Palladium-catalyzed domino spirocyclization of [60]fullerene: synthesis of diverse [60]fullerene-fused spiro[4,5]/[5,5] derivatives†
Jinliang
Ma
,
Tong-Xin
Liu
 *,
Pengling
Zhang
,
Chuanjie
Zhang
and
Guisheng
Zhang
*,
Pengling
Zhang
,
Chuanjie
Zhang
and
Guisheng
Zhang
 *
*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Henan Key Laboratory of Organic Functional Molecule and Drug Innovation, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, China. E-mail: liutongxin_0912@126.com; zgs6668@yahoo.com
First published on 18th November 2020
Abstract
Herein a new, general and practical method for the spirocyclization of [60]fullerene through a palladium-catalyzed domino Heck/C–H activation reaction is presented. A wide range of novel [60]fullerene-fused spirocyclic derivatives can be easily and flexibly synthesized with a broad substrate scope and excellent functional-group tolerance. A plausible mechanism involving an alkyl Pd(II) species as a key intermediate has been proposed.
Fullerene derivatives have received great attention from different fields, such as materials science,1 biomedicine,2 supramolecular chemistry,3 organic photovoltaics (OPVs) and perovskite solar cells (PSCs).4 In this context, functionalization of fullerenes has become an important research area. Considerable progress has been made for establishing various methods and strategies to generate diverse carbon nanostructures.5,6 Among them, transition-metal-catalyzed addition reactions of fullerenes have received growing attention for the high selectivity and efficiency, atom-economy and diversity in forming organofullerenes.5e,f,h,i,6d,g,k,o,7 Owing to the intimate relationship between photoelectric properties and specific configuration, the exploration of new approaches, including transition-metal-catalyzed protocols, to access fullerene derivatives with novel and unique structures is of great importance.
Among the numerous prepared fullerene derivatives, fullerene-fused spirocyclic structures are quite scarce. Spiroannelated methanofullerenes, derived from the addition reactions of cyclic carbenes with fullerenes, are one of the first reported fullerene-fused spirocyclic derivatives.8 Another class of fullerene-fused spirocyclic scaffolds are [60]fullerene-fused spiroindanes, reported by Wang's group, obtained via palladium-catalyzed C–H activation annulation of [60]fullerene with 2-aryl cyclic 1,3-dicarbonyl compounds.9 However, in these spiroannulation transformations, it is necessary to pre-implant cyclic motifs into the reactive site of the substrates. Thus, the development of new strategies to prepare architecturally interesting fullerene-fused spirocyclic frameworks remains to be addressed. Domino reactions allow the formation of multiple bonds in a single operation, and can conveniently construct complex molecules from simple starting materials.10,11 Recently, we disclosed a solvent-induced N-incorporation domino reaction for the rapid construction of [60]fullerene-fused dihydrocarbolines.12 As a continuation of our interest and inspired by the carbopalladation-initiated chemistry,13 herein, we present a new transition-metal-catalyzed domino protocol for the spirocyclization of [60]fullerene. Under palladium-catalyzed conditions, the transformation undergoes a domino Heck/C–H activation process, and affords a series of novel fullerene-fused spirocyclic derivatives in a general and flexible way. The present chemistry opens up a new synthetic strategy to construct fullerene-fused spirocyclic architectures, and also represents the first synthetic application of alkyl palladium species in functionalized fullerenes.
We began the study with 1-iodo-2-((2-phenylallyl)oxy)benzene 1a as the reaction partner of C60 to optimize the reaction conditions (Table 1). In the presence of Cs2CO3, different Pd(0) complexes, such as Pd(PPh3)4, Pd2(dba)3 and Pd(dba)2, were screened (entries 1–3). The results revealed that Pd(PPh3)4 was an efficient catalyst for the transformation, furnishing the spirocyclization derivative 2a in 40% yield under N2. To improve the yield of 2a, the combination of Pd(II) salts and phosphorus ligands was also examined (entries 4–9). Only the Pd(TFA)2/dppe-catalyzed system resulted in a comparable yield. Investigation of the solvent effects indicated that the product yield could be improved to 46% by using 1.4-dioxane as a co-solvent (entries 10–12). Interestingly and importantly, we found that reducing the amount of catalyst from 10 mol% to 5 mol% did not affect the isolated yield of 2a, even without the assistance of a co-solvent (entries 13 and 14). The other bases including K2CO3, Rb2CO3, K3PO4, tBuOK and DABCO were also screened; however, no better yields were obtained (entries 15–19). To enhance the practicability of this chemistry, the reaction was carried out in air (entry 20). To our delight, the transformation was not sensitive to air, and 2a was delivered in 48% yield. Attempts to further decrease the catalyst loading to 2 mol% led to a reduction in the yield, even when prolonging the reaction time (entries 21 and 22). In addition, we also tested the reaction using phenylbromide instead of 1a, but no product was observed.
| Entry | Cat. [Pd] | Ligand | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: C60 (0.05 mmol), 1a (0.10 mmol), [Pd], ligand, base (0.10 mmol), PhCl (6 mL), 130 °C, 24 h, under a nitrogen atmosphere. b Isolated yield; the values in parentheses are based on the consumed C60. c PhCl (6 mL)/CH3CN (1 mL) as a co-solvent. d PhCl (6 mL)/THF (1 mL) as a co-solvent. e PhCl (6 mL)/1,4-dioxane (1 mL) as a co-solvent. f Reaction was conducted in air. g Reaction for 48 h. | ||||
| 1 | Pd(PPh3)4 (10 mol%) | Cs2CO3 | 40 (70) | |
| 2 | Pd2(dba)3 (10 mol%) | Cs2CO3 | n.r. | |
| 3 | Pd(dba)2 (10 mol%) | Cs2CO3 | Trace | |
| 4 | PdCl2 (10 mol%) | PPh3 (20 mol%) | Cs2CO3 | 11 (85) |
| 5 | Pd(MeCN)2Cl2 (10 mol%) | PPh3 (20 mol%) | Cs2CO3 | 22 (51) |
| 6 | Pd(OAc)2 (10 mol%) | PPh3 (20 mol%) | Cs2CO3 | 25 (89) |
| 7 | Pd(TFA)2 (10 mol%) | PPh3 (20 mol%) | Cs2CO3 | 31 (76) |
| 8 | Pd(TFA)2 (10 mol%) | dppe (20 mol%) | Cs2CO3 | 42 (80) |
| 9 | Pd(TFA)2 (10 mol%) | dppf (20 mol%) | Cs2CO3 | 30 (57) |
| 10 | Pd(PPh3)4 (10 mol%) | Cs2CO3 | 15 (24)c | |
| 11 | Pd(PPh3)4 (10 mol%) | Cs2CO3 | 45 (72)d | |
| 12 | Pd(PPh3)4 (10 mol%) | Cs2CO3 | 46 (82)e | |
| 13 | Pd(PPh3)4 (5 mol%) | Cs2CO3 | 44 (81)e | |
| 14 | Pd(PPh3)4 (5 mol%) | Cs2CO3 | 45 (74) | |
| 15 | Pd(PPh3)4 (5 mol%) | K2CO3 | 14 (31) | |
| 16 | Pd(PPh3)4 (5 mol%) | Rb2CO3 | 26 (53) | |
| 17 | Pd(PPh3)4 (5 mol%) | K3PO4 | 41 (86) | |
| 18 | Pd(PPh3)4 (5 mol%) | tBuOK | 29 (51) | |
| 19 | Pd(PPh3)4 (5 mol%) | DABCO | <5 | |
| 20 | Pd(PPh3)4 (5 mol%) | Cs2CO3 | 48 (90)f | |
| 21 | Pd(PPh3)4 (2 mol%) | Cs2CO3 | 35 (77)f | |
| 22 | Pd(PPh3)4 (2 mol%) | Cs2CO3 | 41 (75)f,g | |
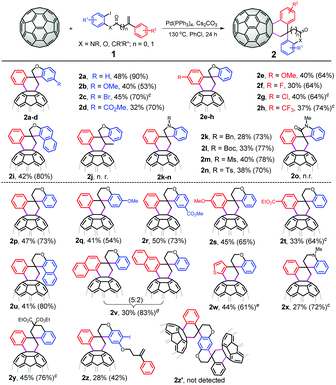
With the optimized reaction conditions in hand, we next evaluated the substrate scope of the domino spirocyclization by varying 1. As shown in Fig. 1, a wide array of 1-iodo-2-((2-arylallyl)oxy)benzenes 1a–h worked well in the reaction, affording the corresponding C60-fused 3′,4′-dihydro-2H,2′H-spiro[benzofuran-3,1′-naphthalene] derivatives 2a–h in moderate to good yields. Various substituents on both phenyl rings, such as MeO, Br, CO2Me, F, Cl and CF3, were tolerated in the transformation. When the phenyl ring with an iodo group was replaced by naphthalene (1i), the desired product 2i was formed in 42% yield. The substrate 1j bearing a nonterminal olefinic unit was unreactive under the reaction conditions. We found that, besides the oxygen-containing aryl iodides, the nitrogen-containing iodides 1k–n with N-Bn/Boc/Ms/Ts substituents were also suitable for the transformation, delivering C60-fused 3′,4′-dihydro-2′H-spiro[indoline-3,1′-naphthalene] adducts in 28%–40% yields. N-(2-iodophenyl)-N-methyl-2-phenylacrylamide 1o was also subjected to the reaction; however, no 2o was obtained and the exact reason was not clear. To our delight, the catalytic spirocyclization protocol was also applicable to 1-iodo-2-((3-arylbut-3-en-1-yl)oxy)benzenes, and the corresponding C60-fused 3′,4′-dihydro-2′H-spiro[chromane-4,1′-naphthalene] products 2p–t were obtained in 33%–50% yields. Substrates containing strong electron-donating/withdrawing groups (MeO and CO2Me) all participated in the transformation, indicating that the electronic effect of the substituent on both phenyl rings had no obvious influence on the reaction outcome. The reaction of 1-iodonaphthalen-2-ol-derived substrate 1u with C60 also successfully produced spirocyclic derivative 2u in 41% yield. When the phenyl group linked to the double bonds was changed to naphthalene (1v) and thiophene (1w), the transformation still proceeded smoothly, leading to spirocyclization products 2v and 2w in 30% and 44% yields, respectively. Expansion of the substrate scope to N-Ms substituted 2-iodo-N-(3-phenylbut-3-en-1-yl)aniline 1x and 2-(2-iodobenzyl)-2-(2-phenylallyl)malonate 1y turned out to be viable, and C60-fused tetrahydro-1′H,2H-spiro[naphthalene-1,4′-quinoline] 2x and -2H,2′H-1,1′-spirobi[naphthalene] 2y were obtained in 27% and 45% yields, respectively. We also explored the possibility of synthesizing a dimer structure with a bis-fullerene skeleton by employing 2,5-diiodobenzene-1,4-diol derived 1z as a substrate. However, the expected dimmer product 2z′ was not observed except for 2z even when changing the reaction parameters, possibly due to the large steric hindrance of the carbon cage itself.
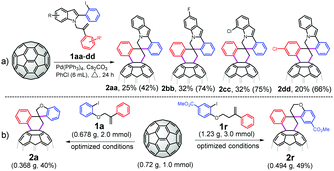
The value of this catalytic domino reaction for constructing diverse C60-fused spirocyclic derivatives was proven by the efficient synthesis of C60-fused 3′,4′-dihydro-2′H,6H-spiro[indolo[2,1-a]isoquinoline-5,1′-naphthalene] derivatives (Scheme 1a). 2-(2-Iodophenyl)-1-(2-phenylallyl)-1H-indoles with different substituents at different positions could be converted into the target products 2aa–dd in 20%–32% yields. Given the wide structural diversity of C60-fused spirocyclic derivatives generated by the present protocol, the practicality of this spirocyclization was further demonstrated by examining a scale-up reaction of 720 mg of C60 (1.0 mmol) with 1a or 1r under the optimized conditions (Scheme 1b). 2a and 2r were obtained in 40% and 49% yields, respectively, thus proving that the method can maintain high efficiency on a large scale.
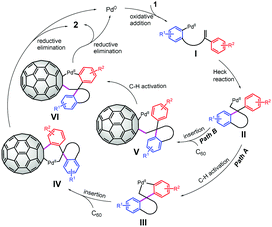
Based on the above experimental results and previous reports, a tentative mechanism for the domino spirocyclization of C60 was proposed (Scheme 2). Aryl iodide 1 undergoes oxidative addition to Pd(0) to form aryl PdIII. Then, I further converts into the key alkyl Pd(II) II through an intramolecular Heck reaction.13,14 In path A, intramolecular C–H activation occurs to generate palladacycle III.13,14 The subsequent insertion of C60 leads to intermediate IV, which undergoes reductive elimination to afford 2 and regenerate Pd(0).5i,7d,9,15 In an alternative path B, the intermolecular insertion of C60 into the alkylpalladium bond forms intermediate V,14c,16 followed by C–H bond activation to yield intermediate VI. VI then undergoes the same reductive elimination process to deliver 2 with regeneration of the Pd(0) catalyst.
The electrochemical properties of novel C60-fused spirocyclic derivatives were also investigated (Table 2). All the selected derivatives exhibit essentially the same electrochemical behaviors and have three reversible redox processes. Compared with PCBM, the first reduction potentials of these compounds are more negative. Particularly, in the cases of 2d, 2u, 2y and 2aa, the first reduction potential decreases by 41, 49, 47 and 67 mV relative to that of PCBM. These results demonstrate that the newly obtained C60-fused spirocyclic derivatives possess higher LUMO energy levels than that of PCBM and have potential as electron-active materials in OPV and PSC fields.
| Compound | E 1 | E 2 | E 3 | LUMO levelb (eV) |
|---|---|---|---|---|
| a Versus ferrocene/ferrocenium; experimental conditions: 1 mM of compound 2 and 0.1 M of (n-Bu)4NClO4 in anhydrous ODCB; reference electrode: SCE; working electrode: Pt; auxiliary electrode: Pt wire; scanning rate: 50 mV s−1. b Estimated using the following equation: LUMO level = −(4.8 + E1) eV. c Scanning rate: 100 mV s−1. | ||||
| 2a | −1.199 | −1.596 | −2.127 | −3.602 |
| 2b | −1.203 | −1.601 | −2.137 | −3.597 |
| 2d | −1.212 | −1.626 | −2.179 | −3.588 |
| 2e | −1.194 | −1.586 | −2.116 | −3.606 |
| 2i | −1.199 | −1.582 | −2.110 | −3.602 |
| 2m | −1.181 | −1.583 | −2.112 | −3.619 |
| 2p | −1.209 | −1.592 | −2.129 | −3.591 |
| 2q | −1.206 | −1.596 | −2.139 | −3.594 |
| 2r | −1.196 | −1.590 | −2.135 | −3.604 |
| 2s | −1.204 | −1.600 | −2.151 | −3.596 |
| 2t | −1.199 | −1.588 | −2.128 | −3.601 |
| 2u | −1.220 | −1.614 | −2.161 | −3.580 |
| 2x | −1.185 | −1.574 | −2.116 | −3.615 |
| 2y | −1.218 | −1.620 | −2.173 | −3.582 |
| 2aa | −1.238 | −1.628 | −2.141 | −3.562 |
| PCBM | −1.171 | −1.571 | −2.090 | −3.629 |
In conclusion, we have developed a new, general and practical palladium-catalyzed domino Heck/C–H activation reaction for the spirocyclization of [60]fullerene. This method can efficiently achieve the synthesis of a wide range of structurally diverse C60-fused spirocyclic derivatives from the readily available starting materials under simple reaction conditions, representing a new synthetic strategy for the construction of fullerene-fused spirocyclic structures. Further studies directed towards extending the scope of the reaction are being undertaken.
We are grateful to the National NSFC (No. U1904181 and U1604285), the Program for Science & Technology Innovation Talents in Universities of Henan Province (18HASTIT006), and the 111 Project (D17007) for financial support.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D. M. Guldi, B. M. Illescas, C. M. Atienza, M. Wielopolski and N. Martín, Chem. Soc. Rev., 2009, 38, 1587 RSC; (b) A. Zieleniewska, F. Lodermeyer, A. Rotha and D. M. Guldi, Chem. Soc. Rev., 2018, 47, 702 RSC; (c) N. Martín and F. Giacalone, Fullerene Polymers: Synthesis, Properties and Applications, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany, 2009 CrossRef; (d) H. Imahori, Bull. Chem. Soc. Jpn., 2007, 80, 621 CrossRef CAS.
- (a) E. Castro, A. H. Garcia, G. Zavala and L. Echegoyen, J. Mater. Chem. B, 2017, 5, 6523 RSC; (b) J.-F. Nierengarten, Chem. Commun., 2017, 53, 11855 RSC; (c) J. Ramos-Soriano, J. J. Reina, B. M. Illescas, N. de la Cruz, L. Laura Rodríguez-Pérez, F. Lasala, J. Rojo, R. Delgado and N. Martín, J. Am. Chem. Soc., 2019, 141, 15403 CrossRef CAS.
- (a) K. Harano and E. Nakamura, Acc. Chem. Res., 2019, 52, 2090 CrossRef CAS; (b) F. Lu, E. A. Neal and T. Nakanishi, Acc. Chem. Res., 2019, 52, 1834 CrossRef CAS; (c) N. Martín and J.-F. Nierengarten, Supramolecular Chemistry of Fullerenes and Carbon Nanotubes, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, Germany, 2012 CrossRef.
- (a) T. Umeyama and H. Imahori, Acc. Chem. Res., 2019, 52, 2046 CrossRef CAS; (b) C. Cui, Y. Li and Y. Li, Adv. Energy Mater., 2017, 7, 1601251 CrossRef; (c) C.-Z. Li, H.-L. Yip and A. K.-Y. Jen, J. Mater. Chem., 2012, 22, 4161 RSC; (d) Y. Matsuo, Chem. Lett., 2012, 41, 754 CrossRef CAS; (e) L. Jia, M. Chen and S. Yang, Mater. Chem. Front., 2020, 4, 2256 RSC; (f) T. Gatti, E. Menna, M. Meneghetti, M. Maggini, A. Petrozza and F. Lamberti, Nano Energy, 2017, 41, 84 CrossRef CAS; (g) L.-L. Deng, S.-Y. Xie and F. Gao, Adv. Electron. Mater., 2017, 1700435 Search PubMed.
- (a) A. Hirsch and M. Brettreich, Fullerenes: Chemistry and Reactions, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2008, 6083 RSC; (c) C. Thilgen and F. Diederich, Chem. Rev., 2006, 106, 5049 CrossRef CAS; (d) Y. Matsuo and E. Nakamura, Chem. Rev., 2008, 108, 3016 CrossRef CAS; (e) M. D. Tzirakis and M. Orfanopoulos, Chem. Rev., 2013, 113, 5262 CrossRef CAS; (f) E. E. Maroto, M. Izquierdo, S. Reboredo, J. Marco-Martínez, S. Filippone and N. Martín, Acc. Chem. Res., 2014, 47, 2660 CrossRef CAS; (g) L. Gan, Acc. Chem. Res., 2019, 52, 1793 CrossRef CAS; (h) K. Itami, Chem. Rec., 2011, 11, 226 CrossRef CAS; (i) G.-W. Wang, Top. Organomet. Chem., 2016, 55, 119 CrossRef CAS; (j) H.-S. Lin and Y. Matsuo, Chem. Commun., 2018, 54, 11244 RSC; (k) S. Yang, I. N. Ioffe and S. I. Troyanov, Acc. Chem. Res., 2019, 52, 1783 CrossRef CAS; (l) S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535 RSC; (m) A. L. Balch and K. Winkler, Chem. Rev., 2016, 116, 3812 CrossRef CAS.
- For selected recent examples, see: (a) Z. Zhou, H. Han, Z. Chen, R. Gao, Z. Liu, J. Su, N. Xin, X. Yang and L. Gan, Angew. Chem., Int. Ed., 2019, 58, 17690 CrossRef CAS; (b) J. Marco-Martínez, S. Vidal, I. Fernández, S. Filippone and N. Martín, Angew. Chem., Int. Ed., 2017, 56, 2136 CrossRef; (c) A. Aghabali, S. Jun, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2016, 138, 16459 CrossRef CAS; (d) M. Ueda, T. Sakaguchi, M. Hayama, T. Nakagawa, Y. Matsuo, A. Munechika, S. Yoshida, H. Yasuda and I. Ryu, Chem. Commun., 2016, 52, 13175 RSC; (e) A.-J. Wu, P.-Y. Tseng, W.-H. Hsu and S.-C. Chuang, Org. Lett., 2016, 18, 224 CrossRef CAS; (f) K.-Q. Liu, J.-J. Wang, X.-X. Yan, C. Niu and G.-W. Wang, Chem. Sci., 2020, 11, 384 RSC; (g) X.-Y. Yang, H.-S. Lin, I. Jeon and Y. Matsuo, Org. Lett., 2018, 20, 3372 CrossRef CAS; (h) A. R. Tuktarov, Z. R. Shakirova and U. M. Dzhemilev, Org. Lett., 2017, 19, 3863 CrossRef CAS; (i) F. Li, Y. Shang, C. Niu, C. Li, X. Huang, G. Xu, J. Xuan, H. Zhou and S. Yang, Chem. Commun., 2020, 56, 9513 RSC; (j) S.-H. Li, Z.-J. Li, W.-W. Yang and X. Gao, Org. Lett., 2018, 20, 2328 CrossRef CAS; (k) A. Artigas, A. Pla-Quintana, A. Lledó, A. Roglans and M. Solà, Chem. – Eur. J., 2018, 24, 10653 CrossRef CAS; (l) H.-W. Liu, H. Xu, G. Shao and G.-W. Wang, Org. Lett., 2019, 21, 2625 CrossRef CAS; (m) L. Ding, B. Jin, Z. Guo, Y. Zhao, J. Chen and R. Peng, Org. Lett., 2019, 21, 9924 CrossRef CAS; (n) M. Yamada, A. Ishitsuka, Y. Maeda, M. Suzuki and H. Sato, Org. Lett., 2020, 22, 3633 CrossRef CAS; (o) C. Wu, T.-X. Liu, P. Zhang, X. Zhu and G. Zhang, Org. Lett., 2020, 22, 7327 CrossRef CAS; (p) O. A. Kraevaya, A. S. Peregudov, I. A. Godovikov, E. V. Shchurik, V. M. Martynenko, A. F. Shestakov, J. Balzarini, D. Schols and P. A. Troshin, Chem. Commun., 2020, 56, 1179 RSC.
- For selected representative examples, see: (a) L. Gan, S. Huang, X. Zhang, A. Zhang, B. Cheng, H. Cheng, X. Li and G. Shang, J. Am. Chem. Soc., 2002, 124, 13384 CrossRef CAS; (b) M. D. Tzirakis and M. Orfanopoulos, Angew. Chem., Int. Ed., 2010, 49, 5891 CrossRef CAS; (c) M. Nambo, R. Noyori and K. Itami, J. Am. Chem. Soc., 2007, 129, 8080 CrossRef CAS; (d) B. Zhu and G.-W. Wang, Org. Lett., 2009, 11, 4334 CrossRef CAS; (e) S. Filippone, E. E. Maroto, A. Martín-Domenech, M. Suarez and N. Martín, Nat. Chem., 2009, 1, 578 CrossRef CAS; (f) S. Lu, T. Jin, M. Bao and Y. Yamamoto, J. Am. Chem. Soc., 2011, 133, 12842 CrossRef CAS; (g) M. Nambo, A. Wakamiya, S. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2009, 131, 15112 CrossRef CAS; (h) Z. Xiao, Y. Matsuo and E. Nakamura, J. Am. Chem. Soc., 2010, 132, 12234 CrossRef CAS; (i) Y. Hashikawa, M. Murata, A. Wakamiya and Y. Murata, J. Am. Chem. Soc., 2017, 139, 16350 CrossRef CAS; (j) S. Lu, T. Jin, E. Kwon, M. Bao and Y. Yamamoto, Angew. Chem., Int. Ed., 2012, 51, 802 CrossRef CAS; (k) E. E. Maroto, A. de Cózar, S. Filippone, A. Martín-Domenech, M. Suárez, F. P. Cossío and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 6060 CrossRef CAS.
- (a) M. Prato, T. Suzuki and F. Wudl, J. Am. Chem. Soc., 1993, 115, 7876 CrossRef CAS; (b) T. Ohno, N. Martín, B. Knight, F. Wudl, T. Suzuki and H. Yu, J. Org. Chem., 1996, 61, 1306 CrossRef CAS; (c) B. Knight, N. Martín, T. Ohno, E. Ortí, C. Rovira, J. Veciana, J. Vidal-Gancedo, P. Viruela, R. Viruela and F. Wudl, J. Am. Chem. Soc., 1997, 119, 9871 CrossRef CAS.
- D.-B. Zhou and G.-W. Wang, Org. Lett., 2016, 18, 2616 CrossRef CAS.
- (a) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS; (b) L. F. Tietze, G. Brasche and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 CrossRef.
- S.-P. Jiang, M. Zhang, C.-Y. Wang, S. Yang and G.-W. Wang, Org. Lett., 2017, 19, 5110 CrossRef CAS.
- T.-X. Liu, S. Yue, C. Wei, N. Ma, P. Zhang, Q. Liu and G. Zhang, Chem. Commun., 2018, 54, 13331 RSC.
- (a) V. P. Mehta and J.-A. García-López, ChemCatChem, 2017, 9, 1149 CrossRef CAS; (b) Y. Ping, Y. Li, J. Zhu and W. Kong, Angew. Chem., Int. Ed., 2019, 58, 1562 CrossRef CAS; (c) J. Ye, Z. Shi, T. Sperger, Y. Yasukawa, C. Kingston, F. Schoenebeck and M. Lautens, Nat. Chem., 2017, 9, 361 CrossRef CAS.
- (a) R. Grigg, P. Fretwell, C. Meerholtz and V. Sridharan, Tetrahedron, 1994, 50, 359 CrossRef CAS; (b) T. Piou, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2012, 51, 11561 CrossRef CAS; (c) M. Pérez-Gómez and J.-A. García-López, Angew. Chem., Int. Ed., 2016, 55, 14389 CrossRef; (d) H. Yoon, A. Lossouarn, F. Landau and M. Lautens, Org. Lett., 2016, 18, 6324 CrossRef CAS; (e) M. Pérez-Gómez, S. Hernández-Ponte, D. Bautista and J.-A. García-López, Chem. Commun., 2017, 53, 2842 RSC; (f) H. Yoon, M. Rölz, F. Landau and M. Lautens, Angew. Chem., Int. Ed., 2017, 56, 10920 CrossRef CAS; (g) C. Shao, Z. Wu, X. Ji, B. Zhou and Y. Zhang, Chem. Commun., 2017, 53, 10429 RSC; (h) R. T. Ruck, M. A. Huffman, M. M. Kim, M. Shevlin, W. V. Kandur and I. W. Davies, Angew. Chem., Int. Ed., 2008, 47, 4711 CrossRef CAS; (i) G. Satyanarayana, C. Maichle-Mössmer and M. E. Maier, Chem. Commun., 2009, 1571 RSC; (j) H. Zheng, Y. Zhu and Y. Shi, Angew. Chem., Int. Ed., 2014, 53, 11280 CrossRef CAS.
- (a) M. Hussain, M. Chen, S. Yang and G.-W. Wang, Org. Lett., 2019, 21, 8568 CrossRef CAS; (b) M. Hussain, C. Niu and G.-W. Wang, Org. Chem. Front., 2020, 7, 1249 RSC; (c) F. Li, J.-J. Wang and G.-W. Wang, Chem. Commun., 2017, 53, 1852 RSC.
- B. Zhu and G.-W. Wang, J. Org. Chem., 2009, 74, 4426 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc07143a |
| This journal is © The Royal Society of Chemistry 2021 |