Open Access Article
Open Access ArticleSynthesis and reactivity of heteroleptic zinc(I) complexes toward heteroallenes†
Bin
Li
 ,
Kevin
Huse
,
Christoph
Wölper
and
Stephan
Schulz
,
Kevin
Huse
,
Christoph
Wölper
and
Stephan
Schulz
 *
*
Institute of Inorganic Chemistry and Center for Nanointegration Duisburg-Essen (CENIDE), University of Duisburg-Essen, 45117 Essen, Germany. E-mail: stephan.schulz@uni-due.de
First published on 23rd November 2021
Abstract
Heteroleptic zinc(I) complexes L1,2Zn–ZnCp* (L1 = HC[C(CF3)NC6F5]21; L2 = HC[C(Me)NDipp]2; Dipp = 2,6-i-Pr2C6H32) are synthesized by reactions of Cp*2Zn2 with L1H and L2ZnH. 2 reacts with t-BuNCO to give unprecedented carbamate complex (4), while reactions with RN3 gave bis-hexazene, triazenide, and trimeric azide complexes (5–7).
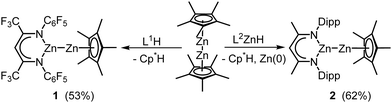
Since the discovery of Cp*2Zn2 (Cp* = C5Me5) by Carmona et al.,1 Zn(I) complexes have received increasing interest.2 In contrast to Cp*2Zn2, which is accessible by reactions of Cp*2Zn with (C2H5)2Zn and by reduction of Cp*2Zn and ZnCl2 with KH,3 the majority of Zn(I) complexes are synthesized by Wurtz-type coupling of organozinc halides RZnX.4 Such reactions also yield homotrinuclear Zn35 and heterotetranuclear Re2Zn2 complexes,6 but this pathway typically suffers from low yields. In contrast, ligand exchange reactions of Cp*2Zn2 with H-acidic ligands or potassium salts are more efficient routes for the synthesis of heteroleptic Zn(I) complexes.7–9 Apart from their bonding nature, Zn(I) complexes are of interest due to their widespread reactivity, i.e. disproportionation,1 acid–base,10 protonation,11 redox,12 and cluster formation reactions,13 respectively. Recently, we reported an isocyanate insertion reaction into one Zn–Cp* bond of Cp*2Zn2,14 revealing a new reaction pattern for Zn(I) complexes. Encouraged by this finding, we became interested in reactions of heteroleptic Zn(I) complexes Cp*ZnZnL with heteroallenes, and report herein on the synthesis of L1ZnZnCp* 1 and L2ZnZnCp* 2, and reactions of 2 with t-BuNCO and three organoazides RN3.
The reaction of L3H (L3 = HC[C(Me)NMes]2, Mes = 2,4,6-Me3C6H2) with Cp*2Zn2 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio yielded the homoleptic Zn(I) complex L32Zn2.7 In contrast, the reaction of Cp*2Zn2 with one or two equiv. of L1H (L1 = HC[C(CF3)NC6F5]2) in toluene at 6 °C gave the heteroleptic complex L1ZnZnCp* 1 (Scheme 1), whereas L2H (L2 = HC[C(Me)NDipp]2, Dipp = 2,6-i-Pr2C6H3) failed to react. Inspired by a Pd(II)-induced homocoupling reaction of RZnH,15 L2ZnH was reacted with Cp*2Zn at 4 °C for 3 days, yielding L2Zn–ZnCp* (2) and other by-products (Fig. S37, ESI†). 2 also formed in the equimolar reaction of L2ZnH with Cp*2Zn2 (Scheme 1), whereas substitution of the second Cp* group by reacting 2 or Cp*2Zn2 with L2ZnH in 1
2 molar ratio yielded the homoleptic Zn(I) complex L32Zn2.7 In contrast, the reaction of Cp*2Zn2 with one or two equiv. of L1H (L1 = HC[C(CF3)NC6F5]2) in toluene at 6 °C gave the heteroleptic complex L1ZnZnCp* 1 (Scheme 1), whereas L2H (L2 = HC[C(Me)NDipp]2, Dipp = 2,6-i-Pr2C6H3) failed to react. Inspired by a Pd(II)-induced homocoupling reaction of RZnH,15 L2ZnH was reacted with Cp*2Zn at 4 °C for 3 days, yielding L2Zn–ZnCp* (2) and other by-products (Fig. S37, ESI†). 2 also formed in the equimolar reaction of L2ZnH with Cp*2Zn2 (Scheme 1), whereas substitution of the second Cp* group by reacting 2 or Cp*2Zn2 with L2ZnH in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio failed, although the desired homoleptic Zn(I) complex L22Zn2 is known.4 In contrast, Cp*2Zn2 reacted with the stronger reductant L2MgH to give zinc metal and L2MgCp* (3, ESI†).
2 molar ratio failed, although the desired homoleptic Zn(I) complex L22Zn2 is known.4 In contrast, Cp*2Zn2 reacted with the stronger reductant L2MgH to give zinc metal and L2MgCp* (3, ESI†).
The 1H and 13C NMR spectra of 1 and 2 show characteristic resonances of the Cp* group and L1 (1) and L2 (2). The 19F NMR spectrum of 1 shows a resonance at −66.40 ppm (CF3) and three resonances of the C6F5 groups in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 intensity ratio.
2 intensity ratio.
Single crystals were grown from toluene solutions at −30 °C (1, Fig. S42, ESI†) and 4 °C (2, Fig. S43, ESI†). The complexes crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (1) and the monoclinic space group P21 (2). The Zn1–Zn2 bond of 1 (2.2883(5) Å) is shorter than that of 2 (2.3008(2) Å) and other Zn(I) complexes, but comparable to those in [Zn]8 clusters (2.27–2.29 Å).16 The Cp* groups are η5-bonded with Zn2–Cp*(centr) distances of 1.8858(4) Å (1) and 1.9215(3) Å (2) and Cp*(centr)–Zn1–Zn2 bond angles of 177.2° (1) and 177.5° (2), which are close to linearity as observed in Zn2Cp*21 and Cp*ZnZnL.8b,14 The Zn1 atoms are three-coordinated, and the Zn1–N1/2 bonds of 1 (1.9917(13), 2.0140(13) Å) are longer than those of 2 (1.9580(10), 1.9598(10) Å), in accordance with the reduced electron donor capacity of the fluorinated L1 ligand,17 but comparable to those of homoleptic complexes L22Zn2 and L32Zn2.4,7
(1) and the monoclinic space group P21 (2). The Zn1–Zn2 bond of 1 (2.2883(5) Å) is shorter than that of 2 (2.3008(2) Å) and other Zn(I) complexes, but comparable to those in [Zn]8 clusters (2.27–2.29 Å).16 The Cp* groups are η5-bonded with Zn2–Cp*(centr) distances of 1.8858(4) Å (1) and 1.9215(3) Å (2) and Cp*(centr)–Zn1–Zn2 bond angles of 177.2° (1) and 177.5° (2), which are close to linearity as observed in Zn2Cp*21 and Cp*ZnZnL.8b,14 The Zn1 atoms are three-coordinated, and the Zn1–N1/2 bonds of 1 (1.9917(13), 2.0140(13) Å) are longer than those of 2 (1.9580(10), 1.9598(10) Å), in accordance with the reduced electron donor capacity of the fluorinated L1 ligand,17 but comparable to those of homoleptic complexes L22Zn2 and L32Zn2.4,7
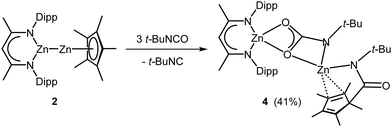
With heteroleptic complex 2 in hand, we explored its reactivity toward heteroallenes. Homoleptic Cp*2Zn2 reacted with RNCO (R = t-Bu, Dipp) at ambient temperature with insertion into one Zn–Cp* bond.14 In contrast, the reaction of heteroleptic Zn(I) complex 2 with t-BuNCO at 70 °C for 4 days gave complex 4 in 41% yield (Scheme 2), whereas no reaction occurred with DippNCO even at 100 °C. The formation of 4 results from insertion of t-BuNCO into the Zn–Zn and Zn–Cp* bonds and cleavage of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond accompanied by the formation of t-BuNC as was confirmed by in situ1H NMR spectroscopy (Fig. S39, ESI†). Any attempts to isolate reaction intermediates by varying the temperature and the molar ratio of the reagents failed. However, an excess of t-BuNCO promotes the reaction, as 4 was not formed in a 1
O bond accompanied by the formation of t-BuNC as was confirmed by in situ1H NMR spectroscopy (Fig. S39, ESI†). Any attempts to isolate reaction intermediates by varying the temperature and the molar ratio of the reagents failed. However, an excess of t-BuNCO promotes the reaction, as 4 was not formed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio reaction at 70 °C. The analogous reaction of L32Zn27 with t-BuNCO also quantitatively gave a zinc carbamate complex 4S (Fig. S40 and S46, ESI†) and t-BuNC.
1 molar ratio reaction at 70 °C. The analogous reaction of L32Zn27 with t-BuNCO also quantitatively gave a zinc carbamate complex 4S (Fig. S40 and S46, ESI†) and t-BuNC.
Complex 4 is thermally stable and decomposes at 210 °C. The 1H NMR spectrum shows two singlets of the t-Bu groups and five singlets of the Cp* group, indicating an asymmetric nature of the complex in solution. The 13C NMR spectrum also shows five singlets of the Me groups of the Cp* ligand as well as two singlets of the tertiary C atom of the t-Bu groups and resonances of the NCO units at 170.1 and 172.8 ppm, respectively.
Single crystals of 4, which crystallize in the monoclinic space group P21/c (Fig. 1), were grown from a saturated toluene solution at 4 °C. Both Zn atoms adopt distorted tetrahedral coordination spheres and are bridged by a carbamate unit.
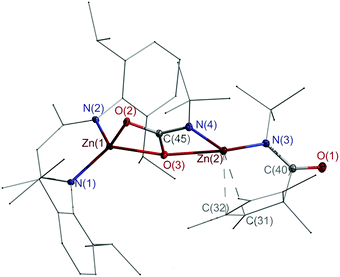 | ||
| Fig. 1 Molecular structure of 4. Thermal ellipsoids are drawn at 30% probability level. Parts of the ligands are drawn in wire/stick model, while H atoms are omitted for clarity. | ||
The Zn1–O2/3 (2.0697(8), 2.0309(8) Å) bond lengths are comparable to those of the zinc carbamate complex L2ZnO2CN(i-Pr)2 (2.028(2), 2.041(1) Å),18 but longer compared to those of the carboxylate complexes [L2Zn(μ,η2-O2CR)]2 (R = H, Me, Ph, Oi-Pr), which range from 1.936 to 2.027 Å.19 The Zn2–N3/4 (1.9000(9), 1.9169(9) Å) bond lengths are virtually identical to that of Cp*Zn–Zn(N(t-Bu)C(Cp*)O) (1.9148(9) Å),14 while the Zn2–O3 distance (2.2780(8) Å) is rather long, indicating a rather weak coordinative interaction. The C–O (1.2819(12), 1.3273(12) Å) and C–N bond lengths (1.3270(13) Å) indicate a delocalized π-electron system within the carbamate unit. The Cp* ligand is η2-coordinated to Zn2, and the Zn–C bonds (2.4906(11), 2.5135(11) Å) are elongated compared to Zn-π complexes with η2 interactions, i.e. alkyne-coordinated ZnBr2 (2.217(5), 2.393(5) Å)20 and [PhC(Nt-Bu)2(Cp*)Si–Zn(Cp*)Cl] (2.2519(26), 2.1224(29) Å),21 but shorter than those in Zn(C6F5)2(tol.) (2.784(2), 2.6847(15) Å)22 and arylacetylene-substituted calix[4]arene zinc complexes (2.7695(37), 3.0667(37) Å).23
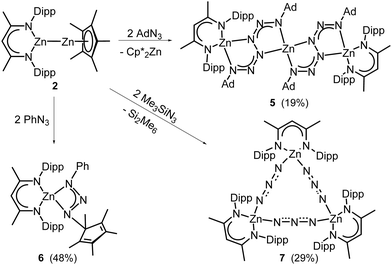
Complex 2 was then reacted with organoazides RN3 (R = Ph, Ad, SiMe3). In contrast to homoleptic L32Zn2, which was found to react with RN3 with formation of zinc hexazene [(L3Zn)2(μ-η2:η2-N6R2)] (R = Ph, 2,6-i-Pr2C6H3) or dimeric zinc azide complexes [(L3Zn)(μ-N3)]2 (R = Me3Si, Me3Sn),12c the reaction of heteroleptic complex 2 with 2 equiv. of AdN3 at 70 °C for 2 days yielded the first bis-hexazene complex 5 (Scheme 3), which is thermally stable in solution up to 100 °C and in the solid state (decomposition temperature > 300 °C), respectively. Complex 5 is likely formed via the Cp*Zn(μ-η2:η2-N6Ad2)ZnL2 intermediate, followed by intramolecular elimination of Cp*2Zn.
In contrast, the reaction of 2 with PhN3 gave the zinc triazenide complex 6 in 48% yield (Scheme 3). Alkaline or alkaline earth metal triazenides are typically formed in reactions of aryl azides and organolithium and magnesium complexes,24 hence the formation of 6 likely results from a nucleophilic attack of the Cp* ligand. Since no reaction was observed in a control experiment of L2ZnCp* with PhN3, we assume that the first reaction step is an insertion reaction of PhN3 into the Zn–Zn bond of 2. Low-valent metal complexes are known to react with RN3 with formation of metal triazenides as was shown for homo- (Al, Cr) and heterobimetallic (In–Zn) complexes,25 while a dinuclear iron complex was formed by the reaction of an Fe–N2 complex with AdN3.26 The reaction of 2 with Me3SiN3 occurred with reductive elimination of Si2Me6 as was reported for the analogous reaction of L32Zn212c and formation of complex 7 featuring a pseudo triangular Zn3N9 moiety (Scheme 3). 7 also formed in 78% yield in the reaction of L2ZnH and Me3SiN3.
1H and 13C NMR spectra of 5 and 6 show resonances of L2 and Ad (5) and Cp* and Ph (6), while 7 shows two sets of resonances of L2 due to two conformers in solution, which form a temperature-dependent equilibrium as confirmed by VT-1H NMR (Fig. S41, ESI†). IR spectra show absorption bands of the hexazene (1265, 1218 cm−1, 5), triazenide (1313, 1255 cm−1, 6) and azide groups (2157, 2124 cm−1, 7).
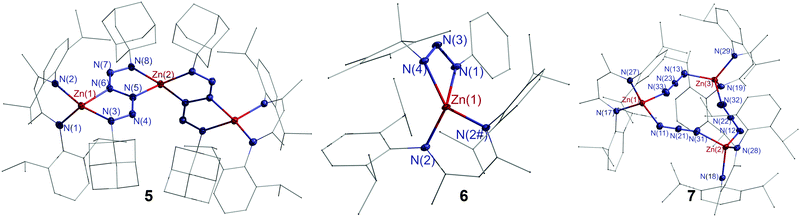
Single crystals of 5–7 were grown from toluene solutions. Complexes 5 and 6 crystallize in the monoclinic space groups P2/n and P21/m and complex 7 in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Fig. 2). Complex 5 contains two bridging hexazene ligands. The Zn1–N1/2 bonds within the C3N2Zn ring are slightly shorter than the Zn1–N3/6 bonds (2.0024(19), 2.0378(18) Å) in the neighbouring N4Zn ring, but comparable to Zn–N3/6 bonds (2.0079(18), 1.9817(19) Å) in the nonadjacent N4Zn ring. The N5–N6 distance (1.400(3) Å) is typical for a single bond, while the other N–N bond lengths (1.297(3)–1.301(3) Å) of the hexazene unit indicate an allyl-like nature as was previously reported for metal hexazene complexes.12c,27 The Zn atom in triazene complex 6 is tetrahedrally coordinated by four N atoms of the L2 ligand and the triazene group. The N–N bond lengths within the ZnN3 metallacycle (1.297(6), 1.307(5) Å) indicate a delocalized π-electron system within the N3 moiety. The Zn–N2 bonds (1.9592(10) Å) are shorter than the Zn–N1/3 bonds (2.0524(13), 2.0869(14) Å). The only structurally characterized zinc triazenide complex [Dipp2N3]2Zn, which was prepared by an ethane elimination reaction of ZnEt2 with Dipp2N3H,28 shows comparable structure parameters.
(Fig. 2). Complex 5 contains two bridging hexazene ligands. The Zn1–N1/2 bonds within the C3N2Zn ring are slightly shorter than the Zn1–N3/6 bonds (2.0024(19), 2.0378(18) Å) in the neighbouring N4Zn ring, but comparable to Zn–N3/6 bonds (2.0079(18), 1.9817(19) Å) in the nonadjacent N4Zn ring. The N5–N6 distance (1.400(3) Å) is typical for a single bond, while the other N–N bond lengths (1.297(3)–1.301(3) Å) of the hexazene unit indicate an allyl-like nature as was previously reported for metal hexazene complexes.12c,27 The Zn atom in triazene complex 6 is tetrahedrally coordinated by four N atoms of the L2 ligand and the triazene group. The N–N bond lengths within the ZnN3 metallacycle (1.297(6), 1.307(5) Å) indicate a delocalized π-electron system within the N3 moiety. The Zn–N2 bonds (1.9592(10) Å) are shorter than the Zn–N1/3 bonds (2.0524(13), 2.0869(14) Å). The only structurally characterized zinc triazenide complex [Dipp2N3]2Zn, which was prepared by an ethane elimination reaction of ZnEt2 with Dipp2N3H,28 shows comparable structure parameters.
In contrast to dimeric [(L3Zn)(μ-N3)]2,29 complex 7 forms a pseudo-triangular Zn3N9 moiety with bridging N3 units, resulting in an almost planar Zn3N9 metallacycle (r.m.s. deviation from the least-squares plane 0.0655 Å), and each Zn atom is further coordinated by one L2 ligand. The Zn–N bonds (1.952(5)–2.027(5) Å) within the Zn3N9 moiety are slightly longer than those in the C3N2Zn rings (1.945(4)–1.959(5) Å). The N–N–N angles of 178.0(7)°, 179.1(7)° and 178.9(7)° are almost linear, and the N–N bond lengths range from 1.147(7) to 1.192(7) Å.
To summarize, heteroleptic Zn(I) complexes L1/2ZnZnCp* (1, 2) were synthesized and reactions of L2ZnZnCp* 2 with heteroallenes are reported. The reaction with t-BuNCO proceeded with insertion into both the Zn–Zn and the Zn–Cp* bonds and formation of carbamate complex 4, whereas reactions with RN3 yielded unprecedented bis-hexazenide, triazenide, and trimeric azide complexes 5–7, respectively.
S. S. acknowledges support by the University of Duisburg-Essen.
Conflicts of interest
There are no conflicts to declare.Notes and references
- I. Resa, E. Carmona, E. Gutierrez-Puebla and A. Monge, Science, 2004, 305, 1136 CrossRef CAS PubMed.
- (a) C.-S. Cao, Y. Shi, H. Xu and B. Zhao, Coord. Chem. Rev., 2018, 365, 122 CrossRef CAS; (b) R. H. Duncan Lyngdoh, H. F. Schaefer and R. B. King, Chem. Rev., 2018, 118, 11626 CrossRef CAS; (c) T. Li, S. Schulz and P. W. Roesky, Chem. Soc. Rev., 2012, 41, 3759 RSC; (d) E. Carmona and A. Galindo, Angew. Chem., Int. Ed., 2008, 47, 6526 CrossRef CAS PubMed; (e) A. Grirrane, I. Resa, A. Rodríguez and E. Carmona, Coord. Chem. Rev., 2008, 252, 1532 CrossRef CAS; (f) R. Ayala and A. Galindo, J. Organomet. Chem., 2019, 898, 120878 CrossRef.
- A. Grirrane, I. Resa, A. Rodríguez, E. Carmona, E. Alvarez, E. Gutierrez-Puebla, A. Monge, A. Galindo, D. del Río and R. A. Andersen, J. Am. Chem. Soc., 2007, 129, 693 CrossRef CAS PubMed.
- (a) M. Ma, L. Shen, H. Wang, Y. Zhao, B. Wu and X.-J. Yang, Organometallics, 2020, 39, 1440 CrossRef CAS; (b) M. Juckel, D. Dange, C. de Bruin-Dickason and C. Jones, Z. Anorg. Allg. Chem., 2020, 646, 603 CrossRef CAS; (c) A. Stasch, Chem. – Eur. J., 2012, 18, 15105 CrossRef CAS PubMed; (d) Y. Liu, S. Li, X.-J. Yang, P. Yang, J. Gao, Y. Xia and B. Wu, Organometallics, 2009, 28, 5270 CrossRef CAS; (e) Z. Zhu, M. Brynda, R. J. Wright, R. C. Fischer, W. A. Merrill, E. Rivard, R. R. Wolf, J. C. Fettinger, M. M. Olmstead and P. P. Power, J. Am. Chem. Soc., 2007, 129, 10847 CrossRef CAS PubMed; (f) I. L. Fedushkin, A. A. Skatova, S. Y. Ketkov, O. V. Eremenko, A. V. Piskunov and G. K. Fukin, Angew. Chem., Int. Ed., 2007, 46, 4302 CrossRef CAS PubMed; (g) Y.-C. Tsai, D.-Y. Lu, Y.-M. Lin, J.-K. Hwang and J.-S. K. Yu, Chem. Commun., 2007, 4125 RSC; (h) X.-J. Yang, J. Yu, Y. Liu, Y. Xie, H. F. Schaefer, Y. Liang and B. Wu, Chem. Commun., 2007, 2363 RSC; (i) Z. Zhu, R. J. Wright, M. M. Olmstead, E. Rivard, M. Brynda and P. P. Power, Angew. Chem., Int. Ed., 2006, 45, 5807 CrossRef CAS PubMed; (j) Y. Z. Wang, B. Quillian, P. R. Wei, H. Y. Wang, X. J. Yang, Y. M. Xie, R. B. King, P. V. Schleyer, H. F. Schaefer and G. H. Robinson, J. Am. Chem. Soc., 2005, 127, 11944 CrossRef CAS PubMed.
- J. Hicks, E. J. Underhill, C. E. Kefalidis, L. Maron and C. Jones, Angew. Chem., Int. Ed., 2015, 54, 10000 CrossRef CAS PubMed.
- T. D. Lohrey, L. Maron, R. G. Bergman and J. Arnold, J. Am. Chem. Soc., 2019, 141, 800 CrossRef CAS PubMed.
- S. Schulz, D. Schuchmann, U. Westphal and M. Bolte, Organometallics, 2009, 28, 1590 CrossRef CAS.
- (a) H. P. Nayek, A. Lühl, S. Schulz, R. Köppe and P. W. Roesky, Chem. – Eur. J., 1773, 2011, 17 Search PubMed; (b) S. Schulz, S. Gondzik, D. Schuchmann, U. Westphal, L. Dobrzycki, R. Boese and S. Harder, Chem. Commun., 2010, 46, 7757 RSC.
- (a) K. Mayer, L. Jantke, S. Schulz and T. F. Fässler, Angew. Chem., Int. Ed., 2017, 56, 2350 CrossRef CAS PubMed; (b) S. Gondzik, D. Bläser, C. Wölper and S. Schulz, Chem. – Eur. J., 2010, 16, 13599 CrossRef CAS.
- (a) D. Schuchmann, U. Westphal, S. Schulz, U. Flörke, D. Bläser and R. Boese, Angew. Chem., Int. Ed., 2009, 48, 807 CrossRef CAS PubMed; (b) M. Carrasco, R. Peloso, I. Resa, A. Rodríguez, L. Sánchez, E. Álvarez, C. Maya, R. Andreu, J. J. Calvente, A. Galindo and E. Carmona, Inorg. Chem., 2011, 50, 6361 CrossRef CAS PubMed; (c) P. Jochmann and D. W. Stephan, Chem. – Eur. J., 2014, 20, 8370 CrossRef CAS PubMed.
- (a) S. Schulz, D. Schuchmann, I. Krossing, D. Himmel, D. Bläser and R. Boese, Angew. Chem., Int. Ed., 2009, 48, 5748 CrossRef CAS PubMed; (b) M. Carrasco, R. Peloso, A. Rodríguez, E. Álvarez, C. Maya and E. Carmona, Chem. – Eur. J., 2010, 16, 9754 CrossRef CAS PubMed; (c) K. Freitag, H. Banh, C. Ganesamoorthy, C. Gemel, R. W. Seidel and R. A. Fischer, Dalton Trans., 2013, 42, 10540 RSC; (d) H. Banh, C. Gemel, R. W. Seidel and R. A. Fischer, Chem. Commun., 2015, 51, 2170 RSC.
- (a) S. Gondzik, D. Bläser, C. Wölper and S. Schulz, J. Organomet. Chem., 2015, 783, 92 CrossRef CAS; (b) S. Gondzik, S. Schulz, D. Bläser and C. Wölper, Chem. Commun., 2014, 50, 1189 RSC; (c) S. Gondzik, S. Schulz, D. Bläser, C. Wölper, R. Haack and G. Jansen, Chem. Commun., 2014, 50, 927 RSC; (d) J. Gao, S. Li, Y. Zhao, B. Wu and X.-J. Yang, Organometallics, 2012, 31, 2978 CrossRef CAS.
- (a) H. Banh, J. Hornung, T. Kratz, C. Gemel, A. Pöthig, F. Gam, S. Kahlal, J.-Y. Saillard and R. A. Fischer, Chem. Sci., 2018, 9, 8906 RSC; (b) K. Freitag, C. Gemel, P. Jerabek, I. M. Oppel, R. W. Seidel, G. Frenking, H. Banh, K. Dilchert and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 4370 CrossRef CAS PubMed; (c) T. Bollermann, K. Freitag, C. Gemel, R. W. Seidel, M. von Hopffgarten, G. Frenking and R. A. Fischer, Angew. Chem., Int. Ed., 2011, 50, 772 CrossRef CAS PubMed; (d) H. Banh, K. Dilchert, C. Schulz, C. Gemel, R. W. Seidel, R. Gautier, S. Kahlal, J.-Y. Saillard and R. A. Fischer, Angew. Chem., Int. Ed., 2016, 55, 3285 CrossRef CAS PubMed.
- B. Li, C. Wölper, K. Huse and S. Schulz, Chem. Commun., 2020, 56, 8643 RSC.
- (a) M. Chen, S. Jiang, L. Maron and X. Xu, Dalton Trans., 2019, 48, 1931 RSC; (b) S. Jiang, M. Chen and X. Xu, Inorg. Chem., 2019, 58, 13213 CrossRef CAS PubMed.
- P. Cui, H.-S. Hu, B. Zhao, J. T. Miller, P. Cheng and J. Li, Nat. Commun., 2015, 6, 6331 CrossRef CAS PubMed.
- (a) K. Huse, C. Wölper and S. Schulz, Eur. J. Inorg. Chem., 2018, 3472 CrossRef CAS; (b) K. Huse, H. Weinert, C. Wölper and S. Schulz, Dalton Trans., 2020, 49, 9773 RSC.
- M. H. Chisholm, J. Gallucci and K. Phomphrai, Inorg. Chem., 2002, 41, 2785 CrossRef CAS PubMed.
- (a) G. Feng, C. Du, L. Xiang, I. del Rosal, G. Li, X. Leng, E. Y.-X. Chen, L. Maron and Y. Chen, ACS Catal., 2018, 8, 4710 CrossRef CAS; (b) C. Scheiper, C. Wölper, D. Bläser, J. Roll and S. Schulz, Z. Naturforsch., B: J. Chem. Sci., 2014, 69, 1365 CrossRef CAS; (c) D. R. Moore, M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2003, 125, 11911 CrossRef CAS PubMed; (d) M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1998, 120, 11018 CrossRef CAS.
- H. Lang, N. Mansilla and G. Rheinwald, Organometallics, 2001, 20, 1592 CrossRef CAS.
- S. Schäfer, R. Köppe, M. T. Gamer and P. W. Roesky, Chem. Commun., 2014, 50, 11401 RSC.
- A. Guerrero, E. Martin, D. L. Hughes, N. Kaltsoyannis and M. Bochmann, Organometallics, 2006, 25, 3311 CrossRef CAS.
- E. Bukhaltsev, I. Goldberg, R. Cohen and A. Vigalok, Organometallics, 2007, 26, 4015 CrossRef CAS.
- (a) S. G. Alexander, M. L. Cole, C. M. Forsyth, S. K. Furfari and K. Konstas, Dalton Trans., 2009, 2326 RSC; (b) S.-O. Hauber, F. Lissner, G. B. Deacon and M. Niemeyer, Angew. Chem., Int. Ed., 2005, 44, 5871 CrossRef CAS PubMed.
- (a) W. Uhl, R. Gerding, S. Pohl and W. Saak, Chem. Ber., 1995, 128, 81 CrossRef CAS; (b) C. Ni, B. D. Ellis, G. J. Long and P. P. Power, Chem. Commun., 2009, 2332 RSC; (c) M. D. Anker, Y. Altaf, M. Lein and M. P. Coles, Dalton Trans., 2019, 48, 16588 RSC.
- R. E. Cowley, N. J. DeYonker, N. A. Eckert, T. R. Cundari, S. DeBeer, E. Bill, X. Ottenwaelder, C. Flaschenriem and P. L. Holland, Inorg. Chem., 2010, 49, 6172 CrossRef CAS PubMed.
- (a) R. E. Cowley, J. Elhaïk, N. A. Eckert, W. W. Brennessel, E. Bill and P. L. Holland, J. Am. Chem. Soc., 2008, 130, 6074 CrossRef CAS PubMed; (b) S. J. Bonyhady, S. P. Green, C. Jones, S. Nembenna and A. Stasch, Angew. Chem., Int. Ed., 2009, 48, 2973 CrossRef CAS PubMed; (c) S. J. Bonyhady, C. Jones, S. Nembenna, A. Stasch, A. J. Edwards and G. J. McIntyre, Chem. – Eur. J., 2010, 16, 938 CrossRef CAS PubMed; (d) J. A. Bellow, P. D. Martin, R. L. Lord and S. Groysman, Inorg. Chem., 2013, 52, 12335 CrossRef CAS PubMed; (e) L. Fohlmeister and C. Jones, Aust. J. Chem., 2014, 67, 1011 CrossRef CAS; (f) S. Gondzik, C. Wölper, R. Haack, G. Jansen and S. Schulz, Dalton Trans., 2015, 44, 15703 RSC; (g) C. Stienen, S. Gondzik, A. Gehlhaar, R. Haack, C. Wölper, G. Jansen and S. Schulz, Organometallics, 2016, 35, 1022 CrossRef CAS.
- N. Nimitsiriwat, V. C. Gibson, E. L. Marshall, P. Takolpuckdee, A. K. Tomov, A. J. P. White, D. J. Williams, M. R. J. Elsegood and S. H. Dale, Inorg. Chem., 2007, 46, 9988 CrossRef CAS PubMed.
- G. Bendt, S. Schulz, J. Spielmann, S. Schmidt, D. Bläser and C. Wölper, Eur. J. Inorg. Chem., 2012, 3725 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, analytical (NMR, IR spectra, elemental analysis) and crystallographic data of 1–7. CCDC 2111218 (1), 2111219 (2), 2111220 (3), 2111221 (4), 2111225 (4S), 2111222 (5), 2111223 (6) and 2111224 (7). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc05617d |
| This journal is © The Royal Society of Chemistry 2021 |