N-Arylated peptide: troponyl residue influences the structure and conformation of N-troponylated-(di/tri)-peptides†
Bibhuti Bhusana
Palai
ab and
Nagendra K.
Sharma
 *ab
*ab
aSchool of Chemical Sciences, National Institute of Science Education and Research (NISER)-Bhubaneswar, Jatni Campus, Bhubaneswar, 752050 Odisha, India. E-mail: nagendra@niser.ac.in; https://www.niser.ac.in/users/nagendra#profile-main; Fax: +91 0674 249 4141; Tel: +91 (674) 2494004
bHBNI-Mumbai, Mumbai, India
First published on 12th November 2020
Abstract
N-arylated peptides as peptoids influence the structural and conformational changes of small peptides that lead to unique foldamers, even in di-/tri-peptides. This report describes the syntheses, structural and conformational studies of rationally designed non-benzenoid N-arylated peptides containing bioactive tropolone molecules such as N-troponylated di-/tri-peptides. This report also demonstrates the role of the troponyl group in influencing the structure and conformation of N-troponylated short peptides (di-/tri-peptides) by single-crystal X-ray data analyses, circular dichroism (CD) studies, and 2D-NMR and DMSO-d61H-NMR titration experiments. The tropolonyl residue has a significant role in the formation of unique supramolecular self-assemblies in dipeptide crystalline solids such as helical/β-sheet/β-turn (antiparallel β-sheet) type secondary structures. The sequence-specific tr-di-/tri-peptides exhibit a characteristic β-strand type of characteristic CD-signal in organic solvents (CH3CN/MeOH). The hydrogen bonding nature of N–H, intra- or intermolecular hydrogen bonding, is also established by DMSO-d61H-NMR titration experiments in the representative tr-di-/tri-peptides. These results explicitly support the role of the troponyl carbonyl group in the formation of secondary structures of peptides, even in di-/tri-peptides. Hence, the N-troponylation of peptides may alter the conformation of peptides to prepare novel peptidomimetics.
Introduction
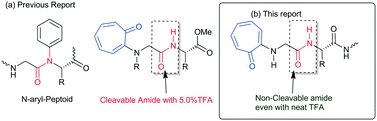
Protein and peptides form secondary structures such as β-sheets/turns and α-helices, which are vital for their biochemical activities.1,2 Non-covalent interactions such as hydrogen bonding and π–π interactions guide the formation of secondary structures in peptides as foldamers.3–5 Generally, the amide carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)/amine (N–H) groups of peptides are involved in the hydrogen bonding and form stable secondary structures.6,7 Sequence-specific structured peptides interact with a specific protein and nucleic acid to maintain the biochemical process. Nowadays, protein–peptide interaction and nucleic acid (DNA/RNA)–peptide interaction studies have emerged as powerful tools for developing peptide-based therapeutic drug candidates. However, naturally occurring peptides and their structures have limitations, such as aqueous solubility, structural stability with peptidase, and cellular uptake affinity, for practical applications in biochemical research. Thus, several structurally modified amino acids are prepared and employed for the synthesis of peptide foldamers. These foldamers are used for the development of novel biomaterials and potential therapeutic drug candidates.8,9 For example, aromatic amino acids, mainly phenylalanine-rich di-/tri-peptides, have shown a high tendency, because of π–π interactions, for the formation of various types of self-assembled materials.10 Aromatic tripeptides containing aromatic amino acids (Tyr & Trp) and methionine (or cysteine) are reportedly known to form fibrous aggregates.11 Bag and co-workers have reported novel scaffolds as β-turn peptidomimetics from the triazole amino acid-containing peptides.12 Gabapentin based hybrid small peptides reportedly form a double turn conformation by C12/C10 hydrogen bonding.13 N-terminal aromatic acid-containing peptides have the ability to spontaneously self-assemble because of hydrogen bonding and π–π interactions14–16 The attachment of an alkyl/aryl group at the N-terminal of peptides alters the structure and function of peptides. Recently, the influence of N-alkylated/arylated peptides or peptoids has been explored in the structural and conformational changes of peptides.17–21N-alkylated peptoids form a unique oligomeric structure and are being tested as potential antifreeze agents for food production and biomedicine.22 Importantly, N-arylated peptoids exhibit atropisomerism and unique foldamer properties.23–25 The synthesis of N-arylated peptides is reported using a metal catalyst and metal-free catalysts.26–29 So far, N-aryl peptides have been prepared from benzenoid aromatic compounds. In nature, non-benzenoid aromatic molecules also occur as bioactive natural products but their link with aminoacid/peptides is poorly explored. Tropolone and related derivatives occur in various natural products such as colchicine, stipitatic acid, α-/β-/γ-thujaplicine, pycnidione, and manicol.30–32 Tropolone related derivatives are natural chromophores, weak fluorophores, and excellent metal chelating agents, mainly with transition metal ions (Cu, Zn, and Ni).33–36 Recently we have introduced a non-benzenoid aromatic molecule, tropolone, into unnatural amino acids/peptides and explored the role of the troponyl residue in hydrogen bonding with adjacent amide-NH (Fig. 1a).37–39 Importantly, their amide bond is cleavable under mildly acidic conditions (∼5.0% TFA). Recently, N-troponylated amino acid esters have been employed to develop small fluorescent molecules such as boron-dipyrromethene (BODIPY) analogues.40,41 These results encourage attachment of the troponyl residue at the N-terminal of peptides, and exploration of the role of troponyl scaffolds in structural changes of native short di-/tri-peptides. Herein we described N-troponylated di-/tri-peptides containing α-/β-amino acids and explored the role of the troponyl group in structural and conformational changes by single crystal X-ray analyses and CD studies.
O)/amine (N–H) groups of peptides are involved in the hydrogen bonding and form stable secondary structures.6,7 Sequence-specific structured peptides interact with a specific protein and nucleic acid to maintain the biochemical process. Nowadays, protein–peptide interaction and nucleic acid (DNA/RNA)–peptide interaction studies have emerged as powerful tools for developing peptide-based therapeutic drug candidates. However, naturally occurring peptides and their structures have limitations, such as aqueous solubility, structural stability with peptidase, and cellular uptake affinity, for practical applications in biochemical research. Thus, several structurally modified amino acids are prepared and employed for the synthesis of peptide foldamers. These foldamers are used for the development of novel biomaterials and potential therapeutic drug candidates.8,9 For example, aromatic amino acids, mainly phenylalanine-rich di-/tri-peptides, have shown a high tendency, because of π–π interactions, for the formation of various types of self-assembled materials.10 Aromatic tripeptides containing aromatic amino acids (Tyr & Trp) and methionine (or cysteine) are reportedly known to form fibrous aggregates.11 Bag and co-workers have reported novel scaffolds as β-turn peptidomimetics from the triazole amino acid-containing peptides.12 Gabapentin based hybrid small peptides reportedly form a double turn conformation by C12/C10 hydrogen bonding.13 N-terminal aromatic acid-containing peptides have the ability to spontaneously self-assemble because of hydrogen bonding and π–π interactions14–16 The attachment of an alkyl/aryl group at the N-terminal of peptides alters the structure and function of peptides. Recently, the influence of N-alkylated/arylated peptides or peptoids has been explored in the structural and conformational changes of peptides.17–21N-alkylated peptoids form a unique oligomeric structure and are being tested as potential antifreeze agents for food production and biomedicine.22 Importantly, N-arylated peptoids exhibit atropisomerism and unique foldamer properties.23–25 The synthesis of N-arylated peptides is reported using a metal catalyst and metal-free catalysts.26–29 So far, N-aryl peptides have been prepared from benzenoid aromatic compounds. In nature, non-benzenoid aromatic molecules also occur as bioactive natural products but their link with aminoacid/peptides is poorly explored. Tropolone and related derivatives occur in various natural products such as colchicine, stipitatic acid, α-/β-/γ-thujaplicine, pycnidione, and manicol.30–32 Tropolone related derivatives are natural chromophores, weak fluorophores, and excellent metal chelating agents, mainly with transition metal ions (Cu, Zn, and Ni).33–36 Recently we have introduced a non-benzenoid aromatic molecule, tropolone, into unnatural amino acids/peptides and explored the role of the troponyl residue in hydrogen bonding with adjacent amide-NH (Fig. 1a).37–39 Importantly, their amide bond is cleavable under mildly acidic conditions (∼5.0% TFA). Recently, N-troponylated amino acid esters have been employed to develop small fluorescent molecules such as boron-dipyrromethene (BODIPY) analogues.40,41 These results encourage attachment of the troponyl residue at the N-terminal of peptides, and exploration of the role of troponyl scaffolds in structural changes of native short di-/tri-peptides. Herein we described N-troponylated di-/tri-peptides containing α-/β-amino acids and explored the role of the troponyl group in structural and conformational changes by single crystal X-ray analyses and CD studies.
Results and discussion
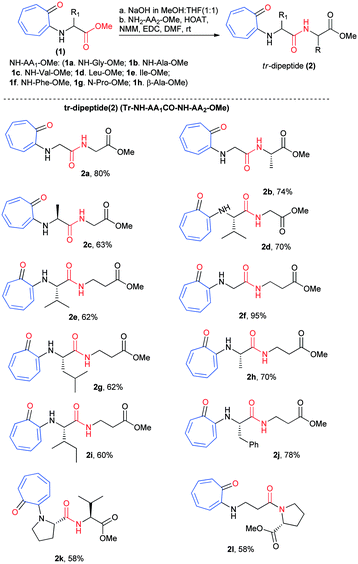
We began the chemical syntheses of rationally designed N-troponyl amino acid ester (1) from tropolone and amino acid ester derivatives by following a previous report (Scheme 1).41 Tropolone was tosylated and then treated with α-/β-amino acid ester derivatives such as NH2-Gly-OMe, NH2-Ala-OMe, NH2-Val-OMe, NH2-Leu-OMe, NH2-Ile-OMe, NH2-Phe-OMe, NH-Pro-OMe, and NH2-β-Ala-OMe. These troponylated amino acid derivatives (1a–1h) were hydrolyzed into respective carboxylic acid derivatives with NaOH (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF) and coupled with α-amino acid esters/β-alanine ester (H2N-AA2-OMe) via an amide bond under peptide coupling reaction conditions. Pleasantly we isolated the N-troponylated-di-peptides esters (2a–2l) in excellent yield (Scheme 1). We used both α-/β-amino acid ester derivatives for peptide coupling that produced N-troponyl-dipeptides (2a–2l). Their characterization data (1H-/13C-NMR/ESI-HRMS) are provided in the ESI† (Fig. S1–S24).
THF) and coupled with α-amino acid esters/β-alanine ester (H2N-AA2-OMe) via an amide bond under peptide coupling reaction conditions. Pleasantly we isolated the N-troponylated-di-peptides esters (2a–2l) in excellent yield (Scheme 1). We used both α-/β-amino acid ester derivatives for peptide coupling that produced N-troponyl-dipeptides (2a–2l). Their characterization data (1H-/13C-NMR/ESI-HRMS) are provided in the ESI† (Fig. S1–S24).
Further, we planned to study the role of troponyl carbonyl in the structural and conformational changes of those peptides in the solid state. We then attempted to crystallize these peptides under different solvent systems. Pleasantly we obtained single crystals of six di-peptides in an EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture and confirmed their chemical structure by X-ray data analyses of their respective crystals. Their crystal data are deposited at the Cambridge Crystallographic Data Centre (CCDC). Their CCDC numbers are CCDC 1889029 for compound (2a), CCDC 1889030 for compound (2b), CCDC 1889031 for compound (2d), CCDC 1889033 for compound (2e), CCDC 1889034 for compound (2f), and CCDC 1889032 compound (2j). To examine the role of tropolone containing peptide conformations in their solid states, we studied the crystal packing arrangement of troponyl peptides (2a, 2b, 2d, 2e, 2f and 2j) by using Diamond visualization software (Diamond version 3.2k). Their X-ray data are summarized in the ESI† (Table S1). We also demonstrate their supramolecular self-assembly structure in the solid state from their respective single-crystal data.
1) solvent mixture and confirmed their chemical structure by X-ray data analyses of their respective crystals. Their crystal data are deposited at the Cambridge Crystallographic Data Centre (CCDC). Their CCDC numbers are CCDC 1889029 for compound (2a), CCDC 1889030 for compound (2b), CCDC 1889031 for compound (2d), CCDC 1889033 for compound (2e), CCDC 1889034 for compound (2f), and CCDC 1889032 compound (2j). To examine the role of tropolone containing peptide conformations in their solid states, we studied the crystal packing arrangement of troponyl peptides (2a, 2b, 2d, 2e, 2f and 2j) by using Diamond visualization software (Diamond version 3.2k). Their X-ray data are summarized in the ESI† (Table S1). We also demonstrate their supramolecular self-assembly structure in the solid state from their respective single-crystal data.
Troponyl-peptide 2a
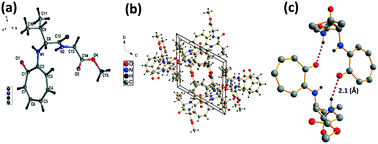
We have performed structural analysis of peptide 2a, which is described in Fig. 2 as the ORTEP diagram, unit cell packing diagram, and self-assembly structure. The tr-Gly-Gly-OMe dipeptide (2a) forms a unique self-assembled supramolecular structure via intermolecular hydrogen bonding between troponyl carbonyl (tr-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and troponyl amine (tr-N–H)/amide N–H with a bond length of 2.1 Å. These interactions lead to an antiparallel double helical type structure along the a axis with a pitch value of 4.8 Å. The formation of such a supramolecular structure from the native dipeptide (NH2-Gly-Gly-OMe) is not known in the literature. Thus, the tropolone residue plays an important role in influencing the structural changes of peptides.
O) and troponyl amine (tr-N–H)/amide N–H with a bond length of 2.1 Å. These interactions lead to an antiparallel double helical type structure along the a axis with a pitch value of 4.8 Å. The formation of such a supramolecular structure from the native dipeptide (NH2-Gly-Gly-OMe) is not known in the literature. Thus, the tropolone residue plays an important role in influencing the structural changes of peptides.
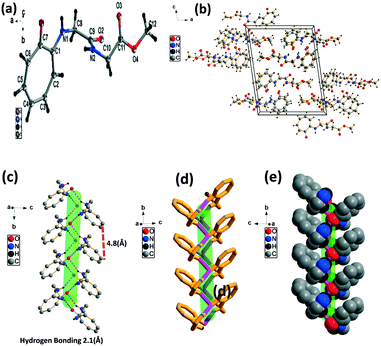 | ||
| Fig. 2 Structural analysis of the N-troponyl-peptide (2a): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c–e) supramolecular self-assembly structure. | ||
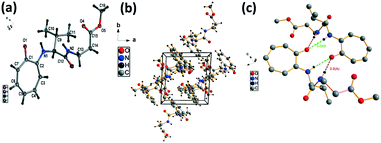
We have performed the structural analysis of troponyl peptide 2b as shown in Fig. 3. Herein we describe the ORTEP diagram (Fig. 3a), unit cell packing diagram (Fig. 2b), and packing diagram (Fig. 3c), and the hydrogen bond self-assembled structure (Fig. 6d–g). The tr-Gly-Ala-OMe dipeptide (2b) forms a unique self-assembled supramolecular structure via intermolecular hydrogen bonding as the Ala-residue (N–H) of one molecule is non-covalently bound to the tropolonyl carbonyl (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-tr) of another molecule with a bond length of 2.1 Å. As a result, dipeptide 2b forms a sheet type structure along the a axis. These β-sheet structures exist at an equal distance along the b axis, leading to a ladder-type supramolecular structure in the solid state of 2b. A similar self-assembly is not noticed with the lone native dipeptide (NH2-Gly-Ala-OMe).
C-tr) of another molecule with a bond length of 2.1 Å. As a result, dipeptide 2b forms a sheet type structure along the a axis. These β-sheet structures exist at an equal distance along the b axis, leading to a ladder-type supramolecular structure in the solid state of 2b. A similar self-assembly is not noticed with the lone native dipeptide (NH2-Gly-Ala-OMe).
 | ||
| Fig. 3 Structural analysis of the troponyl-peptide (2b): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c–g) supramolecular self-assembly structure. | ||
We have performed the structural analysis of troponyl peptide 2f as shown in Fig. 4 which describes the ORTEP diagram (Fig. 4a), unit cell packing diagram (Fig. 4b), and hydrogen bond self-assembled structure (Fig. 4c). The tr-Ala-Gly-OMe dipeptide (4d) forms a β-strand (antiparallel β-sheet) type self-assembled supramolecular structure by non-covalent interactions, two intermolecular hydrogen bonding interactions with a distance of 2.1 Å. In this assembly, the glycine N–H of one molecule is hydrogen-bonded to the tropolonyl carbonyl (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of another molecule that is oriented in an antiparallel fashion in a solid crystal.
C) of another molecule that is oriented in an antiparallel fashion in a solid crystal.
 | ||
| Fig. 4 Structural analysis of troponyl-di-peptide (2d): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c) supramolecular self-assembly structure. | ||
We have performed the structural analysis of troponyl peptide 2e as shown in Fig. 5 which describes the ORTEP diagram (Fig. 5a), unit cell packing diagram (Fig. 5b), and hydrogen bond self-assembled structure (Fig. 5c). The tr-Ala-β-Ala-OMe dipeptide (2e) also forms a β-sheet type self-assembled supramolecular structure in the solid state via two hydrogen bonding interactions with a distance of 2.0 Å as the β-Ala N–H of one molecule is hydrogen-bonded to the tropolonyl group O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of another molecule in an antiparallel manner (Fig. 5). Notably, the π–π interactions between two tropolonyl residues play important role for the formation of supramolecular self-assembly structure.
C of another molecule in an antiparallel manner (Fig. 5). Notably, the π–π interactions between two tropolonyl residues play important role for the formation of supramolecular self-assembly structure.
 | ||
| Fig. 5 Structural analysis of the troponyl-peptide (2e): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c) supramolecular self-assembly structure. | ||
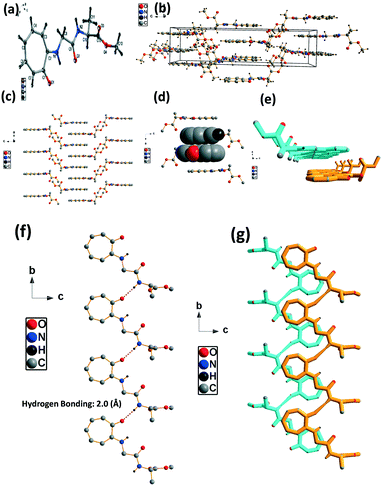
We have performed the structural analysis of troponyl peptide 2f as shown in Fig. 6 which describes the ORTEP diagram (Fig. 6a), unit cell packing diagram (Fig. 6b) and packing diagram (Fig. 6c), and the hydrogen bond self-assembled structure (Fig. 6d–h). The tr-Gly-β-Ala-OMe dipeptide (2f) forms a self-assembled supramolecular complex structure by hydrogen bonding between tropolonyl carbonyl with a β-Ala N–H form with a distance of 2.0 Å (Fig. 6g). Two molecules are oriented in an antiparallel direction. Importantly, this dipeptide forms a chair-form as the planar β-sheet-type structure along the tilted b axis. This sheet is interconnected via hydrogen bonding between πC–H with ester carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Such a kind of structure is not known with the control peptide NH2-Gly-β-Ala-OMe dipeptide. Importantly, this troponyl peptide forms sheets and is separated by weak van der Waals interactions. Each layer (β-sheet) is formed by intermolecular hydrogen bonding.
O. Such a kind of structure is not known with the control peptide NH2-Gly-β-Ala-OMe dipeptide. Importantly, this troponyl peptide forms sheets and is separated by weak van der Waals interactions. Each layer (β-sheet) is formed by intermolecular hydrogen bonding.
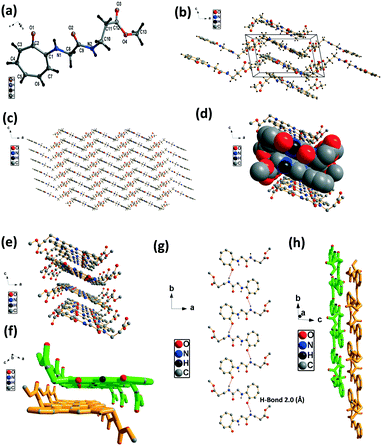 | ||
| Fig. 6 Structural analysis of the troponyl-di-peptide (2f): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c–h) supramolecular self-assembly structure. | ||
We have performed the structural analysis of troponyl peptide 2j as shown in Fig. 7 which describes the ORTEP diagram (a), unit cell packing diagram (b), and hydrogen bond self-assembled structure (c). The tr-Phe-β-Ala-OMe dipeptide (2j) forms a unique self-assembled supramolecular quadrilateral structure via hydrogen bonding as β-Ala-N–H interacts with tropolonyl carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) with a distance of 2.0 Å (Fig. 7c). This self-assembled structure contains two molecules that are oriented in the opposite direction in a twisted manner. Importantly, the tropolonyl residue is almost perpendicular to the linear backbone Phe-β-Ala residue. No such structure is reported with the native dipeptide (NH2-Phe-β-Ala-OMe).
O) with a distance of 2.0 Å (Fig. 7c). This self-assembled structure contains two molecules that are oriented in the opposite direction in a twisted manner. Importantly, the tropolonyl residue is almost perpendicular to the linear backbone Phe-β-Ala residue. No such structure is reported with the native dipeptide (NH2-Phe-β-Ala-OMe).
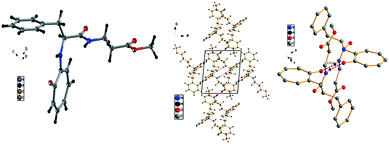 | ||
| Fig. 7 Structural analysis of the troponyl-peptide (2j): (a) structure and ORTEP diagram; (b) unit cell packing diagram; (c) supramolecular self-assembly structure. | ||
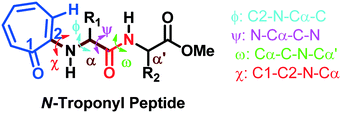
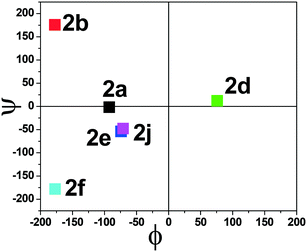
The torsional angles of the amide backbone have a critical role in the conformational changes of peptides to form regular secondary structures such as α-helices and β-strands. The torsional angles around N–Cα (ϕ) and Cα–C (Ψ) of amide backbones mostly depend on the nature of amino acid residues in the native peptide. These angles are employed in the Ramachandran plot (ϕ vs. Ψ) which reveals the role of the amino acid residue in the formation of peptides' secondary structures such as right α-helices, β-strands, and β-turns.42 In the literature, the Ramachandran plot of α-amino acid containing peptides has the following allowed regions: (i) an α-helical region with a torsion angle range −180 < φ < 0°, −100 < ψ < 45°; (ii) β-sheet regions with a torsional angle range −180 < φ < −45°, 45 < ψ < 225; (iii) β-turn regions with a torsional angle range 0 < φ < 180°, −90 < ψ < 90°.42,43 Herein, we also extracted φ/ψ angles of N-troponylated peptides, tr-peptides 2a/2b/2d/2e/2f/2j, from their respective single-crystal structure (Table 1) and plotted them as a φ vs. ψ plot, a Ramachandran plot (Fig. 8). These values and plots are compared with the reported data for α-amino acid peptides. The φ/ψ angles of tr-peptide 2a (tr-Gly-Gly-OMe) do not fall in the allowed region of the Ramachandran plot, so it may not form the regular secondary structure in the peptide. The φ/ψ angles of tr-peptides 2b (tr-Gly-Ala-OMe) and 2f (tr-Gly-β-Ala-OMe) fall in the β-sheet forming allowed region. The φ/ψ angles of tr-peptide 2d (tr-Val-Ala-OMe) fall in the β-turn allowed region. However, the φ/ψ angles of tr-peptides 2e (tr-Val-β-Ala-OMe) and 2j (tr-Phe-β-Al-OMe) fall in the α-helix forming allowed region. Another torsional angle around the amide bond (ω) is close to 180° in native peptides that support the planarity of the amide bond. The ω angles of tr-peptides' crystal are also close to 180° with little deviations (∼8°), so amide bonds of tr-peptides are planar. These results may help in the prediction of secondary structures in long peptides containing a troponyl residue at the N-terminal. In N-aryl peptides, the torsion angle (χ) depends on the nature of the amino acid residue and ortho substituents of the aryl group and guides the orientation of the aryl group peptide backbone, which generates atropoisomers.23 Herein, we also extracted such a torsional angle C1–C2–N–Cα (χ) in tr-peptides (2a/2b/2d/2e/2f/2j) from their respective single crystal structure (Table 1, column 6). The χ-value is negative (∼−175°), while that of 2b/2d is positive (∼+175°). The troponyl carbonyl groups of tr-peptides 2a/2e/2f/2j are oriented in the opposite plane compared to those of tr-peptides 2b/2d. The Cα′ of 2b/2d is non-chiral in nature, in contrast to those of tr-peptides 2a/2b/2d/2e/2f/2j. Thus the Cα′-substituents play an important role in the orientation of the N-troponyl group in tr-peptides.
Herein, the X-ray studies of tr-peptide crystals reveal the ability and importance of the troponyl group in forming a unique self-assembled supramolecular structure in the solid state. We attempted to evaluate the formation of secondary structures in those tr-peptides by the circular dichroism (CD) technique. In the literature, the secondary structures of peptides are well characterized by CD-spectral analyses.44 We recorded the CD spectra of tr-peptides (2a–2l) in two different solvent systems: MeOH (protic polar) and CH3CN (aprotic polar). Their CD spectra in CH3CN are provided in Fig. 9 while those in MeOH are provided in the ESI† (Fig. S69). In the literature, the typical β-strand structure of native peptides exhibits maxima ∼λ195nm and minima at ∼λ220nm. In contrast, their α-helical structure shows maxima at ∼λ190nm and two minima at ∼λ208nm and ∼λ220.45 The CD signals of the native peptide structure occur due to the electronic transition of n–π* and π–π*. The CD spectra of chromophore rich peptides depend on the electronic transition of chromophores.46 Herein, the CD signal of tr-peptides also depends on the electronic transition of the troponyl group. The CD spectra of tr-peptides 2a, 2c, 2f, 2h, 2j, 2k, and 2l exhibit a non-characteristic signal, only a broad minimum at (∼λ260nm) while the CD spectra of 2d/2e/2g/2i exhibit a strong CD signal because of the cotton effect on the troponyl residue. The CD spectra of tr-peptides 2d/2i exhibit maxima at a wavelength of 222 nm (∼λ220nm), and minima at 266 nm (∼λ266nm). The CD spectra of tr-peptides 2e/2g exhibit maxima at a wavelength of 222 nm (∼λ220nm), and two minima with different intensities such as a weak signal at a wavelength of 248 nm (∼λ248nm) and a strong signal at 266 nm (∼λ266nm). Thus the tr-peptides 2d/2i form only a β-strand type structure in solution (CH3CN), and tr-peptides 2e/2g possibly form two types of secondary structures (α-helix and β-sheet and turn) in acetonitrile solution. The CD spectra of tr-dipeptides are similar in MeOH, a protic polar solvent.
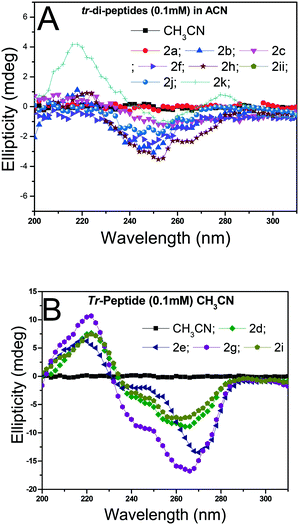 | ||
| Fig. 9 CD spectra of tr-peptides in solvent CH3CN: (A) non-characteristic CD signals in peptides (2a/2b/2c/2f/2h/2j/2k/2l) and (B) characteristic CD signals of peptides (2d/2e/2g/2i). | ||
The formation of a β-turn/helix type secondary structure in the solution state of tr-peptides is mainly due to the intermolecular hydrogen bonding between aminotroponyl-N–H (NH-1)/amide-N–H (NH-2) with aminotroponyl carbonyl/amide carbonyl oxygen as N–H–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–. Generally, a DMSO-d6 titration 1H-NMR experiment reveals the nature of hydrogen bonding as intermolecular or intramolecular.39 It is well known that intra-molecular hydrogen-bonded protons remain unaffected in 1H-NMR spectra with the addition of DMSO-d6, while inter-molecular hydrogen-bonded protons exhibit a significant downfield shift with the addition of DMSO-d6. Thus we assigned the chemical shifts of all protons, including N–H protons, of representative di-/tri-peptides (2a/2b/2d/2e/2f/2j) by 2D-NMR analyses. Their spectra are provided in the ESI† (Fig. S45–S54). We then performed the DMSO-d6 titration experiment with representative tr-peptides such as 2c/2j (exhibit a non-characteristic CD-signal) and 2e (exhibit a β-strand type characteristic CD signal). Their NMR spectra are provided in the ESI† (Fig. S59–S64). We generated a titration plot, DMSO-d6 concentration vs. chemical shift (ppm) from their respective sequential 1H-NMR data. Their NMR-titration plots and outcomes are depicted in Fig. 10. We noticed a significant increment in the chemical shift of amide-N–H (2c/2e/2j) after increasing the concentration of DMSO-d6 in CDCl3. The chemical shifts of NH-1 and NH-2 are sequentially increased after the addition of DMSO-d6 in tr-peptide (2c) CDCl3 solution, though the increment in NH-1 is higher than that in NH-2. It suggests that NH-2 has some sort of intramolecular hydrogen bonding with the carbonyl group. With the help of its NOE data (2D-NMR), we propose the conformation of tr-peptide (2c) as shown in Fig. 10A, (bottom). The CD signal of tr-peptide (2c) is also non-characteristic. In the case of tri-peptide (2e), there is no significant change in the chemical shifts of both NH-1 and NH-2, so both are involved in the intra-molecular hydrogen bonding. With the help of its NOE data, we propose the conformation of tr-peptide (2e) in solution (CDCl3) as shown in Fig. 10B (bottom). This proposed conformation resembles the unique β-turn type structure. Importantly, its CD signals (in both solvents CH3CN & MeOH) strongly support the formation of a β-strand type secondary structure. In the case of tr-peptide (2j), the chemical shift of N–H1 (in CDCl3) is almost constant with addition of DMSO-d6, though the chemical shift of N–H2 (in CDCl3) is increased with DMSO-d6 addition (Fig. 10C). It suggests that only NH-1 is involved in intra-molecular hydrogen bonding. With the help of its NOE data, we propose the conformation of tr-peptide (2j) in solution (CDCl3) as shown in Fig. 10C, (bottom). The CD signal of tr-peptide (2j) is also non-characteristic. Peptides have flexible chemical structures and can adopt various stable conformations that are dependent on the environment. In crystalline solid peptides, one of the stable conformations is crystallized and leads to a unique molecular arrangement. In the solution phase, peptides have alternative stable conformations, including β-turns. The formation of peptides' β-sheet secondary structure competes with the aggregation of peptides, leading to random coils. In some troponylated dipeptides, their CD signals are non-characteristic, although they have a unique self-assembly structure.
C–. Generally, a DMSO-d6 titration 1H-NMR experiment reveals the nature of hydrogen bonding as intermolecular or intramolecular.39 It is well known that intra-molecular hydrogen-bonded protons remain unaffected in 1H-NMR spectra with the addition of DMSO-d6, while inter-molecular hydrogen-bonded protons exhibit a significant downfield shift with the addition of DMSO-d6. Thus we assigned the chemical shifts of all protons, including N–H protons, of representative di-/tri-peptides (2a/2b/2d/2e/2f/2j) by 2D-NMR analyses. Their spectra are provided in the ESI† (Fig. S45–S54). We then performed the DMSO-d6 titration experiment with representative tr-peptides such as 2c/2j (exhibit a non-characteristic CD-signal) and 2e (exhibit a β-strand type characteristic CD signal). Their NMR spectra are provided in the ESI† (Fig. S59–S64). We generated a titration plot, DMSO-d6 concentration vs. chemical shift (ppm) from their respective sequential 1H-NMR data. Their NMR-titration plots and outcomes are depicted in Fig. 10. We noticed a significant increment in the chemical shift of amide-N–H (2c/2e/2j) after increasing the concentration of DMSO-d6 in CDCl3. The chemical shifts of NH-1 and NH-2 are sequentially increased after the addition of DMSO-d6 in tr-peptide (2c) CDCl3 solution, though the increment in NH-1 is higher than that in NH-2. It suggests that NH-2 has some sort of intramolecular hydrogen bonding with the carbonyl group. With the help of its NOE data (2D-NMR), we propose the conformation of tr-peptide (2c) as shown in Fig. 10A, (bottom). The CD signal of tr-peptide (2c) is also non-characteristic. In the case of tri-peptide (2e), there is no significant change in the chemical shifts of both NH-1 and NH-2, so both are involved in the intra-molecular hydrogen bonding. With the help of its NOE data, we propose the conformation of tr-peptide (2e) in solution (CDCl3) as shown in Fig. 10B (bottom). This proposed conformation resembles the unique β-turn type structure. Importantly, its CD signals (in both solvents CH3CN & MeOH) strongly support the formation of a β-strand type secondary structure. In the case of tr-peptide (2j), the chemical shift of N–H1 (in CDCl3) is almost constant with addition of DMSO-d6, though the chemical shift of N–H2 (in CDCl3) is increased with DMSO-d6 addition (Fig. 10C). It suggests that only NH-1 is involved in intra-molecular hydrogen bonding. With the help of its NOE data, we propose the conformation of tr-peptide (2j) in solution (CDCl3) as shown in Fig. 10C, (bottom). The CD signal of tr-peptide (2j) is also non-characteristic. Peptides have flexible chemical structures and can adopt various stable conformations that are dependent on the environment. In crystalline solid peptides, one of the stable conformations is crystallized and leads to a unique molecular arrangement. In the solution phase, peptides have alternative stable conformations, including β-turns. The formation of peptides' β-sheet secondary structure competes with the aggregation of peptides, leading to random coils. In some troponylated dipeptides, their CD signals are non-characteristic, although they have a unique self-assembly structure.
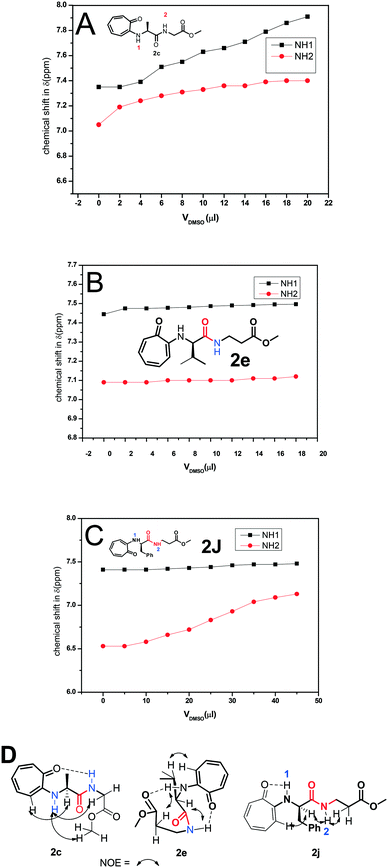 | ||
| Fig. 10 DMSO-d6 titration 1H-NMR experiment of peptides 2c (A), 2e (B) and 2j (C) and NOE and possible intramolecular hydrogen bonding in the respective tr-peptides (D) (Vdmso = volume of DMSO-d6). | ||
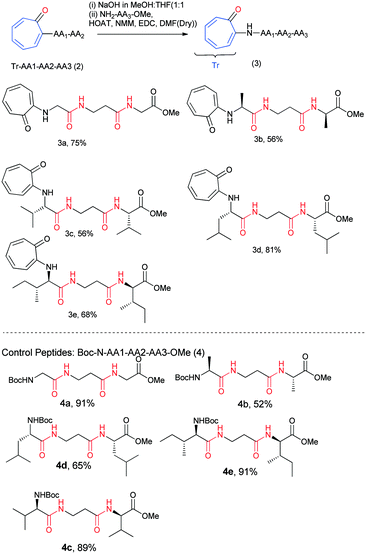
The results of our X-ray, NMR titration, and CD studies reveal that the troponyl substituted di-peptides adopt a β-strand type of secondary structure in a few troponyl di-peptides (tr-di-peptides). We notice that the formation of a β-sheet structure is more pronounced with troponylated hybrid peptides, containing α-amino acid and β-alanine dipeptides. Thus, for practical applications, we synthesized hybrid peptides such as troponyl-α-β-α-hybrid tri-peptides (3a–3e) by following Scheme 2. For control studies, we also synthesized non-troponyl α-β-α-hybrid tri-peptides (4a–4e) by following a similar synthetic procedure (Scheme 2). Their characterization data are provided in the ESI† (Fig. S25–S44). We also synthesized tr-peptides (3a–3e) from the free amine derivative of control peptides by troponylation reaction with O-tosylate tropolone.
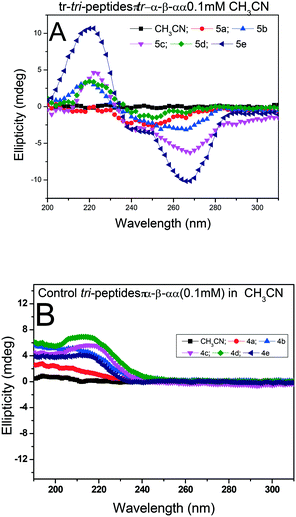
We recorded the CD-spectra of troponyl tri-peptides (tr-tri-peptides) (3a–3e) in two different solvent systems (MeOH & CH3CN). For comparative studies, we also recorded the CD spectra of control peptides (3a–3e). The CD spectra in solvent CH3CN are provided in Fig. 11(A and B), while those in MeOH are given in the ESI† (Fig. S70–S71). In solvent MeOH, the CD spectra of tripeptides 3b, 3c & 3e exhibit a similar pattern to those of tr-di-peptides (maxima at λ220nm and minima ∼λ260nm), which are characteristic of β-strand type secondary structure. The CD spectra of 3e exhibit similar strong maxima (λ220nm) and minima (λ260nm) and are more stable. The CD spectra of the tr-peptide (3d) exhibit a characteristic signal such as broad minima (−λ250nm) and one maximum (λ220nm). These CD data strongly support the formation of helical type structures. However, the CD spectrum of the tr-peptide (3a) exhibits only minima (λ250nm), non-characteristic in the peptide. The comparative CD studies of troponyl tri-peptides (3a–3e) are similar in another solvent MeOH. In contrast, the CD spectra of control hybrid peptides (4a–4e) exhibit only maxima at λ220nm in MeOH, non-characteristic signals. It seems that aggregation occurs in the control peptides. Hence the troponyl residue influences the formation of β-strand/turn type secondary structures in tr-tri-peptides, hybrid peptides.
Nevertheless, we assigned all protons, along with the N–H protons, of tr-tri-peptides (3c/3d) by 2D-NMR analyses (see ESI,† Fig. S55–S58). We then performed DMSO-d61H-NMR titration experiments with tr-peptides 3d/3e and generated a titration plot, DMSO-d6 concentration vs. chemical shift (ppm) from their respective sequential 1H-NMR data (Fig. 12). Meanwhile, subsequent 1H-NMR data are provided in the ESI† (Fig. S65–S68). In the case of the tr-peptide (3d), there is no significant change in the chemical shifts of both NH-2 and NH-3, so both are involved in the intra-molecular hydrogen bonding, though the chemical shift of NH-1 in the tr-peptide (3d) is significantly shifted with the addition of DMSO-d6. With the help of its NOE data, we propose the conformation of the tr-peptide (3d) in solution (CDCl3) as shown Fig. 10B, (bottom). This proposed conformation supports the formation of a helical structure as an i + 8 helix. Importantly, its CD signals (in both solvents CH3CN & MeOH) also support the formation of a helix type secondary structure. In the case of the tr-peptide (3e), there is no significant change in the chemical shifts of NH-1/NH-2, so these are involved in the intra-molecular hydrogen bonding, though the chemical shift of NH-3 in the tr-peptide (3e) is marginally increased with the addition of DMSO-d6. It means that NH-3 is not involved/weakly involved in intramolecular hydrogen bonding. With the help of its NOE data, we propose the conformation of the tr-peptide (3e) in solution (CDCl3) as shown in Fig. 10B, (bottom). This proposed structural conformation supports the formation of β-strand (β-turn) type secondary structures in the tr-peptide (3e). Importantly, its CD signals (in both solvents CH3CN & MeOH) also support the formation of β-strand (β-turn) type secondary structures.
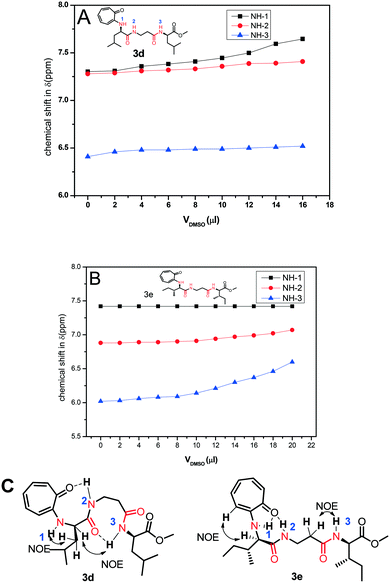 | ||
| Fig. 12 DMSO-d6 titration 1H-NMR experiment of peptides 3c (A)/3d (B) and NOE and possible intramolecular hydrogen bonding in the respective tr-peptides (C) (Vdmso = volume of DMSO-d6). | ||
Conclusions
The synthesis of N-tropolonylated di-/tri-peptides is accomplished containing native α-amino acids and β-alanine. Herein, the role of tropolone residue is demonstrated by forming a unique secondary structure, even in di-/tri-peptides, by NMR, X-ray, and CD-studies. The X-ray studies of di-peptides confirm the establishment of unique self-assembled supramolecular structures such as β-sheets and helical type secondary structures in their respective single crystals. The DMSO-d6 NMR titration studies reveal that aminotroponyl N–H groups are involved in inter/intramolecular hydrogen bonding in the solution state. The CD spectral analysis of tropolonylated di-/tri peptides strongly supports the formation of β-sheet/turn type structures in solution (MeOH and CH3CN) in contrast to that of native control peptides. Hence, the N-troponylation of peptides influences stable secondary structures even in a small peptide, which could help synthesize novel peptidomimetics for practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
BBP is grateful to UGC (JRF & SRF) for fellowship. We thank Tiwari Ranjay Kumar for crystal structure analysis. We thank CSIR-HRDG, New Delhi, for the financial support with grant number 02(0166)/13/EMR-II.References
- D. Margulies and A. D. Hamilton, Curr. Opin. Chem. Biol., 2010, 14, 705–712 CrossRef CAS.
- S. Fahs, Y. Patil-Sen and T. J. Snape, ChemBioChem, 2015, 16, 1840–1853 CrossRef CAS.
- A. M. Kendhale, R. Gonnade, P. R. Rajamohanan, H.-J. Hofmann and G. J. Sanjayan, Chem. Commun., 2008, 2541–2543 RSC.
- D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes and J. S. Moore, Chem. Rev., 2001, 101, 3893–4012 CrossRef CAS.
- T. A. Martinek and F. Fülöp, Chem. Soc. Rev., 2012, 41, 687–702 RSC.
- W. S. Horne and S. H. Gellman, Acc. Chem. Res., 2008, 41, 1399–1408 CrossRef CAS.
- S. H. Gellman, Acc. Chem. Res., 1998, 31, 173–180 CrossRef CAS.
- D. Seebach, S. Abele, K. Gademann and B. Jaun, Angew. Chem., Int. Ed., 1999, 38, 1595–1597 CrossRef CAS.
- A. M. Kendhale, L. Poniman, Z. Dong, K. Laxmi-Reddy, B. Kauffmann, Y. Ferrand and I. Huc, J. Org. Chem., 2011, 76, 195–200 CrossRef CAS.
- P. Singh, S. Misra, N. Sepay, S. Mondal, D. Ray, V. K. Aswal and J. Nanda, Soft Matter, 2020, 6599–6607 RSC.
- H. A. Scheidt, A. Das, A. Korn, M. Krueger, S. Maiti and D. Huster, Phys. Chem. Chem. Phys., 2020, 22, 16887–16895 RSC.
- S. S. Bag, S. Jana, A. Yashmeen and S. De, Chem. Commun., 2015, 51, 5242–5245 RSC.
- M. Konda, B. Kauffmann, D. B. Rasale and A. K. Das, Org. Biomol. Chem., 2016, 14, 4089–4102 RSC.
- K. Tao, A. Levin, L. Adler-Abramovich and E. Gazit, Chem. Soc. Rev., 2016, 45, 3935–3953 RSC.
- S. Xue, N. Zhang, X. Hu, Y. Zeng, J. Zhang, P. Xing and Y. Zhao, J. Phys. Chem. Lett., 2020, 11, 1490–1496 CrossRef CAS.
- S. Bera, B. Xue, P. Rehak, G. Jacoby, W. Ji, L. J. Shimon, R. Beck, P. Král, Y. Cao and E. Gazit, ACS Nano, 2020, 14, 1694–1706 CrossRef CAS.
- B. Yoo and K. Kirshenbaum, Curr. Opin. Chem. Biol., 2008, 12, 714–721 CrossRef CAS.
- S. A. Fowler and H. E. Blackwell, Org. Biomol. Chem., 2009, 7, 1508–1524 RSC.
- A. D. Bautista, C. J. Craig, E. A. Harker and A. Schepartz, Curr. Opin. Chem. Biol., 2007, 11, 685–692 CrossRef CAS.
- N. Delsuc, S. Massip, J.-M. Léger, B. Kauffmann and I. Huc, J. Am. Chem. Soc., 2011, 133, 3165–3172 CrossRef CAS.
- J. R. Stringer, J. A. Crapster, I. A. Guzei and H. E. Blackwell, J. Org. Chem., 2010, 75, 6068–6078 CrossRef CAS.
- M. L. Huang, D. Ehre, Q. Jiang, C. Hu, K. Kirshenbaum and M. D. Ward, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 19922–19927 CrossRef CAS.
- B. Paul, G. L. Butterfoss, M. G. Boswell, M. L. Huang, R. Bonneau, C. Wolf and K. Kirshenbaum, Org. Lett., 2012, 14, 926–929 CrossRef CAS.
- B. Paul, G. L. Butterfoss, M. G. Boswell, P. D. Renfrew, F. G. Yeung, N. H. Shah, C. Wolf, R. Bonneau and K. Kirshenbaum, J. Am. Chem. Soc., 2011, 133, 10910–10919 CrossRef CAS.
- G. L. Butterfoss, B. Yoo, J. N. Jaworski, I. Chorny, K. A. Dill, R. N. Zuckermann, R. Bonneau, K. Kirshenbaum and V. A. Voelz, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 14320–14325 CrossRef CAS.
- L. Zhang, Y. Geng and Z. Jin, J. Org. Chem., 2016, 81, 3542–3552 CrossRef CAS.
- J. Gan and D. Ma, Org. Lett., 2009, 11, 2788–2790 CrossRef CAS.
- M. C. Morejón, A. Laub, B. Westermann, D. G. Rivera and L. A. Wessjohann, Org. Lett., 2016, 18, 4096–4099 CrossRef.
- Z.-H. Du, W.-J. Qin, B.-X. Tao, M. Yuan and C.-S. Da, Org. Biomol. Chem., 2020, 18, 6899–6904 RSC.
- R. Bentley, Nat. Prod. Rep., 2008, 25, 118–138 RSC.
- H. Guo, D. Roman and C. Beemelmanns, Nat. Prod. Rep., 2019, 36, 1137–1155 RSC.
- P. L. Pauson, Chem. Rev., 1955, 55, 9–136 CrossRef CAS.
- B. E. Bryant and W. C. Fernelius, J. Am. Chem. Soc., 1954, 76, 1696–1697 CrossRef CAS.
- B. E. Bryant and W. C. Fernelius, J. Am. Chem. Soc., 1954, 76, 4864–4865 CrossRef CAS.
- B. E. Bryant, W. C. Fernelius and B. E. Douglas, J. Am. Chem. Soc., 1953, 75, 3784–3786 CrossRef CAS.
- B. F. Bryant and W. C. Fernelius, J. Am. Chem. Soc., 1954, 76, 3783–3784 CrossRef CAS.
- C. Balachandra and N. K. Sharma, ACS Omega, 2018, 3, 997–1013 CrossRef CAS.
- C. Balachandra and N. K. Sharma, Org. Lett., 2015, 17, 3948–3951 CrossRef CAS.
- C. Balachandra and N. K. Sharma, Tetrahedron, 2014, 70, 7464–7469 CrossRef CAS.
- C. Balachandra and N. K. Sharma, Dyes Pigm., 2017, 137, 532–538 CrossRef CAS.
- B. B. Palai, R. Soren and N. K. Sharma, Org. Biomol. Chem., 2019, 17, 6497–6505 RSC.
- S. Hovmöller, T. Zhou and T. Ohlson, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2002, 58, 768–776 CrossRef.
- G. J. Kleywegt and T. A. Jones, Structure, 1996, 4, 1395–1400 CrossRef CAS.
- N. J. Greenfield, in Methods in enzymology, Elsevier, 2004, vol. 383, pp. 282–317 Search PubMed.
- N. J. Greenfield, Nat. Protoc., 2006, 1, 2891 CrossRef CAS.
- S. S. Bag, S. Jana, A. Yashmeen, K. Senthilkumar and R. Bag, Chem. Commun., 2014, 50, 433–435 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1889029–1889034 (six structures). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ce01386b |
| This journal is © The Royal Society of Chemistry 2021 |