Thermal desorption part 2: applications in analytical measurement
Analytical Methods Committee AMCTB No. 104
First published on 7th May 2021
Abstract
The past 30 years have seen increasing availability of methods and equipment using thermal desorption for the measurement of airborne substances. These methods offer excellent sensitivity and are well-suited to automation and, in particular, diffusive sampling, a sampling technique which is both easier to use and less obtrusive than pumped sampling. With advances in equipment, thermal desorption is being used in an increasingly wide variety of applications. The capabilities and limitations of thermal desorption equipment and measurement methods were the subject of AMC Technical Brief No. 97. This supplementary Technical Brief aims to illustrate the uses and applications of thermal desorption methods in analytical measurement.
Exposure to chemical compounds, in particular volatile organic compounds (VOCs), is almost unavoidable in the modern world, whether in the workplace, in the environment or even at home. Some of these compounds have the potential to cause adverse health effects, both long term (chronic) and short term (acute) so it is important to be able to detect and measure their concentration. This can be achieved using a variety of portable and laboratory-based measurement methods but, over the past 30 years, methods using thermal desorption (TD) have become increasingly well-established. Such methods, which were the subject of AMC Technical Brief No. 97, can be used to measure concentrations ranging from a few percent to parts per trillion (ppt; 1 part in 1012).1 This capability, together with advances in instrumentation and greater availability of validated methods, has resulted in a wide variety of applications, a selection of which are described here.
Monitoring occupational exposure
The main application of TD-based measurement methods is probably workplace and environmental air monitoring to demonstrate compliance with statutory limits, and safeguard the well-being of workers, the public, and the environment. In Great Britain, Workplace Exposure Limits (WELs) for chemicals are set by the UK Health and Safety Executive (HSE) and listed in EH40/2005. Depending on the toxicity of the chemical, these WELs can range from hundreds of ppm (parts per million) to a few ppb (parts per billion; 1 part in 109). It is important to note that WELs apply to personal samples, i.e. samplers worn by individuals, rather than static samples, i.e. samples taken at fixed sampling points. This is where the benefits of TD-based diffusive samplers are most apparent, being easier to wear and less obtrusive than pumped samplers, which require a sampling pump and associated tubing. The less obtrusive nature of diffusive samplers also means that the wearer is more likely to behave ‘normally’ and the sample result should therefore be more representative of typical levels of exposure.A recent example is the measurement of diacetyl (2,3-butanedione) in the coffee industry where the substance is generated naturally during roasting and grinding of coffee beans. Exposure to diacetyl can result in serious and irreversible lung damage and so, in 2018, diacetyl was assigned an 8 hour WEL of 20 ppb. Existing methods, based on pumped sampling and solvent desorption, were not sufficiently sensitive. A more sensitive TD-based method was therefore devised using diffusive sampling on Tenax® TA sorbent and analysis by gas chromatography-mass spectrometry (GC-MS).2 The sampler was sufficiently unobtrusive to be worn by staff serving customers in coffee shops where wearing a pumped sampler would be out of the question. An example chromatogram from the study is shown in Fig. 1. This illustrates the complex nature of ‘real’ air samples and the necessity of MS detection to achieve the necessary sensitivity and selectivity. The study found the grinding of coffee beans after roasting generated the highest airborne emissions of diacetyl which, if not properly controlled, could lead to production workers being exposed to airborne concentrations above the WEL. However, there was better news for workers and customers in coffee shops as the study found airborne concentrations of diacetyl at the outlets visited to be extremely low.
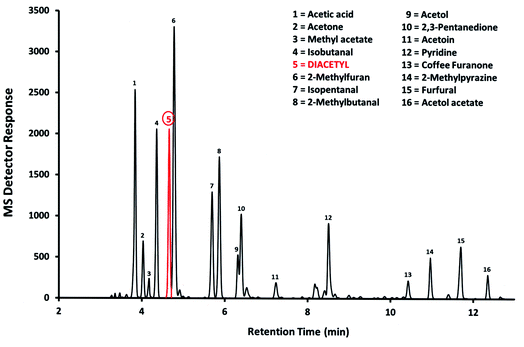 | ||
| Fig. 1 GC-MS chromatogram of an air monitoring sample collected adjacent to a large-scale coffee grinding operation (courtesy HSE). | ||
Another example of the use of TD and diffusive sampling in workplace monitoring is the measurement of occupational exposure to anaesthetic gases such as nitrous oxide and halothanes in medical environments such as hospital operating theatres or dentists. Again, these are environments where samplers need to be unobtrusive and collection of pumped samples would be difficult, if not impossible.
Environmental air monitoring
As previously stated, TD-based methods can also be used for the measurement of airborne chemicals in the environment such as benzene around the perimeters of oil refineries. This procedure, using 2 week diffusive sampling onto TD tubes loaded with Carbopack™ X sorbent and analysis by GC-MS, is described in the United States Environmental Protection Agency (EPA) Method 325.3 The sampling tubes are mounted in a purpose made shelter which provides a ‘standard’ environment and a degree of protection from the elements. The advantage of diffusive sampling is that 2 week samples can be collected without the need for a powered pump, improving reliability and reducing the level of intervention required. The method is capable of detecting benzene in the air at ppt concentrations and other analytes of interest, such as toluene and 1,3-butadiene, can also be measured from the same sample.As well as the outside environment, TD-based methods can also be used for indoor air monitoring, e.g. ISO Methods 16000 (Part 6) and ISO 16017 (Parts 1 and 2). This is an area of increasing interest in modern buildings and links to another application of TD, namely emissions testing of products.
Emissions testing
TD-based methods are widely used in emissions testing, particularly of products used in building products or indoor environments such as paints, adhesives, insulation and wood products. Many standard methods for investigating VOC emissions from such products now exist and more are being developed, for example ISO 10580 for floor coverings, ISO 16000 (Part 9) and ASTM D5116-17 for indoor building materials and furnishings and ASTM D6803-19 for paint products. The ASTM standards use micro-scale chamber equipment which is particularly well suited to sampling using TD-based methods and requires much less sample material than larger scale testing. Micro-scale chamber testing can also be used at elevated temperatures, allowing shorter testing times and investigation of emissions of semi-volatile compounds, for example phthalate esters, a class of potentially harmful compounds commonly used as plasticisers.Emissions testing can be used for both quantitative and qualitative purposes. Quantitative analysis is useful for determining emission rates and how these are affected by environmental factors such as temperature, humidity and air change rates, information which can be of help in the development of products with lower emissions. In some instances emission rates are required for regulatory purposes; France, for example, requires building products to be labelled, from A+ to C, according to their levels of VOC emissions.
Qualitative analysis is used in assessing the potential effects use of a particular product may have on air quality and, in particular, detecting the presence of specific compounds whose use is regulated or prohibited. It can also be used for forensic analysis, for example, detection, even at very low levels, of accelerants such as flammable solvents. The top chromatogram in Fig. 2 shows VOC emissions generated by an artefact recovered from the scene of a fire which contain highly flammable isomers of hexane and heptane. The two lower chromatograms show VOC emissions generated by two solvent-based products recovered from nearby. These show that the VOC emissions from Product 1 are an almost perfect match for those generated by the artefact, i.e. mainly isomers of hexane and heptane, whilst those from Product 2 are completely different. These results provide evidence of the presence of a flammable solvent material, with a VOC composition consistent with that of Product 1, at the scene of the fire.
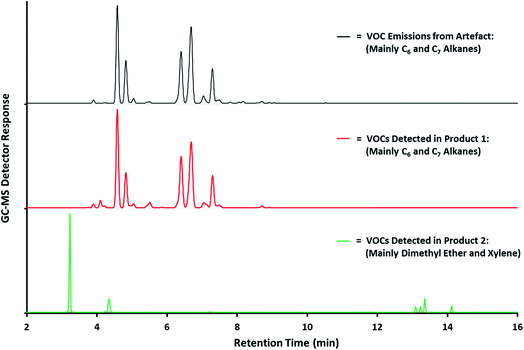 | ||
| Fig. 2 GC-MS chromatogram of VOC emissions obtained from a sample material recovered at the scene of a fire (courtesy HSE). | ||
TD-based emissions testing of small (milligram) amounts of sample can be achieved by placing the whole sample, wrapped in a quartz filter, into an empty TD-tube and analysing the material directly by TD. The sample is typically only heated to a temperature of around 70–100 °C; compared with around 280 °C for desorption of sorbents such as Tenax® TA. This is sufficient to release any residual VOCs but not enough to cause thermal degradation of the sample. Fig. 3 shows an example chromatogram obtained at 100 °C from a fragment of ‘dry’ paint recovered from the interior surface of a container involved in an explosion. This clearly shows the presence of residual solvent compounds in the paint, in particular various alkyl substituted aromatic hydrocarbons, buildup of which, inside the container, may have been a contributory factor in the explosion.
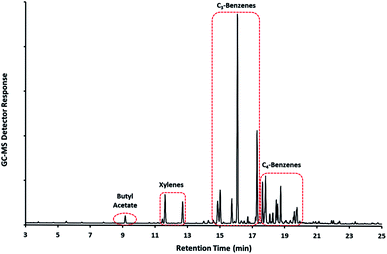 | ||
| Fig. 3 GC-MS chromatogram of residual solvent emissions from a fragment of ‘dry’ paint recovered at the scene of an explosion (courtesy HSE). | ||
Biological monitoring
An application of TD of increasing interest is biological monitoring (BM), most commonly in the form of breath sampling, which allows non-invasive measurement of chemicals present in the human body. This technique can be used for both assessing occupational exposure to harmful chemicals and as a potential means of early diagnosis of cancer and other serious medical conditions.Breath samples are collected by the subject blowing into a sampling device containing one or more TD tubes and analysed by GC-MS. The sampling devices are specifically designed for breath sampling and incorporate features to reduce contamination of the sample with VOCs from the mouth. As this technique measures chemicals present inside the body, it can be used to investigate occupational exposure not just from inhalation but also from ingestion or contact with skin. It is also useful for assessing the effectiveness of personal protective equipment (PPE), such as respirators or gloves; something which is difficult to do by other means.
The use of breath sampling as a non-invasive diagnostic tool for diseases such as cancer is currently a subject of particular interest and ongoing research. Breath samples contain a complex mixture of VOCs and the presence, or absence, of some compounds (when compared with the breath of healthy control subjects) may provide an early indication of the onset of some serious medical conditions, leading to quicker diagnosis, intervention and treatment. For example, a study by Markar et al. identified twelve substances in breath samples for use as biomarkers to provide earlier diagnosis of pancreatic cancer.4
Another study by Trivedi et al. used biomarkers to screen for the onset of Parkinson’s disease.5 In this study the biomarkers were not derived from breath but from sebum, a fluid excreted by glands in the skin, and the VOCs present were not analysed directly by TD, but using a combination of dynamic headspace analysis and TD. This process is similar to small-scale emission testing with the sample, on a gauze swab, being incubated in a controlled flow of inert gas which passes through a sorbent tube. VOCs collected on the tube are then analysed by TD-GC-MS.
Conclusions
TD-based methods have a wide range of potential applications, many of which are described in published methods and international standards. Air sampling, in the workplace, in the environment or in indoor air, represents the main use of this technique, but TD is also used for product testing, forensic analysis and detecting exposure to harmful chemicals. There is also considerable interest in the use of TD for detection of biomarkers to indicate the early stages of conditions such as cancer and Parkinson’s disease. These still developing medical applications have the potential to radically improve diagnosis and treatment of serious medical conditions. The key to this wide range of applications is the flexibility and sensitivity of TD-based methods. A wide range of sorbents is available and the technique is well suited to automation and, in particular, the use of diffusive sampling. The latter allows easy and unobtrusive sampling in environments where pumped methods would not be suitable. Given the continued development of TD equipment it is likely that the range of available applications will only continue to grow.Ian Pengelly (Science Division, Health and Safety Executive, HSE)
This Technical Brief was prepared for the Analytical Methods Committee (AMC), with contributions from members of the AMC Instrumental Analysis Expert Working Group, and approved by the AMC on 7
th
March 2021.
Further reading
- AMC Technical Brief No. 97, Thermal Desorption Part 1: Introduction and Instrumentation, Anal. Methods, 2020, 12, 3425–3428 RSC.
- I. Pengelly, H. O’Shea, G. Smith and M. A. Coggins, Measurement of Diacetyl and 2,3-Pentanedione in the Coffee Industry Using Thermal Desorption Tubes and Gas Chromatography-Mass Spectrometry, Ann. Work Exposures Health, 2019, 63(4), 415–425 CrossRef CAS PubMed.
- Method 325A: Volatile Organic Compounds from Fugitive and Area Sources: Sampler Deployment and VOC Sample Collection, United States Environmental Protection Agency, 2019 Search PubMed.
- S. R. Markar, B. Brodie, S.-T. Chin, A. Romano, D. Spalding and G. B. Hanna, Profile of exhaled breath volatile organic compounds to diagnose pancreatic cancer, Br. J. Surg., 2018, 105(11), 1493–1500 CrossRef CAS PubMed.
- D. K. Trivedi, E. Sinclair, Y. Xu, D. Sarkar, C. Walton-Doyle, C. Liscio, P. Banks, J. Milne, M. Silverdale, T. Kunath, R. Goodacre and P. Barran, Discovery of Volatile Biomarkers of Parkinson’s Disease from Sebum, ACS Cent. Sci., 2019, 5, 599–606 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |


