Lanthanide-based molecular alloys with hydroxyterephthalate: a versatile system†
Jinzeng
Wang
,
Yan
Suffren
,
Carole
Daiguebonne
,
Kevin
Bernot
 ,
Guillaume
Calvez
,
Guillaume
Calvez
 ,
Stéphane
Freslon
and
Olivier
Guillou
,
Stéphane
Freslon
and
Olivier
Guillou
 *
*
Univ Rennes, INSA Rennes, CNRS UMR 6226 “Institut des Sciences Chimiques de Rennes”, F-35708 Rennes, France. E-mail: Olivier.guillou@insa-rennes.fr
First published on 6th November 2020
Abstract
Reactions in water between lanthanide chlorides and the disodium salt of 2-hydroxyterephthalic acid (H2hbdc) lead to six families of lanthanide-based coordination polymers depending on the lanthanide ion and the crystal growth method. Compounds that constitute family F1 have the general chemical formula [Ln(Hhbdc)(hbdc)·9H2O]∞ with Ln = La–Nd and have been obtained by slow evaporation. [Ln2(hbdc)3(H2O)6·4H2O]∞ with Ln = Sm–Eu constitute family F2 and have been obtained by solvothermal synthesis. Family F3 includes compounds, obtained by a solvothermal method, with the general chemical formula [Ln2(hbdc)3(H2O)4·4H2O]∞ with Ln = Ho–Lu plus Y and compounds obtained by slow diffusion through gels with Ln = Eu–Tb. [Ln(Hhbdc)(hbdc)(H2O)3·H2O]∞ with Ln = Tb–Dy have been obtained by solvothermal methods and constitute family F4. [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5) has been obtained by slow evaporation. The last family (F6) includes compounds with the general chemical formula [Ln2(hbdc)3(H2O)8·2H2O]∞ with Ln = Nd–Tb that have been obtained by slow diffusion through gel media. Gd-Based micro-crystalline powders can be obtained by direct mixing of aqueous solutions of Gd3+ and hbdc2−. Unexpectedly, the micro-crystalline powder belongs to F5 when the lanthanide solution is added to the ligand one and to F6 when the opposite occurs. This phenomenon is also observed for the Tb- and/or Eu-based heterolanthanide coordination polymers. Their optical properties have been studied in detail.
Introduction
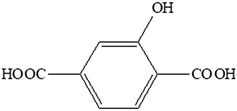
For almost two decades, there has been growing interest in luminescent lanthanide-based coordination polymers because of their potential applications in various technological fields1–4 such as chemical sensing,5–7 lighting and displays,8,9 thermometry10,11 and the fight against counterfeiting,12 for example. Because lanthanide ions can be considered as hard acids according to Pearson's theory,13 the choice of the ligand is of first importance as far as structural organization is concerned. Lanthanide-based coordination polymers with terephthalate have been extensively studied because of their great structural diversity14 and their high optical application potential.15,16 Their hydroxy-derivatives are appealing because of the potential influence of the hydroxyl group on the optical properties and/or the crystal structure. Indeed, the donor hydroxyl function can induce a photon-induced electron transfer (PET) mechanism17 and can be involved in H-bond networks. Therefore, we have undertaken a study of lanthanide-based coordination polymers with the 2-hydroxyterephthalate (hbdc2−) ligand (Scheme 1).To the best of our knowledge, only one lanthanide-based coordination polymer with this ligand has been reported to date: [Eu2(hbdc)3(H2O)]∞.18 This compound, obtained by solvo-thermal techniques, presents a 3D crystal structure. It shows good efficiency in ratiometric Fe3+ ion sensing.
In this paper, six new families of lanthanide based coordination polymers with 2-hydroxyterephthalate as well as their luminescence properties are described.
Experimental section
2-Hydroxyterephthalic acid was purchased from TCI and used without further purification. Lanthanide oxides (4 N) were purchased from Ampère. Lanthanide chlorides were prepared according to established procedures.19 Tetramethylorthosilicate was purchased from Acros Organics. Gels were prepared according to previously described procedures.20–22Synthesis of single-crystals of the coordination polymers
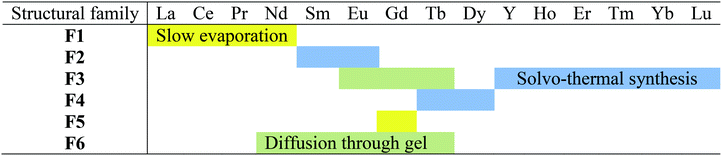
Three different synthetic methods have been used to grow single crystals: solvothermal, slow evaporation and diffusion through gel methods. The results of the crystal growth experiments are summarized in Table 1.Membership of a structural family or another has been assumed on the basis of powder X-ray diffraction diagrams recorded on microcrystalline powders made of crushed single crystals (Fig. S2–S5†) or on the basis of cell parameters when single crystals were grown in gel medium.
Preparation of the lanthanide coordination polymer microcrystalline powders
A solution of a lanthanide chloride (Ln = La–Lu plus Y except Pm) (0.2 mmol in 5 mL of water) was added to an aqueous solution of Na2hbdc·H2O (0.3 mmol in 5 mL of water). The mixture was maintained under stirring during 48 hours to ensure better crystallinity. The obtained white precipitate was filtered and dried under ambient conditions (the yield was about 90%). The microcrystalline powders have been classified into four structural families on the basis of their powder X-ray diffraction diagrams (Table 2 and Fig. S6†).| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Structural family | F1 | F2 | F5 | Unknown phase | |||||||||||
| F6 | |||||||||||||||
It is noticeable that depending on the order one reactant is added to the other, the Gd-derivative crystallizes with structural type F5 or F6 (Fig. S7†).
In order to target brightness and color tuning, some heterolanthanide coordination polymers have also been prepared simply replacing, in the above described synthesis, the lanthanide solution by a solution that contains an appropriate mixture of lanthanide chlorides. Membership of one or another structural family was assumed on the basis of the powder X-ray diffraction diagrams of the microcrystalline powders (Fig. S8–S11† and Table 3). Their relative metallic content was measured by EDS and is listed in Table S1.† Identical to that of the Gd-based coordination polymers, the structural type adopted by the Gd/Tb, Gd/Eu and Tb/Eu-based heterolanthanide coordination polymers depends on the reactant addition order: when the lanthanide solution is added to the ligand solution, the structural type is F5; in contrast, F6 is obtained when the ligand solution is added to the lanthanide solution.
Syntheses of the disodium salt of 2-hydroxyterephthalic acid and the single-crystals of the coordination polymers are available in the ESI.† Powder X-ray diffraction, thermal analysis, electron dispersive spectroscopy and optical measurements have been performed according to procedures that have been previously reported.23 These are available in the ESI.†
Single-crystal X-ray diffraction
Selected crystal and final structure refinement data are gathered in Table 4. Some other details are available in the ESI.†| F1 | F2 | F3 | F4 | F5 | F6 | |
|---|---|---|---|---|---|---|
| Molecular formula | PrC16H19O15 | Sm2C24H32O25 | Y2C24H28O23 | TbC16H17O14 | Gd4C48H80O58 | Gd2C24H32O25 |
| Formula weight (g mol−1) | 592.22 | 1021.19 | 862.28 | 592.21 | 2214.12 | 1034.99 |
| System | Cubic | Triclinic | Triclinic | Triclinic | Monoclinic | Monoclinic |
| Space group (no.) |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (206) (206) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (2) (2) |
C2/c(15) | P21/c(14) |
| a (Å) | 26.9120(7) | 7.9806(8) | 9.3864(7) | 9.4609(8) | 16.982(2) | 10.9491(14) |
| b (Å) | 26.9120(7) | 9.9540(9) | 10.0102(7) | 10.3877(9) | 10.8156(15) | 12.8463(16) |
| c (Å) | 26.9120(7) | 10.8426(11) | 10.3408(8) | 11.0504(8) | 20.008(2) | 11.5619(14) |
| α (°) | 90 | 74.620(3) | 107.622(3) | 115.254(3) | 90 | 90 |
| β (°) | 90 | 74.092(3) | 99.379(3) | 107.794(3) | 105.231(4) | 102.493(4) |
| γ (°) | 90 | 73.803(3) | 110.080(3) | 90.967(3) | 90 | 90 |
| V (Å3) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491.2(4) 491.2(4) |
778.67(13) | 830.17(11) | 921.44(13) | 3545.8(7) | 1587.7(3) |
| Z | 24 | 1 | 1 | 2 | 2 | 2 |
| D calc (g cm−3) | 1.211 | 2.178 | 1.725 | 2.134 | 2.074 | 2.165 |
| R (%) | 0.0907 | 0.0202 | 0.0280 | 0.0210 | 0.0272 | 0.0244 |
| R w (%) | 0.2862 | 0.0522 | 0.0756 | 0.0531 | 0.0688 | 0.0632 |
| GoF | 1.353 | 1.153 | 1.053 | 1.068 | 1.061 | 1.078 |
| CCDC entry | 1913183 | 1913130 | 1913137 | 1913124 | 1913145 | 1913142 |
Results and discussion
Family F1: [Ln(Hhbdc)(hbdc)·9H2O]∞ with Ln = La–Nd
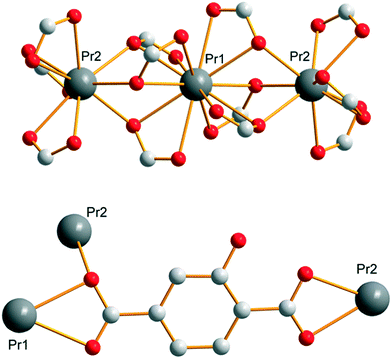
Compounds that constitute this family (F1) have the general chemical formula [Ln(Hhbdc)(hbdc)·9H2O]∞ with Ln = La–Nd. They have been obtained as microcrystalline powders by direct precipitation and as single-crystals by slow evaporation of the filtrates. Their crystal structures have been solved on the basis of the Pr-derivative. Isostructurality of the other compounds has been assumed on the basis of their powder X-ray diffraction diagrams (Fig. S2†). [Pr(Hhbdc)(hbdc)·9H2O]∞ crystalizes in the cubic system, space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 206). It is noticeable that these compounds are isomorphous to [Ln(abdc)(Habdc)·nH2O]∞ with Ln = La–Eu and 8 ≤ n ≤ 11, where H2abdc stands for 2-aminoterephthalic acid, which have been previously described.24,25 In this crystal structure, half of the ligands are once deprotonated as confirmed by IR spectroscopy (Fig. S12†). Therefore, there are two types of ligands, Hhbdc− and hbdc2−, in the chemical formula of the compound. There are two Pr3+ ions in the asymmetric unit. Pr13+ is twelve coordinated by six chelating carboxylate groups from six different ligands to form an icosahedron (Fig. 1). Pr23+ is coordinated by nine oxygen atoms: three out of them from three different monodentate ligands and the six other from three different bidentate ligands. The coordination polyhedron can be described as a distorted tricapped trigonal prism (Fig. 1).
(no. 206). It is noticeable that these compounds are isomorphous to [Ln(abdc)(Habdc)·nH2O]∞ with Ln = La–Eu and 8 ≤ n ≤ 11, where H2abdc stands for 2-aminoterephthalic acid, which have been previously described.24,25 In this crystal structure, half of the ligands are once deprotonated as confirmed by IR spectroscopy (Fig. S12†). Therefore, there are two types of ligands, Hhbdc− and hbdc2−, in the chemical formula of the compound. There are two Pr3+ ions in the asymmetric unit. Pr13+ is twelve coordinated by six chelating carboxylate groups from six different ligands to form an icosahedron (Fig. 1). Pr23+ is coordinated by nine oxygen atoms: three out of them from three different monodentate ligands and the six other from three different bidentate ligands. The coordination polyhedron can be described as a distorted tricapped trigonal prism (Fig. 1).
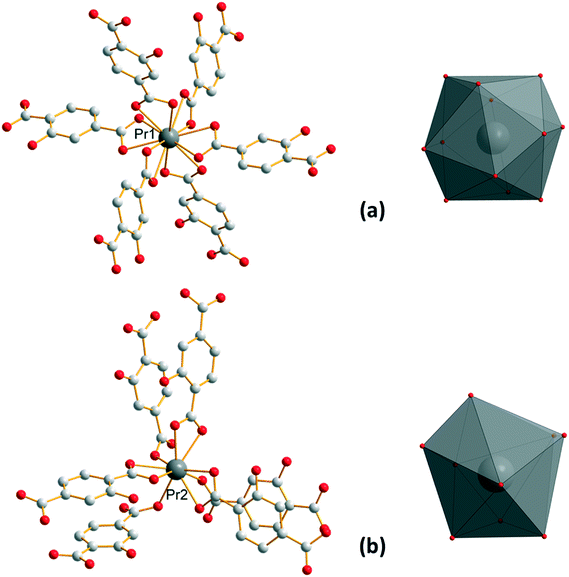 | ||
| Fig. 1 Coordination environments and coordination polyhedra of Pr13+ (a) and Pr23+ (b) in [Pr(Hhbdc)(hbdc)·9H2O]∞ (F1). | ||
Each Pr13+ is bound to two Pr23+ through carboxylate groups to form a trinuclear unit (Fig. 2) which prevents the entrance of coordinated water molecules in the coordination spheres. The shortest distance between Pr13+ and Pr23+ inside a trinuclear unit is 3.9407(5) Å. The Pr–Pr distance between Pr3+ ions that belong to adjacent trinuclear units is 11.6346(5) Å. All the ligands adopt the same coordination mode (Fig. 2).
Each trinuclear unit is linked to twelve ligands which can be seen as six bridges if pairs of ligands are regarded as connections. This connection mode generates a cubic molecular skeleton (Fig. 3). This crystal structure presents large square-section (11 × 11 Å2) channels in which crystallization water molecules are localized. These crystallization water molecules were not precisely localized by single X-ray diffraction (which explains the bad GoF value). However, the overall number of crystallization water molecules was confirmed by thermogravimetric analysis (Fig. S13†).
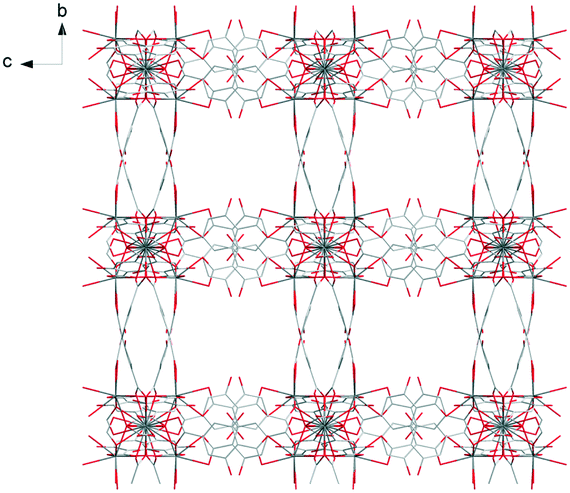 | ||
| Fig. 3 Projection view along the a-axis of [Pr(Hhbdc)(hbdc)·9H2O]∞ (F1). Crystallization water molecules are omitted for clarity. | ||
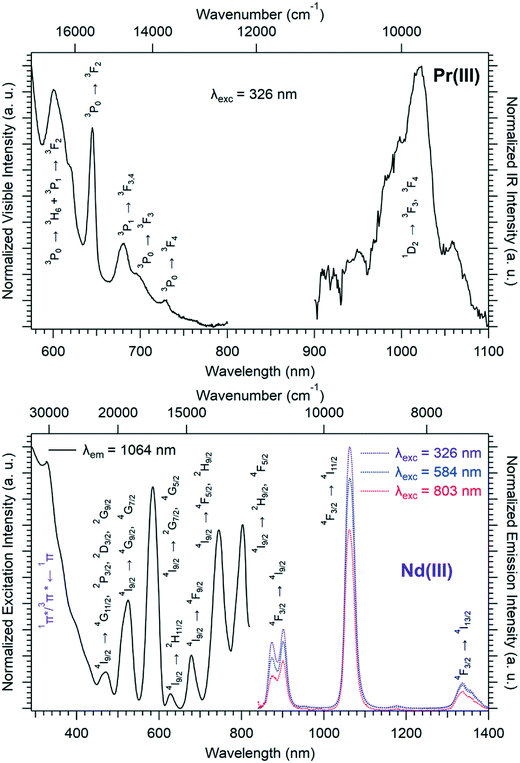
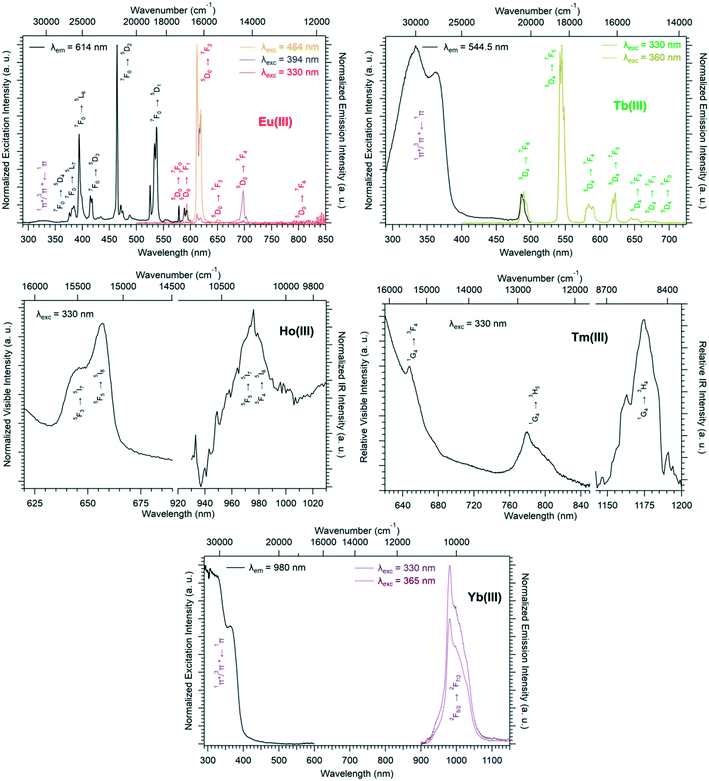
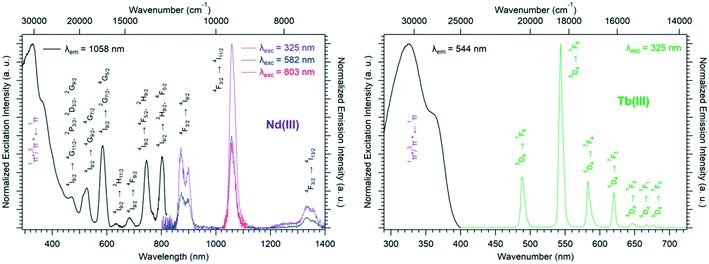
Solid-state excitation and emission spectra of [Ln(Hhbdc)(hbdc)·9H2O]∞ (Ln = La–Nd) have been recorded at room temperature. The Pr3+-derivative shows a weak emission in the visible and near infrared domains (Fig. 4 top). The Nd3+-derivative presents luminescence properties in the NIR region (Fig. 4 bottom) under direct f–f excitation of the Nd3+ ion (λexc = 584 nm and λexc = 803 nm) as well as by the antenna effect26 (λexc = 326 nm).
The emission intensity under excitation at 326 nm is only a little bit stronger than that observed under excitation at 584 nm and 803 nm, which indicates a weakly efficient antenna effect.
Family F2: [Sm2(hbdc)3(H2O)6·4H2O]∞
Microcrystalline powders of the Sm3+- and Eu3+-derivatives are isostructural to [Sm2(hbdc)3(H2O)6·4H2O]∞ (F2). Single-crystals have been obtained by both a solvothermal method and diffusion in gel media with Sm3+. [Sm2(hbdc)3(H2O)6·4H2O]∞ crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . It is two-dimensional. Each Sm3+ ion is coordinated by six oxygen atoms from four different hbdc2− ligands and three oxygen atoms from three coordination water molecules. The coordination polyhedron of the Sm3+ ion can be described as a spherical tricapped trigonal prism (Fig. 5). The ligand hbdc2− presents two different coordination modes (Fig. 5).
. It is two-dimensional. Each Sm3+ ion is coordinated by six oxygen atoms from four different hbdc2− ligands and three oxygen atoms from three coordination water molecules. The coordination polyhedron of the Sm3+ ion can be described as a spherical tricapped trigonal prism (Fig. 5). The ligand hbdc2− presents two different coordination modes (Fig. 5).
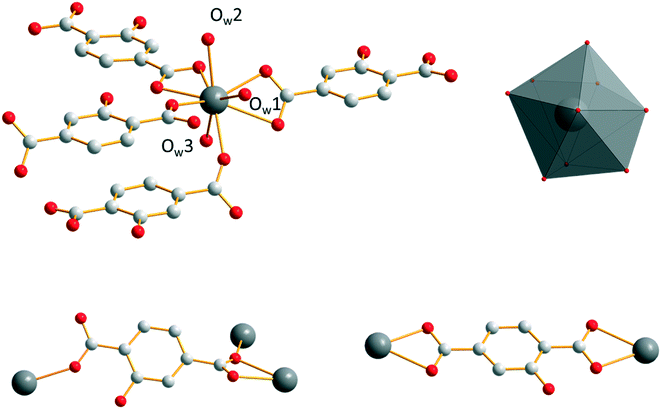 | ||
| Fig. 5 Coordination environment and coordination polyhedron of Sm3+ (top) and the two different coordination modes of the hbdc2− ligands (bottom) in [Sm2(hbdc)3(H2O)6·4H2O]∞ (F2). | ||
The closest Sm3+ ions are bridged by the tridentate carboxylate group of the first coordination mode and form Sm3+-based binuclear units. The intermetallic distance inside a binuclear unit is 4.357(1) Å. The binuclear units are connected to each other by ligands in the second coordination mode through bidentate carboxylate groups, forming a 1D molecular chain. The molecular chains are linked to each other through the second carboxylate group of the ligands that adopt the first coordination mode to generate 2D molecular layers. The intermetallic distances between Sm3+ ions that belong to different binuclear units inside a molecular layer are about 11.5 Å. Between two adjacent layers, the shortest Sm–Sm intermetallic distances are 10.843(1) Å (Fig. 6).
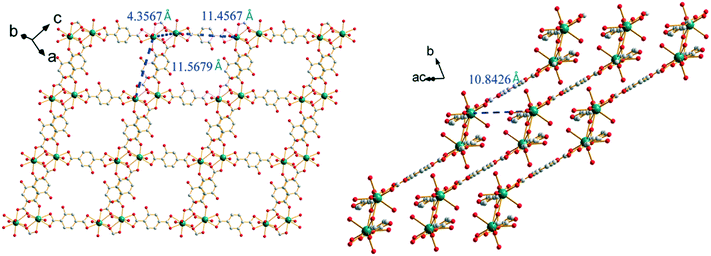 | ||
| Fig. 6 Projection views of a 2D molecular layer (left) and the stacking of the molecular layers (right) of [Sm2(hbdc)3(H2O)6·4H2O]∞ (F2). Characteristic intermetallic distances are indicated. | ||
Crystallization water molecules are located in the channels that spread along the b-axis. These crystallization water molecules are strongly bound to the molecular framework via a hydrogen bond network that involves crystallization water molecules, oxygen atoms of the carboxylic groups and hydroxyl groups of the ligand (Fig. 7). They are removed in two steps: between 120 °C and 170 °C (Fig. S14†).
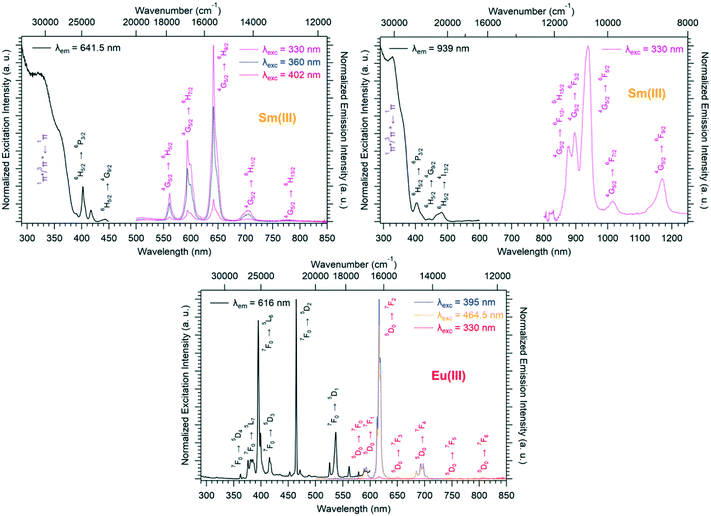
Room temperature solid-state excitation and emission spectra and luminescence decay curves of [Ln2(hbdc)3(H2O)6·4H2O]∞ (F2) with Ln = Sm and Eu have been recorded (Fig. 8 and S15†).
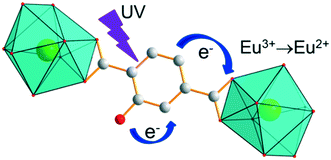
The Sm-derivative exhibits strong luminescence under 330 nm excitation that corresponds to the 1π*/3π* ← 1π absorption band of the ligand. In contrast, the excitation spectrum of [Eu2(hbdc)3(H2O)6·4H2O]∞ (F2) (λem = 616 nm) presents no excitation band at this wavelength. This can be related to a photo-induced electron transfer (PET) mechanism that is commonly observed when an easily reducible lanthanide ion (such as Eu3+) is in the vicinity of a donor group (such as –OH group) (Scheme 2).7,17,27
Family 3: [Y2(hbdc)3(H2O)4·4H2O]∞
Single-crystals of the Ho3+- to Lu3+- and Y3+-derivatives have been obtained by a solvothermal method while those of the Eu- to Tb-derivatives have been grown by slow diffusion through gel medium. All of them are isostructural. Their general chemical formula is [Ln2(hbdc)3(H2O)4·4H2O]∞ with Ln = Eu–Tb and Ho–Lu plus Y (F3). Hereafter, the crystal structure is described on the basis of the Y3+-derivative. [Y2(hbdc)3(H2O)4·4H2O]∞ (F3) crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Each Y3+ is eight coordinated in a bi-augmented trigonal prism coordination environment, defined by six oxygen atoms from five different hbdc2− ligands and two oxygen atoms from two coordination water molecules (Fig. 9). On the one hand, the hbdc2− ligand connects two independent Y3+ ions through bidentate carboxylate groups which leads to an 11.4519(9) Å Y–Y distance. On the other hand, four independent Y3+ ions are bridged by four oxygen atoms from the carboxylate groups of a ligand which leads to 4.7603(5) Å and 10.0102(8) Å Y–Y distances.
. Each Y3+ is eight coordinated in a bi-augmented trigonal prism coordination environment, defined by six oxygen atoms from five different hbdc2− ligands and two oxygen atoms from two coordination water molecules (Fig. 9). On the one hand, the hbdc2− ligand connects two independent Y3+ ions through bidentate carboxylate groups which leads to an 11.4519(9) Å Y–Y distance. On the other hand, four independent Y3+ ions are bridged by four oxygen atoms from the carboxylate groups of a ligand which leads to 4.7603(5) Å and 10.0102(8) Å Y–Y distances.
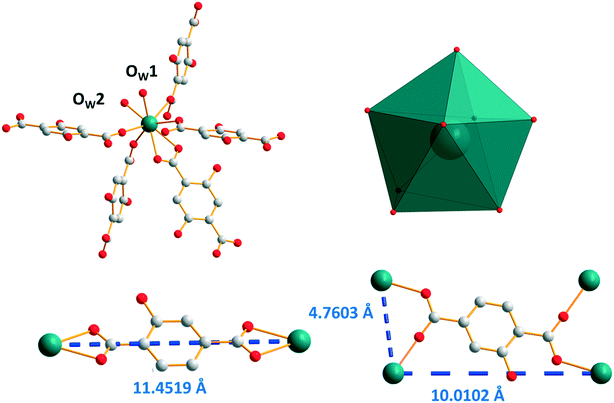 | ||
| Fig. 9 Coordination environment (top left) and coordination polyhedron (top right) of Y3+ ions in [Y2(hbdc)3(H2O)4·4H2O]∞ (F3). The two different coordination modes with Y–Y distances (bottom). | ||
The closest Y3+ ions are bridged by one of the carboxylate groups of the ligand that adopts the second coordination mode to form molecular chains that spread along the a-axis. These molecular chains are bound to each other through the connection of the other carboxylate groups of the ligands that adopt the second coordination mode. This coordination mode generates a 3-dimensionnal network with 10 × 10 Å2 square cross-section channels. At last, these square cross-section channels are divided into two halves by ligands that adopt the first coordination mode (Fig. 10). There are four coordination water molecules and four crystallization water molecules per formula unit (Fig. S16†). It can be noticed that these compounds are isomorphous to compounds with the general chemical formula [Ln1.5(abdc)2·2H2O]∞ with Ln = Nd, Eu, Tb, Dy and Y (abdc2− stands for 2-aminoterephthalate) that have been reported previously.28–31
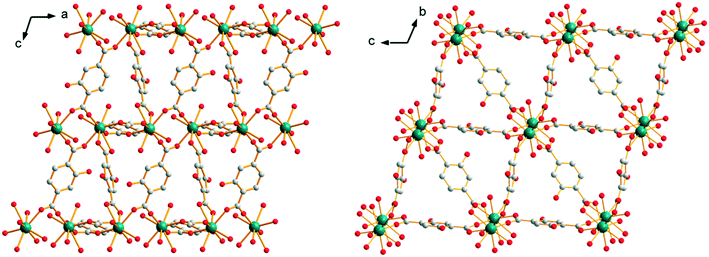 | ||
| Fig. 10 Projection views along the b-axis (left) and a-axis (right) of [Y2(hbdc)3(H2O)4·4H2O]∞ (F3). | ||
Absorption, excitation and emission spectra of [Gd2(hbdc)3(H2O)4·4H2O]∞ (F3) have been recorded at room temperature (Fig. S17† left for absorption) and at room temperature and at 77 K (Fig. S17† right for exc/em), in order to estimate the energy of the first excited singlet and triplet states. These measurements show that ΔE(1π* ← 1π) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 cm−1 and ΔE(3π* ← 1π) = 27
390 cm−1 and ΔE(3π* ← 1π) = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cm−1. The luminescence decay curve of [Gd2(hbdc)3(H2O)4·4H2O]∞ (F3) has been recorded at 77 K as well (Fig. S18†).
400 cm−1. The luminescence decay curve of [Gd2(hbdc)3(H2O)4·4H2O]∞ (F3) has been recorded at 77 K as well (Fig. S18†).
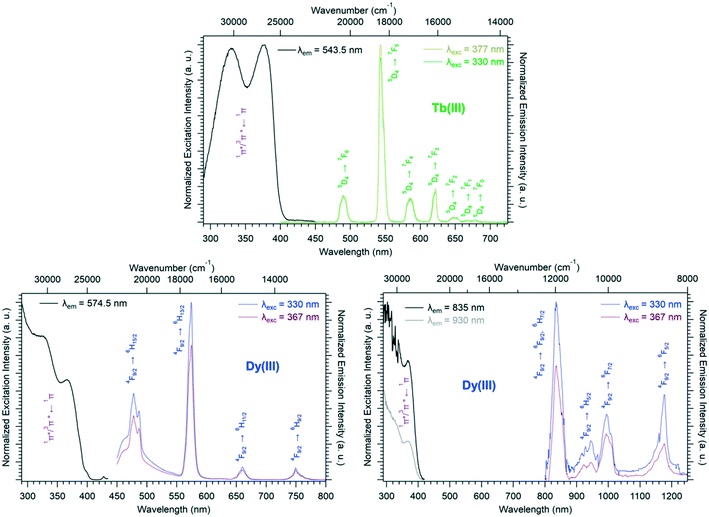
Room temperature solid-state excitation and emission spectra as well as luminescence decay curves were recorded for the Eu3+- and Tb3+-based compounds (Fig. 11 and S19†). The excitation spectrum of the Tb-derivative shows a broad band that corresponds to the ligand 1π*/3π* ← 1π transitions. This indicates that the ligands present an efficient antenna effect toward Tb3+ ions. In contrast, because of a PET mechanism, there is no antenna effect toward Eu3+ ions. Room temperature solid-state emission spectra have also been measured on the Ho- and Tm-derivatives and show emission in both the visible (centered at 650 nm for Ho and 648 and 780 nm for Tm) and infrared domains (976 for Ho and 1175 nm for Tm) (Fig. 11). For the Yb-based compounds, the infrared emission at 980 nm has been observed with a good antenna effect.
Family F4: [Tb(Hhbdc)(hbdc)(H2O)3·H2O]∞
Isostructural Tb3+- and Dy3+-derivatives with the chemical formula [Ln(Hhbdc)(hbdc)(H2O)3·H2O]∞ (F4) with Ln = Tb–Dy have been obtained by a solvothermal method. Their crystal structure is described on the basis of the Tb3+-derivative [Tb(Hhbdc)(hbdc)(H2O)3·H2O]∞, which crystallizes in the triclinic system, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The Tb3+ ion is nine-coordinated by six oxygen atoms from four different ligands and three oxygen atoms from three coordination water molecules which leads to a spherical capped square antiprism geometry (Fig. 12). The IR spectrum, as well as the crystal structure of family F1, evidences that one of the ligand is once protonated (Fig. S20†). There are three different coordination modes of the ligands in the crystal structure. In the first one, four different Tb3+ ions are connected by two tridentate carboxylate groups of the hbdc2− ligand. In the second, bidentate carboxylate groups of the hbdc2− ligand bridge two different Tb3+ ions. In the third, one carboxylate group is bound to one Tb3+. The other carboxylic group is protonated (Fig. 12).
. The Tb3+ ion is nine-coordinated by six oxygen atoms from four different ligands and three oxygen atoms from three coordination water molecules which leads to a spherical capped square antiprism geometry (Fig. 12). The IR spectrum, as well as the crystal structure of family F1, evidences that one of the ligand is once protonated (Fig. S20†). There are three different coordination modes of the ligands in the crystal structure. In the first one, four different Tb3+ ions are connected by two tridentate carboxylate groups of the hbdc2− ligand. In the second, bidentate carboxylate groups of the hbdc2− ligand bridge two different Tb3+ ions. In the third, one carboxylate group is bound to one Tb3+. The other carboxylic group is protonated (Fig. 12).
The crystal structure can be described on the basis of 2-dimensional molecular layers that spread parallel to the bc plane (Fig. 13). The Tb–Tb distance in the binuclear units generated by the first coordination mode is 4.3496(4) Å. The Tb–Tb distance between metallic ions that belong to adjacent binuclear units and are connected by a ligand that adopts the first coordination mode (along the b-axis) is 11.6195(9) Å and the distance between lanthanide ions that are connected via the second coordination mode is 11.5109(9) Å (parallel to the a-axis). The shortest intermetallic distance between lanthanide ions that belong to different molecular layers is 7.4407(6) Å.
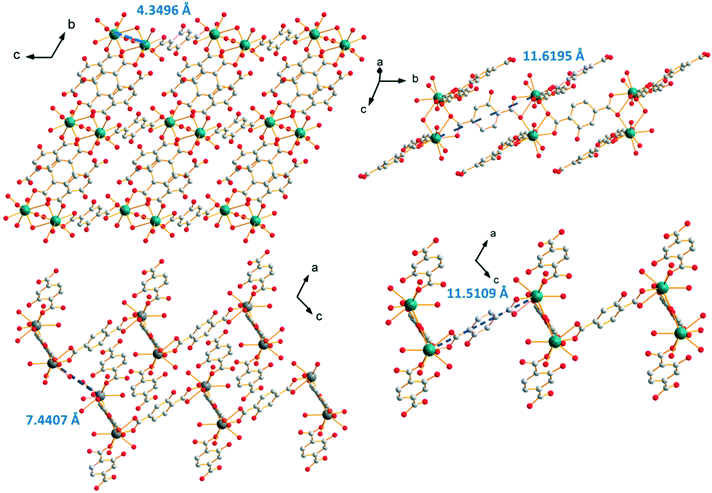 | ||
| Fig. 13 Projection view of a 2-dimensional molecular framework (top left). Projection views that highlight the different Tb–Tb distances in [Tb(Hhbdc)(hbdc)(H2O)3·H2O]∞ (F4). | ||
As confirmed by thermal analyses, there are three coordination water molecules and one crystallization water molecule per formula unit in this crystal structure (Fig. S21†).
Solid-state excitation and emission spectra were recorded, at room temperature, for both the Tb3+- and Dy3+-based compounds of family F4 (Fig. 14). Both excitation spectra exhibit two broad bands assigned to the 1π*/3π* ← 1π transitions of the ligand centered at 330 nm and 377 nm, respectively, which indicates that the ligand presents an efficient antenna effect toward both lanthanide ions. The Dy3+-derivative emission can be observed in both the visible and NIR regions under 330 nm excitation. The luminescence decay curve of the Tb3+-derivative has been recorded at room temperature as well (Fig. S22†).
Family F5: [Gd2(hbdc)3(H2O)8·6H2O]∞
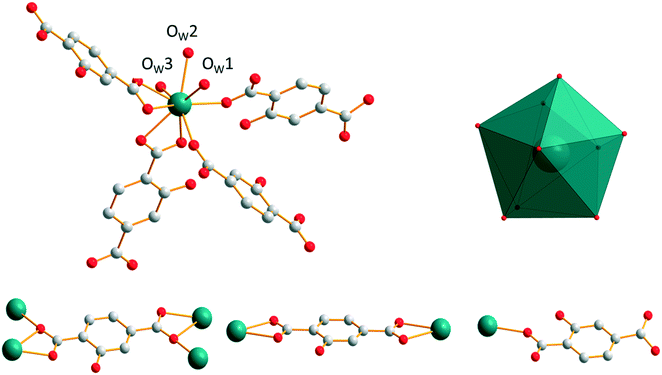
[Gd2(hbdc)3(H2O)8·6H2O]∞, which has been obtained by slow evaporation, crystallizes in the monoclinic system, space group C2/c. The asymmetric unit contains two Gd3+ ions, three hbdc2− ligands, eight coordination water molecules and six crystallization water molecules (Fig. S23†). Each Gd3+ is coordinated by nine oxygen atoms: five from the carboxylate groups of the ligands and four from coordination water molecules. The coordination polyhedron is best described by a muffin geometry (Fig. 15).The ligand presents two different coordination modes. In the first one, the hbdc2− ligand coordinates with two Gd3+ ions through monodentate and bidentate carboxylate groups, respectively. In the second one, two Gd3+ ions are bridged by bidentate carboxylate groups on both sides of the hbdc2− ligand. Six nearby Gd3+ ions are connected by six different carboxylate groups with two different coordination modes forming a 2D honeycomb-like molecular layer that spread parallel to the ab plane (Fig. 16). The area of the hexagons is 19 × 11 Å2. The shortest Gd–Gd distances are about 11.5(1) Å.
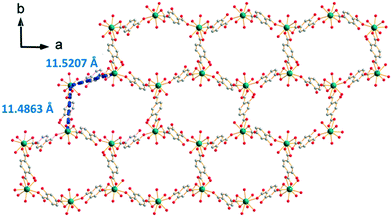 | ||
| Fig. 16 Projection view along the c-axis of a 2D honeycomb-like molecular layer of [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5). Gd–Gd distances are indicated. | ||
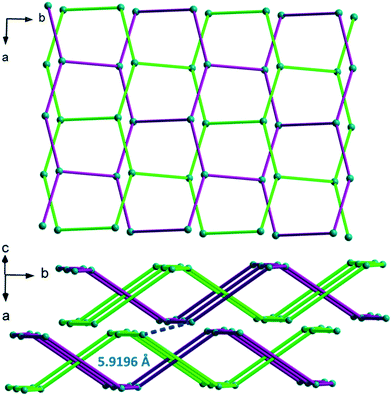
The crystal packing can be described as two-fold interpenetrating layers (Fig. 17). The shortest Gd–Gd distance between adjacent interpenetrating layers is 5.919(1) Å. The crystallization water molecules are localized in the cavities and bound to the molecular skeleton by hydrogen bonds that ensure the crystal structure stability.
Solid-state absorption, excitation and emission spectra of [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5) have been recorded at room temperature (Fig. S24† left) and room temperature and 77 K (Fig. S24† right), respectively. These spectra allow the estimation of the first excited singlet and triplet states of the ligand.32 The energy of the first excited singlet state of the ligand (1π*) can be estimated from the lowest energy-edge of the UV-absorption spectrum of the Gd3+ compounds (∼26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1). The energy of the first excited triplet state (3π*) can be assumed from the highest energy edge of the emission spectrum of the Gd3+ compounds at 77 K (∼25
000 cm−1). The energy of the first excited triplet state (3π*) can be assumed from the highest energy edge of the emission spectrum of the Gd3+ compounds at 77 K (∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1).
000 cm−1).
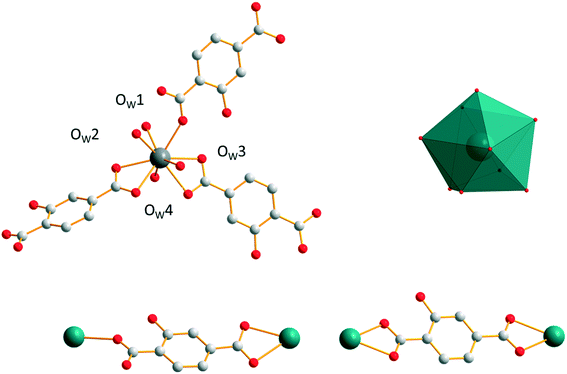
Family F6: [Gd2(hbdc)3(H2O)8·2H2O]∞
Compounds that constitute this structural family have the chemical formula [Ln2(hbdc)3(H2O)8·2H2O]∞ with Ln = Nd–Tb (F6). They have been obtained by slow diffusion through gel media. The coordination environment of [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) is similar to that of [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5). In both crystal structures, each Gd3+ ion is coordinated by nine oxygen atoms from three different ligands and four coordination water molecules (Fig. 18) and the coordination geometry is best described as a muffin geometry. In structural type F6, the hbdc2− ligand adopts two different coordination modes (Fig. 18). In the first coordination mode, both carboxylate groups bridge one Gd3+ ion in a monodentate way. In the second one, bidentate carboxylate groups connect to one Gd3+ ion on both sides.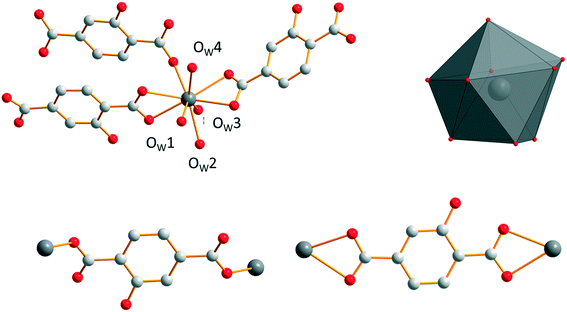 | ||
| Fig. 18 Coordination environment (top left) and coordination polyhedron (top right) of Gd3+ ions and the two coordination modes (bottom) of the hbdc2− ligand in [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6). | ||
The crystal structure can be described on the basis of 2-dimensional molecular layers made of hexanuclear rings (Fig. 19). Two different Gd–Gd distances with two different coordination modes of the ligand can be observed in these rings: 11.0526(11) Å and 11.3667(13) Å. Compared with those observed in the crystal structure of F5, the hexanuclear rings are smaller and narrower. Their area is 15 × 5 Å2. This can be related to the fact that there are only two crystallization water molecules per formula unit in [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) instead of six in [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5) (Fig. S25†). The 2-dimensional molecular layers can be described as wrapped planes made of cyclohexane-like rings (Fig. 20).
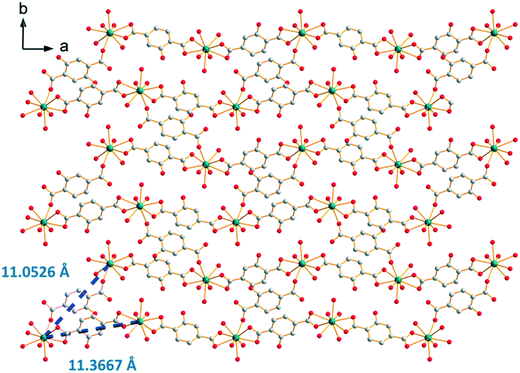 | ||
| Fig. 19 Projection view along the c-axis of a 2-dimensional molecular layer in [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) with the shortest Gd–Gd distances. | ||
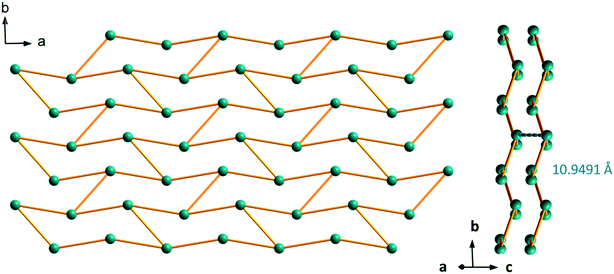 | ||
| Fig. 20 Schematic (bars symbolize the ligands) projection view along the c-axis (left) and packing of the molecular layers (right) of [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6). | ||
Absorption and emission spectra of [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) have been recorded at room temperature and at 77 K, respectively (Fig. S26†), in order to estimate the energy of the first excited singlet and triplet states. These measurements show that ΔE(1π* ← 1π) ∼24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 cm−1 and ΔE(3π* ← 1π) ∼25
390 cm−1 and ΔE(3π* ← 1π) ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.
000 cm−1.
Room temperature solid-state excitation and emission spectra have been recorded for the Nd3+- and Tb3+-derivatives of family F6 (Fig. 21). The excitation spectra of both compounds exhibit two broad bands centered at 325 nm and 360 nm, strongly suggesting an efficient antenna effect of the ligand toward both lanthanide ions. The luminescence decay curve of the Tb-derivative has been recorded at room temperature as well (Fig. S27†).
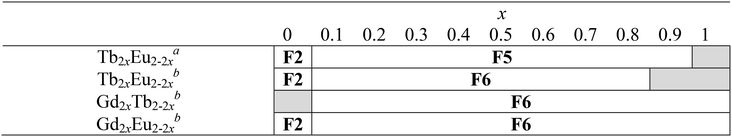
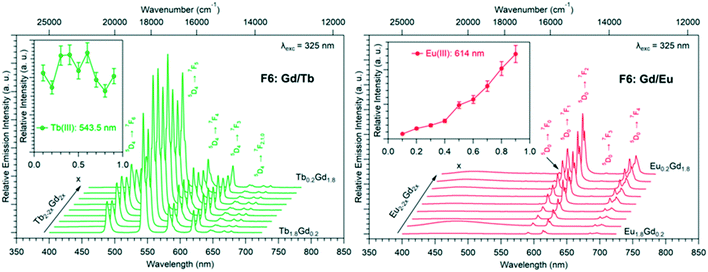
Luminescence properties of the heterolanthanide compounds: [Tb2xEu2−2x(hbdc)3(H2O)8·6H2O]∞ (F5) and [Tb2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) (0 < x < 1)
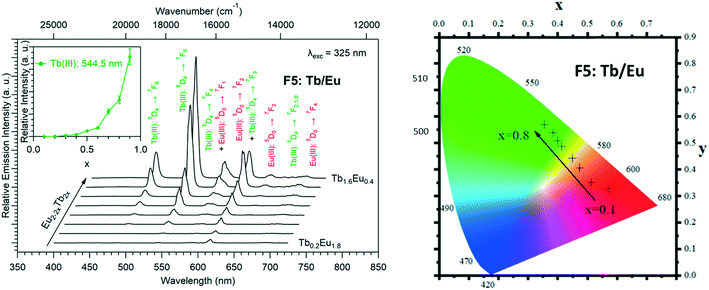
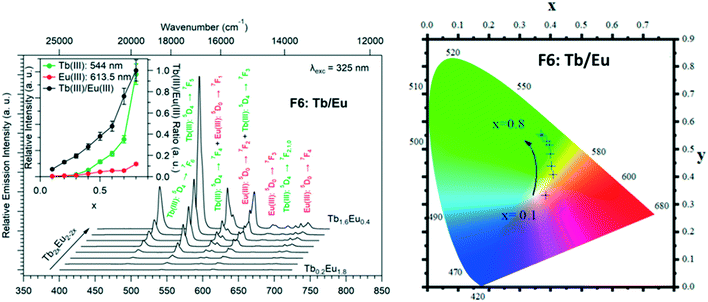
Because Tb3+- and Eu3+-derivatives usually present interesting luminescence properties in the visible domain, we have prepared heterolanthanide Tb3+/Eu3+compounds for investigating the intermetallic energy transfers and modulating the emission color. In this system, whatever the crystal structures of the microcrystalline powders of the homo-Tb3+ and Eu3+-derivatives are, the microcrystalline powders of the Tb3+/Eu3+ heterolanthanide compounds always crystallize in the crystal structure of the Gd3+-derivatives (F5 or F6). For the microcrystalline powder of the Gd3+-derivative, the crystal structure depends on the order of addition of the reactants: adding the solution of gadolinium chloride into the solution of Na2hbdc leads to [Gd2(hbdc)3(H2O)8·6H2O]∞ (F5). In contrast, [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) is obtained when the solution of Na2hbdc is added to the gadolinium chloride solution. The same happens for the Tb3+/Eu3+ mixtures.This phenomenon provides a platform to synthesize two different series of Tb3+/Eu3+ heterolanthanide compounds with the respective chemical formulas [Tb2xEu2−2x(hbdc)3(H2O)8·6H2O]∞ (F5) and [Tb2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) (0 < x < 0.9).
The solid-state emission spectra of the two series of Tb3+/Eu3+ heterolanthanide compounds are reported in Fig. 22 and 23. Corresponding colorimetric coordinates have also been calculated on the basis of the emission spectra.
For both series of compounds, emission spectra show the characteristic emission peaks of Tb3+ and Eu3+ ions. In both cases, the increase of the Tb3+concentration induces an increase of the luminescence intensity of the Tb3+ ions and that of the Eu3+ ions as well. This strongly suggests Tb-to-Eu intermetallic energy transfers. However, the intermetallic energy transfer processes are different in the two series of compounds. For [Tb2xEu2−2x(hbdc)3(H2O)8·6H2O]∞ (F5), the emission color progressively changes from red to orange to yellow to green, whereas for [Tb2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6), the emission color directly changes from orange to yellow and then green. Indeed, with the same Tb3+ content (x = 0.1), the emission color of the compound that belongs to F5 is in the red region, and in the orange region for the compound that belongs to F6.
In order to further compare these two series of compounds, we have measured the luminance under UV irradiation of both series of compounds (Fig. 24).
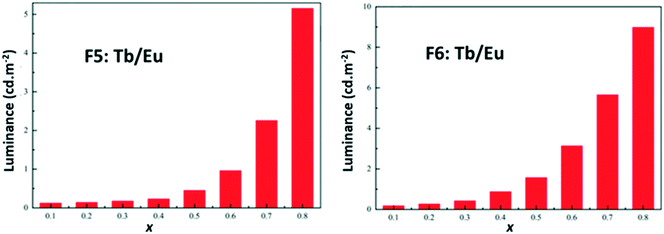 | ||
| Fig. 24 Luminance versus x (right) of [Tb2xEu2−2x(hbdc)3(H2O)8·6H2O]∞ (F5) (left) and [Tb2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) (right) under UV irradiation (flux 0.68(1) mW cm−2; λexc = 312 nm). | ||
Fig. 22–24 show that the two series present quite different behaviors versus x and suggest that intermetallic energy transfers are more efficient in F5 than in F6. The excited singlet and triplet energy levels of the ligand are essentially identical (Fig. S24 and S26†) and therefore cannot be responsible of the discrepancy between the optical behaviors.
From the point of view of their crystal structure, these compounds are close to each other: both present eight coordination water molecules per formula unit, the coordination polyhedra of the lanthanide ions are similar and both crystal structures can be described on the basis of molecular layers made of hexagonal rings. Moreover, the intermetallic distances inside a given molecular layer are similar (around 11 Å) and the mean intermetallic distances estimated with a rough model that have been previously described33 are almost identical: 12.8 Å and 12.5 Å, respectively. The main difference between the two crystal structures is the packing of the molecular layers. Indeed, the shortest intermetallic distances between lanthanide ions that belong to different layers is 10.9 Å in the F6 structural type and only 5.9 Å in the F5 one. It has already been shown that this can be of first importance as far as luminescence is concerned.34
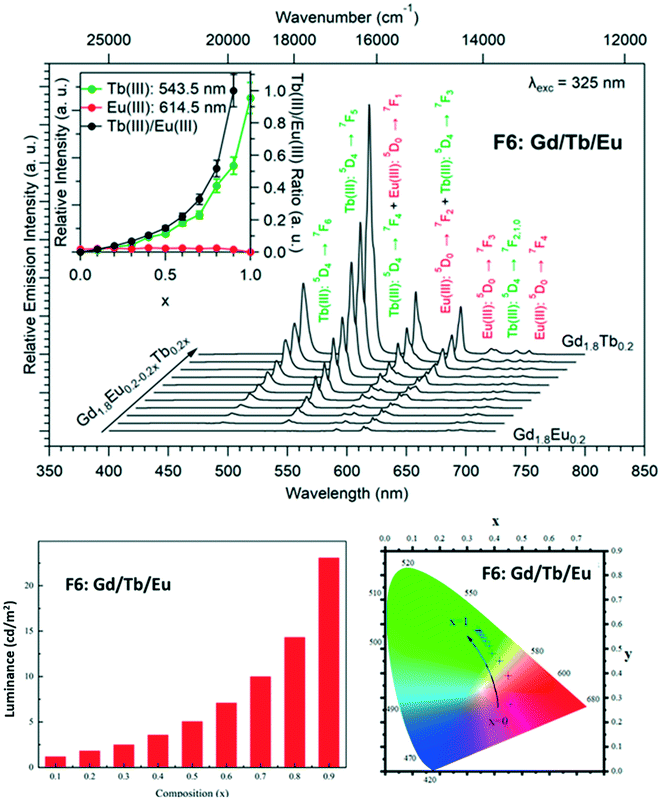
Dilution effect in the F6 structural type: [Gd2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) and [Gd2xTb2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) with 0 < x < 1
In order to more deeply investigate the unexpected luminescence behavior of the compounds that belong to family F6, we have synthesized two more series of compounds: [Gd2xEu2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) and [Gd2xTb2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) with 0 < x < 1, by adding the solution of Na2hbdc to the lanthanide chloride solutions. As expected, all these compounds are isostructural to [Gd(hbdc)3(H2O)8·2H2O]∞ (F6) (Fig. S10 and S11†). The relative metallic content is gathered in Table S1.†Room temperature solid state emission spectra have been recorded (λexc = 325 nm) and luminance measurements have been performed (flux 0.68(1) mW cm−2; λexc = 312 nm) (Fig. 25 and S28†).
Fig. 25 and S28† show that despite the big intermetallic distances, inter-metallic energy transfers are still present in the F6 structural type. Indeed, the luminance of the compounds with the chemical formula [Gd2xTb2−2x(hbdc)3(H2O)8·2H2O]∞ (F6) is almost constant over the whole x range, whereas in the absence of intermetallic energy transfer, it is expected to decrease proportionally to the Tb3+ concentration. This was expected because even if it is commonly admitted that 10 Å is the threshold above which intermetallic energy transfer becomes less efficient,35 they are still present at bigger distances. In contrast, Eu luminescence, under excitation of the ligand, appears and increases as x increases. This strongly suggests that dilution with optically inactive ions (Gd3+) not only reduces intermetallic energy transfers but also allows the occurrence of an antenna effect. This could be related to the number of ligands available for transferring their energy per Eu3+ ion that increases upon dilution with Gd3+. This was unexpected and, to the best of our knowledge, never observed before for lanthanide-based coordination polymers.
Nevertheless, it must be noticed that dilution with optically inactive lanthanide ions provokes only a weak increase of the luminance (×5 for x = 0.9 – Fig. S24†) whereas dilution with Tb3+ ions induces a greater increase (×50 for x = 0.8 – Fig. 24). This confirms that Tb-to-Eu inter-metallic energy transfers are efficient and feed the emitting levels of the Eu3+ ion. On the basis of this observation, we have decided to try to prepare a series of heterolanthanide coordination polymers that would present the structural type F6 and exhibit tunable emission.
Modulation of the luminescence: [Gd1.8Tb0.2xEu0.2−0.2x(hbdc)3(H2O)8·2H2O]∞ (F6) (0 < x < 1)
In order to observe considerable Eu3+ emission and to prevent PET, the Gd3+ concentration has been set to 90% and the relative content of Eu3+ and Tb3+ was allowed to vary. The prepared compounds have the general chemical formula [Gd1.8Tb0.2xEu0.2−0.2x(hbdc)3(H2O)8·2H2O]∞ with 0 < x < 1 and are isostructural to [Gd2(hbdc)3(H2O)8·2H2O]∞ (F6) (Fig. S29 and Table S2†). Their emission spectra (λexc = 325 nm) have been recorded and their colorimetric coordinates and luminance (λexc = 312 nm) have been measured (Fig. 26).Fig. 26 evidences that it is possible to design compounds with tunable color emission and brightness. However, even if some of these compounds exhibit emission spectra that contain a red component despite the presence of the PET mechanism, one must notice that the emission color and brightness don't vary independently. Indeed, it is possible to design compounds with quite significant luminance that emit in the green region but not in the red region.
Conclusion and outlook
Reactions in water between lanthanide chlorides and the disodium salt of 2-hydroxyterephthalic acid have led to six new series of lanthanide-based coordination polymers. It is noticeable that two out of the six crystal structures are isomorphous to 2-aminoterephthalic acid-based analogues and that the –OH group doesn't participate in lanthanide coordination. This system is very versatile and it is possible to prepare compounds with an identical metallic composition but different crystal structures, under similar experimental conditions, by just changing the order of addition of reactants. As anticipated, the ligand induces a very efficient quenching of the Eu3+ luminescence because of the PET mechanism. However, this study evidences that it is possible to overcome this mechanism by dilution with optically inactive ions and to obtain a series of compounds with tunable emission color and intensity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Chinese Scholarship Council is acknowledged for its financial support in the frame of the Chinese-INSA/UT network.References
- Y. Cui, J. Zhang, H. He and G. Qian, Photonic functional metal–organic frameworks, Chem. Soc. Rev., 2018, 47, 5740–5785 CAS.
- Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Metal-organic frameworks as platforms for functionnal materials, Acc. Chem. Res., 2016, 49, 483–493 CAS.
- Y. Cui, J. Zhang, B. Chen and G. Qian, Lanthanide Metal-Organic Frameworks for Luminescent Applications, Handbook on the Physics and Chemistry of Rare Earths, 2016, vol. 50, pp. 243–268 Search PubMed.
- B. Li, H.-M. Wen, Y. Cui, G. Qian and B. Chen, Multifunctionnal lanthanide coordination polymers, Prog. Polym. Sci., 2015, 48, 40–84 CAS.
- J. He, J. Xu, J. Yin, N. Li and X.-H. Bu, Recent advances in luminescent metal-organic frameworks for chemical sensors, Sci. China Mater., 2019, 62, 1655–1678 CAS.
- S. V. Eliseeva and J. C. G. Bünzli, Lanthanide luminescence for functionnal materials and bio-sciences, Chem. Soc. Rev., 2010, 39, 189–227 CAS.
- J.-C. G. Bünzli, Lanthanide luminescence for biomedical analyses and imaging, Chem. Rev., 2010, 111, 2729–2755 Search PubMed.
- Y. Han, P. Yan, J. Sun, G. An, X. Yao, Y. Li and G. Li, Luminescence and white-light emitting luminescent sensor of tetrafluoroterephthalatelanthanide metal–organic frameworks, Dalton Trans., 2017, 46, 4642–4653 CAS.
- J. C. G. Bünzli, Rising stars in science and technology : Luminescent lanthanide materials, Eur. J. Inorg. Chem., 2017, 5058–5063 Search PubMed.
- K.-M. Wang, L. Du, Y.-L. Ma, J.-S. Zhao, Q. Wang, T. Yan and Q.-H. Zhao, Multifunctionnal chemical sensors and luminescent thermometers based on lanthanide metal-organic framework materials, CrystEngComm, 2016, 18, 2690–2700 CAS.
- D. S. C. Brites, A. Millan and L. D. Carlos, Lanthanides in Luminescent Thermometry, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner, J. C. G. Bünzli and V. K. Pecharsky, Elsevier, 2016, vol. 49, pp. 339–427 Search PubMed.
- O. Guillou, C. Daiguebonne, G. Calvez and K. Bernot, A long journey in lanthanide chemistry : from fundamental crystallogenesis studies to commercial anti-counterfeiting taggants, Acc. Chem. Res., 2016, 49, 844–856 CAS.
- R. G. Pearson, Hard and soft acids and bases - the evolution of a chemical concept, Coord. Chem. Rev., 1990, 100, 403–425 CAS.
- F. Le Natur, G. Calvez, S. Freslon, C. Daiguebonne, K. Bernot and O. Guillou, Extending the lanthanide terephthalate system : isolation of an unprecedented Tb(III)-based coordnation polmer with high potential porosity and luminescence properties, J. Mol. Struct., 2015, 1086, 34–42 CAS.
- N. Kerbellec, D. Kustaryono, V. Haquin, M. Etienne, C. Daiguebonne and O. Guillou, An Unprecedented Family of Lanthanide-Containing Coordination Polymers with Highly Tunable Emission Properties, Inorg. Chem., 2009, 48, 2837–2843 CAS.
- C. Daiguebonne, N. Kerbellec, O. Guillou, J. C. G. Bünzli, F. Gumy, L. Catala, T. Mallah, N. Audebrand, Y. Gérault, K. Bernot and G. Calvez, Structural and luminescent properties of micro-sized and nano-sized particles of lanthanide terephthalate coordination polymers, Inorg. Chem., 2008, 47, 3700–3708 CAS.
- S. Freslon, Y. Luo, G. Calvez, C. Daiguebonne, O. Guillou, K. Bernot, V. Michel and X. Fan, Influence of photo-induced electron transfer on lanthanide-based coordination polymers luminescence : A comparison between two pseudo-isoreticular molecular networks, Inorg. Chem., 2014, 53, 1217–1228 CAS.
- H. Xu, Y. Dong, Y. Wu, W. Ren, T. Zhao and S. Wang, An -OH group functionalized MOF for ratiometric Fe3+ sensing, J. Solid State Chem., 2018, 258, 441–446 CAS.
- J. F. Desreux, in Lanthanide Probes in Life, Chemical and Earth Sciences, ed. G. R. Choppin and J. C. G. Bünzli, Elsevier, Amsterdam, 1989, p. 43 Search PubMed.
- H. K. Henisch, Crystals in Gels and Liesegang Rings, Cambridge University Press, Cambridge, 1988 Search PubMed.
- H. K. Henisch and R. Rustum, Crystal Growth in Gels, The Pennsylvania State University Press, 1970, pp. 1–196 Search PubMed.
- C. Daiguebonne, A. Deluzet, M. Camara, K. Boubekeur, N. Audebrand, Y. Gérault, C. Baux and O. Guillou, Lanthanide-based molecular materials : gel medium induced polymorphism, Cryst. Growth Des., 2003, 3, 1015–1020 CAS.
- A. Abdallah, S. Freslon, X. Fan, A. Rojo, C. Daiguebonne, Y. Suffren, K. Bernot, G. Calvez, T. Roisnel and O. Guillou, Lanthanide based coordination polymers with 1,4 carboxyphenylboronic ligand: multi emissive compounds for multi sensitive luminescent thermometric probes, Inorg. Chem., 2019, 58, 462–475 CAS.
- Y. Luo, G. Calvez, S. Freslon, C. Daiguebonne, T. Roisnel and O. Guillou, A new family of lanthanide containing molecular open frameworks with high porosity : [Ln(abdc)(Habdc),2H2O] with Ln = La -Eu and 8<n<11, Inorg. Chim. Acta, 2011, 368, 170–178 CAS.
- X. Y. Chen, B. Zhao, W. Shi, J. Xia, P. Cheng, D. Z. Liao, S. P. Yan and Z. H. Jiang, Microporous metal-organic frameworks built on a Ln3 cluster as a six connecting node, Chem. Mater., 2005, 17, 2866–2874 CAS.
- S. I. Weissman, Intramolecular energy transfer - The fluorescence of complexes of europium, J. Chem. Phys., 1942, 10, 214–217 CAS.
- C. Galaup, J.-M. Couchet, S. Bedel, P. Tisnès and C. Picard, Direct access to terpyridine-containing polyazamacrocycles as photosensitizing ligands for Eu(III) luminescence in aqueous media, J. Org. Chem., 2005, 70, 2274–2284 CAS.
- X. Haitao, Z. Nengwu, J. Xianglin, Y. Ruyi, W. Yonggang, Y. Enyi and L. Zhengquan, Assembly of lanthanide coordination polymers with one dimensional channels, J. Mol. Struct., 2003, 655, 339–342 Search PubMed.
- S. Petrosyants, Z. V. Dobrokhotova, A. Ilyukhin, A. Gavrikov, N. Efimov and V. Novotortsev, Coordination polymers of rare-earth elements with 2-aminoterephthalic acid, Russ. J. Coord. Chem., 2017, 43, 770–779 CAS.
- H. T. Xu, N. W. Zheng, X. L. Jin, R. Y. Yang and Z. Q. Li, Channel structure of diaquasesqui(2-aminoterephthalato)dysprosium(III) dihydrate, J. Mol. Struct., 2003, 646, 197–199 CAS.
- H. T. Xu, N. W. Zheng, X. L. Jin, R. Y. Yang and Z. Q. Li, A new microporous structure constructed by a lanthanide-carboxylate coordination polymer, J. Mol. Struct., 2003, 654, 183–186 CAS.
- J. C. G. Bünzli and S. V. Eliseeva, Basics of lanthanide photophysics, in Lanthanide Luminescence, ed. P. Hänninen and H. Härmä, Springer Berlin Heidelberg, 2010, vol. 7, pp. 1–45 Search PubMed.
- Y. Luo, Y. Zheng, G. Calvez, S. Freslon, K. Bernot, C. Daiguebonne, T. Roisnel and O. Guillou, Synthesis, crystal structure and luminescent properties of new lanthanide-containing coordination polymers Involving 4,4′-oxy-bis-benzoate as Ligand, CrystEngComm, 2013, 15, 706–720 CAS.
- I. Badiane, S. Freslon, Y. Suffren, C. Daiguebonne, G. Calvez, K. Bernot, M. Camara and O. Guillou, High britness and easy color modulation in lanthanide-based coordination polymers with 5-methoxyisophthalate as ligand: Toward emission colors additive strategy, Cryst. Growth Des., 2017, 17, 1224–1234 CAS.
- C. Piguet, J. C. G. Bünzli, G. Bernardinelli, G. Hopfgatner and A. F. Williams, Self-assembly and photophysical properties of lanthanide dinuclear triple-helical complexes, J. Am. Chem. Soc., 1993, 115, 8197–8206 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures for syntheses of Na2(hbdc)·H2O and single-crystals of the coordination polymers, single crystal and powder X-ray diffraction, thermal analyses, electron dispersive spectroscopy and optical measurements. It also contains additional TG/TD, PXRD, FTIR, EDS and optical measurements. CCDC 1913124, 1913130, 1913137, 1913142, 1913145 and 1913183. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce00947d |
| This journal is © The Royal Society of Chemistry 2021 |