 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reply to the ‘Comment on “Thermal effects – an alternative mechanism for plasmon-assisted photocatalysis”’ by P. Jain, Chem. Sci., 2020, 11, DOI: 10.1039/D0SC02914A
Yonatan
Dubi
 ad,
Ieng Wai
Un
bc and
Yonatan
Sivan
ad,
Ieng Wai
Un
bc and
Yonatan
Sivan
 *bd
*bd
aDepartment of Chemistry, Ben-Gurion University, Israel. E-mail: jdubi@bgu.ac.il
bSchool of Electrical and Computer Engineering, Ben-Gurion University of the Negev, Israel
cJoan and Irwin Jacobs TIX Institute, National Tsing Hua University, Taiwan
dIlse Katz Center for Nanoscale Science and Technology, Ben-Gurion University, Israel
First published on 13th August 2020
In his Comment to our paper “Thermal effects – an alternative mechanism for plasmon-assisted photocatalysis”,1 Jain correctly points out that using an Arrhenius fit to the reaction rate is not enough to distinguish thermal from non-thermal effects. The reason is that the Arrhenius form contains only the ratio εa/(kBT), where εa is the reaction activation energy, and T is the catalysts (average) temperature (kB is the Boltzmann constant). Illumination causes an increase of the temperature, but some claim that it may also reduce the activation energy (due to the generation of nonthermal, “hot”, carriers). It might also do both, simultaneously. Thus, there is a continuum of possible fits to the reaction rate (as a function of temperature, say), ranging from the limit where only the activation energy changes, to the limit where the activation energy remains constant and only the temperature changes (as done in our manuscript). Mathematically, this implies that if T = Tdark + aIinc (where Tdark is the ambient temperature, Iinc is the incident illumination intensity and a is the photothermal conversion coefficient), then a can range from a maximal value all the way down to zero, and still yielding a good fit to the data.Jain is in fact correct, which is why, for example, we used the term “alternative explanation” in our title, to demonstrate the caution practiced in our manuscript. Furthermore, this precise point was raised in our recent publication (Y. Sivan, J. H. Baraban and Y. Dubi, OSA Continuum, 2020, 3, 483–497),2 Section 4.8. In Fig. 1 below (taken from ref. 2) we show fits to the data of Zhou et al.3 (ref. 48 in ref. 1), where the photo-thermal conversion coefficient ranges from a = 0 to a = 180 K W−1 cm−2 (left panels). As can be clearly seen, the fits are excellent (in fact indistinguishable). On the right panels of the figure we plot the resulting dependence of activation energy on illumination intensity. Clearly, if one assumes no heating (a = 0) then the activation energy strongly depends on intensity, while it is essentially constant if a is maximal.
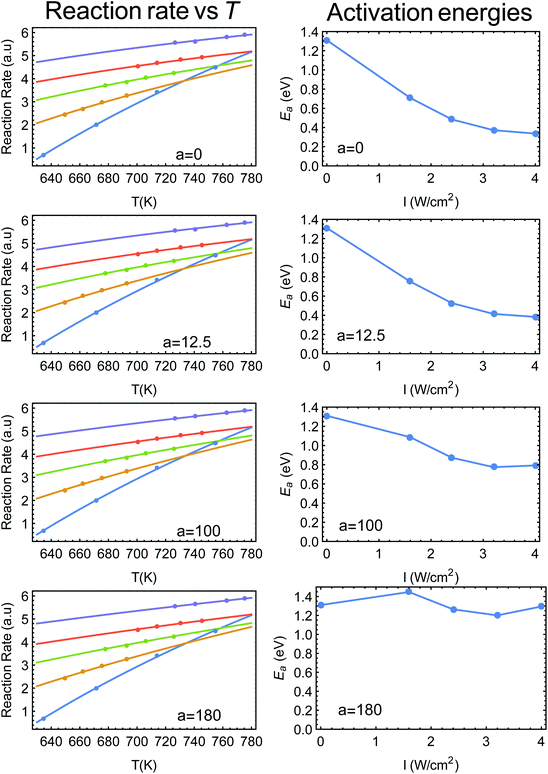 | ||
| Fig. 1 (Left panels) Reaction rate as a function (inverse) temperature, points are data from ref. 3 (ref. 48 in ref. 1). The solid lines are fits to an Arrhenius form with varying values of a. (Right panels) The resulting activation energy as a function of intensity, going from a strongly intensity-dependent activation energy (this is what is plotted in Fig. 2C of ref. 3), all the way to an essentially intensity-independent activation energy for a = 180 K W−1 cm−2. This figure is taken from ref. 2. | ||
Why, then, in our paper1 we seemingly discuss only the limit of constant activation energy? This is discussed in Section II of ref. 1. The key point is that, as our theoretical work demonstrated,4 two points seem to contradict the idea that “hot” electrons somehow contribute to the reaction rates in the papers we discuss in ref. 1. The first is that only a tiny fraction of the illumination power (∼10−7 to 10−10) actually goes to generating hot electrons, and the second is that the number of “hot” electrons nevertheless increases by many orders of magnitude, but reaction rates only rise by a moderate 1–2 orders of magnitude.5
Moreover, in ref. 1 we evaluate the photothermal conversion coefficient independently from both fitting to the data (Fig. 5) and from any direct calculation (Section II of the SI to ref. 4), and find very similar values to those found in the constant activation energy fit. So, by virtue of Occam's razor, the explanation of thermal effects, which is simpler and corroborated by independent quantitative calculations, is far more likely to be the correct one (compared with the speculative claim for “hot” electron action which is not backed up by any sort of theory).
Finally, in the last paragraph of his comment, Jain reminds the readers of the importance of temperature gradients. The importance of thermal gradients was already discussed in great detail in both Section IV of ref. 1, and in our ref. 2. In this context, we point that Jain includes ref. 3 in the list of “practitioners acknowledging the importance of temperature gradients”, while (as we show in ref. 2 and 6), the authors of ref. 3 may have measured them incorrectly, and do not acknowledge their importance.
Conflicts of interest
There are no conflicts of interests to declare.References
- Y. Dubi, I. W. Un and Y. Sivan, Thermal effects – an alternative mechanism for plasmon-assisted photocatalysis, Chem. Sci., 2020, 11, 5017–5027 RSC.
- Y. Sivan, J. Baraban and Y. Dubi, Experimental practices required to isolate thermal effects in plasmonic photo-catalysis – lessons from recent experiments, OSA Continuum, 2020, 3, 483–497 CrossRef.
- L. Zhou, et al., Quantifying hot carrier and thermal contributions in plasmonic photocatalysis, Science, 2018, 362, 69 CrossRef CAS PubMed.
- Y. Dubi and Y. Sivan, “hot electrons” in metallic nanostructures – non-thermal carriers or heating?, Light: Sci. Appl., 2019, 8, 89 CrossRef PubMed.
- Y. Sivan, I. W. Un and Y. Dubi, Assistance of plasmonic nanostructures to photocatalysis just a regular heat source, Faraday Discuss., 2019, 214, 215–233 RSC.
- Y. Sivan, J. Baraban, I. W. Un and Y. Dubi, Comment on “Quantifying hot carrier and thermal contributions in plasmonic photocatalysis”, Science, 2019, 364, eaaw9367 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
