Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rhodium catalysed C-3/5 methylation of pyridines using temporary dearomatisation†
Alexandru
Grozavu
 a,
Hamish B.
Hepburn
a,
Hamish B.
Hepburn
 a,
Elliot P.
Bailey
a,
Peter J.
Lindsay-Scott
b and
Timothy J.
Donohoe
a,
Elliot P.
Bailey
a,
Peter J.
Lindsay-Scott
b and
Timothy J.
Donohoe
 *a
*a
aChemistry Research Laboratory, University of Oxford, Oxford, OX1 3TA, UK. E-mail: timothy.donohoe@chem.ox.ac.uk
bEli Lilly and Company, Erl Wood Manor, Windlesham, Surrey, GU20 6PH, UK
First published on 10th August 2020
Abstract
Pyridines are ubiquitous aromatic rings used in organic chemistry and are crucial elements of the drug discovery process. Herein we describe a new catalytic method that directly introduces a methyl group onto the aromatic ring; this new reaction is related to hydrogen borrowing, and is notable for its use of the feedstock chemicals methanol and formaldehyde as the key reagents. Conceptually, the C-3/5 methylation of pyridines was accomplished by exploiting the interface between aromatic and non-aromatic compounds, and this allows an oscillating reactivity pattern to emerge whereby normally electrophilic aromatic compounds become nucleophilic in the reaction after activation by reduction. Thus, a set of C-4 functionalised pyridines can be mono or doubly methylated at the C-3/5 positions.
Introduction
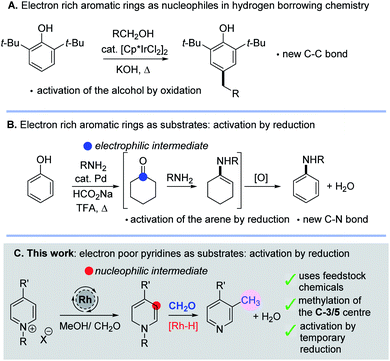
The idea of reversibly altering the oxidation state of a molecule to enable reactive intermediates to be formed transiently is an attractive one for constructing both C–C and C–N bonds; it is most commonly found in hydrogen borrowing methodology.1 Typically new bonds are formed by a sequence that entails selective oxidation of an alcohol, nucleophilic addition to a carbonyl, loss of water and subsequent reduction of the organic substrate. This method has become extremely useful for the alkylation of amines and carbonyl compounds using alcohols.1 Intriguingly, aromatic rings have also been shown to participate in hydrogen borrowing chemistry in a number of different ways. For example, electron-rich aromatic rings such as phenols or indoles can be utilized as nucleophiles towards aldehydes, themselves generated in situ by oxidation of an alcohol (Scheme 1A).2 Intriguingly, work by Li has also shown that phenols could become activated (by an arene reduction reaction), thus turning an electron rich arene into an electrophilic intermediate allowing subsequent C–N bond formation and ultimately reoxidation (Scheme 1B).3In this work, we have found new methodology which shows that electron deficient pyridine rings can also be activated by in situ reduction caused by metal hydride addition; in this case nucleophilic enamine intermediates are formed which enable new C–C bond formation. Pleasingly, this sequence leads to derivatisation of the C-3 position of the pyridine and so mono- and di-methylated pyridines are formed using methanol, formaldehyde and a transition metal catalyst (Scheme 1C). Note that the C-5-silylation of pyridines through a dearomatization/rearomatization sequence has been previously reported by Oestreich4 as has the alkylation of naphthyridines and quinolines with higher alcohols.5,6 In addition, there is a report on pyridine alkylation using methanol with Ni–Co ferrites at high temperatures (ca. 400 °C) in a down-flow vapour phase reactor.7
Interestingly, the temporary participation of dearomatised intermediates, formed via a reduction process involving hydride addition from a metal–H species, makes this approach complementary to the C–H activation and photocatalyst driven approaches that currently exist for pyridine functionalisation.8,9 The direct methylation of pyridines at C-3/5 is a valuable new reaction, and it is noted that the methylation of an aromatic ring is a fundamentally important process in areas ranging from DNA manipulation in vivo10 through to the magic methyl effect discovered in pharmaceutical research.11 Pyridine is the most common heteroaromatic motif found in small molecule FDA approved pharmaceutical drugs,12 making this methylation reaction all the more valuable.
Results and discussion
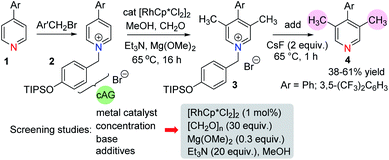
We began our work with an examination of 4-aryl pyridines because these substrates have previously been shown to participate in related catalytic reductive hydroxymethylation reactions.13 In order to provide electrophilic activation for the pyridine towards metal hydride reduction (Scheme 2) it was necessary to quaternarise the nitrogen with a benzyl halide. Therefore, we developed a cleavable activating group (cAG) that can be easily installed before methylation and removed at the end of the reaction simply by the addition of fluoride. This protocol avoids the difficult purification of pyridinium salts and allows isolation of methylated pyridines 4 in one pot. Taking the conditions required for the reductive hydroxymethylation of 4-aryl pyridines as a starting point,13 the variables that we examined in an extensive screening exercise (see ESI, Tables S1–S4†) were the following: (i) metal catalyst – here we found that Rh was consistently better at promoting methylation than other metals; (ii) dilution – running the reaction at a dilute concentration (0.1 M) was beneficial; (iii) base – the addition of a magnesium alkoxide base, together with an added amine gave the highest yields of methylation products; (iv) additives – generally, the addition of additives such as iodide14 was detrimental to the methylation reaction, although iodide did provide a small increase in yields when very electron deficient pyridines were used.In these early studies we were not able to optimise the reaction to form the C-3 mono methyl pyridine because the reaction always gave significant amounts of C-3,5-dimethylated derivatives. Therefore, we found it beneficial to run the reaction to full conversion so that the dimethylated arene could be isolated cleanly.
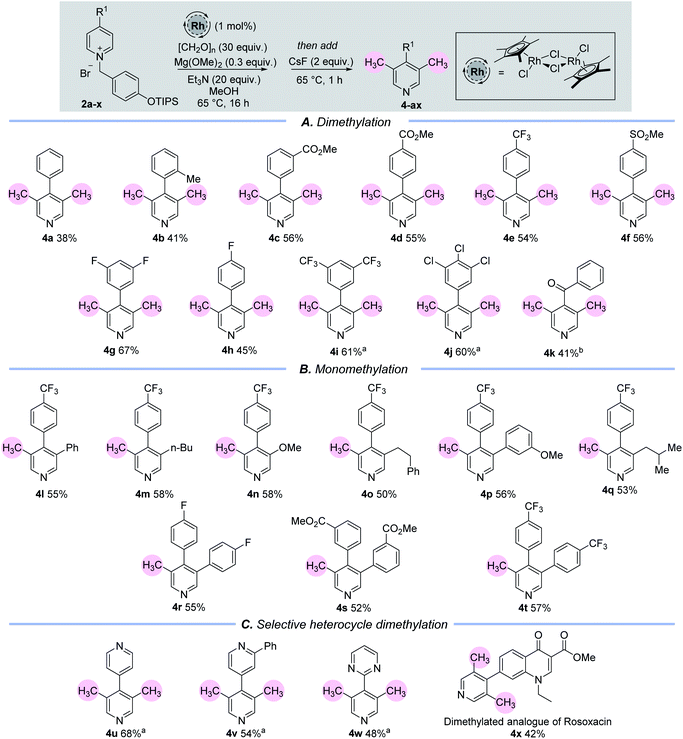
Once a set of suitable conditions were identified, we examined the C-3/5 dimethylation of a set of C-4 substituted pyridine rings (Table 1(A)). 4-Aryl substituted pyridines containing electron neutral or electron withdrawing aryl groups are well tolerated under this methodology (4a–4j) and C-4 keto-substituted substrates are also viable (4k). In terms of scope, the current methodology allows for a substitution pattern that is complementary to those already established in the literature,15 and produces a set of 3,4,5-substituted pyridines. Importantly, it allows for the instalment of a methyl group adjacent to a non-coordinating substituent. Although the yields of this reaction range from 38–68%, this is simply a reflection of the significant number of chemical steps involved, vide infra. The remaining mass from these experiments comprised of a complex mixture of unidentified compounds.
Experiments showed that the C-4 position of the pyridine must be substituted because otherwise multi-component mixtures were formed, which we attribute to over addition by the metal hydride at the unencumbered C-4 position. Moreover, we examined other C-4 substituents in addition to aryl, keto and heteroaryl groups. However, in each case the reaction was unsuccessful because of presumed deprotonation in situ (C-4 alkyl) or SNAr processes (C-4 heteroatom), see ESI.†
Pleasingly, when the 3-position of the starting pyridine is already substituted with electron rich or electron deficient groups, for example with an alkyl, aryl or ether group (1l–1t), then mono-methylation takes place at the C-5 position to generate the 3,4,5-substituted pyridines, 4l–4t (Table 1(B)).
We also sought to utilise the selective cAG activation of a pyridine nitrogen in the presence of other heteroarenes to enable selective catalytic arene methylation (Table 1(C)). In the first instance, this was accomplished through the selective mono-alkylation of symmetrical bis-pyridine, which then delivered product 4u whereby only one pyridine ring had been doubly methylated in the presence of another. Steric and electronic effects can also be exploited to allow differentiation between different heteroarene N atoms. Note the selective activation and dimethylation sequence leading to the bis-arenes 4v and 4w in which the deactivating effects of an ortho-Ph group and a pyrimidine nitrogen were used to deter installation of the cAG and subsequent methylation. Finally, a more complex example lay in the selective activation and dimethylation of the ester of rosoxacin,16 a prescription antibiotic compound. The selective formation of 4x shows clearly that this methodology has significant potential for the selective and late-stage introduction of one, or two, methyl groups onto pharmaceutically important molecules.
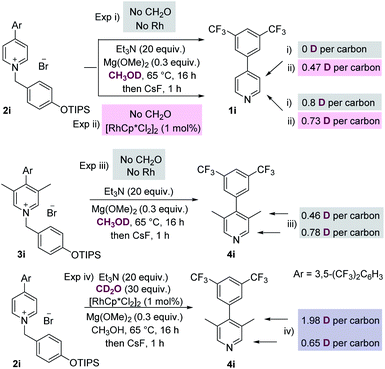
We then conducted a number of control and labelling experiments in order to probe the reaction mechanism (Scheme 3). As expected, the presence of both rhodium catalyst and formaldehyde were essential for the methylation process (see ESI† for further details). The results given in labelling experiment (i), Scheme 3, show that proton exchange at the C-2 position of starting material 2i occurs in the presence of base, presumably via a benzylic deprotonation/reprotonation process. Interestingly, the presence of rhodium catalyst (see experiment (ii)) also promoted exchange at the C-3 position of the recovered starting material 1i.
Labelling experiment (iii) was conducted on the initial product of the methylation reaction 3i, and reveals that the C-3 methyl groups of this product are themselves susceptible to proton exchange with the protic solvent by acid/base reactions, which complicates analysis. Finally, experiment (iv) which isolated and analysed the methylated pyridine product, shows that most of the deuterium present in added CD2O is present at the C-3 methyl group, which is consistent with this reagent being the source of the methyl group itself.
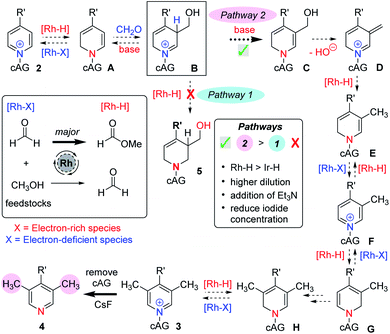
Our earlier work on the reductive hydroxymethylation of similar pyridines using iridium catalysis13 had investigated the in situ formation of metal hydrides via oxidation of the feedstocks formaldehyde and methanol,17 together with reversible Ir–H addition to the C-2 position of a pyridinium ion. Using these basic steps and the mechanistic experiments described above, we now propose the following mechanism (Scheme 4).
We think that a key intermediate in this sequence is iminium salt B, which in our earlier work was reduced to the dearomatised product 5 in situ by a metal hydride species (Pathway 1). We reason that by diverting the electrophilic intermediate B from this iminium reductive process and favouring a deprotonation instead, we have followed a pathway that regains aromaticity later on (Pathway 2). Starting from pyridinium 2 and following the mechanism (Pathway 2, Scheme 4), it can be seen that partial and temporary transition metal mediated dearomatisation of the pyridine (see A), followed by reaction with formaldehyde, which is conceivably activated by the magnesium cation, (B), deprotonation (C), loss of water (D), exocyclic reduction by metal hydride (E) and rearomatisation would lead to C-3 methylated pyridine derivative F. Note that it is also possible for the transformation of D→F to involve a transition metal free tautomerisation process.13b
When both C-3 positions are free on the starting pyridine, we suggest that there is an iterative process that leads to the formation of a C-3,5 dimethylated pyridinium 3 (see F→3). At this point any Rh–H catalysed dearomatisation of the product (3) would lead to sterically hindered enamine H, which does not react with formaldehyde and instead can revert back to pyridinium 3.
This mechanism is consistent with the labelling experiments shown in Scheme 3. In particular, the exchange at C-3 promoted by the rhodium catalyst (see experiment (ii), Scheme 3) can be rationalised by invoking oxidation of methanol (no CH2O is present) by the rhodium catalyst, and the Rh–H species thus generated adding reversibly to the pyridinium C-2 position forming intermediate A (see Scheme 4). Intermediate A, in the absence of added excess formaldehyde electrophile, can then exchange deuterium at C-3 by reversible deuteration from the CH3OD solvent.
Finally, the relatively high amount of D incorporated at C-2 in experiment (iv), Scheme 3, is also consistent with reversible Rh–D (this time originating from the oxidation of CD2O) addition at C-2, which competes with the background deprotonation/reprotonation from CH3OH.
Conclusions
In conclusion, we have developed a set of mild catalytic conditions that allow di- and mono-methylation of pyridines at the relatively unreactive C-3 and C-5 positions. This reaction utilises readily available formaldehyde as the source of the methyl group and does not require a directing group. Moreover, the selective activation and methylation of multi-arene ring systems has also been demonstrated and this holds many opportunities for use in medicinal chemistry lead optimisation programmes in both academia and industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge funding from Eli-Lilly, the EPSRC (EP/L023121/1), and the European Commission (Marie Skłodowska-Curie Actions fellowship: 792562 H2020-MSCA-IF-2017). We also acknowledge the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1), generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB, and Vertex.Notes and references
- For reviews of hydrogen borrowing catalysis: (a) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS; (b) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed; (c) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853–1864 CrossRef; (d) S. Pan and T. Shibata, ACS Catal., 2013, 3, 704–712 CrossRef CAS; (e) C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed; (f) J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Angew. Chem., Int. Ed., 2014, 53, 9142–9150 CrossRef CAS; (g) Y. Obora, ACS Catal., 2014, 4, 3972–3981 CrossRef CAS; (h) Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305–2329 RSC; (i) A. Nandakumar, S. P. Midya, V. G. Landge and E. Balaraman, Angew. Chem., Int. Ed., 2015, 54, 11022–11034 CrossRef CAS; (j) J. Leonard, A. J. Blacker, S. P. Marsden, M. F. Jones, K. R. Mulholland and R. Newton, Org. Process Res. Dev., 2015, 19, 1400–1410 CrossRef CAS; (k) A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed; (l) M. Holmes, L. A. Schwartz and M. J. Krische, Chem. Rev., 2018, 118, 6026–6052 CrossRef CAS PubMed; (m) B. G. Reed-Berendt, K. Polidano and L. C. Morrill, Org. Biomol. Chem., 2019, 17, 1595–1607 RSC; (n) T. Kwok, O. Hoff, R. J. Armstrong and T. J. Donohoe, Chem.–Eur. J., 2020 DOI:10.1002/chem.202001253.
- See (a) Y. Li, T. Yan and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 10476–10480 CrossRef CAS PubMed; (b) S. -J. Chen, G. -P. Lu and C. Cai, RSC Adv., 2015, 5, 70329 RSC; (c) J. R. Frost, C. B. Cheong and T. J. Donohoe, Synthesis, 2017, 49, 910–916 CAS; (d) S. M. A. H. Siddiki, A. S. Touchy, M. A. R. Jamil and T. Toyao, ACS Catal., 2018, 8, 3091–3103 CrossRef CAS.
- (a) Z. Chen, H. Zeng, S. A. Girard, F. Wang, N. Chen and C.-J. Li, Angew. Chem., Int. Ed., 2015, 54, 14487–14491 CrossRef CAS PubMed; (b) T. Cuypers, P. Tomkins and D. E. De Vos, Catal. Sci. Technol., 2018, 8, 2519–2523 RSC; (c) Z. Qiu, L. Lv, J. Li, C.-C. Li and C.-J. Li, Chem. Sci., 2019, 10, 4775–4781 RSC; (d) A. Dominquez-Huerta, I. Perepichka and C.-J. Li, ChemSusChem, 2019, 12, 2999–3002 CrossRef PubMed; (e) P. Tomkins, C. Valgaeren, K. Adriaensen, T. Cuypers and D. E. De Vos, J. Catal., 2019, 371, 207–213 CrossRef CAS.
- S. Wübbolt and M. Oestreich, Angew. Chem., Int. Ed., 2015, 54, 15876–15879 CrossRef PubMed.
- B. Xiong, S. Zhang, H. Jiang and M. Zhang, Org. Lett., 2016, 18, 724–727 CrossRef CAS PubMed.
- (a) F. Xie, R. Xie, J. X. Zhang, H. F. Jiang, L. Du and M. Zhang, ACS Catal., 2017, 7, 4780–4785 CrossRef CAS; (b) Z. Tan, C. Ci, J. Yang, L. Cao, H. Jiang and M. Zhang, ACS Catal., 2020, 10, 5243–5249 CrossRef CAS.
- K. Sreekumar, T. Mathew, R. Rajgopal, R. Vetrivel and B. S. Roa, Catal. Lett., 2000, 65, 99–105 CrossRef CAS.
- (a) L. Yang, N. Uemura and Y. Nakao, J. Am. Chem. Soc., 2019, 141, 7972–7979 CrossRef CAS PubMed; for related C–H activation reactions see, (b) C. Li, S.-M. Wang and H.-L. Qin, Org. Lett., 2018, 20, 4699–4703 CrossRef CAS PubMed; (c) S.-M. Wang, B. Moku, J. Leng and H.-L. Qin, Eur. J. Org. Chem., 2018, 32, 4407–4410 CrossRef; (d) S.-M. Wang, C. Li, J. Leng, S. N. A. Bukhari and H.-L. Qin, Org. Chem. Front., 2018, 5, 1141–1415 Search PubMed.
- R. S. J. Proctor, H. J. Davis and R. J. Phipps, Science, 2018, 360, 419–422 CrossRef CAS PubMed.
- Y. Umezawa and M. Nishio, Nucleic Acids Res., 2002, 30, 2183–2192 CrossRef CAS PubMed.
- H. Schönherr and T. Cernak, Angew. Chem., Int. Ed., 2013, 52, 12256–12267 CrossRef PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- (a) A. Grozavu, H. B. Hepburn, P. J. Smith, H. K. Potukuchi, P. J. Lindsay-Scott and T. J. Donohoe, Nat. Chem., 2019, 11, 242–247 CrossRef CAS PubMed; (b) B. M. Reeves, H. B. Hepburn, A. Grozavu, P. J. Lindsay-Scott and T. J. Donohoe, Angew. Chem., Int. Ed., 2019, 58, 15697–15701 CrossRef CAS PubMed; (c) H. B. Hepburn and T. J. Donohoe, Chem.–Eur. J., 2020, 26, 1963–1967 CrossRef CAS PubMed.
- (a) J. Wu, W. Tang, A. Pettman and J. Xiao, Adv. Synth. Catal., 2013, 355, 35–40 CrossRef CAS; (b) J. Wu, C. Wang, W. Tang, A. Pettman and J. Xiao, Chem.–Eur. J., 2012, 18, 9525–9529 CrossRef CAS.
- For representative examples see, (a) J. Jin and D. W. C. MacMillan, Nature, 2015, 525, 87–90 CrossRef CAS PubMed; (b) J. Tauber, D. Imbri and T. Opatz, Molecules, 2014, 19, 16190–16222 CrossRef PubMed; (c) D. A. DiRocco, K. Dykstra, S. Krska, P. Vachal, D. V. Conway and M. Tudge, Angew. Chem., Int. Ed., 2014, 53, 4802–4806 CrossRef CAS PubMed.
- G. S. Bisacchi, J. Med. Chem., 2015, 58, 4874–4882 CrossRef CAS PubMed.
- B. Sam, B. Breit and M. J. Krische, Angew. Chem., Int. Ed., 2015, 54, 3267–3274 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization data for new compounds. See DOI: 10.1039/d0sc02759f |
| This journal is © The Royal Society of Chemistry 2020 |