Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic generation of alkoxy radicals from unfunctionalized alcohols
Elaine
Tsui
 ,
Huaiju
Wang
,
Huaiju
Wang
 and
Robert R.
Knowles
and
Robert R.
Knowles
 *
*
Department of Chemistry, Princeton University, Princeton, NJ 08544, USA. E-mail: rknowles@princeton.edu
First published on 21st September 2020
Abstract
Alkoxy radicals have long been recognized as powerful synthetic intermediates with well-established reactivity patterns. Due to the high bond dissociation free energy of aliphatic alcohol O–H bonds, these radicals are difficult to access through direct homolysis, and conventional methods have instead relied on activation of O-functionalized precursors. Over the past decade, however, numerous catalytic methods for the direct generation of alkoxy radicals from simple alcohol starting materials have emerged and created opportunities for the development of new transformations. This minireview discusses recent advances in catalytic alkoxy radical generation, with particular emphasis on progress toward the direct activation of unfunctionalized alcohols enabled by transition metal and photoredox catalysis.
Introduction
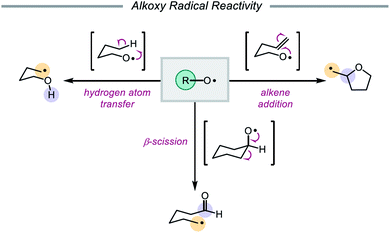
In 1911, Heinrich Wieland published one of the earliest reports that implicated the intermediacy of alkoxy radicals in an organic reaction, suggesting that alkoxy radicals were necessary in the formation of tetraphenyldiphenoxyethane from bis(triphenylmethyl)peroxide.1 Since then, alkoxy radicals have been recognized as versatile reactive intermediates and have been exploited in a wide variety of synthetic transformations.2 These electrophilic oxygen-centered radicals, consisting of an alkyl group bound to an oxygen radical center, lack the stabilization provided by mesomeric effects and spin density delocalization found in other O-centered radicals (e.g. aryloxy radicals). As a result, alkoxy radicals are a particularly high-energy species among heteroatom-centered radicals. Spectroscopic, mechanistic, and synthetic studies over the years have probed the reactivity of O-radicals,2c,3 finding that alkoxy radicals generally participate in one of three elementary reactions: (1) hydrogen atom transfer,4,5 (2) β-scission,6,7 and (3) alkene addition (Scheme 1).8,9Of these three reaction classes, perhaps the most common application of alkoxy radicals is hydrogen atom transfer (HAT).10,11 Due to the propensity of O-centered radicals to form strong bonds to hydrogen atoms (O–H BDFE ≈ 105 kcal mol−1), hydrogen atom abstraction from comparatively weaker aliphatic C–H bonds (BDFE ≈ 98–102 kcal mol−1) is frequently observed.12,13 In particular, intramolecular 1,5-HAT allows for the direct abstraction of δ-C–H bonds and enables selective C–H functionalization, a synthetic strategy that has been exploited since the development of the Barton nitrite ester reaction14 and continues to be explored in modern catalytic methods.2e,2f Alkoxy radicals can also undergo homolytic cleavage of β-C–C bonds to furnish carbonyl products and C-centered radicals. As β-scission tends to favor cleavage of the C–C bond that will generate the most stabilized alkyl radical, the regioselectivity of such C–C cleavage events in unsymmetrical substrates is often both high and predictable.15–17 Lastly, alkoxy radicals engage in olefin addition reactions to form C–O bonds, most commonly in intramolecular cyclization reactions to afford cyclic ether scaffolds.8 While competition between these pathways does occur, careful design of substrates and control of reaction conditions can be utilized to bias one reaction type over another.3,18,19 Indeed, alkoxy radicals have long been successfully employed in the syntheses of complex molecules.14,20
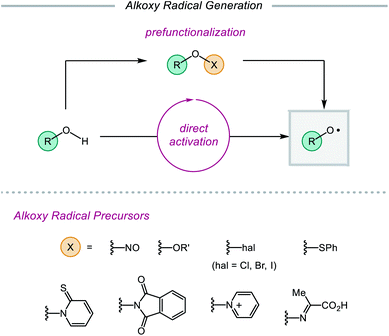
Despite their synthetic potential, the generation of alkoxy radicals is nontrivial. Direct homolytic activation of alkanol O–H bonds has historically been challenging due to their high bond dissociation free energies and the frequent presence of weaker aliphatic C–H bonds in the same substrates. To circumvent these limitations, chemists have developed indirect strategies to access alkoxy radicals through the installation of weak oxygen-heteroatom bonds, such as those in nitrite esters,14,21 peroxides,15a,22 hypohalites,15c,23 sulfenates,24N-alkoxypyridine-2-thiones,25 and N-alkoxyphthalimides26 (Scheme 2). Prefunctionalization of native alcohols is not without drawbacks, from the inevitable loss of atom economy to the instability of many O-functionalized precursors. Chemo- and regioselective installation of such functionality in complex substrates bearing numerous reactive sites can also be challenging and generally necessitates the use of protecting groups. Moreover, these O–X bonds are homolyzed using thermolysis or UV photolysis, conditions that often limit functional group compatibility and the scope of subsequent transformations, preventing use of these surrogates in late-stage manipulations of complex molecules. While methods using milder radical initiators have been employed for O–X bond homolysis, these strategies often require efficient radical chain processes to function effectively.
Recently, modern catalytic methods have been developed to allow for alkoxy radical generation from bench-stable prefunctionalized substrates under milder conditions. For example, while classical strategies for activating the O–N bond of N-alkoxyphthalimide precursors require stoichiometric organotin reagents in the presence of a radical initiator,26 recently developed photocatalytic approaches have enabled the generation of alkoxy radicals from N-alkoxyphthalimides using a transient reductant that can be generated by low intensity irradiation with visible light.27,28 Analogous catalytic systems have also been reported that facilitate the reductive cleavage of N-alkoxypyridinium salts29–32 or the oxidative decomposition of oxyimino acids to unmask alkoxy radical intermediates.33
In spite of major advances in the installation and activation of alkoxy radical surrogates, prefunctionalization of native alcohols necessitates circuitous synthetic steps and generates stoichiometric byproducts. The direct catalytic activation of free alcohols, which bypasses prefunctionalization, has inherent advantages. The development of photoredox catalysis and the expansion of transition-metal catalysis over the last decade have led to the advent of new methodologies that have overcome limitations of classical alkoxy radical generation. Many aspects of alkoxy radicals have been reviewed over the years, with recent reviews examining different types of alkoxy radical-mediated transformations.2d–f The purpose of this review is to highlight recent advances in the development of catalytic methods for alkoxy radical generation from unfunctionalized alcohols, including (1) in situ generation of O–I species, (2) transition metal-mediated homolysis, (3) ligand-to-metal charge transfer (LMCT), and (4) proton-coupled electron transfer (PCET). Across these examples, we showcase powerful strategies for O–H bond activation, emphasizing how these mild methods for alkoxy radical generation have facilitated the development of myriad transformations mediated by alkoxy radicals, each with increasing complexity and functional-group compatibility. Ultimately, this review endeavors to summarize the advances in this area and to discuss current challenges and opportunities that lie ahead.
In situ generation of O–I species
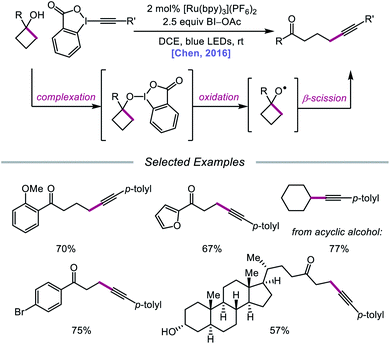
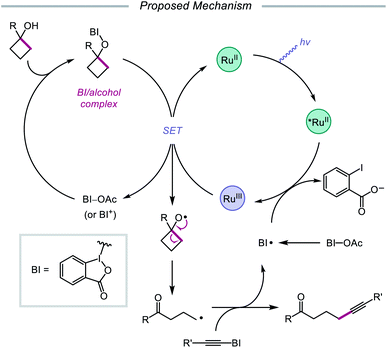
The use of organohypervalent iodine compounds for the generation of alkoxy radicals from alcohols has been investigated since the development of the Suárez system.2c,34 Comprising PhI(OAc)2 and I2, this reagent combination has been used as a strong oxidant, enabling in situ formation of a weak O–I bond that is generally photolyzed under visible-light irradiation to furnish an alkoxy radical intermediate. Various iodine(III) compounds have been utilized to facilitate 1,5-HAT, β-scission, and alkene addition reactions, particularly in the context of steroidal and carbohydrate derivatives.35 However, in the same vein as classical reagents for O-radical generation, such as Pb(OAc)4, Ag2S2O8, HgO/I2, and (NH4)2[Ce(NO3)6], the PhI(OAc)2/I2 system has traditionally suffered from limited functional group compatibility, as the use of strong oxidants results in competitive oxidations that are deleterious to reaction efficiency. In recent years, interest in integrating iodane chemistry with photoredox catalysis has emerged, and new strategies have been developed to generate alkoxy radicals while circumventing the shortcomings of classical systems.In 2016, Chen and coworkers disclosed a method for the activation of alcohols for C–C bond cleavage and subsequent alkynylation and alkenylation reactions.36 Central to this strategy is the use of cyclic iodine(III) reagents (CIRs) under photoredox conditions to trigger formation of the requisite alkoxy radical, even though traditional non-cyclic iodine(III) reagents, such as PhI(OAc)2, failed to give any desired product. A Ru(II) photocatalyst and stoichiometric acetoylbenziodoxole (BI–OAc) are employed in the presence of a cycloalkanol and an alkynyl benziodoxole radical acceptor to furnish a variety of alkynyl ketones (Scheme 3). Cyclopropanols substituted with electron-rich or electron-deficient aryl groups are competent substrates, and electron-rich cyclobutanols are also smoothly converted to functionalized ketones. Steroidal cycloalkanols, including those with a free hydroxy group on the steroid core structure, are well tolerated under these reaction conditions, with ring-opening occurring only at the strained cycloalkanol moiety. Notably, linear alcohols also undergo β-scission and addition to not only alkynyl benziodoxoles but also vinyl carboxylates, which serve as precursors to alkenylated products. The authors propose that photoexcited Ru(II) is initially oxidized to Ru(III), a species that then oxidizes the O–I bond of an in situ formed benziodoxole/alcohol complex (Scheme 4). The alkoxy radical formed after oxidation undergoes C–C bond cleavage to give a distal alkyl radical that can add to a radical acceptor, releasing the alkynylation or alkenylation adduct and benziodoxole radical. The latter is expected to reoxidize excited-state Ru(II) back to Ru(III).
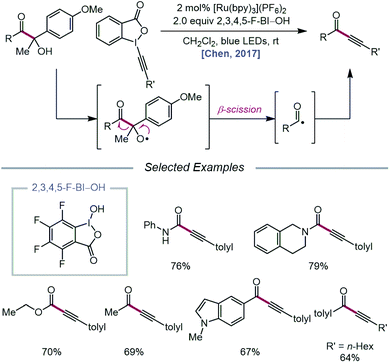
In 2017, the Chen group extended the use of CIRs to achieve carbonyl–C(sp3) bond cleavage and alkynylation of β-amide, β-ester, and β-ketone alcohols under photoredox conditions (Scheme 5).37 Designed around a similar mechanism to that proposed in their earlier work, this protocol for selective cleavage of carbonyl–C(sp3) bonds, which previously required UV light irradiation38 or transition-metal catalysts under high temperatures,39 utilizes β-carbonyl alcohols as substrates for alkoxy radical-mediated β-scission. Unlike their 2016 report, it was found that for many cases, BI–OAc does not give the optimal yield of ynamide, ynoate, or ynone products, but 2,3,4,5-F-BI–OH as the CIR results in higher yields with shorter reaction times. The authors postulate that electron-deficient CIRs have more positive reduction potentials relative to BI–OAc, which in turn enables more efficient quenching of photoexcited Ru(II) to Ru(III). Additionally, electron-deficient benziodoxoles are demonstrated to decrease the oxidation potential of the CIR/alcohol complex (Eoxp = 0.93 V vs. SCE in MeCN for 3,4-F-BI–OAc/alcohol) relative to the BI/alcohol complex (Eoxp = 1.06 V vs. SCE in MeCN) and the free β-carbonyl alcohol (Eoxp = 1.69 V vs. SCE in MeCN), suggesting that the decreased oxidation potentials correlate with superior alcohol activation and alkoxy radical generating ability. The use of CIRs for O–H activation was similarly applied to P–C(sp3) bond cleavage in α-phosphorus alcohols to synthesize phosphonoalkynes.40
 | ||
| Scheme 5 Carbonyl–C(sp3) bond cleavage and alkynylation for the synthesis of ynoates, ynamides, and ynones. | ||
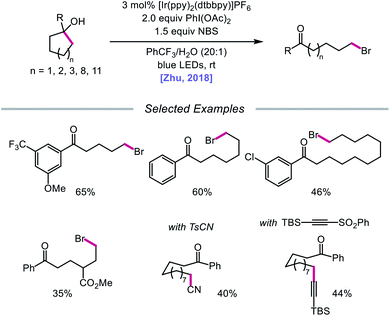
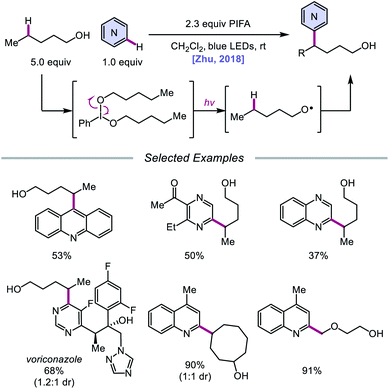
In addition to oxidative cleavage of an alkoxyiodo intermediate, direct homolysis of an in situ formed O–I bond has been invoked in more recent mechanisms involving alkoxy radical formation. Zhu and coworkers demonstrated in 2018 that ring-opening bromination of unstrained cycloalkanols can be accomplished using an Ir(III) photocatalyst, PhI(OAc)2, and N-bromosuccinimide (NBS) as the bromine source under visible light irradiation (Scheme 6).41 This protocol can be employed to construct distally brominated ketones from cyclopentanol, cyclohexanol, cycloheptanol, cyclododecanol, and cyclopentadecanol derivatives via alkoxy radical-mediated β-scission and bromination of the ring-opened C-centered radical. Additionally, substitution of NBS with tosyl cyanide and alkynyl sulfone allows for distal cyanation and alkynylation, respectively, through an analogous ring-opening mechanism. The authors propose that two pathways for O–H activation are operative (Scheme 7): (1) proton-coupled electron transfer involving Ir(IV) as an oxidant and succinimide anion as a weak base, and (2) formation of an alkoxyiodobenzene complex and visible light-induced O–I bond homolysis. Later that year, the Zhu group reported a related metal-free system for the alcohol-directed heteroarylation of C(sp3)–H bonds using phenyliodine bis(trifluoroacetate) (PIFA) as the only reagent (Scheme 8).42 While not catalytic, this strategy serves as an example of direct visible light-promoted O–I bond cleavage in the absence of a photoredox catalyst. It is postulated that a mixture of free alcohol and PIFA leads to formation of dialkoxyiodobenzene, which is confirmed by NMR experiments, and direct irradiation of this intermediate results in O–I bond homolysis to form the desired alkoxy radical. Intramolecular HAT affords the alkyl radical that can add into an N-heteroarene to furnish Minisci-type products, including functionalized acridine, pyrazine, quinoxaline, and drug molecules, such as voriconazole.
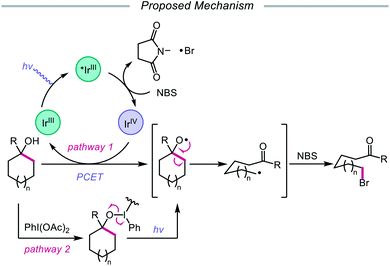 | ||
| Scheme 7 Postulated reaction pathways for ring-opening bromination using Ir(III) photocatalyst and PhI(OAc)2. | ||
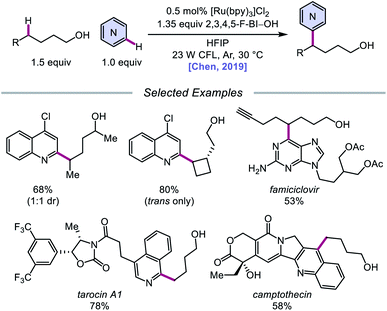
Alternatively, in 2019, Chen and coworkers reported a photocatalytic strategy for the C(sp3)–H bond heteroarylation of alcohols using a Ru(II) catalyst and 2,3,4,5-F-BI–OH (Scheme 9).43 This method enables efficient alkoxy radical formation and δ-C–H functionalization of primary and secondary alcohols, even when using just 1.5 equivalents of alcohol substrate (as opposed to the system reported by Zhu et al.), and has allowed for the alkylation of complex drug molecules, such as famciclovir, tarocin A1, and camptothecin. Mechanistic investigations suggest that 2,3,4,5-F-BI–OH can undergo alcoholysis with 1-butanol to give isolable 2,3,4,5-F-BI–OBu, an intermediate which gives the expected alkylated product under reaction conditions and has been shown to quench excited-state Ru(II). It is, therefore, proposed that upon in situ formation, the new 2,3,4,5-F-BI–OR complex is activated via one-electron reduction by photoexcited Ru(II) to give a benzoate anion and the alkoxy radical (Scheme 10). 1,5-HAT furnishes an alkyl radical, which reacts with N-heteroarenes through a Minisci-type reaction, and Ru(III) oxidizes the resulting adduct to regenerate Ru(II) and give the final product. This protocol for alkoxy radical generation was later extended to a related β-scission-promoted C–H bond alkylation of various N-heteroarenes.44
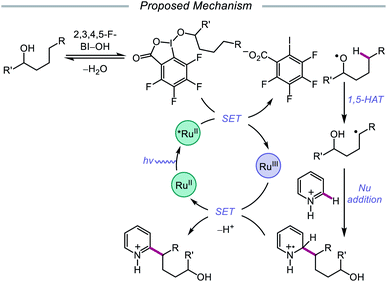 | ||
| Scheme 10 Proposed mechanism for the remote heteroarylation of free alcohols via photoredox catalysis. | ||
Transition metal-mediated homolysis
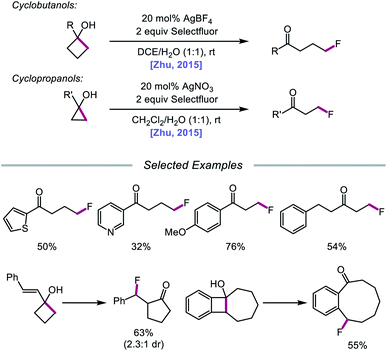
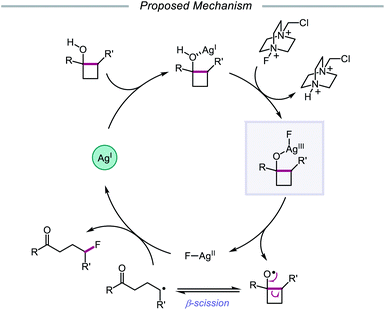
Stoichiometric transition metal salts have been employed for single-electron oxidations of alcohols since the 1960s, usually apropos the oxidations of strained cycloalkanols, releasing ring strain through the formation of ring-opened products. For example, Schaafsma and coworkers reported that Cu(II) and Fe(III) salts can induce the oxidative ring-opening of cyclopropanone hemiacetal, forming β-propionate radical, which can then dimerize or react with a radical acceptor.45 Moreover, Roček found that Mn(III), V(V), and Ce(IV) can serve as one-electron oxidizing agents for cyclobutanol,46 and in 1991, Narasaka disclosed the use of Mn(III) tris(2-pyridinecarboxylate) for the generation of β-keto radicals through O–H activation of cyclopropanol derivatives, demonstrating their efficient addition to silyl enol ethers.47 More recently, focus has shifted from stoichiometric methods to the development of transition metal-catalyzed strategies for accessing alkoxy radicals. The key to a metal catalyst's success is its ability to turn over—namely, its capacity to be reoxidized to its active form as ligand exchange with the alcohol substrate occurs, necessitating the use of oxidants in the reaction. Early examples of such systems include AgNO3/Na2S2O8, used by Minisci and Surzur to activate acyclic alcohols for heteroarylation,48 and AgNO3/(NH4)2S2O8, employed by Narasaka for ring-opening and C(sp3)–C(sp3) bond functionalization of cyclopropanols.49 Since then, catalysts such as Ag(I), Mn(III), and Cu(II) have shown promise in the homolytic activation of alcohol O–H bonds and have been exploited in C–C bond cleavage, C–H bond functionalization, and C–O bond-forming reactions.In 2015, Zhu and coworkers reported a protocol for the ring-opening and fluorination of cyclopropanols and cyclobutanols that relies on a Ag(I) catalyst, Selectfluor as the fluorinating reagent and oxidant, and a biphasic solvent system (Scheme 11).50,51 These conditions can accommodate electron-rich and electron-deficient tertiary cyclobutanols, furnishing γ-fluorinated ketones, such as thienyl and pyridyl ketones. Additionally, this protocol has been adapted for the ring expansion of cyclobutanol to cyclopentanone and the expansion of a fused bicyclic framework to the corresponding nine-membered fluorinated ketone. Upon switching the optimal Ag(I) salt from AgBF4 to AgNO3, the authors report that facile ring-opening and fluorination of cyclopropanols can also be achieved. Mechanistic studies rule out base-assisted ring-opening via a polar mechanism as well as silver-assisted C–C bond cleavage through a Ag(I) insertion and reductive elimination pathway, suggesting the relevance of a silver-catalyzed radical mechanism. Accordingly, the authors propose that the alcohol substrate coordinates with the Ag(I) salt and reacts with Selectfluor to afford a Ag(III)–F complex (Scheme 12). Homolysis of the Ag–O bond releases Ag(II) and the alkoxy radical, which undergoes β-fragmentation to the distal alkyl radical. This C-centered radical intercepts Ag(II)–F to form the fluoroketone product, regenerating Ag(I) in the process. At the time of this report, the groups of Loh and Murakami independently disclosed similar catalytic systems.52,53 Loh and coworkers additionally demonstrated that these conditions can be used for the ring-opening and fluorination of less strained cycloalkanols, including cyclopentanol and cyclohexanol, at the cost of reaction efficiency. Zhu and coworkers later extended silver-catalyzed ring-opening to chlorination reactions through the use of K2S2O8 as an oxidant and N-chlorosuccinimide as the chlorinating reagent; more recently, the Parsons group employed a similar AgNO3/K2S2O8 reaction system to achieve C–C bond cleavage and demethylation of azetidinols, forming α-amino ketone products as part of this alkoxy radical-mediated deconstructive protocol.54
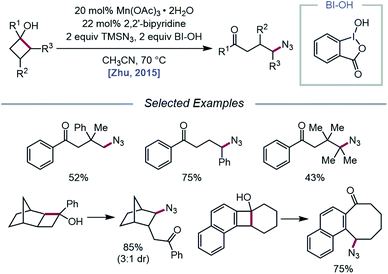
The Zhu group developed a manganese-catalyzed oxidative azidation of cyclobutanols later that year, enabling access to a diverse scope of linear and cyclic alkyl azides.55 During reaction optimization using 1-phenylcyclobutanol, it was found that all attempts at using a silver catalyst afforded undesired 1-tetralone, a product of intramolecular cyclization of the C-centered radical formed after ring-opening.56 Catalytic Mn(OAc)3, in the presence of BI–OH as the oxidant, 2,2′-bipyridine as a ligand, and TMSN3 as the azide source, was instead determined to give the optimal yield of ring-opened azide product (Scheme 13). Notably, the reaction can accommodate multisubstituted cyclobutyl rings as well as bicyclic scaffolds, which give rise to secondary or tertiary azides. In the absence of bipyridine ligand, medium-sized benzocyclic azides can also be formed by using a deconstructive ring-expansion strategy via cleavage of the C–C bond of the cyclobutanol moiety. The authors propose that after ligand exchange between Mn(OAc)3, bipyridine, and TMSN3, BI–OH oxidizes the metal complex to a high-valent Mn(V)–N3 species (Scheme 14). Complexation with cyclobutanol, followed by single-electron transfer, yields Mn(IV)–N3 and the cyclobutyloxy radical, which then undergoes β-scission to form a ring-opened alkyl radical. The nascent C-centered radical is intercepted by Mn(IV)–N3 to form the alkyl azide product, and further reactions with another molecule of TMSN3 and BI–OH turn over the catalytic cycle. Analogous catalyst systems have been developed for cyanation and alkynylation,57 chlorination,58 hydrazination,59 thiolation,60 and selenation.61
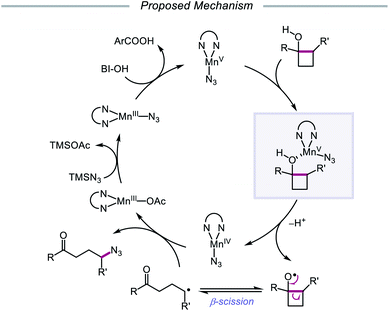 | ||
| Scheme 14 Prospective catalytic cycle for manganese-catalyzed ring-opening and azidation of cyclobutanols. | ||
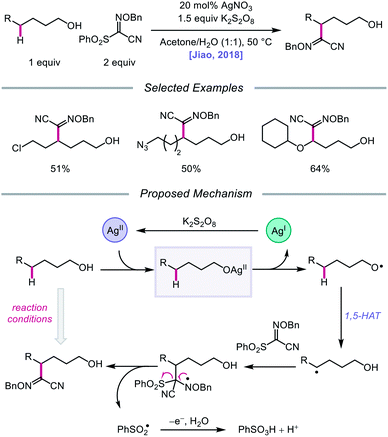
Silver catalysis was again leveraged for alkoxy radical formation in a report by the Jiao group in 2018, this time to promote 1,5-HAT and subsequent C(sp3)–H bond functionalization to form oxime ether products.62 The authors identify AgNO3 and K2S2O8 as the optimal metal/oxidant pair to use for selective δ-C–H abstraction and a sulfonyl oxime ether reagent as the radical acceptor for the transformation (Scheme 15). The reaction tolerates primary and secondary alcohols, including substrates bearing halogen and azide substituents. Additionally, the presence of an oxygen atom at the ε position of the alkanol is critical to obtaining higher yields, presumably due to added stability and nucleophilicity conferred to the adjacent C-centered radical. When a β-substituted alkanol was judiciously subjected to the reaction conditions, a product resulting from β-scission and addition to the oxime ether acceptor was observed, consistent with an alkoxy radical-mediated pathway. As a result, during the course of the reaction, AgNO3 is postulated to be oxidized by K2S2O8 to Ag(II), which coordinates with the alcohol substrate, and the resulting Ag(II) complex undergoes homolysis to give the alkoxy radical and Ag(I) (Scheme 15). The O-centered radical participates in 1,5-HAT, furnishing an alkyl radical that adds to the acceptor, which induces fragmentation and release of a sulfonyl radical and the functionalized product. Jiao and coworkers recently applied similar alcohol activation conditions to an aqueous catalyst system for the regioselective O–H homolysis of 1,3-diols in the presence of DMSO as a hydrogen-bond acceptor, enabling distal C–C cleavage and 1,4-aryl migration.63
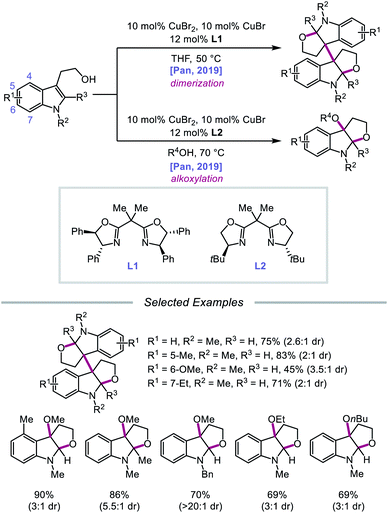
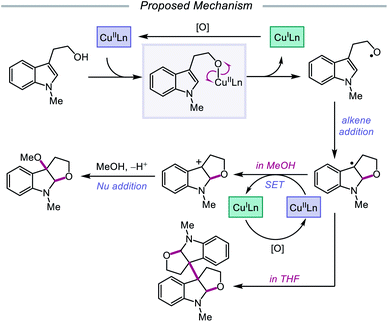
In 2019, Pan and coworkers disclosed a protocol encompassing a copper and oxazoline catalyst system for alkoxy radical generation and C–O bond formation to access 3a,3a′-bisfuro[2,3-b]indoline scaffolds and 3-alkoxy furoindolines from tryptophol derivatives (Scheme 16).64 Remarkably, this method does not require exogenous base, initiator, or oxidant, but the use of a dual Cu(I)/Cu(II) catalyst combination and a bisoxazoline ligand has proven to be essential to reaction efficiency. When run under THF, the protocol affords good yields and moderate diastereoselectivities of C-radical dimerization products, tolerating electron-rich substituents at the 5-, 6-, and 7-positions of the indole ring. In contrast, when the reaction is run in a dilute protic alcohol solvent, such as methanol, the solvent can serve as a trapping reagent to generate a variety of substituted 3-alkoxy furoindolines. Results from mechanistic studies suggest that ligand exchange occurs between the alcohol substrate and the Cu(II) complex to generate a Cu(II)-alkoxide,65 followed by homolysis to form a Cu(I) species and an alkoxy radical (Scheme 17). Intramolecular alkene addition forms the cyclized intermediate containing an adjacent C-centered radical, which undergoes radical–radical dimerization in THF. Alternatively, the alkyl radical can be further oxidized by Cu(II) to generate a carbocation, trapping the protic alcohol solvent to deliver the 3-functionalized furoindoline. While the role of CuBr in accelerating the reaction remains unclear, Cu(I) generated during the course of the reaction is proposed to be reoxidized to Cu(II) by O2.
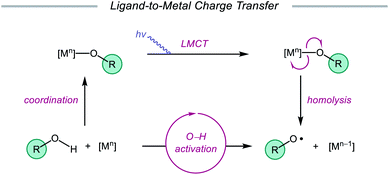
Ligand-to-metal charge transfer
Advances made in visible-light photoredox catalysis over the past decade have opened new avenues for the generation and utilization of open-shell intermediates under mild reaction conditions.66 Many of the new technologies developed in this area rely on accessing the triplet excited state of a late transition-metal polypyridyl photocatalyst via photoinduced metal-to-ligand charge transfer (MLCT). An MLCT transition results in donation of an electron from a metal-centered orbital to a ligand-centered anti-bonding orbital. This excited-state complex, which can serve as both an oxidant or reductant, typically engages in single-electron transfer (SET) with the organic substrate, generating a radical intermediate that can be exploited in subsequent transformations. An alternative photoexcitation manifold, ligand-to-metal charge transfer (LMCT) involves complexes of transition metals with an empty valence shell, wherein an electron from a high-energy ligand orbital is excited to a low-lying metal-centered orbital.67 From the LMCT state, the metal–ligand bond is primed to homolyze, resulting in a formal reduction of the metal center and generation of a ligand-based radical. LMCT catalysis proceeds through sequential substrate coordination to the metal-based catalyst, LMCT excitation, and homolysis of the metal–ligand bond (Scheme 18). This method represents a strategy for substrate activation distinct from processes involving outer-sphere electron transfer events from an excited-state photocatalyst. In the case of O–H activation, LMCT promotes direct oxidation via the excitation event itself, providing a pathway for targeted oxidation of the transiently coordinated metal-alkoxide while leaving other functionality intact. Alkoxy radicals can, therefore, be selectively generated from simple alcohols. The Zuo group has found LMCT to be a powerful platform for alkoxy radical generation and has demonstrated the application of this strategy in a number of reports, a survey of which will be presented in this section of the review.Drawing inspiration from seminal work by Anna, Schelter, and coworkers,68 the Zuo group reported in 2016 the realization of photoinduced LMCT for O–H activation using a cerium-based catalyst system.69 In the presence of CeCl3 precatalyst and Bu4NCl under visible-light irradiation, cyclic alcohols undergo C–C bond β-scission and amination with azodicarboxylates to form distally aminated ketones (Scheme 19). Cycloalkanols of varying ring sizes are well tolerated in the reaction, including steroid- and terpenoid-derived cycloalkanols containing polar functionality. Remarkably, secondary alcohols, such as 2-indanol, are aminated using this protocol to furnish functionalized aldehydes, and this strategy can be employed for the synthesis of molecules with pharmaceutically relevant diazepine scaffolds. The authors propose the following mechanism2d (Scheme 20): Ce(III) is initially oxidized to Ce(IV) by photoexcited azodicarboxylate, followed by in situ formation of a Ce(IV)–OR complex from the alcohol starting material.70 This Ce(IV)–alkoxide species then undergoes LMCT photoexcitation and subsequent homolysis to generate Ce(III) and an alkoxy radical intermediate. C–C bond cleavage and ring-opening provide a carbonyl and distal C-centered radical, the latter of which couples with azodicarboxylate to form a nascent N-centered radical. It is postulated that this N-centered radical is reduced by Ce(III) to afford the product and to turn over the catalytic cycle. Recently, the Zuo lab merged LMCT-mediated alkoxy radical generation with Lewis acid catalysis to achieve a one-step protocol for the C–C bond cleavage of ketones.71
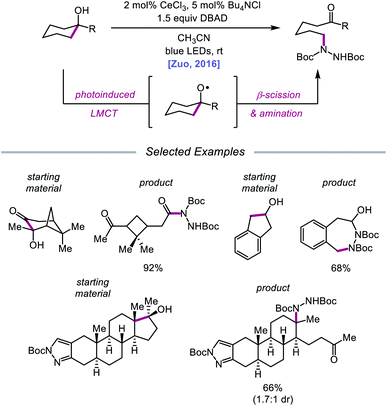 | ||
| Scheme 19 C–C bond cleavage and amination of cycloalkanols via cerium catalysis. DBAD, di-tert-butyl azodicarboxylate. | ||
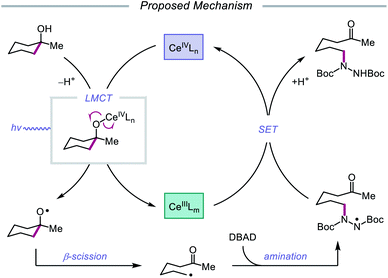 | ||
| Scheme 20 Proposed mechanism for C–C bond cleavage and amination of cycloalkanols through LMCT activation of O–H bonds. | ||
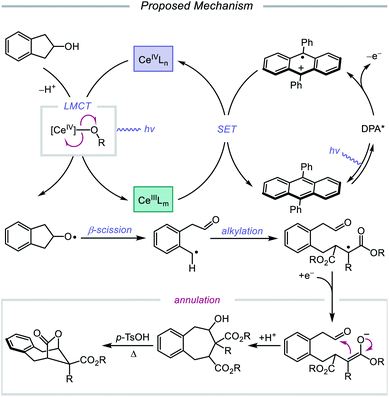
In 2018, Zuo and coworkers extended this method of alkoxy radical generation to the synthesis of bridged lactones by leveraging cerium-catalyzed β-scission and ring expansion of cycloalkanols.72 Given the redox potential of their Ce(III) catalyst (E1/2 (CeIV/CeIII) = 0.40 V vs. SCE in MeCN), regeneration of photoactive Ce(IV) to turn over the catalytic cycle requires a sufficiently oxidizing radical intermediate, such as the N-centered radical employed in the authors' earlier alkoxy radical-mediated amination work. As a result, any catalytic system relying on a single cerium catalyst is constrained to a narrow selection of electron acceptors and transformations and cannot accommodate the single-electron reduction of common radical intermediates such as α-acyl radicals (E1/2 = −0.60 V vs. SCE in MeCN). To overcome this limitation, an electron shuttle with enough oxidizing power to oxidize Ce(III) to Ce(IV) and enough reducing potential to quench the radical intermediate was investigated. The authors concluded that 9,10-diphenylanthracene (DPA) can serve as an efficient electron transfer catalyst and, together with the cerium photocatalyst, can enable the ring expansion of cycloalkanols with electron-deficient alkenes to form bridged lactones (Scheme 21). Results from UV-Vis absorption spectroscopy and Stern–Volmer quenching studies suggest that excited-state DPA plays a catalytic role in the reaction, rendering the reaction a two-photon process. More specifically, it is postulated that irradiation first triggers LMCT, promoting Ce–OR bond homolysis to form the alkoxy radical intermediate (Scheme 22). Cleavage of the α-hydroxy C–C bond furnishes an alkyl radical that then adds into an electron-deficient alkene, forming an α-acyl radical. Reduction of this nascent radical is achieved using photoexcited DPA (*E1/2 = −1.77 V vs. SCE in MeCN) to give an enolate and DPA˙+. An intramolecular aldol reaction facilitates annulation to a ring-expanded intermediate, which is then converted to a bridged lactone upon treatment with p-TsOH, while DPA˙+ (E1/2 = 1.13 V vs. SCE in MeCN) oxidizes Ce(III) back to catalytically active Ce(IV). Substituted indanols, including those containing functional groups prone to transition-metal-catalyzed insertions, are amenable to the reaction, forming [4.2.1]-bridged lactones, while cyclobutanol derivatives are easily converted to a diverse range of [3.2.1] ring systems.
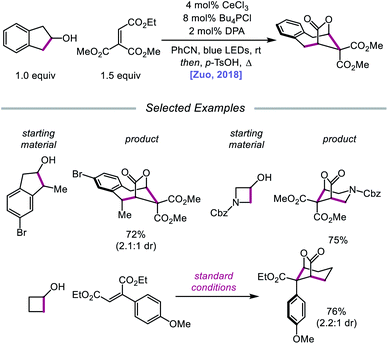 | ||
| Scheme 21 Cerium-catalyzed β-scission and ring-expansion of cycloalkanols for the formation of bridged lactones. | ||
The Zuo lab later disclosed an application of cerium-catalyzed β-scission to the dehydroxymethylation of alcohols and functionalization of these nucleophilic alkyl synthons in radical-mediated conjugate additions (Scheme 23).73 Through a double-excitation mechanism, C–C bond β-cleavage can be exploited to form alkyl radicals with concomitant extrusion of formaldehyde. A catalyst system comprising CeBr3 with Bu4NBr under 365 nm irradiation was found to be integral to the efficiency of the reaction, allowing for excitation of both the in situ formed Ce(IV)–alkoxide species to the LMCT excited state and the Ce(III)–alkoxide complex. The excited-state of the Ce(III)–alkoxide serves as a strong reductant and is capable of reducing the α-acyl radical to the desired product following alkyl radical addition to an electron-deficient alkene. Moreover, the authors note that for dehydroxymethylation to occur with primary alcohols, the corresponding alkoxy radicals would have to undergo β-scission faster than competitive 1,5-HAT. Interestingly, this cerium-based catalyst system generally favors dehydroxymethylation, as demonstrated in selectivity studies, and it is suggested that the weak interaction between alkoxy radicals and surrounding Lewis acidic cerium salts can promote the desired β-scission pathway. A variety of primary alcohols embedded in complex substrates, including protected uridine and adenosine, are amenable to the reaction; surprisingly, primary hydroxy groups are selectively dehydroxymethylated in the presence of secondary and tertiary alcohols in simple carbocycles. Beyond C(sp3)–C(sp3) bond formation, this protocol further leverages the generated alkyl radicals for hydrogenation, amination, alkenylation, and oxidation reactions.
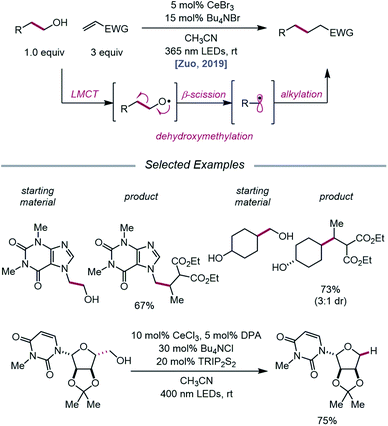 | ||
| Scheme 23 Cerium-catalyzed dehydroxymethylation of primary alcohols enabled by LMCT. TRIP2S2, 1,2-bis(2,4,6-triisopropylphenyl)disulfane. | ||
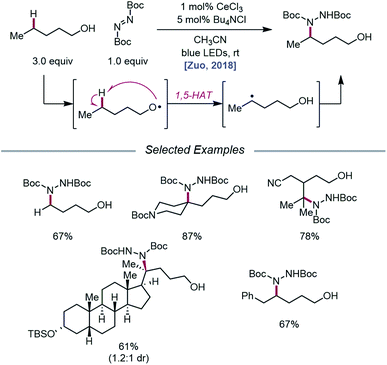
As LMCT was emerging as a method for realizing β-scission reactions, the Zuo group demonstrated in 2018 the advantages LMCT can provide for achieving remote C–H bond functionalization.74 This simple protocol employs catalytic CeCl3 and Bu4NCl to promote alkoxy radical formation via a photoexcited Ce(IV)–alkoxide species. Instead of undergoing β-scission, the resulting primary alkoxy radical engages in facile 1,5-HAT to form a δ-C-centered radical, which can couple with di-tert-butyl azodicarboxylate (DBAD) to construct a new C–N bond and deliver the desired amination product (Scheme 24). A variety of free alcohols, from 4-butanol to lithocholanyl alcohol, are functionalized in this manner. Notably, β-fragmentation is not a competitive pathway for the alcohol substrates studied, likely because these substrates lack substituents at the α- and β-carbons and are not generally prone to β-scission.
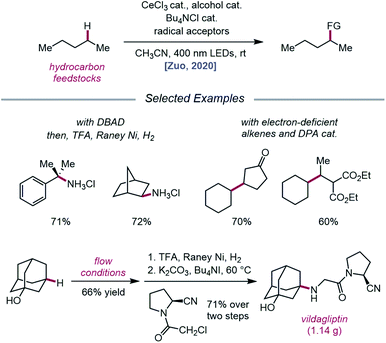
Later that year, it was reported that alkoxy radicals generated from LMCT not only participate in intramolecular HAT but can also be used as intermolecular HAT catalysts for the C(sp3)–H bond functionalization of methane and other gaseous alkanes (Scheme 25).75 Recognizing that simple alcohols, such as methanol or 2,2,2-trichloroethanol (TCE), can serve as precursors to highly electrophilic alkoxy radicals, the authors propose that these catalysts can abstract an H atom from a methane C–H bond (C–H BDFE ≈ 97 kcal mol−1) to form an alkyl radical that proceeds to couple with an acceptor, such as DBAD.12,13 It is demonstrated that 0.5 mol% (Bu4N)2CeCl6 and 20 mol% TCE are effective at providing 63% yield for the amination of methane, while the use of 0.01 mol% CeCl3 and the same TCE catalyst leads to a remarkable yield of 97% aminated product with ethane. Propane and butane pose an interesting challenge with regards to selectivity between the slightly weaker methylene C–H bonds and the more numerous methyl C–H bonds. The authors observe that selectivity can be induced by modulating the electrophilicity of the alcohol HAT catalyst, with methanol generally favoring HAT from methylene C–H bonds. This mixed-phase gas/solution reaction has been adapted to a continuous flow platform, facilitating the scalable production of functionalized alkanes from gaseous hydrocarbon feedstocks.
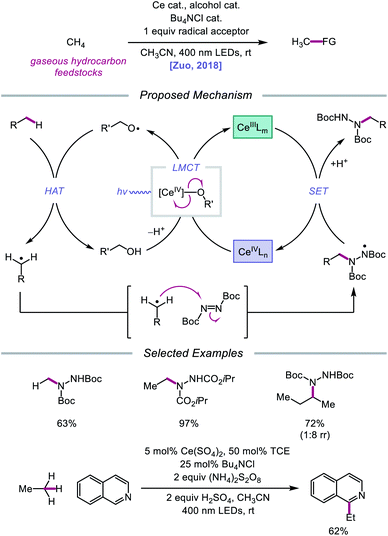 | ||
| Scheme 25 Cerium-catalyzed C–H functionalization of gaseous hydrocarbon feedstocks using alcohols as HAT catalysts. | ||
Expanding upon this protocol for intermolecular HAT, Liu, Zuo, and coworkers in 2020 again utilized simple alcohols as tunable HAT catalysts for the C–H amination and alkylation of liquid hydrocarbons (Scheme 26).76 Extensive selectivity studies reveal that methanol-catalyzed conditions generally favor abstraction from weaker C–H bonds, while sterically bulky alcohols, such as tert-butanol or 1-adamantanol, promote abstraction from less sterically encumbered bonds. Electron-deficient alcohols, such as TCE or 2,2,2-trifluoroethanol, possess stronger O–H bonds and, thus, decrease selectivity for weaker C–H bonds when used as HAT catalysts. Guided by these principles, a variety of hydrocarbons, including cumene and norbornane, are successfully aminated with high selectivity for weak C–H bonds, while 1-adamantanol is converted to 3-amino-1-adamantanol on gram-scale via continuous flow and transformed into antidiabetic drug vildagliptin after two additional synthetic steps. Using DPA as a co-catalyst, C–H bond alkylation is also achieved with electron-deficient alkenes, from enones to substituted methylene-malonates. The LMCT event essential to the Zuo group's method for alkoxy radical generation was then investigated in detail using steady-state homolysis experiments and transient absorption spectroscopy. From this work, the quantum yield of cerium methoxide homolysis (38.8%) and the lifetime of the LMCT state (∼0.73 ps for cerium methoxide) are determined, collectively ruling out LMCT as the rate-determining step in this reaction.
Proton-coupled electron transfer
As highlighted thus far, most modern methods for the activation of O–H bonds require the intermediacy of an alkoxyiodo or a metal–alkoxide species. In principle, direct HAT from the hydroxy group of alcohols would provide straightforward access to the desired alkoxy radical. However, the strength of such bonds (O–H BDFE ≈ 105 kcal mol−1) precludes the use of common hydrogen atom abstractors, which lack the necessary driving force to enable O–H activation.10,77 Moreover, unfavorable polar effects further limit the realization of HAT activation mechanisms—common H-atom acceptors are electrophilic in character and are kinetically slow to abstract from polarity-mismatched protic O–H bonds, instead preferentially activating weaker C–H bonds prevalent in organic molecules.78In light of these limitations, the Knowles group sought to use proton-coupled electron transfer (PCET) as an alternative mechanism for direct O–H bond homolysis to provide catalytic access to alkoxy radicals from simple alcohol starting materials. Multisite PCET involves the concerted movement of a proton and an electron in a single elementary step to site-separated acceptors (Scheme 27).79 Unlike classical HAT, PCET allows access to a broader thermodynamic range through the use of tunable oxidant/base pairs that together serve as formal H-atom acceptors. Each catalyst pair can be described by an effective BDFE that represents the strength of the bond that can be broken in a thermoneutral reaction using this reagent system, calculated from the pKa value of the conjugate acid (B–H) of the employed base, the reduction potential of the oxidant, and the reduction potential of H+ to H˙.12,79d,e,80 By modulating the pKa of B–H and the reduction potential of the one-electron oxidant, effective BDFEs can be achieved that are thermodynamically competent to activate aliphatic O–H bonds, overcoming the energetic limitations of classical HAT reagents. Additionally, the selectivity of multi-site PCET events, which are dictated by hydrogen bonding between the Brønsted base and polar functional group, is expected to be orthogonal to HAT processes, as C–H bonds are poor hydrogen bond donors. Using a separated oxidant/base catalyst pair can enable polar O–H bonds to be homolyzed selectively. Along with these advantages, concerted PCET processes are often kinetically faster than the stepwise electron transfer or proton transfer steps. This is due to the fact that the kinetics of PCET are a function of the reaction's thermodynamic driving force.81 Since the products formed via a concerted pathway are lower in energy than the intermediates formed in the stepwise pathways, PCET activation of O–H bonds is expected to be more efficient than stepwise deprotonation/oxidation steps when relatively weak oxidants and bases are employed. In fact, PCET has long been implicated in the activation of phenols, particularly in biological systems,82 but prior to 2016, PCET activation of aliphatic O–H bonds had largely remained elusive.83
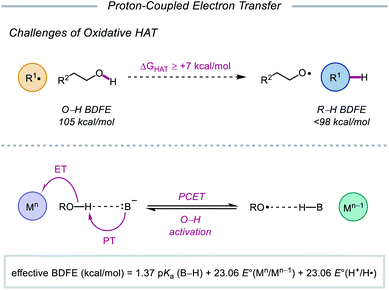 | ||
| Scheme 27 Challenges of conventional HAT, and multisite PCET mechanism for homolytic O–H activation. | ||
In 2016, Knowles and coworkers leveraged this PCET framework for the direct catalytic activation of tertiary alcohol O–H bonds.84 Inspired by mechanistic studies from Baciocchi, Bietti, Lanzalunga, and Steenken,85 the authors achieved a redox-neutral isomerization of cyclic aryl alkanols to linear ketones through a C–C bond scission step mediated by an alkoxy radical intermediate (Scheme 28). This protocol for alkoxy radical generation relies on an intramolecular PCET event comprising deprotonation of a hydroxyl group by a Brønsted base in concert with one-electron reduction of a proximal arene radical cation. In particular, Baciocchi's studies suggest that this reaction design enables alcohol O–H bonds to be selectively deprotonated over C–H bonds at near diffusion-controlled rates,85 despite modest driving forces. Various tertiary cycloalkanols across a range of ring sizes can be accommodated, indicating that strained ring systems are not a requirement for β-scission under these conditions; linear alcohols that can extrude free alkyl radical fragments are also amenable to the reaction. Notably, this ring-opening strategy can also tolerate polycyclic structures without compromising proximal stereocenters. However, this catalytic system is limited to substrates decorated with a pendent electron-rich arene in order to facilitate PCET reactivity.
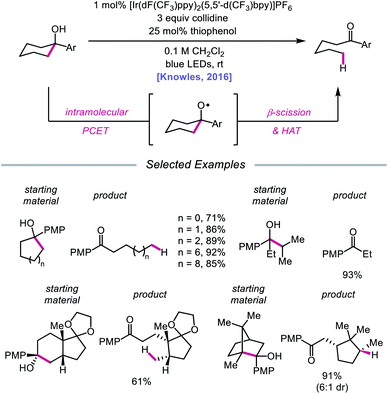 | ||
| Scheme 28 Ring-opening of cycloalkanols enabled by PCET activation of O–H bonds. PMP, p-methoxyphenyl. | ||
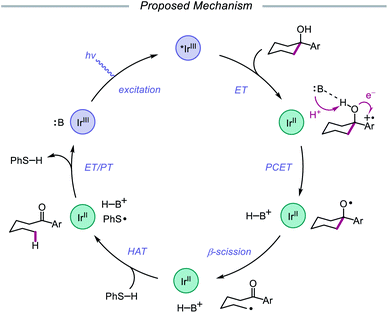
Results from mechanistic studies are consistent with the proposed catalytic cycle (Scheme 29), wherein an excited-state Ir(III) photoredox catalyst oxidizes an electron-rich arene on the substrate to furnish a radical cation. An intramolecular PCET event then occurs, during which concerted deprotonation by collidine as the Brønsted base and oxidation by the proximal aryl radical cation as an internal oxidant affords the alkoxy radical. Subsequent β-fragmentation of the adjacent C–C bond results in ring-opening to generate an aryl ketone and a distal alkyl radical, which is then reduced by catalytic thiophenol. Finally, single electron reduction of the thiyl by the Ir(II) species and protonation by collidinium turns over the catalytic cycle. Support for a PCET event in the formation of the alkoxy radical is drawn from an examination of the reaction efficiency for eight substrates bearing arenes with different oxidation potentials in the presence of four different Brønsted bases. These studies demonstrate that effective bond strength considerations can successfully predict the feasibility of alkoxy radical generation across the entire series. In 2018, the Yang group elaborated on this PCET system, demonstrating that the nascent alkyl radical formed after β-scission can be captured by radical acceptors as part of an allylation protocol or oxidized by O2 in air for subsequent formylation.86
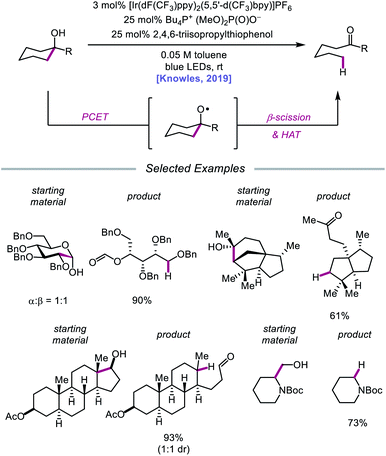
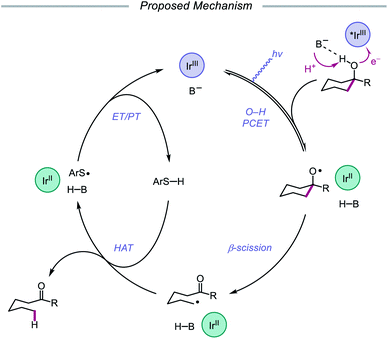
In 2019, the Knowles group expanded upon the use of PCET as a catalytic platform for O–H activation by reporting a general procedure for the ring-opening isomerization of cyclic aliphatic alcohols (Scheme 30). Unlike the previous protocol, this strategy no longer requires that substrates bear an oxidizable arene.87 Instead, direct PCET activation of O–H bonds in primary, secondary, and tertiary aliphatic alcohols can be achieved with a key change—substituting the neutral collidine base for an anionic tetrabutylphosphonium dimethyl phosphate base. The authors hypothesize that the improved reaction efficiency may result from the enhanced ability of the anionic phosphate base to form a more favorable pre-PCET hydrogen bond complex with the alcohol substrate (Scheme 31). A wide range of alcohols and hemiacetals, including hexose and pentose derivatives, are viable substrates under these conditions, as well as more complex polycyclic scaffolds which can be isomerized with high regioselectivity. Interestingly, computational studies reveal that many of the cyclic alcohol starting materials are thermodynamically more stable than their ring-opened carbonyl products, even though the reaction is a redox-neutral isomerization that consumes no stoichiometric reagents other than photons. These observations demonstrate how excited-state electron transfer events can be employed to drive reactions against a thermodynamic gradient in a manner that cannot be accomplished using conventional ground-state catalysts. The PCET strategy described here for C–C bond scission was adapted independently by the groups of Zhang and Knowles for the depolymerization of lignin models and biomass-derived polymeric lignin.88,89
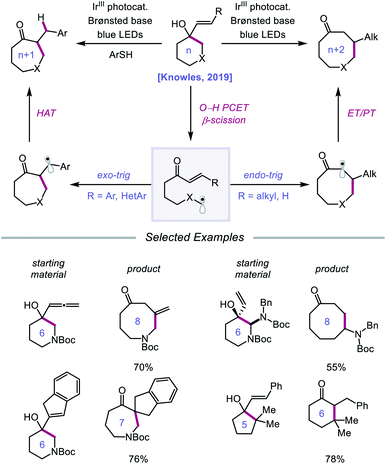
Following their 2019 report, the Knowles lab developed a strategy for the catalytic ring expansion of cyclic alcohols to n + 1 or n + 2 expanded ketones by taking advantage of the propensity of alkoxy radicals to undergo C–C cleavage.90 By employing a similar series of elementary steps as in their prior work, O–H PCET is leveraged to generate an alkoxy radical intermediate from cyclic allylic alcohols (Scheme 32). After β-scission, an α,β-unsaturated ketone and tethered alkyl radical are formed, which recombine to give a new C–C bond as part of a ring-expanded ketone product. The regioselectivity of this enone addition step can be varied to allow access to ring systems homologated by two of the olefinic carbons (n + 2) or by only one carbon (n + 1). The selectivity of this C–C bond-forming step is controlled by the nature of the substituent on the exocyclic olefin acceptor, with alkyl substituents favoring endo-trig cyclization to generate an α-acyl radical and aryl substituents preferring exo-trig cyclization to furnish a stabilized benzylic radical. While the Ir(II) state of the photocatalyst can adequately reduce the α-acyl radical and close the catalytic cycle, the authors note that in the latter exo-trig case, the potential of Ir(II) is insufficient to reduce the benzylic radical. To overcome this complication, 2,4,6-triisopropylthiophenol is employed catalytically to mediate HAT reduction of the benzylic radical.
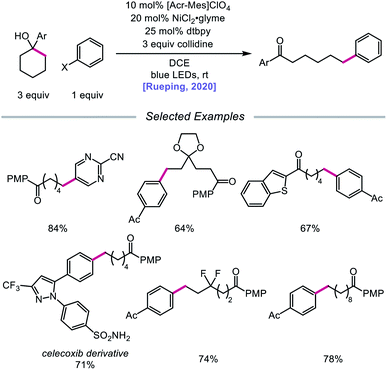
In 2020, the Rueping group reported a strategy using PCET-promoted β-scission to achieve site-specific nickel-catalyzed arylation of ketones (Scheme 33).91 The authors propose a mechanism wherein a highly oxidizing acridinium-derived organophotocatalyst and collidine base are used to activate the O–H bond in an arene-containing tertiary alcohol through a radical-cation redox relay PCET event (Scheme 34). Subsequent C–C bond cleavage provides a distal carbon-centered radical that can be intercepted by Ni(0) to give an alkylnickel(I) intermediate. Oxidative addition with an aryl halide affords a Ni(III) species that can then undergo reductive elimination to furnish the cross-coupled product, creating a new C(sp3)–C(sp2) bond in the process. By merging PCET with nickel photoredox catalysis, Rueping and coworkers demonstrate that the site of arylation can be modulated by using carbocycles of various ring sizes, from 3-membered rings to 15-membered ring derivatives, regardless of the ring strain. Bridged-rings and tertiary linear alcohols are also competent radical precursors in this cross-coupling system. Following this report, the authors disclosed a related intramolecular PCET system that achieves trifluoromethylthiolation of ring-opened ketone products.92
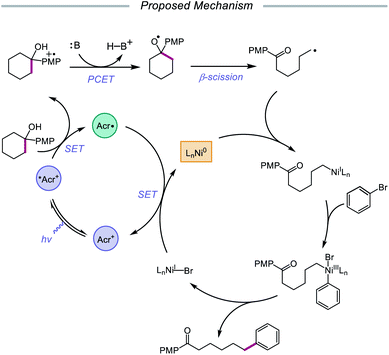 | ||
| Scheme 34 Postulated mechanism for site-specific arylation of ring-opened ketones enabled by PCET and nickel catalysis. | ||
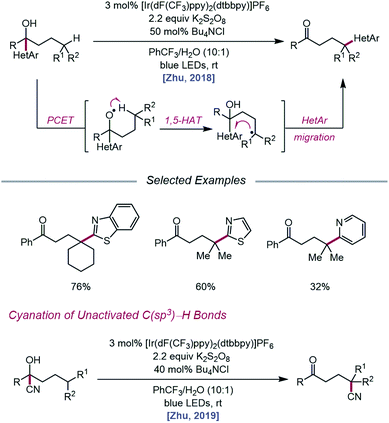
As methods for the ring-opening and functionalization of cycloalkanols continued to be explored, Zhu and coworkers reported a strategy for the heteroarylation of remote C(sp3)–H bonds via an alkoxy radical intermediate.93 Starting from a heteroarene-containing tertiary alcohol in the presence of an Ir(III) photocatalyst, K2S2O8, and Bu4NCl, a variety of isomeric heteroaryl ketones are formed, with γ-C–H bonds functionalized with migrated benzothiazolyl, thiazolyl, and pyridyl groups (Scheme 35). Stern–Volmer studies indicate that [Ir(dF(CF3)ppy)2(dtbbpy)]PF6 can be oxidatively quenched by K2S2O8. The inability of the resulting Ir(IV) complex (E1/2(IV/III) = 1.69 V vs. SCE in MeCN) to engage the alcohol substrate in direct electron transfer (Ep/2 = 2.06 V vs. SCE in MeCN) leads the authors to postulate that a ground-state PCET process involving Ir(IV) as the electron acceptor may be operative. Once the alkoxy radical is formed, it triggers successive 1,5-HAT and intramolecular heteroaryl migration to furnish a ketone product. In a similar vein, the Zhu group disclosed a PCET process for cyanation of remote C–H bonds via intramolecular cyano group migration (Scheme 35).94 A PCET mechanism comprising Ir(IV) as the oxidant and sulfate as the base is invoked, although the authors note that direct HAT from the alcohol O–H bond by a sulfate radical anion cannot be ruled out.
In an effort to extend the synthetic applications of PCET activation of O–H bonds beyond β-fragmentation reactions, Knowles and coworkers reported in 2020 a catalytic system for the intramolecular hydroetherification and carboetherification of alkenols (Scheme 36).95 The authors propose a transformation in which the nascent electrophilic alkoxy radical generated from PCET adds into a pendent olefin, subsequently producing an adjacent C-centered radical that can be quenched with either an HAT co-catalyst to form tetrahydrofuran products or intercepted by an electron-deficient alkene to generate alkylated tetrahydrofurans. Trisubstituted, disubstituted, styrenyl, and monosubstituted alkenes are competent under the reaction conditions, and groups such as alkyl halides, heterocycles, sulfonamides, and thioethers are shown to be compatible. Notably, the versatility of this transformation gives rise to fused, bridged, and spirocyclic tetrahydrofuran scaffolds from simple alkenol starting materials. In addition to 5-exo-trig cyclizations, this method can also be utilized to achieve 6-endo-trig and 6-exo-trig ring closures with trisubstituted alkenes to provide tetrahydropyran products, with the latter outcompeting 1,5-HAT from allylic C–H bonds.
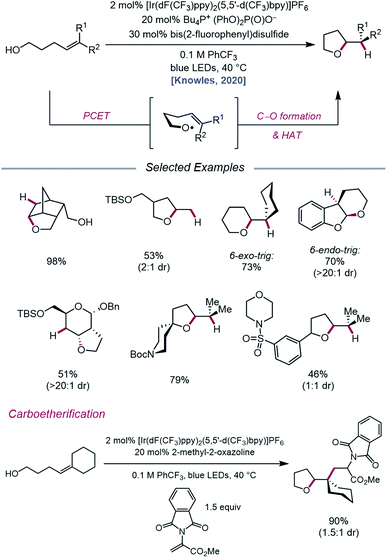 | ||
| Scheme 36 Catalytic hydroetherification and carboetherification of unactivated alkenes enabled by PCET. | ||
Against this backdrop of new methods for PCET activation of alcohol O–H bonds, Shi and coworkers proposed in 2018 a related but alternative mechanism for alkoxy radical generation enabled by direct HAT.96 Using stoichiometric phthaloyl peroxide (PPO) and Bu4NBr under blue light irradiation, radical ring-opening and bromination of a variety of cycloalkanols are accomplished (Scheme 37). Based on kinetic isotope effect studies and DFT computations, the authors suggest that, rather than PCET, PPO facilitates the formation of an alkoxy radical intermediate through a direct HAT event aided by hydrogen-bonding interactions. PPO, in equilibrium with its diradical form, engages with a bromide anion to form a radical-anion intermediate, which has an enhanced ability to promote HAT from O–H bonds due to H-bonding between the carboxyl groups on PPO and the alcohol substrate. It is proposed that 5- to 8-membered cyclic alcohols undergo HAT to the PPO-derived radical-anion, producing an alkoxy radical that then undergoes β-scission to the ring-opened intermediate. Coupling with a bromine radical furnishes the distally brominated ketone product.
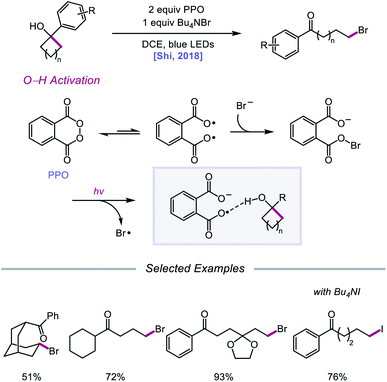 | ||
| Scheme 37 Ring-opening halogenation of cycloalkanols via HAT induced by Brønsted base-tethered acyloxy radicals. | ||
Conclusions
Significant advances in catalytic alcohol O–H activation, largely enabled by the development of light-driven and transition metal-catalyzed mechanisms, have facilitated straightforward access to alkoxy radicals from free alcohols and have provided a solution to a long-standing challenge in radical chemistry. By precluding the need for prefunctionalized substrates and stoichiometric reagents, this diverse repertoire of methods has been utilized for the development of myriad transformations mediated by alkoxy radicals, including C–C bond cleavage, remote C–H bond functionalization, and C–O bond formation. While these strategies for alkoxy radical generation have expanded the scope of new transformations and inspired novel disconnections, several challenges remain. The next steps in the advancement of these catalytic systems are to control the fate of the alkoxy radical by modulating reactivity toward HAT, β-scission, or alkene addition via catalyst control, expanding the scope of intermolecular reactivity, and developing technologies for stereocontrol in alkoxy radical-mediated transformations. We anticipate that progress in these areas can be achieved as we continue to see innovations in alkoxy radical chemistry in the years to come.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Institutes of Health (R35 GM134893).References
- H. Wieland, Ber. Dtsch. Chem. Ges., 1911, 44, 2550–2556 CrossRef CAS
.
- For reviews of alkoxy radicals, see:
(a) P. Gray and A. Williams, Chem. Rev., 1959, 59, 239–328 CrossRef CAS
; (b) C. Walling, Pure Appl. Chem., 1967, 15, 69–80 CAS
; (c) J. Hartung, T. Gottwald and K. Špehar, Synthesis, 2002, 1469–1498 CrossRef CAS
; (d) J. J. Guo, A. Hu and Z. Zuo, Tetrahedron Lett., 2018, 59, 2103–2111 CrossRef CAS
; (e) K. Jia and Y. Chen, Chem. Commun., 2018, 54, 6105–6112 RSC
; (f) X. Wu and C. Zhu, Chem. Commun., 2019, 55, 9747–9756 RSC
.
- M. Rueda-Becerril, J. C. T. Leung, C. R. Dunbar and G. M. Sammis, J. Org. Chem., 2011, 76, 7720–7729 CrossRef CAS
.
- G. Majetich and K. Wheless, Tetrahedron, 1995, 51, 7095–7129 CrossRef CAS
.
- Ž. Čeković, Tetrahedron, 2003, 59, 8073–8090 CrossRef
.
-
E. Suárez and M. S. Rodriguez, in Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2001, pp. 440–454 Search PubMed
.
- M. Murakami and N. Ishida, Chem. Lett., 2017, 46, 1692–1700 CrossRef CAS
.
- J. Hartung, Eur. J. Org. Chem., 2001, 619–632 CrossRef CAS
.
-
J. Hartung, in Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2001, pp. 427–439 Search PubMed
.
-
L. Feray, N. Kuznetsov and P. Renaud, in Radicals in Organic Synthesis, ed. P. Renaud and M. P. Sibi, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2001, pp. 246–278 Search PubMed
.
- M. Salamone and M. Bietti, Acc. Chem. Res., 2015, 48, 2895–2903 CrossRef CAS
.
- J. J. Warren, T. A. Tronic and J. M. Mayer, Chem. Rev., 2010, 110, 6961–7001 CrossRef CAS
.
- S. J. Blanksby and G. B. Ellison, Acc. Chem. Res., 2003, 36, 255–263 CrossRef CAS
.
-
(a) D. H. R. Barton, J. M. Beaton, L. E. Geller and M. M. Pechet, J. Am. Chem. Soc., 1960, 82, 2640–2641 CrossRef CAS
; (b) D. H. R. Barton and J. M. Beaton, J. Am. Chem. Soc., 1960, 82, 2641 CrossRef CAS
; (c) D. H. R. Barton, J. M. Beaton, L. E. Geller and M. M. Pechet, J. Am. Chem. Soc., 1961, 83, 4076–4083 CrossRef CAS
.
-
(a) J. K. Kochi, J. Am. Chem. Soc., 1962, 84, 1193–1197 CrossRef CAS
; (b) C. Walling and A. Padwa, J. Am. Chem. Soc., 1963, 85, 1593–1597 CrossRef CAS
; (c) C. Walling and R. T. Clark, J. Am. Chem. Soc., 1974, 96, 4530–4534 CrossRef CAS
; (d) M. Bietti, O. Lanzalunga and M. Salamone, J. Org. Chem., 2005, 70, 1417–1422 CrossRef CAS
; (e) A. L. J. Beckwith, R. Kazlauskas and M. R. Syner-Lyons, J. Org. Chem., 1983, 48, 4718–4722 CrossRef CAS
.
- S. P. Morcillo, Angew. Chem., Int. Ed., 2019, 58, 14044–14054 CrossRef CAS
.
- X. Y. Yu, J. R. Chen and W. J. Xiao, Chem. Rev., 2020 DOI:10.1021/acs.chemrev.0c00030
.
- H. Zhu, J. C. T. Leung and G. M. Sammis, J. Org. Chem., 2015, 80, 965–979 CrossRef CAS
.
- J. H. Horner, S. Y. Choi and M. Newcomb, Org. Lett., 2000, 2, 3369–3372 CrossRef CAS
.
- For selected examples, see:
(a) P. de Armas, C. G. Francisco and E. Suárez, J. Am. Chem. Soc., 1993, 115, 8865–8866 CrossRef CAS
; (b) A. Martin, J. A. Salazar and E. Suárez, J. Org. Chem., 1996, 61, 3999–4006 CrossRef CAS
; (c) N. Hussain, D. O. Morgan, C. R. White and J. A. Murphy, Tetrahedron Lett., 1994, 35, 5069–5072 CrossRef CAS
; (d) J. Hartung and R. Kneuer, Tetrahedron: Asymmetry, 2003, 14, 3019–3031 CrossRef CAS
; (e) H. Zhu, J. G. Wickenden, N. E. Campbell, J. C. T. Leung, K. M. Johnson and G. M. Sammis, Org. Lett., 2009, 11, 2019–2022 CrossRef CAS
; (f) S. Yamashita, A. Naruko, Y. Nakazawa, L. Zhao, Y. Hayashi and M. Hirama, Angew. Chem., Int. Ed., 2015, 54, 8538–8541 CrossRef CAS
.
- J. M. Surzur, M. P. Bertrand and R. Nouguier, Tetrahedron Lett., 1969, 48, 4197–4200 CrossRef
.
-
(a) Z. Čeković and M. Cvetković, Tetrahedron Lett., 1982, 23, 3791–3794 CrossRef
; (b) Z. Čeković and M. Cvetković, Tetrahedron Lett., 1982, 23, 3791–3794 CrossRef
; (c) R. Kundu and Z. T. Ball, Org. Lett., 2010, 12, 2460–2463 CrossRef CAS
; (d) H. Guan, S. Sun, Y. Mao, L. Chen, R. Lu, J. Huang and L. Liu, Angew. Chem., Int. Ed., 2018, 57, 11413–11417 CrossRef CAS
.
-
(a) C. Walling and A. Padwa, J. Am. Chem. Soc., 1961, 83, 2207–2208 CrossRef CAS
; (b) C. Walling and D. Bristol, J. Org. Chem., 1972, 37, 3514–3516 CrossRef CAS
; (c) C. Walling and J. A. Mcguinness, J. Am. Chem. Soc., 1969, 91, 2053–2058 CrossRef CAS
; (d) K. Heusler and J. Kalvoda, Angew. Chem., Int. Ed., 1964, 3, 525–538 CrossRef
.
-
(a) A. L. J. Beckwith, B. P. Hay and G. M. Williams, J. Chem. Soc., Chem. Commun., 1989, 1202–1203 RSC
; (b) D. J. Pasto and F. Cottard, Tetrahedron Lett., 1994, 35, 4303–4306 CrossRef CAS
; (c) G. Petrović, R. N. Saičić and Ž. Čeković, Synlett, 1999, 635–637 CrossRef
.
-
(a) A. L. J. Beckwith and B. P. Hay, J. Am. Chem. Soc., 1988, 110, 4415–4416 CrossRef CAS
; (b) J. Hartung and F. Gallou, J. Org. Chem., 1995, 60, 6706–6716 CrossRef CAS
.
-
(a) S. Kim, T. A. Lee and Y. Song, Synlett, 1998, 471–472 CrossRef CAS
; (b) M. Zlotorzynska, H. Zhai and G. M. Sammis, Org. Lett., 2008, 10, 5083–5086 CrossRef CAS
.
- J. Zhang, Y. Li, F. Zhang, C. Hu and Y. Chen, Angew. Chem., Int. Ed., 2016, 55, 1872–1875 CrossRef CAS
.
- C. Wang, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 13495–13498 CrossRef CAS
.
- A. L. Barthelemy, B. Tuccio, E. Magnier and G. Dagousset, Angew. Chem., Int. Ed., 2018, 57, 13790–13794 CrossRef CAS
.
- I. Kim, B. Park, G. Kang, J. Kim, H. Jung, H. Lee, M.-H. Baik and S. Hong, Angew. Chem., Int. Ed., 2018, 57, 15517–15522 CrossRef CAS
.
- X. Bao, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2019, 58, 2139–2143 CrossRef CAS
.
- L. Capaldo and D. Ravelli, Chem. Commun., 2019, 55, 3029–3032 RSC
.
- A. N. Herron, D. Liu, G. Xia and J.-Q. Yu, J. Am. Chem. Soc., 2020, 142, 2766–2770 CrossRef CAS
.
- H. Togo and M. Katohgi, Synlett, 2001, 565–581 CrossRef CAS
.
- For selected examples, see:
(a) J. I. Concepción, C. G. Francisco, R. Hernández, J. A. Salazar and E. Suárez, Tetrahedron Lett., 1984, 25, 1953–1956 CrossRef
; (b) R. Freire, J. J. Marrero, M. S. Rodríguez and E. Suárez, Tetrahedron Lett., 1986, 27, 383–386 CrossRef CAS
; (c) P. de Armas, C. G. Francisco and E. Suárez, Angew. Chem., Int. Ed., 1992, 31, 772–774 CrossRef
; (d) H. Togo, T. Muraki, Y. Hoshina, K. Yamaguchi and M. Yokoyama, J. Chem. Soc., Perkin Trans. 1, 1997, 787–793 RSC
.
- K. Jia, F. Zhang, H. Huang and Y. Chen, J. Am. Chem. Soc., 2016, 138, 1514–1517 CrossRef CAS
.
- K. Jia, Y. Pan and Y. Chen, Angew. Chem., Int. Ed., 2017, 56, 2478–2481 CrossRef CAS
.
- R. G. W. Norrish and C. H. Bamford, Nature, 1936, 138, 1016 CrossRef CAS
.
-
(a) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410–9464 CrossRef CAS
; (b) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613–8661 CrossRef CAS
.
- K. Jia, J. Li and Y. Chen, Chem. - Eur. J., 2018, 24, 3174–3177 CrossRef CAS
.
- D. Wang, J. Mao and C. Zhu, Chem. Sci., 2018, 9, 5805–5809 RSC
.
- X. Wu, H. Zhang, N. Tang, Z. Wu, D. Wang, M. Ji, Y. Xu, M. Wang and C. Zhu, Nat. Commun., 2018, 9, 3343 CrossRef
.
- G.-X. Li, X. Hu, G. He and G. Chen, Chem. Sci., 2019, 10, 688–693 RSC
.
- X. Hu, G.-X. Li, G. He and G. Chen, Org. Chem. Front., 2019, 6, 3205–3209 RSC
.
-
(a) S. E. Schaafsma, H. Steinberg and T. J. de Boer, Recl. Trav. Chim. Pays-Bas, 1966, 85, 70–72 CrossRef CAS
; (b) S. E. Schaafsma, H. Steinberg and T. J. de Boer, Recl. Trav. Chim. Pays-Bas, 1966, 85, 73–74 CrossRef CAS
; (c) S. E. Schaafsma, R. Jorritsma, H. Steinberg and T. J. de Boer, Tetrahedron Lett., 1973, 14, 827–830 CrossRef
.
- K. Meyer and J. Roček, J. Am. Chem. Soc., 1972, 94, 1209–1214 CrossRef CAS
.
-
(a) N. Iwasawa, S. Hayakawa, K. Isobe and K. Narasaka, Chem. Lett., 1991, 20, 1193–1196 CrossRef
; (b) N. Iwasawa, M. Funahashi, S. Hayakawa and K. Narasaka, Chem. Lett., 1993, 22, 545–548 CrossRef
.
- A. Clerici, F. Minisci, K. Ogawa and J. M. Surzur, Tetrahedron Lett., 1978, 19, 1149–1152 CrossRef
.
-
(a) S. Chiba, Z. Cao, S. A. A. El Bialy and K. Narasaka, Chem. Lett., 2006, 35, 18–19 CrossRef CAS
; (b) S. Chiba, M. Kitamura and K. Narasaka, J. Am. Chem. Soc., 2006, 128, 6931–6937 CrossRef CAS
.
- H. Zhao, X. Fan, J. Yu and C. Zhu, J. Am. Chem. Soc., 2015, 137, 3490–3493 CrossRef CAS
.
- For recent work on silver-catalyzed β-scission and radical fluorination, see: X. Zhou, H. Ding, P. Chen, L. Liu, Q. Sun, X. Wang, P. Wang, Z. Lv and M. Li, Angew. Chem., Int. Ed., 2020, 59, 4138–4144 CrossRef CAS
.
- S. Ren, C. Feng and T. P. Loh, Org. Biomol. Chem., 2015, 13, 5105–5109 RSC
.
- N. Ishida, S. Okumura, Y. Nakanishi and M. Murakami, Chem. Lett., 2015, 44, 821–823 CrossRef CAS
.
-
(a) X. Fan, H. Zhao, J. Yu, X. Bao and C. Zhu, Org. Chem. Front., 2016, 3, 227–232 RSC
; (b) R.-C. Raclea, P. Natho, L. A. T. Allen, A. J. P. White and P. J. Parsons, J. Org. Chem., 2020, 85, 9375–9385 CrossRef CAS
; see also:; (c) F.-Q. Huang, J. Xie, J.-G. Sun, Y.-W. Wang, X. Dong, L.-W. Qi and B. Zhang, Org. Lett., 2016, 18, 684–687 CrossRef CAS
.
- R. Ren, H. Zhao, L. Huan and C. Zhu, Angew. Chem., Int. Ed., 2015, 54, 12692–12696 CrossRef CAS
.
- J. Yu, H. Zhao, S. Liang, X. Bao and C. Zhu, Org. Biomol. Chem., 2015, 13, 7924–7927 RSC
.
- R. Ren, Z. Wu, Y. Xu and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 2866–2869 CrossRef CAS
.
-
(a) L. Huan and C. Zhu, Org. Chem. Front., 2016, 3, 1467–1471 RSC
; (b) B. D. W. Allen, M. D. Hareram, A. C. Seastram, T. McBride, T. Wirth, D. L. Browne and L. C. Morrill, Org. Lett., 2019, 21, 9241–9246 CrossRef CAS
.
- D. Wang, R. Ren and C. Zhu, J. Org. Chem., 2016, 81, 8043–8049 CrossRef CAS
.
- R. Ren, Z. Wu and C. Zhu, Chem. Commun., 2016, 52, 8160–8163 RSC
.
- M. Wang, Z. Wu and C. Zhu, Org. Chem. Front., 2017, 4, 427–430 RSC
.
- Y. Zhu, K. Huang, J. Pan, X. Qiu, X. Luo, Q. Qin, J. Wei, X. Wen, L. Zhang and N. Jiao, Nat. Commun., 2018, 9, 2625 CrossRef
.
- Y. Zhu, Z. Zhang, R. Jin, J. Liu, G. Liu, B. Han and N. Jiao, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202007187
.
- H. Ren, J.-R. Song, Z.-Y. Li and W.-D. Pan, Org.
Lett., 2019, 21, 6774–6778 CrossRef CAS
.
- For complementary Cu(II)-catalyzed etherification reactions via an oxycupration mechanism, see:
(a)
D. Chen, I. A. Berhane and S. R. Chemler, Org. Lett., 2020, DOI:10.1021/acs.orglett.0c01691;
(b) M. T. Bovino, T. W. Liwosz, N. E. Kendel, Y. Miller, N. Tyminska, E. Zurek and S. R. Chemler, Angew. Chem., Int. Ed., 2014, 53, 6383–6387 CrossRef CAS
; (c) Y. Miller, L. Miao, A. S. Hosseini and S. R. Chemler, J. Am. Chem. Soc., 2012, 134, 12149–12156 CrossRef CAS
.
- For reviews of photoredox catalysis, see:
(a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS
; (b) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS
; (c) M. D. Kärkäs, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef
.
-
V. Balzani, P. Ceroni and A. Juris, Photochemistry and Photophysics: Concepts, Research, Applications, Wiley-VCH, Weinheim, Germany, 2014 Search PubMed
.
-
(a) H. Yin, P. J. Carroll, J. M. Anna and E. J. Schelter, J. Am. Chem. Soc., 2015, 137, 9234–9237 CrossRef CAS
; (b) H. Yin, P. J. Carroll, B. C. Manor, J. M. Anna and E. J. Schelter, J. Am. Chem. Soc., 2016, 138, 5984 CrossRef CAS
.
- J.-J. Guo, A. Hu, Y. Chen, J. Sun, H. Tang and Z. Zuo, Angew. Chem., Int. Ed., 2016, 55, 15319–15322 CrossRef CAS
.
-
(a) L. B. Young and W. S. Trahanovsky, J. Am. Chem. Soc., 1969, 91, 5060–5068 CrossRef CAS
; (b) M. J. Zhang, G. M. Schroeder, Y.-H. He and Z. Guan, RSC Adv., 2016, 6, 96693–96699 RSC
.
- Y. Chen, J. Du and Z. Zuo, Chem, 2020, 6, 266–279 Search PubMed
.
- A. Hu, Y. Chen, J. J. Guo, N. Yu, Q. An and Z. Zuo, J. Am. Chem. Soc., 2018, 140, 13580–13585 CrossRef CAS
.
- K. Zhang, L. Chang, Q. An, X. Wang and Z. Zuo, J. Am. Chem. Soc., 2019, 141, 10556–10564 CrossRef CAS
.
- A. Hu, J.-J. Guo, H. Pan, H. Tang, Z. Gao and Z. Zuo, J. Am. Chem. Soc., 2018, 140, 1612–1616 CrossRef CAS
.
- A. Hu, J.-J. Guo, H. Pan and Z. Zuo, Science, 2018, 361, 668–672 CrossRef CAS
.
- Q. An, Z. Wang, Y. Chen, X. Wang, K. Zhang, H. Pan, W. Liu and Z. Zuo, J. Am. Chem. Soc., 2020, 142, 6216–6226 CrossRef CAS
.
- M. T. Green, J. H. Dawson and H. B. Gray, Science, 2004, 304, 1653–1656 CrossRef CAS
.
-
(a) B. P. Roberts, Chem. Soc. Rev., 1999, 28, 25–35 RSC
; (b) J. M. Tedder, Angew. Chem., Int. Ed., 1982, 21, 401–410 CrossRef
.
- For reviews on PCET, see:
(a) D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS
; (b) S. Hammes-Schiffer and A. A. Stuchebrukhov, Chem. Rev., 2010, 110, 6939–6960 CrossRef CAS
; (c) S. Y. Reece and D. G. Nocera, Annu. Rev. Biochem., 2009, 78, 673–699 CrossRef CAS
; (d) D. C. Miller, K. T. Tarantino and R. R. Knowles, Top. Curr. Chem., 2016, 374, 30 CrossRef
; (e) E. C. Gentry and R. R. Knowles, Acc. Chem. Res., 2016, 49, 1546–1556 CrossRef CAS
.
- F. G. Bordwell, J.-P. Cheng and J. A. Harrelson, J. Am. Chem. Soc., 1988, 110, 1229–1231 CrossRef CAS
.
- J. M. Mayer, Acc. Chem. Res., 2011, 44, 36–46 CrossRef CAS
.
-
(a) S. Y. Reece and D. G. Nocera, Annu. Rev. Biochem., 2009, 78, 673–699 CrossRef CAS
; (b) S. Y. Reece, J. M. Hodgkiss, J. Stubbe and D. G. Nocera, Philos. Trans. R. Soc., B, 2006, 361, 1351–1364 CrossRef CAS
.
- For an example of PCET activation of aliphatic O–H bonds, see: D. Wang, E. R. Farquhar, A. Stubna, E. Münck and L. Que, Nat. Chem., 2009, 1, 145–150 CrossRef CAS
.
- H. G. Yayla, H. Wang, K. T. Tarantino, H. S. Orbe and R. R. Knowles, J. Am. Chem. Soc., 2016, 138, 10794–10797 CrossRef CAS
.
-
(a) E. Baciocchi, M. Bietti and S. Steenken, J. Am. Chem. Soc., 1997, 119, 4078–4079 CrossRef CAS
; (b) E. Baciocchi, M. Bietti, O. Lanzalunga and S. Steenken, J. Am. Chem. Soc., 1998, 120, 11516–11517 CrossRef CAS
; (c) E. Baciocchi, M. Bietti and O. Lanzalunga, Acc. Chem. Res., 2000, 33, 243–251 CrossRef CAS
.
- J. Wang, B. Huang, C. Shi, C. Yang and W. Xia, J. Org. Chem., 2018, 83, 9696–9706 CrossRef CAS
.
- E. Ota, H. Wang, N. L. Frye and R. R. Knowles, J. Am. Chem. Soc., 2019, 141, 1457–1462 CrossRef CAS
.
- Y. Wang, Y. Liu, J. He and Y. Zhang, Sci. Bull., 2019, 64, 1658–1666 CrossRef CAS
.
- S. T. Nguyen, P. R. D. Murray and R. R. Knowles, ACS Catal., 2020, 10, 800–805 CrossRef CAS
.
- K. Zhao, K. Yamashita, J. E. Carpenter, T. C. Sherwood, W. R. Ewing, P. T. W. Cheng and R. R. Knowles, J. Am. Chem. Soc., 2019, 141, 8752–8757 CrossRef CAS
.
- L. Huang, T. Ji and M. Rueping, J. Am. Chem. Soc., 2020, 142, 3532–3539 CrossRef CAS
.
- T. Ji, X. Y. Chen, L. Huang and M. Rueping, Org. Lett., 2020, 22, 2579–2583 CrossRef CAS
.
- X. Wu, M. Wang, L. Huan, D. Wang, J. Wang and C. Zhu, Angew. Chem., Int. Ed., 2018, 57, 1640–1644 CrossRef CAS
.
- M. Wang, L. Huan and C. Zhu, Org. Lett., 2019, 21, 821–825 CrossRef CAS
.
- E. Tsui, A. J. Metrano, Y. Tsuchiya and R. R. Knowles, Angew. Chem., Int. Ed., 2020, 59, 11845–11849 CrossRef CAS
.
- R. Zhao, Y. Yao, D. Zhu, D. Chang, Y. Liu and L. Shi, Org. Lett., 2018, 20, 1228–1231 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2020 |