Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A fully bio-based wood adhesive valorising hemicellulose-rich sidestreams from the pulp industry†
Tijana
Todorovic
a,
Emelie
Norström
a,
Farideh
Khabbaz
a,
Jörg
Brücher
b,
Eva
Malmström
 ac and
Linda
Fogelström
ac and
Linda
Fogelström
 *ac
*ac
aKTH Royal Institute of Technology, School of Engineering Sciences in Chemistry, Biotechnology and Health, Department of Fibre and Polymer Technology, Division of Coating Technology, Teknikringen 56-58, SE-100 44 Stockholm, Sweden. E-mail: lindafo@kth.se; Tel: +46 8 790 97 58
bHolmen Development, SE-89180 Örnsköldsvik, Sweden
cKTH Royal Institute of Technology, School of Engineering Sciences in Chemistry, Biotechnology and Health, Department of Fibre and Polymer Technology, Wallenberg Wood Science Center, Teknikringen 56-58, SE-100 44 Stockholm, Sweden
First published on 15th April 2021
Abstract
Today, most wood adhesives are prepared from fossil-based polymers and contain hazardous components, e.g., formaldehyde. With the growing environmental concern there is an urge to develop bio-based and harmless substitutes. In this study, the ambition is to explore and valorise hemicelluloses, a biproduct from pulping, as the main component in wood adhesives. Wood adhesives were prepared from different sources: xylan from beech wood, hemicellulose-rich liquids obtained from hydrolysis of hardwood, and ultrafiltered softwood hemicellulose recovered from the process water of a thermomechanical pulp mill. Hemicelluloses themselves do not exhibit sufficient bonding performance, but excellent bond strength and water resistance were obtained in combination with poly(vinyl amine). It was also demonstrated that chitosan can be used as a bio-based amino-functional alternative to synthetic poly(vinyl amine), with similar or superior properties. Hemicelluloses alone show insufficient water resistance, but hemicelluloses in combination with chitosan exhibit exceptionally good bonding performance, especially regarding water resistance. Adhesives prepared from liquids rich in hardwood- and softwood hemicelluloses showed similar bond strength in combination with amino-functional polymers (poly(vinyl amine) and chitosan), regardless of their differences in structure. The current study constitutes an example on how sidestreams from the pulp industry in combination with chitosan can be used to substitute fossil-based materials in the quest for a more sustainable society.
1. Introduction
Wood adhesives are irreplaceable in many large-scale applications such as load-bearing constructions, flooring, furniture, windows, and doors, and are produced in huge amounts. In 2018, the global wood adhesives market was estimated to USD 4.60 billion, and the predicted annual growth rate until 2025 is 4.7%.1Historically, wood adhesives were prepared from various bio-based polymers such as starch and proteins from blood or milk.2 In the middle of the 20th century, synthetic adhesives became available and have since then dominated over bio-based counterparts due to their superior properties regarding bonding performance, easy handling, and favourable price.3 Today's fossil-based wood adhesives have excellent performance but the fossil resource is limited and non-renewable. Moreover, many adhesives also contain harmful substances such as isocyanates and formaldehyde; the latter being recognised by the World Health Organization as carcinogenic to humans.4 A rapidly growing environmental awareness, combined with the increasingly sharper formaldehyde regulations,5,6 urges the industry to develop new environment-friendly and less fossil-dependent adhesives. To effectively compete with fossil-derived adhesives, the bio-based alternatives must be cost-effective and have similar adhesive properties as the existing, non-sustainable, adhesives or bring new or superior properties to the adhesive.
Various bio-based polymers have been suggested as binders for wood adhesives,3,7 but it has proven difficult to fully replace synthetic adhesives with bio-based alternatives as they often exhibit poor bonding performance, particularly regarding water resistance, and/or are not cost competitive. Another challenge when replacing synthetic polymers, having well-known and reproducible characteristics, is the broad property variation of bio-based polymers, emanating from locus, type of source, growth season, growth conditions, and extraction process.8 Furthermore, protein and starch, which are among the bio-based polymers that have been suggested for industrial-scale adhesive applications, are also possible food sources, which should be taken into account since food supply is considered a worldwide problem.9 In those cases where serious attempts have been made to re-commercialize bio-based wood adhesives, the formulations most often include synthetic and harmful components, such as isocyanates or epoxides, that cannot be considered “green”.10
Polysaccharides have been suggested as potential candidates for adhesive preparation.11 Extensive research has been conducted on starch as previously reviewed in literature. Because of its poor water resistance and, as mentioned above, food-source deficiency, commercialization of starch-based wood adhesives is challenging.7,12,13 Also gums, polysaccharides obtained from seeds, plants, or microorganisms, have been evaluated as wood adhesives.14,15 Locust bean gum, a galactomannan obtained from seeds from the carob tree, dispersed in water shows promising results with very good bond strength, water resistance, and heat resistance.16
Another interesting group of polysaccharides are hemicelluloses, which are found in biomass such as wood, grass, and cereals. Wood hemicelluloses are heteropolysaccharides whose composition and structure vary with species. The most important hemicelluloses in softwood are galactoglucomannans and arabinoglucuronoxylans, and in hardwood glucuronoxylan. Wood hemicelluloses have low molecular weight; the average degree of polymerization (DP) is typically below 200, compared with gums or cellulose with DPs of several thousands.17,18 In nature, the most important role of hemicellulose is to act as a “binder” between cellulose and lignin in the cell wall; the alcohol side groups interact with cellulose fibres, and the acetyl- and methyl side groups are improving the interactions between hemicelluloses and lignin.19,20 This interaction with both cellulose and lignin could be useful in wood adhesives.
Sidestreams from forestry and agriculture are valuable sources of hemicelluloses, but so far mostly combusted to recover energy. With the rapidly growing environmental awareness, there is an urge to transform the traditional pulp mill into a biorefinery which can produce high-value components for sustainable chemicals, materials, and fuels. Xylan, extracted and purified from beech wood, has previously been evaluated in wood-adhesive applications. Xylan alone did not show sufficient bonding performance, but with the addition of dispersing agents and/or crosslinkers the bonding performance was significantly improved, and particularly xylan in combination with poly(vinyl amine) (PVAm) demonstrated surprisingly good bonding performance.21 PVAm is a well-known chemical in the forest-products industry, traditionally used as an efficient wet-strength additive in paper, proven to form both covalent bonds (imine- and aminal bonds with aldehydes) and physical bonding through electrostatic interactions.22,23 However, commercially available, purified, xylan is far too costly to constitute a plausible alternative for cheap wood adhesives.
Inspired by the promising results obtained for purified xylan and PVAm, we decided to investigate less defined hemicellulose-rich sources, more realistic for wood-adhesive applications. In this study, pulping liquids (sidestreams), rich in hardwood (HW) or softwood (SW) hemicelluloses, have been explored with minimum work-up; hardwood hydrolysate was produced using a pilot cooker, simulating an industrial pulping process, and softwood hemicellulose was obtained by ultrafiltration of process water from a thermomechanical pulp mill. Moreover, instead of using fossil-based PVAm as dispersing agent, we here explored the use of chitosan, an amino-functional bio-based polymer, to achieve a fully bio-based adhesive. Chitosan consists of β-(1,4)-linked 2-amino-2-deoxy-D-glucopyranose (glucosamine) and 2-acetamido-2-deoxy-D-glucopyranose (N-acetyl glucosamine) and is obtained by deacetylation of chitin.24 Annually, chitin is produced by crustaceans and other living organisms in a comparable scale to cellulose.25 Chitosan is insoluble in most organic solvents and in water at neutral and alkaline pH, but soluble under acidic conditions due to protonation of the amino side-groups.26,27 Chitosan has shown promising results as binder in wood adhesives in several studies, mainly in combination with other components and only in small scale, using purified, well-defined, raw materials.28–37
The present study, utilizing sidestreams from pulping in combination with chitosan, demonstrates a fully bio-based wood adhesive with the potential of large-scale production, and with properties on par, or superior to, commercial fossil-based counterparts.
2. Experimental
2.1 Materials
Low molecular weight chitosan, with a viscosity of 92 mPa s and degree of deacetylation of 77% (product number: 448869), xylan (≥90% HPLC) from beech wood, and acetic acid (ReagentPlus®, ≥90%,) were purchased from Sigma Aldrich. Lupamin® 9095, 20 wt% solution of poly(vinyl amine), PVAm, with a molar mass of 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 was supplied by BASF.
000 g mol−1 was supplied by BASF.
Cascol® 3304, a poly(vinyl acetate)-based (PVAc) wood adhesive, with 50 wt% dry content, was kindly supplied by AkzoNobel Adhesives AB and used as a reference. Cascol is a thermoplastic wood adhesive suitable for indoor applications.
Beech wood veneers were purchased from Holgers Stugmaterial AB, Sweden.38
Hardwood hydrolysate was kindly supplied by Holmen, Sweden, in collaboration with MoRe Research, Sweden. Ultrafiltered softwood hemicellulose was kindly supplied by Stora Enso, Kvarnsveden, Sweden.
2.2 Composition analysis
The content of the saccharides in hemicellulose-rich liquids (HW and SW) was analysed by ion-exchange chromatography (IC). Prior to the analysis, samples were hydrolysed under acid conditions according to SCAN-CM 71.39 HW and SW samples (200 mg dry weight) were mixed with H2SO4, 72 wt% (3 mL) and kept in a vacuum desiccator for an hour. Deionized water (84 mL) was added and hydrolysis was run in an autoclave for 60 min at 125 °C. After the hydrolysis, the solutions were filtered through a glass-fibre filter; saccharide solution filtrates were further diluted and analysed by IC, and lignin residues (Klason lignin) were dried at 105 °C for 12 h. Lignin content was then determined gravimetrically. High-performance anion-exchange chromatogram (Dionex, Sunnyvale, CA, USA) was equipped with pulsed amperometric detector (HPAEC-PAD), and a CarboPac PA-1 column. Eluent was ultrapure water, and the flow rate was 1 mL min−1. Calibration was performed with six monosaccharides solutions: xylose, galactose, rhamnose, mannose, arabinose, and glucose. Data was processed with Chromeleon 7.1 software.2.3 Size exclusion chromatography
2.3.1.1 Hardwood hydrolysate and softwood ultrafiltrate. The molecular-size distribution of the hemicellulose fractions in the hemicellulose-rich liquids was analysed using size exclusion chromatography with combined pulsed amperometric-(PAD), UV- and refractive index (RI) detection. Separation was performed using a combined column set-up SB-802.5 HQ, SB-803 HQ and SB-804 HQ from Shodex with water as mobile phase. Calibration was performed by injection of pullulan standards (150–212
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). Prior to analysis, all samples were dissolved in water under stirring and thereafter filtered.
000 g mol−1). Prior to analysis, all samples were dissolved in water under stirring and thereafter filtered.
2.3.1.2 Xylan from beech wood. The xylan from beech wood was analysed using size exclusion chromatography SEC (SECcurity 1260, Polymer Standard Services, PSS, Mainz, Germany) coupled to a refractive index detector (SECcurity 1260, Polymer Standard Services, Mainz, Germany) thermostated to 45 °C. Separations were performed using a combined column set-up with a GRAM pre-column, and two GRAM 1000 analytical columns in series (Polymer Standard Services, Mainz, Germany) with 0.5 mL min−1 of dimethyl sulfoxide, DMSO, (LiBr 0.5% w/w) as mobile phase at 60 °C. The samples (100 μL) were injected with a 65 min interval. Calibration of the detectors and the column set-up was performed by injection of pullulan standards (342–708
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). Prior to analysis, all samples were dissolved in DMSO at 70 °C for 48 h. Data collection and analysis from SEC separations with light-scattering detection were performed using WinGPC software (Polymer Standard Services, Mainz, Germany).
000 g mol−1). Prior to analysis, all samples were dissolved in DMSO at 70 °C for 48 h. Data collection and analysis from SEC separations with light-scattering detection were performed using WinGPC software (Polymer Standard Services, Mainz, Germany).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). Prior to analysis, all samples were dissolved and diluted in a borate-buffer with 10% methanol at pH 10.3.
000 g mol−1). Prior to analysis, all samples were dissolved and diluted in a borate-buffer with 10% methanol at pH 10.3.
2.4 Adhesive preparation
All adhesives were prepared on a 4 g scale to a final dry content of 20 wt%. Adhesives containing both hemicellulose and PVAm or chitosan were prepared in different ratios (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 based on dry weight), Tables S1 and S2.†
3 based on dry weight), Tables S1 and S2.†
The adhesives containing PVAm were prepared not more than 2 h before bonding with wood. Hemicellulose and PVAm were blended with a spoon and with a vortex for approximately 2 min.
Chitosan was dissolved in 10 wt% acetic acid solution (aq.), pH 2, by blending with a spoon and with a vortex for approximately 2 min and left on a shaking table overnight. The chitosan-containing dispersions were prepared the day before bonding wood. Hemicellulose was added to the chitosan dispersion, blended with a spoon and with a vortex for approximately 2 min where after bonding was conducted.
2.5 pH measurement
The pH of the dispersions was measured with a pH glass electrode (JENWAY) connected to a pH meter (3510 pH meter JENWAY). The measurements were performed before adhesive application.2.6 Specimen preparation
Bond strength of the prepared adhesives was tested according to modified standards SS EN 204 and SS EN 205;40,41 wood specimens were thinner compared with standard thickness, and additional hot-water resistance was determined for some adhesives.42Beech wood veneers (1.5 mm thickness) were cut into specimens with the dimensions 100 × 20 mm and were conditioned in standard atmosphere (23 ± 2 °C temperature and 50 ± 5% relative humidity) before bonding. Adhesive dispersions were applied and spread as a thin glue line (360 g m−2) between two veneers with 20 mm overlap, maximum 2 h after preparation. The bonded veneers were hot-pressed at 120 °C and 1.67 MPa for 2.5 min in a Fontijne Grotnes Lab Pro 400.
After pressing, veneers were conditioned according to methods shown in Table 1, for determination of dried-, redried-, wet-, and wet- and warm strength. Three samples were prepared for each conditioning method. For the wet- and warm durability class, only adhesives containing hardwood hydrolysate and chitosan were tested.
| Conditioning method | Durability class | |
|---|---|---|
| a Standard atmosphere: 23 ± 2 °C temperature and 50 ± 5% relative humidity. | ||
| Dry strength | 7 days in standard atmospherea | Dried |
| Water resistance | 7 days in standard atmospherea, 3 h in water at 20 ± 5 °C, 7 days in standard atmospherea | Redried |
| 7 days in standard atmospherea, 4 days in water at 20 ± 5 °C | Wet | |
| Water resistance at 60 °C | 7 days in standard atmospherea, 3 h in water at 60 ± 5 °C | Wet and warm |
Additionally, adhesives containing hardwood hydrolysate and chitosan were pressed at room temperature for 2 h.
2.7 Tensile shear strength measurement
The strength of the bonded veneers was assessed using a low force universal testing system Instron 5566, with a 10 kN load cell and a crosshead speed of 1 mm min−1. The measurements were conducted in standard atmosphere. Bluehill software was used to collect data. The tensile shear strength was calculated as the ratio between the maximum force before failure [N] and the bonded area [mm2].2.8 Solubility test
Samples containing HW, CS, and their mixtures with ratios 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and with dry content of 20 wt% were pressed at 120 °C and 1.67 MPa for 2.5 minutes. Films (100 mg, ca. 1 mm thickness) were immersed in dilute acetic acid, pH = 2 (10 mL) for 24 hours. The insoluble- and soluble fractions were obtained after centrifugation and determined gravimetrically.
3, and with dry content of 20 wt% were pressed at 120 °C and 1.67 MPa for 2.5 minutes. Films (100 mg, ca. 1 mm thickness) were immersed in dilute acetic acid, pH = 2 (10 mL) for 24 hours. The insoluble- and soluble fractions were obtained after centrifugation and determined gravimetrically.
2.9 FTIR
Fourier-transform infrared spectroscopy (FTIR) analysis was performed using a PerkinElmer Spectrum 100 equipped with an attenuated total reflection (ATR) accessory unit (Golden Gate) from Specac LTD (Kent, England). Spectra were based on 16 scans in the range of 600–4000 cm−1. The data were analyzed with the Spectrum software from PerkinElmer.3. Results and discussion
In this study, two hemicellulose-rich liquids from the pulp industry; hydrolysate from pre-hydrolysis of hardwood wood chips (HW) and ultrafiltered process-water from a thermomechanical pulp mill using softwood (SW) have been evaluated as components in wood adhesives (Fig. 1). Commercial xylan (Xyl) from beech wood was also investigated as a reference hemicellulose. These hemicelluloses were combined with poly(vinyl amine) (PVAm) or chitosan (CS), carrying primary amino-groups, to improve bond strength (Fig. 2). A commercial wood adhesive, poly(vinyl acetate) (PVAc), was used as a reference.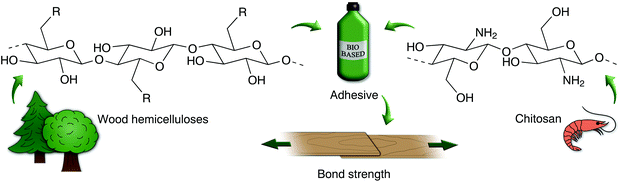 | ||
| Fig. 1 Overview of the conducted study, assessing the use of wood hemicelluloses in wood adhesive applications. | ||
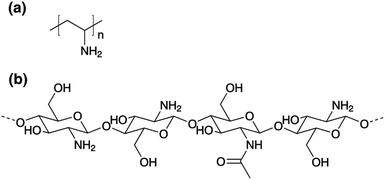 | ||
| Fig. 2 Representative chemical structures of amino-functional polymers: (a) poly(vinyl amine) (PVAm), and (b) chitosan (CS). | ||
3.1 Chemical composition of hemicellulose-rich fractions
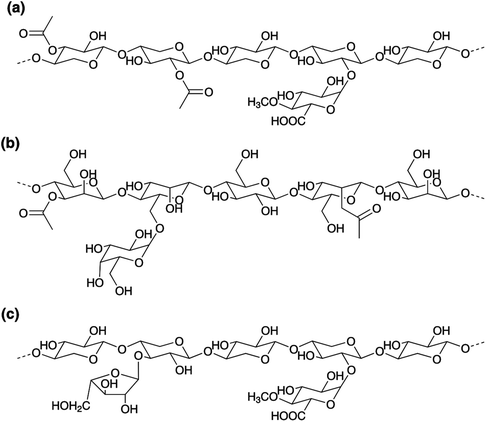
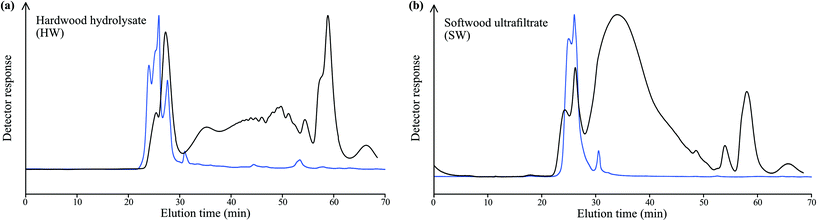
The compositions of the hemicellulose-rich fractions were analysed by carbohydrate analysis, Table S3.† As expected, HW contains mainly xylose, whereas SW contains mainly mannose and glucose. The main parts of the hemicelluloses in the HW and SW are in their polymeric form, which was deduced from SEC results. Representative chemical structures of the most common hemicelluloses in hardwood (O-acetyl-4-O-methylglucuronoxylan, AcGX), and softwood (O-acetyl-galactoglucomannan, GGM, and arabino-4-O-methylglucuronoxylan, AGX) are shown in Fig. 3. Both HW and SW also contain lignin, 3.6 and 1.3% by weight, respectively.The molecular weights of the samples were evaluated by SEC using dual detectors; a PAD-detector to detect carbohydrates and a UV-detector to assess the aromatic lignin-containing fractions, Fig. 4. As can be observed, there are responses from both the PAD-detector (carbohydrates, black line) and the UV-detector (lignin, blue line) for both HW and SW. Based on the chromatograms it can be concluded that the lignin-content resides in the fractions of the samples with largest hydrodynamic volume, i.e., higher molecular weights (peaks between 20 and 30 min). However, no information whether there are covalent bonds between the carbohydrates and the lignin-residues, or if it is a physical mixture of carbohydrates, lignin, and carbohydrate–lignin complexes, is obtained.
The SW-sample has higher molecular weight and less broad molecular weight distribution, Table 2. Note that the highest molecular weight compounds in both samples exceed the range of the calibration standards used (Mw = 212![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) resulting in unreliable numbers.
000 g mol−1) resulting in unreliable numbers.
| M n [g mol−1] | M w [g mol−1] | Polydispersity Đ | |
|---|---|---|---|
| Hardwood hydrolysate (HW) | 400 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
77 |
| Softwood ultrafiltrate (SW) | 1200 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35 |
The RI-traces of the chromatograms can also be compared, Fig. 5. Both the HW- and SW-samples show peaks at low molecular weight values (peak A), attributed to monosaccharides. Apart from that, SEC does not provide conclusive information other than that the majority of the SW-sample is composed of molecules of intermediate molecular weights in contrast to HW which is mainly composed of a low and a high molecular weight fraction with little in between. HW and SW have higher molecular weights than expected for hemicelluloses obtained from wood. This can be explained by the reaction that may occur between hemicellulose and lignin in native wood, and which can also be enabled by high-temperature treatment or acidic conditions during pulping.43 These conditions may lead to the formation of lignin–carbohydrate complexes, resulting in higher molecular weight fractions; from Fig. 4 it can be seen that the highest molecular weight fractions in both the HW- and SW-samples contain lignin. SW has less of the highest molecular weight fraction; the lower amount of lignin in SW gives less opportunity for complexing into higher molecular weight aggregates.
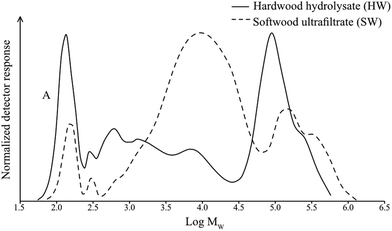 | ||
| Fig. 5 Size exclusion chromatograms of molecular weight distributions of hemicelluloses in the hardwood hydrolysate (HW, solid line) and softwood ultrafiltrate (SW, dashed line). | ||
The molecular weights and molecular weight distributions of the lignin fractions in HW and SW are shown in Fig. S1 and Table S4.† The molecular weights of lignin are similar in the two samples but the polydispersity is higher for SW (Đ = 7.0), compared with HW (Đ = 2.5).
In our previous work,21 commercial xylan from beech wood (≥96%),44 that has been used for comparison in this study, was characterized with respect to sugar content using ion-exchange chromatography; it was found to contain mainly xylose but traces of arabinose, galactose, glucose and rhamnose were also detected. The molecular weight was approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 according to SEC. The lignin content, determined by the Klason method, was approximately 1% by weight.
000 g mol−1 according to SEC. The lignin content, determined by the Klason method, was approximately 1% by weight.
3.2 Bond strength
Adhesives were formulated using Xyl, HW, or SW, in combination with PVAm or CS. Wood veneers were bonded, and bond properties were determined by evaluation of dry, redried, and wet tensile shear strengths. A commercial wood adhesive, PVAc, was used as a reference.None of the hemicelluloses (Xyl, HW, SW) by themselves exhibited sufficient bonding performance, Fig. 6. The bond lines were very brittle, and the dry bond strengths low. All bonded wood veneers delaminated when immersed in water. Increasing the dry content of the HW- and SW-dispersions had no effect on the bonding performance. These observations were in accordance to what has already been reported for Xyl.21
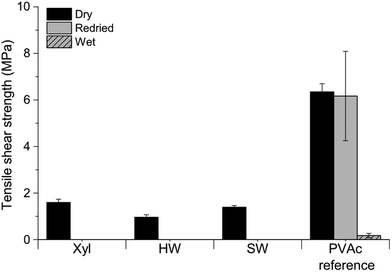 | ||
| Fig. 6 Tensile shear strength of wood veneers bonded with xylan (Xyl), hardwood hydrolysate (HW), and softwood ultrafiltrate (SW). PVAc was used as a reference. | ||
Inspired by the results of our previous work, showing improved bonding performance when Xyl was combined with PVAm,21 we further investigated HW- and SW–PVAm and compared them with Xyl–PVAm. As PVAm is a non-renewable polymer, we identified CS as a sustainable bio-based amino-functional alternative. Veneers were bonded and tensile shear strengths were evaluated, Fig. 7a and b.
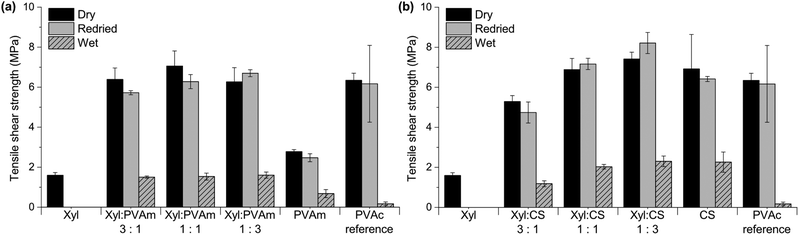 | ||
| Fig. 7 Tensile shear strength of wood veneers bonded with (a) xylan (Xyl) and PVAm, and (b) xylan (Xyl) and chitosan (CS). PVAc was used as a reference. | ||
All adhesives containing PVAm were gel-like and quite difficult to apply evenly on the veneer, but could be smeared out with a spatula. Attempts were made to measure the viscosity of these adhesives using a cone and plate viscometer, but the results were inconclusive. This is most likely due to the experimental setup not allowing measurements over the whole viscosity range. Images showing the appearance of the adhesives can be found in the ESI in Fig. S2.† From Fig. 7a it can be seen that joints glued with Xyl–PVAm had similar strengths to the reference PVAc adhesive, but that the combination of Xyl and PVAm resulted in significantly higher dry and redried strengths than both Xyl and PVAm alone, suggesting a non-linear additive effect. The wet strength of Xyl–PVAm was improved compared with PVAm and the PVAc reference. All improvements brought by the addition of PVAm were independent of the ratio Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVAm used in this study, as these adhesive formulations exhibited similar strengths.
PVAm used in this study, as these adhesive formulations exhibited similar strengths.
Fig. 7b shows tensile shear strengths of veneers bonded with Xyl and CS. CS, a sustainable alternative to PVAm, showed good bonding properties, both alone and in combination with Xyl. The CS dispersion had a high viscosity, but it was possible to apply with a spatula. Images showing the appearance of the adhesives can be found in the ESI in Fig. S2.† Wood veneers bonded with CS alone exhibited similar results as for the PVAc reference regarding bond strength of the dry and redried samples. Interestingly though, the wet strength was significantly higher, more than ten times; as in the case of Xyl–PVAm, the combination of Xyl and CS also brings about superior wet strength. Unlike the Xyl–PVAm adhesives, the Xyl–CS adhesives exhibited a more linear addition effect upon variation of component ratio; the bond strengths were increased with increasing amounts of CS. With Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS ratio 3
CS ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the adhesive became easier to apply on veneers, but the dry and redried strengths were lower in comparison with the PVAc reference. With higher amount of CS, ratios 1
1, the adhesive became easier to apply on veneers, but the dry and redried strengths were lower in comparison with the PVAc reference. With higher amount of CS, ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the bond strengths were increased and higher than for both CS alone and for the PVAc reference, but on the other hand the viscosity was unfavourably high for these compositions.
3, the bond strengths were increased and higher than for both CS alone and for the PVAc reference, but on the other hand the viscosity was unfavourably high for these compositions.
Fig. 7a and b show that it is possible to achieve good bond strength, even in wet state, when Xyl is mixed with amino-functional components (PVAm and CS). The goal of this work was to investigate if also dispersions containing less well-defined hemicelluloses can be used as wood adhesives when combined with PVAm or CS.
Fig. 8 and 9 show tensile shear strengths of veneers bonded with HW and SW mixed with either PVAm or CS, and both HW and SW showed promising bonding performance in combination with these amino-functional polymers.
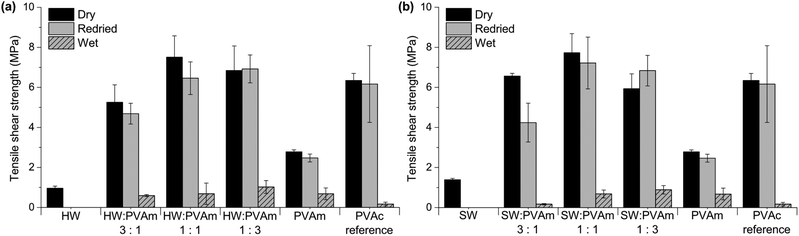 | ||
| Fig. 8 Tensile shear strength of wood veneers bonded with (a) hardwood hydrolysate (HW) and PVAm and (b) softwood ultrafiltrate (SW) and PVAm. PVAc was used as a reference. | ||
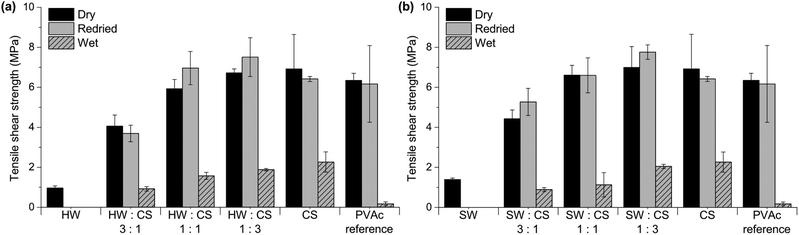 | ||
| Fig. 9 Tensile shear strength of wood veneers bonded with (a) hardwood hydrolysate (HW) and chitosan (CS) and (b) softwood ultrafiltrate (SW) and chitosan (CS). PVAc was used as a reference. | ||
Fig. 8a shows tensile shear strengths of veneers bonded with HW and PVAm, and Fig. 8b those bonded with SW and PVAm. Both systems showed similar trends regarding dry and redried strengths, with higher strengths for both HW–PVAm and SW–PVAm mixtures compared with the strengths of HW or SW alone. Overall, HW and SW performed similarly as adhesives, irrespectively of having different carbohydrate compositions, and slightly different molecular weights and lignin contents.
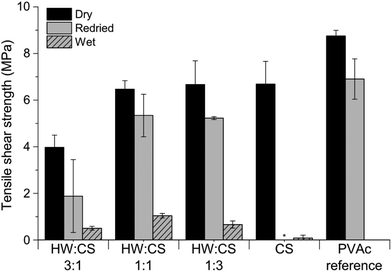
The tensile shear strengths of veneers bonded with HW– and SW–CS adhesives are shown in Fig. 9. CS had a positive effect on the bonding performance of both HW and SW adhesives, and with increasing amount of CS, strengths were also increasing. With the highest amount of HW or SW (ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the bond strengths of the dry and redried samples were not as high as the PVAc reference, but the wet strengths were superior. With equal amounts of HW or SW, and CS (ratio 1
1) the bond strengths of the dry and redried samples were not as high as the PVAc reference, but the wet strengths were superior. With equal amounts of HW or SW, and CS (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and with more CS (ratio 1
1), and with more CS (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) the bonding performance was improved, being superior to the PVAc reference and on par to CS alone. Overall, the bond strengths of the CS-containing samples in the wet state were exceptionally good; wet veneers even showed significant fibre tear after the tensile shear strength test, which is an indication of a strong adhesive. There were no significant differences between the adhesives containing HW or SW, but the commercial Xyl showed slightly better bonding performance than HW or SW, in combination with CS. However, the viscosity of Xyl–CS dispersions is higher than HW- and SW–CS and therefore more difficult to spread on the wood substrate, which is an important parameter when considering possible adhesive applications. A dispersion of CS alone exhibits good bonding properties, but its viscosity is also too high, why application on the wood veneer is difficult. By combining CS with hemicellulose-rich liquids (HW or SW), viscosities are lower which allows for easier application, and the adhesives maintain good bond strengths.
3) the bonding performance was improved, being superior to the PVAc reference and on par to CS alone. Overall, the bond strengths of the CS-containing samples in the wet state were exceptionally good; wet veneers even showed significant fibre tear after the tensile shear strength test, which is an indication of a strong adhesive. There were no significant differences between the adhesives containing HW or SW, but the commercial Xyl showed slightly better bonding performance than HW or SW, in combination with CS. However, the viscosity of Xyl–CS dispersions is higher than HW- and SW–CS and therefore more difficult to spread on the wood substrate, which is an important parameter when considering possible adhesive applications. A dispersion of CS alone exhibits good bonding properties, but its viscosity is also too high, why application on the wood veneer is difficult. By combining CS with hemicellulose-rich liquids (HW or SW), viscosities are lower which allows for easier application, and the adhesives maintain good bond strengths.
For most compositions containing HW or SW, and CS, the bond strength of the veneers was preserved or higher after water treatment and redrying. Inspired by the surprisingly good water resistance of all CS-containing adhesives, a water-resistance test at elevated temperature was performed. Since there was no significant difference between the tensile strengths of HW– and SW–CS adhesives, only the systems with HW were evaluated in the wet-60 °C test. From Fig. 10 it can be seen that all HW–CS adhesives showed remarkable bonding performance and the veneers held together in hot water. The wood specimens bonded with the PVAc reference delaminated in the hot water, as expected. For adhesives containing large amounts of water, which is the case with all adhesive systems we tested, except for the PVAc reference, a higher press temperature is usually required. PVAc is a thermoplastic wood adhesive, normally pressed at room temperature, and not at 120 °C as in this study. To compare the HW–CS adhesives with the PVAc reference under “normal PVAc-adhesive conditions”, HW–CS adhesives were pressed at room temperature and the tensile shear strengths are presented in Fig. 11. Remarkably good bonding performance was obtained even though the water content of the adhesives containing HW and CS was very high, 80 wt%. CS itself showed good dry bond strength and the veneers held together in water, but the edges started to delaminate during drying and therefore the redried samples could not be tested. The trend for the RT-pressed samples was similar to the HW–CS adhesives pressed at 120 °C; with lower strengths for ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and higher for ratios 1
1, and higher for ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, but not as high as the strengths of the PVAc-reference adhesive. The wet strength, on the other hand, of all HW–CS adhesives was significantly higher compared with the PVAc reference, which showed no wet strength.
3, but not as high as the strengths of the PVAc-reference adhesive. The wet strength, on the other hand, of all HW–CS adhesives was significantly higher compared with the PVAc reference, which showed no wet strength.
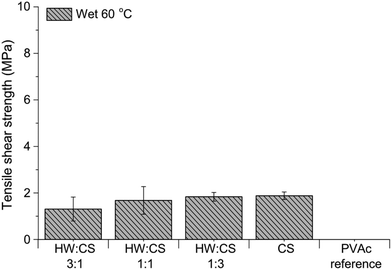 | ||
| Fig. 10 Tensile shear strengths of wood veneers bonded with hardwood hydrolysate (HW) and chitosan (CS), and PVAc reference, after conditioning in water at 60 °C for 3 h. | ||
3.3 Interactions between adhesive components
In this study, the underlying adhesion mechanisms in the adhesives are not understood in detail; the bonding performance is most likely a combined effect of mechanical interlocking, secondary forces and covalent bonds (Fig. 12). The presence of small amounts of lignin is expected to add value to the adhesives’ performance, being a hydrophobic component with a potential to improve the water resistance and possibly forming covalent bonds.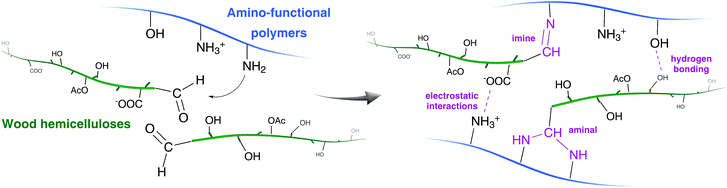 | ||
| Fig. 12 Overview of possible interactions between amino-functional polymers and wood hemicelluloses. | ||
HW and SW together with the amino-functional polymers were found to provide very good bonding performance regarding bond strength and water resistance. Pelton and coworkers have proposed, when discussing the role of PVAm as a wet strength additive in wood pulp, that the amino groups in PVAm can form covalent bonds with the aldehyde groups at the reducing chain ends of cellulose, resulting in imine- and aminal bonds.22,45,46 Also chitosan has been reported to improve the strength of wood-pulp webs.47–49 As the hemicelluloses in HW and SW have significantly lower molecular weights than cellulose in wood, the number of chain ends, and thereby also the number of aldehyde groups, is substantially higher. Imine bond formation can also be facilitated by longer pressing times and higher temperatures, as utilized in this study, compared with paper making as in the Pelton studies mentioned above.22,45,46 However, even though only a small amount of covalent imine bonds is formed in an adhesive, they can have a substantial impact on the bonding performance. According to Pelton et al., there are also contributions to the wet strength from physical bonding through electrostatic interactions between amines in PVAm and carboxyls on oxidized cellulose.22 John and coworkers adsorbed a PVAm-based polymer on wood veneers, suggesting that adsorption is possible due to hydrogen bonds and electrostatic interactions between wood and PVAm.50 In our study, PVAm adhesives showed moderate adhesive strengths, but they were improved when Xyl, HW or SW were added. These improvements are suggested to be due to the hydroxyl- and carboxyl groups in hemicelluloses which increase the possibility for hydrogen bonds and electrostatic interactions. CS has both amino- and hydroxyl groups, leading to a higher strength compared with PVAm, lacking hydroxyl groups.
FTIR analysis was performed to elucidate the possible formation of chemical interactions in the adhesive. A thin bond line in glued veneer samples is difficult to analyse by FTIR; therefore, films were hot-pressed from HW, CS, and mixtures thereof by using the same conditions as for the veneer samples. The FTIR spectra of samples from the film with HW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS ratio 3
CS ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown in Fig. S3.† Hydrogen bonding between HW and CS could be confirmed by the shift of the carbonyl peak of HW acetyl groups (from 1731 to 1721 cm−1) which is in accordance with what has been observed previously for the amide peak in CS when mixed with cellulose.51 It was not possible to confirm further electrostatic interactions and hydrogen bonding due to the overlapping of HW and CS peaks. A peak in the region for imine bonds (1630–1690 cm−1) was present in both the FTIR spectra of the HW–CS film and the pure CS film. It is difficult to confirm if this peak in the HW–CS spectrum emanates from imine-bond formation between HW and CS or between CS chains alone. Moreover, the imine region is close to the amide I band; therefore, we cannot rule out that the peak in this region is the amide I peak from CS that has shifted due to secondary interactions after pressing and mixing with HW. The formation of imine bonds between chitosan and aldehydes has been shown previously by others.52 To corroborate the formation of covalent bonds between HW and CS, solubility tests of hot-pressed films of HW, CS, and their mixtures in dilute acetic acid (pH = 2) were conducted (Fig. 13). Pure films of HW were almost completely soluble, while pure films of CS had a small insoluble fraction. When the two components were mixed the insoluble fractions were larger, being largest for the HW
1 are shown in Fig. S3.† Hydrogen bonding between HW and CS could be confirmed by the shift of the carbonyl peak of HW acetyl groups (from 1731 to 1721 cm−1) which is in accordance with what has been observed previously for the amide peak in CS when mixed with cellulose.51 It was not possible to confirm further electrostatic interactions and hydrogen bonding due to the overlapping of HW and CS peaks. A peak in the region for imine bonds (1630–1690 cm−1) was present in both the FTIR spectra of the HW–CS film and the pure CS film. It is difficult to confirm if this peak in the HW–CS spectrum emanates from imine-bond formation between HW and CS or between CS chains alone. Moreover, the imine region is close to the amide I band; therefore, we cannot rule out that the peak in this region is the amide I peak from CS that has shifted due to secondary interactions after pressing and mixing with HW. The formation of imine bonds between chitosan and aldehydes has been shown previously by others.52 To corroborate the formation of covalent bonds between HW and CS, solubility tests of hot-pressed films of HW, CS, and their mixtures in dilute acetic acid (pH = 2) were conducted (Fig. 13). Pure films of HW were almost completely soluble, while pure films of CS had a small insoluble fraction. When the two components were mixed the insoluble fractions were larger, being largest for the HW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS ratio 1
CS ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The FTIR peak in the region of imine bonds was present for the insoluble fractions, but absent for the soluble fractions. The combined results of FTIR and the solubility tests indicate the formation of covalent imine bonds when HW and CS are mixed. Since HW has similar structure to the hemicellulose component in wood, it is likely that interactions proposed for the HW–CS films also could occur between the adhesive and the wood substrate.
3. The FTIR peak in the region of imine bonds was present for the insoluble fractions, but absent for the soluble fractions. The combined results of FTIR and the solubility tests indicate the formation of covalent imine bonds when HW and CS are mixed. Since HW has similar structure to the hemicellulose component in wood, it is likely that interactions proposed for the HW–CS films also could occur between the adhesive and the wood substrate.
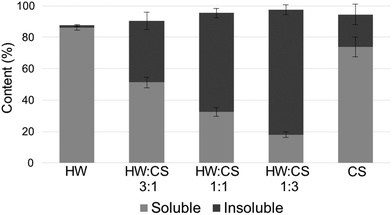 | ||
Fig. 13 Results from the solubility tests of films from hardwood hydrolysate (HW), chitosan (CS), and their mixtures (HW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS) in dilute acetic acid (pH = 2). CS) in dilute acetic acid (pH = 2). | ||
The HW- and SW formulations showed comparable appearance and bonding performance even though they differ with respect to chemical composition, and somewhat in molecular weight and lignin content. The extraction- and work-up procedures are also different for these samples. These observations suggest that the range (i.e., regarding hemicellulose source, extraction–fractionation-work-up procedures, etc.) of hemicellulose-rich fractions that can be used in adhesive applications is very broad, and that pure or well-defined hemicelluloses are not required to obtain adhesives with sufficient bonding performance. This is attractive from a commercialisation point of view, as hemicelluloses can be retrieved from (the sidestreams of) a broad range of forest products-related processes.
In the beginning of the project, we speculated that chitin could form from chitosan in the presence of acetic acid at elevated press temperatures, which could increase the water resistance and strength of the adhesive, since chitin is more insoluble in water and less hydrophilic than chitosan. However, neither XRD or FTIR showed any indication of the formation of chitin in our adhesives.
4. Conclusions
In this study, we have demonstrated that it is possible to attain a fully bio-based wood adhesive, valorising hemicellulose-rich sidestreams from pulping processes without the need for formaldehyde or any other hazardous chemicals. The performance of hemicellulose-based wood adhesives was evaluated with different sources of hemicellulose, a commercial xylan from beech wood and two hemicellulose-rich liquids: hydrolysate from pre-hydrolysis of wood chips from hardwood, and ultrafiltered process-water from a thermomechanical pulp mill using softwood. The addition of an amino-functional polymer, PVAm, results in an adhesive with good dry bond strength and water resistance. The bond strengths of the dry- and redried samples are comparable to the commercial PVAc reference while the wet strengths of the hemicellulose-based adhesives are superior. This is remarkable, considering that the hemicellulose-based adhesives only have 20 wt% dry content, which is significantly lower compared with the PVAc of 50 wt% dry content. By using chitosan as a bio-based alternative to PVAm, fully bio-based wood adhesives were achieved with superior bonding performance, especially regarding water resistance. Wood veneers bonded with hemicellulose-chitosan adhesives can withstand immersion in water for four days at room temperature. Wood veneers bonded with hardwood hydrolysate-chitosan also show remarkable water resistance at elevated temperature, withstanding water immersion at 60 °C. Hemicellulose-chitosan adhesives can even be pressed at room temperature, while reaching almost the same bonding performance. The softwood and hardwood hemicellulose-rich liquids performed similarly, regardless of their structural differences. These results show that the source of hemicellulose and the extraction process are not critical parameters for their application as wood adhesives in this context. This is advantageous since it opens up possibilities for the adhesive raw material to be retrieved from a broad range of forest products-related processes, increasing the availability and profitability significantly, while at the same time making more efficient and sustainable use of the biomass.Abbreviations
| PVAm | Poly(vinyl amine) |
| PVAc | Poly(vinyl acetate) |
| Xyl | Xylan |
| HW | Hardwood hydrolysate |
| SW | Softwood ultrafiltrate |
| CS | Chitosan |
Author contributions
Tijana Todorovic: Methodology, validation, investigation, writing – original draft, writing – review & editing, visualization; Emelie Norström: Methodology, validation, investigation, writing – original draft, visualization; Farideh Khabbaz: Methodology, supervision; Jörg Brücher: Methodology, resources, writing – review & editing, supervision; Eva Malmström: Conceptualization, methodology, validation, investigation, resources, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition; Linda Fogelström: Conceptualization, methodology, validation, investigation, resources, writing – original draft, writing – review & editing, visualization, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The VINN Excellence Centre BiMaC Innovation is acknowledged for financial support. Parts of this work has also been carried out within the national platform Treesearch and is funded through the strategic innovation programme BioInnovation, a joint effort by Vinnova, Formas and the Swedish Energy Agency. The authors would also like to acknowledge funding from the Knut and Alice Wallenberg foundation through Wallenberg Wood Science Center.References
- GrandViewResearch, Wood Adhesives Market Size, Share & Trend Analysis Report By Product (UF, MUF, Soy-Based), By Substrate (OSB, Plywood, MDF), By Application, By Region, And Segment Forecasts, 2019–2025, https://www.grandviewresearch.com/industry-analysis/wood-adhesives-market (accessed 2020-08-03, 2020).
- C. R. Frihart, in Handbook of wood chemistry and wood composites, ed. R. M. Rowell, CRS Press, Florida, 2005, pp. 215–278 Search PubMed.
- V. Hemmilä, S. Adamopoulos, O. Karlsson and A. Kumar, RSC Adv., 2017, 7, 38604–38630 RSC.
- IARC, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 100F, Lyon, France, 2012 Search PubMed.
- KIFS 2017:7 om kemiska produkter och biotekniska organismer, Swedish Chemical Agency KEMI, 2017.
- EPA United States Environmental Protection Agency, Formaldehyde Emission Standards for Composite Wood Products, https://www.epa.gov/formaldehyde/formaldehyde-emission-standards-composite-wood-products, (accessed 2018-03-13).
- E. Norström, D. Demircan, L. Fogelström, F. Khabbaz and E. Malmström, in Applied Adhesive Bonding in Science and Technology, ed. H. Özer, InTech, Rijeka, 2018, ch. 04, DOI: DOI:10.5772/intechopen.72072.
- A. Teleman, in The Ljungberg Textbook Wood Chemistry and Wood Biotechnology, ed. G. Gellerstedt, Stockholm, 2009, ch. 5, pp. 87–107 Search PubMed.
- Global Report on Food Crises 2019, FSIN Food Security Information Network, 2019.
- L. A. Heinrich, Green Chem., 2019, 21, 1866–1888 RSC.
- M. G. D. Baumann and A. H. Conner, in Handbook of adhesive technology, ed. A. Pizzi and K. Mittal, Marcel Dekker, Inc., New York, 2nd edn, 2003, ch. 22, pp. 495–510 Search PubMed.
- P. A. Kumar, J.-D. Mathias and P. Michaud, Rev. Adhes. Adhes., 2013, 1, 312–345 CrossRef.
- R. V. Gadhave, P. A. Mahanwar and P. T. Gadekar, Open J. Polym. Chem., 2017, 7, 19–32 CrossRef CAS.
- A. Abuarra, R. Hashim, S. Bauk, S. Kandaiya and E. T. Tousi, Mater. Des., 2014, 60, 108–115 CrossRef CAS.
- D. Paiva, C. Gonçalves, I. Vale, M. Bastos and F. D. Magalhães, Polymers, 2016, 8, 259 CrossRef PubMed.
- E. Norström, L. Fogelström, P. Nordqvist, F. Khabbaz and E. Malmström, Ind. Crops Prod., 2014, 52, 736–744 CrossRef.
- E. Sjöström, in Wood Chemistry (Second Edition), ed. E. Sjöström, Academic Press, San Diego, 2nd edn, 1993, pp. 51–70, DOI:10.1016/B978-0-08-092589-9.50007-3.
- R. P. Roger, M. Rowell, J. S. Han, J. S. Rowell and M. A. Tshabalala, in Handbook of Wood Chemistry and Wood Composites, ed. R. M. Rowell, CRC Press, Florida, 1st edn, 2005, ch. 3, pp. 35–74 Search PubMed.
- C. M. Hansen and A. Björkman, Holzforschung, 1998, 52, 335–344 CrossRef CAS.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- E. Norström, L. Fogelström, P. Nordqvist, F. Khabbaz and E. Malmström, Eur. Polym. J., 2015, 67, 483–493 CrossRef.
- J. L. DiFlavio, R. Bertoia, R. Pelton and M. Leduc, Pulp and S. Paper Fundamental Research, Bury, 2005 Search PubMed.
- R. Pelton, Langmuir, 2014, 30, 15373–15382 CrossRef CAS PubMed.
- T. Chandy and C. P. Sharma, Biomater., Artif. Cells, Artif. Organs, 1990, 18, 1–24 CrossRef CAS PubMed.
- G. S. Dhillon, S. Kaur, S. K. Brar and M. Verma, Crit. Rev. Biotechnol., 2013, 33, 379–403 CrossRef CAS PubMed.
- K. M. Vårum, M. W. Antohonsen, H. Grasdalen and O. Smidsrød, Carbohydr. Res., 1991, 211, 17–23 CrossRef.
- M. Rinaudc, G. Pavlov and J. Desbrières, Int. J. Polym. Anal. Charact., 1999, 5, 267–276 CrossRef.
- X. Ji, Y. Dong, T. T. Nguyen, X. Chen and M. Guo, R. Soc. Open Sci., 2018, 5, 172002 CrossRef PubMed.
- X. Ji and M. Guo, Int. J. Adhes. Adhes., 2018, 82, 8–13 CrossRef CAS.
- J. Shang, H. Liu, C. Qi, X. Chen and K. Guo, J. Adhes. Sci. Technol., 2015, 29, 2334–2344 CrossRef CAS.
- J. Shang, H. Liu, C. Qi, K. Guo and V. C. Tran, J. Appl. Polym. Sci., 2015, 132, 42202 Search PubMed.
- K. Umemura, A. Inoue and S. Kawai, J. Wood Sci., 2003, 49, 221–226 CrossRef CAS.
- K. Umemura, A. Mihara and S. Kawai, J. Wood Sci., 2010, 56, 387–394 CrossRef CAS.
- A. K. Patel, P. Michaud, E. Petit, H. de Baynast, M. Grediac and J.-D. Mathias, J. Appl. Polym. Sci., 2013, 127, 5014–5021 CrossRef CAS.
- S. Peshkova and K. Li, J. Biotechnol., 2003, 102, 199–207 CrossRef CAS PubMed.
- V. Ibrahim, G. Mamo, P.-J. Gustafsson and R. Hatti-Kaul, Ind. Crops Prod., 2013, 45, 343–348 CrossRef CAS.
- K. Umemura, Y. Iijima and S. Kawai, J. Adhes. Soc. Jpn., 2005, 41, 216–222 CrossRef CAS.
- Holgers , Holgers Stugmaterial AB, holgers.se, (accessed 09.11.2020.).
- SCAN-CM71:09, Carbohydrate Composition, Scandinavian Pulp, Paper and Board Testing Committee, 2009.
- EN204, European Standard EN 204:2016. Classification of thermoplastic wood adhesives for non-structural applications, European Committee for Standardization, 2016.
- EN205, European Standard EN 205:2016. Adhesives – Wood adhesives for non-structural applications – Determination of tensile shear strength of lap joints, European Committee for Standardization, 2016.
- Inspired by Chinese Standard GB/T17657-2013, Chinese National Standardization Menagement Committee, 2013.
- D. Tarasov, M. Leitch and P. Fatehi, Biotechnol. Biofuels, 2018, 11, 269 CrossRef PubMed.
- SigmaAldrich, Certificate of Analysis Xylan from beechwwod BCBL2915V, 2013 Search PubMed.
- R. Pelton, D. Yang and E. Gustafsson, BioResources, 2019, 14, 2389–2419 Search PubMed.
- D. Yang, T. C. Stimpson, J. Soucy, A. Esser and R. H. Pelton, Cellulose, 2019, 26, 341–353 CrossRef CAS.
- L. Makhlouf and I. P. Ivan, Nord. Pulp Pap. Res. J., 1991, 6, 99–103 CrossRef.
- L. Markhlorf and I. P. Ivan, Nord. Pulp Pap. Res. J., 1992, 7, 174–180a CrossRef.
- Z. Song, G. Li, F. Guan and W. Liu, Polymers, 2018, 10, 389 CrossRef PubMed.
- R. John, K. Trommler, K. Schreiter, C. Siegel, F. Simon, A. Wagenführ and S. Spange, BioResources, 2017, 12, 8134–8159 CAS.
- C. Stefanescu, W. H. Daly and I. I. Negulescu, Carbohydr. Polym., 2012, 87, 435–443 CrossRef CAS.
- L. Marin, B. Simionescu and M. Barboiu, Chem. Commun., 2012, 48, 8778–8780 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc04273k |
| This journal is © The Royal Society of Chemistry 2021 |