Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature
Shun
Zhang
,
Zhengpeng
Wan
and
Roger D.
Kamm
 *
*
Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA. E-mail: rdkamm@mit.edu
First published on 15th January 2021
Abstract
Human organoids, self-organized and differentiated from homogenous pluripotent stem cells (PSC), replicate the key structural and functional characteristics of their in vivo counterparts. Despite the rapid advancement of organoid technology and its diverse applications, major limitations in achieving truly in vivo like functionality have been the lack of matured structural organization and constraints on tissue size, both of which are direct consequences of lacking a functional vasculature. In the absence of perfusable vessels, a core region within organoids quickly becomes necrotic during development due to increased metabolic demands that cannot be met by diffusion alone. Thus, incorporating functional vasculature in organoid models is indispensable for their growth in excess of several hundred microns and maturaturation beyond the embryonic and fetal phase. Here, we review recent advancements in vascularizing organoids and engineering in vitro capillary beds, and further explore strategies to integrate them on a microfluidic based platform, aiming for establishing perfused vasculature throughout organoids in vitro.
1 Introduction
Organoids represent a major technological breakthrough attained over the last decade. They are now widely utilized as models of development and disease, and demonstrate tremendous potential in regenerative therapy and drug development.1,2 With the most general definition of ‘similar to an organ’, the effort of creating organoids can be traced back in time to the early 1900's when Wilson demonstrated that dissociated sponge cells are able to self-organize and generate an entire organism.3 The successful isolation of human embryonic stem cells (ESCs) and establishment of human induced pluripotent stem cells (iPSCs) open new frontiers for organoid technology, marked by the groundbreaking work from the Sasai and Clevers groups during the past decade. Sasai and his team demonstrated that ESCs can self-aggregate and differentiate into cortical tissue and optical cup4 whereas Clevers and his colleagues demonstrated that intestinal organoids with in vivo like crypt–villus structures could be generated from intestinal adult stem cells (ASCs).5 Since then, the definition of organoids has been widely accepted as self-organized 3D cell aggregates with multiple organ specific cell types derived from ESCs, ASCs or iPSCs, recapitulating their in vivo counterpart to varying degrees in structural organization, cell composition, physiology and functionality. Currently, a variety of organoids have been successfully generated mimicking organs developed from all three primary germ layers, most with multiple generation protocols available.With their inherent ability to differentiate and self-organize from initially homogenous PSCs, organoids recapitulate many key processes in organogenesis, some of which are unique to humans, and have thus been widely used as models in developmental biology to reveal mechanisms central to human organogenesis.4–6 Moreover, organoids have been extensively utilized to investigate various genetic disorders and diseases, including birth defects, cancer, infectious disease, and degenerative disease.2 During the current COVID-19 pandemic, human organoid models have played a crucial role in understanding pathogenesis in various organs that are susceptible to SARS-COV-2 infection, which is the key for developing corresponding therapeutics.7,8 Moreover, the successful application of organoids to disease modelling has opened the door to new approaches to drug screening and personalized medicine.
Despite these tremendous achievements and rapid evolution, this field is still in its infancy with a few limitations and unmet needs that hinder its wider application and limit broader impact. One major limitation is the lack of a perfusable vasculature. Even though vasculature plays a key role in many physiological events beyond just serving as conduits, its major function is still to transport nutrients, oxygen, metabolic wastes and various cells. Without a perfusable vasculature, organoids in 3D culture rely solely on passive diffusion to exchange nutrients, oxygen and metabolic waste. Although depending on the cellular density and metabolic activity of the cells, the effective distance of diffusion, however, is usually no more than approximately 300 microns, which gradually leads to a developed necrotic core.9 By incorporating vasculature, the overall size and life span of an organoid could be significantly increased, which might be the key for organoids to resemble organs beyond their embryonic and fetal phase, and potentially open the possibility of use in organ replacement. Moreover, the correct regional patterning and crosstalk can be established by avoiding the necrotic core, thus make it possible for organoids to recapitulate in vivo physiology and functionality at a higher level. Furthermore, the endothelium of vasculature provides for paracrine signaling and allows basement membrane interactions with other cells types, which could potentially improve organoid maturation. Consequently, even those organoids with self-organized lumen structures, such as intestinal and colon organoids, where insufficient nutrients and oxygen are not limiting factors, could still benefit from incorporating a vascular network.
Recently, efforts have increasingly focused on developing the capability to vascularize organoids drawing upon techniques ranging from co-culture with endothelial cells (ECs), co-differentiation with mesodermal progenitors, in vivo transplantation, to incorporating mechanical stimuli or inducible genetic circuits. Various levels of success have been achieved with brain, liver, and kidney organoids, among others.10–12 To date, perfusable vasculature within organoid has only been demonstrated through transplantation into host animals, where native vasculature was seen to penetrate into the ectopic implant or anastomose with pre-vascularized organoids.11,13 Whether it is necessary to pre-vascularize organoids or co-culture with ECs before transplantation is still under debate, but there is no doubt that under appropriate conditions, implanted organoids will eventually become vascularized by the host. In many cases, transplanted organoids show improved maturation along with host vasculature, further demonstrating the importance of incorporating functional vasculature in organoids.11,13,14 However, although various degrees of vascularization have been induced within organoids, no in vitro approach has yet successfully demonstrated intravascular perfusion within organoids. With rapid advances in organoid technology, an in vitro platform with functional vasculature throughout the hosted organoid is an urgent need to study complex biological events. For example, organ-specific metastases, immune cell trafficking can be investigated by perfusing corresponding cells through functional vessels. By hosting multiple vascularized organoids connected directly with vascular bed, it could be employed to study interactions between organs. Furthermore, more mature and physiologically relevant organoids can be generated with such platform to provide better organoid models.
In parallel with the advancement of organoids technology, researchers have established multiple methodologies to successfully construct functional vasculature in vitro with various hydrogels, from natural materials like fibrin, collagen and Matrigel to synthetic biocompatible materials including PEG and its derivatives.15–17 Based on the design principles, the existing in vitro vascular platforms can be broadly classified into two categories, self-organizing or ‘emergent’ and pre-patterning or ‘top-down engineered’.18 The first often involves microfluidic devices with 3 channels, a central gel region with 2 media channels flanked along each side.17,19 ECs with or without stromal cells can be introduced into the gel channel together with hydrogel solution before polymerization, or adhered to the walls of gel channel after hydrogel polymerization. Within days, ECs will self-organize and form networks with perfusable lumens, mimicking the in vivo vasculogenesis and angiogenesis processes. In contrast to self-organizing, the pre-patterning approach often starts with constructing hydrogel scaffolds, either by utilizing various 3D printing methods or involving sacrificial materials.20,21 The pre-defined channels are then populated with ECs to form functional vascular networks. Unlike the self-organized vasculature, the structure, orientation, and geometry are predefined, offers the ability to form either simple macroscale channel, or complex microscale vessels. Inspired by those efforts, more and more in vitro vascularized tissues have been constructed in recent years.22–24
These techniques have been extensively incorporated in tissue engineering with the efforts to engineer in vivo like bulk tissues and organs, leading to organ-on-a-chip systems, which evolved in parallel with organoids technology, with the same goal: to build multicellular in vitro organotypic models recapitulating physiology and functionality of corresponding organs.25 However, fundamental differences exist between organoids and organ-on-a-chip platforms despite the similarities in their names. Organs-on-a-chip typically integrates only those elements deemed essential for the physiological functions of interest into a microfluidic device, where cell types, structure organization and microenvironment are precisely controlled. In contrast, organoids often start with homogenous PSCs that spontaneously self-organize and differentiate into 3D structures mimicking their in vivo counterparts. Due to the self-organizing characteristic, it is not feasible to directly engineer perfusable vasculature throughout organoids at this time. Nevertheless, advances in making vascularized hydrogels provide promising strategies to vascularize organoids. Furthermore, vascularized hydrogels are potentially suitable as a capillary bed on which to grow pre-vascularized organoids in vitro, with the long-standing goal of obtaining functional intravascular perfusion within organoids through anastomosis of internal and external vasculatures.
Here, we review state of the art in vascularizing PSC-derived organoids and engineering in vitro capillary beds, and further discuss possible strategies to integrate these two systems aiming for engineering a platform with functional microvascular network (MVN) throughout the embedded organoids, while highlighting current challenges and future directions.
2 Techniques to pre-vascularize organoids
Living organisms naturally possess the ability to stimulate vascularization. Based on previous work, it has been shown that organoids, even without pre-developed internal vasculature, can be successfully vascularized by the host after in vivo transplantation. One particular example is the brain organoids, which generated by promoting the development of ectoderm without any noticeable vessel network structure. Mansour et al. engrafted hPSC-derived brain organoids into a murine model, demonstrated functional vasculature, decreased apoptosis and improved neuronal differentiation in organoid grafts.13 Based on this example, it seems that a feasible strategy to incorporate functional vasculature within organoids is to simply enable this natural process to occur, by induced angiogenesis from neighboring vessels after embedding organoids in an engineered vascular bed in vitro. However, except with fibroblast spheroid models and cancer models,26,27 such strategy has not been proven to be successful to form fully perfusable vasculature throughout and within any kind of PSC-derived organoids. On top of that, a few groups have demonstrated significantly improved survival rate and maturation of pre-vascularize organoids after transplantation,11,12,28 indicating the importance of timely establishment of functional vasculature through anastomosis between host and organoid grafts. Taken together, this evidence suggests that organoid pre-vascularization, even without demonstrated perfusion, is an indispensable part towards a truly perfusable organoid platform in vitro.In this section, we discuss possible pre-vascularizing strategies of PSC-derived organoids. Instead of organizing by each type of organoids, we categorize according to methodology since we believe these strategies to be relatively generic and thus applicable to various types of organoids.
2.1 Co-culture with endothelial cells
Perhaps the most straight forward and intuitive way to vascularize organoids is by co-culture with endothelial cells (ECs), which are single layered squamous cells that line the interior surface of blood and lymphatic vessels. This methodology has been applied to organoids generated from all three germ layers and achieved various degrees of vascularization.11,14,28 However, successful implementation requires a careful plan with regard to the timing of co-culture, which strongly depends on the organoid generation protocols. Today, the large number of available protocols for generating PSC-derived organoids can be broadly classified into two categories. The first involves the initial formation of an embryonic body (EB), a 3D aggregate of PSCs that undergo initial developmental specification of three primary germ layers in much the same manner as the pre-gastrulating embryo. The development of one specific germ layer is then promoted by biochemical cues, and subsequently differentiated into a specific organoid. The other approach starts with differentiating PSCs into progenitor cells in 2D monoculture, followed by mixing of multiple cell types that are essential for proper functionality of a specific organ, and eventually growth into the desired organoid. For organoids generated with the latter approach, ECs are often incorporated in the mixing step, where progenitor cells, ECs and other supporting cells are mixed to form 3D aggregates. For instance, Takebe et al. developed vascularized liver buds (LBs) by seeding iPSC derived hepatic endoderm, human umbilical vein endothelial cells (HUVECs) and human bone marrow mesenchymal stem cells (MSCs) on Matrigel11 (Fig. 1A1). The contractile nature of MSCs initiated condensation within the heterotypic cell mixtures and resulted in the formation of self-organized 3D spheroids, within which HUVECs formed nascent endothelial networks.29 Human iPSC-LBs underwent further maturation upon transplantation, with the production of high level human albumin. Notably, functional anastomosis was established within 48 hours of transplantation, confirmed by perfusion of dextran dye from host vessels into human vessels within the transplant. Similar techniques also have been utilized to vascularize pancreatic14 and liver organoids.30 In all these studies, the control organoids without ECs failed to vascularize and engraft, further highlighting the importance of incorporating ECs to pre-vascularize organoids before transplantation.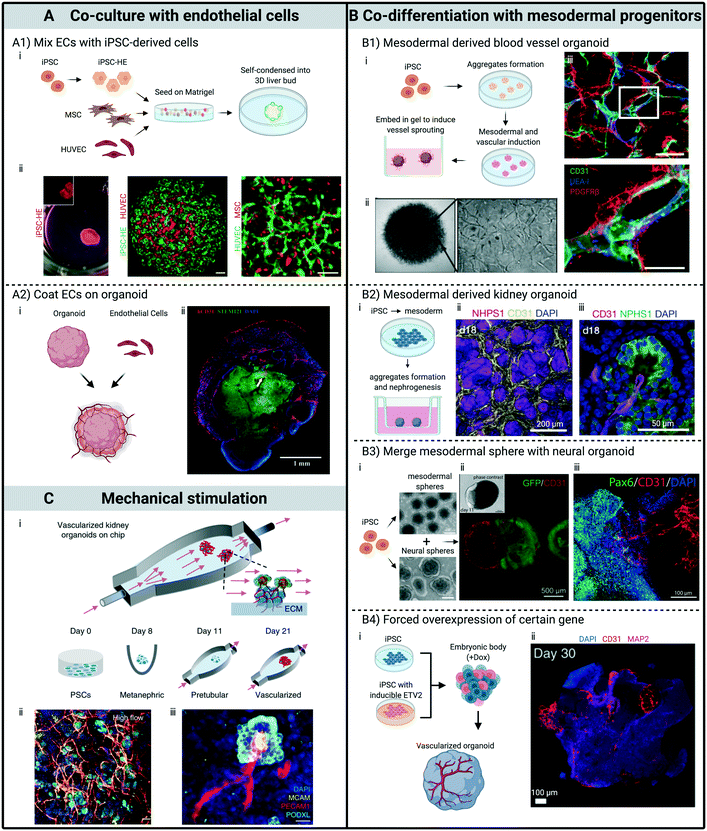 | ||
| Fig. 1 Strategies to pre-vascularize organoids. All subfigures (i) are schematic illustrations of processes for developing various pre-vascularized organoids. (A) Through co-culture with ECs. (A1) Mixing ECs with iPSC-derived cells to form organoids. ECs self-organized into networks within organoid. Scale bars, 100 μm. (A2) Coating ECs on organoid. Immunostaining showing penetration of capillaries into the outer layers of a brain organoid 20 days after coating and co-culturing with ECs. (B) Through co-differentiation with mesodermal progenitor cells. (B1) Human blood vessel organoids formed by modulating mesoderm development and vascular specification. Mature organoids contain endothelial tubes (CD31 and UEA-I) covered by pericytes (PDGFRβ). Scale bars, 50 μm. (B2) Kidney organoids developed by induction of mesoderm-derived progenitor populations. Immunofluorescent images showing (ii) CD31+ endothelial network within the renal interstitium and (iii) invasion into glomeruli at day 18 of culture. (B3) Pre-vascularized neural organoid developed by merging with mesodermal progenitor spheroid. Immunostaining showing (iii) CD31+ endothelial networks infiltrate Pax6+ neural part of the organoid at day 180. (B4) Induce vascular formation within brain organoid through forced ETV2 over-expression. Immunostaining indicates abundant vasculature-like structures at day 30 in a brain organoid with forced ETV2 overexpression in 20% of the cells. (C) Through mechanical stimulation. Kidney organoids exhibit enhanced vascularization and maturation when cultured under external flow. Confocal 3D renderings for vascular markers reveal (ii) abundant vascular structures, which (iii) further extend to and invade glomerular compartments when renal organoids were subjected to flow with controlled wall shear stress. (A1) Adapted from ref. 11, with permission of Springer Nature. (A2) Adapted from ref. 28, with permission of Wolters Kluwer Health, Inc. (B1) Adapted from ref. 41, with permission of Springer Nature. (B2) Adapted from ref. 40, with permission of Springer Nature. (B3) Adapted from ref. 45 and 46, with permission under Creative Commons license. (B4) Adapted from ref. 12, with permission of Springer Nature. (C) Adapted from ref. 10, with permission of Springer Nature. | ||
For organoids that are derived following the EB formation protocol, where PSCs are co-differentiated toward multiple organ specific cell types, a direct mixing of PSCs and ECs initially to form 3D aggregates often leads to phase separation of the different cell populations or failure to differentiate into proper cell lineages.31 In this case, researchers often turn to a co-differentiation approach to vascularize organoids, which will be discussed next. However, ECs can still be incorporated at a later time point to vascularize organoids that are generated based on the EB formation protocol. Pham et al. coated iPSC derived ECs on day 34 onto brain organoids derived from the same iPSC line. After two more weeks of in vitro culture, there were abundant CD31+ structures surrounding the organoid, with capillary-like structures growing into its outer layers. Organoids were subsequently engrafted into mouse brain, and only the pre-vascularized brain organoids survived 2 weeks after transplantation28 (Fig. 1A2). Song et al. assembled hiPSC-derived cortical spheroids and isogenic EC spheroids at D14. The hybrid spheroids exhibited elevated gene expression levels associated with blood brain barrier (BBB) proteins. However, there is no evidence in this work that ECs developed a vascular network in the cortical spheroid through angiogenesis.32
Depending on the in vivo counterpart that a specific organoid is mimicking, the type of ECs should be carefully selected as well. It is well known that ECs exhibit large heterogeneity between organs, and this specificity is critical for organ function.33 For example, cerebral ECs together with astrocytes and pericytes form tight barriers typical of the brain microvasculature.34 The tight barrier allows the passage of some molecules by passive diffusion, as well as the selective transport of various nutrients and macromolecules, but prevents most solutes, especially harmful substances, from entering the central nervous system.35 In contrast, liver sinusoidal capillaries have a discontinuous fenestrated endothelium and underlying basal lamina. The porous barrier assures delivery of solutes and colloids to the liver lobules, as well as contributing to the proper execution of liver functions such as gas-exchange, nutrient absorption, scavenging and detoxification.36
A wide variety of ECs are currently available for co-culturing with organoids. HUVECs, among all other types of ECs, are most extensively utilized in these efforts because the ease with which they can be obtained and cultured. Furthermore, since HUVECs are harvested from fetal umbilical cord, their use is compatible with the fetal or embryonic phase tissues that PSC-derived organoids resemble. iPSC-derived ECs are another common choice. Compared with using primary ECs, iPSCs provide an unlimited source to generate large numbers of ECs. Another advantage of iPSC-derived ECs is that they can be co-differentiated with all other organ specific cell types in 3D aggregates, which closely mimicking in vivo organogenesis. Due to the microenvironmental cues provided by a specific organoid during its development, iPSC-ECs have the potential to further differentiate into organ specific ECs to adapt to the needs of the organoid.37–39 Furthermore, using patient-specific iPSC cells to generate both ECs and corresponding organoids might be most promising in translational applications such as regenerative medicine, since they would avoid the complex immune response of host to xenografts.
2.2 Co-differentiation with mesodermal progenitor cells
The development of embryonic vasculature starts with the differentiation of mesoderm-derived angioblasts, and coordinates closely with organogenesis to provide sufficient nutrients and oxygen, as well as growth factors to support the rapid growth of embryonic organs in vivo. Since mesoderm is the exclusive source of EC progenitors during embryogenesis, promoting the development of mesoderm to further differentiate into ECs coincides with organoid growth is another possible strategy to pre-vascularize organoids. Compared with EC co-culture, however, the timing of introduction and the number of resulting ECs can not easily be controlled. But since the co-differentiation approach more closely mimics the development of nascent vasculature during organogenesis, it is often preferred in the studies of organogenesis and developmental events that lead to tissue formation. Furthermore, co-differentiating ECs together with the development of organoids adds a fascinating level of complexity in this system, providing opportunities to study intricate interactions among different cell types including ECs during stages of development.Organoids developed from mesoderm often yield PSC-derived ECs spontaneously, likely due to their shared mesoderm origin. In 2015, Takasato et al. generated kidney organoids containing nephrons associated with a collecting duct network by utilizing known developmental mechanisms regulating the preferential induction of collecting duct versus kidney mesenchyme progenitors.40 Remarkably, endothelial networks with lumen formed after 18 days of culture, with PDGFRα+ perivascular cells located along the endothelium and early mesangial cells invaginating the glomeruli, as observed in human fetal kidney (Fig. 1B2). The human blood vessel organoids reported by Wimmer et al. represent another excellent example. The authors developed a multi-step protocol to induce mesoderm development and later vascular specification of human iPSC aggregates. The engineered human vascular organoids, which were comprised of pericytes, endothelium, mesenchymal stem-like cells as well as haematopoietic cells, enclosed self-assembled capillary networks that are enveloped by a basement membrane (Fig. 1B1). Upon transplantation, vascular organoids formed a stable and perfused vascular tree including arteries, arterioles and venules. When exposed in vivo to a diabetic milieu in mice, the human vessels faithfully recapitulate structural and functional changes of human blood vessels found in patients with diabetes, thus can be used as a reliable tool for modelling and identifying the regulators of diabetic vasculopathy.41
With the appropriate cocktail of growth factors and culture media, development of mesoderm can be induced along with other germ layers to generate vascularized organoids originated from endoderm or ectoderm. Wu et al. reported a method to generate functional hepatobiliary organoids from human iPSCs with simultaneous inducement of endoderm and a small part of mesoderm by inclusion of 25% mTeSR into hepatic differentiation medium.42 This treatment mildly delayed early hepatic differentiation, but turned on biliary specification. Even though their goal was to co-differentiate hepatocyte-like cells and cholangiocyte-like cells within the organoid, mesoderm-derived endothelial cells appeared as tubular structures across the organoids by day 15. On the other hand, promoting the co-development of mesoderm and ectoderm is still challenging because it is known that activating certain pathways to direct cells to ectodermal fate will restrict mesoderm differentiation.43 Ham et al. supplemented vascular endothelial growth factor (VEGF) in the process of generating cerebral organoids, and observed vascular structures expressing EC markers. However, those structures were sparse and did not interconnect to form a vascular network.44 Wörsdörfer et al. took a different path to fuse separately cultured neural and mesodermal spheres at a later stage, with CD31+ structures invading the neural tissue from the interfaces between neural and mesodermal parts of the aggregate after 180 days of co culture (Fig. 1B3). However, despite the extensive vascular network in the mesodermal part, there were few vessel sprouts in the neural part.45,46
Advances in gene engineering offer possible solutions to vascularize organoids via co-differentiation that circumvent the tedious work of finding appropriate cocktails of growth factors. After Morita et al. reported their method for converting human fibroblast into functional endothelial cells via ETS transcription factor 2 (ETV2) in 2015,47 the role of ETV2 in specifying cells to endothelial and hematopoietic lineages has been extensively studied and utilized. Through temporal delivery of modified mRNA encoding ETV2 at an intermediate mesodermal stage of differentiation, Wang et al. achieved exceedingly high efficiency (>90%) in differentiating 13 different human iPSC lines to functional ECs.48 Cakir et al. were the first to take advantage of this technique to vascularize organoids. By incorporating 20% of iPSCs that were transduced with a doxycycline (Dox) inducible ETV2 gene in the initial 3D aggregates, they successfully induced the development of vascular structures within organoids following traditional cerebral organoid culture protocol. The vascularized organoids exhibited higher neuronal activity with more mature neurons than the avascular ones. Furthermore, once transplanted, pre-vascularized brain organoids showed increased perfusion with less permeable vessels than control organoids12 (Fig. 1B4). One striking finding from this study is that the subset of iPSCs with overexpressed ETV2 can still robustly differentiate into ECs, even in the culture media with growth factors that direct the differentiation towards ectodermal fate. The result essentially suggests that overexpression of internal transcriptional factors can work independently of, or override, the differentiating signals in external microenvironment. This should be a rather generic methodology to incorporate ECs in a system, and has the potential to be broadly applied on all kinds of organoids. Compared with activating ETV2, which serves the specific function of inducing differentiation towards ECs, overexpression of less specific transcriptional factors sometimes leads to multiple progenitors that can include endothelial progenitor cells. Guye et al. generated liver-bud like tissue from engineered iPSCs with heterogenic expression of GATA6. Through a transient forced GATA6 expression, progenitor cells of both endodermal and mesodermal origin rapidly emerged as a complex function of GATA6 expression levels and tissue context. Within two weeks, CD31+ and CD34+ cells emerged and formed vascular-like network, surrounded by micro-structures composed of cells with hepatic fate expressing markers of CEBPα, HNF4α, secreted alpha-1 antitrypsin (AAT), etc. The fetal liver like function has also been confirmed with steady production of AAT, albumin and fibrinogen. Moreover, small spherical cells expressing CD45 or haemoglobin budded off from endothelial tubes after day 14, together with significantly upregulated hemoglobin gamma at day 15, suggesting definitive fetal erythropoiesis.49
2.3 Mechanical stimulation
A developing vasculature is subjected to various mechanical cues in vivo, ranging from viscoelastic properties of neighboring extracellular matrix (ECM) to traction stresses generated by peri-vasculature cells. Since ECs formed the interface between circulating blood flow and the surrounding tissue, they are in addition subjected to shear stresses induced by pulsatile or unidirectional blood flow, transmural flow and interstitial flow. Various microfluidic platforms that have been engineered to investigate the effects of mechanical factors on angiogenesis, vasculogenesis and 3D capillary morphogenesis in vitro, confirmed the vital role of mechanical cues in inducing and maintaining physiologically functional vasculature.15,50,51 As for the development of organoids, the importance of mechanical factors is also increasingly appreciated. The benefits of subjecting developing organoids to continuous media flow have been reported with islet and intestinal organoid models in microfluidic devices.52,53However, even though mechanical factors may be as important as biochemical factors in development of vasculature and organoids, they are much less studied, possibly due to the complexity of engineering suitable platforms to investigate or implement individual mechanical factors. The lack of biomechanical control has thus been recognized as a crucial limitation to the further development of mature organoids and more physiologically relevant models. In 2019, Homan et al. reported an in vitro method for culturing kidney organoids on top of a customized hydrogel under flow, which induced the formation of EC networks within and sprouting from the organoids10 (Fig. 1C). To our knowledge, this is the only in vitro approach that demonstrated an improved vasculature within organoids through mechanical stimulation alone. The shear stresses exerted by flow expanded the endogenous pool of endothelial progenitor cells, which further differentiated and self-organized into vascular networks, and in some cases, grew into glomerular structures in close association with tubules. Compared with control organoids in a static, no-flow condition, organoids subjected to 10 days of flow exhibited enhanced maturation, with upregulation in tubular epithelial transporters, adult transcription factors, and enhanced glomerular vascularization and foot process maturation. Although the composition of ECM used in this study and the addition of fetal bovine serum in the culture media seems to contribute as well to expansion of the endothelial network, the incorporation of shear stresses by flow played a crucial role in the improved development of vascular structure and maturation.
Methodologies described in this section could potentially work in a synergistic manner with integration of microfluidic techniques, which offer the advantage of precise control over the in vitro micro-environment. That includes spatial–temporal distribution of growth factors, morphogens, and mechanical factors including stiffness of ECM, stresses and pressure gradients induced by interstitial as well as convectional flow. It should be noted that although various degrees of vascularization have been achieved in organoid models, none of these have yet been demonstrated to have fully perfusable function without in vivo transplantation. Thus, organoids cannot be considered as truly vascularized in vitro yet.
3 Engineering functional capillary beds in vitro
One major limitation to achieve perfusion in an organoid model is to develop functional anastomosis between internal vasculature and available external functional vessels in the surrounding ECM. Advances in related fields of engineering perfusable in vitro capillary beds provide various potentially suitable platforms to host pre-vascularized organoids, and thus bring us closer to the ultimate goal of a fully perfusable in vitro system throughout the developed organoid. In this section, we focus on the available techniques for engineering vascularized hydrogels and associated microfluidic devices, which can be classified into two broad categories: self-organized and pre-patterned.3.1 Self-organizing vascular networks
These designs draw upon natural self-organizing and self-assembly principles by promoting conditions in which vasculogenesis or angiogenesis, two major ways of forming and developing vascularization in vivo, can occur. A major advantage of self-organizing approach is its similarity to the in vivo processes to grow and develop vasculature, thus leading to spontaneous formation of vessels by ECs in hydrogel mimicking in vivo counterparts in both function and morphology. In vivo, vasculogenesis occurs in developing embryos to form the earliest primary vascular plexus, which starts with the differentiation of mesodermal cells to angioblasts. In response to local cues, the angiogenic cells further differentiate into ECs, migrate and coalescence to form early capillary-like networks.54In vitro vasculogenesis models typically utilize microfluidics which can be seeded with various types of hydrogels containing ECs.16,17,55 Following this approach, Whisler et al. seeded HUVECs in fibrin gels co-cultured with human lung fibroblasts (HLFs) in the middle region of microfluidic chip with three parallel fluidic channels separated by precisely spaced micro-posts, which confined the liquid gel in the central channel before polymerization (Fig. 2A1). Culture medium was subsequently introduced into the outside channels to support the development of vasculature. This results in a perfusable network in 4–7 days, as confirmed by perfusion of fluorescent beads or dextran dyes. Multiple factors, including cell seeding densities, fibrinogen concentration, additional VEGF and sphingosine-1-phsophate (S1P) were found to affect the morphological properties of the microvascular networks.17 Remarkably, co-culture with supporting cells was found to be critical in avoiding network regression and maintaining stable vessels beyond one week. Besides HLFs, other studies have also demonstrated the supporting role of adipose tissue derived stromal cells56 and MSCs57 to maintain long lasting MVNs in vitro. Similar functional capillary beds have been obtained through various designs of microfluidic devices. For example, Moya et al. cultured human capillary bed on multiple diamond shape gel regions that have been integrated into a high-throughput microfluidic platform.15 Xiaolin Wang et al. further integrated the diamond shape microfluidic devices with EC lining strategies and demonstrated the anastomosis between capillary networks and large EC-lined microfluidic channels.58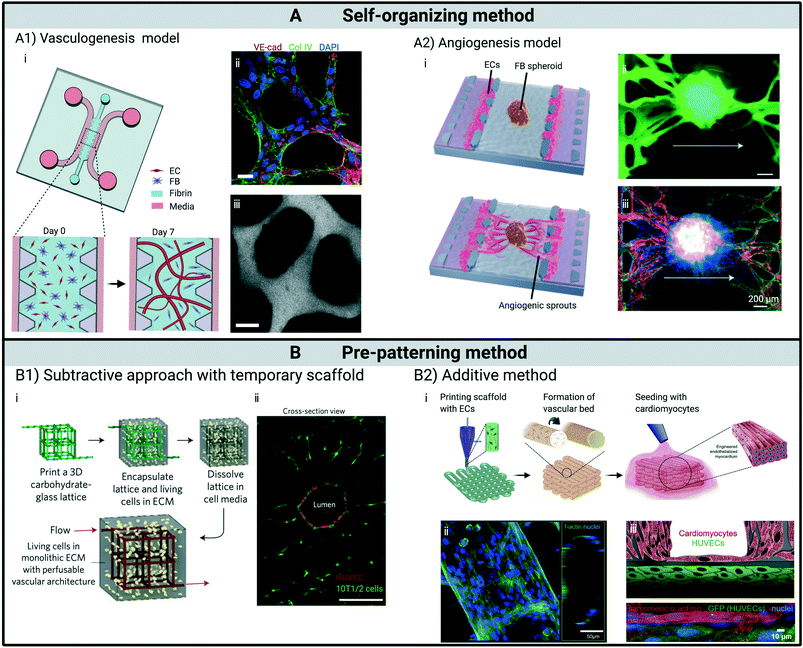 | ||
| Fig. 2 State-of-the-art in engineering in vitro capillary beds. (A) Vasculature formed by self-organization. (A1) Vascular bed formed through vasculogenesis. (i) Schematics illustrate the formation of perfusable vasculature within a week by injecting mixture of ECs and fibroblasts into central gel channel of a microfluidic device. (ii) At day 7, ECs self-organized into in vivo-like capillaries with expression of continuous cell–cell junction protein VE-cadherin, and deposition of collagen IV basement membrane around the lumen. (iii) Patent lumen is confirmed by perfusion with 70 kDa dextran. Scale bars, 20 μm. (A2) Vascular bed formed through angiogenesis and subsequent integration with embedded spheroid. (i) Schematics showing fibroblast spheroid vascularized by angiogenic sprouts induced from ECs seeded on the interface of central gel channel. Perfusion within spheroid is verified with introduction of (ii) dextran dye and (iii) microbeads. (B) Vasculature fabricated through pre-patterning techniques. (B1) Functional vascular network formed around temporary scaffold. (i) Schematics showing cell–laden matrix is introduced surrounding a temporary scaffold, which is removed or dissolved afterwards. ECs are subsequently introduced into the open channels, yielding a vascular structure that matches the original scaffold. (ii) Cross-section imaging of a representative channel demonstrates endothelialized patent lumen. Scale bar, 200 μm. (B2) Vascular structures fabricated through an additive bioprinting method by directly depositing cell–laden bioinks following pre-designed patterns. (i) Schematics showing the process of fabricating endothelialized myocardium by bioprinting. (ii) Confocal fluorescent images demonstrate tubular structure formed by ECs. (iii) Schematic and confocal fluorescent image showing endothelialized myocardial tissue formed by seeding cardiomyocytes onto the bioprinted endothelialized vascular structures. (A1) Adapted from ref. 55, with permission of Oxford University Press. (A2) Adapted from ref. 26, with permission of Oxford University Press. (B1) Adapted from ref. 20, with permission of Springer Nature. (B2) Adapted from ref. 21, with permission of Elsevier. | ||
The other vital physiological process to generate new vessels is angiogenesis. Unlike vasculogenesis, angiogenesis initiates angiogenic sprouts from pre-existing vessels. In response to pro-angiogenic growth factors, ECs degrade ECM by proteases to escape from the parent vessel wall, enabling the growth of new vessels into avascular regions.59,60 Numerous microfluidic models and hydrogel based assays have been designed to extensively investigate this process, which includes enzymatic degradation of ECM, EC proliferation, invasion, tubulogenesis and vessel stabilization.50,61,62In vitro angiogenesis is usually initiated from EC monolayers seeded onto the surface of various hydrogels63 or coated beads.64,65 Further integration of such hydrogels in PDMS-based microfluidics allows for tip cell migration and lumen formation across much of the gel region within a week, sometimes leading to a functional vascular bed.66 One approach that has proven successful involves coating HUVEC monolayers on opposite ends of hydrogel in a microfluidic device with a centrally-located spheroid. HUVECs sprouting into the gel from opposite sides fused with each other, which spontaneously resulted in perfusable MVN through angiogenesis26,27 (Fig. 2A2). Functional vessels can be obtained through either vasculogenesis or angiogenesis in the same microfluidic platform through different seeding strategies. For instance, Kim et al. engineered a microfluidic chip with three parallel gel regions separated by two media channels.19 Micro-posts have also been positioned to generate enough surface tension to confine the gels in corresponding channels. By seeding HUVECs in the central gel channel and HLFs in outer gel regions, or seeding HUVECs on the left side-wall of the acellular fibrin matrix that filled the central channel and HLFs on the opposite side in the outer channel, perfusable vessels can be established through vasculogenesis or angiogenesis, respectively.
Similarities between self-organized in vitro MVNs and in vivo capillaries have been demonstrated by numerous works, in terms of both form and function. Immunofluorescence is commonly used to demonstrate connectivity and maturity of networks, including CD31, vascular endothelial (VE)-cadherin, tight junction proteins zonula occuldins-1 (ZO-1), and basement membrane proteins like laminin and collagen IV,15,19,66 although perfusion is best determined by introducing a fluorescent dye to the medium and imaging vessel filling. Moreover, normal function has also been shown in response to inflammatory cues and perfusion of whole blood.19,67,68 Furthermore, integrity and proper barrier function of in vitro self-organized 3D networks have been characterized by the measurement of permeability, which was shown to be up to two orders of magnitude lower than that measured in transwells using the same cells, and was comparable to values observed in vivo.19,55,69,70 Transcytosis across the endothelium of large molecules has also been demonstrated and measured with such 3D perfusable microvascular networks.70,71 To further replicate in vivo functionality, organotypic vasculatures have been engineered through self-organizing approach by co-culturing relevant types of cells. Campisi et al. developed a human BBB microvascular model with a tri-culture system consisting of iPSC-derived endothelial cells and primary brain pericytes and astrocytes in fibrin gel.34 The model exhibited perfusable and selective microvasculature, with permeability lower than conventional in vitro models, and similar to in vivo measurements in rat brain. When contrasted with the vasculature developed from HUVEC and supporting cells, organotypic vasculatures are potentially more beneficial for hosted organoids since they provide a more physiologically relevant microenvironment.
Recently, efforts have also integrated such self-organized perfusable vessels with large tissue in microfluidic devices. Nashimoto et al. demonstrated this idea by seeding a spheroid, made from a mixture of HUVECs and HLFs, in the central region of a similar 3-channel device. HUVECs were subsequently seeded on both side walls of the central gel26 (Fig. 2A2). Driven by angiogenic factors secreted by the co-cultured spheroid, ECs on the side wall invaded into the gel as angiogenic sprouts, reaching toward the spheroid. Around day 4, vessels originating from the co-culture spheroid and from side walls met and anastomosed near the periphery of the spheroid. Remarkably, these functional anastomoses led to a fully perfusable network across the central gel, including the spheroid, as evidenced by successful perfusion of fluorescent dextran dye into the core of the spheroid. Using the same platform and strategy, they recently demonstrated fully perfusable cancer spheroids, which were formed by mixing ECs, HLFs and cancer cells.27 Although the same approach has not yet been implemented on PSC-derived organoids, such work provides a promising strategy to achieve fully-functional vasculature through an organoid and peripheral capillary bed in vitro.
3.2 Pre-patterning
Distinct functional vascular beds with pre-defined geometries, ranging from single vessel to complex in vivo like vasculature, have also been demonstrated via various pre-patterning techniques, which can be further classified into subtractive and additive approaches.The subtractive approach usually involves forming a network of 3D channels within a hydrogel by casting pre-polymerized hydrogels around a temporary mold, which is subsequently dissolved or removed, leaving hollow channels for perfusion. ECs are then seeded into the channels to form a monolayer on the channel walls (Fig. 2B1). Through this method, numerous rod-based techniques have been employed to generate perfusable single vessels.72–74 For instance, Chrobak et al. engineered perfusable endothelial tubes by seeding ECs inside a hollow channel formed within a collagen gel by removal of a stainless steel needle. Such endothelial tubes displayed a strong barrier function over 5 days, and reacted quickly to inflammatory stimuli.72 Molding with sacrificial materials offers the possibility to construct complex vascular networks beyond uniaxial single vessels. In one example, Miller et al. reported a method to create vascularized tissues with a 3D printed carbohydrate glass lattice, which is used as biocompatible sacrificial template embedded in ECM containing living cells.20 The lattice was easily dissolved by immersing in complete medium for 10 minutes, leaving behind interconnecting cylindrical channels that could be populated with ECs (Fig. 2B1). In this work, the authors successfully demonstrated perfusable networks with diameters ranging from 150 to 750 μm. In addition to carbohydrate glass, numerous biocompatible materials have been utilized to create temporary sacrificial templates within hydrogels.3,75 Recently, functional vessel networks made from a sacrificial scaffold have been employed to support the development of organoid-based tissues. Skylar-Scott et al. created vascularized tissue with high cellular density by condensing a large number of PSC-derived organoids in a solution of collagen and Matrigel.76 A scaffold was subsequently printed into the solution with sacrificial ink made of gelatin, which was then dissolved and seeded with ECs. With this approach, they created perfusable cardiac tissue that fused and beat synchronously when perfused over long durations. However, the printed vessels were of large diameter, so the construct lacked a microvasculature, which could partially explain the relatively low contractility observed in the engineered cardiac tissue. Another less popular strategy to generate complex channels within hydrogel in a subtractive manner involves boring holes directly with laser cutting systems.77,78 However, in order to take full advantage of this method, the hydrogel material must have appropriate properties to accommodate laser wavelength, which limits the choice of available materials. In all of these methods, however, one limitation is that small diameter channels are difficult to endothelialize, so most networks formed in this way lack capillary-sized vessels.
In vitro vascular beds can also be obtained in an additive manner, for example through a layer by layer deposition of hydrogels incorporating cells of interest via 3D bioprinting. Through this method, various vascularized in vitro tissues have been engineered by depositing ECs together with other cell types in pre-designed patterns and sequences.21,79–81 In one example, Zhang et al. fabricated endothelialized myocardium by first printing the vasculature scaffold with customized bioink containing ECs21 (Fig. 2B2). The scaffold was further stabilized through crosslinking by exposure to UV light after printing. Afterward, ECs migrated towards the peripheries of the microfibers and formed a confluent endothelium, generally by around day 15. Cardiomyocytes were then seeded in the 3D endothelial bed to generate aligned myocardium capable of spontaneous and synchronous contraction. ECs can also be printed with other cells altogether in specific patterns via printing of coaxial filaments, where ECs reside in the core while the external layer is composed of tissue-specific cells. Leong et al. fabricated patterned vascularized hepatic or adipose tissue constructs by assembling individually tailored cell-laden polyelectrolyte hydrogel fibres, with ECs in the center and surrounded by hepatocytes or adipocyte precursor cells, respectively.81 A more detailed discussion in applying pre-patterning techniques to vascularize organoids can be found in ref. 82.
Each of the approaches discussed above has its pros and cons. Geometry of vascular beds engineered through pre-patterning techniques can be fully controlled. However, the diameters of vessels that can be formed and endothelialized (>100 μm) are usually an order of magnitude larger than that of a in vivo capillary, due to physical limitations of the materials to make temporary mold, the size of nozzles used for bioprinting, and the difficulties of inducing ECs to migrate into small-diameter holes. On the other hand, self-organized MVNs mimic in vivo capillaries in both form and function, but the geometry of spontaneously formed vasculature cannot be pre-defined. Fortunately, a combined method can benefit from both strategies by first forming a vascular scaffold through temporary molding, followed by induction of angiogenic sprouts to grow from EC-lined endothelium into the surrounding gel to form in vivo like capillaries.83,84
4 Establishing anastomoses between an in vitro vascular bed and pre-vascularized organoids
Before we have discussed numerous techniques to obtain pre-vascularized organoids that could be derived from all three germ layers, as well as various engineered vascular beds that recapitulate both form and functions of in vivo capillaries. However, challenges still exist in combing these two approaches to create a fully perfusable system throughout the vascular bed and organoid. The main hurdles lie in fully integrating the pre-vascularized organoids with a capillary bed in vitro to achieve functional anastomoses, as well as constructing a suitable microenvironment to support the development of this co-culture system. In this section, we highlight some of the crucial factors that need to be considered, and further discuss possible strategies to establish functional anastomoses between organoids and a vascular bed in vitro.The choice of hydrogel might be the most important aspect to consider when co-culturing organoids with a capillary bed. Hydrogel not only offers supporting matrix as scaffold, but also supplements with numerous beneficial growth factors, and adhesion molecules, while also providing appropriate mechanical support and signaling to facilitate the development of organoids and vasculature.85 By far, natural materials are most commonly employed for culturing organoids and vascular bed, due to their superior biocompatibility, excellent cell affinity and ease to obtain. However, it must be noted that organoids and a developing vascular bed often require different culture conditions and different hydrogels. During their development, organoids are most often embedded in Matrigel, which is derived from Engelbreth–HolmSwarm mouse sarcomas and closely mimicks the natural ECM. Even though Matrigel has also been utilized in developing various angiogenic assays, ECs embedded within Matrigel cannot self-organize into a perfusable vessel network, likely due to the difficulties in remodeling Matrigel by the ECs.86 Instead, functional in vitro MVNs are generally grown within collagen or fibrin, where local matrix is remodeled by ECs and FBs and result in patent lumens,15,17,67 and the initial matrix is gradually degraded and replaced by a cell-generated ECM. In the experimental design, the compatibility of supporting matrix must be optimized to ensure a successful co-culture. Very recently, Rajasekar et al. engineered a microfluidic chamber, where patient-derived colon organoids were co-cultured within a self-assembled vascular network.86 Similarly, they found ECs self-organized to form functional vessels in fibrin, but not in Matrigel, while colon organoids properly developed in Matrigel, but were unable to grow in fibrin. This dilemma was solved by co-culturing in a hydrogel mixture of fibrin with 10% Matrigel, which has been shown to support the development of both organoids and vasculature. The device was further placed on a programmable rocker to maintain cyclic luminal flow across capillary bed. Compared with conventional static condition, colon organoids grew significantly better in such a platform enabling constant perfusion. However, even though perfusable vessels were found in very close proximity to the colon organoids, which was confirmed by histology and perfusion of fluorescent particles, there was still no direct evidence of intravascular perfusion into the organoid or functional anastomosis with internal vasculature.
In addition to the supporting matrix, culture media need to be optimized as well. Media for ECs contains crucial elements for growth of ECs such as VEGF, EGF, FGF, and are typically used to support the development and maintain functional vasculature. Organoid medium, on the other hand, contains different growth factors to promote efficient differentiation towards specific cell lineages. Usually, titrating with different combination ratios of the endothelial and organoid growth media is sufficient to determine an optimized culture media mixture that supports the co-culture system, provided the mixed medium does not contain inhibitors that hinder the development of either the organoid or the MVNs. In most cases, organoids were cultured separately following the standard organoid-forming protocol initially, up to the time at which the homogenous PSCs differentiated into progenitors with self-organized cellular structures. Co-culturing at an earlier stage with an undifferentiated PSC aggregate will likely disrupt the proper development of organoid due to the presence of vascular growth factors in the media and paracrine signals from ECs. Although a Dox-inducible system can be utilized to override the differentiation signals in the microenvironment by forced overexpression of internal transcriptional factors, there are few gene circuits available at this time.
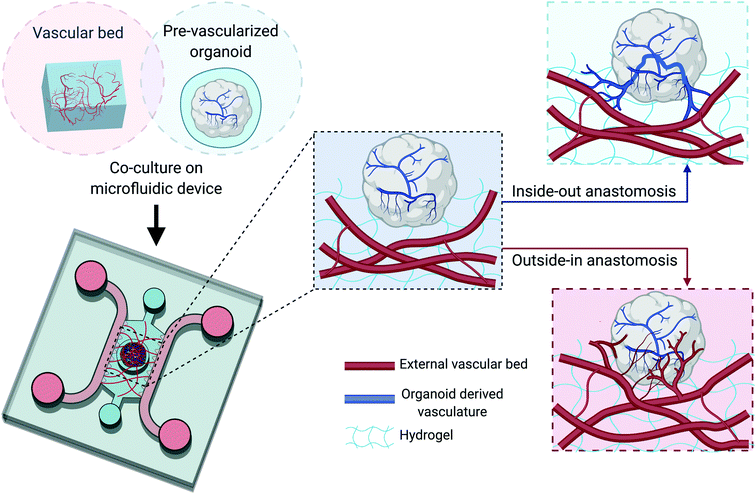
Most current co-culture methods begin by seeding organoids on top of a pre-established vascular bed, or embedding organoids in a hydrogel mixture together with ECs and other supporting cells. This runs counter to some organoid culture protocols, where organoids are grown in suspension without encapsulation inside a hydrogel. However, the choice of the specific microenvironment in which to grow organoids, either by embedding in hydrogel or culturing them directly in low adhesive plate or in suspension, is still largely driven by empirical considerations and many approaches can and have been tried. Those organoids that are not encapsulated inside hydrogel during development may still be compatible with the co-culture methods. Using the previously discussed kidney organoid as an example, Homan et al. generated kidney organoids generally following an established protocol from Morizane et al.87 However, instead of placing the developing organoids in round bottomed ultra-low-attachment plates, they were instead seeded on top of an optimized hydrogel composed of gelatin and fibrin in a microfluidic chamber.10 The direct contact with matrix led to enhanced vascularization with elevated peripheral expression of the vascular marker PECAM1, as well as the outgrowth of the vascular network into the adjacent ECM, which was further improved when subjecting the top portion of the organoid to external shear stresses. Thus, a fully perfusable system might be established with this ‘inside-out’ approach, where vasculature networks formed within the organoid expand and grow into the surrounding matrix, either reaching a perfusable channel in a microfluidic device, or merging with a neighboring MVN (Fig. 3). This mechanism can be more robustly established by precise control over the spatiotemporal distribution of pro-angiogenic factors in the matrix with advanced bioengineering techniques.88
Alternatively, functional anastomosis could be obtained through an opposite ‘outside-in’ mechanism, which is driven by angiogenesis as well (Fig. 3). This involves inducing angiogenic sprouts from ECs that make up an external vascular bed to penetrate into the organoids and connect with the vessels formed by ECs or endothelial progenitor cells presented in organoids. Such mechanism closely mimics the process that occurs after in vivo transplantation of pre-vascularized organoids, where host vasculature invades the graft establishing a vascularized organoid through functional connections.11 To activate this mechanism, pro-angiogenic factors such as VEGF are secreted by the parenchymal cells within the organoid, a natural response to the hypoxic conditions found in the core. Indeed, it has been shown that PSC-derived organoids are capable of producing VEGF themselves.89 Moreover, it has been demonstrated that spheroids can develop an internal vasculature that be perfused through similar approaches, as was discussed in previous section.26,27 Collectively, these previous works suggest that several strategies exist to produce perfused PSC-derived organoids in vitro.
5 Conclusions and perspectives
Despite rapid advances in the field of organoid technology in the past decade, the lack of functional vasculature has been widely regarded as one of the major barriers hindering the effective recapitulation of in vivo physiology. To date, fully functional intravascular perfusion of organoids has only been reported through transplantation, which negates many of the perceived advantages of using organoids as a convenient in vitro model of organ function and disease. Therefore, engineered in vitro platforms incorporating functional vasculature throughout the embedded organoids are in urgent need to broaden the biomedical applications of organoid technology. In this review, we discussed necessary steps and possible means by which our community might achieve this objective, including engineering pre-vascularized organoids, embedding organoids in pre-established capillary bed, as well as establishing functional anastomosis between organoids and external MVNs.Enormous effort has been directed toward the incorporation of vasculature within organoids. Despite numerous available protocols for various types of organoids, many of those strategies are rather generic thus have the potential to extend beyond the specific examples we discussed. At this point, each type of organoid is usually associated with multiple distinct generation protocols, most of which are still driven by empirical considerations. Detailed comparisons among different protocols in terms of functional characterization, relative cellular composition, efficiency of derivation are scarce. Moreover, our knowledge is still rather limited in identifying diverse mechanisms and factors that promote the development of vasculature, including complex interactions with parenchymal cells, appropriate microenvironment, related cytokines and growth factors, to name a few. With a renewed focus on understanding the underlying biology associated with organoid vascularization, we foresee the discovery of additional mechanisms to form a vasculature, especially through the use of mechanical cues and new genetic circuits. Taken together, vasculature formation will be induced within organoid through a more robust and effective manner, by integrating multiple factors that can work synergistically with most appropriate organoid derivation protocols.
Notably, vascular beds that are driven by distinct design principles have been successfully engineered, typically by either seeding a pre-formed channel network, or by natural self-organization and self-assembly. At this moment, self-organized vessel networks within a 3D hydrogel matrix through angiogenesis or vasculogenesis of seeded ECs is the method of choice for hosting pre-vascularized organoids, since the self-organized vasculatures closely recapitulate in vivo capillaries in both form and function. However, with the rapid technological advancement of bioprinting and scaffolding, we foresee the capability to fabricate endothelial-lined vessels on capillary scale to be realized in the near future. This promises to facilitate the fabrication of a physiologically relevant and fully controlled vascular system with specified geometry, cell compositions and pre-patterned spatiotemporal distributions of relevant growth factors. Whether or not this approach will prove more effective than natural self-assembly remains to be seen. In long-term cultures, this also raises the question of the importance of network remodeling, which would presumably be necessary as the organoids grow and develop.
To ensure a successful co-culture, the external supporting matrix must be optimized to support the development and growth of both organoids and engineered vasculature. Currently, most approaches employ natural hydrogels because of their biocompatibility and convenience. However, natural hydrogels are not easily modified with mechanical properties and ligand presentation individually regulated. In addition, the composition of some animal derived matrices such as Matrigel, for example, is poorly defined and subject to batch to batch variation thereby limiting the reproducibility between experiments. Conversely, fully defined synthetic hydrogels have the demonstrated potential to serve as suitable 3D matrices to improve the reliability with fully controlled physical and biomolecular properties of microenvironment,90 although it is important to recognize that under long-term culture, the initial matrix will gradually be degraded and replaced with cell-secreted matrix.
Another advance that is certain to come is the use of PSC-derived ECs from the same source as the organoid, which should tend to increase the chance of successful co-culture in forming and connecting the internal and external vascular beds. iPSC-ECs have demonstrated the capability of forming functional vessels, just as primary ECs.68,91 This is potentially beneficial because PSC-derived ECs will likely remodel and evolve to form organotypic vasculature in the presence of embedded organoids through complex interactions.92,93 Detailed characterization will be needed to demonstrate the evolution of organotypic vasculature under the long-term co-culture with organoids. Once combined with the capacity of intravascular perfusion, it will bring truly functional vasculature to organoid models, thus greatly improve the physiological relevance of organoids to mimic their in vivo counterparts.
The successful intravascular perfusion of organoids achieved through in vivo transplantation clearly demonstrates that organoids of most types, given the appropriate microenvironment, can become vascularized via an existing vascular bed. Further effort is required to understand and identify the key steps in this process, and ultimately implement in vitro the necessary biochemical and mechanical cues to engineer a fully perfused system. This will require a concerted effort on the part of scientists and engineers from diverse multidisciplinary fields. We envision that the convergence of various advancement from biomaterials, microfluidic engineering and stem cell biology will eventually lead us to fully vascularized organoids with intravascular perfusion in vitro. When this goal is realized, it will become an invaluable tool to further expand the application of organoid technology.
Conflicts of interest
RDK is co-founder of and holds a significant financial interest in AIM Biotech, a company that produces microfluidic devices. RDK also receives research support from Amgen, Biogen, and W. L. Gore.References
- J. Kim, B.-K. Koo and J. A. Knoblich, Human organoids: model systems for human biology and medicine, Nat. Rev. Mol. Cell Biol., 2020, 19, 1–14 Search PubMed.
- M. A. Lancaster and J. A. Knoblich, Organogenesis in a dish: Modeling development and disease using organoid technologies, Science, 2014, 345(6194), 1247125 CrossRef.
- H. V. Wilson, A new method by which sponges may be artificially reared, Science, 1907, 25(649), 912–915 CrossRef CAS.
- M. Eiraku, N. Takata, H. Ishibashi, M. Kawada, E. Sakakura and S. Okuda, et al., Self-organizing optic-cup morphogenesis in three-dimensional culture, Nature, 2011, 472(7341), 51–56 CrossRef CAS.
- T. Sato, R. G. Vries, H. J. Snippert, M. Van De Wetering, N. Barker and D. E. Stange, et al., Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche, Nature, 2009, 459(7244), 262–265 CrossRef CAS.
- E. Karzbrun, A. Kshirsagar, S. R. Cohen, J. H. Hanna and O. Reiner, Human Brain Organoids on a Chip Reveal the Physics of Folding, Nat. Phys., 2018, 14(5), 515–522 Search PubMed.
- J. Zhou, C. Li, X. Liu, M. C. Chiu, X. Zhao and D. Wang, et al., Infection of bat and human intestinal organoids by SARS-CoV-2, Nat. Med., 2020, 1–7 Search PubMed.
- V. Monteil, H. Kwon, P. Prado, A. Hagelkrüys, R. A. Wimmer and M. Stahl, et al., Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, 2020, 181(4), 905–913.e7 CrossRef CAS.
- M. Geiger, Fundamentals of Vascular Biology, Springer, 2019 Search PubMed.
- K. A. Homan, N. Gupta, K. T. Kroll, D. B. Kolesky, M. Skylar-Scott and T. Miyoshi, et al., Flow-enhanced vascularization and maturation of kidney organoids in vitro, Nat. Methods, 2019, 16(3), 255–262 CrossRef CAS.
- T. Takebe, K. Sekine, M. Enomura, H. Koike, M. Kimura and T. Ogaeri, et al., Vascularized and functional human liver from an iPSC-derived organ bud transplant, Nature, 2013, 499(7459), 1–5 CrossRef.
- B. Cakir, Y. Xiang, Y. Tanaka, M. H. Kural, M. Parent and Y.-J. Kang, et al., Engineering of human brain organoids with a functional vascular-like system, Nat. Methods, 2019, 16(11), 1169–1175 CrossRef CAS.
- A. A. Mansour, J. T. Gonçalves, C. W. Bloyd, H. Li, S. Fernandes and D. Quang, et al., An in vivo model of functional and vascularized human brain organoids, Nat. Biotechnol., 2018, 36(5), 432–441 CrossRef CAS , Available from: http://www.nature.com/articles/nbt.4127.
- Y. Takahashi, K. Sekine, T. Kin, T. Takebe and H. Taniguchi, Self-Condensation Culture Enables Vascularization of Tissue Fragments for Efficient Therapeutic Transplantation, Cell Rep., 2018, 23(6), 1620–1629 CrossRef CAS.
- M. L. Moya, Y.-H. Hsu, A. P. Lee, C. C. W. Hughes and S. C. George, In VitroPerfused Human Capillary Networks, Tissue Eng., Part C, 2013, 19(9), 730–737 CrossRef CAS.
- A. Brown, H. He, E. Trumper, J. Valdez, P. Hammond and L. G. Griffith, Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments, Biomaterials, 2020, 119921 CrossRef CAS.
- J. A. Whisler, M. B. Chen and R. D. Kamm, Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System, Tissue Eng., Part C, 2014, 20(7), 543–552 CrossRef CAS.
- R. D. Kamm, R. Bashir, N. Arora, R. D. Dar, M. U. Gillette and L. G. Griffith, et al., Perspective: The promise of multi-cellular engineered living systems, APL Bioeng., 2018, 2(4), 040901 CrossRef.
- S. Kim, H. Lee, M. Chung and N. L. Jeon, Engineering of functional, perfusable 3D microvascular networks on a chip, Lab Chip, 2013, 13(8), 1489–1500 RSC.
- J. S. Miller, K. R. Stevens, M. T. Yang, B. M. Baker, D.-H. T. Nguyen and D. M. Cohen, et al., Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues, Nat. Mater., 2012, 11(9), 768–774 CrossRef CAS.
- Y. S. Zhang, A. Arneri, S. Bersini, S.-R. Shin, K. Zhu and Z. Goli-Malekabadi, et al., Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials, 2016, 110(c), 45–59 CrossRef CAS.
- S. Guo, I. Redenski, S. Landau, A. Szklanny, U. Merdler and S. Levenberg, Prevascularized Scaffolds Bearing Human Dental Pulp Stem Cells for Treating Complete Spinal Cord Injury, Adv. Healthcare Mater., 2020, 9(20), 2000974 CrossRef CAS.
- L. Perry, U. Merdler, M. Elishaev and S. Levenberg, Enhanced Host Neovascularization of Prevascularized Engineered Muscle Following Transplantation into Immunocompetent versus Immunocompromised Mice, Cell, 2019, 8(12), 1472 CrossRef CAS.
- S. Ben-Shaul, S. Landau, U. Merdler and S. Levenberg, Mature vessel networks in engineered tissue promote graft-host anastomosis and prevent graft thrombosis, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(8), 2955–2960 CrossRef CAS.
- S. N. Bhatia and D. E. Ingber, Microfluidic organs-on-chips, Nat. Biotechnol., 2014, 32(8), 760–772 CrossRef CAS.
- Y. Nashimoto, T. Hayashi, I. Kunita, A. Nakamasu, Y.-S. Torisawa and M. Nakayama, et al., Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device, Integr. Biol., 2017, 9(6), 506–518 CrossRef.
- Y. Nashimoto, R. Okada, S. Hanada, Y. Arima, K. Nishiyama and T. Miura, et al., Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid, Biomaterials, 2020, 229, 119547 CrossRef CAS.
- M. T. Pham, K. M. Pollock, M. D. Rose, W. A. Cary, H. R. Stewart and P. Zhou, et al., Generation of human vascularized brain organoids, NeuroReport, 2018, 29(7), 588–593 CrossRef.
- T. Takebe, M. Enomura, E. Yoshizawa, M. Kimura, H. Koike and Y. Ueno, et al., Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation, Cell Stem Cell, 2015, 16(5), 556–565 CrossRef CAS.
- G. Pettinato, S. Lehoux, R. Ramanathan, M. M. Salem, L.-X. He and O. Muse, et al., Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells, Sci. Rep., 2019, 9(1), 8920–8921 CrossRef.
- M. S. Steinberg, On the mechanism of tissue reconstruction by dissociated cells, I. Population kinetics, differential adhesiveness, and the absence of directed migration, Proc. Natl. Acad. Sci. U. S. A., 1962, 48(9), 1577 CrossRef CAS.
- L. Song, X. Yuan, Z. Jones, K. Griffin, Y. Zhou and T. Ma, et al., Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues, Sci. Rep., 2019, 9(1), 5977 CrossRef.
- W. C. Aird, Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms, Circ. Res., 2007, 100(2), 158–173 CrossRef CAS.
- M. Campisi, Y. Shin, T. Osaki, C. Hajal, V. Chiono and R. D. Kamm, 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes, Biomaterials, 2018, 180, 117–129 CrossRef CAS.
- N. J. Abbott, A. A. Patabendige, D. E. Dolman, S. R. Yusof and D. J. Begley, Structure and function of the blood–brain barrier, Neurobiol. Dis., 2010, 37(1), 13–25 CrossRef CAS.
- K. K. Sørensen, J. Simon Santamaria, R. S. McCuskey and B. Smedsrød, Liver sinusoidal endothelial cells, Compr. Physiol., 2011, 5(4), 1751–1774 Search PubMed.
- C. Praça, S. C. Rosa, E. Sevin, R. Cecchelli, M.-P. Dehouck and L. S. Ferreira, Derivation of brain capillary-like endothelial cells from human pluripotent stem cell-derived endothelial progenitor cells, Stem Cell Rep., 2019, 13(4), 599–611 CrossRef.
- G. Sriram, J. Y. Tan, I. Islam, A. J. Rufaihah and T. Cao, Efficient differentiation of human embryonic stem cells to arterial and venous endothelial cells under feeder- and serum-free conditions, Stem Cell Res. Ther., 2015, 6(1), 261 CrossRef.
- G. I. Uenishi, H. S. Jung, A. Kumar, M. A. Park, B. K. Hadland and E. McLeod, et al., NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells, Nat. Commun., 2018, 9(1), 1–14 CrossRef CAS.
- M. Takasato, P. X. Er, H. S. Chiu, B. Maier, G. J. Baillie and C. Ferguson, et al., Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis, Nature, 2015, 526(7574), 564–568 CrossRef CAS.
- R. A. Wimmer, A. Leopoldi, M. Aichinger, N. Wick, B. Hantusch and M. Novatchkova, et al., Human blood vessel organoids as a model of diabetic vasculopathy, Nature, 2019, 565(7740), 505–510 CrossRef CAS.
- F. Wu, D. Wu, Y. Ren, Y. Huang, B. Feng and N. Zhao, et al., Generation of hepatobiliary organoids from human induced pluripotent stem cells, J. Hepatol., 2019, 70(6), 1145–1158 CrossRef.
- N. Sasai, R. Yakura, D. Kamiya, Y. Nakazawa and Y. Sasai, Ectodermal factor restricts mesoderm differentiation by inhibiting p53, Cell, 2008, 133(5), 878–890 CrossRef CAS.
- O. Ham, Y. B. Jin, J. Kim and M.-O. Lee, Blood vessel formation in cerebral organoids formed from human embryonic stem cells, Biochem. Biophys. Res. Commun., 2020, 521(1), 84–90 CrossRef CAS.
- P. Wörsdörfer, N. Dalda, A. Kern, S. Krüger, N. Wagner and C. K. Kwok, et al., Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells, Sci. Rep., 2019, 9(1), 15663 CrossRef.
- P. Wörsdörfer, A. Rockel, Y. Alt, A. Kern and S. Ergün, Generation of Vascularized Neural Organoids by Co-culturing with Mesodermal Progenitor Cells, STAR Protoc., 2020, 1(1), 100041 CrossRef.
- R. Morita, M. Suzuki, H. Kasahara, N. Shimizu, T. Shichita and T. Sekiya, et al., ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(1), 160–165 CrossRef CAS.
- K. Wang, R.-Z. Lin, X. Hong, A. H. Ng, C. N. Lee and J. Neumeyer, et al., Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA, Sci. Adv., 2020, 6(30), eaba7606 CrossRef CAS , Available from: http://advances.sciencemag.org/content/6/30/eaba7606.abstract.
- P. Guye, M. R. Ebrahimkhani, N. Kipniss, J. J. Velazquez, E. Schoenfeld and S. Kiani, et al., Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6, Nat. Commun., 2019, 7(1), 1–12 Search PubMed.
- J. W. Song and L. L. Munn, Fluid forces control endothelial sprouting, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(37), 15342–15347 CrossRef CAS.
- V. Vickerman and R. D. Kamm, Mechanism of a flow-gated angiogenesis switch: early signaling events at cell–matrix and cell–cell junctions, Integr. Biol., 2012, 4(8), 863–874 CrossRef CAS.
- M. J. Workman, J. P. Gleeson, E. J. Troisi, H. Q. Estrada, S. J. Kerns and C. D. Hinojosa, et al., Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips, Cell. Mol. Gastroenterol. Hepatol., 2018, 5(4), 669–677.e2 CrossRef.
- T. Tao, Y. Wang, W. Chen, Z. Li, W. Su and Y. Guo, et al., Engineering human islet organoids from iPSCs using an organ-on-chip platform, Lab Chip, 2019, 19(6), 948–958 RSC.
- J. D. Coffin, J. Harrison, S. Schwartz and R. Heimark, Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo, Dev. Biol., 1991, 148(1), 51–62 CrossRef CAS.
- M. B. Chen, J. A. Whisler, J. S. Jeon and R. D. Kamm, Mechanisms of tumor cell extravasation in an in vitro microvascular network platform, Integr. Biol., 2013, 5(10), 1262–1271 CrossRef CAS.
- B. Andrée, H. Ichanti, S. Kalies, A. Heisterkamp, S. Strauß and P.-M. Vogt, et al., Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels, Sci. Rep., 2019, 9(1), 5437 CrossRef.
- T. Takebe, N. Koike, K. Sekine, M. Enomura, Y. Chiba and Y. Ueno, et al., Generation of functional human vascular network, Transplant. Proc., 2012, 44(4), 1130–1133 CrossRef CAS.
- X. Wang, D. T. T. Phan, A. Sobrino, S. C. George, C. C. W. Hughes and A. P. Lee, Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels, Lab Chip, 2016, 16(2), 282–290 RSC.
- W. Risau, Mechanisms of angiogenesis, Nature, 1997, 386(6626), 671–674 CrossRef CAS.
- P. Carmeliet and R. K. Jain, Molecular mechanisms and clinical applications of angiogenesis, Nature, 2011, 473(7347), 298–307 CrossRef CAS.
- W. A. Farahat, L. B. Wood, I. K. Zervantonakis, A. Schor, S. Ong and D. Neal, et al., Ensemble analysis of angiogenic growth in three-dimensional microfluidic cell cultures, PLoS One, 2012, 7(5), e37333 CrossRef CAS.
- D.-H. T. Nguyen, S. C. Stapleton, M. T. Yang, S. S. Cha, C. K. Choi and P. A. Galie, et al., Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(17), 6712–6717 CrossRef CAS.
- L. L. Bischel, E. W. K. Young, B. R. Mader and D. J. Beebe, Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels, Biomaterials, 2013, 34(5), 1471–1477 CrossRef CAS.
- V. Nehls and D. Drenckhahn, A microcarrier-based cocultivation system for the investigation of factors and cells involved in angiogenesis in three-dimensional fibrin matrices in vitro, Histochem. Cell Biol., 1995, 104(6), 459–466 CrossRef CAS.
- M. N. Nakatsu, R. C. Sainson, J. N. Aoto, K. L. Taylor, M. Aitkenhead and S. Pérez-del-Pulgar, et al., Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1☆, Microvasc. Res., 2003, 66(2), 102–112 CrossRef CAS.
- J. H. Yeon, H. R. Ryu, M. Chung, Q. P. Hu and N. L. Jeon, In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices, Lab Chip, 2012, 12(16), 2815–2822 RSC.
- C. B. Weinberg and E. Bell, A blood vessel model constructed from collagen and cultured vascular cells, Science, 1986, 231(4736), 397–400 CrossRef CAS.
- D. G. Belair, J. A. Whisler, J. Valdez, J. Velazquez, J. A. Molenda and V. Vickerman, et al., Human Vascular Tissue Models Formed from Human Induced Pluripotent Stem Cell Derived Endothelial Cells, Stem Cell Rev. Rep., 2014, 11(3), 511–525 CrossRef.
- J. P. Morgan, P. F. Delnero, Y. Zheng, S. S. Verbridge, J. Chen and M. Craven, et al., Formation of microvascular networks in vitro, Nature, 2013, 8(9), 1820–1836 Search PubMed.
- G. S. Offeddu, K. Haase, M. R. Gillrie, R. Li, O. Morozova and D. Hickman, et al., An on-chip model of protein paracellular and transcellular permeability in the microcirculation, Biomaterials, 2019, 212, 115–125 CrossRef CAS.
- G. S. Offeddu, L. Possenti, J. T. Loessberg Zahl, P. Zunino, J. Roberts and X. Han, et al., Application of Transmural Flow Across In Vitro Microvasculature Enables Direct Sampling of Interstitial Therapeutic Molecule Distribution, Small, 2019, 15(46), 1902393 CrossRef CAS.
- K. M. Chrobak, D. R. Potter and J. Tien, Formation of perfused, functional microvascular tubes in vitro, Microvasc. Res., 2006, 71(3), 185–196 CrossRef CAS.
- N. Sadr, M. Zhu, T. Osaki, T. Kakegawa, Y. Yang and M. Moretti, et al., SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures, Biomaterials, 2011, 32(30), 7479–7490 CrossRef CAS.
- H. Yoshida, M. Matsusaki and M. Akashi, Multilayered Blood Capillary Analogs in Biodegradable Hydrogels for In Vitro Drug Permeability Assays, Adv. Funct. Mater., 2012, 23(14), 1736–1742 CrossRef.
- A. P. Golden and J. Tien, Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element, Lab Chip, 2007, 7(6), 720–725 RSC.
- M. A. Skylar-Scott, S. G. M. Uzel, L. L. Nam, J. H. Ahrens, R. L. Truby and S. Damaraju, et al., Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Sci. Adv., 2019, 5(9), eaaw2459 CrossRef CAS.
- M. Radisic, H. Park, F. Chen, J. E. Salazar-Lazzaro, Y. Wang and R. Dennis, et al., Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds, Tissue Eng., 2006, 12(8), 2077–2091 CrossRef CAS.
- S. N. Nazhat, E. A. Abou Neel, A. Kidane, I. Ahmed, C. Hope and M. Kershaw, et al., Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers, Biomacromolecules, 2007, 8(2), 543–551 CrossRef CAS.
- C. Xu, Z. Zhang, K. Christensen, Y. Huang, J. Fu and R. R. Markwald, Freeform Vertical and Horizontal Fabrication of Alginate-Based Vascular-Like Tubular Constructs Using Inkjetting, J. Manuf. Sci. Eng., 2014, 136(6), 157–158 CrossRef.
- A. Sala, P. Hänseler, A. Ranga, M. P. Lutolf, J. Vörös and M. Ehrbar, Engineering 3D cell instructive microenvironments by rational assembly of artificial extracellular matrices and cell patterning, Integr. Biol., 2011, 3(11), 1102–1110 CrossRef.
- M. F. Leong, J. K. C. Toh, C. Du, K. Narayanan, H. F. Lu and T. C. Lim, et al., Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres, Nat. Commun., 2013, 4(1), 2353 CrossRef.
- S. Grebenyuk and A. Ranga, Engineering Organoid Vascularization, Front. Bioeng. Biotechnol., 2019, 7, 82 CrossRef.
- D.-H. T. Nguyen, S. C. Stapleton, M. T. Yang, S. S. Cha, C. K. Choi and P. A. Galie, et al., Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(17), 6712–6717 CrossRef CAS.
- J. Paek, S. E. Park, Q. Lu, K.-T. Park, M. Cho and J. M. Oh, et al., Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues, ACS Nano, 2019, 13(7), 7627–7643 CrossRef CAS.
- H. Liu, Y. Wang, K. Cui, Y. Guo, X. Zhang and J. Qin, Advances in Hydrogels in Organoids and Organs-on-a-Chip, Adv. Mater., 2019, 31(50), 1902042 CrossRef CAS.
- S. Rajasekar, D. S. Y. Lin, L. Abdul, A. Liu, A. Sotra and F. Zhang, et al., IFlowPlate—A Customized 384-Well Plate for the Culture of Perfusable Vascularized Colon Organoids, Adv. Mater., 2020, 30, 2002974 CrossRef.
- R. Morizane, A. Q. Lam, B. S. Freedman, S. Kishi, M. T. Valerius and J. V. Bonventre, Nephron organoids derived from human pluripotent stem cells model kidney development and injury, Nat. Biotechnol., 2015, 33(11), 1193–1200 CrossRef CAS.
- F. E. Freeman, P. Pitacco, L. H. van Dommelen, J. Nulty, D. C. Browe and J.-Y. Shin, et al., 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration, Sci. Adv., 2020, 6(33), eabb5093 CrossRef CAS.
- C. W. van den Berg, L. Ritsma, M. C. Avramut, L. E. Wiersma, B. M. van den Berg and D. G. Leuning, et al., Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo, Stem Cell Rep., 2018, 10(3), 751–765 CrossRef.
- E. Cambria, K. Renggli, C. C. Ahrens, C. D. Cook, C. Kroll and A. T. Krueger, et al., Covalent modification of synthetic hydrogels with bioactive proteins via sortase-mediated ligation, Biomacromolecules, 2015, 16(8), 2316–2326 CrossRef CAS.
- S. L. Natividad-Diaz, S. Browne, A. K. Jha, Z. Ma, S. Hossainy and Y. K. Kurokawa, et al., A combined hiPSC-derived endothelial cell and in vitro microfluidic platform for assessing biomaterial-based angiogenesis, Biomaterials, 2019, 194, 73–83 CrossRef CAS.
- P. N. Ingram, L. E. Hind, J. A. Jiminez Torres, A. Huttenlocher and D. J. Beebe, An Accessible Organotypic Microvessel Model Using iPSC-Derived Endothelium, Adv. Healthcare Mater., 2018, 7(2), 1700497 CrossRef.
- Y. Koui, T. Kido, T. Ito, H. Oyama, S.-W. Chen and Y. Katou, et al., An in vitro human liver model by iPSC-derived parenchymal and non-parenchymal cells, Stem Cell Rep., 2017, 9(2), 490–498 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |