Open Access Article
Open Access ArticleHelix-constructed polar rare-earth iodate fluoride as a laser nonlinear optical multifunctional material†
Guang
Peng
 ab,
Yi
Yang
c,
Tao
Yan
*ab,
Dan
Zhao
ab,
Yi
Yang
c,
Tao
Yan
*ab,
Dan
Zhao
 d,
Bingxuan
Li
ab,
Ge
Zhang
ab,
Zheshuai
Lin
d,
Bingxuan
Li
ab,
Ge
Zhang
ab,
Zheshuai
Lin
 c and
Ning
Ye
c and
Ning
Ye
 *ab
*ab
aKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: nye@fjirsm.ac.cn; yantao@fjirsm.ac.cn
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350002, China
cBeijing Center for Crystal R&D, Key Lab of Functional Crystals and Laser Technology of Chinese Academy of Sciences, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
dCollege of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo, Henan 454000, China
First published on 23rd June 2020
Abstract
The first trivalent rare-earth iodate fluoride nonlinear optical (NLO) crystal, Y(IO3)2F (YIF), was successfully designed and synthesized, featuring polarization-favorable helical chains constructed from trans-YO6F2 polyhedra and IO3 groups. It exhibited a suitable balance of a wide transparency range of 0.26–10.0 μm, high laser damage threshold (LDT) of 39.6 × AgGaS2, and moderate second harmonic generation (SHG) effect of 2 × KDP. A series of doped RE:YIF (RE = Pr, Nd, Dy, Ho, Er, Tm, and Yb) crystals were easily synthesized benefiting from the spring-shaped helix structure, which possess wide absorption and emission peaks as well as long lifetime, especially in the visible and near-infrared regions. Particularly, the remarkable fluorescence properties of Nd and Yb doped YIF crystals are comparable to and even better than those of traditional self-frequency doubling (SFD) crystals such as YAB, YCOB, and GdCOB. Thus, these RE-doped YIF crystals are promising laser SFD crystals. This work also indicated that constructing helical chains should be an effective strategy for the design of inorganic polar materials.
A laser self-frequency doubling (SFD) crystal is a type of excellent multifunctional crystal, which has both the stimulated emission function of a laser crystal and the second harmonic generation (SHG) properties of a nonlinear optical (NLO) crystal, so that it can achieve functions such as self-activation and SFD.1 By using laser SFD crystals, all-solid-state lasers can achieve advantages such as a small size and compact structure, low cost, easy adjustability, high stability, and so on. Generally, a laser SFD crystal is composed of a host crystal and one or more types of active ions which mainly include trivalent lanthanide rare-earth ions except those with a full-shell or semi-full-shell (Sc3+, Y3+, La3+, Lu3+, and Gd3+) and some transition ions.2 However, only a few host crystals are practically available at present, mainly including LiNbO3 (LN), YAl3(BO3)4 (YAB), YCa4O(BO3)3 (YCOB), GdCa4O(BO3)3 (GdCOB), etc.3 Therefore, there is still an urgent demand to explore new laser NLO multifunctional crystals.
A laser SFD host crystal must be a NLO crystal with a noncentrosymmetric (NCS) or polar structure and easily doped with active ions. However, it is known that minerals and synthetic crystals tend to crystallize into centrosymmetric (CS) structures. It is still of great technical interest but challenging to effectively synthesize polar crystals.
To accelerate the discovery of new NLO materials, the introduction of the largest electronegative element F into oxysalts such as borates, carbonates, nitrates, and phosphates has been recognized as an effective strategy.4 Besides, F can usually improve crystals' optical properties such as broadening the band gaps and enlarging laser damage thresholds (LDTs). In recent years, metal iodate fluorides have become a research hotspot. Several metal iodate fluoride NLO crystals have been reported, mainly including Bi(IO3)F2,5 Bi3OF3(IO3)4,6 ABi2(IO3)2F5 (A = K, Rb, Cs, NH4),7 β-Ba[VFO2(IO3)2],8 α/β-Ba2[VO2F2(IO3)2]IO3,8 α/β-Ba2[GaF4(IO3)2](IO3),9 K5(W3O9F4)(IO3),10 CsVO2F(IO3),11 Ce(IO3)2F2·H2O,12 Sn(IO3)2F2,13etc. These metal iodate fluoride NLO crystals generally exhibit a wide transparent range covering the visible to mid-IR region, large SHG effects, and a high laser damage threshold (LDT). Notably, a trivalent rare-earth iodate fluoride has not been reported yet. Considering the varied and flexible coordination patterns of rare-earth ions, and that crystals containing trivalent rare-earth ions are often easily doped with other rare-earth active ions for excitation luminescence, it is meaningful to explore the potential trivalent rare-earth iodate fluoride photoelectric functional materials.
In this study, we have successfully designed and synthesized the first trivalent rare-earth iodate fluoride, Y(IO3)2F (YIF), which exhibited a good balance of a wide UV transmission range, high LDT and relatively large SHG effect. In particular, active ion doped RE:YIF (RE = Pr, Nd, Dy, Ho, Er, Tm, and Yb) crystals were also synthesized easily, showing wide absorption and emission peaks as well as long lifetime, especially in the visible and near-infrared regions. Specifically, the remarkable fluorescence properties of Nd and Yb doped YIF crystals are superior to those of several popular commercial laser SFD crystals such as YAB, YCOB, and GdCOB, indicating their potential application as laser SFD crystals. Most importantly, the novel helical structure will provide important guidance for the design of polar inorganic NLO crystal materials. Herein, we will report on the synthesis, crystal structure, and linear and nonlinear optical properties of YIF, as well as the fluorescence performance of RE doped YIF.
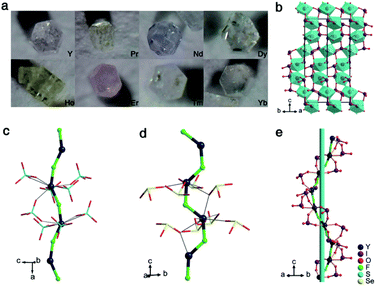
The YIF and RE-doped YIF crystals (Fig. 1a) were all synthesized by a mild hydrothermal method at a temperature of 240 °C. As plotted in Fig. S1 in the ESI,† powder X-ray diffraction (XRD) analysis confirmed the purity of YIF and the structure-nondestructive doping of RE doped YIF. The energy dispersive X-ray spectroscopy (EDS) analysis confirmed the existence of Y, I, O, and F elements in YIF (Fig. S2†). The synthesized crystals exhibited good chemical stability. Thermogravimetric analysis (TGA) showed that the YIF was thermally stable up to about 380 °C (Fig. S3†). The weight loss of 73.5% (cal. 72.9%) at around 380–1100 °C is attributed to the release of I2O5, and the final residue at 1100 °C was confirmed to be YOF by powder XRD (Fig. S4†).
YIF crystallizes in the hexagonal polar space group P65 (the detailed crystallographic data are listed in Tables S1–S4 in the ESI†), and it has one Y atom, one F atom, two I atoms, and six O atoms in an asymmetric unit. The bond valence calculations14 revealed the valence states of 5.13 or 5.46 for I5+ and 3.08 for Y3+, being consistent with their ideal valence. Unlike most reported metal iodate fluorides or other Y-containing oxysalt fluorides, the YIF features a novel spring-shaped one-dimensional (1D) helical chains constructed from F-bridged trans-YO6F2 polyhedra with IO3 groups hung on (Fig. 1b and e). The bond lengths of Y–O, Y–F, and I–O bonds are varied in the region of 2.33(2)–2.55(2), 2.14(2)–2.22(2), and 1.77(2)–1.84(2) Å, respectively. These 1D helical chains are further connected to each other into a 3D structure by sharing IO3, and the dangling bonds on IO3 groups are therefore eliminated, which is favorable for the enlargement of the band gap. Benefitting from the polarization-induction of the helical chains, the IO3 groups were configured to a polar arrangement. The local dipole moment of an individual IO3 group was calculated to be 14.62–14.90 D (Debye), which is consistent with other iodates.5–13 The x and y components from the twelve IO3 groups within one unit cell cancel each other out completely, while the z components add up to a net dipole moment of −3.13 D, indicating the formation of a polar arrangement.
Notably, the helical structure has received extensive attention in the field of metal organic coordination chemistry but rarely been mentioned in the field of inorganic NLO crystals.15 Therefore, it will be meaningful and interesting to study the helical chain formation of YIF by comparing with Y(SO4)F (Pnma),16 Y(SeO3)F (P21/c),17 and NaYF(PO4) (C2/m).18 All three crystals possess YO6F2 polyhedra and CS space groups. The YO6F2 polyhedra in NaYF(PO4) are cis which hinder the formation of single chains. As for Y(SO4)F and Y(SeO3)F, their trans-YO6F2 polyhedra promote the formation of single YF(SO4) and YF(SeO3) chains. Unfortunately these chains are all centric linear chains, instead of helical, which then induce SHG-groups (SO4 and SeO3) packed with inversion centers (Fig. 1c and d). It can be inferred that the trans-YO6F2 polyhedra, rather than the cis-YO6F2 polyhedra, are essential for the formation of single chains. In YIF, the density of Y (6.78 × 10−3 Å3) is much smaller than those of Y(SO4)F (10.87 × 10−3 Å3) and Y(SeO3)F (12.45 × 10−3 Å3). The lower density means that the space to be filled between Y3+ is larger, and the formation of the helical chains are conducive to tight filling. Meanwhile, the IO3 groups with a lower valence are more favorable to achieve the charge balance of the framework with fewer positive charges but more lacunae. The molar ratio of the SHG-groups to Y may be a simple but effective parameter to guide the synthesis of polar crystals with helical chains. Obviously, the value of the IO3/Y molar ratio in YIF is 2, which is twice that of Y(SO4)F and Y(SeO3)F. Hence, we deem that trans-YO6F2 polyhedra, SHG-groups with a lower valence, and a large molar ratio of SHG-groups to Y are all positive factors for helical chain formation. This rule may be applied to other cationic systems.
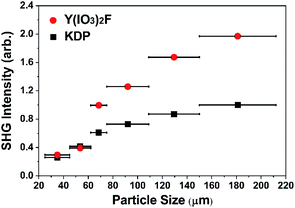
The polar structure of YIF prompted us to study its SHG properties under 1064 nm laser radiation via the Kurtz–Perry method.19 As shown in Fig. 2, the YIF exhibited a moderate SHG response of 2.0 × KDP (particle size 150–212 μm) and a type-I phase-matching behavior. Because the SHG response is proportional to the square of the effective nonlinear coefficient, the derived effective NLO coefficient for YIF is 0.55 pm V−1 according to that of KDP (d36 = 0.39 pm V−1).
The UV-vis-NIR diffuse reflectance and IR spectra showed that YIF was transparent over a broad spectrum range from about 0.26 to 10.0 μm (Fig. S5 and S6†), which covered the important entire mid-infrared range of atmospheric windows (3–5 μm) and half of the thermal infrared atmospheric window (8–13 μm). The experimental optical band gap of YIF was 3.91 eV (Fig. S7†), which is not only much larger than that of the commercialized IR NLO crystal AgGaS2 (2.62 eV),20 but also comparable to those of outstanding Bi(IO3)F2 (3.97 eV), Bi3OF3(IO3)4 (3.7 eV), ABi2(IO3)2F5 (A = K, Rb, Cs, NH4) (3.75–3.88 eV), K5(W3O9F4)(IO3) (3.83 eV), β-Ba2[GaF4(IO3)2](IO3) (4.35 eV), and Sn(IO3)2F2 (4.08 eV).
Because of the large optical band gap, YIF was inferred to possess a large LDT. Therefore, a powder LDT measurement for YIF was performed together with AgGaS2 as a reference under the same conditions (1064 nm, 10 ns, and 10 Hz). The results showed that YIF indeed possessed a large LDT value of 87.61 MW cm−2, which is about 39.6 times that of AgGaS2 (2.21 MW cm−2). It is noteworthy that this value is also larger than those of α/β-Ba2[GaF4(IO3)2](IO3) (29.7 and 28.3 × AgGaS2, respectively), despite their larger band gaps. Thus, we deem that both F and helical chains may be conducive to improving the LDT.
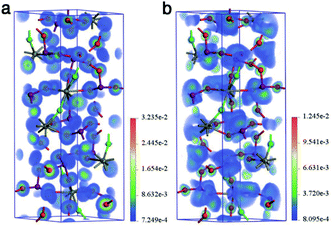
First-principles calculations for YIF based on density functional theory (DFT) were performed to elucidate the relationship between the electronic structure and optical properties.21 The calculated band gap was 3.50 eV (Fig. S8†), inherently underestimated by the GGA-PBE functionals.21c A scissor correction22 of 0.41 eV was adopted in the subsequent calculations of linear and NLO properties. The partial densities of states (PDOS) of YIF showed that the top of valence band (VB) was mainly occupied by O-2p orbitals, and the bottom of conduction band (CB) was mainly occupied by I-5p and O-2p orbitals (Fig. S9†). It indicates that the electronic behavior in IO3 groups dominates the linear and NLO properties. According to the restriction of Kleinman's symmetry, YIF has two nonzero independent SHG coefficient tensors. The calculated values were d31 = −0.64 pm V−1 and d33 = 2.04 pm V−1 at 1064 nm (1.165 eV), respectively. For compounds in the 6 point group, the effective tensor is d31, which is why our experimental value is consistent with the smaller d31. The SHG-weighted charge density analysis tool and the real-space atom-cutting technique were adopted to further intuitively and quantificationally evaluate the SHG-contribution of IO3 groups and YO6F2 polyhedra.23 The SHG-weighted electron densities of the occupied and unoccupied states in the virtual electron (VE) process have been calculated for the VE process dominantly contributing to the overall SHG effects. As shown in Fig. 3a, the SHG-weighted electron density of the occupied states is mainly concentrated on O2−. In the unoccupied states (Fig. 3b), the orbitals on both O2− and I5+ were the major source of SHG-weighted electron densities. It means that the IO3 groups have made the dominant contribution to the whole SHG effects. This is also confirmed by the real-space atom-cutting technique (see Table S5†), where the contribution of IO3 groups (−0.83 pm V−1) to the SHG coefficient d31 was very large, while the YO6F2 polyhedra made a negligible negative contribution (0.07 pm V−1). Besides, the birefringence was calculated to be 0.041 at a wavelength of 1064 nm, which is large enough for satisfy the phase-matching condition at 1064 nm. And the atom-cutting result also exhibited the dominant contribution of IO3 groups to the birefringence.
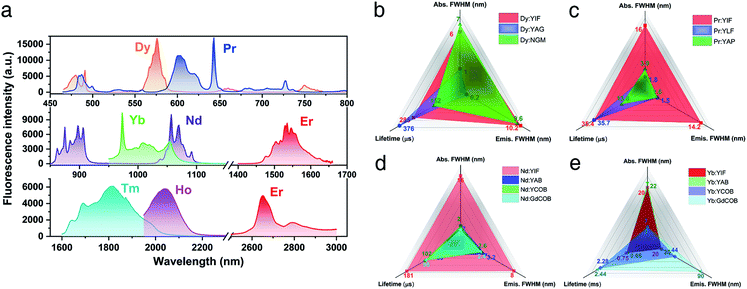
A series of RE (RE = Pr, Nd, Dy, Ho, Er, Tm, and Yb) doped YIF crystals were synthesized in order to confirm its great potential for laser application. The synthesis process is quite easy, which should be attributed to the high compatibility of the spring-shaped helix structure with doped ions with different ionic radii and concentrations. Fig. 4 shows the fluorescence spectra of RE doped YIF crystals at room temperature. The detailed spectral properties are shown in Table S6,† including the main absorption peak and width, the typical emission peak and width, and the fluorescence lifetime. As demonstrated in Fig. 4b and c, the full width at half maximum (FWHM) of absorption and emission peaks for Dy:YIF was much wider than that of the traditional laser crystal Y3Al5O12 (YAG)24 and nearly the same as that of the newly developed Na2Gd4(MoO4)7 (NGM) crystal.25 And the Pr:YIF sample exhibited a very broad absorption and emission peak in comparison with commercial YLiF4 (YLF)26 and YAlO3 (YAP)27 crystals. Besides, the fluorescence lifetime for both Dy and Pr doped YIF were comparable to that of the aforementioned crystals. Therefore, both Dy and Pr doped YIF are very promising laser gain media in the visible range. As for the Nd:YIF sample (Fig. 4d), its fluorescence properties far exceeded those of the well-known traditional self-frequency-doubling crystals YAB, YCOB and GdCOB, including much broader absorption and emission peaks as well as longer lifetime.28 As plotted in Fig. 4e, the fluorescence properties of Yb:YIF were nearly the same as those of Yb:YAB. Besides, the NLO effect of YIF was close to those of the abovementioned crystals (1.1–1.3 pm V−1).29 Therefore, it can be inferred that both Nd and Yb doped crystals would be new remarkable SFD crystals in the near-infrared region. With regard to the samples doped with Er, Tm and Ho, one can see the significant feature of broadening absorption and emission peaks, which should be attributed to the varied and flexible coordination patterns of rare earth ions in RE:YIF. In brief, from the viewpoint of fluorescence, YIF is an excellent laser SFD host crystal particularly in the field of the Dy and Pr activated visible region as well as Nd and Yb activated near-infrared region.
Conclusions
In conclusion, via the introduction of F−, we successfully obtained the first trivalent rare-earth iodate fluoride nonlinear optical (NLO) crystal Y(IO3)2F (YIF). It featured unusual helical chains constructed from trans-YO6F2 polyhedra and IO3 groups, which are favorable for polar arrangement of the IO3 groups. Apart from the suitable balance of a wide transparency range of 0.26–10.0 μm, high laser damage threshold (LDT) of 39.6 × AgGaS2, and moderate second harmonic generation (SHG) effect of 2 × KDP, YIF showed great potential as a laser self-frequency doubling (SFD) host crystal. Benefiting from the spring-shaped helix structure, a series of doped RE:YIF (RE = Pr, Nd, Dy, Ho, Er, Tm, and Yb) crystals were easily synthesized, which possessed wide absorption and emission peaks as well as long lifetime, especially in the visible and near-infrared regions. Particularly, compared with those famous laser and SFD crystals including YAG, NGM, YLF, and YAP as well as YAB, YCOB, and GdCOB, the Dy, Pr, Nd and Yb doped YIF all exhibited more remarkable and comparable fluorescence properties. Hence, it can be concluded that YIF is a promising NLO and SFD host multifunctional crystal. Besides, constructing helical chains should be an effective strategy for the design of inorganic polar materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (51890862, 21921001, U1605245, 51761135115, and 51902308), the National Key Re-search and Development Plan of Ministry of Science and Technology (2016YFB0402104), the Strategic Priority Re-search Program of the Chinese Academy of Sciences (XDB20000000), and the China Postdoctoral Science Foundation (2019M652267).Notes and references
- (a) J. H. Du, J. Y. Wang, H. H. Yu and H. J. Zhang, Opt. Lett., 2020, 45, 327–330 CrossRef CAS; (b) M. Kowalczyk, X. Z. Zhang, X. Mateos, S. Y. Gao, Z. P. Wang, X. G. Xu, P. Loiko, F. Rotermund, J. Sotor, U. Griebner and V. Petrov, Opt. Express, 2019, 27, 590–596 CrossRef CAS PubMed.
- A. A. Kaminskii, Laser Photonics Rev., 2007, 1, 93–177 CrossRef CAS.
- (a) D. Z. Wang, D. H. Sun, X. L. Kang, Y. H. Sang, B. X. Yan, H. Liu and Y. Bi, Opt. Express, 2015, 23, 17727–17738 CrossRef CAS PubMed; (b) P. Dekker, J. M. Dawes, J. A. Piper, Y. G. Liu and J. Y. Wang, Opt. Commun., 2001, 195, 431–436 CrossRef CAS; (c) F. Khaled, P. Loiseau, G. Aka and L. Gheorghe, Opt. Lett., 2016, 41, 3607–3610 CrossRef CAS PubMed.
- (a) D. Cyranoski, Nature, 2009, 457, 953–955 CrossRef CAS PubMed; (b) T. T. Tran, H. Yu, J. M. Rondinelli, K. R. Poeppelmeier and P. S. Halasyamani, Chem. Mater., 2016, 28, 5238–5258 CrossRef CAS; (c) M. Mutailipu and S. Pan, Angew. Chem., Int. Ed., 2019 DOI:10.1002/anie.201913974; (d) G. Peng, N. Ye, Z. S. Lin, L. Kang, S. L. Pan, M. Zhang, C. S. Lin, X. F. Long, M. Luo, Y. Chen, Y. H. Tang, F. Xu and T. Yan, Angew. Chem., Int. Ed., 2018, 57, 8968–8972 CrossRef CAS PubMed; (e) S. Zhao, L. Kang, Y. Shen, X. Wang, M. A. Asghar, Z. Lin, Y. Xu, S. Zeng, M. Hong and J. Luo, J. Am. Chem. Soc., 2016, 138, 2961–2964 CrossRef CAS PubMed; (f) H. Liu, Y. Wang, B. Zhang, Z. Yang and S. Pan, Chem. Sci., 2020, 11, 694–698 RSC; (g) H. Wu, H. Yu, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215–4218 CrossRef CAS PubMed; (h) G. Zou, N. Ye, L. Huang and X. Lin, J. Am. Chem. Soc., 2011, 133, 20001–20007 CrossRef CAS PubMed; (i) T. T. Tran, J. Young, J. M. Rondinelli and P. S. Halasyamani, J. Am. Chem. Soc., 2017, 139, 1285–1295 CrossRef CAS PubMed; (j) L. Kang, S. Luo, H. Huang, N. Ye, Z. Lin, J. Qin and C. Chen, J. Phys. Chem. C, 2013, 117, 25684–25692 CrossRef CAS; (k) G. Peng, Y. Yang, Y.-H. Tang, M. Luo, T. Yan, Y. Zhou, C. Lin, Z. Lin and N. Ye, Chem. Commun., 2017, 53, 9398–9401 RSC; (l) E. J. Cho, S.-J. Oh, H. Jo, J. Lee, T.-S. You and K. M. Ok, Inorg. Chem., 2019, 58, 2183–2190 CrossRef CAS PubMed; (m) J. Lu, J.-N. Yue, L. Xiong, W.-K. Zhang, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2019, 141, 8093–8097 CAS; (n) B. Zhang, G. Han, Y. Wang, X. Chen, Z. Yang and S. Pan, Chem. Mater., 2018, 30, 5397–5403 CrossRef CAS.
- F. Mao, C.-L. Hu, X. Xu, D. Yan, B.-P. Yang and J.-G. Mao, Angew. Chem., Int. Ed., 2017, 129, 2183–2187 CrossRef.
- M. Zhang, X. Su, M. Mutailipu, Z. Yang and S. Pan, Chem. Mater., 2017, 29, 945–949 CrossRef CAS.
- (a) H. Liu, Q. Wu, X. Jiang, Z. Lin, X. Meng, X. Chen and J. Qin, Angew. Chem., Int. Ed., 2017, 56, 9492–9496 CrossRef CAS PubMed; (b) H. Fan, C. Lin, K. Chen, G. Peng, B. Li, G. Zhang, X. Long and N. Ye, Angew. Chem., Int. Ed., 2020, 59, 5268–5272 CrossRef CAS PubMed.
- H. Yu, M. L. Nisbet and K. R. Poeppelmeier, J. Am. Chem. Soc., 2018, 140, 8868–8876 CrossRef CAS PubMed.
- J. Chen, C.-L. Hu, F.-F. Mao, J.-H. Feng and J.-G. Mao, Angew. Chem., Int. Ed., 2019, 131, 2120–2124 CrossRef.
- C. Wu, L. Lin, X. Jiang, Z. Lin, Z. Huang, M. G. Humphrey, P. S. Halasyamani and C. Zhang, Chem. Mater., 2019, 31, 10100–10108 CrossRef CAS.
- J. Chen, C.-L. Hu, X.-H. Zhang, B.-X. Li, B.-P. Yang and J.-G. Mao, Angew. Chem., Int. Ed., 2020, 59, 5381–5384 CrossRef CAS PubMed.
- T. Abudouwufu, M. Zhang, S. Cheng, Z. Yang and S. Pan, Chem. – Eur. J., 2019, 25, 1221–1226 CrossRef CAS PubMed.
- M. Luo, F. Liang, X. Hao, D. Lin, B. Li, Z. Lin and N. Ye, Chem. Mater., 2020, 32, 2615–2620 CrossRef.
- (a) I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244–247 CrossRef; (b) N. E. Brese and M. Okeeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192–197 CrossRef.
- (a) P. A. Maggard, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2001, 123, 7742–7743 CrossRef CAS PubMed; (b) L. Han and M. Hong, Inorg. Chem. Commun., 2005, 8, 406–419 CrossRef CAS; (c) X.-D. Zheng and T.-B. Lu, CrystEngComm, 2010, 12, 324–336 RSC.
- X. Wang, L. Liu, K. Ross and A. Jacobson, J. Solid State Sci., 2000, 2, 109–118 CrossRef CAS.
- L. Christian and S. Thomas, Z. Anorg. Allg. Chem., 2008, 657–661 Search PubMed.
- M. G. Zhizhin, A. V. Olenev, F. M. Spiridonov, L. N. Komissarova and O. G. D’yachenko, J. Solid State Chem., 2001, 157, 8–12 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- G. C. Bhar and R. C. Smith, Phys. Status Solidi A, 1972, 13, 157–168 CrossRef CAS.
- (a) S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS; (b) M. C. Payne, M. P. Teter, D. C. Allan, T. A. Arias and J. D. Joannopoulos, Rev. Mod. Phys., 1992, 64, 1045–1097 CrossRef CAS; (c) A. M. Rappe, K. M. Rabe, E. Kaxiras and J. D. Joannopoulos, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1227–1230 CrossRef PubMed.
- R. W. Godby, M. Schluter and L. J. Sham, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10159–10175 CrossRef PubMed.
- (a) J. Lin, M. H. Lee, Z. P. Liu, C. T. Chen and C. J. Pickard, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 13380–13389 CrossRef CAS; (b) R. He, Z. S. Lin, M. H. Lee and C. T. Chen, J. Appl. Phys., 2011, 109, 103510 CrossRef; (c) Z. Lin, X. Jiang, L. Kang, P. Gong, S. Luo and M.-H. Lee, J. Phys. D: Appl. Phys., 2014, 47, 253001 CrossRef.
- S. R. Bowman, S. O'Connor and N. J. Condon, Opt. Express, 2012, 20, 12906–12911 CrossRef CAS PubMed.
- T. Jiang, Y. Chen, Y. Lin, X. Gong, J. Huang, Z. Luo and Y. Huang, J. Lumin., 2018, 199, 133–137 CrossRef CAS.
- F. Cornacchia, A. Di Lieto, M. Tonelli, A. Richter, E. Heumann and G. Huber, Opt. Express, 2008, 16, 15932–15941 CrossRef CAS PubMed.
- M. Fibrich, H. Jelínková, J. Šulc, K. Nejezchleb and V. Škoda, Appl. Phys. B, 2009, 97, 363–367 CrossRef CAS.
- (a) J. Bartschke, K.-J. Boller, R. Wallenstein, I. V. Klimov, V. B. Tsvetkov and I. A. Shcherbakov, J. Opt. Soc. Am. B, 1997, 14, 3452–3456 CrossRef CAS; (b) Q. Ye, L. Shah, J. Eichenholz, D. Hammons, R. Peale, M. Richardson, A. Chin and B. H. T. Chai, Opt. Commun., 1999, 164, 33–37 CrossRef CAS; (c) F. Mougel, G. Aka, A. Kahn-Harari, H. Hubert, J. M. Benitez and D. Vivien, Opt. Mater., 1997, 8, 161–173 CrossRef CAS; (d) M. J. Lederer, M. Hildebrandt, V. Z. Kolev, B. Luther-Davies, B. Taylor, J. Dawes, P. Dekker, J. Piper, H. H. Tan and C. Jagadish, Opt. Lett., 2002, 27, 436–438 CrossRef CAS PubMed; (e) H. Zhang, X. Meng, L. Zhu, P. Wang, X. Liu, R. Cheng, J. Dawes, P. Dekker, S. Zhang and L. Sun, J. Cryst. Growth, 1999, 200, 335–338 CrossRef CAS; (f) H. Jiang, J. Wang, H. Zhang, X. Hu, B. Teng, C. Zhang and P. Wang, Chem. Phys. Lett., 2002, 357, 15–19 CrossRef CAS.
- (a) H. Liu, X. Chen, L. X. Huang, X. Xu, G. Zhang and N. Ye, Mater. Res. Innovations, 2011, 15, 140–144 CrossRef CAS; (b) T. Sasaki, Y. Mori, M. Yoshimura, Y. K. Yap and T. Kamimura, Mater. Sci. Eng., R, 2000, 30, 1–54 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1962423. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02789h |
| This journal is © The Royal Society of Chemistry 2020 |