Open Access Article
Open Access ArticleHeterobimetallic Eu(III)/Pt(II) single-chain nanoparticles: a path to enlighten catalytic reactions †
Nicolai D.
Knöfel
 a,
Hannah
Rothfuss
b,
Pavleta
Tzvetkova
a,
Hannah
Rothfuss
b,
Pavleta
Tzvetkova
 d,
Bragavie
Kulendran
a,
Christopher
Barner-Kowollik
d,
Bragavie
Kulendran
a,
Christopher
Barner-Kowollik
 *bc and
Peter W.
Roesky
*bc and
Peter W.
Roesky
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstrasse 15, 76131 Karlsruhe, Germany. E-mail: roesky@kit.edu
bMacromolecular Architectures, Institute for Chemical Technology and Polymer Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76131 Karlsruhe, Germany. E-mail: christopher.barner-kowollik@kit.edu
cCentre for Materials Science, School of Chemistry and Physics, Queensland University of Technology (QUT), 2 George Street, Brisbane, Queensland 4000, Australia. E-mail: christopher.barnerkowollik@qut.edu.au
dInstitute of Organic Chemistry, Institute for Biological Interfaces 4 – Magnetic Resonance, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany
First published on 24th August 2020
Abstract
We introduce the formation and characterization of heterometallic single-chain nanoparticles entailing both catalytic and luminescent properties. A terpolymer containing two divergent ligand moieties, phosphines and phosphine oxides, is synthesized and intramolecularly folded into nanoparticles via a selective metal complexation of Pt(II) and Eu(III). The formation of heterometallic Eu(III)/Pt(II) nanoparticles is evidenced by size exclusion chromatography, multinuclear NMR (1H, 31P{1H}, 19F, 195Pt) as well as diffusion-ordered NMR and IR spectroscopy. Critically, we demonstrate the activity of the SCNPs as a homogeneous and luminescent catalytic system in the amination reaction of allyl alcohol.
Introduction
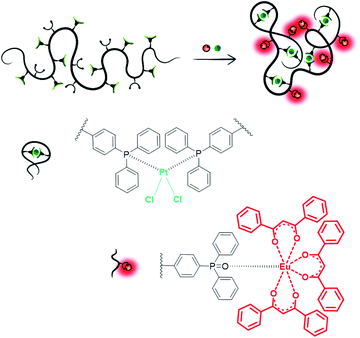
Single-chain nanoparticles (SCNPs) are single polymer chains, which – via defined introduction of functionalized motifs – can be intramolecularly folded into highly specific nanoparticles.1–4 Employing metal ions as linker molecules allows for the design of task-specific pockets within the SCNP architecture in addition to the introduction of unique properties, such as catalytic activity or luminescent behavior. To date, a series of metal-folded SCNP systems has been examined in catalytic studies,5–7 showing promising results, e.g. enhanced selectivity, catalyst recyclability as well as enzyme-mimetic behavior.8–16 Synthetically challenging, yet opening advanced prospects in SCNP chemistry, the enclosure of different metal species within one chain may enable the formation of macromolecular materials with versatile functionalities. The design of compartmentalized areas within a SCNP, containing metal ions of different characteristics, may permit access to advanced catalytic reactions, such as multi-step or tandem catalysis.13As a rare example, Lemcoff and colleagues designed heterobimetallic single-chain nanoparticles via the introduction of catalytically active Ir(I) and Rh(I) ions into a diene-functionalized polymer chain.11 However, no targeted metal placement was carried out, as merely one type of ligand moiety was present, suitable for both metal species. Thus, the introduction of orthogonal linker moieties into the polymer chain is mandatory for a selective and controlled incorporation of different metal ions. To form heterometallic SCNP structures, at least one of the metal precursors needs to induce the chain collapse, whereas a second metal species coordinating to an orthogonal ligand system can add additional functionality. Striving for a SCNP system, which exhibits both luminescent and catalytic properties, we herein report the synthesis and in-depth characterization of a bifunctional terpolymer, featuring both phosphine and phosphine oxide ligand moieties. The terpolymer enables SCNP formation via orthogonal complexation of platinum(II) and europium(III), providing novel catalytic material (Fig. 1). The combinatorial design of catalytic and luminescent properties within a SCNPs system allows i.e. the visualization and tracking of the polymer nanoparticles during catalysis and helps to monitor a successful catalyst separation.
Results and discussion
Polymer synthesis and characterization
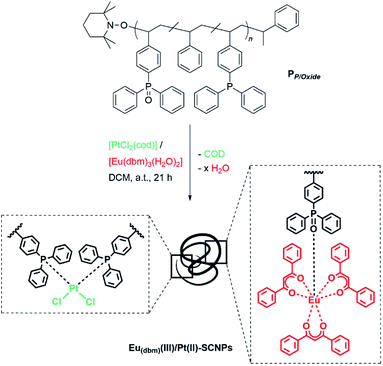
Ligand systems applied in metal–SCNP chemistry, mostly containing oxygen- and nitrogen-donor functionalities,9,17–19 tend to coordinate non-selectively to metal ions. In recent studies, our teams investigated the introduction of phosphine moieties into polymer chains, which enabled subsequent SCNP formation via the addition of Pd(II) as well as Pt(II) ions.8,20 Advantageously, phosphines can readily be oxidized, thereby changing their coordination behavior significantly due to the ionic character of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.21 In a terpolymer exhibiting both phosphine and phosphine oxide moieties, the soft phosphine ligands can selectively coordinate to Pt(II) centers, inducing the single chain collapse, whereas the phosphine oxide functionalities act as a hard acid counterpart for the complexation of luminescent Eu(III). For the synthesis of a suitable bifunctional terpolymer, the monomers 4-(diphenyl-phosphino)styrene, 4-(diphenylphosphino oxide)styrene and styrene, working as a spacer monomer, were copolymerized via nitroxide mediated polymerization (NMP),22 yielding PP/Oxide (Scheme 1). Hereby, the phosphine and phosphine oxide functionalities are statistically distributed along the chain. The monomer composition of terpolymer PP/Oxide was determined by 1H and 31P{1H} NMR spectroscopy (in CDCl3), comparing the resonance integrals of the respective monomer species (ESI, Fig. S2 and S3†).
O bond.21 In a terpolymer exhibiting both phosphine and phosphine oxide moieties, the soft phosphine ligands can selectively coordinate to Pt(II) centers, inducing the single chain collapse, whereas the phosphine oxide functionalities act as a hard acid counterpart for the complexation of luminescent Eu(III). For the synthesis of a suitable bifunctional terpolymer, the monomers 4-(diphenyl-phosphino)styrene, 4-(diphenylphosphino oxide)styrene and styrene, working as a spacer monomer, were copolymerized via nitroxide mediated polymerization (NMP),22 yielding PP/Oxide (Scheme 1). Hereby, the phosphine and phosphine oxide functionalities are statistically distributed along the chain. The monomer composition of terpolymer PP/Oxide was determined by 1H and 31P{1H} NMR spectroscopy (in CDCl3), comparing the resonance integrals of the respective monomer species (ESI, Fig. S2 and S3†).
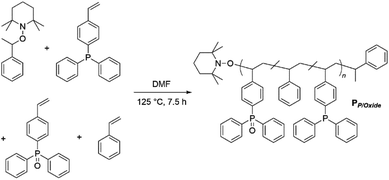 | ||
| Scheme 1 Synthesis of bifunctional terpolymer PP/Oxidevia nitroxide mediated polymerization of styrene, 4-(diphenylphosphino)styrene and 4-(diphenylphosphino oxide)styrene. | ||
Analysis resulted in a monomer ratio of approx. 3.5 mol% phosphine and 1.7 mol% phosphine oxide functionalities in the terpolymer, in agreement with the feed ratio. The given values are – within the margin of error of NMR spectroscopy – estimates which are additionally hampered by the solvent resonance in the 1H NMR spectrum. Therefore, 31P{1H} NMR experiments, applying bis(diphenyl-phosphino)methane (DPPM) as an external standard, were additionally performed and resulted in 0.17 mmol phosphine oxide and 0.35 mmol phosphine species per 1 g of terpolymer PP/Oxide (Fig. S21†), which is in agreement with the priorly determined values. Accordingly, two resonances, in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
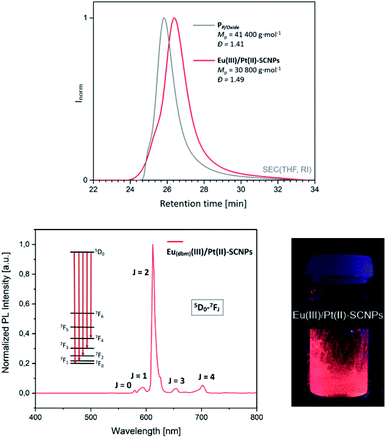
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, are observed at δ = −6.2 ppm and δ = 29.2 ppm in the 31P{1H} NMR spectrum of PP/Oxide, attributed to the implemented triarylphosphine and triarylphosphine oxide species (Fig. 4A). The characterization of PP/Oxidevia SEC (THF, RI) resulted in Mp = 41 400 g mol−1 (average molecular weight 25
1, are observed at δ = −6.2 ppm and δ = 29.2 ppm in the 31P{1H} NMR spectrum of PP/Oxide, attributed to the implemented triarylphosphine and triarylphosphine oxide species (Fig. 4A). The characterization of PP/Oxidevia SEC (THF, RI) resulted in Mp = 41 400 g mol−1 (average molecular weight 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 ) with a dispersity of Đ = 1.4 (Fig. 3, top). Combining the results of the SEC and NMR analysis, approx. 8–9 phosphine and 4 phosphine oxide moieties are estimated per chain. In addition, PP/Oxide was analyzed by IR spectroscopy (ESI, Fig. S22†). Among others, characteristic bands at
300 g mol−1 ) with a dispersity of Đ = 1.4 (Fig. 3, top). Combining the results of the SEC and NMR analysis, approx. 8–9 phosphine and 4 phosphine oxide moieties are estimated per chain. In addition, PP/Oxide was analyzed by IR spectroscopy (ESI, Fig. S22†). Among others, characteristic bands at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1202 and
= 1202 and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1118 cm−1 are attributed to the P
= 1118 cm−1 are attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational stretching and C–H deformation mode of the triarylphosphine oxide units.23,24
O vibrational stretching and C–H deformation mode of the triarylphosphine oxide units.23,24
SCNPs formation and characterization
As phosphine ligands are capable of forming stable 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with Pt(II)-ions via coordinative bond formation, the intramolecular collapse of terpolymer PP/Oxide into SCNPs was induced by addition to the platinum complex [PtCl2(cod)], (cod = cyclooctadiene) in high dilution (Scheme 2).
1 complexes with Pt(II)-ions via coordinative bond formation, the intramolecular collapse of terpolymer PP/Oxide into SCNPs was induced by addition to the platinum complex [PtCl2(cod)], (cod = cyclooctadiene) in high dilution (Scheme 2).
Herein, cod is readily substituted by two phosphine ligands of PP/Oxide, as already established in previous studies on Pt(II)-linked SCNPs.8 The orthogonal properties of the phosphine oxide ligands, which do not coordinate to Pt(II), allow for the simultaneous complexation of a second suitable metal species. Thus, the europium precursor [Eu(dbm)3(H2O)2] (dbm = dibenzoyl-methanide) was employed, coordinating exclusively to the phosphine oxides, via release of H2O, yielding Eu(dbm)(III)/Pt(II)–SCNPs. Hereby, the phosphine oxide Eu(III) coordination can be realized either prior or subsequent to the Pt induced chain folding, resulting in an analogue Eu(III)–Pt(II)–SCNP system. Thus, the nanoparticle formation is independent from the sequence of metal ion addition, allowing dual functionalization and single-chain collapse in one step. Additional experiments, investigating stepwise metal addition and selective coordination behavior of platinum and europium towards the disparate ligand systems, are presented in the ESI (Schemes S4 and S5†). In theory, the attachment of either one or two phosphine oxides to a [Eu(β-diketonate)3] species is possible.25,26 However, the small number of approx. four phosphine oxide units per chain, combined with a high steric demand of the rigid polymer backbone suggests a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination ratio. In addition, the coordination of [Eu(dbm)3] to a sole triarylphosphine oxide functionalized copolymer (styrene based) was investigated at high concentrations. Yet, no network formation was observed, as it is expected in case of a 2
1 coordination ratio. In addition, the coordination of [Eu(dbm)3] to a sole triarylphosphine oxide functionalized copolymer (styrene based) was investigated at high concentrations. Yet, no network formation was observed, as it is expected in case of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand to Eu(III) coordination. For this reason, an Eu(III) coordination ratio of 1
1 ligand to Eu(III) coordination. For this reason, an Eu(III) coordination ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is presumed. This assumption was further confirmed by DOSY NMR measurements (see below).
1 is presumed. This assumption was further confirmed by DOSY NMR measurements (see below).
Following an analogous reaction procedure, different Eu(III) complexes can be applied, emphasizing the versatile applicability of the bifunctional polymer system. In case of the precursor [Eu(tta)3(H2O)] (tta = thenoyltrifluoroacetonate) heterometallic Eu(tta)(III)/Pt(II)–SCNPs were obtained, exhibiting e.g. a convenient 19F NMR sensor (Fig. 2 and ESI, Scheme S3†).
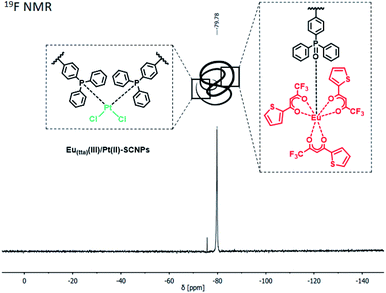 | ||
| Fig. 2 19F NMR spectrum of Eu(tta)(III)/Pt(II)–SCNPs in CDCl3. The tta moiety of the Eu(III) moiety allows tracking of the SCNPs in solution via19F NMR spectroscopy. | ||
To confirm the assumption of an orthogonal metal complexation and verify the metal-induced chain compaction, the obtained particles were thoroughly investigated (for detailed analysis of the Eu(tta)(III)/Pt(II)–SCNPs refer to ESI†). The expected collapse into a more compact structure was verified by SEC analysis (THF, RI).27 In comparison to the terpolymer PP/Oxide, the SEC trace for the Eu(dbm)(III)/Pt(II)–SCNPs is shifted towards longer retention times (Fig. 3, top), indicating a smaller hydrodynamic radius and thus pointing to the formation of nanoparticles without intermolecular side reactions. Further, the curve indicates the existence of a small molecular species (not shown in the spectrum), which most likely originates from unbound europium(III) complexes. To some extent the phosphine oxide coordination is apparently not sufficiently strong to withstand SEC measurement conditions. Consequently, the depicted SEC elugram rather represents a trace of Pt(II)-linked SCNPs. Furthermore, the transition of the linear terpolymer into a more compact nanoparticle was demonstrated by diffusion ordered spectroscopy (DOSY).28,29 Based on the obtained diffusion coefficients, the hydrodynamic radii of the particles were calculated, applying the Stokes–Einstein equation (see ESI†). The mean hydrodynamic radius of PP/Oxide resulted in rH = 8.0 nm, whereas a radius of rH = 1.9 nm was determined for the Eu(dbm)(III)/Pt(II)–SCNPs. Interestingly, sole addition of [Eu(dbm)3(H2O)2] to PP/Oxide, resulting in the intermediate metallopolymer ‘PP/Oxide–Eu(dbm)3’, affording a slightly larger hydrodynamic radius of rH = 11.2 nm. Hence, the addition of an Eu species to PP/Oxide does not seem to induce chain compaction. The results are in line with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination ratio of Eu(III) to the phosphine oxide ligands of PP/Oxide, confirming the orthogonal behavior of the two ligand moieties.
1 coordination ratio of Eu(III) to the phosphine oxide ligands of PP/Oxide, confirming the orthogonal behavior of the two ligand moieties.
To determine the photophysical properties, photoluminescent emission (PL) spectra of the Eu(dbm)(III)/Pt(II)–SCNPs were recorded in the solid state (Fig. 3, bottom). After excitation at λ = 350 nm, the spectrum features characteristic bands resulting from 4f–4f transitions (5D0–7FJ) and is dominated by a band at λ = 612 nm (5D0–7F2 transition).25,30 The resulting bright red luminescence is typical for europium(III) compounds.17,31 The coordination of phosphine oxide moieties to Eu(III)-diketonates is reported to further enhance the luminescence, working as an antenna ligand.21,25 As 4f–4f transitions are virtually unaffected by the surrounding ligands of Eu(III), an almost identical emission spectrum is observed for the Eu(tta)(III)/Pt(II)–SCNPs (see ESI, Fig. S26†). However, the non-symmetric tta moiety of the Eu(III) species results in a visibly more intense luminescent behavior of the corresponding nanoparticles.
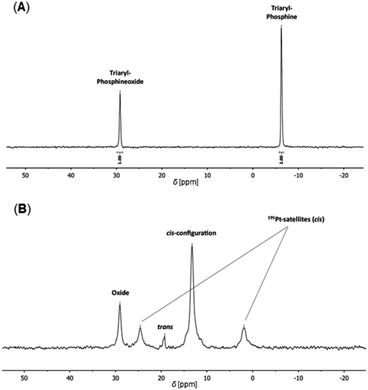
Furthermore, the Eu(dbm)(III)/Pt(II)–SCNPs were characterized by multinuclear NMR spectroscopy, primarily to gain closer insights into the metal coordinated folding units. In the 31P{1H} NMR spectrum (CDCl3) of the SCNPs, the resonance of the triarylphosphines at δ = −6.2 ppm (Fig. 4A) is not detected anymore. Instead, an intense resonance at δ = 13.5 ppm is observed, accompanied by platinum(II) satellites (d, 1JP,Pt = 3661 Hz), which are attributed to square planar cis-[PtCl2(PPh2Ar)2] folding units (Fig. 4B).
A minor resonance at δ = 19.5 ppm is assigned to the corresponding trans-species. The obtained data are in agreement with isostructural Pt(II)–SCNPs and analogous model complexes.8,32 The high preference for a cis-geometry of the Pt(II) centers, approx. 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (cis/trans), is rationalized by the predetermined arrangement of the precursor complex cis-[PtCl2(cod)] as well as the steric constraints of the polymer backbone. Furthermore, the resonance at δ = 29.1 ppm, corresponding to the phosphine oxide moieties, is still present. However, in comparison to PP/Oxide, the resonance is broadened, resulting in a decrease in its integral value. This is presumably caused by the coordination to the paramagnetic europium(III) cores.33 This effect is also observed in the alike 31P{1H} NMR spectrum of the Eu(tta)(III)/Pt(II)–SCNPs (ESI, Fig. S8†). In the 195Pt NMR spectrum of the Eu(dbm)(III)/Pt(II)–SCNPs, a triplet resonance for the cis-[PtCl2(PPh2Ar)2] folding units is detected at δ = −4420 ppm (t, 1JPt,P = 3715 Hz), confirming the obtained 31P{1H} NMR data (ESI, Fig. S6†). The coordination of [Eu(dbm)3] to the phosphine oxide moieties of PP/Oxide is additionally evidenced by 1H NMR spectroscopy. In the corresponding spectrum a characteristic high field resonance at δ = 16.9 ppm is attributed to the methine group of the dbm ligands (ESI, Fig. S4†). Further information was obtained by IR spectroscopy. In the IR spectrum of the Eu(dbm)(III)/Pt(II)–SCNPs bands for the dbm ligands are detected at
1 (cis/trans), is rationalized by the predetermined arrangement of the precursor complex cis-[PtCl2(cod)] as well as the steric constraints of the polymer backbone. Furthermore, the resonance at δ = 29.1 ppm, corresponding to the phosphine oxide moieties, is still present. However, in comparison to PP/Oxide, the resonance is broadened, resulting in a decrease in its integral value. This is presumably caused by the coordination to the paramagnetic europium(III) cores.33 This effect is also observed in the alike 31P{1H} NMR spectrum of the Eu(tta)(III)/Pt(II)–SCNPs (ESI, Fig. S8†). In the 195Pt NMR spectrum of the Eu(dbm)(III)/Pt(II)–SCNPs, a triplet resonance for the cis-[PtCl2(PPh2Ar)2] folding units is detected at δ = −4420 ppm (t, 1JPt,P = 3715 Hz), confirming the obtained 31P{1H} NMR data (ESI, Fig. S6†). The coordination of [Eu(dbm)3] to the phosphine oxide moieties of PP/Oxide is additionally evidenced by 1H NMR spectroscopy. In the corresponding spectrum a characteristic high field resonance at δ = 16.9 ppm is attributed to the methine group of the dbm ligands (ESI, Fig. S4†). Further information was obtained by IR spectroscopy. In the IR spectrum of the Eu(dbm)(III)/Pt(II)–SCNPs bands for the dbm ligands are detected at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1519 cm−1, 1549 cm−1 and 1600 cm−1 (ESI, Fig. S23†), pointing towards its successful encapsulation in PP/Oxide.
= 1519 cm−1, 1549 cm−1 and 1600 cm−1 (ESI, Fig. S23†), pointing towards its successful encapsulation in PP/Oxide.
Catalytic application
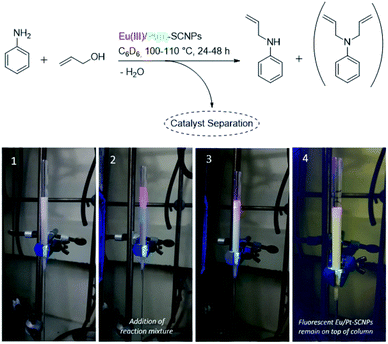
As a proof-of-principle, both synthesized heterometallic Eu(III)/Pt(II)–SCNPs were subsequently investigated as homogeneous catalytic systems in the amination reaction of allyl alcohol (for details refer to ESI†).34 A similar catalytic study has previously been reported for sole Pt(II)–SCNPs, which proved to be an active and recyclable system for this reaction.8 Yet in the current approach, the additional Eu(III) functionalities of the heterometallic SCNPs enable a photophysical detection of the catalyst via illumination with UV-light. This allows e.g. a facile verification of catalyst separation. As mentioned before, the nanoparticles featuring the tta modified Eu(III) complexes exhibited a visibly more intense luminescence, thus being more suitable as a luminescent sensor for the catalytic reaction. Aniline and allyl alcohol were employed as starting material, using 4 mol% of catalyst (equals 2 mol% of Eu species) and ferrocene as an internal standard (Fig. 5, top). These comparatively high catalyst loadings were applied mainly with regards to the amount of Eu(III) species, as the luminescence is partially quenched by the starting materials. The reaction was performed in an NMR Young tube and the respective conversion was determined by 1H NMR spectroscopy (ESI, Fig. S28–S32†). A temperature of approx. 100 °C led to a near quantitative allyl alcohol conversion within less than 48 h, whereat merely small differences were observed for the [Eu(tta)3] and [Eu(dbm)3] modified SCNP systems. In each case, the mono allyl-substituted amine was predominantly generated (>80%).The results are similar to the data obtained for the literature-known Pt(II)–SCNPs as well as the monomeric platinum complex cis-[Pt(PPh3)2Cl2], yet a different reaction procedure and catalyst loading need to be considered when compared in detail.8 After the catalytic reaction, the SCNPs were readily isolated by precipitation in methanol, or subsequent column chromatography (e.g. acetone, neural aluminum oxide). In the latter case, they remained on top of the column and were readily detected when irradiated with UV light (Fig. 5, applying Eu(tta)(III)/Pt(II)–SCNPs as catalyst). Thus, for this specific reaction a simple detection mode for the catalyst's separation was achieved. UV illumination of the filtrate after column chromatography showed no luminescence, thus indicating a complete catalyst separation. However, the photoluminescence of the catalyst is visibly reduced during catalysis, most likely due to the formation of H2O and thermal decomposition, leading to partial quenching of the Eu(III) species over time (ESI, Fig. S33†). Thus, recycling of the catalyst, as it has been previously studied for Pt(II)–SCNPs,8 was not further pursued, as the luminescence was significantly reduced in the second cycle. Additionally, in case of the Eu(tta)(III)/Pt(II)–SCNPs19F NMR studies revealed that the diketonate ligands of the Eu(III) complex are partially removed with increasing reaction temperature and time. Additionally, in the eluant of the chromatography a weak resonance in the 19F NMR spectrum is detected, attributed to unbound tta ligands, confirming partial decomposition during catalysis.
Advantageously, the amount of phosphine and phosphine oxide moieties can readily be adjusted. Thus, for future studies, an introduction of task-specific quantities of Pt(II) and Eu(III) centers, as well as the implementation of other metal combinations, is feasible.
Conclusions
In summary, a bifunctional terpolymer containing two orthogonal ligand moieties was synthesized via NMP, giving way to the facile formation of heterometallic Eu(III)/Pt(II)–SCNPs. The SCNP synthesis proved to be selective in metal coordination and independent from the sequence of metal addition. In addition, featuring catalytic and luminescent centers, the SCNPs were employed as a homogeneous catalytic system. The selective coordination of two metal species exhibiting different functionalities represents, to this date, an unprecedented concept in the realm of single-chain nanoparticles. The combinatorial design of catalytically active and luminescent properties within a SCNP system may not only allow the visualization of the catalyst (and its separation), yet tracking for specific systems seems practicable.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. B.-K. and P. W. R. acknowledge support from the SFB 1176 (project A2) funded by the German Research Council (DFG). H. R.'s and N. K.'s PhD studies were additionally supported by the Fonds der Chemischen Industrie (FCI). C. B. -K. acknowledges key support from the Australian Research Council (ARC) in the context of a Laureate Fellowship enabling his photochemical research program as well as by the Queensland University of Technology (QUT) for continued support via its Centre for Materials Science. C. B. -K. additionally acknowledges continued support by the Helmholtz association via the STN and BIFTM programs. C. Zovko is thanked for her help regarding the PL measurements and E. Rosas Valdez is acknowledged for the synthesis of [Eu(tta)3(H2O)2].Notes and references
- S. Mavila, O. Eivgi, I. Berkovich and N. G. Lemcoff, Chem. Rev., 2016, 116, 878 CrossRef CAS PubMed.
- A. M. Hanlon, C. K. Lyon and E. B. Berda, Macromolecules, 2016, 49, 2 CrossRef CAS.
- C. K. Lyon, A. Prasher, A. M. Hanlon, B. T. Tuten, C. A. Tooley, P. G. Frank and E. B. Berda, Polym. Chem., 2015, 6, 181 RSC.
- I. Berkovich, S. Mavila, O. Iliashevsky, S. Kozuch and N. G. Lemcoff, Chem. Sci., 2016, 7, 1773 RSC.
- Y. Liu, P. Turunen, B. F. M. de Waal, K. G. Blank, A. E. Rowan, A. R. A. Palmans and E. W. Meijer, Mol. Syst. Des. Eng., 2018, 3, 609 RSC.
- J. Rubio-Cervilla, E. González and J. Pomposo, Nanomaterials, 2017, 7, 341 CrossRef PubMed.
- H. Rothfuss, N. D. Knöfel, P. W. Roesky and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 5875 CrossRef CAS PubMed.
- N. D. Knöfel, H. Rothfuss, J. Willenbacher, C. Barner-Kowollik and P. W. Roesky, Angew. Chem., Int. Ed., 2017, 56, 4950 CrossRef PubMed.
- A. Sanchez-Sanchez, A. Arbe, J. Colmenero and J. A. Pomposo, ACS Macro Lett., 2014, 3, 439 CrossRef CAS.
- T. Terashima, T. Mes, T. F. A. De Greef, M. A. J. Gillissen, P. Besenius, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 4742 CrossRef CAS PubMed.
- S. Mavila, I. Rozenberg and N. G. Lemcoff, Chem. Sci., 2014, 5, 4196 RSC.
- S. Thanneeru, J. K. Nganga, A. S. Amin, B. Liu, L. Jin, A. M. Angeles-Boza and J. He, ChemCatChem, 2017, 9, 1157 CrossRef CAS.
- J. Chen, K. Li, J. S. L. Shon and S. C. Zimmerman, J. Am. Chem. Soc., 2020, 142, 4565 CrossRef CAS PubMed.
- J. Chen, J. Wang, K. Li, Y. Wang, M. Gruebele, A. L. Ferguson and S. C. Zimmerman, J. Am. Chem. Soc., 2019, 141, 9693 CrossRef CAS PubMed.
- Y. Liu, S. Pujals, P. J. M. Stals, T. Paulöhrl, S. I. Presolski, E. W. Meijer, L. Albertazzi and A. R. A. Palmans, J. Am. Chem. Soc., 2018, 140, 3423 CrossRef CAS PubMed.
- J. P. Cole, A. M. Hanlon, K. J. Rodriguez and E. B. Berda, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 191 CrossRef CAS.
- H. Rothfuss, N. D. Knöfel, P. Tzvetkova, N. C. Michenfelder, S. Baraban, A.-N. Unterreiner, P. W. Roesky and C. Barner-Kowollik, Chem.–Eur. J., 2018, 24, 17475 CrossRef CAS PubMed.
- N. D. Knöfel, H. Rothfuss, C. Barner-Kowollik and P. W. Roesky, Polym. Chem., 2019, 10, 86 RSC.
- Y. Liu, T. Pauloehrl, S. I. Presolski, L. Albertazzi, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2015, 137, 13096 CrossRef CAS PubMed.
- J. Willenbacher, O. Altintas, V. Trouillet, N. Knöfel, M. J. Monteiro, P. W. Roesky and C. Barner-Kowollik, Polym. Chem., 2015, 6, 4358 RSC.
- A. W. G. Platt, Coord. Chem. Rev., 2017, 340, 62 CrossRef CAS.
- C. J. Hawker, G. G. Barclay and J. Dao, J. Am. Chem. Soc., 1996, 118, 11467 CrossRef CAS.
- L. Daasch and D. Smith, Anal. Chem., 1951, 23, 853 CrossRef CAS.
- M. Halmann and S. Pinchas, J. Chem. Soc., 1958, 3264 RSC.
- N. B. D. Lima, S. M. C. Gonçalves, S. A. Júnior and A. M. Simas, Sci. Rep., 2013, 3, 2395 CrossRef PubMed.
- H. Yasuchika, T. Shiori, Y. Masanori, N. Takayuki, K. Yuichi, S. Tomohiro, I. Hajime and F. Koji, Chem. - Eur. J., 2017, 23, 2666 CrossRef PubMed.
- J. Engelke, J. Brandt, C. Barner-Kowollik and A. Lederer, Polym. Chem., 2019, 10, 3410 RSC.
- E. Blasco, B. T. Tuten, H. Frisch, A. Lederer and C. Barner-Kowollik, Polym. Chem., 2017, 8, 5845 RSC.
- P. Groves, Polym. Chem., 2017, 8, 6700 RSC.
- E. Moretti, L. Bellotto, M. Basile, C. Malba, F. Enrichi, A. Benedetti and S. Polizzi, Mater. Chem. Phys., 2013, 142, 445 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1 CrossRef CAS.
- L. Bemi, H. C. Clark, J. A. Davies, C. A. Fyfe and R. E. Wasylishen, J. Am. Chem. Soc., 1982, 104, 438 CrossRef CAS.
- N. B. D. Lima, A. I. S. Silva, P. C. Gerson Jr, S. M. C. Gonçalves and A. M. Simas, PLoS One, 2016, 10, e0143998 CrossRef PubMed.
- S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: NMR- and IR-spectra. See DOI: 10.1039/d0sc03579c |
| This journal is © The Royal Society of Chemistry 2020 |