (Fig. S18, ESI†), 1H DOSY NMR spectra (Fig. S19, ESI†) and 1H–13C HSQC NMR (Fig. S21, ESI†) of 3. The 1H NMR spectrum shows three singlets at δ 1.79, 1.66 and 1.50 ppm in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can be attributed to three types of Cp* groups, reflecting the formation of a new compound with an intricate structure. It also showed six doublets at δ 5.17, 4.51, 4.40, 4.34, 4.17 and 3.84 ppm for the protons of –CH2– groups of L ligands. Another three singlets at δ 7.86, 6.75, 5.95 ppm and five doublets at 5.08, 5.06, 4.59, 4.15, 3.81 ppm display the protons of PDM groups of L ligands. Besides, there are two multiplet peaks at δ 8.80–8.77 and 7.37–7.33 ppm, corresponding to the protons of pyridyl groups of ligands L. The prominent doublets at δ 8.43, 8.20, 8.12, 8.07, 7.93, 7.19 ppm, triplets at δ 7.51, 7.03 ppm could be attributed to multiple types of BiBzIm groups. In addition, the 1H DOSY NMR spectrum of 3 shows that the aromatic and Cp* signals are associated with a single diffusion constant (D = 2.36 × 10−10 m2 s−1), suggesting that only one stoichiometry of assembly is formed in methanol (Fig. S19, ESI†). Along with this NMR spectroscopic data, ESI-MS also indicated the presence of complex 3 in solution: [3–2OTf−]2+ (m/z = 2296.23) and [3–3OTf−]3+ (m/z = 1480.84) (Fig. 5). The abnormal trefoil knot topology of 3 in the solid state was confirmed by single-crystal X-ray analysis. However, despite great efforts, the crystal data of 3 is not ideal (Fig. S3†).
1, which can be attributed to three types of Cp* groups, reflecting the formation of a new compound with an intricate structure. It also showed six doublets at δ 5.17, 4.51, 4.40, 4.34, 4.17 and 3.84 ppm for the protons of –CH2– groups of L ligands. Another three singlets at δ 7.86, 6.75, 5.95 ppm and five doublets at 5.08, 5.06, 4.59, 4.15, 3.81 ppm display the protons of PDM groups of L ligands. Besides, there are two multiplet peaks at δ 8.80–8.77 and 7.37–7.33 ppm, corresponding to the protons of pyridyl groups of ligands L. The prominent doublets at δ 8.43, 8.20, 8.12, 8.07, 7.93, 7.19 ppm, triplets at δ 7.51, 7.03 ppm could be attributed to multiple types of BiBzIm groups. In addition, the 1H DOSY NMR spectrum of 3 shows that the aromatic and Cp* signals are associated with a single diffusion constant (D = 2.36 × 10−10 m2 s−1), suggesting that only one stoichiometry of assembly is formed in methanol (Fig. S19, ESI†). Along with this NMR spectroscopic data, ESI-MS also indicated the presence of complex 3 in solution: [3–2OTf−]2+ (m/z = 2296.23) and [3–3OTf−]3+ (m/z = 1480.84) (Fig. 5). The abnormal trefoil knot topology of 3 in the solid state was confirmed by single-crystal X-ray analysis. However, despite great efforts, the crystal data of 3 is not ideal (Fig. S3†).
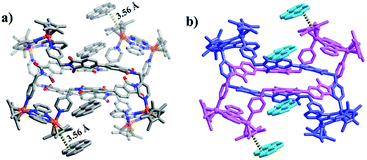
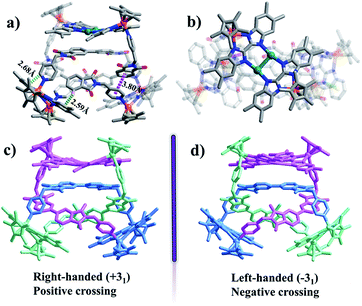
Fortunately, single crystals more suitable for X-ray diffraction were obtained by slow vapor diffusion of diethyl ether into a saturated solution of 3′ in CH3OH under ambient conditions for four days. The structure was definitively revealed by X-ray crystallographic analysis to have an abnormal trefoil knot topology. Complex 3′ crystallises in space group C2/c with the topology of a 31 knot according to the Alexander–Briggs notation. The observation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of both enantiomers (ΛΛΛ and ΔΔΔ) is consistent with other reported trefoil knot structures16 (Fig. 6c and d). In the refined structure, two different binuclear units are connected via ligands L featuring two different geometries (U and Z shapes), making up an abnormal trefoil knot form. The diagonal centroid–centroid distance of benzene groups of the newly-formed Ag(I)-containing bridging building block E3′ (10.40 Å) is similar to the separation of two nitrogen-atoms of phthalic diimide groups of L (10.59 Å), which allows for the face-to-face placement of phthalic diimide groups of L and the benzene rings of E3′. The distances between phthalic diimide groups of L and the opposing benzene rings of E3′ are ca. 3.66 and 3.93 Å, indicating the formation of π–π interactions between the TmBiBzIm group of E3′ and L. Edge-to-face-type CH⋯π interactions (ca. 2.46, 2.68 Å) between the phenyl moieties of L and TmBiBzIm were observed, in addition to parallel-displaced π–π interactions (of interlayer distance 3.77 Å) between benzene rings and adjacent pyridyl moieties of another L (Fig. S2, ESI†). Thus it is likely that the stabilization of the abnormal trefoil knot structure is due to a combination of π–π and CH⋯π interactions (Fig. 6a and b). As the abnormal trefoil knot structure of 3′ is achieved by the formation of only one Ag(I)-containing bridging building block E3′ with the two other E3 building blocks remaining Ag(I)-free, we wondered if additional Ag(I) ions would result in further structural transformation of 3′ to other intricate structures. In a series of exploratory experiments, different amounts of silver(I) triflate were added to solutions of 3′ in CH3OH. However, in all of these experiments, only the abnormal trefoil knot 3′ was observed by NMR spectroscopy (Fig. S22–S24†), ESI-MS (Fig. S38†), and single-crystal X-ray crystallographic analysis.
1 ratio of both enantiomers (ΛΛΛ and ΔΔΔ) is consistent with other reported trefoil knot structures16 (Fig. 6c and d). In the refined structure, two different binuclear units are connected via ligands L featuring two different geometries (U and Z shapes), making up an abnormal trefoil knot form. The diagonal centroid–centroid distance of benzene groups of the newly-formed Ag(I)-containing bridging building block E3′ (10.40 Å) is similar to the separation of two nitrogen-atoms of phthalic diimide groups of L (10.59 Å), which allows for the face-to-face placement of phthalic diimide groups of L and the benzene rings of E3′. The distances between phthalic diimide groups of L and the opposing benzene rings of E3′ are ca. 3.66 and 3.93 Å, indicating the formation of π–π interactions between the TmBiBzIm group of E3′ and L. Edge-to-face-type CH⋯π interactions (ca. 2.46, 2.68 Å) between the phenyl moieties of L and TmBiBzIm were observed, in addition to parallel-displaced π–π interactions (of interlayer distance 3.77 Å) between benzene rings and adjacent pyridyl moieties of another L (Fig. S2, ESI†). Thus it is likely that the stabilization of the abnormal trefoil knot structure is due to a combination of π–π and CH⋯π interactions (Fig. 6a and b). As the abnormal trefoil knot structure of 3′ is achieved by the formation of only one Ag(I)-containing bridging building block E3′ with the two other E3 building blocks remaining Ag(I)-free, we wondered if additional Ag(I) ions would result in further structural transformation of 3′ to other intricate structures. In a series of exploratory experiments, different amounts of silver(I) triflate were added to solutions of 3′ in CH3OH. However, in all of these experiments, only the abnormal trefoil knot 3′ was observed by NMR spectroscopy (Fig. S22–S24†), ESI-MS (Fig. S38†), and single-crystal X-ray crystallographic analysis.
Structural transformations between abnormal trefoil knot 3 and ring-in-ring complex 2 or tetranuclear macrocycle 1
The solvent-dependent formation of tetranuclear macrocycle and ring-in-ring complexes encouraged us to explore their interconversion starting from the respective pure entities. We speculated that alteration of the solvent might promote a unique and reversible conversion between ring-in-ring complex 2 and tetranuclear rectangle 1. Upon dissolution of crystals of the ring-in-ring complex 2 in CD3CN, a set of simple 1H NMR spectroscopic signals was observed, clearly indicating the formation of tetranuclear rectangle 1. The initial 1H NMR spectrum of tetranuclear rectangle 1 in CD3OD also showed simple peaks, however, over time, precipitation began to appear along with the reduction of the intensity of the 1H NMR signals. Accordingly, we grew single crystals of this species by slow vapor diffusion of diethyl ether into a saturated solution of 1 in CH3OH under ambient conditions. X-ray crystallographic data obtained for these crystals were consistent with those of 2, confirming the ability of complex 1 to convert gradually to a ring-in-ring complex in CH3OH (Scheme 3).Meanwhile, upon addition of AgOTf (2.0 equiv.) to a turbid solution of ring-in-ring complex 2 in CD3OD, the solid gradually dissolved and a complicated set of 1H NMR signals appeared, that were consistent with the 1H NMR spectrum of complex 3, confirming the transformation from ring-in-ring complex to an abnormal trefoil knot. Likewise, upon combining AgOTf (2.0 equiv.) and tetranuclear rectangle 1 in CD3OD, clear changes in the 1H NMR spectra were observed that indicated the gradual transformation from tetranuclear rectangle to an abnormal trefoil knot.
It is well documented that Ag+ ions are extremely sensitive toward light and can react with Cl− ions to form insoluble precipitates in solution. This opened the possibility of transformation between the silver-containing abnormal trefoil knots and ring-in-ring architectures or tetranuclear rectangles either by exposure to sunlight or addition of Cl− ions. As expected, in CH3OH, trefoil knot 3 transformed smoothly into ring-in-ring architecture 2 upon removal of both Ag(Ι) ions by exposure to with sunlight, which was additionally supported by X-ray crystallographic analysis. Correspondingly, adding [Cp*RhCl2]2, AgOTf and L in appropriate amounts to a methanol solution of 3 also led to formation of the ring-in-ring complex. Thereby, light-induced degradation led to removal of the Ag(I) ions in complex 3, providing a clear yellow solution after filtration to which silver triflate (4.0 equiv.), [Cp*RhCl2]2 (1.0 equiv.) and ligand L (1.0 equiv.) were added. Single crystals suitable for X-ray diffraction were subsequently obtained by slow vapor diffusion of diethyl ether into the yellow solution under ambient conditions for three days. The structure was confirmed to be ring-in-ring structure 2. In another reaction, silver triflate (2.0 equiv.) was added to a solution of [Cp*RhCl2]2 (1.0 equiv.) in CH3OH in the dark, at which point ligand L (1.0 equiv.) and complex 3 (1.0 equiv.) were added to the filtered solution. A yellow solution was acquired after 12 h. Subsequently, single-crystal X-ray crystallography confirmed this species to be the ring-in-ring complex 2. Additionally, a similar experimental process were also attempted in CH3CN, providing compound 1, confirming that the removal of silver ions with sunlight or Cl− ions can facilitate the transformation of trefoil knot 3 to macrocycle 1 in CH3CN. This results provide the first confirmation of interconversion between ring-in-ring, trefoil knot and tetranuclear rectangle architectures, which is an important milestone for the development of field of supramolecular chemistry.
Conclusions
In summary, we have demonstrated a selective and reversible transformation between molecular ring-in-ring structures and abnormal trefoil knots for the first time by addition of Ag+ ions under ambient condition. Both structures were further proven to be convertible to the corresponding tetranuclear macrocycle. New molecular ring-in-ring structures, abnormal trefoil knots and tetranuclear macrocycles bearing semi-rigid ligands were synthesised in high yields, and these structures were confirmed by X-ray crystallographic analysis, NMR spectroscopy and ESI-MS. π–π stacking and C–H⋯π interactions were shown to play important roles in the stabilization of the ring-in-ring and abnormal trefoil knot architectures. A deeper understanding of supramolecular transformations induced by the addition and removal of Ag+ ions reaction and solvent effects will be helpful to construct bespoke architectures and responsive supramolecular systems, opening up new insights into the field of smart materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (21531002, 21720102004) and the Shanghai Science Technology Committee (19DZ2270100).Notes and references
- R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434–5464 CrossRef CAS PubMed.
- T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed.
- W. L. Shan, Y. J. Lin, F. E. Hahn and G.-X. Jin, Angew. Chem., Int. Ed., 2019, 58, 5882–5886 CrossRef CAS PubMed.
- J. F. Nierengarten, C. O. Dietrich-Buchecker and J.-P. Sauvage, J. Am. Chem. Soc., 1994, 116, 375–376 CrossRef CAS PubMed.
- N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658–4683 RSC.
- H. Lee, P. Elumalai, N. Singh, H. Kim, S. U. Lee and K. W. Chi, J. Am. Chem. Soc., 2015, 137, 4674–4677 CrossRef CAS PubMed.
- X.-Y. Chang, G.-T. Xu, B. Cao, J.-Y. Wang, J.-S. Huang and C.-M. Che, Chem. Sci., 2017, 8, 7815–7820 RSC.
- T. B. Gasa, C. Valente and J. F. Stoddart, Chem. Soc. Rev., 2011, 40, 57–78 RSC.
- J. E. M. Lewis, R. J. Bordoli, M. Denis, C. J. Fletcher, M. Galli, E. A. Neal, E. M. Rochette and S. M. Goldup, Chem. Sci., 2016, 7, 3154–3161 RSC.
- T. H. Ngo, J. Labuta, G. N. Lim, W. A. Webre, F. D'Souza, P. A. Karr, J. E. M. Lewis, J. P. Hill, K. Ariga and S. M. Goldup, Chem. Sci., 2017, 8, 6679–6685 RSC.
- E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chem. Soc. Rev., 2018, 47, 5266–5311 RSC.
- M. Gaedke, F. Witte, J. Anhauser, H. Hupatz, H. V. Schroder, A. Valkonen, K. Rissanen, A. Lutzen, B. Paulusa and C. A. Schalley, Chem. Sci., 2019, 10, 10003–10009 RSC.
- A. W. Heard and S. M. Goldup, Chem, 2020, 6, 994–1006 CAS.
- C. O. Dietrich-Buchecker and J.-P. Sauvage, Angew. Chem., Int. Ed., 1989, 28, 189–192 CrossRef.
- N. Ponnuswamy, F. B. L. Cougnon, G. D. Pantos and J. K. M. Sanders, J. Am. Chem. Soc., 2014, 136, 8243–8251 CrossRef CAS PubMed.
- S. D. P. Fielden, D. A. Leigh and S. L. Woltering, Angew. Chem., Int. Ed., 2017, 56, 11166–11194 CrossRef CAS PubMed.
- J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080–11093 CrossRef CAS PubMed.
- L. Zhang, A. J. Stephens, A. L. Nussbaumer, J. F. Lemonnier, P. Jurček, I. J. Vitorica-Yrezabal and D. A. Leigh, Nat. Chem., 2018, 10, 1083–1088 CrossRef CAS PubMed.
- J. J. Danon, D. A. Leigh, S. Pisano, A. Valero and I. J. Vitorica-Yrezabal, Angew. Chem., Int. Ed., 2018, 130, 14029–14033 CrossRef.
- L. Zhang, A. J. Stephens, J. F. Lemonnier, L. Pirvu, I. J. Vitorica-Yrezabal, C. J. Robinson and D. A. Leigh, J. Am. Chem. Soc., 2019, 141, 3952–3958 CrossRef CAS PubMed.
- L.-L. Dang, Z.-B. Sun, W.-L. Shan, Y.-J. Lin, Z.-H. Li and G.-X. Jin, Nat. Commun., 2019, 10, 2057 CrossRef PubMed.
- Y. Segawa, M. Kuwayama, Y. Hijikata, M. Fushimi, T. Nishihara, J. Pirillo, J. Shirasaki, N. Kubota and K. Itami, Science, 2019, 365, 272–276 CrossRef CAS PubMed.
- M. Fujita and K. Ogura, Coord. Chem. Rev., 1996, 148, 249–264 CrossRef CAS.
- F. Ibukuro, M. Fujita, K. Yamaguchi and J.-P. Sauvage, J. Am. Chem. Soc., 1999, 121, 11014–11015 CrossRef CAS.
- N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658–4683 RSC.
- C. Schouwey, J. J. Holstein, R. Scopelliti, K. O. Zhurov, K. O. Nagornov, Y. O. Tsybin, O. S. Smart, G. Bricogne and K. Severin, Angew. Chem., Int. Ed., 2014, 53, 11261–11265 CrossRef CAS PubMed.
- Y. H. Song, N. Singh, J. Jung, H. Kim, E. H. Kim, H. K. Cheong, Y. Kim and K. W. Chi, Angew. Chem., Int. Ed., 2016, 55, 2007–2011 CrossRef CAS PubMed.
- J. C. Loren, M. Yoshizawa, R. F. Haldimann, A. Linden and J. S. Siegel, Angew. Chem., Int. Ed., 2003, 42, 5702–5705 CrossRef CAS PubMed.
- K. S. Chichak, S. J. Cantrill, A. R. Pease, S.-H. Chiu, G. W. V. Cave and J. L. Atwood, Science, 2004, 304, 1308–1312 CrossRef CAS PubMed.
- Y. Lu, Y.-X. Deng, Y.-J. Lin, Y.-F. Han, L.-H. Weng, Z.-H. Li and G.-X. Jin, Chem, 2017, 3, 110–121 CAS.
- C. A. Schalley, Angew. Chem., Int. Ed., 2004, 43, 4399–4401 CrossRef CAS PubMed.
- L. Zhang, L. Lin, D. Liu, Y. J. Lin, Z. H. Li and G.-X. Jin, J. Am. Chem. Soc., 2017, 139, 1653–1660 CrossRef CAS PubMed.
- M. Fujita, N. Fujita, K. Ogura and K. Yamaguchi, Nature, 1999, 400, 52–55 CrossRef CAS.
- M. Fukuda, R. Sekiya and R. Kuroda, Angew. Chem., Int. Ed., 2008, 47, 706–710 CrossRef CAS PubMed.
- A. Mishra, A. Dubey, J. W. Min, H. Kim, P. J. Stang and K. W. Chi, Chem. Commun., 2014, 50, 7542–7544 RSC.
- W.-X. Gao, H.-J. Feng, Y.-J. Lin and G.-X. Jin, J. Am. Chem. Soc., 2019, 141, 9160–9164 CrossRef PubMed.
- H.-J. Feng, W.-X. Gao, Y.-J. Lin and G.-X. Jin, Chem.–Eur. J., 2019, 25, 14785–14789 CrossRef PubMed.
- H.-N. Zhang, W.-X. Gao, Y.-J. Lin and G.-X. Jin, J. Am. Chem. Soc., 2019, 141, 16057–16063 CrossRef CAS PubMed.
- J. J. Danon, A. Krüger, D. A. Leigh, J. F. Lemonnier, A. J. Stephens, I. J. Vitorica-Yrezabal and S. L. Woltering, Science, 2017, 355, 159–162 CrossRef CAS PubMed.
- Y. Inomata, T. Sawada and M. Fujita, Chem, 2020, 6, 294–303 Search PubMed.
- R. Zhu, J. Lübben, B. Dittrich and G. H. Clever, Angew. Chem., Int. Ed., 2015, 54, 2796–2800 CrossRef CAS PubMed.
- C. S. Wood, T. K. Ronson, A. M. Belenguer, J. J. Holstein and J. R. Nitschke, Nat. Chem., 2015, 7, 354–358 CrossRef CAS PubMed.
- J. Singh, D. H. Kim, E. H. Kim, N. Singh, H. Kim, R. Hadiputra, J. Jung and K. W. Chi, Chem. Commun., 2019, 55, 6866–6869 RSC.
- T. Sawada, Y. Inomata, K. Shimokawa and M. Fujita, Nat. Commun., 2019, 10, 5687 CrossRef CAS PubMed.
- J. Singh, D. H. Kim, E. H. Kim, H. Kim, R. Hadiputra, J. Jung and K. W. Chi, J. Am. Chem. Soc., 2020, 142, 9327–9336 CrossRef CAS PubMed.
- T. Feng, X. Li, Y. Y. An, S. Bai, L. Y. Sun, Y. Li, Y. Y. Wang and Y. F. Han, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202004112.
- L.-L. Dang, X. Gao, Y.-J. Lin and G.-X. Jin, Chem. Sci., 2020, 11, 1226–1232 RSC.
- M. Schmittel, A. Ganz and D. Fenske, Org. Lett., 2002, 4, 2290–2292 CrossRef PubMed.
- R. S. Forgan, D. C. Friedman, C. L. Stern, C. J. Bruns and J. F. Stoddart, Chem. Commun., 2010, 46, 5861–5863 RSC.
- J. K. Klosterman, J. Veliks, D. K. Frantz, Y. Yasui, M. Loepfe, E. Zysman-Colman, A. Linden and J. S. Siegel, Org. Chem. Front., 2016, 3, 661–666 RSC.
- M. C. Lipke, T. Cheng, Y. L. Wu, H. Arslan, H. Xiao, M. R. Wasielewski, W. A. Goddard and J. F. Stoddart, J. Am. Chem. Soc., 2017, 139, 3986–3998 CrossRef CAS PubMed.
- K. Cai, M. C. Lipke, Z. C. Liu, J. Nelson, T. Cheng, Y. Shi, C. Y. Cheng, D. K. Shen, J. M. Han, S. Vemuri, Y. N. Feng, C. L. Stern, W. A. Goddard, M. R. Wasielewski and J. F. Stoddart, Nat. Commun., 2018, 9, 5275 CrossRef PubMed.
- V. Vajpayee, Y. H. Song, T. R. Cook, H. Kim, Y. Lee, P. J. Stang and K. W. Chi, J. Am. Chem. Soc., 2011, 133, 19646–19649 CrossRef CAS PubMed.
- X.-Q. Lü, J.-J. Jiang, L. Zhang, C.-L. Chen, C.-Y. Su and B.-S. Kang, Cryst. Growth Des., 2005, 5, 419–421 CrossRef.
- Q.-X. Cheng, J.-J. Lan, Y.-Q. Chen, J. Lin, R. C. K. Reddy, X. M. Lin and Y.-P. Cai, Inorg. Chem. Commun., 2018, 96, 30–33 CrossRef CAS.
- M. Fourmigué and P. Batail, Chem. Rev., 2004, 104, 5379–5418 CrossRef PubMed.
- X. G. Yang, X. M. Lu, Z. M. Zhai, Y. Zhao, X. Y. Liu, L. F. Ma and S. Q. Zang, Chem. Commun., 2019, 55, 11099–11102 RSC.
- T. Kim, N. Singh, J. Oh, E. H. Kim, J. Jung, H. Kim and K. W. Chi, J. Am. Chem. Soc., 2016, 138, 8368–8371 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2002844 (1′), 2002845 (2), 2002846 (2′), 2002856 (2-pyrene) and 2002847 (3′) and 2002843 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02733b |
| This journal is © The Royal Society of Chemistry 2020 |