The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: a systematic review and meta-analysis†
Yali
Wei
a,
Yan
Meng
b,
Na
Li
 c,
Qian
Wang
b and
Liyong
Chen
c,
Qian
Wang
b and
Liyong
Chen
 *ab
*ab
aDepartment of Toxicology and Nutrition, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China. E-mail: chenle73@sina.com; weiyali0811@163.com; Tel: (+86)15168867157
bDepartment of Nutrition, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China. E-mail: mengsandy@aliyun.com; wangqian.0821@163.com
cInstitute of Agro-Food Science and Technology, Shandong Academy of Agricultural Sciences/Shandong Provincial Food for Special Medical Purpose Engineering Technology Research Center/Key Laboratory of Agro-Products Processing Technology of Shandong Province/Key Laboratory of Novel Food Resources Processing, Ministry of Agriculture and Rural Affairs, Jinan, China. E-mail: 2803729865@qq.com
First published on 24th November 2020
Abstract
Objective: The purpose of the systematic review and meta-analysis was to determine if low-ratio n-6/n-3 long-chain polyunsaturated fatty acid (PUFA) supplementation affects serum inflammation markers based on the current studies. Methods: PubMed, Embase and The Cochrane library databases were systematically searched to find randomized controlled trials (RCTs) on the effect of low-ratio n-6/n-3 PUFA intervention on inflammation markers up to July 2020. Data were pooled using standardized mean difference (SMD) and 95% confidence intervals (95% CI), with P value ≦ 0.05 as statistical significance. Results: Thirty-one RCTs were included in the meta-analysis. The analysis indicated that increasing low-ratio n-6/n-3 PUFA supplementation decreased the level of tumor necrosis factor-α (TNF-α) (SMD = −0.270; 95% CI: −0.433, −0.106; P = 0.001) and interleukin 6 (IL-6) (SMD = −0.153; 95% CI: −0.260, −0.045; P = 0.005). There were no significant effects on C-reactive protein (CRP) (SMD = −0.027; 95% CI: −0.189: 0.135; P = 0.741). Subgroup analysis indicated that there was a significant reduction in TNF-α serum concentration in subjects from Asia (SMD: −0.367; 95% CI: −0.579, −0.155; P = 0.001) and in subjects with diseases (SMD: −0.281; 95% CI: −0.436, −0.127; P < 0.001). In the subgroup of the n-6/n-3 ratio ≦5, low-ratio n-6/n-3 PUFA supplementation could decrease the level of TNF-α (SMD: −0.335; 95% CI: −0.552, −0.119; P = 0.002). Serum IL-6 decreased significantly in patients from the Europe subgroup (SMD: −0.451; 95% CI: −0.688, −0.214; P < 0.001), but not in Asia (SMD: −0.034; 95% CI: −0.226, 0.157; P = 0.724), North America (SMD: −0.115; 95% CI: −0.274, 0.044; P = 0.157) and Oceania (SMD: 0.142; 95% CI: −0.557, 0.842; P = 0.690). Conclusion: Low-ratio n-6/n-3 PUFA supplementation could decrease significantly the concentration of serum TNF-α and IL-6, but not decrease CRP concentration.
Introduction
The inflammatory response is an important part of the human immune system. The human body resists external stimulation by producing massive inflammatory cells and secreting inflammatory factors. Inflammatory mediators play a dominant and antagonistic role in the homeostasis of organisms. However, strong inflammatory responses impair normal physiological activities. Chronic inflammation is considered an underlying pathological condition.1 Inflammation is a key factor in the pathogenesis of many chronic diseases.2 Inflammatory cells secrete excessive inflammatory mediators, such as TNF-α, IL-6 and CRP involved in the inflammatory response. When inflammatory mediators are present for a long time, they can cause a variety of chronic diseases and even cancer.3,4As important members of PUFA, omega-6 fatty acid (ω-6 fatty acid or n-6 fatty acid) and omega-3 fatty acid (ω-3 fatty acid or n-3 fatty acid) have attracted much attention in recent years. Among omega-3 fatty acids, there are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-6 fatty acids include linoleic acid (LA), γ-linolenic acid (GLA) and arachidonic acid. In recent years, many scholars have studied the effects of omega-3 and omega-6 fatty acids on inflammatory factors. Many studies have documented that EPA and DHA are anti-inflammatory and promote inflammation regression.5–7 Some studies have found no effects of omega-3 fatty acid on inflammatory markers.8 A higher ratio of n-6/n-3 PUFA increases the risk of obesity, further increasing the levels of inflammatory factors.9 A previous RCT indicated that supplementation with a low n-6/n-3 PUFA ratio had no effect on inflammatory markers.10 There is no consensus on the effects of supplementation with omega-3 and omega-6 fatty acids on inflammatory markers.
There is a competitive inhibition relationship between derivatives of omega-6 and omega-3 fatty acids. The mechanism of interactions is unclear between omega-3 and omega-6 fatty acids in the human body. It is more practical to research the effects of the ratio of omega-6 to omega-3 fatty acids on inflammation. The purpose of this study was to investigate whether supplementation with a low n-6/n-3 PUFA ratio was beneficial to reduce the level of inflammatory markers.
Methods
Search strategy
Three databases (PubMed, Embase and the Cochrane Library) were exhaustively retrieved to find relevant articles using the following term: “n-6/n-3 fatty acid ratio” OR “n-6/n-3 PUFA” OR “omega-3 fatty acid” OR “n-3 PUFA” OR “n-6 PUFA” OR “omega-6 fatty acid” OR “Docosahexaenoic Acid” OR “DHA” OR “eicosapentaenoic acid” OR “EPA” OR “α-linolenic acid” OR “fish oil” paired with the following: inflammation OR inflammatory OR “inflammatory factors” OR “C reactive protein” OR “C-reactive protein” OR “high-sensitivity C-reactive protein” OR hs-CRP OR interleukin-6 OR “interleukin 6” OR IL-6 OR “tumor necrosis factor-”OR “tumor necrosis factor” OR TNF-α. References from experimental papers and review were searched to find the missing target studies.Inclusion and exclusion criteria
The inclusion and exclusion criteria of selected articles were as follows: (1) the study was a randomized controlled or randomized crossover population trial; (2) the subjects were adults, excluding infants and children; (3) in terms of intervention type, oral feeding was included, whereas enteral nutrition and intravenous input were excluded; (4) the n-6/n-3 ratio was provided in the article, or the ratio could be obtained by calculation; (5) at least one of three inflammatory factors TNF-α, IL-6 and CRP, the mean and standard deviation were provided in the article; (6) non-original studies, repetitive articles and non-English articles were excluded.Data extraction and quality assessment
Data extraction was carried out by two researchers independently according to the inclusion criteria. If there was any controversy, the original literature was checked again, and the third researcher would jointly negotiate to solve the problem when no consensus could be reached. The basic information was extracted, including the author, year, region, population and basic information about the subjects (age, sex, body mass index (BMI)). For trails, the following information was extracted in detail: the number of participants in the experimental group and control group, whether the participants were healthy and what diseases they suffered from, intervention duration and n-6/n-3 PUFA ratio.Cochrane Collaboration's tool was used to evaluate the quality of the included articles. The content of assessment included seven aspects: random sequence generation, allocation concealment, blinding, observation bias, loss to follow-up, selective reporting and other bias.
Statistical analysis
Stata (version 12.0; Stata Corporation, College Station, TX) and RevMan software (version 5.1; Cochrane Collaboration, Oxford, United Kingdom) were used to evaluate the effects of low-ratio n-6/n-3 PUFA intervention on the indicators of three inflammatory factors. SMD and 95% CI were used to compare effect sizes between trials due to various measurements applied in the included studies. Specifically, the result was considered significant at P ≤ 0.05. I2 statistics were conducted to evaluate the degree of heterogeneity. I2 > 50% suggested significant heterogeneity between the selected studies, and the random-effects model was used for data analysis. Otherwise, the fixed-effects model was used. Sensitivity analyses were carried to identify sources of heterogeneity.11 In this study, subgroup analyses were conducted according to the different classifications of region, physical condition and n-6/n-3 PUFA ratio. Meta-regression was used to investigate whether there was any significant linear relationship between effects and duration or ratio. Egger's test and Begg's funnel plots were used to assess potential publication bias.12Results
Search results and study characteristics
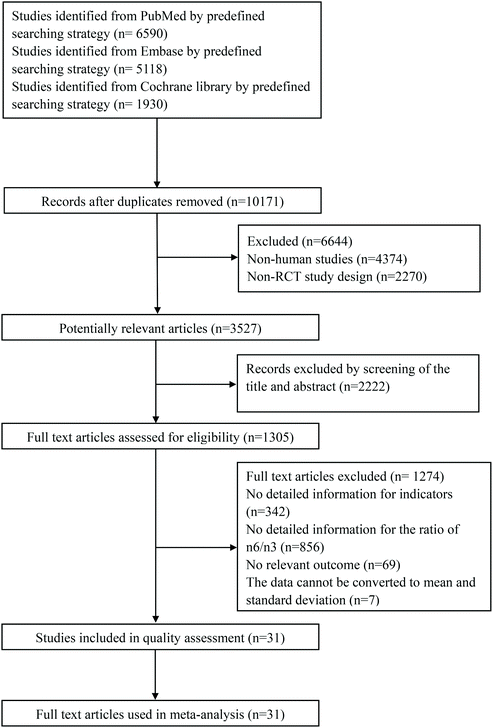
A flow chart of the retrieval strategy is shown in Fig. 1. After searching three databases and removing repeated papers, 10,171 papers were found. There were 3527 potentially relevant articles after excluding non-human studies and non-RCT, and 1305 articles assessed for eligibility by screening of the title and abstract. After the full text was read and a quality assessment conducted, 31 studies finally met the inclusion and exclusion criteria.The detailed features of the included studies are presented in Table 1. Selected articles were published from 2003 to 2019: five articles from the USA;13–17 nine articles from Canada,18–20 Iran21–23 and Greece24–26 (three articles each); 12 articles from Spain,27,28 Denmark,29,30 Germany,31,32 UK,33,34 Australia35,36 and China37,38 (two articles each); five articles from Japan,39 New Zealand,40 Norway,41 Italy42 and Croatia43 (one article each). The 31 included trials included a total of 1450 participants ranging in age from 18 to 76. Some subjects were healthy, and some suffered from diseases. The types of disease included metabolic syndrome, hyperlipidemia, rheumatoid arthritis, coronary artery disease, gestational diabetes, polycystic ovary syndrome, type 2 diabetes, hemodialysis, dyslipidemia, obesity, ischemic stroke, hypertriacylglycerolemia and coronary heart disease. Duration ranged from 35 days to 24 weeks. There were two comparisons in the paper by Chiang, Y. L. et al.13 and Baril-Gravel, L. et al.,18 respectively. Kaul, N. et al.,20 Wallace, Fiona A. et al.34 and Zhang, J. et al.37 each had three randomized, parallel controlled trials. Four groups of RCTs involving 123 subjective were carried out simultaneously in the paper by Zhou, Q. et al.38
| Study | Year | Country | Design | Population | Participants | Duration | n-6/n-3 | Indicators |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: CAD: coronary artery disease; CHD: coronary heart disease; CRP: c-reactive protein; D: day; DM2: type 2 diabetes mellitus; GDM: gestational diabetes; HD: hemodialysis; HLP: hypercholesterolemia; IL-6: interleukin-6; MetS: metabolic syndrome; PCOS: polycystic ovary syndrome; RA: rheumatoid arthritis; RCCT: randomized controlled cross trial; RCrT: randomized crossover trial; RCT: randomized controlled trial; RPT: randomized parallel trial. | ||||||||
| Baril-Gravel | 2014 | Canada | RCCT | 114/114 | MetS | 4 W | 1.6/46.7;2.4/46.7 | IL-6, CRP |
| Capo | 2014 | Spain | RCT | 9/6 | Healthy | 8 W | 1.079/8.39 | TNF-α, IL-6 |
| Chiang | 2012 | USA | RCCT | 25/25 | HLP | 4 W | 4.7/9.4; 5.1/9.4 | TNF-α, IL-6, CRP |
| Cornish | 2018 | Canada | RCT | 11/12 | Healthy | 12 W | 6.49/9.16 | TNF-α, IL-6 |
| Damsgaard | 2008 | Denmark | RPT | 14/17 | Healthy | 8 W | 1.51/7.33 | IL-6, CRP |
| Dawczynski | 2018 | Germany | RCCT | 25/25 | RA | 10 W | 0.92/5.66 | CRP |
| Dawczynski | 2013 | Germany | RCT | 17/24; 17/14 | HLP | 10 W | 4.1/7.21;1.91/7.21 | CRP |
| Agh | 2017 | Iran | RCT | 24/21 | CAD | 8 W | 94.88/145.45 | CRP |
| Hallund | 2010 | Denmark | RPT | 23/22 | Healthy | 8 W | 0.16/3.33 | IL-6, CRP |
| Han | 2012 | USA | RCrT | 18/18 | High LDL | 35 D | 2.73/7.81 | CRP |
| Jamilian | 2016 | Iran | RCT | 27/27 | GDM | 6 W | 18.54/123.5 | CRP |
| Kalgaonkar | 2011 | USA | RCT | 17/14 | PCOS | 6 W | 4.61/22.06 | TNF-α, IL-6, CRP |
| Kaul | 2008 | Canada | RCT | 22/22 | Healthy | 12 W | 0.05/46;0.3/46;3/46 | TNF-α, CRP |
| Kondo | 2014 | Japan | RCrT | 23/23 | DM2 | 4 W | 2.2/6.4 | CRP |
| Kontogianni | 2013 | Greece | RCrT | 37/37 | Healthy | 6 W | 1.4/8.3 | TNF-α, CRP |
| Kooshki | 2011 | Iran | RCT | 17/17 | HD | 10 W | 4.65/128.57 | TNF-α, IL-6, CRP |
| Martorell | 2014 | Spain | RCT | 9/6 | Healthy | 8 W | 1.079/8.39 | CRP |
| Minihane | 2005 | UK | RPT | 15/14 | Healthy | 6 W | 9/16 | CRP |
| Munro | 2012 | Australia | RCT | 18/14 | Obesity | 4 W | 4.33/8.47 | TNF-α, IL-6, CRP |
| Murphy | 2007 | Australia | RCT | 38/36 | Overweight | 24 W | 3.97/6.72 | CRP |
| Paschos | 2007 | Greece | RPT | 18/17 | Dyslipidemic | 12 W | 0.27/148.8 | TNF-α |
| Poppitt | 2009 | New Zealand | RCT | 47/48 | Ischemic stroke | 12 W | 0.28/8.97 | CRP |
| Rallidis | 2003 | Greece | RPT | 50/26 | Dyslipidemic | 12 W | 1.3/13.2 | IL-6, CRP |
| Seierstad | 2005 | Norway | RPT | 20/19 | CHD | 6 W | 0.1/1.64 | TNF-α, IL-6, CRP |
| Sofi | 2013 | Italy | RCrT | 20/20 | Healthy | 10 W | 0.44/1.27 | TNF-α, IL-6 |
| Stupin | 2018 | Croatia | RCT | 20/16 | Healthy | 3 W | 2.63/7.29 | CRP |
| Lee | 2014 | USA | RPT | 21/16 | DM2 | 8 W | 1.3/10.2 | CRP |
| Vargas | 2011 | USA | RCT | 17/17 | PCOS | 6 W | 0.01/7.27 | CRP |
| Wallace | 2007 | UK | RCT | 8/8 | Healthy | 12 W | 3.03/8.13;5.53/8.13;3.6/8.13 | TNF-α, IL-6 |
| Zhang | 2012 | China | RCT | 32/33; 32/33; 29/33 | Hypertriacylglycerolemia | 8 W | 4.6/15.1; 5.4/15.1; 7.3/15.1 | TNF-α, IL-6, CRP |
| Zhou | 2019 | China | RCT | 23/24; 25/24; 25/24; 26/24 | HLP | 12 W | 6.98/14.93; 4.53/14.93; 3.5/14.93; 2.03/14.93 | TNF-α, IL-6 |
The quality-assessment results are presented in Table 2. All 31 articles used a randomized controlled approach, most using single- or double-blind studies and concealed supplement allocation. Observation bias and loss to follow-up bias were not found in most studies. No other sources of bias were identified in most studies.
| Study | Random sequence generation | Allocation concealment | Blinding | Observation bias | Loss to follow-up | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Baril-Gravel | Yes | Unclear | Unclear | No | Yes | No | No |
| Capo | Yes | Yes | Unclear | Unclear | Yes | No | No |
| Chiang | Yes | Unclear | Yes | No | Yes | No | No |
| Cornish | Yes | Yes | Yes | No | Yes | No | No |
| Damsgaard | Yes | Yes | Yes | No | Yes | No | No |
| Dawczynski | Yes | Yes | Yes | No | Yes | No | No |
| Dawczynski | Yes | Yes | Yes | No | Yes | No | No |
| Agh | Yes | Yes | Yes | No | Yes | No | No |
| Hallund | Yes | Yes | Yes | No | Yes | No | No |
| Han | Yes | Yes | Yes | No | Unclear | Unclear | Unclear |
| Jamilian | Yes | Yes | Yes | No | Yes | No | No |
| Kalgaonkar | Yes | Yes | Yes | No | Yes | No | No |
| Kaul | Yes | Yes | Yes | No | Yes | No | No |
| Kondo | Yes | No | No | Unclear | Yes | No | Unclear |
| Kontogianni | Yes | Yes | Yes | Unclear | Yes | No | Unclear |
| Kooshki | Yes | Yes | Yes | No | Unclear | Unclear | Unclear |
| Martorell | Yes | Yes | Unclear | Unclear | Yes | No | No |
| Minihane | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear |
| Munro | Yes | Yes | Yes | No | Yes | No | No |
| Murphy | Yes | Yes | Yes | No | Yes | No | No |
| Paschos | Yes | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| Poppitt | Yes | Yes | Yes | No | Yes | No | No |
| Rallidis, | Yes | No | No | Yes | No | No | Yes |
| Seierstad | Yes | Yes | Yes | No | Yes | No | No |
| Sofi | Yes | Unclear | Unclear | Unclear | Yes | No | No |
| Stupin | Yes | Yes | Yes | No | Unclear | Unclear | No |
| Lee | Yes | Unclear | Yes | No | Yes | No | No |
| Vargas | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Unclear |
| Wallace | Yes | Yes | Yes | No | Yes Yes | No | No |
| Zhang | Yes | Yes | Yes | No | Yes | No | No |
| Zhou | Yes | Yes | Yes | No | Yes | No | No |
Effects of low-ratio n-6/n-3 PUFA on inflammatory biomarkers
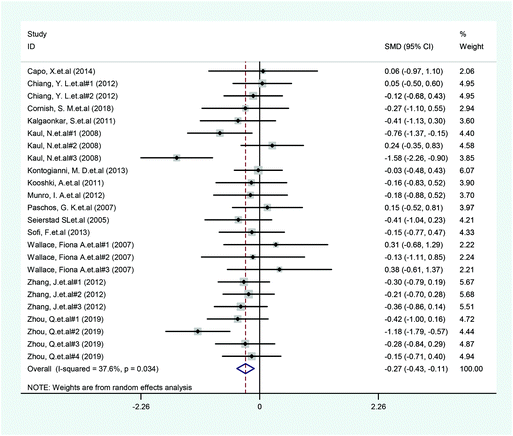
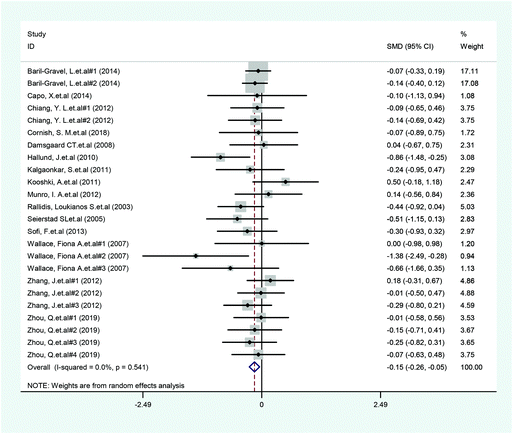
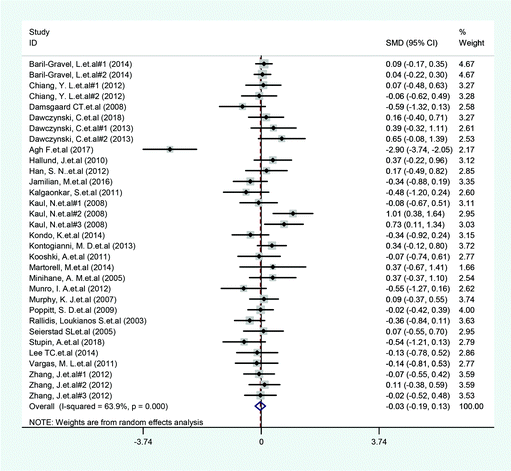
A total of 14 included articles with 24 effect values investigated the effect of low-ratio n-6/n-3 PUFA supplementation on TNF-α. As shown in Fig. 2, the experimental group with low-ratio n-6/n-3 PUFA supplementation could significantly reduce the serum concentration of TNF-α compared with the control group with placebo (SMD = −0.270; 95% CI: −0.433, −0.106; P = 0.001), with a lower heterogeneity (I2 = 37.6%). Results for IL-6 were reported in 15 eligible studies, which resulted in a total of 24 comparisons (Fig. 3). Overall, the meta-analysis showed that serum IL-6 concentration in the experimental group was significantly lower than that in the control group, with statistical significance (SMD = −0.153; 95% CI: −0.260, −0.045; P = 0.005). In addition, IL-6 did not reveal any significant heterogeneity among the trials (I2 = 0.0%). However, 24 trails with 32 comparisons showed that low-ratio n-6/n-3 PUFA supplementation did not affect the level of CRP (SMD = −0.027; 95% CI: −0.189, 0.135; P = 0.741). The tests for heterogeneity were highly significant (I2 = 63.9%) (Fig. 4).Sensitivity analysis and subgroup analysis
A sensitivity analysis was performed to identify the sources of high heterogeneity. As shown in the ESI,† the sensitivity analysis did not find that the removal of any study could change the effect of low-n-6/n-3 PUFA supplementation on CRP. A subgroup analysis was carried out by stratifying region, duration, health status and n-6/n-3 PUFA ratio to investigate further the effects of low-ratio n-6/n-3 PUFA supplementation on the three inflammatory markers (Table 3).| Variables | Subgroups | Number | SMD (95% CI) | P | |
|---|---|---|---|---|---|
| TNF-α | Region | Europe | 8 | −0.035 (−0.283, 0.214) | 0.784 |
| Asia | 8 | −0.367 (−0.579, −0.155) | 0.001 | ||
| North America | 7 | −0.394 (−0.842, 0.054) | 0.085 | ||
| Oceania | 1 | −0.175 (−0.875, 0.524) | 0.623 | ||
| Participants | Health | 10 | −0.230 (−0.614, 0.147) | 0.232 | |
| Diseases | 14 | −0.281 (−0.436, −0.127) | <0.001 | ||
| Duration | ≦8 weeks | 10 | −0.194 (−0.374, −0.015) | 0.034 | |
| >8 weeks | 14 | −0.320 (−0.608, −0.032) | 0.029 | ||
| Ratio | >5 | 8 | −0.137 (−0.357, 0.084) | 0.224 | |
| ≦5 | 16 | −0.335 (−0.552, −0.119) | 0.002 | ||
| IL-6 | Region | Europe | 9 | −0.451 (−0.688, −0.214) | <0.001 |
| Asia | 8 | −0.034 (−0.226, 0.157) | 0.724 | ||
| North America | 6 | −0.115 (−0.274, 0.044) | 0.157 | ||
| Oceania | 1 | 0.142 (−0.557, 0.842) | 0.690 | ||
| Participants | Health | 8 | −0.394 (−0.715, −0.073) | 0.016 | |
| Diseases | 16 | −0.111 (−0.228, 0.005) | 0.060 | ||
| Duration | ≦8 weeks | 13 | −0.135 (−0.264, −0.007) | 0.039 | |
| >8 weeks | 11 | −0.194 (−0.404, 0.016) | 0.071 | ||
| Ratio | >5 | 8 | −0.204 (−0.421, 0.013) | 0.065 | |
| ≦5 | 16 | −0.128 (−0.284, 0.027) | 0.106 | ||
| CRP | Region | Europe | 12 | 0.077 (−0.137, 0.290) | 0.482 |
| Asia | 7 | −0.460 (−1.022, 0.102) | 0.109 | ||
| North America | 11 | 0.108 (−0.088, 0.304) | 0.280 | ||
| Oceania | 2 | −0.172 (−0.793, 0.449) | 0.588 | ||
| Participants | Health | 9 | 0.228 (−0.114, 0.571) | 0.191 | |
| Diseases | 23 | −0.112 (−0.291, 0.067) | 0.220 | ||
| Duration | ≦8 weeks | 22 | −0.143 (−0.344, 0.058) | 0.164 | |
| >8 weeks | 10 | 0.210 (−0.050, 0.471) | 0.114 | ||
| Ratio | >5 | 15 | 0.064 (−0.081, 0.209) | 0.388 | |
| ≦5 | 17 | −0.144 (−0.444, 0.156) | 0.347 |
A subgroup analysis of the TNF-α by region classification showed significant differences in the studies from Asia and no differences from other continents. In Asia, low-ratio n-6/n-3 PUFA supplementation significantly reduces the level of serum TNF-α (SMD: −0.367; 95% CI: −0.579, −0.155; P = 0.001). However, in Europe, North America, and Oceania, the serum TNF-α concentration did not show a significant reduction (SMD: −0.035, 95% CI: −0.283, 0.214; P = 0.784; SMD: −0.394, 95% CI: −0.842, 0.054; P = 0.085; SMD: −0.175, 95% CI: −0.875, 0.524; P = 0.623). The included studies stratified by health status indicated that low-ratio n-6/n-3 PUFA supplementation led to lower serum levels of TNF-α in participants who suffered from disease (SMD: −0.281; 95% CI: −0.436, −0.127; P < 0.001), with statistically significance. However, supplementation did not reduce TNF-α levels in healthy subjects (SMD: −0.230; 95% CI: −0.614, 0.147; P = 0.232). A stratified analysis was conducted according to whether the ratio of n-6/n-3 PUFA supplementation was >5. The results showed that when the ratio of n-6/n-3 PUFA was ≦5, the difference between the control group and the experimental group was statistically significant (SMD: −0.335; 95% CI: −0.552, −0.119; P = 0.002). When the ratio was >5, there was no statistical significance (SMD: −0.137; 95% CI: −0.357, 0.084; P = 0.224) (Table 3).
The studies stratified by region indicated that the pooled effect showed a significant reduction in the IL-6 level in Europe (SMD: −0.451; 95% CI: −0.688, −0.214; P < 0.001), not in Asia, North America and Oceania (SMD: −0.034, 95% CI: −0.226, 0.157, P = 0.724; SMD: −0.115, 95% CI: −0.274, 0.044, P = 0.157; SMD: 0.142, 95% CI: −0.557, 0.842, P = 0.690). A subgroup analysis showed that the effect of low-ratio n-6/n-3 PUFA on the reduction in IL-6 level in the healthy population (SMD: −0.394; 95% CI: −0.715, −0.073; P = 0.016) was more obvious than in patients (SMD: −0.111; 95% CI: −0.228, −0.005; P = 0.060). As for the stratification of duration, the extraction results showed that there was a significant difference between the experimental group with low-ratio n-6/n-3 PUFA and the control group with placebo when the duration was ≦8 weeks (SMD:-0.135; 95% CI: −0.264, −0.007; P = 0.039). There was no significant difference between the two groups when the duration was >8 weeks (SMD: −0.194; 95% CI: −0.404, 0.016; P = 0.071) (Table 3). The subgroup analysis of CRP was not statistically significant (Table 3).
Meta-regression and publication bias
The duration of low-ratio n-6/n-3 PUFA supplementation ranged from 35 days to 24 weeks in the included studies. We conducted a meta-regression analysis and found that there was no linear relationship between TNF-α concentration and duration. Similarly, IL-6 was not linearly related to duration. The same results were found in the meta-regression of ratio and inflammation markers (Table 4).| b | SE | t | P | 95% CI | |
|---|---|---|---|---|---|
| TNF-α | |||||
| Duration | −0.030 | 0.282 | −1.07 | 0.295 | (−0.089, 0.028) |
| Ratio | 0.000 | 0.000 | −0.56 | 0.584 | (−0.001, −0.000) |
| IL-6 | |||||
| Duration | −0.013 | 0.017 | −0.82 | 0.423 | (−0.048, 0.021) |
| Ratio | 0.002 | 0.005 | 0.31 | 0.761 | (0.009, 0.012) |
Begg's test and Egger's test found no publication bias in TNF-α (PBegg = 0.785, PEgger = 0.729), IL-6 (PBegg = 0.333, PEgger = 0.307) and CRP (PBegg = 0.466, PEgger = 0.337) (ESI†).
Discussion
To the best of our knowledge, this article is the first meta-analysis to investigate the effects of low-ratio n-6/n-3 PUFA supplementation on inflammatory factors. A total of 31 RCTs involving 1450 participants were included in this meta-analysis. The results showed that low-ratio n-6/n-3 PUFA supplementation significantly reduced serum TNF-α and IL-6 concentrations.The subgroup analysis indicated that the effect of low-ratio n-6/n-3 PUFA on the reduction in TNF-α level in Asian countries was more obvious than in other countries. Low-ratio n-6/n-3 PUFA supplementation significantly decreased serum IL-6 concentration in Europe, but not in other regions. Eating habits vary from region to region, not only in terms of nutrients, but also in terms of dietary patterns that affect changes in markers of inflammation. The effects of dietary habits on markers of inflammation are also inconsistent.44 Meta-analyses have also found that supplementation with omega-3 fatty acids has different effects on blood sugar between Asians and Europeans, which may be due not only to dietary habits, but also to ethnic and environmental differences.45 Similarly, the effect of low-ratio n-6/n-3 PUFA supplementation on serum inflammatory markers may also vary from region to region.
In the subgroup analysis, participants were divided into a healthy group and a disease group according to their physical health status. A diet with low-ratio n-6/n-3 PUFA supplementation significantly reduced TNF-α levels in sick individuals but not in healthy individuals. Studies have shown that supplementation with DHA and EPA significantly reduced CRP concentrations, especially in subjects with dyslipidemia and higher baseline CRP concentrations.46 We speculated that in the patients, the inflammatory factor level was higher, and the effect of low-ratio n-6/n-3 PUFA supplementation was more obvious. The pooled effect of serum TNF-α concentration stratified by health status could further illustrate the effect of low-ratio n-6/n-3 PUFA supplementation on inflammatory markers. Therefore, in an inflammatory state, the interaction between omega-3 and omega-6 is complex. High levels of n-6 fatty acids could counteract the anti-inflammatory effects of n-3 fatty acids.47 Omega-6 and omega-3 fatty acids compete for the biological synthase, causing different physiological effects on the body. There is a balance between n-6 and n-3 PUFA.48,49 With respect to IL-6, there was a statistically significant decrease in healthy individuals, but not in patients. A meta-analysis showed that supplementation of omega-3 fatty acids alone could not reduce inflammation levels in patients with renal disease.50 Omega-3 fatty acids reduced CRP levels but did not reduce IL-6 levels in patients undergoing dialysis.51 The anti-inflammatory effects on colorectal cancer were also different at different doses and durations.52 It takes more than 1 g d−1 of omega-3 fatty acids to reduce inflammation in patients with heart failure.53 The effects of low n-6/n-3 PUFA supplementation on inflammatory factors need to be refined further for different diseases, as well as for different doses of intake. On the other hand, it may also be shown that diets with a low-ratio n-6/n-3 PUFA can help reduce the levels of inflammatory factor and prevent inflammation-related diseases in healthy men.
The studies stratified by duration indicated that TNF-α was significantly reduced regardless of whether the duration was longer than or less than 8 weeks, whereas the change in CRP level was not statistically significant. There was a significant decrease in serum IL-6 levels within 8 weeks of low-ratio n-6/n-3 PUFA supplementation, although there was no significant decrease when the duration was beyond 8 weeks. Molecular biology studies have shown that n-3 PUFA, n-6 PUFA and their derivatives can target transcription factors to regulate gene expression and participate in the process of inflammation regression by modifying cell-membrane composition.54 An RCT showed that supplementation with a low ratio of n-6/n-3 PUFA at 26 weeks altered gene expression and reduced the expression of inflammation-related genes, which suggests that long-term supplementation of low-ratio n-6/n-3 PUFA could reduce the incidence of inflammation.55 Regarding the effect of IL-6 stratified by duration, further analysis revealed that most of the studies of >8 weeks’ duration came from Asian. The pooled effect of a significant reduction in the ≦8 weeks subgroup may be due to confounding factors caused by regional differences. Subgroup analysis showed that there was a significant decrease in TNF-α when the ratio of n-6/n-3 PUFA was no higher than 5. Some derivatives of n-6 fatty acids, such as endogenous cannabinoids, target NF-κB to participant in the inflammation response. The concentration of endocannabinoids is influenced by a dietary intake of omega-6 and omega-3 fatty acids. Diets with a high omega-6 fatty acid content can lead to an overactive endocannabinoid system. Diets with a high omega-6/omega-3 ratio lead to an increase in endocannabinoid signaling and related agents, leading to an inflammatory state.56 It is particularly important to find an appropriate ratio of n-6/n-3 PUFA. The results in this paper suggest that a ratio of no higher than 5 is more conducive to reducing the level of inflammatory markers. A number of RCTs are needed to further explore the optimal ratio of n-6/n-3 PUFA.
Conclusion
Previous studies have focused on the effects of EPA and DHA on inflammation, or on the effects of single n-6 or n-3 PUFA on inflammatory markers. This paper is a novel study on the effect of the ratio of n-6/n-3 PUFA on inflammatory markers. The type of fatty acids in the diet is complex, and it is rarely a single intake of n-6 or n-3 PUFA.The study of the effect of the n-6/n-3 PUFA ratio on inflammatory biomarkers has practical significance for inflammation-related diseases. In conclusion, this meta-analysis provides evidence that low-ratio n-6/n-3 PUFA supplementation has obvious effects on lowering TNF-α and IL-6 levels.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was funded in full by the Major project of Shandong Province, China; grant number 2018YYSP020 (To Liyong Chen).References
- M. E. Kotas and R. Medzhitov, Homeostasis, inflammation, and disease susceptibility, Cell, 2015, 160(5), 816–827, DOI:10.1016/j.cell.2015.02.010.
- R. Medzhitov, Origin and physiological roles of inflammation, Nature, 2008, 454(7203), 428–435, DOI:10.1038/nature07201.
- K. Suzuki, Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise, Biomolecules, 2019, 9(6), 223, DOI:10.3390/biom9060223.
- K. T. Feehan and D. W. Gilroy, Is Resolution the End of Inflammation?, Trends Mol. Med., 2019, 25(3), 198–214, DOI:10.1016/j.molmed.2019.01.006.
- A. G. Aslıhan, F. W. Rossi, S. Bellando-Randone, N. Prevete, A. Tufan, M. Manetti, A. de Paulis and M. Matucci-Cerinic, The Role of Endogenous Eicosapentaenoic Acid and Docosahexaenoic Acid-Derived Resolvins in Systemic Sclerosis, Front. Immunol., 2020, 11, 1249, DOI:10.3389/fimmu.2020.01249.
- H. J. Lee, Y. M. Han and J. An, et al., Role of omega-3 polyunsaturated fatty acids in preventing gastrointestinal cancers: current status and future perspectives, Expert Rev. Anticancer Ther., 2018, 18(12), 1189–1203, DOI:10.1080/14737140.2018.1524299.
- D. Sacks, B. Baxter and B. C. V. Campbell, et al., Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke., Int. J. Stroke, 2018, 13(6), 612–632, DOI:10.1177/1747493018778713.
- S. M. Mirhashemi, F. Rahimi and A. Soleimani, et al., Effects of Omega-3 Fatty Acid Supplementation on Inflammatory Cytokines and Advanced Glycation End Products in Patients With Diabetic Nephropathy: a Randomized Controlled Trial., Iran J. Kidney Dis., 2016, 10(4), 197–204 Search PubMed.
- A. P. Simopoulos, An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity, Nutrients, 2016, 8(3), 128, DOI:10.3390/nu8030128.
- B. Burns-Whitmore, E. Haddad and J. Sabaté, et al., Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study, Nutr. J., 2014, 13(29), 1475–2891, DOI:10.1186/1475-2891-13-29.
- J. P. Higgins, S. G. Thompson and J. J. Deeks, et al., Measuring inconsistency in meta-analyses, Br. Med. J., 2003, 327(7414), 557–560, DOI:10.1136/bmj.327.7414.557.
- J. A. Sterne and M. Egger, Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis, J. Clin. Epidemiol., 2001, 54(10), 1046–1055, DOI:10.1016/s0895-4356(01)00377-8.
- Y. L. Chiang, E. Haddad and S. Rajaram, et al., The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: a randomized, controlled crossover trial, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2012, 87(4–5), 111–117, DOI:10.1016/j.plefa.2012.07.007.
- S. N. Han, A. H. Lichtenstein and L. M. Ausman, et al., Novel soybean oils differing in fatty acid composition alter immune functions of moderately hypercholesterolemic older adults, J. Nutr., 2012, 142(12), 2182–2187, DOI:10.3945/jn.112.164335.
- S. Kalgaonkar, R. U. Almario and D. Gurusinghe, et al., Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS, Eur. J. Clin. Nutr., 2011, 65(3), 386–393, DOI:10.1038/ejcn.2010.266.
- T. C. Lee, P. Ivester and A. G. Hester, et al., The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population., Lipids Health Dis., 2014, 13(196), 13–196, DOI:10.1186/1476-511X-13-196.
- M. L. Vargas, R. U. Almario and W. Buchan, et al., Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome, Metabolism, 2011, 60(12), 1711–1718, DOI:10.1016/j.metabol.2011.04.007.
- L. Baril-Gravel, M. E. Labonte and P. Couture, et al., Docosahexaenoic acid-enriched canola oil increases adiponectin concentrations: a randomized crossover controlled intervention trial, Nutr. Metab. Cardiovasc. Dis., 2015, 25(1), 52–59, DOI:10.1016/j.numecd.2014.08.003.
- S. M. Cornish, S. B. Myrie and E. M. Bugera, et al., Omega-3 supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men, Nutr. Res., 2018, 60, 87–95, DOI:10.1016/j.nutres.2018.09.005.
- N. Kaul, R. Kreml and J. A. Austria, et al., A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers, J. Am. Coll. Nutr., 2008, 27(1), 51–58, DOI:10.1080/07315724.2008.10719674.
- F. Agh, H. M. Niyaz and M. Djalali, et al., Omega-3 Fatty Acid Could Increase One of Myokines in Male Patients with Coronary Artery Disease: A Randomized, Double-Blind, Placebo-Controlled Trial., Arch. Iran. Med., 2017, 20(1), 28–33, DOI:0172001/AIM.007.
- M. Jamilian, M. Samimi and F. Kolahdooz, et al., Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial, J. Matern.-Fetal Neonat. Med., 2016, 29(4), 669–675, DOI:10.3109/14767058.2015.1015980.
- A. Kooshki, F. A. Taleban and H. Tabibi, et al., Effects of marine omega-3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients, Ann. Nutr. Metab., 2011, 58(3), 197–202, DOI:10.1159/000329727.
- M. D. Kontogianni, A. Vlassopoulos and A. Gatzieva, et al., Flaxseed oil does not affect inflammatory markers and lipid profile compared to olive oil, in young, healthy, normal weight adults, Metabolism, 2013, 62(5), 686–693, DOI:10.1016/j.metabol.2012.11.007.
- G. K. Paschos, A. Zampelas and D. B. Panagiotakos, et al., Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men, Eur. J. Nutr., 2007, 46(6), 315–320, DOI:10.1007/s00394-007-0668-5.
- L. S. Rallidis, G. Paschos and G. K. Liakos, et al., Dietary α-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients, Atherosclerosis, 2003, 167(2), 237–242, DOI:10.1016/s0021-9150(02)00427-6.
- X. Capo, M. Martorell and I. Llompart, et al., Docosahexanoic acid diet supplementation attenuates the peripheral mononuclear cell inflammatory response to exercise following LPS activation, Cytokine, 2014, 69(2), 155–164, DOI:10.1016/j.cyto.2014.05.026.
- M. Martorell, X. Capo and A. Sureda, et al., Effect of DHA on plasma fatty acid availability and oxidative stress during training season and football exercise, Food Funct., 2014, 5(8), 1920–1931, 10.1039/c4fo00229f.
- C. T. Damsgaard, H. Frøkiaer and A. D. Andersen, et al., Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men., J. Nutr., 2008, 138(6), 1061–1066, DOI:10.1093/jn/138.6.1061.
- J. Hallund, B. O. Madsen and S. H. Bugel, et al., The effect of farmed trout on cardiovascular risk markers in healthy men, Br. J. Nutr., 2010, 104(10), 1528–1536, DOI:10.1017/S0007114510002527.
- C. Dawczynski, M. Dittrich and T. Neumann, et al., Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil, Clin. Nutr., 2018, 37(2), 494–504, DOI:10.1016/j.clnu.2017.02.021.
- C. Dawczynski, K. A. Massey and C. Ness, et al., Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors, Clin. Nutr., 2013, 32(5), 686–696, DOI:10.1016/j.clnu.2012.12.010.
- A. M. Minihane, L. M. Brady and S. S. Lovegrove, et al., Lack of effect of dietary n-6:n-3 PUFA ratio on plasma lipids and markers of insulin responses in Indian Asians living in the UK, Eur. J. Nutr., 2005, 44(1), 26–32, DOI:10.1007/s00394-004-0488-9.
- F. A. Wallace, E. A. Miles and P. C. Calder, Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects, Br. J. Nutr., 2007, 89(5), 679–689, DOI:10.1079/bjn2002821.
- I. A. Munro and M. L. Garg, Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet, Br. J. Nutr., 2012, 108(8), 1466–1474, DOI:10.1017/S0007114511006817.
- K. J. Murphy, B. J. Meyer and T. A. Mori, et al., Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors, Br. J. Nutr., 2007, 97(4), 749–757, DOI:10.1017/S000711450747252X.
- J. Zhang, C. Wang and L. Li, et al., Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women, Br. J. Nutr., 2012, 108(8), 1455–1465, DOI:10.1017/S0007114511006866.
- Q. Zhou, Z. Zhang and P. Wang, et al., EPA+DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial, Mol. Nutr. Food Res., 2019, 63(10), e1801157, DOI:10.1002/mnfr.201801157.
- K. Kondo, K. Morino and Y. Nishio, et al., A fish-based diet intervention improves endothelial function in postmenopausal women with type 2 diabetes mellitus: a randomized crossover trial, Metabolism, 2014, 63(7), 930–940, DOI:10.1016/j.metabol.2014.04.005.
- S. D. Poppitt, C. A. Howe and F. E. Lithander, et al., Effects of moderate-dose omega-3 fish oil on cardiovascular risk factors and mood after ischemic stroke: a randomized, controlled trial, Stroke, 2009, 40(11), 3485–3492, DOI:10.1161/STROKEAHA.109.555136.
- S. L. Seierstad, I. Seljeflot and O. Johansen, et al., Dietary intake of differently fed salmon; the influence on markers of human, Eur. J. Clin. Invest., 2005, 35(1), 52–59, DOI:10.1111/j.1365-2362.2005.01443.x.
- F. Sofi, G. Giorgi and F. Cesari, et al., The atherosclerotic risk profile is affected differently by fish flesh with a similar EPA and DHA content but different n-6/n-3 ratio, Asia Pac. J. Clin. Nutr., 2013, 22(1), 32–40, DOI:10.6133/apjcn.2013.22.1.12.
- A. Stupin, L. Rasic and A. Matic, et al., Omega-3 polyunsaturated fatty acids-enriched hen eggs consumption enhances microvascular reactivity in young healthy individuals, Appl. Physiol., Nutr., Metab., 2018, 43(10), 988–995, DOI:10.1139/apnm-2017-0735.
- L. Galland, Diet and inflammation, Nutr. Clin. Pract., 2010, 25(6), 634–640, DOI:10.1177/0884533610385703.
- O. Coelho, B. P. da Silva and D. Rocha, et al., Polyunsaturated fatty acids and type 2 diabetes: Impact on the glycemic control mechanism., Crit. Rev. Food Sci. Nutr., 2017, 57(17), 3614–3619, DOI:10.1080/10408398.2015.1130016.
- X. F. Guo, K. L. Li and J. M. Li, et al., Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials., Crit. Rev. Food Sci. Nutr., 2019, 59(20), 3380–3393, DOI:10.1080/10408398.2018.1492901.
- P. C. Calder, Omega-3 fatty acids and inflammatory processes: from molecules to man, Biochem. Soc. Trans., 2017, 45(5), 1105–1115, DOI:10.1042/BST20160474.
- A. P. Simopoulos, The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases., Exp. Biol. Med., 2008, 233(6), 674–688, DOI:10.3181/0711-MR-311.
- A. P. Simopoulos, Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet, World Rev. Nutr. Diet., 2011, 102, 10–21, DOI:10.1159/000327785.
- C. Hu, I. D. Orcid and M. Yang, et al., Effects of Omega-3 Fatty Acids on Markers of Inflammation in Patients With Chronic Kidney Disease: A Controversial Issue., Ther. Apheresis Dial., 2018, 22(2), 124–132, DOI:10.1111/1744-9987.12611.
- P. K. Wu, S. C. Yeh, S. J. Li and Y. N. Kang, Efficacy of Polyunsaturated Fatty Acids on Inflammatory Markers in Patients Undergoing Dialysis: A Systematic Review with Network Meta-Analysis of Randomized Clinical Trials., Int. J. Mol. Sci., 2019, 20(15), 3645, DOI:10.3390/ijms20153645.
- M. C. Mocellin, C. Q. Camargo and E. A. Nunes, et al., A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer., Clin. Nutr., 2016, 35(2), 359–369, DOI:10.1016/j.clnu.2015.04.013.
- W. Xin, W. Wei and X. Li, Effects of fish oil supplementation on inflammatory markers in chronic heart: a meta-analysis of randomized controlled trials., BMC Cardiovasc. Disord., 2012, 12(77), 1471–2261, DOI:10.1186/1471-2261-12-77.
- R. Marion-Letellier, G. Savoye and S. Ghosh, Polyunsaturated fatty acids and inflammation, IUBMB Life, 2015, 67(9), 659–667, DOI:10.1002/iub.1428.
- M. Bouwens, O. van de Rest and N. Dellschaft, et al., Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells., Am. J. Clin. Nutr., 2009, 90(2), 415–424, DOI:10.3945/ajcn.2009.27680.
- S. Banni and V. Di Marzo, Effect of dietary fat on endocannabinoids and related mediators: consequences on energy homeostasis, inflammation and mood., Mol. Nutr. Food Res., 2010, 54(1), 82–92, DOI:10.1002/mnfr.200900516.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo01976c |
| This journal is © The Royal Society of Chemistry 2021 |