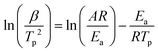A high performance fully bio-based epoxy thermoset from a syringaldehyde-derived epoxy monomer cured by furan-derived amine†
Hafezeh
Nabipour
,
Xin
Wang
 *,
Lei
Song
and
Yuan
Hu
*,
Lei
Song
and
Yuan
Hu
 *
*
State Key Laboratory of Fire Science, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China. E-mail: yuanhu@ustc.edu.cn; wxcmx@ustc.edu.cn; Fax: +86-551-63601664; Tel: +86-551-63601664
First published on 26th November 2020
Abstract
With the growing awareness of global warming and depletion of petroleum resources, the development of bio-based epoxy thermosets as an alternative to petroleum-based diglycidyl ether of bisphenol A (DGEBA) epoxy thermosets has generated extensive interest over the past decade. However, most of the bio-based epoxy thermosets studied to date are bio-based epoxy monomers or bio-based curing agents. In order to increase the biomass content in the resultant epoxy thermoset as high as possible, it is desirable to fabricate fully bio-based epoxy thermosets. In this study, we synthesized a bio-based epoxy monomer (SA-GA-EP) from syringaldehyde, as well as a bio-based curing agent (DIFFA) from furfurylamine. The fully bio-based epoxy thermoset was obtained through curing SA-GA-EP by DIFFA, while the commercial DGEBA epoxy thermoset was cured by 4,4′-diaminodiphenylmethane (DDM) as a contrast sample. The glass transition temperature (Tg) of the cured SA-GA-EP/DIFFA thermoset was as high as 204 °C, which was much higher than that of the DGEBA/DDM thermoset (143 °C). The tensile strength and elongation at break of SA-GA-EP/DIFFA were 57.4 MPa and 2.9%, respectively, which were comparable to those of DGEBA/DDM. Additionally, the cured SA-GA-EP/DIFFA thermoset displayed excellent intrinsic flame retardancy with UL-94 V-0 classification and a relatively high limiting oxygen index of 40.0%. The results of the cone calorimetry test also manifested that the peak heat release rate, the total heat release and the smoke production rate of SA-GA-EP/DIFFA significantly declined by 85%, 86% and 38%, respectively, compared to those of DGEBA/DDM. Moreover, bio-based epoxy exhibited antibacterial activities against Gram-positive S. aureus. Owing to these outstanding performances (including mechanical strength and modulus, high Tg, intrinsic flame resistance and antibacterial properties), this fully bio-based epoxy thermoset is a green and promising substitute for DGEBA-based thermoset in high performance fire safe applications.
Introduction
Epoxy resins as the commonly used thermosets have a wide application in the aerospace and electronics industries, and in adhesives, structural composites, insulation materials, and coatings, due to their considerable electrical and chemical resistance, high mechanical efficiency, slight shrinkage during curing, processability, and adhesive properties.1–3 Unfortunately, approximately 90% commercially available epoxy resin is diglycidyl ether of bisphenol A (DGEBA), which is synthesized from non-renewable resources.4 Epichlorohydrin and bisphenol A (BPA) are the main reactants for the preparation of DGEBA. Epichlorohydrin is prepared from bio-based glycerol on a commercial scale.5 BPA-derived resins are prohibited substances in the food packaging industry of some developed countries, with respect to the leakage of BPA as a risk factor.6 Since BPA is similar to estrogen, it can closely attach to estrogen receptors and affect bodily functions such as growth, cell regeneration, embryo development, level of energy, and reproduction. BPA may also interact with the receptors of other hormones e.g., thyroid. Moreover, it plays a decisive role in the pathogenesis of metabolic disorders such as polycystic ovary syndrome and of endocrine disorders with hormone-dependent tumors such as prostate and breast cancers.7,8 An increasing interest, along with environmental concerns and health issues necessitates the use of nontoxic sustainable biomass resources to produce bio-based epoxy resins as a substitute for BPA-based DGEBA. Many studies have been conducted on bio-based epoxy resin synthesis from biomaterials, including seed oils, eugenol, lignin, tannins, vanillin, hemicellulose, cardanol, and cellulose4,9–12 whose properties are in no way comparable to those of DGEBA.However, similar to DGEBA, most of the bio-based epoxy resins suffer from flammability, which hampers their applications. In order to overcome this inherent flammability, several bio-based intrinsic flame retardant epoxy monomers have been synthesized from fatty acids,13,14 eugenol,15–20 cardanol,19 vanillin,21,22 daidzein,23 furan,20,24 itaconic acid,25etc. For example, Wang et al. prepared two vanillin-based epoxy thermosets cured by 4,4′-diaminodiphenylmethane (DDM), which displayed superior flame retardancy over DGEBA/DDM. These two vanillin-based epoxy thermosets achieved a high LOI value of more than 31% and UL-94 V-0 rating.21 Ecochard et al. synthesized a phosphorylated cardanol-based epoxy thermoset (TECP) cured by meta-xylylenediamine (MXDA).19 The peak heat release rate of TECP/MXDA was 52% lower than that of DGEBA/MXDA, but the total heat release of the former was quite close to that of the latter owing to the long aliphatic chains of cardanol. Xie et al.26 synthesized a Schiff base epoxy monomer (PH-ODA-EP) from protocatechualdehyde and epichlorohydrin. The cured PH-ODA-EP thermoset with DDM exhibited higher mechanical strength and better fire-safety performance than DGEBA/DDM. However, most of the bio-based epoxy thermosets studied to date use either bio-based epoxy monomers or bio-based curing agents. In order to increase the biomass content in the resultant epoxy thermoset as high as possible, it is desirable to fabricate fully bio-based epoxy thermosets with a bio-based epoxy monomer cured by bio-based curing agent. Additionally, the development of bio-based epoxy thermosets with fire safety features and a combination of essential physical properties is an ongoing challenge. Syringaldehyde is a phenolic aldehyde found in fruits, nuts, and plants that synthesize lignin-related compounds.27 The structure of syringaldehyde is similar to that of vanillin, and it contains functional groups, making it a favorable biomass resource for developing Schiff base epoxy thermosets with good performance.28
Microbial contamination has recently turned into an important problem globally, and seriously influences human safety and health. Pathogenic microbes causing cross-infection or microbial infections are present in different environments and materials used in water purification systems, hospitals, food packaging, and medical devices.29 There have been many studies on the development of efficient antimicrobial agents for inhibiting the colonization and spread of microorganisms. Various studies have investigated antimicrobial applications of antibacterial agents, such as amine compounds,30 phosphonium salts,31 and heavy ions and metal oxides.32 The damages caused by heavy metals to human health and environment have been considerable in recent years. Research findings have shown great antibacterial properties of Schiff base due to its combination with the lipophilic layer, which enhances the bacterial membrane permeability. A cell is surrounded by a lipid membrane that enhances the passage of lipid-soluble materials and results in the cell membrane breakdown. Thus, it facilitates the easy entrance of the Schiff base into the cell membrane for damaging its DNA and killing the bacteria.33 However, the integration of the outstanding performances including mechanical strength and modulus, high Tg, intrinsic flame resistance and antibacterial properties into a high performance fully bio-based epoxy thermoset, has seldom been reported.
In this study, we proposed a fully bio-based epoxy thermoset with fire-safety, mechanical strength, and antibacterial features through the molecular design of a new syringaldehyde-based Schiff base epoxy monomer and a furan-based curing agent. The Schiff base epoxy monomer (SA-GA-EP) was synthesized through the reaction of syringaldehyde and 3,5-diamino-1,2,4-triazole, followed by epoxidation with epichlorohydrin, and the furan-based curing agent (DIFFA) was fabricated from furfurylamine. The fully bio-based epoxy thermoset was obtained by thermal curing of SA-GA-EP with DIFFA. The chemical structure, thermal stability, curing kinetics, mechanical properties, fire-retardant performance, and antibacterial characteristics of the cured SA-GA-EP/DIFFA were characterized, and further compared with those of the commercial DGEBA cured with DDM. It is anticipated to develop a high performance fully bio-based epoxy thermoset as a replacement for the DGEBA-based thermoset.
Experimental
Materials
4,4′-Diaminodiphenylmethane (DDM), syringaldehyde, 3,5-diamino-1,2,4-triazole, anhydrous magnesium sulfate, epichlorohydrin, and furfurylamine were bought from Aladdin Chemical Reagent Co., Ltd (Shanghai, China). Ethanol, acetone, absolute ether, dichloromethane, acetic acid, hydrochloric acid, tetrabutylammonium bromide, sodium hydroxide and ethyl acetate were provided by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). DGEBA-type epoxy pre-polymer was commercially obtained from Hefei Jiangfeng Chemistry Co. Ltd (Anhui, China).Synthesis of syringaldehyde-derived Schiff base epoxy monomer (SA-GA-EP)
In a 500 mL three-necked glass flask equipped with a reflux condenser, a nitrogen inlet and a mechanical stirrer, syringaldehyde (36.42 g, 0.20 mol) and 3,5-diamino-1,2,4-triazole (9.91 g, 0.10 mol) were dissolved in ethanol (250 mL). The mixture was stirred at room temperature for 1 h, and then three drops of acetic acid were added to the mixture. The reaction system was kept stirring at 90 °C for 48 h under a nitrogen atmosphere. After this, the precipitate was filtered, and washed three times with acetone and absolute ether to remove the impurity. Finally, the intermediate (SA-GA) powder was obtained after drying at 60 °C for 12 h in a vacuum oven.In a 250 mL three-necked glass flask equipped with a reflux condenser, a nitrogen inlet and a mechanical stirrer, tetrabutylammonium bromide (1.61 g, 5 mmol), SA-GA (21.37 g, 0.05 mol), epichlorohydrin (46.3 g, 0.50 mol), and 50 mL of ethanol were mixed uniformly and kept stirring under a nitrogen atmosphere at 80 °C for 2 h. Then, the reaction system was cooled to 0 °C in an ice bath, followed by the drop-wise addition of 15 mL of sodium hydroxide aqueous solution (40.0 wt%) within 1 h. The reaction was further conducted at 25 °C for 6 h. Then, the resulting product was filtered, and the filtrate was mixed with 50 mL of dichloromethane. The resulting product was washed with purified water seven times. Anhydrous magnesium sulfate was used for drying the organic layer, and it was then filtered. The solvent was removed using a rotary evaporator to yield a yellow viscous product (yield: 72%).
Synthesis of the furan-based curing agent (DIFFA)
A three-necked round-bottom flask equipped with a reflux condenser was used for introducing furfurylamine (20 g, 0.206 mol), and it was cooled to 0 °C in an ice bath. Then, an 18.0 wt% aqueous solution of hydrochloric acid (208 g) was added to furfurylamine in a drop-wise manner. After the addition of this solution, the temperature was increased to 25 °C, followed by stirring the resulting product for 15 min. Afterward, acetone (5.68 g, 0.098 mol) was added. The temperature was increased to 40 °C. An additional aliquot of acetone (4 g) was added again to the mixture. After one week, the reaction was ceased, and it was required to add two additional aliquots of acetone for increasing the conversion. Then, the product was cooled to 25 °C, followed by the addition of deionized water (600 mL). The pH was adjusted to 10 using a 15.0 wt% aqueous solution of sodium hydroxide. The solution was extracted thrice with ethyl acetate. Afterward, the organic layer was collected, and washed with brine. They were dried over anhydrous magnesium sulfate, and ethyl acetate was eliminated using a rotary evaporator for obtaining DIFFA as a brown liquid (yield: 63%).Preparation of the cured SA-GA-EP/DIFFA thermoset
A stoichiometric mixture of DIFFA and SA-GA-EP (N–H/epoxy group molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was mixed at 60 °C, and then poured to polytetrafluoroethylene molds, degassed at 70 °C for 24 h and at 90 °C for 24 h. Subsequently, the mixture was cured at 120 °C (2 h), 140 °C (2 h), 160 °C (2 h), and 180 °C (2 h) for obtaining the SA-GA-EP/DIFFA thermoset. DGEBA/DDM (N–H/epoxy group molar ratio of 1
1) was mixed at 60 °C, and then poured to polytetrafluoroethylene molds, degassed at 70 °C for 24 h and at 90 °C for 24 h. Subsequently, the mixture was cured at 120 °C (2 h), 140 °C (2 h), 160 °C (2 h), and 180 °C (2 h) for obtaining the SA-GA-EP/DIFFA thermoset. DGEBA/DDM (N–H/epoxy group molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was prepared using the same curing procedures as mentioned above.
1) was prepared using the same curing procedures as mentioned above.
Characterization
The Fourier transform infrared (FTIR) spectrum was recorded between 400 and 4000 cm−1 using a Nicolet 6700FTIR spectrometer (Thermo Electron Scientific Instruments Co. Ltd, USA) with KBr pellets. Scanning electron microscopy (SEM) images were obtained by an EVO MA15 (Japan) at an acceleration voltage of 20 kV. The thermo-gravimetric analysis/differential thermal gravimetric analysis (TGA/DTG) was carried out on a Q5000 thermogravimetric analyzer (TA, USA) in both nitrogen and air atmospheres with a temperature range of 25–800 °C at a heating rate of 20 °C min−1. 1H and 13C nuclear magnetic resonance (NMR) spectroscopy was performed using an AVANCE NEO 600 WB spectrometer (Bruker, Germany) using DMSO-d6 as the solvent. Differential scanning calorimetry (DSC) analyses were conducted on a Q2000 instrument (TA, USA) under a nitrogen atmosphere at four heating rates of 5, 10, 15, and 20 °C min−1 (from 50 to 250 °C) for non-isothermal curing behavior. The tensile test was performed on an INSTRON 5966 (J Lloyd, UK) according to the ASTM D3039-08 method with a crosshead speed of 2.0 mm min−1. Dynamic mechanical analysis (DMA) was performed using a Q800 apparatus at a heating rate of 5 °C min−1 from 30–200 °C with the dimension of 50 × 10 × 3 mm3. X-ray photoelectron spectroscopy (XPS) was conducted on an ESCALAB 250 XPS spectrometer (Thermo Scientific, USA) with a monochromatic Al Kα source (hv = 1253.6 eV). The UL-94 burning test was carried out using a CZF-3 chamber (China) following the ASTM D3801-19 standard procedure, and the samples applied were 125 × 13 × 3 mm3 in dimension. The limiting oxygen index (LOI) was determined using a HC-2 LOI tester following the ASTM D2863-17a standard, and the samples used were 100 × 10 × 3 mm3 in dimension. The heat release rate test was carried out on a cone calorimeter (FTT, UK) following the ISO 5660 standard. The samples (at dimensions of 100 × 100 × 3 mm3) were horizontally exposed to thermal radiation of 50 kW m−2.Antibacterial assay
The disk-diffusion technique was used for the measurement of antibacterial functioning of DGEBA/DDM and SA-GA-EP/DIFFA. Two pathogenic bacteria, S. aureus ATCC 25923 and E. coli ATCC 8739, were studied. Firstly, about 20 mL of sterilized nutrient agar was poured into the Petri dish, and it was left for 30 min for solidification before inoculation. 500 μL bacterial suspensions were spread in a concentration of 1 × 105 colony-forming unit (CFU) mL−1 on the surface of the sterile agar plate, creating two holes in each plate. We reserved EP samples within the holes with a diameter of 3 mm and incubation was conducted at 37 °C for 24 h. A Vernier caliper was used to measure the inhibition diameter.34Results and discussion
The bio-based Schiff base epoxy monomer (SA-GA-EP) was synthesized by a two-step method: (1) 3,5-diamino-1,2,4-triazole undergoes nucleophilic addition with syringaldehyde to give Schiff base intermediate SA-GA, as shown in Fig. 1. The reaction was carried out in acidic buffer to activate the carbonyl group; (2) the second step involves the reaction of epichlorohydrin with the hydroxyl group of SA-GA under alkaline conditions to obtain a bio-based epoxy monomer. These above-mentioned steps are highly efficient and simple synthetic steps, offering a practical approach for preparing bio-based epoxy resins sustainably and innovatively.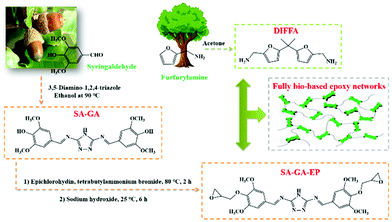 | ||
| Fig. 1 Synthetic route to the fully bio-based epoxy thermoset from a syringaldehyde-derived epoxy monomer cured by a furan-derived amine. | ||
FTIR was used for verifying the Schiff base SA-GA and SA-GA-EP monomer (Fig. 2a). In the FTIR spectrum of SA-GA, the absorption peaks at 1612 cm−1 (–OH stretching) and 3494 cm−1 (N–H stretching) appear. The absence of the characteristic absorption peaks at 1670 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) attributed to syringaldehyde35 and those at 3390 cm−1 (–NH asymmetric stretching vibration of C3) and 3310 cm−1 (–NH symmetric stretching vibration of C3) attributed to 3,5-diamino-1,2,4-triazole36 indicates the successful synthesis of the Schiff based intermediate SA-GA from 3,5-diamino-1,2,4-triazole and syringaldehyde. In the FTIR spectrum of SA-GA-EP, the appearance of a new peak at 1006 cm−1 (C–O–C stretching of the epoxy group) as well as the absence of the absorption peak at 1612 cm−1 (–OH stretching) suggest the successful conversion of SA-GA to SA-GA-EP.
O stretching) attributed to syringaldehyde35 and those at 3390 cm−1 (–NH asymmetric stretching vibration of C3) and 3310 cm−1 (–NH symmetric stretching vibration of C3) attributed to 3,5-diamino-1,2,4-triazole36 indicates the successful synthesis of the Schiff based intermediate SA-GA from 3,5-diamino-1,2,4-triazole and syringaldehyde. In the FTIR spectrum of SA-GA-EP, the appearance of a new peak at 1006 cm−1 (C–O–C stretching of the epoxy group) as well as the absence of the absorption peak at 1612 cm−1 (–OH stretching) suggest the successful conversion of SA-GA to SA-GA-EP.
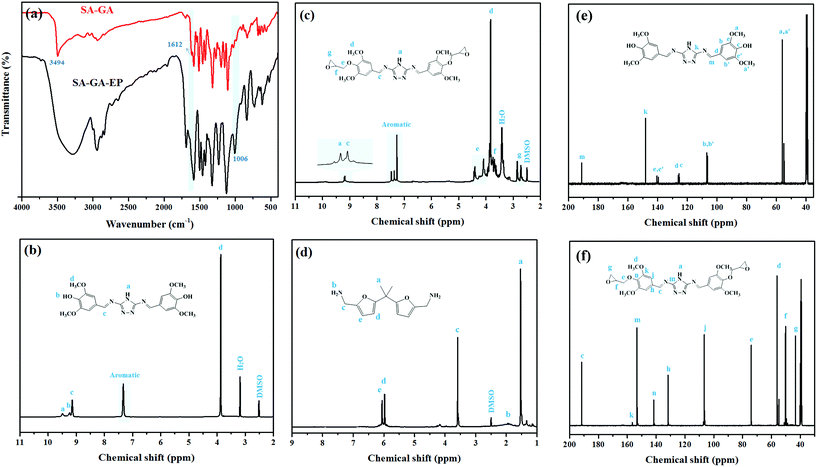 | ||
| Fig. 2 (a) FTIR spectra of syringaldehyde, 3,5-diamino-1,2,4-triazole, SA-GA, and SA-GA-EP; 1H-NMR spectra of (b) SA-GA, (c) SA-GA-EP and (d) DIFFA; 13C-NMR spectra of (e) SA-GA and (f) SA-GA-EP. | ||
Fig. 2b displays the 1H-NMR spectrum of SA-GA. The peaks at 9.48, 9.24 and 9.14 ppm are assignable to –NH, –OH and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, respectively. The signals of the aromatic protons are found at 7.31 and 7.21 ppm, and the peak at 3.87 ppm is assigned to –OCH3. Furthermore, the characteristic peaks of protons at 2.73, 2.86, 3.69, 4.10, and 4.41 ppm in the 1H-NMR spectrum of SA-GA-EP (Fig. 2c) also confirm the formation of the epoxy group. Besides, there is a good agreement between the chemical shifts of the observed peaks and the target product. Fig. 2d exhibits the 1H-NMR spectrum of DIFFA. The synthesized DIFFA shows a singlet at 1.50 ppm assigned to –CH3 (6H), a broad peak at 1.85 ppm belonging to –NH2 (4H), a singlet at 3.58 ppm assigned to –CH2– (4H), and a doublet at 5.97 and 6.08 ppm ascribed to 2H attached to each carbon on the furan ring. In addition, in the 13C-NMR spectra of SA-GA and SA-GA-EP (Fig. 2e and f), the chemical shift of all the peaks accords well with the carbon atoms in the molecular structures of SA-GA and SA-GA-EP. These results confirm that the target products have been synthesized successfully.
N, respectively. The signals of the aromatic protons are found at 7.31 and 7.21 ppm, and the peak at 3.87 ppm is assigned to –OCH3. Furthermore, the characteristic peaks of protons at 2.73, 2.86, 3.69, 4.10, and 4.41 ppm in the 1H-NMR spectrum of SA-GA-EP (Fig. 2c) also confirm the formation of the epoxy group. Besides, there is a good agreement between the chemical shifts of the observed peaks and the target product. Fig. 2d exhibits the 1H-NMR spectrum of DIFFA. The synthesized DIFFA shows a singlet at 1.50 ppm assigned to –CH3 (6H), a broad peak at 1.85 ppm belonging to –NH2 (4H), a singlet at 3.58 ppm assigned to –CH2– (4H), and a doublet at 5.97 and 6.08 ppm ascribed to 2H attached to each carbon on the furan ring. In addition, in the 13C-NMR spectra of SA-GA and SA-GA-EP (Fig. 2e and f), the chemical shift of all the peaks accords well with the carbon atoms in the molecular structures of SA-GA and SA-GA-EP. These results confirm that the target products have been synthesized successfully.
The non-isothermal curing kinetics of the SA-GA-EP/DIFFA and DGEBA/DDM systems are investigated by DSC at different heating rates (5, 10, 15 and 20 °C min−1) and shown in Fig. S1.† It can be observed that both SA-GA-EP/DIFFA and DGEBA/DDM systems display an exothermic peak. Table 1 summarizes the onset, peak, and end curing temperatures (Tonset, Tp and Tend), and exothermic enthalpy (ΔH) during the curing process at different heating rates. The onset, peak, and end curing temperatures increase gradually with the increase in the heating rate. The apparent curing activation energy (Ea) is calculated by Kissinger's method:
| Sample | β (°C min−1) | T onset (°C) | T p (°C) | T end (°C) | ΔH (J g−1) | E a (kJ mol−1) |
|---|---|---|---|---|---|---|
| SA-GA-EP/DIFFA | 5 | 62.1 | 94.2 | 147.6 | 74.6 | 43.66 |
| 10 | 67.4 | 109.2 | 162.4 | 78.6 | ||
| 15 | 76.0 | 119.5 | 173.4 | 87.5 | ||
| 20 | 80.5 | 128.5 | 182.4 | 91.1 | ||
| DGEBA/DDM | 5 | 74.9 | 128.7 | 211.0 | 120.7 | 37.35 |
| 10 | 80.6 | 149.6 | 228.5 | 129.3 | ||
| 15 | 92.3 | 162.5 | 232.0 | 135.4 | ||
| 20 | 105.4 | 175.0 | 241.5 | 138.6 |
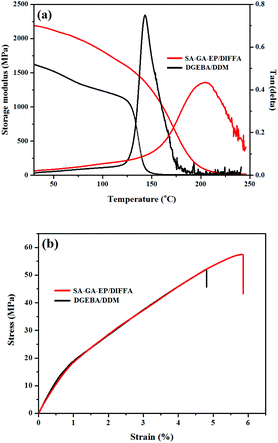
Fig. 3a indicates the thermo-mechanical properties of DGEBA/DDM and SA-GA-EP/DIFFA. The storage modulus (E′) at 30 °C of DGEBA/DDM and SA-GA-EP/DIFFA are 1620.7 and 2188.7 MPa, respectively, indicating higher stiffness of SA-GA-EP/DIFFA. Besides, the glass transition temperature (Tg) of SA-GA-EP/DIFFA is 204 °C, which is significantly higher than that of DGEBA/DDM (143 °C). Generally, the structural rigidity and the cross-linking density of the polymer are the main determinants of Tg. The aromatic structure and rigid Schiff base are the factors that restrict molecular chain movements. Hence, the Tg of SA-GA-EP/DIFFA networks is increased. Moreover, using the rubber elasticity model, the cross-linking density (υe) can be calculated37 by eqn (1):
| υe = E′/3 RT | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak of SA-GA-EP/DIFFA is broader than that of DGEBA/DDM, because the intermolecular interactions are stronger in SA-GA-EP/DIFFA, restricting the motion of chain segments. Hence, a broader temperature range is required for triggering chain mobility.
δ peak of SA-GA-EP/DIFFA is broader than that of DGEBA/DDM, because the intermolecular interactions are stronger in SA-GA-EP/DIFFA, restricting the motion of chain segments. Hence, a broader temperature range is required for triggering chain mobility.
| Sample | T g (°C) | E′ (30 °C) (MPa) | E′ at Tg + 30 °C (MPa) | υe × 103 (mol m−3) | Tensile strength (MPa) | Elongation at break (%) | Young's modulus (GPa) |
|---|---|---|---|---|---|---|---|
| SA-GA-EP/DIFFA | 204 | 2188.7 | 20.7 | 3.5 | 57.4 | 5.8 | 2.6 |
| DGEBA/DDM | 143 | 1620.7 | 7.6 | 0.6 | 51.8 | 4.8 | 2.5 |
Tensile tests were conducted to investigate the mechanical properties of the cured DGEBA/DDM and SA-GA-EP/DIFFA systems. Fig. 3b illustrates the tensile stress–strain curve of the SA-GA-EP/DIFFA and DGEBA/DDM, and Table 2 presents the corresponding parameters including elongation at break, Young's modulus and tensile strength. It can be seen that both Young's modulus (2.6 GPa) and tensile strength (57.4 MPa) of SA-GA-EP/DIFFA are comparable to or slightly higher than those of DGEBA/DDM (2.5 GPa and 51.8 MPa). These results demonstrate that SA-GA-EP/DIFFA has excellent mechanical performance as DGEBA/DDM.
TGA was performed for evaluating the thermal stability of DGEBA/DDM and SA-GA-EP/DIFFA under a nitrogen and an air atmosphere. Both the temperature for the maximum decomposition rate (Tmax) and the temperature equivalent to the weight loss of 10.0 wt% (Td10%, described as the initial decomposition temperature) are crucial parameters for the evaluation of thermal stability. Fig. 4a shows that the Td10% and Tmax under a nitrogen atmosphere are 300 and 317 °C, respectively, for SA-GA-EP/DIFFA, while they are 389 and 406 °C for DGEBA/DDM. The initial thermal stability of SA-GA-EP/DIFFA is lower than that of DGEBA/DDM, which is primarily due to the thermal degradation of the imine bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in the cross-linked network, release of tiny molecules such as NH3 or N2 gases during degradation and increases of char formation.26 It should be noted that when thermal degradation of DGEBA/DDM begins, it degrades rapidly with a Tmax at 406 °C and a decomposition rate as high as 1.58%/°C. By comparison, SA-GA-EP/DIFFA has a much lower thermal decomposition rate (0.60%/°C) at 316.5 °C compared to DGEBA/DDM. Consequently, for SA-GA-EP/DIFFA, the residual char yield at 800 °C is as high as 40.3%, which was approximately 3.8 times higher than that of DGEBA/DDM (10.5%). The major reason for the low degradation rate and high char yield of SA-GA-EP/DIFFA is the presence of imine structures in SA-GA-EP, which promotes char formation during the degradation of the epoxy matrix. Moreover, the TGA test under an air atmosphere also shows similar results (Fig. 4b). A two-stage thermal decomposition process in air is observed for both DGEBA/DDM and SA-GA-EP/DIFFA systems. In the first stage, the DGEBA/DDM exhibits a much higher thermal decomposition rate (1.04%/°C at 392 °C) compared to the SA-GA-EP/DIFFA (0.52%/°C at 552 °C). The second decomposition stage is attributed to the oxidized decomposition of the formed char. The char yield at 800 °C of SA-GA-EP/DIFFA is 2.4% higher than that of DGEBA/DDM. The high char yield of SA-GA-EP/DIFFA primarily originates from the carbonization induced by the Schiff base structure26 as well as the excellent thermal resistance of the triazole structure. The high residual char yield at high temperatures is usually conducive to favorable flame retardancy.
N) in the cross-linked network, release of tiny molecules such as NH3 or N2 gases during degradation and increases of char formation.26 It should be noted that when thermal degradation of DGEBA/DDM begins, it degrades rapidly with a Tmax at 406 °C and a decomposition rate as high as 1.58%/°C. By comparison, SA-GA-EP/DIFFA has a much lower thermal decomposition rate (0.60%/°C) at 316.5 °C compared to DGEBA/DDM. Consequently, for SA-GA-EP/DIFFA, the residual char yield at 800 °C is as high as 40.3%, which was approximately 3.8 times higher than that of DGEBA/DDM (10.5%). The major reason for the low degradation rate and high char yield of SA-GA-EP/DIFFA is the presence of imine structures in SA-GA-EP, which promotes char formation during the degradation of the epoxy matrix. Moreover, the TGA test under an air atmosphere also shows similar results (Fig. 4b). A two-stage thermal decomposition process in air is observed for both DGEBA/DDM and SA-GA-EP/DIFFA systems. In the first stage, the DGEBA/DDM exhibits a much higher thermal decomposition rate (1.04%/°C at 392 °C) compared to the SA-GA-EP/DIFFA (0.52%/°C at 552 °C). The second decomposition stage is attributed to the oxidized decomposition of the formed char. The char yield at 800 °C of SA-GA-EP/DIFFA is 2.4% higher than that of DGEBA/DDM. The high char yield of SA-GA-EP/DIFFA primarily originates from the carbonization induced by the Schiff base structure26 as well as the excellent thermal resistance of the triazole structure. The high residual char yield at high temperatures is usually conducive to favorable flame retardancy.
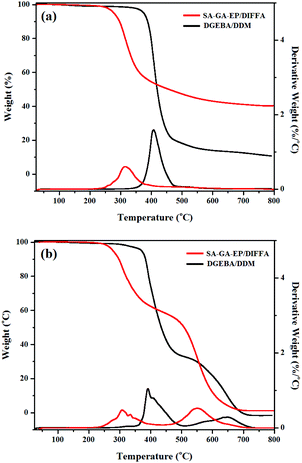 | ||
| Fig. 4 TGA/DTG curves of SA-GA-EP/DIFFA and DGEBA/DDM (a) under a nitrogen atmosphere and (b) under an air atmosphere. | ||
The thermal properties in terms of Tg and char yield at 700 °C under nitrogen of the cured SA-GA-EP/DIFFA system are selected and compared with several recent reports on bio-based epoxy thermosets.38–46 The cured SA-GA-EP/DIFFA system exhibits both high Tg (204 °C) and char yield at 700 °C under nitrogen (41.1%) compared to other bio-based epoxy systems (Fig. S3†). It is believed that the abundant aromatic species and the Schiff base structure of the SA-GA-EP/DIFFA system result in a better performance.
UL-94 vertical-burning test and LOI test are performed to systematically investigate the fire safety of SA-GA-EP/DIFFA. Table 3 gives the corresponding values. In the UL-94 vertical-burning test, SA-GA-EP/DIFFA achieves a V-0 rating, whereas DGEBA/DDM does not achieve any rating and it is fully burned. DGEBA/DDM burns violently, with molten dripping in the combustion process. In contrast, SA-GA-EP/DIFFA self-extinguishes immediately after the first ignition and self-extinguishes again within 3 s after the second ignition. The LOI test results indicate that SA-GA-EP/DIFFA has a much higher LOI value (40.0%) than DGEBA/DDM (23.5%). According to these findings, the SA-GA-EP/DIFFA thermoset has superior flame retardancy, while DGEBA/DDM is highly flammable. The excellent flame retardancy of SA-GA-EP/DIFFA is mostly attributed to the outstanding charring capacity induced by the aromatic triazole and the Schiff base structures in the SA-GA-EP molecule.26
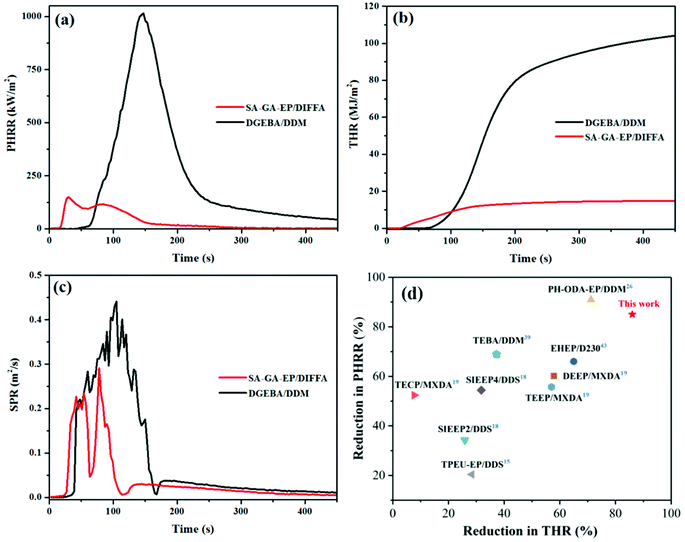
Cone calorimeter test was further employed for evaluating the burning behavior in the epoxy resins in a simulated fire disaster by comparing the peak values of the heat release rate (PHRR), smoke production rate (SPR), time to ignition (TTI), and total heat release (THR). Table 4 lists the typical values of SA-GA-EP/DIFFA and DGEBA/DDM. The curves of PHRR, THR and SPR as a function of time are shown in Fig. 5a–c. The TTI of SA-GA-EP/DIFFA is 21 s, which is shorter than that of DGEBA/DDM. This is most likely because the low molecular fragment, which promotes ignition, was eliminated from the 3,5-diamino-1,2,4-triazole-containing and Schiff base structure of SA-GA-EP at low temperatures. This phenomenon agrees well with the earlier decomposition behavior from the TGA result. It is noteworthy that the PHRR value of SA-GA-EP/DIFFA decreased significantly to 149.5 kW m−2 from 1014.9 kW m−2 for DGEBA/DDM, corresponding to an approximately 85% reduction. This significant PHRR reduction is attributed to the high char yield of SA-GA-EP/DIFFA which serves as a shielding barrier to prevent the underlying material from heat attack during combustion. Strikingly, the THR value of SA-GA-EP/DIFFA also decreased to 14.8 MJ m−2 from 104.2 MJ m−2 for DGEBA/DDM, corresponding to an approximately 86% reduction, indicating significantly suppressed flammability. Another extreme reduction is observed in the SPR of SA-GA-EP/DIFFA that shows a 38% decrease compared to that of DGEBA/DDM. Fig. 5d compares the reduction in the PHRR and THR values of SA-GA-EP/DIFFA with several recent reports on bio-based epoxy thermosets.15,18,19,26,39,43 It can be observed that the cured SA-GA-EP/DIFFA system is located at the upper right corner, demonstrating superior fire safety features over other bio-based epoxy thermosets in previous reports. In summary, the results of UL-94, LOI and cone calorimeter tests manifest the superior flame retardancy of SA-GA-EP/DIFFA when exposed to fire, benefitting from its superior charring capacity in the condensed phase.
| Sample | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | SPR (m2 s−1) |
|---|---|---|---|---|
| SA-GA-EP/DIFFA | 21.0 | 149.5 ± 1.4 | 14.8 ± 0.6 | 0.25 ± 0.04 |
| DGEBA/DDM | 54.0 | 1014.9 ± 20.7 | 104.2 ± 0.6 | 0.40 ± 0.05 |
Fig. 6a and c show the digital photographs of the residual chars after the cone calorimeter test. The DGEBA/DDM produces little residual char, with the molten underlying aluminum foil (Fig. 6a). The char of SA-GA-EP/DIFFA is apparently intumescent in contrast to that of DGEBA/DDM (Fig. 6c). It is clear that the yield of residual char of SA-GA-EP/DIFFA is much more compared to that of DGEBA/DDM. These findings show the outstanding charring ability and intumescent char structure of SA-GA-EP/DIFFA, which is supposed to significantly retard fire propagation. The micromorphology of the char residues of DGEBA/DDM and SA-GA-EP/DIFFA was further observed by SEM. A large number of pores can be detected on the surface of the char residue of DGEBA/DDM (Fig. 6b). By contrast, the char residue of SA-GA-EP/DIFFA is denser and more continuous than DGEBA/DDM (Fig. 6d), which can effectively hinder the exchange of heat and combustible gases between the molten polymer and flames.
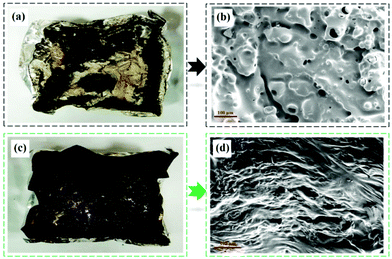 | ||
| Fig. 6 Photographs and SEM images of char residues of (a, b) DGEBA/DDM and (c, d) SA-GA-EP/DIFFA after the cone calorimeter test. | ||
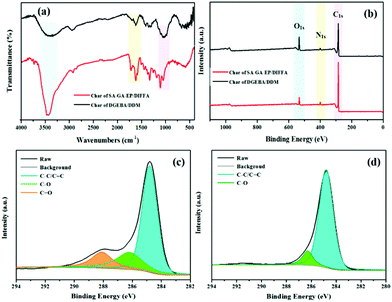
The mechanism of fire resistance and smoke suppression is also elucidated by FTIR and XPS analysis of the char residues of SA-GA-EP/DIFFA and DGEBA/DDM after cone calorimeter test. The peaks at 3423, 1719, and 1612 cm−1 can be attributed to the hydroxyl of water, carbonyl group, and aromatic compounds, respectively. The peaks at 1089 and 1048 cm−1 in the char residues of SA-GA-EP/DIFFA and DGEBA/DDM arise from the C–O band (Fig. 7a). The full scan XPS spectra of the char residues are depicted in Fig. 7b. The char residues of the DGEBA/DDM and SA-GA-EP/DIFFA contain carbon, nitrogen, and oxygen elements. The high-resolution C 1s XPS spectra of the char residues are presented in Fig. 7c and d. The C 1s XPS spectra can be sub-divided into three peaks at 284.9, 286.7, and 288.5 eV, which are attributed to C–C in aliphatic and aromatic carbons (Ca), C–O in ether or hydroxyl groups, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carbonyl or carboxylic groups, respectively.47 The C–O and C
O in carbonyl or carboxylic groups, respectively.47 The C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups can be considered as oxidized carbons (Cox). The thermal oxidative resistance can be evaluated by the calculation of the Cox/Ca ratio,48,49 as listed in Table 5. The Cox/Ca ratio is 0.14 and 0.54 for the residual char of SA-GA-EP/DIFFA and DGEBA/DDM, respectively. The Cox/Ca value of SA-GA-EP/DIFFA is much lower than that of DGEBA/DDM, implying better thermal-oxidative resistance of the residual char. The char layer with superior thermal-oxidative resistance could provide better flame retardancy by inhibiting the diffusion of oxygen and combustible gases, and retarding mass and heat transfer. From XPS analysis, the chemical constituents of the char residues of DGEBA/DDM and SA-GA-EP/DIFFA are also obtained. The N content in the char residues of SA-GA-EP/DIFFA and DGEBA/DDM is found to be 3.9% and 3.1%, respectively. Compared to DGEBA/DDM, the increased N content of SA-GA-EP/DIFFA is attributed to the formation of thermally stable nitrogen-rich char originating from the aromatic triazole structure. Based on the results, the unique chemical structure of SA-GA-EP/DIFFA contributes to the formation of a higher thermally stable char layer, which accounts for excellent flame retardancy in the condensed phase.
O groups can be considered as oxidized carbons (Cox). The thermal oxidative resistance can be evaluated by the calculation of the Cox/Ca ratio,48,49 as listed in Table 5. The Cox/Ca ratio is 0.14 and 0.54 for the residual char of SA-GA-EP/DIFFA and DGEBA/DDM, respectively. The Cox/Ca value of SA-GA-EP/DIFFA is much lower than that of DGEBA/DDM, implying better thermal-oxidative resistance of the residual char. The char layer with superior thermal-oxidative resistance could provide better flame retardancy by inhibiting the diffusion of oxygen and combustible gases, and retarding mass and heat transfer. From XPS analysis, the chemical constituents of the char residues of DGEBA/DDM and SA-GA-EP/DIFFA are also obtained. The N content in the char residues of SA-GA-EP/DIFFA and DGEBA/DDM is found to be 3.9% and 3.1%, respectively. Compared to DGEBA/DDM, the increased N content of SA-GA-EP/DIFFA is attributed to the formation of thermally stable nitrogen-rich char originating from the aromatic triazole structure. Based on the results, the unique chemical structure of SA-GA-EP/DIFFA contributes to the formation of a higher thermally stable char layer, which accounts for excellent flame retardancy in the condensed phase.
| Sample | Area (%) | Cox/Ca ratio | N1s (%) | ||
|---|---|---|---|---|---|
| C1s (C–C) | C1s (C–O) | C1s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|||
| SA-GA-EP/DIFFA | 88.1 | 11.9 | - | 0.14 | 3.9 |
| DGEBA/DDM | 64.9 | 17.1 | 18.0 | 0.54 | 3.1 |
Developing an antibacterial material with the ability to bind to the catheter surface with long-lasting antibacterial properties is an interesting research subject. Currently, three main strategies have been proposed to design antibacterial materials: contact-killing, anti-adhesion/bacteria-repelling, and antibacterial agent release. Contact-killing includes combining efficient antibacterial materials on the material surface for killing bacteria via direct contact. In this study, the antimicrobial activity of the Schiff base SA-GA-EP/DIFFA was examined using S. aureus (Gram-positive bacteria) and E. coli (Gram-negative bacteria) as the bacterium models. The outer surface of E. coli is made of an outer membrane, a peptidoglycan layer, and an inner membrane. The periplasm is located between the inner and the outer membrane, which surrounds the cytoplasm of E. coli. The outer membrane of E. coli consists of lipopolysaccharides, lipoproteins, and other outer membrane proteins.50,51Fig. 8 shows the antibacterial activities of SA-GA-EP/DIFFA and DGEBA/DDM against S. aureus and E. coli. There are no inhibition zones surrounding the DGEBA/DDM (Fig. 8a and b), indicating the absence of antibacterial properties of DGEBA/DDM. By comparison, a small inhibition zone with a diameter of 5 mm is observed in SA-GA-EP/DIFFA against E. coli (Fig. 8c). SA-GA-EP/DIFFA also exhibits a clear and large inhibition zone with a diameter of 16 mm against S. aureus (Fig. 8d). These results reveal that the SA-GA-EP/DIFFA has antibacterial properties that originate from the Schiff base structure (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in SA-GA-EP.52 The antibacterial feature of SA-GA-EP/DIFFA can be explained as follows: a thin peptidoglycan cell wall surrounds Gram-negative bacteria and the thickness of the peptidoglycan layer is more than that in Gram-positive bacteria. For killing these bacteria, fully bio-based epoxy should come in contact with the bacterial cells. However, it is more difficult for the epoxy to come in contact with Gram-negative bacteria than Gram-positive bacteria, since there is an additional barrier in the outer membrane preventing its entrance to the inner membrane. However, contact is easier in Gram-positive organisms since there is no outer membrane in S. aureus (Fig. 8e). According to the results, this fully bio-based Schiff base epoxy thermoset can be potentially employed as a high-performance antibacterial material.
N) in SA-GA-EP.52 The antibacterial feature of SA-GA-EP/DIFFA can be explained as follows: a thin peptidoglycan cell wall surrounds Gram-negative bacteria and the thickness of the peptidoglycan layer is more than that in Gram-positive bacteria. For killing these bacteria, fully bio-based epoxy should come in contact with the bacterial cells. However, it is more difficult for the epoxy to come in contact with Gram-negative bacteria than Gram-positive bacteria, since there is an additional barrier in the outer membrane preventing its entrance to the inner membrane. However, contact is easier in Gram-positive organisms since there is no outer membrane in S. aureus (Fig. 8e). According to the results, this fully bio-based Schiff base epoxy thermoset can be potentially employed as a high-performance antibacterial material.
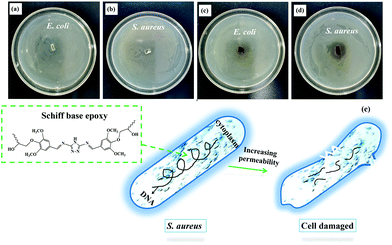 | ||
| Fig. 8 Antibacterial activities of (a and b) DGEBA/DDM and (c and d) SA-GA-EP/DIFFA against E. coli and S. aureus; and (e) antibacterial mechanism of SA-GA-EP/DIFFA. | ||
Conclusions
A fully bio-based intrinsically flame-retardant epoxy thermoset was successfully synthesized from a syringaldehyde-derived epoxy monomer (SA-GA-EP) and a furan-based curing agent (DIFFA). The cured SA-GA-EP/DIFFA system exhibited a relatively high Tg of up to 204 °C, which was 61 °C higher than that of the cured DGEBA/DDM system. The tensile strength, elongation at break and young's modulus of SA-GA-EP/DIFFA were found to be 57.4 MPa, 5.8% and 2.6 GPa, respectively, which were comparable to those of DGEBA/DDM. More impressively, superior flame-retardant properties were detected for the cured SA-GA-EP/DIFFA system, as demonstrated by the UL-94 V-0 classification and LOI value of 40.0%. Benefitting from the excellent charring ability induced by the aromatic triazole and Schiff base structures, the cured SA-GA-EP/DIFFA system showed an 85%, 86% and 38% decrease in PHRR (149.5 versus 1014.9 kW m−2), THR (14.8 versus 104.2 MJ m−2) and TSP (0.25 versus 0.40 m2 s−1), respectively, compared with the cured DGEBA/DDM system. Additionally, the SA-GA-EP/DIFFA also displayed good antibacterial activity against Gram-positive bacteria in comparison with the DGEBA/DDM. In summary, a strategy for preparing high performance fully bio-based epoxy thermosets with high glass transition temperature, good mechanical strength and modulus, intrinsic flame resistance and antibacterial activity is revealed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of China (no.: 22050410269) and the CAS President's International Fellowship (no.: 2019PE0014).Notes and references
- Q. H. Kong, T. Wu, J. H. Zhang and D. Y. Wang, Compos. Sci. Technol., 2018, 154, 136–144 CrossRef CAS.
- C. Gioia, G. Lo Re, M. Lawoko and L. Berglund, J. Am. Chem. Soc., 2018, 140, 4054–4061 CrossRef CAS PubMed.
- X. Wang, Y. Hu, L. Song, W. Y. Xing, H. D. A. Lu, P. Lv and G. X. Jie, Polymer, 2010, 51, 2435–2445 CrossRef CAS.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J. P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- S. Q. Ma, X. Q. Liu, L. B. Fan, Y. H. Jiang, L. J. Cao, Z. B. Tang and J. Zhu, ChemSusChem, 2014, 7, 555–562 CrossRef CAS PubMed.
- J. Y. Dai, N. Teng, J. K. Liu, J. X. Feng, J. Zhu and X. Q. Liu, Composites, Part B, 2019, 179, 107523 CrossRef CAS.
- A. Konieczna, A. Rutkowska and D. Rachoń, Rocz. Panstw. Zakl. Hig., 2015, 66, 5–11 Search PubMed.
- H. Okada, T. Takatoshi, L. Xiaohui, T. Sayaka, M. Ayami and Y. Shimohigashi, Environ. Health Perspect., 2008, 116, 32–38 CrossRef CAS PubMed.
- S. Zhao and M. M. Abu-Omar, ACS Sustainable Chem. Eng., 2017, 5, 5059–5066 CrossRef CAS.
- X. Y. Jian, Y. He, Y. D. Li, M. Wang and J. B. Zeng, Chem. Eng. J., 2017, 326, 875–885 CrossRef CAS.
- S. Kumar, S. Krishnan, S. Mohanty and S. K. Nayak, Polym. Int., 2018, 67, 815–839 CrossRef CAS.
- S. Kumar, S. K. Samal, S. Mohanty and S. K. Nayak, Polym.-Plast. Technol. Eng., 2018, 57, 133–155 CrossRef CAS.
- G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 6717–6727 CrossRef CAS.
- G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5630–5644 CrossRef CAS.
- J. T. Wan, B. Gan, C. Li, J. Molina-Aldareguia, Z. Li, X. Wang and D. Y. Wang, J. Mater. Chem. A, 2015, 3, 21907–21921 RSC.
- I. Faye, M. Decostanzi, Y. Ecochard and S. Caillol, Green Chem., 2017, 19, 5236–5242 RSC.
- J. T. Miao, L. Yuan, Q. Guan, G. Liang and A. Gu, Polym. Int., 2018, 67, 1194–1202 CrossRef CAS.
- C. Li, H. Fan, T. Aziz, C. Bittencourt, L. Wu, D.-Y. Wang and P. Dubois, ACS Sustainable Chem. Eng., 2018, 6, 8856–8867 CrossRef CAS.
- Y. Ecochard, M. Decostanzi, C. Negrell, R. Sonnier and S. Caillol, Molecules, 2019, 24, 1818 CrossRef CAS PubMed.
- J. T. Miao, L. Yuan, Q. Guan, G. Liang and A. Gu, ACS Sustainable Chem. Eng., 2017, 5, 7003–7011 CrossRef CAS.
- S. Wang, S. Q. Ma, C. X. Xu, Y. Liu, J. Y. Dai, Z. B. Wang, X. Q. Liu, J. Chen, X. B. Shen, J. J. Wei and J. Zhu, Macromolecules, 2017, 50, 1892–1901 CrossRef CAS.
- J. K. Liu, J. Y. Dai, S. P. Wang, Y. Y. Peng, L. J. Cao and X. Q. Liu, Composites, Part B, 2020, 190, 107926 CrossRef CAS.
- J. Y. Dai, Y. Y. Peng, N. Teng, Y. Liu, C. C. Liu, X. B. Shen, S. Mahmud, J. Zhu and X. Q. Liu, ACS Sustainable Chem. Eng., 2018, 6, 7589–7599 CrossRef CAS.
- J. Meng, Y. Zeng, G. Zhu, J. Zhang, P. Chen, Y. Cheng, Z. Fang and K. Guo, Polym. Chem., 2019, 10, 2370–2375 RSC.
- S. Ma, X. Liu, Y. Jiang, L. Fan, J. Feng and J. Zhu, Sci. China: Chem., 2014, 57, 379–388 CrossRef CAS.
- W. Xie, S. Huang, D. Tang, S. Liu and J. Zhao, Chem. Eng. J., 2019, 394, 123667 CrossRef.
- D. Yancheva, E. Velcheva, Z. Glavcheva, B. Stamboliyska and A. Smelcerovic, J. Mol. Struct., 2016, 1108, 552–559 CrossRef CAS.
- S. Wang, S. Q. Ma, Q. Li, X. W. Xu, B. B. Wang, W. C. Yuan, S. H. Zhou, S. S. You and J. Zhu, Green Chem., 2019, 21, 1484–1497 RSC.
- N. Y. Pan, Y. F. Wang, X. H. Ren, T. S. Huang and I. S. Kim, Mater. Sci. Eng., C, 2019, 103, 109877 CrossRef CAS PubMed.
- D. T. Nguyen, L. T. Pham, H. T. T. Le, M. X. Vu, H. T. M. Le, H. T. M. Le, N. H. Pham and L. T. Lu, RSC Adv., 2018, 8, 19707–19712 RSC.
- D. Zhu, H. H. Cheng, J. N. Li, W. W. Zhang, Y. Y. Shen, S. J. Chen, Z. C. Ge and S. G. Chen, Mater. Sci. Eng., C, 2016, 61, 79–84 CrossRef CAS.
- M. Fiedot-Tobola, M. Ciesielska, I. Maliszewska, O. Rac-Rumijowska, P. Suchorska-Wozniak, H. Teterycz and M. Bryjak, Materials, 2018, 11, 707 CrossRef PubMed.
- Y. J. Xu, Y. Shi, F. Lei and L. Dai, Carbohydr. Polym., 2020, 230, 115671 CrossRef CAS.
- H. Nabipour, S. Ghammamy and A. Rahmani, Micro Nano Lett., 2011, 6, 217–220 CrossRef CAS.
- S. G. de Brito, J. K. D. Maciel, Y. C. Teles, M. M. M. de Souza Fernandes, O. S. Chaves, M. D. Ferreira, P. D. Fernandes, L. P. Felix, I. C. D. Cirino, J. P. Siqueira, R. Braz and M. D. V. de Souza, Rev. Bras. Farmacogn., 2017, 27, 453–458 CrossRef.
- H. Nabipour, X. Wang, M. Z. Rahman, L. Song and Y. Hu, J. Cleaner Prod., 2020, 273, 122832 CrossRef CAS.
- Y. Z. Tian, Q. Wang, Y. J. Hu, H. Sun, Z. C. Cui, L. L. Kou, J. Cheng and J. Y. Zhang, Polymer, 2019, 178, 121592 CrossRef CAS.
- R. Menard, S. Caillol and F. Allais, Ind. Crops Prod., 2017, 95, 83–95 CrossRef CAS.
- Z. Y. Chi, Z. W. Guo, Z. C. Xu, M. J. Zhang, M. Li, L. Shang and Y. H. Ao, Polym. Degrad. Stab., 2020, 176, 109151 CrossRef CAS.
- W. Q. Xie, D. L. Tang, S. M. Liu and J. Q. Zhao, Polym. Test, 2020, 86, 106466 CrossRef CAS.
- M. Janvier, L. Hollande, A. S. Jaufurally, M. Pernes, R. Menard, M. Grimaldi, J. Beaugrand, P. Balaguer, P. H. Ducrot and F. Allais, ChemSusChem, 2017, 10, 738–746 CrossRef CAS PubMed.
- G. H. M. de Kruijff, T. Goschler, N. Beiser, A. Stenglein, O. M. Turk and S. R. Waldvogel, Green Chem., 2019, 21, 4815–4823 RSC.
- J. H. Liu, Z. J. He, G. H. Wu, X. Q. Zhang, C. Zhao and C. H. Lei, Chem. Eng. J., 2020, 390, 124620 CrossRef CAS.
- S. Q. Ma, X. Q. Liu, Y. H. Jiang, Z. B. Tang, C. Z. Zhang and J. Zhu, Green Chem., 2013, 15, 245–254 RSC.
- E. Savonnet, E. Grau, S. Grelier, B. Defoort and H. Cramail, ACS Sustainable Chem. Eng., 2018, 6, 11008–11017 CrossRef CAS.
- X. Xu, S. Ma, J. Wu, J. Yang, B. Wang, S. Wang, Q. Li, J. Feng, S. You and J. Zhu, J. Mater. Chem. A, 2019, 7, 15420–15431 RSC.
- W. Guo, S. Nie, E. N. Kalali, X. Wang, W. Wang, W. Cai, L. Song and Y. Hu, Composites, Part B, 2019, 176, 107261 CrossRef CAS.
- X. Wang, L. Song, H. Y. Yang, W. Y. Xing, B. Kandola and Y. Hua, J. Mater. Chem., 2012, 22, 22037–22043 RSC.
- X. Wang, S. Zhou, W. Y. Xing, B. Yu, X. M. Feng, L. Song and Y. Hu, J. Mater. Chem. A, 2013, 1, 4383–4390 RSC.
- B. Soltani, H. Nabipour and N. A. Nasab, J. Inorg. Organomet. Polym., 2018, 28, 1090–1097 CrossRef CAS.
- M. Park, G. Yoo, J. H. Bong, J. Jose, M. J. Kang and J. C. Pyun, Biochim. Biophys. Acta, Biomembr., 2015, 1848, 842–847 CrossRef CAS PubMed.
- S. M. El-Megharbel, A. S. Megahed and M. S. Refat, J. Mol. Liq., 2016, 216, 608–614 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc03451g |
| This journal is © The Royal Society of Chemistry 2021 |