Open Access Article
Open Access ArticleScandium calix[n]arenes (n = 4, 6, 8): structural, cytotoxicity and ring opening polymerization studies†
Abdullah Fahad A.
Alshamrani
ab,
Orlando
Santoro
a,
Timothy J.
Prior
 a,
Mohammed A.
Alamri
a,
Graeme J.
Stasiuk
a,
Mohammed A.
Alamri
a,
Graeme J.
Stasiuk
 c,
Mark R. J.
Elsegood
c,
Mark R. J.
Elsegood
 d and
Carl
Redshaw
d and
Carl
Redshaw
 *a
*a
aPlastics Collaboratory, Department of Chemistry, The University of Hull, Cottingham Road, Hull, HU6 7RX, UK. E-mail: c.redshaw@hull.ac.uk
bDepartment of Diagnostic Radiology Technology, College of Applied Medical Sciences, Taibah University, Madina, Saudi Arabia
cDepartment of Imaging Chemistry and Biology, School of Biomedical Engineering and Imaging, King's College London, London, SE1 7EH, UK
dChemistry Department, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK
First published on 1st June 2021
Abstract
Interaction of [Sc(OR)3] (R = iPr or triflate) with p-tert-butylcalix[n]arenes, where n = 4, 6, or 8, affords a number of intriguing structural motifs, which are relatively non-toxic (cytotoxicity evaluated against cell lines HCT116 and HT-29) and a number were capable of the ring opening polymerization (ROP) of cyclohexene oxide.
Ring opening polymerization (ROP) catalyzed by metal alkoxides is one of the promising avenues being pursued to access more environmentally friendly polymeric materials.1,2 For the metal catalyst, it is desirable if the ligands employed can be readily modified to allow for a degree of control, that they are of low toxicity, and that the catalyst can operate under relatively mild conditions. Chelating ligands, including macrocyclic systems are proving to be suitable options.3 The family of phenolic macrocycles called calix[n]arenes, where n represents the number of phenolic groups, has found widespread use in a variety of catalytic applications, partly due to facile synthesis and modification.4 In the case of the ROP of cyclic esters, a number of titanocalix[4]arenes have been successfully employed,5 whilst the use of other metals has produced mixed results.6,7 Given the success of scandium-based systems in a variety of catalytic processes,8 we have initiated investigations into the use of scandium-based metallocalix[n]arenes for the ROP of epoxides, and herein focus on cyclohexene oxide (CHO). Interest in this polyether stems from the favorable characteristics displayed by poly(CHO), e.g. high insulating properties, resistance to UV light, and high glass transition temperature.9 Moreover, given CHO is a waste product of the petroleum industry, there is also interest in CHO/CO2 co-polymerizations.10 Reports of scandium calix[n]arenes are scant,11 as are structural reports on oxo-alkoxide/hydroxide scandium clusters.12 In terms of ROP, a variety of non-calixarene scandium catalysts have been employed with some success.13 Scandium triflate is an effective catalyst for the ROP of CHO in the ionic liquid 1-n-butyl-3-methylimidazolium tetrafluoroborate.14 We note that a calix[6]arene scandium complex has been employed in the ROP of 2,2′-dimethyltrimethylene carbonate,15 although the molecular structure of the complex, as determined by X-ray diffraction, was not reported. Our entry points into this chemistry are the commercially available reagents [Sc(OTf)3] (Tf = triflate) and [Sc(OiPr)3] (iPr = isopropyl, see Schemes S1 and S2†), and we have investigated their interaction with the p-tert-butylcalix[n]arenes, where n = 4 (L4H4), 6 (L6H6), and 8 (L8H8).
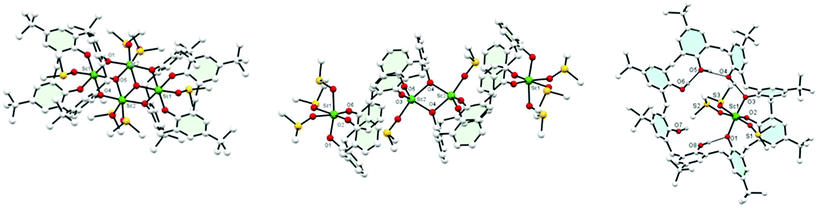
Use of [Sc(OTf)3]: treatment of L4H4 with two equivalents of the triflate in the presence of triethylamine in DMSO/acetone afforded, following work-up, the complex [(Sc4O2)L42(DMSO)6] (1) in moderate yield. The molecular structure is shown in Fig. 1 (left), with selected geometry given in the ESI.† The four pseudo-octahedral Sc ions form part of an 8-membered metallocyclic core, which can be viewed as a distorted square comprising an Sc2O2 unit, capped above and below by a Sc ion linked to the square via the bridging oxygens. Each of the central Sc ions is bound by two DMSO ligands, whilst the capping Sc ions are bound by one DMSO ligand. The central core (Fig. S1, ESI†) is further capped by two fully deprotonated L1 ligands, which are rotated by about 180° to each other and encapsulate within each bowl one of the ‘Sc-capped’ DMSO ligands.16 Increasing the size of the calix[n]arene to n = 6 (L6H6) led, on interaction with the triflate precursor [Sc(OTf)3], in the presence of Et3N in DMSO/acetone, to the complex [(L6)2Sc4(DMSO)4]·2DMSO·2acetone (2·2DMSO·2acetone). The molecular structure is shown in Fig. 1 (centre); selected bond lengths/angles are given in the ESI.† A central Sc2O2 square formed from trigonal bipyramidal 5-coordinate Sc ions (τ = 0.742)17 joins two calix[6]arenes; each Sc is bonded to three oxygens from one calix[6]arene, one bridging oxygen (O4) from the other, and a molecule of DMSO. The other three oxygen atoms of each calix[6]arene coordinate to another Sc ion; coordination about this distorted octahedral Sc is completed by three molecules of DMSO. Each L6 macrocycle is severely twisted to accommodate the bulky Sc(DMSO) fragments. The whole arrangement is centrosymmetric.
Similar use of L8H8 led to the formation of pale-yellow [Sc(L8H5)(DMSO)3]·½DMSO·4½MeCN (3·½DMSO·4½MeCN), (see Fig. 1 right; selected bond lengths/angles in ESI†). Three calixarene phenolate oxygens are bound to the Sc(III) centre, balancing the charge, in a mer conformation. Remaining calixarene phenolic oxygens form hydrogen bonds to their neighbours in a group of two and a group of three.
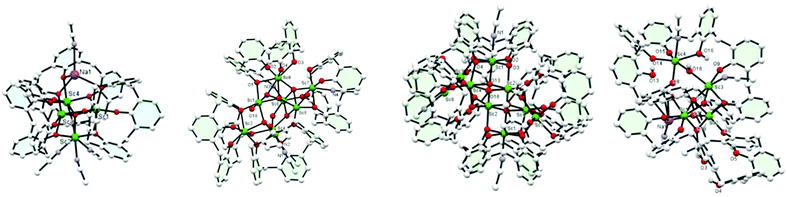
Use of [Sc(OiPr)3]: the use of [Sc(OiPr)3] led to very different products. In the case of reaction with L4H4 in an equimolar ratio, following work-up (refluxing MeCN), the complex {Sc3O(L4H1.5)2[L4H(Na(NCMe)1.5)0.5]Sc(NCMe)3}·19MeCN (4·19MeCN) (4·19MeCN) was isolated (Fig. 2, left). The core comprises a tetrahedral cluster of four Sc ions around a central oxide. About this cluster lie three calix[4]arenes. Three of the Sc ions lie in a distorted octahedral environment bound to an oxide ion and 5 oxygen atoms from calix[4]arenes, and the other Sc is 7 coordinate, bound by oxide, three oxygen atoms of calixarene and three molecules of acetonitrile. Attached to one end of this cluster is a sodium ion. There are two polymorphs of this compound. For further discussion of these structures, see ESI.† On changing the molar ratio to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Sc
1 (Sc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) calix), the product, following work-up, was found to possess the composition [Sc8(L4)3(L4H)1(O)3(OH)3(OH2)2]·17MeCN (5·17MeCN), Fig. 2, second left. A view of the core is shown in Fig. S5 (ESI†) along with selected bond lengths/angles. Interest here is that this compound contains an oxo cluster containing 8 Sc ions in various coordination geometries. 7 of them are 6-coordinate but one is 7-coordinate. There is oxide or hydroxide too, and bound to this cluster are 4 L4 molecules. Three of these are in a normal bowl configuration but the fourth is twisted so that one side of the bowl lies flattened. The aromatic ring subtends an angle of ∼74° to the central plane of the L4, much more open than the normal angle of 23°. Acetonitrile is bound to complete the coordination about the Sc.
calix), the product, following work-up, was found to possess the composition [Sc8(L4)3(L4H)1(O)3(OH)3(OH2)2]·17MeCN (5·17MeCN), Fig. 2, second left. A view of the core is shown in Fig. S5 (ESI†) along with selected bond lengths/angles. Interest here is that this compound contains an oxo cluster containing 8 Sc ions in various coordination geometries. 7 of them are 6-coordinate but one is 7-coordinate. There is oxide or hydroxide too, and bound to this cluster are 4 L4 molecules. Three of these are in a normal bowl configuration but the fourth is twisted so that one side of the bowl lies flattened. The aromatic ring subtends an angle of ∼74° to the central plane of the L4, much more open than the normal angle of 23°. Acetonitrile is bound to complete the coordination about the Sc.
From the acetonitrile extraction, a small amount of colourless prisms was isolated that were found to possess a huge unit cell (volume ∼28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Å) with a large asymmetric unit, Z = 8 in space group C2/c. Interestingly, this product revealed high nuclearity for which the structural formula is [(L4)2(L4H)4Sc9(OH)7(H2O)(MeCN)4]·11MeCN (6·11MeCN, Fig. 2, third left). It is not clear from the diffraction data exactly how many of the hydroxide anions should be formulated as oxide. The cluster is centrosymmetric and contains a central core of four ScO6 distorted octahedra and two distorted ScO5N octahedra that share edges. These are capped by further ScO6 distorted octahedra that share corners with the other six. There are further Sc ions surrounding this block that are partially occupied and have a coordination number four. The cluster is surrounded by six calix-4 molecules, four of which are completely deprotonated and the oxygen atoms coordinate to the Sc ions; the other two have a single proton only, and coordinate through the oxygen atoms. To emphasise the structure one might formulate this as [(calix-4)6(Sc(OH))8]Sc1(MeCN)4·11MeCN where the cluster is enclosed in square brackets. The coordination about the Sc is completed by MeCN. Reaction of [Sc(OiPr)3] (two equivalents) with L6H6 afforded, following work-up (MeCN), the complex [(L6H4)Sc2(OH)2(NCMe)2]2·12MeCN (7·12MeCN), see Fig. S7.† There are two different types of symmetry-related, distorted octahedral Sc centre present, linked via two asymmetric hydroxo bridges O(7)/O(8); the coordination sphere for Sc(2) also comprises an acetonitrile ligand which is involved in H-bonding to O(3) and O(6).
600 Å) with a large asymmetric unit, Z = 8 in space group C2/c. Interestingly, this product revealed high nuclearity for which the structural formula is [(L4)2(L4H)4Sc9(OH)7(H2O)(MeCN)4]·11MeCN (6·11MeCN, Fig. 2, third left). It is not clear from the diffraction data exactly how many of the hydroxide anions should be formulated as oxide. The cluster is centrosymmetric and contains a central core of four ScO6 distorted octahedra and two distorted ScO5N octahedra that share edges. These are capped by further ScO6 distorted octahedra that share corners with the other six. There are further Sc ions surrounding this block that are partially occupied and have a coordination number four. The cluster is surrounded by six calix-4 molecules, four of which are completely deprotonated and the oxygen atoms coordinate to the Sc ions; the other two have a single proton only, and coordinate through the oxygen atoms. To emphasise the structure one might formulate this as [(calix-4)6(Sc(OH))8]Sc1(MeCN)4·11MeCN where the cluster is enclosed in square brackets. The coordination about the Sc is completed by MeCN. Reaction of [Sc(OiPr)3] (two equivalents) with L6H6 afforded, following work-up (MeCN), the complex [(L6H4)Sc2(OH)2(NCMe)2]2·12MeCN (7·12MeCN), see Fig. S7.† There are two different types of symmetry-related, distorted octahedral Sc centre present, linked via two asymmetric hydroxo bridges O(7)/O(8); the coordination sphere for Sc(2) also comprises an acetonitrile ligand which is involved in H-bonding to O(3) and O(6).
Reaction of [Sc(OiPr)3] (four equivalents) with L8H8 led, following work-up, to the complex [Sc4Na(L8H3)2(OiPr)(OH)2(NCMe)4]·6.14MeCN (8·6.14MeCN). Each Sc3+ is 6-coordinate octahedral, but the coordination environments are all different, see Fig. 2, right. For a fuller description of this structure, see Fig. S10 (ESI†). The sodium present in 8 is likely to derive from the drying agent of the solvents used for the reaction, and is serendipitously incorporated into the structure.
Polymerization studies
Complexes 1, 4, and 8, along with their [Sc(OR)3] precursors, have been tested as catalysts in the ROP of ε-caprolactone (ε-CL), δ-valerolactone (δ-VL), r-lactide (r-LA) and cyclohexene oxide (CHO) (Table 1). While [Sc(OiPr)3], 1 and 8 proved completely inactive regardless of the monomer, [Sc(OTf)3] was shown to efficiently convert ε-CL and CHO into their corresponding polymers under solvent-free conditions within 24 h. This was consistent with previous reports describing the catalytic activity of [Sc(OTf)3] in the ROP of cyclic esters and CHO.18 Complex 4 proved active in the ROP of ε-CL and CHO, albeit affording low molecular weight oligomers. Finally, 8 allowed for 65% conversion towards a poly-cyclohexene oxide having a Mn of 5770 Da and polydispersity of 1.61. For comparison purposes, the Sc-oxacalix[3]arene complex reported by Hampton et al.11a was tested in the ROP of CHO. Interestingly, although complete monomer conversion was achieved, the Mn of the polymer (1050 Da) was somewhat lower than that isolated with 8. Moreover, MALDI-ToF analysis of the sample indicated the formation of both α-H-ω-OH-terminated and cyclic species (see ESI†).| Run | Mon. | Cat. | Mon.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sc Sc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
Conv.a (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (kDa)
(kDa) |
|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy on the crude reaction mixture. b Determined by GPC. c Reaction in toluene. d Reaction performed at 130 °C. e Reaction under solvent-free conditions. f Reaction performed at 75 °C. n.d. = not determined. Oligom. = low molecular weight oligomers. | |||||
| 1c,d | ε-CL | 1 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — |
| 2d,e | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11 | n.d. | ||
| 3d,e | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — | ||
| 4d,e | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | n.d. | ||
| 5 , | 4 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
77 | Oligom. | |
| 6c,d | 8 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — | |
| 7c,d | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
None | — | ||
| 8d,e | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — | ||
| 9d,e | [Sc(OiPr)3] | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
10 | n.d. | |
| 10d,e | [Sc(OTf)3] | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
>99 | 3.70 | |
| 11d,e | δ-VL | 8 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — |
| 12d,e | r-LA | 8 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — |
| 13e,f | CHO | 4 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
70 | Oligom. |
| 14e,f | 8 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
65 | 5.77 | |
| 15e,f | [Sc(OiPr)3] | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
None | — | |
| 16e,f | [Sc(OTf)3] | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
>99 | 2.07 | |
| 17e,f | Sc-oxacalix11a | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
>99 | 1.05 | |
Cell viability studies
Given there is often catalyst contamination in polymers, we have tested a number of the complexes (1, 2, and 7) against the cancerous cell lines HCT116 and HT-29 in order to evaluate their cytotoxicity. These experiments were carried out over a multi-range of concentrations to determine the amount of compound required to reduce cell growth by 50%. The results showed that the complexes are non-toxic at concentrations likely to persist as contamination in resultant polymers. The IC50 for all complexes fell in the range between 2.23 μM and 14.96 μM (Table 2) which were determined using the cell viability assay; MTS graphs for treatment of HCT116 and HT-29 cells with 1, 2, and 7 are shown in Fig. S13.† To obtain more information on the mechanism of cell death, colorimetric MTS tests were performed. It is worth noting that all complexes at higher concentrations (1.59 to 780 μM) are significantly toxic. However, at lower concentrations (0.78 to 0.00156 μM), all complexes exhibit non-toxicity to all cells tested.| Compound | HCT116 | HT-29 |
|---|---|---|
| 1 | 2.23 ± 3.55 | 11.13 ± 4.63 |
| 2 | 11.27 ± 2.40 | 14.96 ± 1.37 |
| 7 | 2.36 ± 1.74 | 9.50 ± 1.26 |
The relatively higher number of the counted viable cells found at these concentrations after 24 h of treatment indicates that the complexes are safe and can be used for catalytic purposes. From the IC50, it is clear that compounds 1 and 7 are more toxic for colorectal cancer HCT116 than compound 2, evidenced by the IC50 values of 2.36, 2.23, and 11.27, respectively. The IC50 values obtained for compounds 1, 2, and 7 against HT-29 are, 11.13, 14.96, and 9.50 μM respectively. From the obtained cytotoxicity data, it was concluded that these complexes, at specific concentrations, can be safely used for catalysis. For a toxicity comparison versus other metallocalixarenes and cisplatin, see ESI.†
In conclusion, we have isolated and structurally characterized a number of rare examples of scandium calix[n]arenes (n = 4, 6, 8) using either [Sc(OTf)3] or [Sc(OiPr)3] as the entry point. A number of unusual structures have been identified, particularly when employing the tris(isopropoxide) as starting material. The products are relatively non-toxic, and in the case of 4 and 8, are capable of the efficient ROP of cyclohexene oxide, affording poly(CHO) of molecular weight ca. 5700 and with reasonable control (PDI ca. 1.8).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The EPSRC (PRIF grant EP/S025537/1) and the Whitelaw Frater Cancer Trust are thanked for financial support. The Saudi Cultural Bureau is thanked for sponsorship (of AFA and MA). GJS would like to thank both the Medical Research Council (MR/T002573/1) and the Engineering and Physical Sciences Research Council (EP/V027549/1 and EP/T026367/1) for financial support. We also thank the EPSRC National Crystallographic Service Centre at Southampton for data.Notes and references
- A. King, Chem. Ind., 2019, 11, 26–29 Search PubMed
.
- For reviews, see.
(a) B. J. O'Keefe, M. A. Hillmeyer and W. B. Tolman, J. Chem. Soc., Dalton Trans., 2001, 2215–2224 RSC
; O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 Search PubMed
; (b) M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC
; (c) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC
; (d) A. Arbaoui and C. Redshaw, Polym. Chem., 2010, 1, 801–826 RSC
; (e) Y. Sarazin and J.-F. Carpentier, Chem. Rev., 2015, 115, 3564–3614 CrossRef CAS PubMed
and references therein.
- C. Redshaw, Catalysts, 2017, 7, 165–178 CrossRef
.
- D. H. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086–5130 CrossRef CAS PubMed
.
-
(a) M. Frediani, D. Sémeril, A. Marrioti, L. Rosi, P. Frediani, L. Rosi, D. Matt and L. Toupet, Macromol. Rapid Commun., 2008, 29, 1554–1560 CrossRef CAS
; (b) M. Frediani, D. Sémeril, D. Matt, L. Rosi, P. Frediani, F. Rizzolo and A. M. Papini, Int. J. Polym. Sci., 2010, 2010, 490724 Search PubMed
; (c) J. D. Ryan, K. J. Gagnon, S. J. Teat and R. D. McIntosh, Chem. Commun., 2016, 52, 9071–9073 RSC
.
- Y. Li, K.-Q. Zhao, C. Feng, M. R. J. Elsegood, T. J. Prior, X. Sun and C. Redshaw, Dalton Trans., 2014, 43, 13612–13619 RSC
.
- Z. Sun, Y. Zhao, T. J. Prior, M. R. J. Elsegood, K. Wang, T. Xing and C. Redshaw, Dalton Trans., 2019, 48, 1454–1466 RSC
.
- P. M. Zeimentz, S. Arndt, B. R. Elvidge and J. Okuda, Chem. Rev., 2006, 106, 2404–2433 CrossRef CAS PubMed
.
-
(a) Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef CAS
; (b) A. Oral, M. A. Tasdelen, A. L. Demirel and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5328–5335 CrossRef CAS
.
-
(a) S. Klaus, M. W. Lehenmeier, C. E. Anderson and B. Rieger, Coord. Chem. Rev., 2011, 255, 1460–1479 CrossRef CAS
; (b) M. Taherimehr and P. P. Pescarmona, J. Appl. Polym. Sci., 2014, 131, 41141 CrossRef
; (c) G. Trott, P. K. Saini and C. K. Williams, Philos. Trans. R. Soc., A, 2016, 374, 20150085 CrossRef PubMed
; (d) Y. Wang and D. J. Darensbourg, Coord. Chem. Rev., 2018, 372, 85–100 CrossRef CAS
; (e) C. M. Kozak, K. Ambrose and T. S. Anderson, Coord. Chem. Rev., 2018, 376, 565–587 CrossRef CAS
.
-
(a) P. D. Hampton, C. E. Daitch and E. N. Duesler, Inorg. Chem., 1995, 34, 5641–5645 CrossRef
; (b) Y. Masuda, Y. Zhang, C. Yan and B. Li, J. Alloys Compd., 1998, 275–277, 872–876 CrossRef CAS
; (c) H. R. Webb, M. J. Hardie and C. L. Raston, Chem. – Eur. J., 2001, 7, 3616–3620 CrossRef CAS PubMed
; (d) F. Estler, E. Herdtweck and R. Anwander, J. Chem. Soc., Dalton Trans., 2002, 3088–3089 RSC
.
- See. M. C. Bernini, N. Snejko, E. Gutierrez-Puebla and A. Monge, CrystEngComm, 2011, 13, 1797–1800 RSC
and references therein.
-
(a) H. Ma, T. P. Spaniol and J. Okuda, Angew. Chem., Int. Ed., 2006, 45, 7818–7821 CrossRef PubMed
; (b) A. Takasu, M. Oshimura and T. Hirabayashi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2300–2304 CrossRef CAS
; (c) M. Oshimura, A. Takasu and K. Nagata, Macromolecules, 2009, 42, 3086–3091 CrossRef CAS
; (d) E. Grunova, E. Kirillov, T. Roisnel and J.-F. Carpentier, Dalton Trans., 2010, 39, 6739–6752 RSC
; (e) J.-C. Buffet and J. Okuda, Dalton Trans., 2011, 40, 7748–7754 RSC
; (f) Y. Chapurina, J. Klitzke, O. d. L. Casagrande Jr., M. Awada, V. Dorcet, E. Kirillov and J.-F. Carpentier, Dalton Trans., 2014, 43, 14322–14333 RSC
; (g) C. Bakewell, A. J. P. White, N. J. Long and C. K. Williams, Inorg. Chem., 2015, 54, 2204–2212 CrossRef CAS PubMed
; (h) Y. Cui, W. Gu, Y. Wang, B. Zhao, Y. Yao and Q. Shen, Catal. Sci. Technol., 2015, 5, 3302–3312 RSC
; (i) J. El Haj Hassan, V. Radkov, V. Dorcet, J.-F. Carpentier and E. Kirillov, J. Organomet. Chem., 2016, 823, 34–39 CrossRef CAS
; (j) H. Xie, C. Wu, D. Cui and Y. Wang, J. Organomet. Chem., 2018, 875, 5–10 CrossRef CAS
; (k) T. P. Seifert, T. S. Brunner, T. S. Fischer, C. Barner-Kowollik and P. W. Roesky, Organometallics, 2018, 37, 4481–4487 CrossRef CAS
; Y. Pan, W. Li, N.-N. Wei, Y.-M. So, X. Lai, Y. Li, K. Jiang and G. He, Dalton Trans., 2019, 48, 9079–9088 Search PubMed
.
- J. Ling, L. You, Y. Wang and Z. Shen, J. Appl. Polym. Sci., 2012, 124, 2537–2540 CrossRef CAS
.
-
(a) J. Ling, W. Zhu and Z. Shen, Macromolecules, 2004, 37, 758–763 CrossRef CAS
; (b) W. Zhu, J. Ling and Z. Shen, Polym. Bull., 2004, 52, 185–189 CrossRef CAS
; (c) P. F. Gou, P. W. Zhu and Z. Q. Shen, Sci. China, Ser. B: Chem., 2007, 50, 648–653 CrossRef CAS
.
- The same structural motif is commonly found in other first row TM calix[4]arene complexes. See for example:
(a) S. M. Taylor, R. D. McIntosh, C. M. Beavers, S. J. Teat, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 1440–1442 RSC
; (b) S. M. Taylor, G. Karotsis, R. D. McIntosh, S. Kennedy, S. J. Teat, C. M. Beavers, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. – Eur. J., 2011, 17, 7521–7530 CrossRef CAS PubMed
.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC
.
-
(a) N. Nomura, A. Taira, T. Tomioka and M. Okada, Macromolecules, 2000, 33, 1497–1499 CrossRef CAS
; (b) N. Nomura, A. Taira, A. Nakase, T. Tomioka and M. Okada, Tetrahedron, 2007, 63, 8478–8484 CrossRef CAS
; (c) M. Möller, R. Kånge and J. L. Hedrick, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2067–2074 CrossRef
; (d) J. Ling, W. Zhu and Z. Shen, Macromolecules, 2004, 37, 758–763 CrossRef CAS
; (e) M. Oshimura, A. Takasu and K. Nagata, Macromolecules, 2009, 42, 3086–3091 CrossRef CAS
; (f) J. Ling, L. You, Y. Wang and Z. Shen, J. Appl. Polym. Sci., 2012, 124, 2537–2540 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Chem Draws, alternative views and crystallographic data for 1–8; synthetic details; MTS graphs for 1, 2, and 7. CCDC 2050782–2050789 and 2051060 for 1–8. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt01330k |
| This journal is © The Royal Society of Chemistry 2021 |