The determination of the thermodynamic constants of MgUO2(CO3)32− complex in NaClO4 and NaCl media by time-resolved luminescence spectroscopy, and applications in different geochemical contexts†
Abstract
The formation constants and specific ion interaction coefficients of MgUO2(CO3)32− complex were determined in 0.1 to 1.0 mol kgw−1 NaCl and 0.10 to 2.21 mol kgw−1 NaClO4 media in the framework of the specific ion interaction theory (SIT), by time-resolved laser-induced luminescence spectroscopy. The upper limits of ionic strength were chosen in order to limit luminescence quenching effects generated by high concentrations of Cl− and ClO4− already observed during our earlier studies on CanUO2(CO3)3(4−2n)− complexes (Shang & Reiller, Dalton Trans., 49, 466; Shang et al., Dalton Trans., 49, 15443). The cumulative formation constant determined is 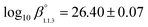 , and the specific ion interaction coefficients are ε(MgUO2(CO3)32−, Na+) = 0.19 ± 0.11 kgw mol−1 in NaClO4 and ε(MgUO2(CO3)32−, Na+) = 0.09 ± 0.16 kgw mol−1 in NaCl. Two gratings of 300 and 1800 lines per mm were used to acquire MgUO2(CO3)32− luminescence spectra, where the high-resolution 1800 lines per mm grating detected slight spectral shifts for the principal luminescent bands relative to CanUO2(CO3)3(4−2n)−. The applications of the consistent set of thermodynamic constants and ε values for MnUO2(CO3)3(4−2n)− (M = Mg and Ca) were examined in different geochemical contexts, where Mg over Ca concentration ratio varies to help defining the relative importance of these species.
, and the specific ion interaction coefficients are ε(MgUO2(CO3)32−, Na+) = 0.19 ± 0.11 kgw mol−1 in NaClO4 and ε(MgUO2(CO3)32−, Na+) = 0.09 ± 0.16 kgw mol−1 in NaCl. Two gratings of 300 and 1800 lines per mm were used to acquire MgUO2(CO3)32− luminescence spectra, where the high-resolution 1800 lines per mm grating detected slight spectral shifts for the principal luminescent bands relative to CanUO2(CO3)3(4−2n)−. The applications of the consistent set of thermodynamic constants and ε values for MnUO2(CO3)3(4−2n)− (M = Mg and Ca) were examined in different geochemical contexts, where Mg over Ca concentration ratio varies to help defining the relative importance of these species.



 Please wait while we load your content...
Please wait while we load your content...