Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Provenancing of cement using elemental analyses and isotope techniques – the state-of-the-art and future perspectives
Anera
Kazlagić
 *a,
Jochen
Vogl
*a,
Jochen
Vogl
 a,
Gregor J. G.
Gluth
a,
Gregor J. G.
Gluth
 b and
Dietmar
Stephan
b and
Dietmar
Stephan
 c
c
aFederal Institute for Materials Research and Testing, Division 1.1 Inorganic Trace Analysis, Richard-Willstäter-Straße 11, 12489 Berlin, Germany. E-mail: anera.kazlagic@bam.de
bFederal Institute for Materials Research and Testing, Division 7.4 Technology of Construction Materials, Unter den Eichen 87, 12205 Berlin, Germany
cTechnische Universität Berlin, Department of Civil Engineering, Building Materials and Construction Chemistry, Gustav-Meyer-Allee 25, 13355 Berlin, Germany
First published on 3rd September 2021
Abstract
With the aim of identifying the origin and the manufacturer of a cement, a reliable procedure that provides unambiguous results is needed. Such procedure could resolve practical issues in damage research, liability issues and forensic investigations. A substantial number of attempts for fingerprinting of building materials, including cement, has already been carried out during the last decades. Most of them were based on concentration analysis of the main elements/components. This review provides an overview of provenance studies of cement and the main approaches commonly used. Provenance studies of cement via isotope techniques are also presented and discussed as representatives of the state-of-the-art in the field. Due to the characteristic properties and the occurrence of carefully selected isotope ratios, unique fingerprints of different kinds of materials can be provided by these methods. This property has largely been explored in various scientific fields such as geo- and cosmochemistry, food forensics, archaeology, geochronology, biomedical studies, and climate change processes. However, the potential of isotope techniques in cement and concrete research for provenance studies has barely been investigated. Therefore, the review outlines a suitable approach using isotope ratios, which could lead to reliable provenancing of cementitious materials in the future.
Introduction
The built environment is one of the most significant achievements of human civilisation, because with buildings it provides protection against environmental influences and the basis for the production of goods, but with transport systems, it also provides the basis for all forms of traffic. Concrete is the basis for this in most cases, which is why concrete has become the material that is artificially produced in the largest quantity by human hands during the last hundred years. The concrete properties are dominated by the reactive component cement, which is the synthetic chemical product produced in the largest quantity. Due to the ageing of concrete as a building material due to environmental influences, but also due to application errors caused by faulty constructions or wrong mix compositions of concrete, various structural damages occur worldwide, which cause high costs and, in severe cases, even endanger human lives. After their occurrence, damages are being preventively investigated and researched. Intending to minimise the consequences of damages, it is crucial to identify the source and the cause that lead to such events. Therefore, one of the main reasons for cement provenancing is identifying the causes of failure in concrete structures. Poor and substandard building material, primarily steel reinforcement, structural steel, and cement represent the main causes of building failure and collapse.1 Concrete roof collapses are occurring worldwide.2 According to a recent review by Proske and Schmid,3 the annual collapse frequency of buildings is 2.4 × 10−7 per year in industrialised countries, and 4.7 × 10−6 per year in developing countries, in many cases caused by insufficient quality of the employed building materials. The authors also determined mortality rate values of building collapses, ranging from 9.8 × 10−8 to 1.5 × 10−6 per year, for industrialised and developing countries, respectively, which is in agreement with the value obtained by Blockley.4 Furthermore, bridge collapse frequencies worldwide have been reported to be on the order of magnitude of 10−4 per year.5Many government agencies tried to develop a protocol and a guideline to reduce and prevent the risk of such catastrophes. The type and origin of the construction and raw materials used, and the manufacturer of the components are of great relevance not only for avoidance and damage assessment but also for the resulting liability issues. This is also of interest when considering the stability of concrete,6,7 marble,8,9 and stone10–15 in historic structures and investigating the origin of the raw materials from which those structures were made. For example, previous studies emphasized the investigation of raw materials from which buildings in Portugal were constructed.16 In most structural failure cases, analysis of the building materials, including cement as the major component, is one of the key issues for understanding the quality and the strength of the structure. Testing institutes are also interested in provenance studies, since they are sometimes asked whether two cementitious samples are identical or are of the same origin.17
Recycling of rubble may also require provenance studies in some cases. Furthermore, the determination of rubble provenance can contribute to expand the knowledge about this material, and also about possible ways to return the material to the construction processes.
Beyond this, cement provenancing is required in forensic investigations. Given the variety of possible circumstances that may be encountered on the crime scene, any building material, such as cement, mortar, or concrete, may hold evidentiary significance. This includes cement particles or dust found at a crime scene. Those particles can serve as a physical evidence linking a suspect(s) to the victim or the crime scene. Knowing the origin of the cement and concrete particles can be beneficial for locating the crime scene, tracking back the particles to a packaging unit or providing further evidence for use in court.
Geographical provenancing of any kind of material can be described as an act of identifying the place of origin or the source of the material. Knowing the geographical origin of a specific material has been very useful in fields such as forensics and archaeology for unravelling residence histories of suspects or trading routes between past cultures. The basis of provenance studies is that there are specific, recurrent and definable interdependences between raw materials and the final products derived from them.18
Therefore, we can summarise three main cases:
(1) Finished product correlates to a raw material used in the production, e.g. marble.
(2) Processed material, within which one component dominates, either in terms of the mass fraction or in other terms, e.g. curse tablets made of pure lead.
(3) Complex mixtures or a highly processed material, e.g., alloys, cement.
The provenancing of any material can be determined by utilising specific properties of the material under investigation and it's preceding (original) raw material.
Cements for general purposes are defined in the standard EN 197-1:2011,19 which differentiates between five main categories: CEM I Portland cement, CEM II Portland-composite cement, CEM III Blast furnace cement, CEM IV Pozzolanic cement, CEM V Composite cement. Portland cement clinker, the main component of ordinary Portland cement (OPC; CEM I according to EN 197-1:2011), is produced by sintering a finely ground raw meal consisting mainly of limestone and clay or the natural mixture marl from local materials. Other natural raw materials, such as quartz sand, or secondary raw materials, such as foundry sands, slags, etc., can also be used to adjust the composition. In the rotary kiln, the mixture is heated to a temperature of approximately 1450 °C. In addition to hard coal and lignite, a complex mix of different secondary fuels is usually used in clinker production to achieve high temperatures. The OPC clinker is mixed with a certain amount of different calcium sulphate minerals and then finely ground to give OPC. The calcium-rich components of OPC that do not originate from local sources are primarily the calcium sulphates which either come from industrial by-products such as gypsum from flue gas desulphurisation plants (FGD gypsum20) or partly from natural sources such as natural anhydrite and gypsum. Cements other than OPC contain additional constituents, such as limestone powder, ground granulated blast furnace slag, natural pozzolana, fly ash or burnt oil shale. Due to this broad range of components with different geographic origin, the provenancing of cement is not straightforward.
This paper provides an overview of published provenance studies of cement and concrete and lists the main approaches usually adopted. Provenance studies of cement utilising isotope techniques are also presented and discussed as representatives of the state-of-the-art in the field.
Previous attempts for cement provenancing
Elemental fingerprinting
Trace elements analysis is being performed in different scientific fields and for provenance studies of various inorganic materials. Trace elemental pattern was used for establishing the provenance of pottery,21 archaeological mortars,22,23 as well as for clinker and cement, which is described in this section.For element to be used in cement provenance analysis, the local raw materials must be the predominant source. It should not originate from the ancillary components, the used fuel, or the furnace. Furthermore, its concentration must be independent of the temperature fluctuations in the kiln. For the concept to be transferred from cement to concrete, the elements must be highly immobilised in the alkaline pore solution.24 While reviewing the literature, we noticed that many authors investigated major, minor and trace elements in the studies. However, trace element label in these studies partially differ from the definition put forward by IUPAC.25 Therein, trace element is considered as any element having an average mass fraction of less than about 100 μg g−1.
The first paper on the provenancing of cement was published in 1993 by Goguel and St John in two parts. In the first part,24 major, minor and trace elements present in New Zealand cements were investigated by semiquantitative Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and quantitative Atomic Absorption (AA), Atomic Emission (AE) and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Cement samples were prepared by digestion in a mixture of HF and HClO4, followed by dissolution in diluted HNO3. The authors concluded that Ca/Sr, Ca/Ba and Ca/Mn ratios have the greatest potential for identifying cements in hardened concretes and that the Ca/Sr ratio seems to be the most accurately quantifiable measurand. The variation of the Ca/Sr ratio of cement from the same production site over a long period is small, making this ratio suitable for discriminating between production sites. Rare earth elements (REE) distribution pattern, which were analysed as well, showed more limited identification potential. In the second part,26 the authors discussed Ca, Sr and Mn concentration leached from the concrete. By performing selective acid cement leaching from hardened New Zealand concretes, authors have tried to determine its source of manufacture. Crushing the concrete and selecting the fragments allowed them to exclude coarse aggregate from leaching, while a small portion of fine aggregates could not be excluded. The authors tested four types of aggregates to determine their contribution to leachates from concrete. It was concluded that it is essential to assess the contributions from the aggregates to the leaching solution. The application of REE distribution pattern to provenance the cement in a hardened concrete is limited by contamination of REEs released from aggregates during leaching. These two articles triggered further research in this field. Tamas and co-authors noted that cement's origin determination is both scientifically and practically a challenge which could be solved by analytical determination of certain elements contained in cements, and statistical processing of analytical data. In their study they stated that a three-element approach based on Sr–Ba–Mn is not fully adequate, especially in case of sulphate resistant clinkers having high iron content.27
Therefore, they added Mg, Ti and Zr, finally ending up with a six-element approach, realised by ICP-OES measurements and statistical processing of the data, using “pattern recognition” methods.28
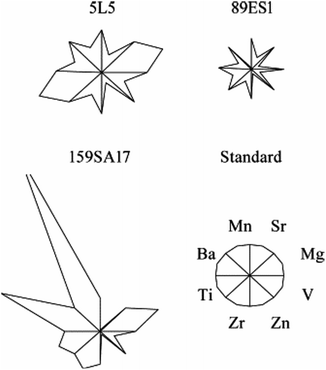
In another project, Tamas and co-authors analysed minor and trace elements in fifteen clinker sorts to classify the clinkers produced in different factories in Spain. For the qualitative identification of clinkers a rule base classifier was designed - trained by C4.5 algorithm.29 In 2001 and 2002, Tamas and Abonyi investigated the content of minor and trace elements in OPC clinkers, and their use for provenancing. They determined the Mg, Sr, Ba, Mn, Ti, Zr, Zn and V content in several hundred clinkers. The maximum and minimum values, the standard deviation of data, as well as the real and rounded averages were used for plotting (Table 1). The authors concluded that the first six elements come from the local raw materials, while Zn and V mainly come from fuel and are not useful for provenance determination. They considered Ba, Sr and Mg as the most relevant elements for clinker fingerprinting.29 The sample preparation was made by dissolving the clinker in HCl, followed by SiO2 precipitation, filtration and washing steps. The filtrate was analysed by ICP-OES, statistically processed via MATLAB© and the “star plots” were constructed (Fig. 1). Star plots are a graphical method used to simplify and to visualize the minor and trace element content in clinker, by comparing it with the proposed standard. The area of the star in a star plot is proportional to the total amount of the considered elements, and the eight rays of the star represent the eight elements. However, among other elements, Zn and V were still used for constructing “star plots”. The trace elements were selected based on the obtained clusters by the modified version of the Fisher interclass separability method. For clinker identification a specific software was developed30 which is available on internet.31 The software relies on unsupervised fuzzy clustering, identified by a fuzzy classifier using a control algorithm.32
| Element | Maximum value | Minimum value | Standard deviation | Average | Rounded average |
|---|---|---|---|---|---|
| Ba | 442 | 47 | 122.74 | 192.68 | 200 |
| Mn | 6538 | 15 | 995.06 | 507.02 | 500 |
| Sr | 2972 | 19 | 677.63 | 560.28 | 500 |
| Ti | 1691 | 175 | 289.78 | 1189.13 | 1000 |
| Zr | 149 | 4 | 17.33 | 47.32 | 50 |
| Mg | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 125 |
1751 | 6453.27 | 8976.93 | 8950 |
| Zn | 559 | 11 | 112.89 | 113.7 | 100 |
| V | 297 | 17 | 67.11 | 85.69 | 100 |
In 2004, Abonyi and co-authors presented a paper in which data presentation box and quantile–quantile plots were proposed to analyse the relationships between different factories and different minor and trace elements in clinkers.33 The authors concluded that exploratory data analysis can be useful in the qualitative analysis of elemental content in clinker. According to Lin and co-authors,34 a standard pattern of each origin must be established prior to the identification of the clinker origin. Since each clinker origin shows a broad range of trace element patterns, the uncertainty resulting from defining a distinctive compound pattern from its various forms makes these techniques difficult for usage. Therefore, it is not easy to define a precise standard pattern of a clinker origin. Commonly applied mathematical models for solving these uncertainty issues are the random model, the fuzzy model35 and the Gray model.
Proposed by Deng in 1989, the Gray relational analysis is a geometric method that can measure the approachability or similarity between series.36 Lin and co-authors' study integrated both concepts of Gray number and Gray relational analysis to set up a model for qualitative identification of clinker. The results showed that proposed model was successful in identifying the origin of clinker. The authors concluded that the methodology of the model can be extended to various areas with similar uncertain information.
In his doctoral thesis entitled “Chemical identification of Portland cements produced in Poland on the basis of the content of trace elements”, D. Kalarus determined Cr, Zn, Cd, Pb, Co, Ni, Co, Sr, Ba, Mn, Ti and Mg. The analytical method which the author used for the research was ICP-OES and scanning electron microscope (SEM). Based on the concentrations of the elements in clinkers, cements and raw materials, it was concluded that Mg, Sr and Mn serve as “markers” for the chemical identification of CEM I produced in Poland.37 The author concluded that the concentration of these elements in cement depends almost exclusively on their content in the raw materials, and not on the substitute fuels or waste materials such as used car tires and municipal waste, which is in agreement with the statement by Tamas and Abonyi. Other studies on cement and clinker provenancing were mostly presented as a conference proceedings or conference papers.38–41 Sawaki and co-authors estimated the chemical composition of cement in hardened concrete via an electron probe microanalyser, but the sensitivity turned out to be insufficient for discrimination.42 Kasamatsu and co-authors43 examined elemental (Cu, Rb, Zn, Sr, Zr, Ba, La, Ce, Nd and Pb) discrimination in nitric acid soluble components of concrete. According to the authors, this method can be used for forensic discrimination. However, Goguel and co-authors26 concluded that the nitric acid leaching approach is not applicable to aggregates consisting substantially of limestone. Therefore, nitric acid soluble component of concrete cannot represent a cement sample, but rather a mix of cement and calcareous additives. Moreover, dissolving concrete in nitric acid results in significant extraction of the lanthanides from aggregates, which removes the potential of lanthanides for cement identification in concrete.26
In several papers, MATLAB© and the star plot method were used to potentially pinpoint the origin of cement and, in some cases, mortars.44–46 A summary of the previous attempts for clinker, cement and concrete provenancing is shown in Table 2, where “elemental fingerprint” includes algorithm, pattern recognition and geometric methods, considering that for its realisation and interpretation, elemental analysis is a prerequisite. “Isotope ratio” implies the application of one isotopic ratio, e.g.87Sr/86Sr. In summary, the elemental fingerprint approach requires a large number of samples and the selection of appropriate (major, minor and trace) elements for establishing a standard pattern of clinker and later cement. Most of the previous research relied on multivariate statistical analysis. Such methods cannot be used for evaluation of all elements. This is because these elements either have no relation to the geographic origin or they are related to additives, whose origin is not connected to the origin of the major components, such as fuel used in production (used tires, heavy fuel oil etc.). Besides that, to have a meaningful result, many samples and parameters should be considered. Sufficiently large numbers of the target elements and as well of the samples are needed to achieve statistical significance; otherwise, the results are meaningless due to high standard errors. In several studies, these requirements were met and the origin of clinker for certain locations was determined via different approaches. Clinker is an intermediate product, which is available only at the production site and therefore, the practical relevance is rather limited. The identification of clinker in cement and hardened concrete, however, remains an open question.
| Clinker | Cement | Concrete | Elemental fingerprint | Isotope ratio | Year | Reference |
|---|---|---|---|---|---|---|
| x | x | 1993 | 24 | |||
| x | x | x | 1994 | 26 | ||
| x | x | 1996 | 27 | |||
| x | x | 1997 | 28 | |||
| x | x | 1997 | 38 | |||
| x | 1998 | 39 | ||||
| x | x | x | x | 2000 | 48 | |
| x | x | 2001 | 29 | |||
| x | x | 2002 | 30 | |||
| x | x | 2002 | 35 | |||
| x | x | 2003 | 44 | |||
| x | x | 2003 | 45 | |||
| x | x | 2003 | 40 | |||
| x | x | 2004 | 33 | |||
| x | 2005 | 17 | ||||
| x | x | 2007 | 49 | |||
| x | x | 2007 | 46 | |||
| x | x | x | 2007 | 37 | ||
| x | x | 2008 | 34 | |||
| x | x | 2018 | 43 |
Isotope studies
One of the most suitable techniques which can be used in the provenancing of building material is isotope ratio analysis of metal elements by means of mass spectrometry. The basic principle of all mass spectrometers is ionisation of a sample in an ion source, separation of the ions via a mass separator according to their m/z (mass/charge) ratio and finally, their detection at a detection unit.47Thermal Ionisation Mass Spectrometry (TIMS) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) are after Isotope-ratio mass spectrometry (IRMS), the most used techniques for authentication purposes.
Besides TIMS, both single-collector (SC) ICP-MS50–53 and MC-ICP-MS51,54 have been widely used for measuring isotope ratios. Due to the high measurement precision, the large natural isotope variation and the comparatively high contents in different sample types, strontium isotope ratios have found wide application outside of geochemistry and have been successfully used to determine the origin of inorganic materials, archaeological artefacts55–57 and glass.58,59 Therefore, Sr isotope ratios can have high significance in cement provenancing. Since the main raw material for cement production is limestone, and plenty of limestone deposits in Europe originate from geological formations containing calcite or dolomite, the different geological age of limestones and the original mineral composition combined with geological history are reflected in 87Sr/86Sr isotope ratio of cement. 87Rb decays to 87Sr with a half-life of 4.8 × 1010 years, while emitting a beta− particle.60 Depending on the age, and the initial Rb/Sr ratio, different geochemical reservoirs and rocks therein developed a large variation in today's 87Sr/86Sr ratios, ranging from approximately 0.702 for the depleted mantle to >0.943 for old continental crust.61
For the light element isotope systems, such as S, H, N, O and C, the mass-dependent effects are significant. Since their isotope composition can be changed in individual process steps (e.g. burning, annealing) by isotope fractionation, they have limited suitability for determining the origin of cement and concrete. Contrastingly, for the heavy element isotope systems, such as Sr, Nd and Pb, the mass dependent effects are mostly within the noise of the measurement and surely much smaller than the regional natural variations that are observed in provenancing applications, therefore, scientists can get a unique insight into the material's geographic source and/or production history.47,62 For the elements like Sr, which have more than one non-radiogenic isotope, the relative abundance of the radiogenic isotope (87Sr expressed as 87Sr/86Sr) can be normalised to the fixed nominal abundance ratio of a pair of stable isotopes of that element (86Sr/88Sr = 0.1194 (ref. 63)). Therefore, for Sr, only the radiogenic portion of a certain isotope is considered, and this special internal calibration corrects for any stable isotope fractionation (for example, burning) independently whether it occurs in the mass spectrometer, during sample preparation or even before in the history of the sample.
The first idea for using Sr isotopes as tracer for the geographic origin of cements dates to the year 2000 when Graham and co-authors have published a case study from New Zealand. They combined chemical and strontium isotopic analysis, and showed that New Zealand cements carry geochemical fingerprints from their raw materials to the final product, the concrete.48 The authors demonstrated that there are measurable differences in the 87Sr/86Sr values of most of the cements analysed, which can be used to pinpoint the geographic origin. The number of cement plants, however, is rather low in New Zealand and neighbouring countries play no role in this context. Additionally, the investigated cement and concrete samples date back to the 1980s and earlier, which might not be comparable to modern cement.
Kosednar-Legenstein, in her PhD thesis,49 and in studies with co-authors64,65 investigated the mineralogical, chemical and isotopic composition of historic mortars and plasters (with burnt lime as binder) from Austria. Among other results, the authors reported 87Sr/86Sr ratios of the mortars and plasters to be between 0.7091 and 0.7115, whereas Sr/Ca ratios did not exceed 0.003. In addition, burning and setting experiments were conducted to investigate the behaviour of REE and the Sr isotopes. The authors concluded that burning has a negligible influence on Sr/Ca, 87Sr/86Sr and REE distribution, and that Sr/Ca and 87Sr/86Sr signatures can thus, in principle, be used to identify natural deposits used for manufacturing of the materials. However, variations of the 87Sr/86Sr ratios in some of the investigated deposits as well as the possibility that limestone from different deposits was used for the binder and the aggregates, respectively, made unequivocal provenancing difficult.
All other publications that link isotope analysis and cement research were focused on isotope applications other than geographical origin determination. A study performed by Pierkes and co-authors investigated the influence of cement hydration on the distribution of oxygen and hydrogen isotopes.66 Another example is the application of stable isotopes (elements H, C, N, O, S) to investigate reactions during curing or concrete damage.67–70 Recently, Grengg and co-authors investigated the deterioration mechanism of alkali-activated cements in sulfuric acid, using oxygen-isotope signatures, among other methods and tools.71
However, if we take a look at the other examples of inorganic material's provenancing, we would surely notice that isotope techniques were frequently used, mostly for studying different categories of archaeological materials72,73 and ceramics provenancing.74–76 In ceramics provenancing, with the aim of assigning a production location to a specific ceramic artefact of unknown origin, the first step is the characterisation, followed by identification of their chemical, physical or structural “fingerprint”, and the last step is comparison. This comparison is made by using collected fingerprints and advanced statistical techniques to search for similarities and differences. This procedure allows the collection of all ceramics with similar features and differentiating groups of ceramics with different properties.77 In archaeology, lead isotopes have been used as tracers for provenancing of metal samples and artefacts since its introduction by Brill and Wampler in 1967.78–80 By combining the isotopic information with additional information on the sources and the artefacts being independent of the isotope data, it is possible to overcome limitations such as overlapping lead isotopic compositions of ore deposits, large spreads within one ore deposit or a missing overlap with (un)known mining locations.57,81
A promising approach for cement provenancing is the application of isotope ratios as described above for archaeological and ceramics provenance studies. In principle, the transfer of these approaches to cement is possible, but works best for approaches which have been established for glass and ceramics due to the similarity of the materials.
The advantage of isotopic over elemental fingerprinting
Provenancing of building materials through isotope techniques are based on the principle that every material, with a different geographical origin, has its unique isotopic fingerprint. The major advantage of isotope techniques is that some isotope systems which contain radiogenic isotope, such as 87Sr/86Sr, can be linked to the geographic origin, as they reflect the mineral composition and geological history of the raw material. Moreover, radiogenic isotope systems do not change, while the elemental concentration are exposed to changes in environment as well as in technical processes. If strontium is considered as an element of interest in cement, the isotope ratio depends on its origin from each of these raw materials, predominantly in limestone or marl. The current literature reveals that various methods can be employed for the provenancing of cements and clinkers. Of particular interest is the tendency of using elemental concentrations towards less unequivocal data, and consequently, the need for a higher number of features (appropriate elements) in the elemental fingerprint. Elemental fingerprints seem easier to determine but would, subsequently, require knowledge in multivariant statistics and data analysis to be interpreted. However, isotope ratio determination allows simpler isotopic fingerprints, which are unique and unambiguous. Finally, isotope fingerprinting approach offers reduced number of measurands, leading to a more straight forward statistical evaluation and interpretation of data.Other techniques
In addition to the above-mentioned analytical techniques, X-ray diffraction (XRD) and X-ray fluorescence (XRF) spectrometry are important techniques for provenance studies. Their usefulness was proven in various fields, e.g. the analysis of dust from a shipment of contraband ivory to determine the original location where the ivory was packed,82 or solving a double murder case in Australia,83 where X-ray diffraction allowed to identify soils on a tool and from a quarry, but also for solving other forensic cases.84 In principle, this capacity could be also used for provenancing of concrete particles found on a crime scene, and their possible agreement with samples from a suspected quarry. For example, quartz gravels and sands, used as aggregate for concrete, differ as regards crystallinity,85 which can possibly be exploited for provenance studies. Besides XRD, XRF was very often used for characterising and provenancing of different types of building materials,86,87 predominantly old stones8,15,88 and marbles9,89 in buildings or artifacts with historical significance. Both XRD and XRF were used for establishing the provenance of limestone.90 With the purpose of collecting both XRD and XRF data on the same spot of an object, a portable XRD/XRF91 instrument was developed. Another technique which was used for limestone,92,93 but also for pottery94–96 provenancing was Instrumental Neutron Activation Analysis (INAA). Meyers and Vanzelts used INAA in their pilot-study97 to distinguish between objects made of limestone from different sources.Another technique worth mentioning is laser ablation (LA) ICP-MS. Van Ham-Meert and co-authors124 published an article about LA-ICP-MS for Pb and Sr isotopic determination in archaeological glass. This technique was also used for provenancing of ceramics,98,99 as well as for cultural heritage research.100,101 The study of using portable LA for sampling solid materials, with subsequent Sr and Nd measurements on TIMS was reported by Knaf and co-authors.102
An additional promising method for isotopic microanalysis is laser ablation molecular isotopic spectrometry103 (LAMIS). In their article,104 Bol'shakov and co-authors evaluated LAMIS for rapid optical analysis of isotopes of different elements. Application of LAMIS for optical isotopic analysis of solid samples was also investigated in a paper published by Mao and co-authors,105 where measurement of strontium isotopes showed promising results. In forensic analysis, for an unknown soil sample, scanning electron microscopy with energy dispersive X-ray spectrometry (SEM-EDX) allows recognition of the presence or absence of man-made particles, including concrete and cement.106 Therefore, automated mineralogy is amenable to distinguish and recognise the particles derived from common construction material classes. Indubitably, all these techniques can provide valuable information for elemental and phase identification and quantification in cement, which can be very beneficial for an overall picture in provenance study.
Since cement and concrete are both highly processed materials, IRMS is not covered within this review, since it relies on analysis of light element isotope systems whose composition can be strongly changed by isotope fractionation, as explained in the section “Isotope studies” and thus tracing back cement to the source materials is impossible.
The combined approach for provenancing
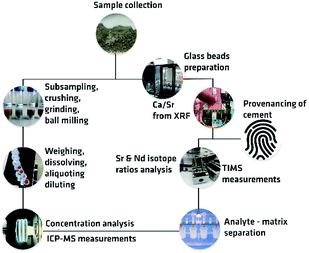
The combined approach for provenancing, as proposed here, incorporates the use of 87Sr/86Sr and 143Nd/144Nd isotope ratios and of Ca/Sr elemental ratios to provide a unique fingerprint of cement. The whole pathway for cement provenancing is shown in Fig. 2.Structure of the combined approach
First, a sample should be subsampled for mass fraction determination and isotope ratio analysis. For isotope ratio analysis, a selection of the sample preparation procedure is very important.For trace elements like Sr and Nd, prior to isotope ratio analysis, mass fraction determination should take place preferably on ICP-MS or ICP-OES. The following step is matrix separation, to purify the small quantity of analytes. To obtain accurate and precise isotope ratios, it is recommended to perform the 87Sr/86Sr and 143Nd/144Nd measurements by TIMS, on a single and on a double or triple filament geometry, respectively. When both Sr and Nd isotopic fingerprints are not capable of completely resolving the different cement origins, a third parameter should be introduced. Here, Ca/Sr concentration ratio could be a promising measurand which could help distinguishing between samples with very similar 87Sr/86Sr–143Nd/144Nd isotopic fingerprint. This parameter is promising and has already been studied by Goguel and co-authors.26 To measure total concentration, an element must be fully dissolved. For this purposes, total digestion in HF and HNO3 are often used. Due to the many reasons, such as presence of calcium as a major constituent in cement and the formation of CaF2 precipitate while dissolving cement in HF and HNO3, numerous interferences on ICP-MS, as well as time-consuming sample preparation, these measurements are recommended to be carried out using X-Ray Fluorescence (XRF) on cement glass beads. Once prepared and properly stored, fused (glass) beads are very stable and can be used several times.
Improvement by adding another isotope system
In the past mostly one isotope system (isotope ratios of one chemical element) has been used for provenancing. For complex materials such as alloys or glass, which consist of several components of different geographical origin, one isotope system, however, often resulted in large overlaps and insufficient resolution of the measurement space. To solve this, additional isotope systems were introduced for glass provenancing.58,59,107,108 In 2009, Henderson and co-authors published a paper on the provenancing of glass in the Islamic Middle East by using oxygen, strontium, and neodymium isotope systems. They concluded that the determination of Nd and Sr isotope ratios for selected samples would be a suitable approach for providing independent geographical information.58Elements such as Sr and Nd display variations in their isotopic composition since one or more of their isotopes is the final product of the decay of naturally occurring and long-lived radionuclides. Consequently, the isotopic composition of such radiogenic isotopes is governed by the initial parent/daughter ratio and the time these nuclides have spent together.
Therefore, the isotopic composition of these elements is not only used for geochronological dating purposes but also for tracing the geographical origin.
Neodymium is a classical isotope tracer in geochemistry and has been used widely for dating and the study of petrogenesis, alteration, and weathering processes.109–111 Due to its affinity to silicate phases, the Nd isotope system has been applied in addition to the Sr isotope system in provenance studies of glass.59,108,112–116147Sm decays to 143Nd while emitting an alpha particle with a half-life of 1.06 × 1011 years.117 Besides using this decay system for dating purposes, it can be used as a natural tracer since the isotopic composition of Nd as measured today is time-integrated results of Sm/Nd ratio in a specific environment.118 The assumption is that 143Nd/144Nd ratios are different in the raw materials from different (geographically located) plants used for clinker, and therefore also for cement production. Sr is the tracer for the calcium carbonate-bearing raw material (limestone or marl), and Nd for the silicate-bearing raw material (clay). The addition of neodymium isotopes as a second parameter offers the potential to achieve results that provide more information about the geographical origin and empower the new approach for provenancing. This is already demonstrated by Henderson and co-authors, who applied both Sr and Nd isotope ratios for glass provenance studies. When it comes to cement a similar approach might be feasible, because cement and glass have much in common. Both materials are complex mixtures of a silicate and a carbonate component and contain a suite of minor additives. After mixing, both materials are exposed to temperatures above 1400 °C hampering the application of classical stable isotopes of elements such as H, C and O. In Fig. 3 the 87Sr/86Sr and 143Nd/144Nd isotope measurements in Bronze Age glass are plotted versus each other. This diagram clearly shows that Mesopotamian glasses can be differentiated from Egyptian and Greek glasses based on their combined 87Sr/86Sr and 143Nd/144Nd signatures, which is not possible based on the 87Sr/86Sr signatures or the 143Nd/144Nd signatures alone.107 Samples which cannot be resolved by using one isotope system, because the data overlap within the measurement uncertainty, might be resolved by a second isotope system, adding a third or fourth measurand, whether it is an isotope ratio, an interelement ratio or an element concentration, will even increase the resolution, provided the measurand is correlated with the origin of one of the raw material.
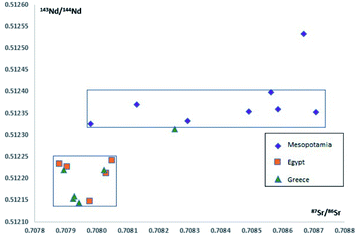 | ||
| Fig. 3 A plot of 87Sr/86Sr vs.143Nd/144Nd isotope measurements in Bronze Age glass from Mesopotamia (lozenges), Egypt (squares) and Greece (triangles). Adapted from Henderson and co-authors, 2010.107 | ||
General limitations
The selection of a sample preparation procedure has an important role in cement provenancing. If we consider provenancing of CEM I, the total analyte's content and isotopic fingerprint in minor additional constituent must be assessed. Gypsum present in cement is exclusively from non-local sources, and Ca bearing minerals always carry a certain amount of Sr, which may complicate origin determination. Besides gypsum, CEM I can contain other constituents, included in maximum 5% of minor additional constituents. Since they may have an influence on the Sr isotopic fingerprint, all the mineral additions which are present in cement should be considered while performing sample preparation. Furthermore, due to the different fuels used in rotary kilns for clinker production, the possible contribution regarding the contamination from fuels must be assessed. Since only clinker carries the local raw materials correlated to the Sr fingerprint and therefore the origin, one must ensure that while preparing the sample, only the clinker fraction is obtained. Finally, the measured isotope ratios should correspond to those of the clinker fraction. To exclude everything but the analyte while performing the measurement, it is necessary to check and afterwards validate the right analytical procedure, including matrix separation. Another limitation of isotope analysis is the duration, which is mainly attributed to the sample preparation. To isolate the analyte of interest (e.g. Sr), matrix separation via cation-exchange or extraction chromatography must be performed. To date, the column preparation, resin cleaning and analyte-matrix separation is being performed manually at the expenses of working time. A few years ago, however, a new way of fully automated sample purification system has been offered as an option. The prepFAST MC® systems can overcome this time-consuming issue in sample preparation.119–123 An even higher degree of automation might be allowed by laser ablation coupled to ICP-MS when an automated sample changer and corresponding software packages are available.Summary and outlook
Provenance studies of cement are of great importance for failure research, damage assessment and resulting liability issues related to concrete structures and in investigating ancient building materials and in forensic science.In this paper, the origin determination of cement has been reviewed, and the main approaches from past and current research have been presented. The majority of the published work was related to trace elemental contents of cement and clinkers, mostly combined with either the “star plot method”, the “gray rational analysis” or other statistical and pattern recognition methods. Even though the sample preparation and the analysis presented in the publications were straightforward, the data interpretation requires different visualisation methods, such as star graphs to intuitively compare the data. An advanced statistical method like PCA and clustering algorithms, which require solid statistical knowledge and computational power, restrict the number of potential users. Besides this, two (Zn, V) from a maximum of eight elements have proven useless because they originate from a fuel deployed in the production of the clinker. Finally, the published literature revealed that mostly clinkers were analysed. Clinker, however, only occurs within the production process before other components are admixed. Therefore, the provenancing of clinker is of almost no practical relevance. The application of isotope ratios in the provenancing of cement is scarce and is limited to Graham and co-authors48 and Kosednar-Legenstein and co-authors,64,65 who showed that Sr isotopes, combined with elemental analysis, are useful tools for provenancing of cement in a restricted area concerning time and space. When expanding this approach to a larger region such as Europe or even worldwide, a second isotope system is required to increase the resolution of the provenancing approach. Here, Nd being another radiogenic isotope system, is selected in addition to Sr. Both elements, Sr and Nd, have their main occurrence in different components of the raw mix of the cement. Thus, they act as tracers for different raw materials. By measuring both Sr and Nd isotope ratios, a characteristic isotopic fingerprint of cement can be established, which then can be used to identify concrete of unknown origin. The addition of the interelement ratio of Ca/Sr to the strontium and neodymium isotope ratios will increase the analytical resolution of the approach and most likely lead to the geographical origin of the material, and ideally to its manufacturer. The main advantage of the Sr–Nd isotope analysis with or without the Ca/Sr ratio over the statistical and pattern recognition approaches is a reduced number of measurands leading to a more straight forward statistical evaluation, which can be visualized in 2D or 3D plots. The measurands themselves, the Sr and the Nd isotope ratio and the Ca/Sr ratio, show a stronger correlation with the origin of the raw materials, thus enabling a more precise mapping of the cement origin. However, sample preparation needs to be improved to avoid interferences from mineral additions that do not originate from the clinker but also in terms of time consumption. The use of automated matrix separation facilitates the sample preparation and reduces chemicals as well as time consumption. Finally, the expansion of the analytical approach, e.g. addition of Ca/Sr ratios, needs to be considered to enable the transfer of this approach to the provenance determination of concrete samples.
Future research should focus on three main topics: first, optimisation and validation of the proposed wet-chemical method for cement provenancing should take place. Second, an in situ method for the determination of 87Sr/86Sr and 143Nd/144Nd isotope ratios based on LA-ICP-MS should be developed for concrete provenancing. The lateral resolution of LA should allow the differentiation between different mineral phases of the concrete, i.e. the cementitious phase and the aggregates. Together with the already determined isotope data and the interelement ratios, the procedure for the determination of cement's origin should be transferable to concrete.
Third, a databank for isotopic fingerprints of cement should be established. Besides answering individual questions regarding the provenance of specific cement samples or concrete structures, provenance studies also contribute to the creation of cement datasets. Provenancing of cement allows the complete documentation of the cement samples combining isotope and concentration ratios along with their identified provenance. As a result, new reference groups of various cements of known origin are generated, while present ones expand with new samples, leading to a reference databank. Such a databank can be of great importance since it could provide geochemists and other scientists with more knowledge and could simplify future cement provenancing.
Abbreviations
| AA | Atomic absorption |
| AE | Atomic emission |
| EDX | Energy dispersive X-ray spectrometry |
| FGD | Flue gas desulphurisation |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| INAA | Instrumental neutron activation analysis |
| IRMS | Isotope ratio mass spectrometry |
| LA | Laser ablation |
| LAMIS | Laser ablation molecular isotopic spectrometry |
| MC | Multi collector |
| OPC: | ordinary Portland cement |
| PCA | Principal component analysis |
| REE | Rare earth elements |
| SC | Single collector |
| SEM | Scanning electron microscope |
| TIMS | Multi collector thermal ionisation mass spectrometry |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
Author contributions
Anera Kazlagic: conceptualisation, methodology, project administration, investigation, writing – original draft, writing – review & editing, visualisation. Jochen Vogl: methodology, supervision, funding acquisition, writing – original draft, writing – review & editing. Gregor J. G. Gluth: methodology, supervision, writing – review & editing. Dietmar Stephan: methodology, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Federal Institute for Material Research and Testing (BAM) for providing funds under the project number MIT1-2019-8.References
- M. Hamma-Adama, Am. J. Eng. Res., 2017, 6, 289–300 Search PubMed.
- G. Campione and G. Giambanco, J. Perform. Constr. Facil., 2020, 34, 04020001 CrossRef.
- D. Proske and M. Schmid, Bautechnik, 2021, 98, 423–432 CrossRef.
- D. I. Blockley, The Nature of Structural Design and Safety, Ellis Horwood Ltd, Chichester, England, 1980 Search PubMed.
- D. Proske, Bridge Collapse Frequencies versus Failure Probabilities, Springer, Cham, 2019 Search PubMed.
- P. S. Reed, K. Schoonees and J. Salmond, Historic Concrete Structures in New Zealand: Overview, Maintenance and Management, Science & Technical Publishing, Department of Conservation, New Zealand, 2008 Search PubMed.
- M. Bostenaru Dan, Materials, Technologies and Practice in Historic Heritage Structures, Springer, Dordrecht, 2014 Search PubMed.
- K. Al-Bashaireh, F. Abudanah and M. Driessen, Archaeol. Anthropol. Sci., 2020, 12, 26 CrossRef.
- S. Columbu, F. Antonelli, M. Lezzerini, D. Miriello, B. Adembri and A. Blanco, J. Archaeol. Sci., 2014, 49, 332–342 CrossRef CAS.
- R. Fort, M. A. de Buergo, E. Perez-Monserrat and M. J. Varas, Eng. Geol., 2010, 115, 149–157 CrossRef.
- A. Torok and R. Prikryl, Eng. Geol., 2010, 115, 139–142 CrossRef.
- E. Galan, M. I. Carretero and E. Mayoral, Eng. Geol., 1999, 54, 287–298 CrossRef.
- R. Dreesen and M. Dusar, Mater. Charact., 2004, 53, 273–287 CrossRef CAS.
- M. Gomez-Heras and R. F. Gonzalez, Mater. Constr., 2004, 54, 33–49 CrossRef.
- L. Carta, D. Calcaterra, P. Cappelletti, A. Langella and M. de'Gennaro, J. Cult. Herit., 2005, 6, 277–286 CrossRef.
- A. Murta, J. Pinto, H. Varum, J. Guedes, J. Lousada and P. Tavares, Portugal Sb10: Sustainable Building Affordable to All – Low Cost Sustainable Solution, 2010, pp. 589–596 Search PubMed.
- F. Schmidt-Döhl, J. Koepke and A. Schimrosczyk, Presented in Part at the 27th International Conference on Cement Microscopy, Victoria, British Columbia, Canada, April 24 to 28, 2005 Search PubMed.
- A. Buko, World Archaeol., 1984, 15, 348–365 CrossRef.
- M. A. Sanjuan and C. Argiz, Mater. Constr., 2012, 62, 425–430 CrossRef CAS.
- F. W. Locher, Cement : Principles of Production and Use, Bau+Technik Gmbh, Düsseldorf, 2006 Search PubMed.
- H. Mommsen, J. Radioanal. Nucl. Chem., 2001, 247, 657–662 CrossRef CAS.
- E. D'Ambrosio, F. Marra, A. Cavallo, M. Gaeta and V. Guido, J. Archaeol. Sci. Rep., 2015, 2, 186–203 Search PubMed.
- L. Ortega, M. Zuluaga, A. Alonso Olazabal, M. Insausti and A. Ibañez Etxeberria, Archaeometry, 2007, 50, 387–408 CrossRef.
- R. L. Goguel and D. A. Stjohn, Cem. Concr. Res., 1993, 23, 59–68 CrossRef CAS.
- A. D. McNaught and A. Wilkinson, IUPAC Compendium of chemical terminology, Oxford, 2nd edn, 1997 Search PubMed.
- R. L. Goguel and D. A. Stjohn, Cem. Concr. Res., 1993, 23, 283–293 CrossRef CAS.
- F. D. Tamas, World Cement Res. Dev. Sect., 1996, 27, 75–79 Search PubMed.
- F. D. Tamas, M. Patkai-Horvath, E. Kristof-Mako and J. Tritthart, Qualitative identification of clinkers and cements - some results and possibilitiest, Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, 1997, vol. 3, p. 3v010 Search PubMed.
- F. D. Tamas, J. Abonyi and F. Puertas, Mater. Constr., 2001, 51, 85–96 CrossRef CAS.
- F. D. Tamas and J. Abonyi, Cem. Concr. Res., 2002, 32, 1319–1323 CrossRef CAS.
- J. Abonyi, Qualitative Identification of Clinkers by Fuzzy Clustering, https://www.mathworks.com/matlabcentral/fileexchange/47162-qualitative-identification-of-clinkers-by-fuzzy-clustering, accessed 18.03.2021 Search PubMed.
- J. Madar, J. Abonyi and F. Szeifert, Eng. Appl. Artif. Intell., 2005, 18, 343–351 CrossRef.
- J. Abonyi, F. D. Tamas and J. Tritthart, Adv. Cem. Res., 2004, 16, 9–15 CrossRef CAS.
- Y. H. Lin, P. C. Lee and T. P. Chang, J. Mater. Civ. Eng., 2008, 20, 539–543 CrossRef CAS.
- F. D. Tamas, J. Abonyi, J. Borszeki and P. Halmos, Cem. Concr. Res., 2002, 32, 1325–1330 CrossRef CAS.
- J. L. Deng, J. Grey Syst., 1989, 1, 1–24 Search PubMed.
- D. Kalarus, PhD thesis, Academia Gorniczo-Hutnicza, 2008.
- J. H. Potgieter, Presented in Part at the 10th International Congress on the Chemistry of Cement, Göteborg, Sweden, 1997 Search PubMed.
- F. D. Tamas, A. Tagnit-Hamou and J. Tritthart, in Materials Science of Concrete Special Volume: the Sidney Diamond Symposium, ed. S. M. M. Cohen and J. P. Skalny, Wiley, 1998, pp. 57–69 Search PubMed.
- F. D. Tamas and J. Abonyi, Presented in Part at the 11th International Congress on the Chemistry of Cement, South Africa, 2003 Search PubMed.
- H. Justnes, Proceedings of the 10th International Congress on the Chemistry of Cement, Amarkai AB and Congrex, Gothenburg, Sweden, June 2–6, 1997 Search PubMed.
- D. Sawaki, K. Kobayashi and E. Sakai, Bunseki Kagaku, 2010, 59, 1051–1064 CrossRef CAS.
- M. Kasamatsu, T. Igawa, S. Suzuki and Y. Suzuki, Anal. Sci., 2018, 34, 729–733 CrossRef CAS PubMed.
- J. Abonyi, F. D. Tamas, S. Potgieter and H. Potgieter, S. Afr. J. Chem., 2003, 56, 15–20 CAS.
- J. H. Potgieter, S. S. Potgieter, R. I. McCrindle and F. D. Tamas, Adv. Cem. Res., 2003, 15, 45–50 CrossRef CAS.
- S. S. Potgieter-Vermaak, J. H. Potgieter, K. Worobiec, R. van Grieken, L. Majanovic and S. Moeketsi, Cem. Concr. Res., 2007, 37, 834–843 CrossRef CAS.
- T. Prohaska, Sector Field Mass Spectrometry for Elemental and Isotopic Analysis, Royal Society of Chemistry, Cambridge, 2015 Search PubMed.
- I. J. Graham, R. L. Goguel and D. A. St John, Cem. Concr. Res., 2000, 30, 1105–1111 CrossRef CAS.
- B. Kosednar-Legenstein, PhD thesis, Technische Universitaet Graz, 2007.
- S. Augagneur, B. Medina and F. Grousset, Fresenius' J. Anal. Chem., 1997, 357, 1149–1152 CrossRef CAS.
- M. Barbaste, L. Halicz, A. Galy, B. Medina, H. Emteborg, F. C. Adams and R. Lobinski, Talanta, 2001, 54, 307–317 CrossRef CAS PubMed.
- R. Larcher, G. Nicolini and P. Pangrazzi, J. Agric. Food Chem., 2003, 51, 5956–5961 CrossRef CAS PubMed.
- B. Medina, S. Augagneur, M. Barbaste, F. E. Grouset and P. Buat-Meard, Food Addit. Contam., 2000, 17, 435–445 CrossRef CAS PubMed.
- M. Barbaste, K. Robinson, S. Guilfoyle, B. Medina and R. Lobinski, J. Anal. At. Spectrom., 2002, 17, 135–137 RSC.
- H. Ma, J. Henderson and J. Evans, Archaeometry, 2016, 58, 68–80 CrossRef CAS.
- H. J. Ma, J. Henderson and J. Evans, J. Archaeol. Sci., 2014, 50, 551–558 CrossRef CAS.
- J. Vogl, M. Rosner, J. Curbera, U. Peltz and B. Peplinski, Archaeol. Anthropol. Sci., 2018, 10, 1111–1127 CrossRef.
- J. Henderson, J. Evans and Y. Barkoudah, Antiquity, 2009, 83, 414–429 CrossRef.
- J. Henderson, J. Evans, P. Bellintani and A. M. Bietti-Sestieri, J. Archaeol. Sci., 2015, 55, 1–8 CrossRef CAS.
- R. Bowen, in Isotopes in the Earth Sciences, Springer Netherlands, Dordrecht, 1994, pp. 162–200, DOI:10.1007/978-94-009-2611-0_4.
- M. Rosner, Food Chem., 2010, 121, 918–921 CrossRef CAS.
- J. A. Hoogewerff, C. Reimann, E. Ueckermann, R. Frei, K. M. Frei, T. van Aswegen, C. Stirling, M. Reid, A. Clayton, A. Ladenberger and G. P. Team, Sci. Total Environ., 2019, 672, 1033–1044 CrossRef CAS PubMed.
- R. H. Steiger and E. Jäger, Earth Planet. Sci. Lett., 1977, 36, 359–362 CrossRef CAS.
- B. Kosednar-Legenstein, M. Dietzel, A. Leis, K. Stingl, B. Wiegand and M. Baumgartner, Geochim. Cosmochim. Acta, 2007, 71, A514 Search PubMed.
- B. Kosednar-Legenstein, M. Dietzel, A. Leis, B. Wiegand, K. Stingl and M. Baumgartner, Presented in Part at the European Geosciences Union General Assembly, Vienna, Austria, 2006 Search PubMed.
- R. Pierkes, H. Förstel and M. Boner, Influence of Cement Hydration on the Distribution of Oxygen and Hydrogen Isotopes, Institut fuer Bauforschung Aachen, IBAC, Rheinisch-Westfaelische Technische Hochschule Aachen, RWTH, Aachen, 2005 Search PubMed.
- G. Macleod, A. E. Fallick and A. J. Hall, Chem. Geol., 1991, 86, 335–343 CAS.
- F. Mittermayr, C. Bauer, D. Klammer, M. E. Bottcher, A. Leis, P. Escher and M. Dietzel, Isot. Environ. Health Stud., 2012, 48, 105–117 CrossRef CAS PubMed.
- K. Pye and N. Schiavon, Nature, 1989, 342, 663–664 CrossRef CAS.
- B. Kosednar-Legenstein, M. Dietzel, A. Leis and K. Stingl, Appl. Geochem., 2008, 23, 2425–2437 CrossRef CAS.
- C. Grengg, G. J. G. Gluth, F. Mittermayr, N. Ukrainczyk, M. Bertmer, A. Guilherme Buzanich, M. Radtke, A. Leis and M. Dietzel, Cem. Concr. Res., 2021, 142, 106373 CrossRef CAS.
- M. Murillo-Barroso, I. Montero-Ruiz, J. M. Nieto, M. D. C. Massieu, D. M. Socas and M. Martinon-Torres, J. Iber. Geol., 2019, 45, 585–608 CrossRef.
- D. J. Killick, J. A. Stephens and T. R. Fenn, Archaeometry, 2020, 62, 86–105 CrossRef CAS.
- M. Guzowska, I. Kuleff, E. Pernicka and M. Satir, in Troia and the Troad: Scientific Approaches, Springer, Heidelberg, 2003, pp. 233–249 Search PubMed.
- B. P. Li, J. X. Zhao, A. Greig, K. D. Collerson, Y. X. Feng, X. M. Sun, M. S. Guo and Z. X. Zhuo, J. Archaeol. Sci., 2006, 33, 56–62 CrossRef.
- B. P. Li, J. X. Zhao, A. Greig, K. D. Collerson, Z. X. Zhuo and Y. X. Feng, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 240, 726–732 CrossRef CAS.
- A. Sarris, Best Practices of Geoinformatic Technologies for the Mapping of Archaeolandscapes, Archaeopress Publishing Ltd., Oxford, 2015 Search PubMed.
- R. H. Brill and J. M. Wampler, Am. J. Archaeol., 1967, 71, 63–77 CrossRef CAS.
- Z. A. Stos-Gale and N. H. Gale, Archaeol. Anthropol. Sci., 2009, 1, 195–213 CrossRef.
- E. Niederschlag, E. Pernicka, T. Seifert and M. Bartelheim, Archaeometry, 2003, 45, 61–100 CrossRef CAS.
- J. Vogl, B. Paz and E. Volling, Archaeol. Anthropol. Sci., 2019, 11, 3267–3277 CrossRef.
- D. Stoney, A. Bowen, V. Bryant, E. A. Caven, M. Cimino and P. L. Stoney, J. Am. Soc. Trace Evid. Examiners, 2019, 2, 13–34 Search PubMed.
- R. W. Fitzpatrick and M. D. Raven, Soil Horiz., 2012, 53, 14–29 CrossRef.
- R. W. Fitzpatrick and M. Raven, Acta Crystallogr., Sect. A: Found. Adv., 2005, 61, C14 Search PubMed.
- N. Marinoni and M. A. T. M. Broekmans, Cem. Concr. Res., 2013, 54, 215–225 CrossRef CAS.
- D. Ergenc and R. Fort, Measurement, 2019, 147, 106876 CrossRef.
- E. Delluniversita, I. M. Muntoni, I. Allegretta, M. Tarantini, A. Monno, P. Maiorano, A. Girone, M. Morsilli, R. Terzano and G. Eramo, Archaeol. Anthropol. Sci., 2019, 11, 6037–6063 CrossRef.
- J. Powers, D. M. Smilgies, E. C. Geil, K. Clinton, N. Dimitrova, M. Peachin and R. E. Thorne, J. Archaeol. Sci., 2009, 36, 343–350 CrossRef.
- D. Magrini, D. Attanasio, S. Bracci, E. Cantisani and W. Prochaska, Archaeol. Anthropol. Sci., 2018, 10, 1141–1152 CrossRef.
- L. Bianco, Rom. J. Phys., 2017, 62, 901 Search PubMed.
- P. Sarrazin, G. Chiari and M. Gailhanou, Adv. X-Ray Anal., 2009, 52, 175–186 CAS.
- J. Zhu, M. D. Glascock, C. S. Wang, X. J. Zhao and W. Lu, J. Archaeol. Sci., 2012, 39, 2568–2573 CrossRef CAS.
- L. A. Dim, J. Adetunji, C. D. Okujeni, S. B. Elegba and S. A. Agaja, J. Radioanal. Nucl. Chem., 1991, 148, 145–153 CrossRef CAS.
- L. S. Bohus, M. M. M. de Antczak, E. D. Greaves, A. Antczak, J. Bermudez, Z. Kasztovszky, T. Poirier and A. Simonits, J. Radioanal. Nucl. Chem., 2005, 265, 247–256 CrossRef.
- P. S. Bedregal, P. A. Mendoza, M. S. Ubillus, W. Yepez, J. Jennings and E. H. Montoya, J. Radioanal. Nucl. Chem., 2015, 306, 729–736 CrossRef CAS.
- J. B. Tandoh, B. J. B. Nyarko, S. B. Dampare, Y. Bredwa-Mensah, O. Gyampo and H. Ahiamadjie, J. Radioanal. Nucl. Chem., 2010, 284, 567–573 CrossRef CAS.
- P. Meyers and L. Vanzelst, Radiochim. Acta, 1977, 24, 197–204 CrossRef CAS.
- J. Pérez-Arantegui, M. Resano, E. García-Ruiz, F. Vanhaecke, C. Roldán, J. Ferrero and J. Coll, Talanta, 2008, 74, 1271–1280 CrossRef PubMed.
- M. Resano, P. Marzo, J. Pérez-Arantegui, M. Aramendía, C. Cloquet and F. Vanhaecke, J. Anal. At. Spectrom., 2008, 23, 1182–1191 RSC.
- B. Giussani, D. Monticelli and L. Rampazzi, Anal. Chim. Acta, 2009, 635, 6–21 CrossRef CAS PubMed.
- M. Vannoorenberghe, T. Van Acker, J. Belza, D. Teetaert, P. Crombe and F. Vanhaecke, J. Anal. At. Spectrom., 2020, 35, 2686–2696 RSC.
- A. C. S. Knaf, J. M. Koornneef and G. R. Davies, J. Anal. At. Spectrom., 2017, 32, 2210–2216 RSC.
- A. A. Bol'shakov, X. L. Mao, J. J. Gonzalez and R. E. Russo, J. Anal. At. Spectrom., 2016, 31, 119–134 RSC.
- A. A. Bol'shakov, X. L. Mao, D. L. Perry and R. E. Russo, Spectroscopy, 2014, 29, 30–39 Search PubMed.
- X. L. Mao, A. A. Bol'shakov, I. Choi, C. P. McKay, D. L. Perry, O. Sorkhabi and R. E. Russo, Spectrochim. Acta, Part B, 2011, 66, 767–775 CrossRef CAS.
- D. Pirrie, A. J. Pidduck, D. E. Crean, T. M. Nicholls and R. P. Awbery, Forensic Sci. Int., 2019, 305, 109974 CrossRef CAS PubMed.
- J. Henderson, J. Evans and K. Nikita, Mediterr. Archaeol. Archaeom., 2010, 10, 1–24 Search PubMed.
- J. Henderson, H. Ma and J. Evans, J. Archaeol. Sci., 2020, 119, 105164 CrossRef CAS.
- J. Veizer, Annu. Rev. Earth Planet. Sci., 1989, 17, 141–167 CrossRef CAS.
- M. R. Walter, J. J. Veevers, C. R. Calver, P. Gorjan and A. C. Hill, Precambrian Res., 2000, 100, 371–433 CrossRef CAS.
- D. J. DePaolo and E. E. Daley, Chem. Geol., 2000, 169, 157–185 CrossRef CAS.
- P. Degryse, J. Henderson and G. Hodgins, Isotopes in Vitreous Materials, Leuven University Press, Leuven, 2017 Search PubMed.
- A. Meek, J. Henderson and J. Evans, J. Anal. At. Spectrom., 2012, 27, 786–795 RSC.
- D. Brems, M. Ganio, K. Latruwe, L. Balcaen, M. Carremans, D. Gimeno, A. Silvestri, F. Vanhaecke, P. Muchez and P. Degryse, Archaeometry, 2013, 55, 449–464 CrossRef CAS.
- M. Ganio, M. Gulmini, K. Latruwe, F. Vanhaecke and P. Degryse, J. Archaeol. Sci., 2013, 40, 4264–4270 CrossRef CAS.
- C. Boschetti, J. Henderson and J. Evans, J. Archaeol. Sci. Rep., 2017, 11, 647–657 Search PubMed.
- M. C. Gupta and R. D. MacFarlane, J. Inorg. Nucl. Chem., 1970, 32, 3425–3432 CrossRef CAS.
- P. Richard, N. Shimizu and C. J. Allègre, Earth Planet. Sci. Lett., 1976, 31, 269–278 CrossRef CAS.
- S. C. Metzger, B. W. Ticknor, K. T. Rogers, D. A. Bostick, E. H. McBay and C. R. Hexel, Anal. Chem., 2018, 90, 9441–9448 CrossRef CAS PubMed.
- A. Retzmann, T. Zimmermann, D. Profrock, T. Prohaska and J. Irrgeher, Anal. Bioanal. Chem., 2017, 409, 5463–5480 CrossRef CAS PubMed.
- A. M. Wefing, J. Arps, P. Blaser, C. Wienberg, D. Hebbeln and N. Frank, Chem. Geol., 2017, 475, 140–148 CrossRef CAS.
- T. G. Enge, M. P. Field, D. F. Jolley, H. Ecroyd, M. H. Kim and A. Dosseto, J. Anal. At. Spectrom., 2016, 31, 2023–2030 RSC.
- S. J. Romaniello, M. P. Field, H. B. Smith, G. W. Gordon, M. H. Kim and A. D. Anbar, J. Anal. At. Spectrom., 2015, 30, 1906–1912 RSC.
- A. Van Ham-Meert, S. M. Chernonozhkin, S. J. M. Van Malderen, T. Van Acker, F. Vanhaecke and P. Degryse, Geostand. Geoanal. Res., 2018, 42(2), 223–238 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |