Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colorimetric and fluorometric probes for the optical detection of environmental Hg(II) and As(III) ions
Tapendu
Samanta
and
Raja
Shunmugam
 *
*
Polymer Research Centre, Department of Chemical Sciences, Indian Institute of Science Education and Research, Kolkata, India. E-mail: sraja@iiserkol.ac.in
First published on 17th November 2020
Abstract
Human exposure to Hg(II) and As(III) can lead to several physiological problems such as liver damage, kidney damage, lung cancer, skin cancer, motion disorder, brain damage, etc. To monitor and identify these harmful ions, throughout the years enormous effort has been put into the development of sensors. In this review, colorimetric and fluorometric chemical compounds (sensors) for Hg(II) (since 2015) and As(III) detection are described and discussed in detail. For Hg(II), sensors are divided on the basis of the compound's nature, such as heteroatom-based ligand-containing small molecules, rhodamine-based small molecules, reaction-based small molecules, and polymers. On the other hand, most As(III) ion sensors are based on H-bonding interactions.
1. Introduction
A highly polluted living environment for humankind is a result of modern globalization and industrialization, where water sources are the most affected part while water is the basic and essential ingredient of human life on earth. Heavy metals are one of the most effective pollutants among other sources. Metals with a high atomic number, high density, and which are poisonous at low concentrations are known as heavy metals.1–3 Heavy metals are harmful due to their tendency to bioaccumulation. Heavy metals can enter the human body through food, drinking water, and air sources. Metals such as arsenic, cadmium, lead, nickel, mercury, chromium, cobalt, zinc, copper and selenium are familiar heavy metals. The accumulation of these metals in our resources is a major concern as many industries discharge their metal wastes into freshwater without any purification.4,5Among the mentioned heavy metals, arsenic (As) and mercury (Hg) are the topmost candidates in the context of toxicity. The World Health Organization (WHO) and Environmental Protection Agency (EPA) have defined the permissible limits of concentrations for both mercury and arsenic.6
Arsenic poisoning in groundwater is a major concern in several parts of the world, such as Bangladesh, West Bengal, the western USA, Mexico, Chile, and Argentina.7 Many electronic component manufacturing industries use trace amounts of arsenic combined with silicon for light-emitting diodes (LEDs) and other devices.8 Although in some cases arsenic is used in drugs, chronic exposure can damage the human body. Long-term exposure and usage of arsenic-contaminated water can cause various health risks, such as kidney damage, liver damage, lung cancer, and skin cancer.9–11 Arsenic can exist in −3 to +5 oxidation states, though the As(III) form is the most toxic to the environment and human health.12 Due to such toxicity, the WHO set a limit of a safe arsenic (As) concentration in drinking water of 10 ppb.13,14
Mercury is another heavy metal that is famous for Minamata disease15 and poisoning in Iraq.16 Mercury can spread in water, soil, and air from many sources, such as coal plants, mercury lamps, gold production, thermometers, barometers, and caustic soda.17 Fish is one of the major sources of mercury in humans.18 Mercury exposure has several harmful effects on health, like kidney failure, motion disorder, and brain damage.19–21 Methylation of mercury promotes lipid solubility and, as a result, it can easily penetrate biological membranes along with the blood–brain barrier to damage the central nervous system. To avoid the harmful effects of mercury, an upper limit for Hg has been set at 10 nM by the U.S. Environmental Protection Agency (EPA).22
The extent of toxicity for arsenic and mercury makes it necessary to monitor them in drinking water and different environmental sources with high selectivity and sensitivity. Techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), atomic fluorescence spectrometry (AFS), high-performance liquid chromatography (HPLC), surface-enhanced Raman scattering, chromatographic techniques, hydride-generation atomic absorption spectroscopy (HG-AAS), and voltammetry studies are used for the detection of arsenic and mercury.23–33 The above-mentioned techniques are well known for their detection ability in very low concentrations, but they need costly instrumentation and extensive sample preparation. Therefore, the development of simple, cost-effective, and rapid detection methods is very much needed for real-time and ‘on-field’ monitoring of industrial, environmental, and biological contaminated samples.
Among different methods, optical detection through changes in fluorescence or color are most advantageous due to their simple nature and low limit of detection.34–37 Colorimetric sensors can be useful in the case of the naked eye and rapid detection of analytes without any prior set-up where fluorogenic probes can detect contaminants at the cellular level. Thanks to such advantages, fluorescent and colorimetric sensors have been developed throughout the last few decades for the detection of toxic elements.
To date, three reviews have reported which have provided collective information about various detection systems for environmental Hg2+. These reviews discussed optical probes developed up to 2015.38–40 Furthermore, there have been several reviews for arsenic detection based on the electrochemical method,41 surface-enhanced Raman spectroscopy (SERS),42,43 and nano-material systems.44–46 However, to the best of our knowledge no review based on fluorogenic and chromogenic compounds for As(III) detection has been reported.
In this review, we discuss in detail fluorescent and colorimetric chemical sensors for mercury and arsenic detection. We have classified the sensors depending upon their chemical behavior, such as interaction-based ligand systems, reaction-based irreversible systems, and polymeric materials for a clear and general overview of available sensing materials that have been developed in recent years. Until 2015, there were not such extensive studies on cellular-level tracking of toxic ions. But the new trend of tracking toxic ions at the cellular level has emerged as an excellent technique for a better understanding of their effects on the biological system. Exclusive selectivity and high sensitivity along with cell imaging have become the better outcomes of the sensing field at the present time for mercury and arsenic. Also, many new strategies have been developed in the field of polymer research, where functionalized polymers with sensing moieties have overcome the solubility issue with respect to small molecules and have triggered an improvement in ‘in-field’ applications for the detection of toxic ions. We have tried to give an overall idea of new ways and new developments in mercury and arsenic detection systems based on typical fluorogenic and chromogenic probes.
2. Fluorescent and colorimetric sensors for Hg2+ detection
2.1 Heteroatom-based ligand-containing small molecules
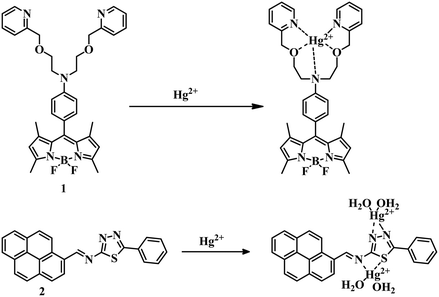
Heteroatom-containing ligands associated with a chromogenic or fluorogenic moiety are excellent candidates for the detection of analytes. Ligand-based sensors have been developed over the years with desirable signals and effectiveness for the detection of Hg2+ ions in different contaminated sources. In this section we will discuss the formation of Hg–O, Hg–S, Hg–N, etc. bonds due to the interaction of Hg and a ligand to promote some change in color or emission as a detection signal.In this regard, an N,N-bis(2-(pyridin-2-ylmethoxy)ethyl)aniline receptor containing a boron-dipyrromethene (BODIPY) molecule 147 acts as a fluorescence ‘turn-off’ to ‘turn-on’ sensor for Hg2+ (Fig. 1). Here 1 can detect mercury in CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium with a limit of detection (LOD) of 1.81 × 10−7 M. In this case due to the presence of an N,N-bis(2-(pyridin-2-ylmethoxy)ethyl)aniline receptor along with BODIPY, the PET process is in the active mode which results in a fluorescence-off mode in the system. But the addition of Hg2+ stopped the photoinduced electron transfer (PET) process by interaction with the N and O atoms of the ligand which triggered the strong emission signal. All these observations made sensor 1 a highly selective and sensitive system for the detection of Hg2+ by the naked eye. A pyrene derivative 248 formed a 1
1) medium with a limit of detection (LOD) of 1.81 × 10−7 M. In this case due to the presence of an N,N-bis(2-(pyridin-2-ylmethoxy)ethyl)aniline receptor along with BODIPY, the PET process is in the active mode which results in a fluorescence-off mode in the system. But the addition of Hg2+ stopped the photoinduced electron transfer (PET) process by interaction with the N and O atoms of the ligand which triggered the strong emission signal. All these observations made sensor 1 a highly selective and sensitive system for the detection of Hg2+ by the naked eye. A pyrene derivative 248 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with Hg2+ in 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer-CH3CN (3
2 complex with Hg2+ in 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer-CH3CN (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, 10 mM buffer, pH = 7.4) medium (Fig. 1). Due to complexation, 2 showed an unusual red fluorescence and a change in color from yellow to orange along with a 36 nM LOD. The change in color as well as in emission is an advantage for 2.
7, v/v, 10 mM buffer, pH = 7.4) medium (Fig. 1). Due to complexation, 2 showed an unusual red fluorescence and a change in color from yellow to orange along with a 36 nM LOD. The change in color as well as in emission is an advantage for 2.
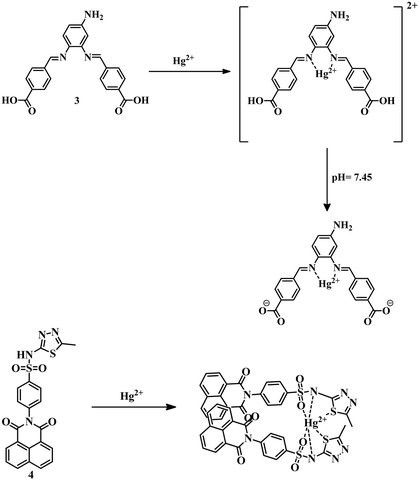
A Schiff-base 349 synthesized from 4-nitro-o-phenylenediamine and 4-formylbenzoic acid showed interesting sensing phenomena for the detection of Hg2+ in DMF–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) medium with an LOD of 0.061 μM. Initially, 3 showed green fluorescence, though upon addition of mercury at pH 7 the fluorescence was quenched, but at pH 7.45 fluorescence was turned on again (Fig. 2). The whole phenomenon was described as due to the reduction of intra-molecular charge transfer (ICT) caused by the formation of a strong 5-membered ring between Hg2+ and two imine nitrogens at pH 7 and again the introduction of ICT with increasing pH. Due to this interesting behavior of 3 in physiological pH, it has been used in cell imaging studies to extend their application. Sensor 450 also exhibits a ratiometric emission changing phenomenon due to the binding of heteroatoms of 1,8-naphthalimide-sulfamethizole compound with Hg2+ in DMSO–water (1
3) medium with an LOD of 0.061 μM. Initially, 3 showed green fluorescence, though upon addition of mercury at pH 7 the fluorescence was quenched, but at pH 7.45 fluorescence was turned on again (Fig. 2). The whole phenomenon was described as due to the reduction of intra-molecular charge transfer (ICT) caused by the formation of a strong 5-membered ring between Hg2+ and two imine nitrogens at pH 7 and again the introduction of ICT with increasing pH. Due to this interesting behavior of 3 in physiological pH, it has been used in cell imaging studies to extend their application. Sensor 450 also exhibits a ratiometric emission changing phenomenon due to the binding of heteroatoms of 1,8-naphthalimide-sulfamethizole compound with Hg2+ in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) medium with an LOD 14.7 nM. Binding of Hg2+ with –SO2 and the thiadiazole ring of the sulfamethizole segment of 4 leads to aggregation-induced emission enhancement (AIEE) which is responsible for the selective detection of mercury (Fig. 2).
99) medium with an LOD 14.7 nM. Binding of Hg2+ with –SO2 and the thiadiazole ring of the sulfamethizole segment of 4 leads to aggregation-induced emission enhancement (AIEE) which is responsible for the selective detection of mercury (Fig. 2).
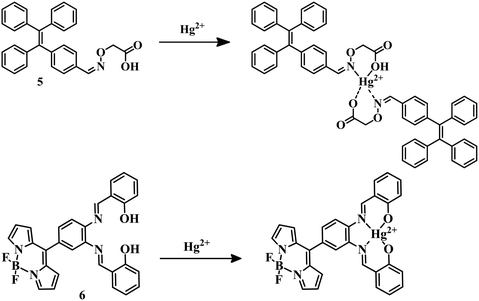
Tetraphenylethene derivatives are known for their aggregation-induced emission property. Using this as an advantageous property, sensor 551 has been developed by the reaction of 2-(aminooxy)acetic acid and 4-(1,2,2-triphenylvinyl)benzaldehyde. With the addition of mercury to sensor 5 in ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) medium, the –CH
7) medium, the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –OH groups coordinate with Hg2+ (Fig. 3) and form an aggregate to give a distinct emission with a low LOD of 45.4 nM. BODIPY derivatives are known to have excellent chromophore photochemical and thermal stability, and salen is an excellent ligand system due to its effective coordination behavior with an electron-deficient Lewis-acid (metal center).52,53 Sensor 654 was designed by the combination of the two above-mentioned advantageous segments for the detection of Hg2+. Sensor 6 selectively detected Hg2+ among other metal ions by complexation in MeCN–H2O (v/v, 1
N and –OH groups coordinate with Hg2+ (Fig. 3) and form an aggregate to give a distinct emission with a low LOD of 45.4 nM. BODIPY derivatives are known to have excellent chromophore photochemical and thermal stability, and salen is an excellent ligand system due to its effective coordination behavior with an electron-deficient Lewis-acid (metal center).52,53 Sensor 654 was designed by the combination of the two above-mentioned advantageous segments for the detection of Hg2+. Sensor 6 selectively detected Hg2+ among other metal ions by complexation in MeCN–H2O (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, HEPES 10 mM, pH = 7.4) medium with an LOD of 1.21 μM (Fig. 3). In this particular case, metal-induced intramolecular charge transfer is the reason behind the change in color from colorless to pink to detect Hg2+ selectively and sensitively.
1, HEPES 10 mM, pH = 7.4) medium with an LOD of 1.21 μM (Fig. 3). In this particular case, metal-induced intramolecular charge transfer is the reason behind the change in color from colorless to pink to detect Hg2+ selectively and sensitively.
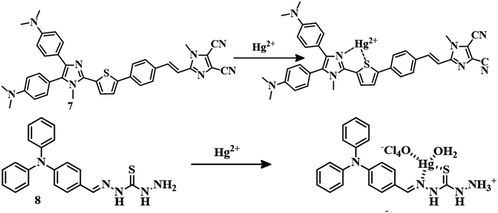
For biimidazole push–pull dye, sensor 755 coordinates with Hg2+ through the thiophene unit (Fig. 4) and stopped the charge transfer process to change the color to colorless from yellow in a CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture with an LOD of 32.8 ppb. With a combination of thiosemicarbazone and 4-(diphenylamino) benzaldehyde, sensor 856 can detect Hg2+ in DMSO/Tris–HCl (8
1) mixture with an LOD of 32.8 ppb. With a combination of thiosemicarbazone and 4-(diphenylamino) benzaldehyde, sensor 856 can detect Hg2+ in DMSO/Tris–HCl (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, pH = 7.0) medium. Hg2+ can bind with the sulfur of thiocarbonyl and the nitrogen of the imine group of 8 (Fig. 4) to induce a chelation-enhanced fluorescence quenching (CHEQ) effect. This fluorescence turn-off sensor 8 can detect Hg2+ up to a low concentration of 3.11 × 10−8 M. Sensor 957 is a coumarin–thiol based receptor that detects Hg2+ in a CH3CN–water (3
2, v/v, pH = 7.0) medium. Hg2+ can bind with the sulfur of thiocarbonyl and the nitrogen of the imine group of 8 (Fig. 4) to induce a chelation-enhanced fluorescence quenching (CHEQ) effect. This fluorescence turn-off sensor 8 can detect Hg2+ up to a low concentration of 3.11 × 10−8 M. Sensor 957 is a coumarin–thiol based receptor that detects Hg2+ in a CH3CN–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, pH = 7) mixture in both colorimetric and fluorometric fashion with an LOD of 5.01 × 10−8 M. In this case binding of Hg2+ with –SH, N of the imine group, and the –C
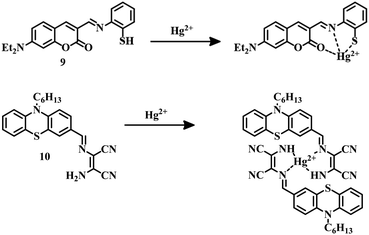
2, v/v, pH = 7) mixture in both colorimetric and fluorometric fashion with an LOD of 5.01 × 10−8 M. In this case binding of Hg2+ with –SH, N of the imine group, and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the coumarin unit of 9 (Fig. 5) induce a color change as well as change in fluorescence. This dual-sensing nature makes 9 a good candidate for mercury detection.
O group of the coumarin unit of 9 (Fig. 5) induce a color change as well as change in fluorescence. This dual-sensing nature makes 9 a good candidate for mercury detection.
Phenothiazine-based sensor 1058 was designed to detect Hg2+ in an ethanol–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
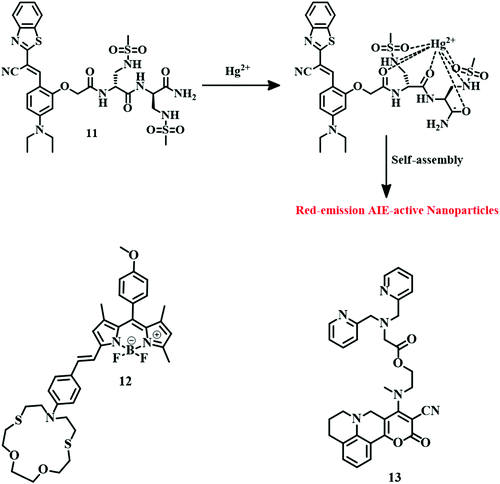
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) mixture with an LOD of 17.8 nM. Complexation of Hg2+ diaminomalenonitrile moieties of 10 (Fig. 5) inhibits the original ICT process; as a result, quenching of fluorescence and a change in color are observed. With this fluorescence change property, 10 can track cellular Hg2+ successfully. Receptor-based sensing models have emerged as a useful tool for the development of Hg2+ sensors. A sulfonamide group containing an unnatural peptide receptor with cyanostilbene-based ratiometric fluorogenic probe 11 has been developed.59 It binds with Hg2+ in such a fashion that it can form an aggregate which is red-emissive (Fig. 6). Sensor 11 is highly selective towards Hg2+ in an aqueous buffered solution (10 mM HEPES, pH = 7.4) with an impressive detection limit of 65 nM. This red-emissive nature of 11 is useful for imaging of HeLa cells with Hg2+ contamination.
4, v/v) mixture with an LOD of 17.8 nM. Complexation of Hg2+ diaminomalenonitrile moieties of 10 (Fig. 5) inhibits the original ICT process; as a result, quenching of fluorescence and a change in color are observed. With this fluorescence change property, 10 can track cellular Hg2+ successfully. Receptor-based sensing models have emerged as a useful tool for the development of Hg2+ sensors. A sulfonamide group containing an unnatural peptide receptor with cyanostilbene-based ratiometric fluorogenic probe 11 has been developed.59 It binds with Hg2+ in such a fashion that it can form an aggregate which is red-emissive (Fig. 6). Sensor 11 is highly selective towards Hg2+ in an aqueous buffered solution (10 mM HEPES, pH = 7.4) with an impressive detection limit of 65 nM. This red-emissive nature of 11 is useful for imaging of HeLa cells with Hg2+ contamination.
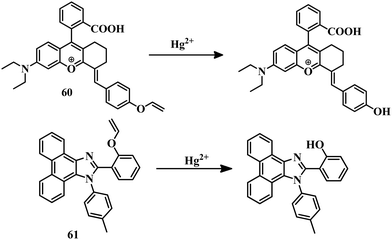
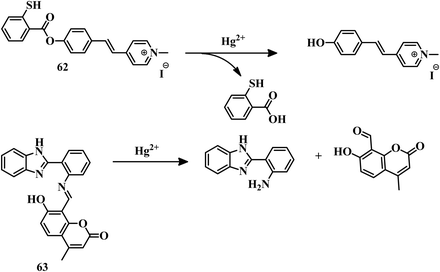
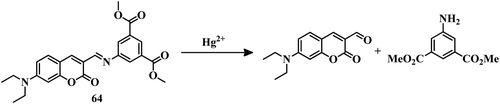
Macrocycles containing sulfur atoms are capable of capturing Hg2+. Also, if the BODIPY unit can be functionalized with receptors in the 3 and 5 positions, then the recognition can induce a variation in both emission and absorption. Combining these facts, sensor 1260 was developed where N-phenyl-1-aza-4,13-dithia-7,10-dioxacyclopentadecane is attached to the 3 positions of the BODIPY unit (Fig. 6). This BODIPY derivative (12) can detect Hg2+ in CH3CN–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) medium by a color change as well as turn-on mode of emission change with an LOD of 99 ppm. The design of a fluorescent probe can detect an analyte depending upon different phenomena like PET, FRET, ICT TICT, etc. for the output emission signal. 4-(Methylthio)-2-oxo-2H-pyrano[3,2-c]julolidine-3-carbonitrile derived sensor 1361 is a twisted intramolecular charge transfer (TICT) active fluorophore, where dipicolyl amine acts as a chelator to bind Hg2+ (Fig. 6). The sensing behavior of 13 has been studied in a methanol–HEPES buffer (7
95, v/v) medium by a color change as well as turn-on mode of emission change with an LOD of 99 ppm. The design of a fluorescent probe can detect an analyte depending upon different phenomena like PET, FRET, ICT TICT, etc. for the output emission signal. 4-(Methylthio)-2-oxo-2H-pyrano[3,2-c]julolidine-3-carbonitrile derived sensor 1361 is a twisted intramolecular charge transfer (TICT) active fluorophore, where dipicolyl amine acts as a chelator to bind Hg2+ (Fig. 6). The sensing behavior of 13 has been studied in a methanol–HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH = 7.2) medium. Sensor 13 detects Hg2+ in a ratiometric fashion by changing the emission from red to green with an excellent detection level of 5.7 × 10−9 M along with tracking of Hg2+ in the MCF-7 cell line. Binding with a dipicolylamine unit of 13 with Hg2+ restricts the TICT activity which is responsible for the drastic change in emission signal.
3, v/v, pH = 7.2) medium. Sensor 13 detects Hg2+ in a ratiometric fashion by changing the emission from red to green with an excellent detection level of 5.7 × 10−9 M along with tracking of Hg2+ in the MCF-7 cell line. Binding with a dipicolylamine unit of 13 with Hg2+ restricts the TICT activity which is responsible for the drastic change in emission signal.
Aggregation induced emission (AIE)-based fluorogenic probes are very useful due to their unique fluorescence phenomenon. The tetrathenylethene unit is a well-established moiety for designing AIE-active molecules. Cationic AIE-active sensor 1462 consists of tetraphenylethene and quinoline units, and can detect Hg2+ by a different method. Initially, 14 showed red fluorescence in aqueous solution (containing 1% DMSO) due to aggregation, but the emission was quenched due to the addition of I− to it. This I−-containing sensor 14 system can now act as an Hg2+ sensor. Addition of Hg2+ to the 14–I− system turns on the red emission again. By this process, 14 can be used as a turn-on fluorescence sensor for Hg2+ with a detection limit of 591.9 nM. Another AIE-active sensor 1563 is developed by the conjugation between tetraphenylethene and pyrido[2,3-b]pyrazine units. Sensor 15 has an excellent AIEE phenomenon itself. In the presence of Hg2+ in the acetonitrile solution sensor, 15 changed its emission color from red-orange to colorless with a moderate LOD of 7.46 × 10−6 M. The chemical structure of sensors 14 and 15 are depicted in Fig. 7.
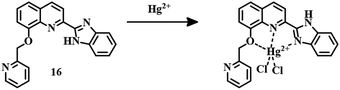
The 8-hydroxyquinoline based sensor 16 was successfully applied for the recognition of Hg2+ (Fig. 8).64 In a solution of MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v), fluorescence quenching was observed for Hg2+ with a simultaneous change in emission from blue to colorless. This is a typical example of a turn-off fluorescent sensor where complexation between the sensor and the analyte is responsible for fluorescence quenching.
4, v/v), fluorescence quenching was observed for Hg2+ with a simultaneous change in emission from blue to colorless. This is a typical example of a turn-off fluorescent sensor where complexation between the sensor and the analyte is responsible for fluorescence quenching.
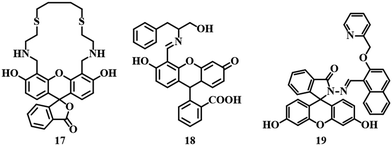
Fluorescein as a fluorophore is widely used due to its high molar extinction coefficient, high quantum yield, and strong absorbance along with strong emission signals in the visible range.65–67 Sensor 1768 is a fluorescein dithia-cyclic skeleton which show selective detection ability for Hg2+ in an MeOH–Tris–HCl (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, pH = 7.2) medium. Upon interaction with Hg2+, a drastic color change from yellow to orange was observed along with quenched fluorescence due to the inherent quenching property of mercury (Fig. 9). Another sensor 18,69 a Schiff base receptor of fluorescein–phenylalaninol conjugate has been successfully applied for the detection and removal of Hg2+. The addition of Hg2+ to 18 in aqueous medium changed the color from yellow to pink with quenching of the fluorescence of the system. Binding of Hg2+ with the –CH
5, v/v, pH = 7.2) medium. Upon interaction with Hg2+, a drastic color change from yellow to orange was observed along with quenched fluorescence due to the inherent quenching property of mercury (Fig. 9). Another sensor 18,69 a Schiff base receptor of fluorescein–phenylalaninol conjugate has been successfully applied for the detection and removal of Hg2+. The addition of Hg2+ to 18 in aqueous medium changed the color from yellow to pink with quenching of the fluorescence of the system. Binding of Hg2+ with the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –OH groups is responsible for the quenching of fluorescence as well as color (Fig. 9). Sensor 18 has impressive LODs of 1.65 μM and 0.34 μM, as calculated from absorption and fluorescence studies, respectively. Another fluorescein-derived chemosensor 19 has been reported for the detection of Hg2+. Sensor 1970 is a fluorescein hydrazide coupled 2-(pyridine-2-ylmethoxy)-naphthalene-1-carbaldehyde moiety which exhibited excellent selectivity and sensitivity towards Hg2+ in buffer solution (pH = 7.2, HEPES buffer). Hg2+–induced spirolactam ring-opening of the fluorescein moiety is the reason behind the drastic enhancement in fluorescence (Fig. 9). An LOD of 1.24 μM and the ability to track Hg2+ at the cellular level make sensor 19 a good candidate in the area of mercury detection.
N and –OH groups is responsible for the quenching of fluorescence as well as color (Fig. 9). Sensor 18 has impressive LODs of 1.65 μM and 0.34 μM, as calculated from absorption and fluorescence studies, respectively. Another fluorescein-derived chemosensor 19 has been reported for the detection of Hg2+. Sensor 1970 is a fluorescein hydrazide coupled 2-(pyridine-2-ylmethoxy)-naphthalene-1-carbaldehyde moiety which exhibited excellent selectivity and sensitivity towards Hg2+ in buffer solution (pH = 7.2, HEPES buffer). Hg2+–induced spirolactam ring-opening of the fluorescein moiety is the reason behind the drastic enhancement in fluorescence (Fig. 9). An LOD of 1.24 μM and the ability to track Hg2+ at the cellular level make sensor 19 a good candidate in the area of mercury detection.
Excited-state intramolecular proton transfer (ESIPT)-active molecules are advantageous due to their excellent photostability, large Stokes shift, and unique emission properties. Naphthalene-derived sensor 2071 is an ESIPT-active probe that has been able to detect Hg2+ in CH3CN–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.0, HEPES buffer) medium in a fluorescence on to off fashion accompanied by a drastic change in color from colorless to yellow. The interaction between the N and –OH groups of 20 and Hg2+ stopped the ESIPT process, resulting in fluorescence quenching (Fig. 10).
1, v/v, pH = 7.0, HEPES buffer) medium in a fluorescence on to off fashion accompanied by a drastic change in color from colorless to yellow. The interaction between the N and –OH groups of 20 and Hg2+ stopped the ESIPT process, resulting in fluorescence quenching (Fig. 10).
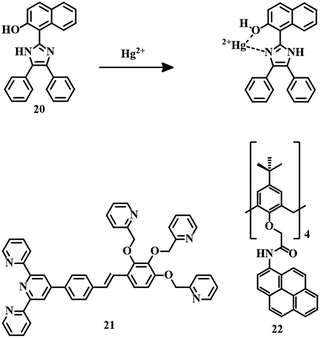 | ||
| Fig. 10 The chemical structures of sensors 20, 21, and 22, and the proposed sensing mechanism of sensor 20. | ||
Terpyridine is a good chromophore that can be introduced to design colorimetric chemical sensors for metal ions. Sensor 21 is a colorimetric fluorescent probe, a combination of a terpyridine unit as a fluorescent moiety and three pyridine rings with ether linkers as receptors (Fig. 10). Sensor 2172 is a typical ratiometric fluorescent sensor for Hg2+ in aqueous medium by changing the fluorescence from blue to green with an LOD of 0.138 ppm. Initial blue fluorescence is due to weak ICT generated from the terpyridine segment. But after the strong complexation of Hg2+ by the receptor, the PET process becomes the driving force for the change in emission color to green. Due to this colorimetric fluorescence change, sensor 21 can be coated on filter paper as paper strips, for the detection of the contaminant in various water sources with the naked eye.
Calix[4]arene-based fluorescent sensing probes are a combination of two parts, i.e.; an ionophore that is responsible for the analyte interaction and a fluorogenic moiety responsible for signal generation. Sensor 2273 is an example of a calix[4]arene-based probe where pyrene is used as a signaling unit (Fig. 10). Sensor 22 can detect Hg2+ selectively in CH3CN–HEPES (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, pH = 7.2) medium with an excellent detection limit of 2.94 × 10−9 M. Complexation between Hg2+ and the carbonyl groups of the amide linkages of 22 is the reason behind the fluorescence quenching.
4, v/v, pH = 7.2) medium with an excellent detection limit of 2.94 × 10−9 M. Complexation between Hg2+ and the carbonyl groups of the amide linkages of 22 is the reason behind the fluorescence quenching.
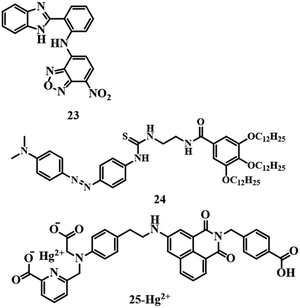
Naked-eye detection of analytes has its uses for cost-effectiveness and simplicity. Sensor 2374 is an NBD-based chemosensor (Fig. 11) which detects Hg2+ in a methanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture by changing the color of the solution from pale yellow to pink with a detection limit of 4.7 × 10−7 M. The formation of a complex by interaction between 23 and Hg2+ triggered the intramolecular charge transfer (ICT) process; as a result, a pink color was observed. Another colorimetric sensor 24,75 an azobenzene gelator, acts as a multianalyte detection system (Fig. 11) by changing color. The addition of Hg2+ to an acetonitrile solution of 24 resulted in a color change to vermeil from yellow due to complexation. Sensor 24 has an LOD of 9.22 × 10−9 M towards Hg2+ ions.
1) mixture by changing the color of the solution from pale yellow to pink with a detection limit of 4.7 × 10−7 M. The formation of a complex by interaction between 23 and Hg2+ triggered the intramolecular charge transfer (ICT) process; as a result, a pink color was observed. Another colorimetric sensor 24,75 an azobenzene gelator, acts as a multianalyte detection system (Fig. 11) by changing color. The addition of Hg2+ to an acetonitrile solution of 24 resulted in a color change to vermeil from yellow due to complexation. Sensor 24 has an LOD of 9.22 × 10−9 M towards Hg2+ ions.
4-Amino-1,8-napthalimide based fluorogenic probe 2576 has been developed to detect Hg2+, and it contains iminodiacetic acid and picolinic acid as receptors. Sensor 25 is highly selective and sensitive towards Hg2+ in HEPES buffer solution (pH = 7.4) with a moderate LOD of 1.03 × 10−7 M. A reduced PET process due to the binding of Hg2+ with the picolinic acid and iminodiacetic acid receptor of 25 (Fig. 11) is responsible for the turn-on fluorescence signal. Due to the turn-on fluorescence feature, 25 is also able to image cells in the presence of Hg2+.
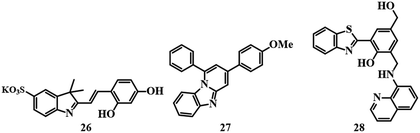
Colorimetric fluorescent sensors have their own advantages due to their dual-mode of signaling, i.e.; by the naked eye and under UV light. Sensor 26,77 which is based on one indole ring (Fig. 12), is a typical example of a colorimetric fluorescent probe. Sensor 26 was applied for the detection of Hg2+ in HEPES buffer solution (pH = 7.0) by changing the color of the system from light yellow to pink and quenching the emission with a detection limit of 1.08 × 10−6 M. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between Hg2+ and 26 resulted in a color change and fluorescence quenching. Sensor 26 can successfully image HeLa cells at pH 7.4 in the presence of Hg2+.
1 binding between Hg2+ and 26 resulted in a color change and fluorescence quenching. Sensor 26 can successfully image HeLa cells at pH 7.4 in the presence of Hg2+.
An imidazo[1,2-a]pyridine-based fluorogenic probe 2778 was developed to detect Hg2+. Sensor 27 (Fig. 12) was able to detect Hg2+ in a fluorescence turn-off fashion in EtOH–water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) medium. A 2
2, v/v) medium. A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry is observed between 27 and Hg2+ with an excellent detection limit of 1 ppb with imaging of HeLa cells. Unfortunately, 27 suffers from interference by Fe3+.
1 binding stoichiometry is observed between 27 and Hg2+ with an excellent detection limit of 1 ppb with imaging of HeLa cells. Unfortunately, 27 suffers from interference by Fe3+.
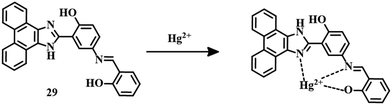
Earlier we discussed one ESIPT sensor. Another ESIPT sensor 2879 is a Schiff base type system where an 8-aminoquinoline moiety acts as the binding site for Hg2+ ions (Fig. 12). In MeCN–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, 10 mM HEPES buffer, pH = 7.0) medium, sensor 28 is able to sense Hg2+ with a detection limit of 0.11 μM. Upon binding of Hg2+ with 28, a strong emission signal is observed due to the disruption of the ESIPT process. Sensor 28 is useful for detecting Hg2+ at the cellular level due to the intense emission signal. Again, an ESIPT probe 2980 was also developed for the detection of Hg2+. Sensor 29 (Fig. 13) differentiates Hg2+ from other competitive ions in a fluorescence turn-on fashion in DMF–HEPES solution (1
2, v/v, 10 mM HEPES buffer, pH = 7.0) medium, sensor 28 is able to sense Hg2+ with a detection limit of 0.11 μM. Upon binding of Hg2+ with 28, a strong emission signal is observed due to the disruption of the ESIPT process. Sensor 28 is useful for detecting Hg2+ at the cellular level due to the intense emission signal. Again, an ESIPT probe 2980 was also developed for the detection of Hg2+. Sensor 29 (Fig. 13) differentiates Hg2+ from other competitive ions in a fluorescence turn-on fashion in DMF–HEPES solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 10 mM, pH = 7.4). The free probe shows a very weak emission as it is in both PET and ESIPT active mode. Upon the addition of mercury, both processes are disrupted to turn on the intense emission. This change in emission from off to on demonstrates the sensing ability of 29 with an LOD of 6.45 × 10−6 M.
1, v/v, 10 mM, pH = 7.4). The free probe shows a very weak emission as it is in both PET and ESIPT active mode. Upon the addition of mercury, both processes are disrupted to turn on the intense emission. This change in emission from off to on demonstrates the sensing ability of 29 with an LOD of 6.45 × 10−6 M.
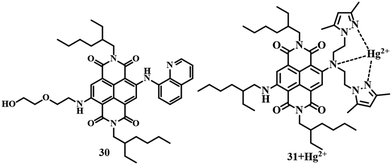
Similar to PET, ESIPT, AIEE, etc., TICT is also a well-known phenomenon in the case of fluorescence spectroscopy. Sensor 3081 is an example of a TICT-active probe, where naphthalene diimide acts as the signaling unit. It can detect Hg2+ in acetone medium with a change in emission from colorless to red with a moderate LOD of 3 μM. The change in emission is attributed to the restriction of the TICT process of 30 due to binding with Hg2+. Another TICT-active molecule 3182 was developed with naphthalene diimide as a signaling unit and bis[2-(3,5-dimethylpyrazole-1-yl)ethyl]amine as an electron donor and ligand for the binding of Hg2+. In an acetone–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium, sensor 31 can recognize Hg2+ (Fig. 14) with a change in fluorescence from colorless to red. The sensing mechanism is quite similar to that of sensor 30. With a 1.3 × 10−6 M detection limit, sensor 31 is useful for carrying out a biological study for the detection of Hg2+ in MCF-7 cells.
1) medium, sensor 31 can recognize Hg2+ (Fig. 14) with a change in fluorescence from colorless to red. The sensing mechanism is quite similar to that of sensor 30. With a 1.3 × 10−6 M detection limit, sensor 31 is useful for carrying out a biological study for the detection of Hg2+ in MCF-7 cells.
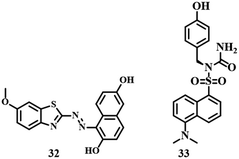
Benzothiazole-based sensor 3283 is an example of a colorimetric and fluorescent probe. The addition of Hg2+ to 32 (Fig. 15) in CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 8.0) medium resulted in a change in color from pink to blue accompanied by a strong emission signal at 425 nm. Thus 32 can be applied to detect Hg2+ with a significant range of 2.5 μM by UV-vis and 1.8 ppb by fluorescence emission. A combination of dansyl group and tyrosine developed a turn-on fluorescence sensor 3384 which was applied to detect Hg2+ in HEPES buffer solution (pH = 7.4). With interaction between two molecules of the sensor 33 (Fig. 15) and one Hg2+, the distance between dansyl units is reduced forming a dimer which is responsible for the enhanced fluorescence signal. Such high selectivity and an impressive LOD of 22.65 × 10−9 M towards Hg2+ make sensor 33 an excellent detection system with cell imaging capability.
1, v/v, pH = 8.0) medium resulted in a change in color from pink to blue accompanied by a strong emission signal at 425 nm. Thus 32 can be applied to detect Hg2+ with a significant range of 2.5 μM by UV-vis and 1.8 ppb by fluorescence emission. A combination of dansyl group and tyrosine developed a turn-on fluorescence sensor 3384 which was applied to detect Hg2+ in HEPES buffer solution (pH = 7.4). With interaction between two molecules of the sensor 33 (Fig. 15) and one Hg2+, the distance between dansyl units is reduced forming a dimer which is responsible for the enhanced fluorescence signal. Such high selectivity and an impressive LOD of 22.65 × 10−9 M towards Hg2+ make sensor 33 an excellent detection system with cell imaging capability.
We have discussed chemosensors which are based on ligand systems attached to some signaling units. Systems based on different phenomena such as ICT, PET, ESIPT, TICT, and AIEE have been used for the detection of Hg2+ by UV-vis and fluorescence spectroscopic techniques. Not all them but many of these sensors can image the cell in the presence of mercury. A summary of the information about the above-mentioned sensors is given in Table 1.
| Compound | Medium | LOD | Type of sensing | Biological study |
|---|---|---|---|---|
| 1 | CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.81 × 10−7 M | Turn-on fluorescence | NA |
| 2 | CH3CN–HEPES (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
36 × 10−9 M | Turn-on fluorescence | NA |
| 3 | DMF–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
0.061 × 10−6 M | Ratiometric fluorescence | Done |
| 4 | DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) 99, v/v) |
14.7 × 10−9 M | Turn-on fluorescence | NA |
| 5 | Ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
45.4 × 10−9 M | Turn-on fluorescence | NA |
| 6 | MeCN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.21 × 10−6 M | Colorimetric | NA |
| 7 | MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
32.8 ppb | Colorimetric | NA |
| 8 | DMSO–Tris–HCl (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
3.11 × 10−8 M | Turn-off fluorescence | NA |
| 9 | MeCN–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
5.01 × 10−8 M | Turn-off fluorescence and colorimetric | NA |
| 10 | EtOH–water (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) 4, v/v) |
17.8 × 10−9 M | Turn-off fluorescence | Done |
| 11 | Buffered solution containing 1% DMSO | 65 × 10−9 M | Turn-off fluorescence | Done |
| 12 | CH3CN–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) 95, v/v) |
99 ppm | Colorimetric and turn-on fluorescence | NA |
| 13 | MeOH–HEPES (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
5.7 × 10−9 M | Turn-on ratiometric fluorescence and colorimetric | Done |
| 14 | DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) 99, v/v) |
591.9 × 10−9 M | Turn-on fluorescence | NA |
| 15 | CH3CN | 7.46 × 10−6 M | Turn-off fluorescence | Done |
| 16 | MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) 4, v/v) |
3.12 × 10−9 M | Turn-off fluorescence | Done |
| 17 | MeOH–Tris–HCl (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95, v/v) 95, v/v) |
7.38 × 10−9 M | Turn-off fluorescence and colorimetric | NA |
| 18 | Water | 0.34 × 10−6 M | Colorimetric and turn-off fluorescence | NA |
| 19 | HEPES buffer | 1.24 μM | Turn-on fluorescence | Done |
| 20 | CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) 9, v/v) |
1.24 × 10−6 M | Turn-off fluorescence | NA |
| 21 | Water | 0.138 ppm | Turn-on ratiometric fluorescence | NA |
| 22 | CH3CN–HEPES (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) 4, v/v) |
2.94 × 10−9 M | Turn-off fluorescence | NA |
| 23 | Methanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
4.7× 10−7 M | Colorimetric | NA |
| 24 | CH3CN | 9.22 × 10−9 M | Colorimetric | |
| 25 | HEPES buffer | 1.03 × 10−7 M | Turn-on fluorescence | Done |
| 26 | HEPES buffer | 1.08 × 10−6 M | Turn-off fluorescence and colorimetric | Done |
| 27 | EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
1 ppb | Turn-off fluorescence | Done |
| 28 | MeCN–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
0.11 × 10−7 M | Turn-on fluorescence | Done |
| 29 | DMF–HEPES solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
6.45 × 10−6 M | Turn-on fluorescence | Done |
| 30 | Acetone | 3 × 10−6 M | Turn-on | NA |
| 31 | Acetone–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.3 × 10−6 M | Turn-on | Done |
| 32 | CH3CN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
2.5 × 10−6 M | Colorimetric and turn-on fluorescence | NA |
| 33 | HEPES buffer | 22.65 × 10−9 M | Turn-on fluorescence | Done |
2.2 Rhodamine-based ligand systems for the detection of Hg2+
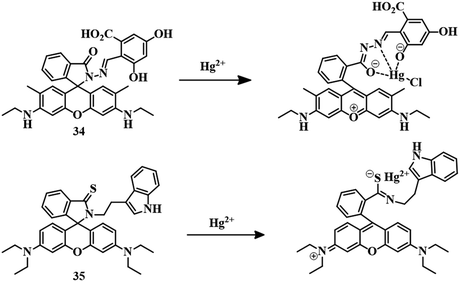
We have discussed heteroatom-based ligand systems (1–33) which consist of different fluorogenic and chromogenic moieties apart from rhodamine, for the detection of Hg2+. Among several dyes available as a fluorophore, rhodamine dyes are well known for their unique features, such as high absorption coefficient, long excitation wavelength, strong absorption, emission signal in the visible range and high quantum yield. Due to such extensive features, rhodamine is being used as the basic unit for the development of fluorescent and colorimetric probed to detect metal ions.85–89 In this section we are going to discuss reported Hg2+ sensors based on rhodamine derivatives with ligand systems.For the binding of Hg2+, a phthalaldehydic has been combined with rhodamine 6G to synthesize sensor 34.90 Spirolactam ring-closed derivative 34 formed a colorless solution in a MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixture with no emission. Upon the addition of Hg2+, the solution instantly changed color to pink with a strong yellow fluorescence. Strong 1
1, v/v) mixture with no emission. Upon the addition of Hg2+, the solution instantly changed color to pink with a strong yellow fluorescence. Strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between 34 and Hg2+ resulted in the spirolactam ring-opening of the rhodamine derivative (Fig. 16), credited with the color and fluorescence change with an excellent lower detection limit of 5 pM, Due to this turn-on fluorescence behavior of 34, it has been used for the detection of Hg2+ in HeLa and macrophage cells.
1 binding between 34 and Hg2+ resulted in the spirolactam ring-opening of the rhodamine derivative (Fig. 16), credited with the color and fluorescence change with an excellent lower detection limit of 5 pM, Due to this turn-on fluorescence behavior of 34, it has been used for the detection of Hg2+ in HeLa and macrophage cells.
Sensor 3591 is a rhodamine–tryptamine-coupled fluorogenic and chromogenic sensor. Tryptamine is analogous to tryptophan and acts as a neurotransmitter. The carbonyl oxygen of rhodamine is replaced by sulfur as Hg2+ has a high affinity towards thio groups. Initially, 35 has no color or fluorescence in MeOH–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) medium, but the sudden appearance of a pink color along with orange-red emission has been observed upon the addition of Hg2+. The high thiophilic affinity of Hg2+ results in spirolactam ring-opening (Fig. 16) and a simultaneous change in color and fluorescence. A strong 1
3) medium, but the sudden appearance of a pink color along with orange-red emission has been observed upon the addition of Hg2+. The high thiophilic affinity of Hg2+ results in spirolactam ring-opening (Fig. 16) and a simultaneous change in color and fluorescence. A strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between Hg2+ and 35 and naked-eye visualization of a change in color as well as fluorescence established the excellent sensing capacity of the sensor with an extremely low detection limit of 2.1 nM.
1 binding stoichiometry between Hg2+ and 35 and naked-eye visualization of a change in color as well as fluorescence established the excellent sensing capacity of the sensor with an extremely low detection limit of 2.1 nM.
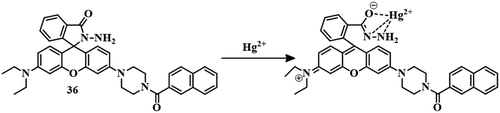
Another rhodamine-derived sensor 36 has been applied for the detection of Hg2+. Slight modification has been undertaken to make the sensor different from the rhodamine B unit, where one side of the xanthene moiety has been substituted by piperazine and further functionalized with naphthyl chloride to develop sensor 36.92 A closed spirolactam ring has been formed by the introduction of hydrazine, which acts as the binding segment for Hg2+. In an MeCN–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
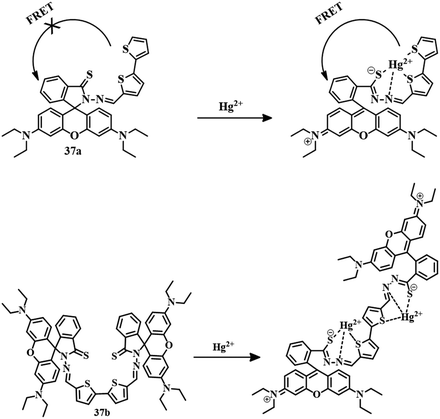
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) mixture, sensor 36 showed no color or emission. But upon the introduction of Hg2+ to the solution, the sudden appearance of a pink color and yellow fluorescence has been observed. Initial binding of Hg2+ (Fig. 17) with 36 induces spirolactam ring-opening followed by hydrolysis for the formation of rhodamine acid, leading to the change in color as well as fluorescence. Sensor 36 has a moderate LOD of 0.38 μM and can be applied to stain living cells. Similarly, a combination of thioxo-rhodamine B hydrazine with [2,2′-bithiophene]-5-carboxaldehyde and [2,2′-bithiophene]-5,5′-dicarboxaldehyde leads to the development of sensors 37a and 37b, respectively.93 These two sensors have different modes of action, although Hg2+–induced spirolactam ring-opening is common to both. The sensing behavior of both sensors was carried out in EtOH–HEPES (1
3, v/v) mixture, sensor 36 showed no color or emission. But upon the introduction of Hg2+ to the solution, the sudden appearance of a pink color and yellow fluorescence has been observed. Initial binding of Hg2+ (Fig. 17) with 36 induces spirolactam ring-opening followed by hydrolysis for the formation of rhodamine acid, leading to the change in color as well as fluorescence. Sensor 36 has a moderate LOD of 0.38 μM and can be applied to stain living cells. Similarly, a combination of thioxo-rhodamine B hydrazine with [2,2′-bithiophene]-5-carboxaldehyde and [2,2′-bithiophene]-5,5′-dicarboxaldehyde leads to the development of sensors 37a and 37b, respectively.93 These two sensors have different modes of action, although Hg2+–induced spirolactam ring-opening is common to both. The sensing behavior of both sensors was carried out in EtOH–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium. Initially, the solutions of both 37a and 37b have no color or emission. Upon the addition of Hg2+, both showed a pink color with reddish-orange emission. Complexation of Hg2+ with 37a triggers spirolactam ring-opening to activate the color and fluorescence of the rhodamine unit upon the excitation wavelength of the bithiophene moiety which suggests a FRET mechanism. In the case of 37b, the sensing mode is different from that of 37a, a traditional Hg2+–induced spirolactam ring-opening upon binding in the cavity formed by thiophene and rhodamine unit is credited with the color change and emission change. The LOD values for 37a and 37b are 3.1 × 10−9 M and 2.92 × 10−9 M, respectively. These naked-eye fluorescent sensors are capable of detecting Hg2+ in living cells. The proposed sensing mechanisms of 37a and 37b are mentioned in Fig. 18.
1) medium. Initially, the solutions of both 37a and 37b have no color or emission. Upon the addition of Hg2+, both showed a pink color with reddish-orange emission. Complexation of Hg2+ with 37a triggers spirolactam ring-opening to activate the color and fluorescence of the rhodamine unit upon the excitation wavelength of the bithiophene moiety which suggests a FRET mechanism. In the case of 37b, the sensing mode is different from that of 37a, a traditional Hg2+–induced spirolactam ring-opening upon binding in the cavity formed by thiophene and rhodamine unit is credited with the color change and emission change. The LOD values for 37a and 37b are 3.1 × 10−9 M and 2.92 × 10−9 M, respectively. These naked-eye fluorescent sensors are capable of detecting Hg2+ in living cells. The proposed sensing mechanisms of 37a and 37b are mentioned in Fig. 18.
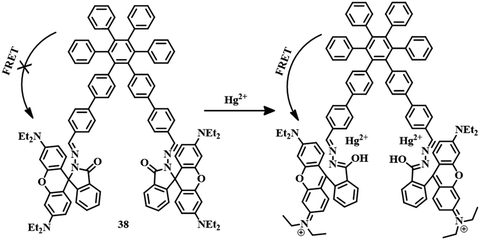
Another sensor 3894 was designed by the attachment of hexaphenylbenzene and rhodamine units (Fig. 19). Initially, sensor 38 showed AIEE in a water–MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and formed fluorescent aggregates. The aggregates showed blue fluorescence due to the stacking of hexaphenylbenzene units. After the addition of Hg2+ to the solution, the fluorescence signal of 475 nm (blue emission) decreased and a new signal at 582 nm (orange-red emission) appeared with an intense pink color. The opening of the spirolactam ring by Hg2+ triggers the FRET process from hexaphenylbenzene (donor) to the rhodamine unit (acceptor). Thus, sensor 38 has evolved as an excellent tool for the detection of Hg2+ both in water sources and at a cellular level with a detection limit of 100 nM.
1) mixture and formed fluorescent aggregates. The aggregates showed blue fluorescence due to the stacking of hexaphenylbenzene units. After the addition of Hg2+ to the solution, the fluorescence signal of 475 nm (blue emission) decreased and a new signal at 582 nm (orange-red emission) appeared with an intense pink color. The opening of the spirolactam ring by Hg2+ triggers the FRET process from hexaphenylbenzene (donor) to the rhodamine unit (acceptor). Thus, sensor 38 has evolved as an excellent tool for the detection of Hg2+ both in water sources and at a cellular level with a detection limit of 100 nM.
Slightly modified rhodamine B derivative 3995 was developed by the reaction between thiocarbonyl-functionalized rhodamine B and different substituted cinnamyl aldehydes. In an EtOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, PBS, Ph 7.4) mixture, all the derivatives of 39 have been applied for the detection of Hg2+ with the same mode of action. Spirolactam ring-opening and binding of Hg2+ through C
1, v/v, PBS, Ph 7.4) mixture, all the derivatives of 39 have been applied for the detection of Hg2+ with the same mode of action. Spirolactam ring-opening and binding of Hg2+ through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C
S and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N segments resulted in a color change and fluorescence change (Fig. 20). All the derivatives are capable of tracking Hg2+ in living cells as well as in animal systems. The sensor consisting of H-functionalized cinnamyl aldehyde, has the maximum efficiency towards Hg2+ detection.
N segments resulted in a color change and fluorescence change (Fig. 20). All the derivatives are capable of tracking Hg2+ in living cells as well as in animal systems. The sensor consisting of H-functionalized cinnamyl aldehyde, has the maximum efficiency towards Hg2+ detection.
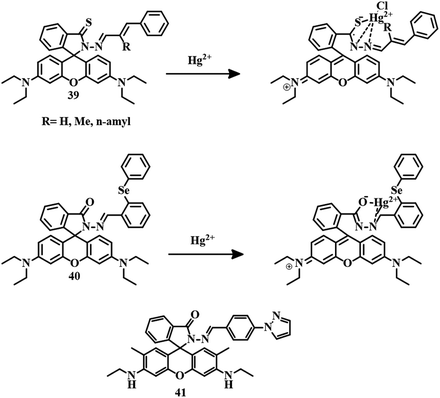 | ||
| Fig. 20 The chemical structures of 39, 40, and 41, and the proposed sensing mechanisms of 39 and 40. | ||
Another sensor 40,96 a combination of diphenyl selenium and rhodamine B hydrazide was applied for the selective and sensitive detection of Hg2+ in MeOH–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium. Traditional Hg2+-induced spirolactam ring-opening followed by complexation with 40 resulted in a color change and fluorescence change (Fig. 20). With an impressive LOD of 12 nM, sensor 40 is capable of detecting Hg2+ in living cells and zebrafish. A similar kind of rhodamine 6G derivative 4197 has been developed for the selective and sensitive detection of Hg2+ in DMSO–water (1
1, v/v) medium. Traditional Hg2+-induced spirolactam ring-opening followed by complexation with 40 resulted in a color change and fluorescence change (Fig. 20). With an impressive LOD of 12 nM, sensor 40 is capable of detecting Hg2+ in living cells and zebrafish. A similar kind of rhodamine 6G derivative 4197 has been developed for the selective and sensitive detection of Hg2+ in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium with an acceptable LOD of 2.07 × 10−8 M. An initial weak emission band at 575 nm of 41 is due to a PET process but after the addition of Hg2+, a drastic enhancement in fluorescence intensity at 575 nm has been observed as a result of a chelation-enhanced fluorescence (CHEF) process (Fig. 20). The visual detection ability of 41 enabled the development of paper strips for the detection of Hg2+.
1) medium with an acceptable LOD of 2.07 × 10−8 M. An initial weak emission band at 575 nm of 41 is due to a PET process but after the addition of Hg2+, a drastic enhancement in fluorescence intensity at 575 nm has been observed as a result of a chelation-enhanced fluorescence (CHEF) process (Fig. 20). The visual detection ability of 41 enabled the development of paper strips for the detection of Hg2+.
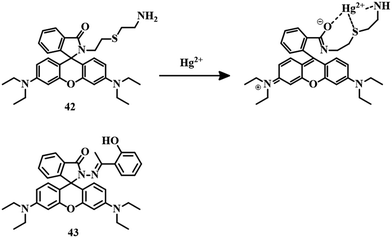
From the condensation of thiobisethylamine with rhodamine B was developed a simply synthesized sensor 42.98 A solution of 42 in MeCN–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) showed no color and no fluorescence, but the addition of Hg2+ resulted in a sudden color change and fluorescence change. Complexation between Hg2+ and 42 through an oxygen atom of the amide carbonyl group, S, and N atoms of the thiobisethylamine unit (Fig. 21) simultaneously opened the spirolactam ring which triggered the color and fluorescence change. With a moderate LOD of 0.14 μM, 42 has the capability of imaging cells in the presence of Hg2+.
99, v/v) showed no color and no fluorescence, but the addition of Hg2+ resulted in a sudden color change and fluorescence change. Complexation between Hg2+ and 42 through an oxygen atom of the amide carbonyl group, S, and N atoms of the thiobisethylamine unit (Fig. 21) simultaneously opened the spirolactam ring which triggered the color and fluorescence change. With a moderate LOD of 0.14 μM, 42 has the capability of imaging cells in the presence of Hg2+.
Another sensor 43,99 a combination of 2-hydroxy acetophenone and rhodamine hydrazine, was applied for the selective and sensitive detection of Hg2+ in an EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) buffer (10 mM, HEPES, pH = 7.2) mixture. The solution of 43 turned pink upon the addition of Cu2+ and Hg2+, though enhanced reddish-orange fluorescence has been observed only in the case of Hg2+. Strong 1
1, v/v) buffer (10 mM, HEPES, pH = 7.2) mixture. The solution of 43 turned pink upon the addition of Cu2+ and Hg2+, though enhanced reddish-orange fluorescence has been observed only in the case of Hg2+. Strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding (Fig. 21) between the ion and 43 is attributed to spirolactam ring-opening and subsequent color and fluorescence change with a detection limit of 150 nM.
1 binding (Fig. 21) between the ion and 43 is attributed to spirolactam ring-opening and subsequent color and fluorescence change with a detection limit of 150 nM.
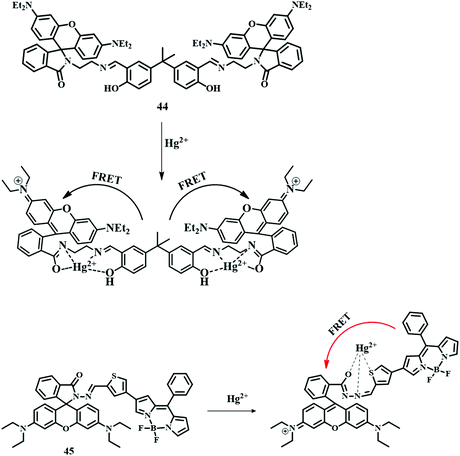
A combination of a dialdehyde derivative of bisphenol A and N-(rhodamine-B) lactam-ethylenediamine developed a dual-channel probe 44,100 which has been applied for the selective detection of Hg2+ by the FRET phenomenon. In an MeCN–water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, HEPES, Ph = 7.0) mixture, 44 showed an excellent pink color along with orange fluorescence upon the addition of Hg2+. Spirolactam ring-opening of the rhodamine unit by Hg2+ forms a new conjugate system and activates the FRET process from the bisphenol (donor) unit to the rhodamine (acceptor) unit to give the orange emission as an output signal (Fig. 22). This emissive property of 44 can be used in the detection of Hg2+ in living cells.
2, v/v, HEPES, Ph = 7.0) mixture, 44 showed an excellent pink color along with orange fluorescence upon the addition of Hg2+. Spirolactam ring-opening of the rhodamine unit by Hg2+ forms a new conjugate system and activates the FRET process from the bisphenol (donor) unit to the rhodamine (acceptor) unit to give the orange emission as an output signal (Fig. 22). This emissive property of 44 can be used in the detection of Hg2+ in living cells.
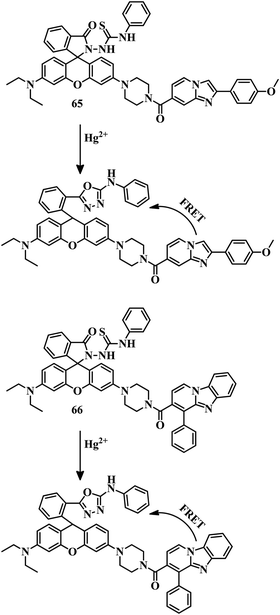
Ratiometric sensor 45,101 a combination of a BODIPY unit and a rhodamine unit, was developed very cleverly. A combination of these two units has been used as a FRET pair, where the BODIPY unit can act as a donor and the rhodamine unit as an acceptor. These two units have been attached by a thiophene unit, which can act as a binding unit of Hg2+. An ethanolic solution of 45 showed only a green fluorescence due to the BODIPY unit. But upon the addition of Hg2+, reddish-orange emission observed upon excitation at 480 nm (BODIPY moiety) due to spirolactam ring-opening followed by FRET activation (Fig. 22). This FRET pair has an excellent LOD value of 1.56 ppb.
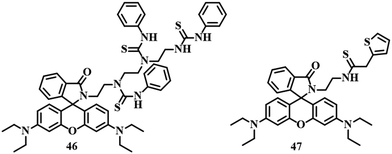
Sensor 46102 was also developed by the sequential reactions between rhodamine B, triethylenetetramine, and phenyl isothiocyanate (Fig. 23). Thiourea segments of 46 are responsible for the interaction with Hg2+. In MeCN–HEPES (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium, 46 and Hg2+ formed a stable 1
1, v/v) medium, 46 and Hg2+ formed a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex with a strong orange emission accompanied by a pink color. Similarly, another rhodamine-based sensor 47 (Fig. 23) has been developed with the combination of rhodamine B and -thiophene acetyl chloride.103 This thiophene-coupled rhodamine derivative 47 was applied for the detection of Hg2+ in an EtOH–water (2
3 complex with a strong orange emission accompanied by a pink color. Similarly, another rhodamine-based sensor 47 (Fig. 23) has been developed with the combination of rhodamine B and -thiophene acetyl chloride.103 This thiophene-coupled rhodamine derivative 47 was applied for the detection of Hg2+ in an EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixture. Spirolactam ring-opening induced by Hg2+, followed by the complexation with 47 resulted in a pink coloration and reddish emission. An LOD of 0.11 μM and the ability to image living cells and zebrafish helped 47 to become a potential tool for the detection of Hg2+.
1, v/v) mixture. Spirolactam ring-opening induced by Hg2+, followed by the complexation with 47 resulted in a pink coloration and reddish emission. An LOD of 0.11 μM and the ability to image living cells and zebrafish helped 47 to become a potential tool for the detection of Hg2+.
In this section, we have discussed reported sensors of Hg2+ based on heteroatom-containing ligands and rhodamine units as the signaling moiety. The rhodamine unit has been successfully used as the signaling unit as it has both colorimetric and fluorometric properties in spirolactam ring-closed form. Rhodamine-based sensors can show turn-off to turn-on fluorescence activity upon interaction with metal ions. Hg2+ can induce the opening of the spirolactam ring in rhodamine derivatives and switch on the color as well an emission. Due to this unique feature, modified rhodamine molecules are one of the most commonly used tools for the detection of Hg2+ ions. All the comparative data are described in tabular form in Table 2.
| Compound | Medium | LOD | Type of sensing | Biological study |
|---|---|---|---|---|
| 34 | MeOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
5 × 10−12 M | Colorimetric and turn-on fluorescence | Done |
| 35 | MeOH–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
2.1 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
| 36 | MeCN–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
0.36 × 10−6 M | Colorimetric and turn-on fluorescence | Done |
| 37a | EtOH–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
3.1 × 10−9 M | Colorimetric and turn-on fluorescence | Done |
| 37b | EtOH–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
2.92 × 10−9 M | Colorimetric and turn-on fluorescence | Done |
| 38 | MeCN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
100 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
| 39 | EtOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, PBS, pH = 7.4) 1, v/v, PBS, pH = 7.4) |
8.26 × 10−9 M, 15.52 × 10−9 M, 23.26 × 10−9 M | Colorimetric and turn-on fluorescence | Done |
| 40 | MeOH–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
12 × 10−9 M | Colorimetric and turn-on fluorescence | Done |
| 41 | DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
2.07 × 10−8 M | Colorimetric and turn-on fluorescence | NA |
| 42 | MeCN–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) 99, v/v) |
0.14 × 10−6 M | Colorimetric and turn-on fluorescence | Done |
| 43 | EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
150 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
| 44 | MeCN–water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, HEPES, Ph = 7.0) 2, v/v, HEPES, Ph = 7.0) |
2.16 × 10−6 M | Colorimetric and turn-on fluorescence | Done |
| 45 | EtOH | 7.8 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
| 46 | MeCN–HEPES (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
3.04 × 10−7 M | Colorimetric and turn-on fluorescence | NA |
| 47 | EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
0.11 × 10−6 M | Colorimetric and turn-on fluorescence | Done |
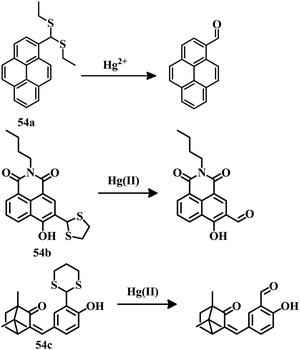
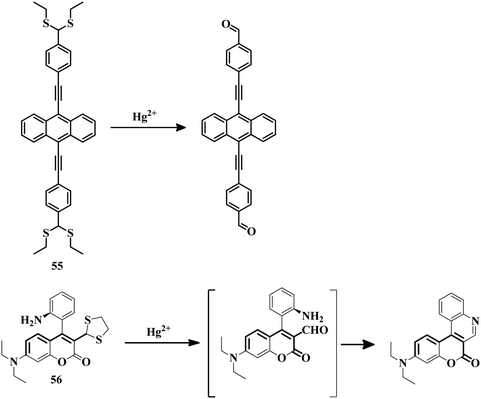
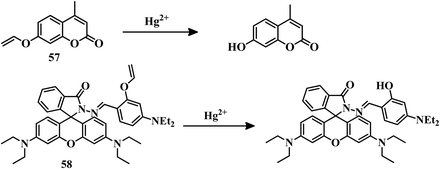
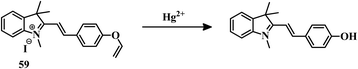
2.3 Reaction-based irreversible sensors for Hg2+
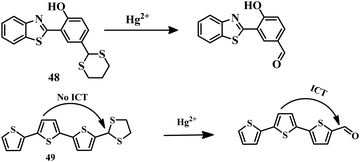
The above-mentioned sensor systems can detect Hg2+ complexation with the ligands and they can also be reversible. These interaction-based systems are one kind of developed tool that can be used for the detection of analytes, but there is always a chance of interference by other metal ions with the same type of chemical properties. But reaction-based detection systems are preferable due to their high selectivity over other competitive metal ions. Thioacetal deprotection, 1,3,4-oxadiazole formation, ester hydrolysis, hydrolysis of an imine bond, and alkyne or vinyl ether oxymercuration are well-known reactions which are preferably carried out by Hg2+. These reactions have been used to design and develop different sensing systems for Hg2+ over the years. In this section, we are going to discuss reaction-based sensing systems that have evolved over the last few years.Similarly, an oligothiophene-based thioacetal system 49105 has been developed as an excellent colorimetric and fluorometric probe for the detection of Hg2+ in EtOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium. Sensor 49 has a strong blue fluorescence due to the stopped ICT process as well as having no color. But upon addition of Hg2+, the color of 49 changed from colorless to yellow accompanied by yellow fluorescence. The formation of an aldehyde group from thioacetal is promoted by Hg2+ (Fig. 24) which activates the ICT process, which is responsible for the color change as well as the fluorescence change. Sensor 49 can detect Hg2+ in water, soil, and seafood with a detection limit of 1.03 × 10−8 M.
1) medium. Sensor 49 has a strong blue fluorescence due to the stopped ICT process as well as having no color. But upon addition of Hg2+, the color of 49 changed from colorless to yellow accompanied by yellow fluorescence. The formation of an aldehyde group from thioacetal is promoted by Hg2+ (Fig. 24) which activates the ICT process, which is responsible for the color change as well as the fluorescence change. Sensor 49 can detect Hg2+ in water, soil, and seafood with a detection limit of 1.03 × 10−8 M.
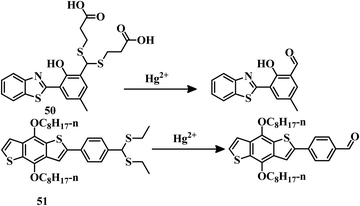
Benzothiazole-based “ESIPT + AIE”-active probe 50106 was designed for the selective detection of Hg2+ in THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, PBS, Ph = 8.5) medium. The aldehyde group was protected by 3-marceptopropionic acid to make it an Hg2+-reactive compound. Hg2+-triggered hydrolysis of 50 (Fig. 25) resulted in the formation of an “ESIPT-AIE”-active molecule which shows a strong yellow fluorescence. This distinct change in emission can be effectively applied for biological study. Sensor 50 has an impressive detection limit of 1.59 × 10−8 M. Similarly a colorimetric and fluorescent probe 51,107 based on a benzo[1,2-b:4,5-b′]dithiophene (BDT) unit has been developed for the detection of Hg2+, where a thioacetal group is used as the reaction site. Sensor 51 has been applied for the detection of Hg2+ in a THF–water (1
1, v/v, PBS, Ph = 8.5) medium. The aldehyde group was protected by 3-marceptopropionic acid to make it an Hg2+-reactive compound. Hg2+-triggered hydrolysis of 50 (Fig. 25) resulted in the formation of an “ESIPT-AIE”-active molecule which shows a strong yellow fluorescence. This distinct change in emission can be effectively applied for biological study. Sensor 50 has an impressive detection limit of 1.59 × 10−8 M. Similarly a colorimetric and fluorescent probe 51,107 based on a benzo[1,2-b:4,5-b′]dithiophene (BDT) unit has been developed for the detection of Hg2+, where a thioacetal group is used as the reaction site. Sensor 51 has been applied for the detection of Hg2+ in a THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture with a change of color to yellow from colorless and yellow emission from blue. Thioacetal deprotection to aldehyde formation (Fig. 25) of sensor 51 promoted by Hg2+ is the reason behind the color change as well as the emission change. Due to its reaction-based sensing mechanism, sensor 51 emerged as a highly selective Hg2+ sensor with a moderate LOD of 3.1 × 10−7 M.
1) mixture with a change of color to yellow from colorless and yellow emission from blue. Thioacetal deprotection to aldehyde formation (Fig. 25) of sensor 51 promoted by Hg2+ is the reason behind the color change as well as the emission change. Due to its reaction-based sensing mechanism, sensor 51 emerged as a highly selective Hg2+ sensor with a moderate LOD of 3.1 × 10−7 M.
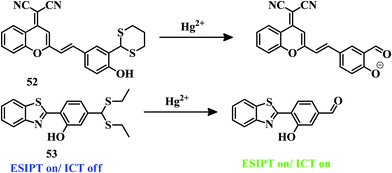
A 1,3-dithiane group has been incorporated into a dicyanomethylene-4H-pyran fluorophore for the design of a dual colorimetric and NIR fluorescent probe 52.108 Sensor 52 has been applied for the detection of Hg2+ in a PBS–DMSO buffer (20 mM, pH 7.4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) mixture. Hg2+–Induced deprotection of the thioacetal segment changes the color to pink from purple with an emission signal around the NIR area (Fig. 26). 52 can successfully detect selectively only Hg2+ among other metal ions due to the thiophilic nature of mercury. This dual-channel detection system has an LOD value of 6.8 × 10−8 M. Due to its prominent and visual color change as well as emission signal around the NIR zone, it can be applied in biological systems and the environment for the detection of Hg2+.
5, v/v) mixture. Hg2+–Induced deprotection of the thioacetal segment changes the color to pink from purple with an emission signal around the NIR area (Fig. 26). 52 can successfully detect selectively only Hg2+ among other metal ions due to the thiophilic nature of mercury. This dual-channel detection system has an LOD value of 6.8 × 10−8 M. Due to its prominent and visual color change as well as emission signal around the NIR zone, it can be applied in biological systems and the environment for the detection of Hg2+.
Another sensor 53109 consists of benzothiazole as a core unit. The presence of the thioacetal group stops the ICT process and activates the ESIPT between –OH and the benzothiazole unit; as a result, blue fluorescence has been observed in a pH 7.4, 10 mM HEPES buffer–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixture. Upon addition of Hg2+ to the mixture of 53, intense greenish-yellow emission was observed by decreasing the blue emission. ICT along with ESIPT has been activated due to the appearance of the –CHO group by Hg2+-triggered deprotection of thioacetal (Fig. 26). Due to the ratiometric nature of the detection and low LOD of 5.8 nM, sensor 53 can act as a good tool for the detection of Hg2+, though it lacks biological application.
1, v/v) mixture. Upon addition of Hg2+ to the mixture of 53, intense greenish-yellow emission was observed by decreasing the blue emission. ICT along with ESIPT has been activated due to the appearance of the –CHO group by Hg2+-triggered deprotection of thioacetal (Fig. 26). Due to the ratiometric nature of the detection and low LOD of 5.8 nM, sensor 53 can act as a good tool for the detection of Hg2+, though it lacks biological application.
A simple pyrene derivative 54a110a containing a bisethylsulfane moiety has been applied to sense Hg2+ in semi-aqueous medium and biological systems. Sensor 54 can detect Hg2+ selectively due to the presence of the bisethylsulfane segment. Hg2+ promotes the removal of bisethylsulfane to produce 1-pyrenecarboxaldehyde (Fig. 27) in MeCN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium with the appearance of blue emission. Sensor 54a has a detection limit of 57 nM and can successfully detect Hg2+ in living cells and zebrafish.
1, v/v) medium with the appearance of blue emission. Sensor 54a has a detection limit of 57 nM and can successfully detect Hg2+ in living cells and zebrafish.
Another dithiolane-based probe 54b110b has been successfully applied to identify Hg(II) in ethanol/water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v; pH = 7.40) medium. Due to the protected form of the –CHO group in the naphthalene moiety, the fluorescence intensity is weak initially. After addition of Hg(II), a colour change to light green from light yellow and strong emission were observed. This change in colour and emission is expected due to the transformation of dithiolane to a formyl group (Fig. 27). Sensor 54b turned out to be an excellent sensor for Hg(II) with a detection limit of 4.0 × 10−8 M and ability for cell imaging.
8, v/v; pH = 7.40) medium. Due to the protected form of the –CHO group in the naphthalene moiety, the fluorescence intensity is weak initially. After addition of Hg(II), a colour change to light green from light yellow and strong emission were observed. This change in colour and emission is expected due to the transformation of dithiolane to a formyl group (Fig. 27). Sensor 54b turned out to be an excellent sensor for Hg(II) with a detection limit of 4.0 × 10−8 M and ability for cell imaging.
Probe 54c110c has been reported as an excellent small molecular probe for Hg(II) detection. In a 99% PBS buffer solution of 54c, with the addition of Hg(II), the solution turned green-emissive from non-emissive. Here also the dithiolane segment has been used as selective reaction site for Hg(II). By virtue of this the formation of a –CHO group triggered by Hg(II) leads to the observed green emission (Fig. 27). As a reaction-based sensor, 54c is highly selective towards Hg(II) and suffered zero interference by other analytes. It has been successfully applied in a paper-strip model and for analysis of Hg(II) contamination in real samples. The limit of detection for 54c is calculated as 19.3 nM.
Another sensor 55 consists of a π-extended anthracene moiety bearing thioacetal segments within it.111 In a solution of a THF–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) mixture, sensor 55 showed weak blue fluorescence. After the addition of mercury, successful deprotection of thioacetal formed the aldehyde. Aldehyde formation from thioacetal (Fig. 28) triggered the intense green emission which contributed to the effectiveness of the chemodosimetric nature of 55 with an LOD of 59 nM. Due to this ratiometric change in emission, 55 was utilized for the detection of Hg2+ in living cells.
1, v/v, pH = 7.4) mixture, sensor 55 showed weak blue fluorescence. After the addition of mercury, successful deprotection of thioacetal formed the aldehyde. Aldehyde formation from thioacetal (Fig. 28) triggered the intense green emission which contributed to the effectiveness of the chemodosimetric nature of 55 with an LOD of 59 nM. Due to this ratiometric change in emission, 55 was utilized for the detection of Hg2+ in living cells.
Coumarin-based fluorescent probe 56112 was developed by introducing a 2-aminophenyl group to the Hg2+/CH3Hg+-reactive thioacetal group. In a PBS buffer solution of 56, the introduction of Hg2+ ions resulted in aldehyde formation from thioacetal followed by condensation with an adjacent –NH2 group to form a heterocyclic aromatic compound with large conjugation (Fig. 28). Due to the formation of the heterocyclic aromatic compound with a coumarin ring, bright green emission was observed with the shifting of the emission spectrum. Sensor 56 is able to detect both Hg2+ and CH3Hg+ with the same mode of sensing mechanism and green emission as an output signal. The LOD values are 27 nM and 5.7 μm for Hg2+ and CH3Hg+, respectively.
In this section, we have discussed thioacetal-based probes for the irreversible detection of Hg2+ in the environment and biological systems.
Fluorescent probe 57113 consisting of coumarin as a fluorophore moiety and vinyl ether as the reactive site was developed for the detection of Hg2+. Sensor 57 in HEPES buffer solution showed no emission but upon addition of Hg2+ the sudden appearance of blue emission was observed. The irreversible hydrolysis reaction of the vinyl ether group promoted by Hg2+ (Fig. 29) is the reason behind the turn-on fluorescence response of 57 with a detection limit of 0.12 μM. Similarly, another sensor 58114 comprises an O–vinyl protected hydroxyl benzaldehyde coupled with rhodamine hydrazone. Initially 58 in CH3CN–PBS buffer (3/7, v/v, 10.0 mM, pH = 7.40) solution exhibited green fluorescence due to the ICT process. After the addition of Hg2+, the green fluorescence of 58 decreased due to the deprotection of the vinyl ether group which inhibited the ICT process (Fig. 29). With the turn-off emission of 58 in the presence of Hg2+, it has a detection limit of 244 ppb.
Sensor 59,115 based on a vinyl ether derivative of hemicyanine, has been developed for the detection of Hg2+. Deprotection of the –OH group of hemicyanine by a vinyl group blocks the ICT process. Hydrolysis of vinyl ether promoted by Hg2+ in 59 (Fig. 30) activates the ICT process in the HEPES buffer solution, which is responsible for the appearance of an orange color as well as reddish-yellow fluorescence. This drastic change in color and fluorescence of 59 appears to be advantageous in the field of Hg2+ detection with biological applications.
An NIR-fluorescent probe 60116 was developed, where 9-(2-carboxyphenyl)-6-(diethylamino)-1,2,3,4-tetrahydroxanthylium was used as a fluorophore and vinyl ether acted as the reactive site for Hg2+. Sensor 60 in ethanol–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v 50 mM HEPES buffer solution, pH = 7.4) solution initially showed a weak red emission at 660 nm but upon the introduction of Hg2+, a drastic enhancement in 660 nm peak with strong red emission was observed. This change in emission was due to the removal of the vinyl ether group and the formation of an –OH group which increases the “push–pull” property to activate the ICT process (Fig. 31). Due to the turn-off to turn-on nature of 60 in the presence of Hg2+, it is very useful in the environment and biological systems. This NIR probe has an LOD value of 3.2 nM.
8, v/v 50 mM HEPES buffer solution, pH = 7.4) solution initially showed a weak red emission at 660 nm but upon the introduction of Hg2+, a drastic enhancement in 660 nm peak with strong red emission was observed. This change in emission was due to the removal of the vinyl ether group and the formation of an –OH group which increases the “push–pull” property to activate the ICT process (Fig. 31). Due to the turn-off to turn-on nature of 60 in the presence of Hg2+, it is very useful in the environment and biological systems. This NIR probe has an LOD value of 3.2 nM.
Another sensor 61117 was developed for the selective and sensitive detection of Hg2+ with the help of a vinyl ether group as the reaction site. Initially, blue fluorescence of 61 was observed in 10 mM PBS buffer (1% CH3CN) solution which was due to the stopped ESIPT process. But after the addition of Hg2+ to the PBS buffer solution, an equivolume of DCM was added and fluorescence was recorded, a drastic red shift of the emission spectrum was observed with a cyan emission. Deprotection of the vinyl ether group promoted by Hg2+ and formation of –OH group (Fig. 31) activate the ESIPT process which was the reason for cyan fluorescence. Sensor 61 is an excellent chemodosimeter due to its ratiometric nature and an impressive LOD of 7.8 × 10−9 M. It can also be applied in biological systems to detect Hg2+ with good efficiency.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the 2-mercapto-benzyl segment to facilitate the hydrolysis reaction to form an –OH group of the fluorophore (Fig. 32). This hydrolysis reaction triggers activation of the ICT process to show a green-yellow emission signal. Sensor 62 acted as a turn-on chemodosimeter for the detection of Hg2+ with a detection limit of 6.5 nM. Another sensor 63,119 a Schiff base type of compound containing benzimidazole and coumarin units as fluorophores, was developed for the selective detection of Hg2+ in HEPES buffer/DMSO (v/v = 9
O groups of the 2-mercapto-benzyl segment to facilitate the hydrolysis reaction to form an –OH group of the fluorophore (Fig. 32). This hydrolysis reaction triggers activation of the ICT process to show a green-yellow emission signal. Sensor 62 acted as a turn-on chemodosimeter for the detection of Hg2+ with a detection limit of 6.5 nM. Another sensor 63,119 a Schiff base type of compound containing benzimidazole and coumarin units as fluorophores, was developed for the selective detection of Hg2+ in HEPES buffer/DMSO (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.2) medium. Initial weak blue emission turned intense blue upon the addition of Hg2+ into the solution of 63. This enhanced emission signal is expected to be due to the cleavage of the imine bond by Hg2+ and the formation of the coumarin part (Fig. 32). With a high sensitivity of 70 nM, 63 can be applied for the detection of Hg2+ in biological systems.
1, pH = 7.2) medium. Initial weak blue emission turned intense blue upon the addition of Hg2+ into the solution of 63. This enhanced emission signal is expected to be due to the cleavage of the imine bond by Hg2+ and the formation of the coumarin part (Fig. 32). With a high sensitivity of 70 nM, 63 can be applied for the detection of Hg2+ in biological systems.
Similarly, sensor 64120 was developed using a coumarin derivative and a 5-aminoisophthalic acid methyl ester unit. Due to the formation of the Schiff base between coumarin dye and the amino isophthalic acid methyl ester derivative in CH3CN–H2O (8/2, v/v, 0.1 M KClO4 buffer, pH = 7.34) medium, no emission was observed. Upon the addition of Hg2+, a highly intense peak at 490 was observed. The resulting emission signal corresponded to the coumarin aldehyde unit which was a result of imine bond cleavage promoted by Hg2+ to suppress the PET process (Fig. 33). Sensor 64 was successfully applied to detect mercury at the nanomolar level with biological applications.
An imidazo[1,2-a]pyridine-rhodamine ratiometric fluorescent probe 65121 was developed for the detection of Hg2+ in PBS/EtOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) medium. Upon addition of Hg2+ to the solution of 65, it turned pink in color with a reddish emission. Hg2+ promoted spirolactam ring-opening followed by the formation of 1,3,4-oxadiazole by thiosemicarbazide (Fig. 34). Due to this new structure formation and extended conjugation, the FRET process was generated to give a unique color and emission. Sensor 65 has an LOD of 9.1 nM and the capability of tracking mercury at the cellular level with excellent efficiency.
1, v/v) medium. Upon addition of Hg2+ to the solution of 65, it turned pink in color with a reddish emission. Hg2+ promoted spirolactam ring-opening followed by the formation of 1,3,4-oxadiazole by thiosemicarbazide (Fig. 34). Due to this new structure formation and extended conjugation, the FRET process was generated to give a unique color and emission. Sensor 65 has an LOD of 9.1 nM and the capability of tracking mercury at the cellular level with excellent efficiency.
Another sensor 66122 was developed as a pyrido[1,2-a]benzimidazole-rhodamine based FRET system. In this system, benzimidazole was used as the energy donor and a piperazine-functionalized rhodamine unit was used as the acceptor of energy. In a solution of EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v), 66 shows blue emission with no color. After the addition of Hg2+, the sudden appearance of a pink color with red emission was observed upon excitation at 380 nm which corresponds to the benzimidazole moiety. The mechanism is similar to that of sensor 65, where the formation of 1,3,4-oxadiazole and spirolactam ring-opening (Fig. 34) is the reason behind the color change and emission change. Sensor 66 with a good LOD of 18.8 nM, can be applied for detecting Hg2+ in biological systems.
8, v/v), 66 shows blue emission with no color. After the addition of Hg2+, the sudden appearance of a pink color with red emission was observed upon excitation at 380 nm which corresponds to the benzimidazole moiety. The mechanism is similar to that of sensor 65, where the formation of 1,3,4-oxadiazole and spirolactam ring-opening (Fig. 34) is the reason behind the color change and emission change. Sensor 66 with a good LOD of 18.8 nM, can be applied for detecting Hg2+ in biological systems.
Sensor 67123 was developed with the combination of rhodamine B, o-phenylenediamine, and phenyl isothiocyanate. Initially a solution of 67 in MeCN–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v, pH = 7.2) medium showed no color and no emission. The addition of Hg2+ to the solution resulted in a pink color and reddish emission. Hg2+ promoted spirolactam ring-opening followed by the formation of a benzimidazole-appended rhodamine intermediate (Fig. 35) to generate the color as well as the emission output. The dual-mode of detection of 67 in a chemodosimetric manner made it much more useful with an LOD of 1.6 nM and biological applicability.
9, v/v, pH = 7.2) medium showed no color and no emission. The addition of Hg2+ to the solution resulted in a pink color and reddish emission. Hg2+ promoted spirolactam ring-opening followed by the formation of a benzimidazole-appended rhodamine intermediate (Fig. 35) to generate the color as well as the emission output. The dual-mode of detection of 67 in a chemodosimetric manner made it much more useful with an LOD of 1.6 nM and biological applicability.
Another sensor 68 was designed with a perimidine moiety for the detection of Hg2+ ions. In a MeCN–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) mixture, 68a124a showed no emission. A strong blue emission was observed for 68a upon the addition of Hg2+. The blue emission signal is attributed to the Hg2+-mediated formation of a double bond in the junction of the coumarin and naphthalin moiety (Fig. 35). Sensor 68a suffered from a low LOD of 1.08 μM although it can be applied in biological systems to detect Hg2+.
7, v/v) mixture, 68a124a showed no emission. A strong blue emission was observed for 68a upon the addition of Hg2+. The blue emission signal is attributed to the Hg2+-mediated formation of a double bond in the junction of the coumarin and naphthalin moiety (Fig. 35). Sensor 68a suffered from a low LOD of 1.08 μM although it can be applied in biological systems to detect Hg2+.
Another azo-based sensor 68b124b has been introduced for the successful colorimetric detection of Hg(II) in a DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) system. Here the Hg(II)-triggered desulfurization and subsequent rearrangement leads to the change in colour for 68b (Fig. 35). This system is an excellent molecule to detect Hg(II) with an LOD of 8.1 nM and can be used as a solid chip to visualize the colour change.
99, v/v) system. Here the Hg(II)-triggered desulfurization and subsequent rearrangement leads to the change in colour for 68b (Fig. 35). This system is an excellent molecule to detect Hg(II) with an LOD of 8.1 nM and can be used as a solid chip to visualize the colour change.
A combination of the dye pyronin and the chelating agent meso-2,3-dimercaptosuccinic acid developed sensor 69125 for the detection and removal of Hg2+. This one of the rare systems which can detect and sense Hg2+ ions. In PBS buffer solution, 69 remained in turn-off fluorescence mode and was colorless to the naked eye. Upon introduction of Hg2+, a solution of 69 turned pink in color and emitted reddish fluorescence. Strong binding of Hg2+ with the ligand and subsequent removal of the chelated complex generated free pyronin dye to which was attributed the appearance of the pink color and reddish emission (Fig. 36). With the excellent dual-sensing signal and high LOD of 300 pM, sensor 69 was successfully applied at the cellular level and in a zebrafish model for tracking Hg2+.
The thiocarbonate-appended fluorescein-based chemodosimeter 70126 was developed for the turn-on fluorescent detection of Hg2+ in a HEPES buffer solution (20 mM, pH 7.4, 1% EtOH). Initially, with no color and emission, 70 showed green emission upon the addition of Hg2+. Hg2+ promotes the hydrolysis of the thiocarbonate segment and forms free fluorescein acid which results in a greenish emission (Fig. 36). Sensor 70 has a detection limit of 40 nM.
Another thiocarbonate-based sensor 71127 was developed, where 2-(2′-hydroxyphenyl)benzothiazole was used as the fluorophore unit. Benzothiazole segments are well known for their ESIPT activity as well as their AIEE activity. Upon functionalization of the –OH group, the ESIPT process stopped for 71. An appearance of yellow fluorescence was observed in an EtOH–water (5/5, v/v, HEPES pH = 7.4) solution of 71 upon the addition of Hg2+ ions. This yellow color was generated due to the Hg2+-triggered hydrolysis of thiocarbonate to form an –OH group and activate the ESIPT process (Fig. 37). Sensor 71 has a detection limit of 55 nM.
Similarly, sensor 72128 was designed based on the ESIPT phenomenon, where 2-(2′-hydroxyphenyl)benzothiazole also acted as a signaling unit and thiophosphate as a reaction site. Blocking of –OH group with thiophosphate deactivated the ESIPT process and turned off the emission. In a solution of CH3CN/HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, 10 mM, pH = 7.4), 72 turned blue-emissive upon addition of Hg2+ ions. Removal of the thiophosphate group resulted in the formation of an –OH group (Fig. 37) which activated the ESIPT to give blue emission. With an LOD of 12 nM, 72 can be applied for the detection of Hg2+ at the cellular level.
4, v/v, 10 mM, pH = 7.4), 72 turned blue-emissive upon addition of Hg2+ ions. Removal of the thiophosphate group resulted in the formation of an –OH group (Fig. 37) which activated the ESIPT to give blue emission. With an LOD of 12 nM, 72 can be applied for the detection of Hg2+ at the cellular level.
In this section, we have successfully discussed Hg2+ sensors based on reactions, which are chemodosimetric in nature as they irreversibly detect the analyte. In comparison to heteroatom-containing ligand systems, reaction-based sensors are superior due to their excellent selectivity towards Hg2+ ions. All the summarised information about reaction-based sensors (48–72), is given in Table 3.
| Compound | Medium | LOD | Type of sensing | Biological study |
|---|---|---|---|---|
| 48 | PBS buffer (pH = 7.4, containing 2% DMSO) | 7.6 × 10−9 M | Turn-on fluorescence | Done |
| 49 | EtOH–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.03 × 10−8 M | Turn-on fluorescence | NA |
| 50 | THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, PBS, pH = 8.5) 1, v/v, PBS, pH = 8.5) |
1.59 × 10−8 M | Turn-on fluorescence | NA |
| 51 | THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
3.1 × 10−7 M | Turn-on fluorescence | NA |
| 52 | PBS–DMSO buffer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v 20 mM, pH = 7.4) 5, v/v 20 mM, pH = 7.4) |
6.8 × 10−8 M | Turn-on fluorescence and colorimetric | Done |
| 53 | Ethanol–HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) 1, v/v, pH = 7.4) |
5.8 × 10−9 M | Turn-on fluorescence | NA |
| 54a | MeCN–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
57 × 10−9 M | Turn-on fluorescence | Done |
| 54b | EtOH–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v, pH = 7.4) 8, v/v, pH = 7.4) |
4.0 × 10−8 M | Turn-on fluorescence | Done |
| 54c | DMS–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v, pH = 7.4) 99, v/v, pH = 7.4) |
19.3 × 10−9 M | Turn-on fluorescence | Done |
| 55 | THF–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) 1, v/v, pH = 7.4) |
59 × 10−9 M | Turn-on fluorescence | Done |
| 56 | PBS buffer | 27 × 10−9 M | Turn-on fluorescence | NA |
| 57 | HEPES buffer | 0.12 × 10−6 M | Turn-on fluorescence | NA |
| 58 | CH3CN–PBS buffer (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, 10.0 mM, pH = 7.4) 7, v/v, 10.0 mM, pH = 7.4) |
244 ppb | Turn off fluorescence | NA |
| 59 | HEPES buffer | NA | Turn-on fluorescence and colorimetric | Done |
| 60 | Ethanol–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v 50 mM HEPES buffer solution, pH = 7.4) 8, v/v 50 mM HEPES buffer solution, pH = 7.4) |
3.2 × 10−9 M | Turn-on fluorescence | Done |
| 61 | PBS buffer (1% CH3CN) | 7.8 × 10−9 M | Turn-on fluorescence | Done |
| 62 | PBS buffer | 6.5 × 10−9 M | Turn-on fluorescence | NA |
| 63 | HEPES buffer/DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.2) 1, v/v, pH = 7.2) |
70 × 10−9 M | Turn-on fluorescence | Done |
| 64 | CH3CN/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, 0.1 M KClO4 buffer, pH = 7.34) 2, v/v, 0.1 M KClO4 buffer, pH = 7.34) |
Nanomolar level | Turn-on fluorescence | Done |
| 65 | PBS/EtOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
9.1 × 10−9 M | Ratiometric fluorescence | Done |
| 66 | EtOH–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) 8, v/v) |
18.8 × 10−9 M | Turn-on fluorescence | Done |
| 67 | MeCN–HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v, pH = 7.2) 9, v/v, pH = 7.2) |
1.6 × 10−9 M | Turn-on fluorescence | Done |
| 68a | MeCN–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
1.08 × 10−6 M | Turn-on fluorescence | Done |
| 68b | DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) 9, v/v) |
8.1 × 10−9 M | Colorimetric | NA |
| 69 | PBS buffer | 300 × 10−12 M | Turn-on fluorescence | Done |
| 70 | HEPES buffer | 40 × 10−9 M | Turn-on fluorescence | NA |
| 71 | EtOH–water (5/5, v/v, HEPES pH = 7.4) | 55 × 10−9 M | Turn-on fluorescence | NA |
| 72 | CH3CN–HEPES (10 mM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) 4, v/v) |
12 × 10−9 M | Turn-on fluorescence | Done |
2.4 Polymer-based sensors for the detection of Hg2+
Above we have discussed small-molecule systems to detect Hg2+ ion in environmental sources and biological systems. Among the sensors (1–72), only a few compounds can detect Hg2+ in pure water or buffer medium. To overcome this issue, polymeric sensors were introduced for this detection process. Not only their water solubility but also the presence of many repeating units throughout the backbone, means polymers can produce highly amplified intensity to which can be attributed their high sensitivity.129–134 Due to these advantageous features, polymeric materials have been developed in recent times for the detection of analytes such as Hg2+. In this section, we are going to discuss some polymeric materials which were applied for the detection of Hg2+ ion under different conditions.A dithioacetal-based conjugated polymeric sensor 73135 was developed for detecting Hg2+ in THF medium with high selectivity and sensitivity. Initial green fluorescence turned into red emission upon the addition of Hg2+ to a solution of 73. The intramolecular charge transfer process was inhibited in the dithioacetal containing 73, but Hg2+ promoted deprotection of dithioacetal and the formation of –CHO group again triggered the ICT process to give red emission (Fig. 38). This visual color change of fluorescence for 73 can be used as a good tool for detecting Hg2+ with a detection limit of 1 μM.
Another polymeric sensor 74,136 consisting of a hydrophilic segment and a functionalized BODIPY segment, was developed for the detection of Hg2+. A 4-amino phenol functionalized BDIPY unit used as the responsible site for Hg2+ sensing. Due to the water solubility of random polymer 74, it can detect Hg2+ in a pure water medium. The appearance of a brown color and intense green emission was observed after the addition of Hg2+ to the solution of 74. This drastic change in color and emission was due to the hydrolysis of the imine bond by Hg2+ and the formation of an aldehyde group (Fig. 38). Sensor 74 has an LOD of 1.10 μM.
Sensor 75137 is a thermoresponsive diblock copolymer with colorimetric detection ability towards Hg2+ ions. Polymeric sensor 75 has an LCST value of ∼55 °C and is molecularly soluble in water. Below the LCST, PHPDEA units are exposed to water. Upon the addition of Hg2+, the exposed PHPDEA unit is hydrolyzed to form the strong electron-withdrawing group cyanide. With this formation of a strong –CN group (Fig. 39), the intramolecular charge transfer process was maximized and an obvious sudden color change to pink from yellow was observed. But above LCST, 75 was unable to detect Hg2+ ions as the HPDEA units were then inside the hydrophobic core of the multi-molecular micelles. Sensor 75 was demonstrated as an excellent highly selective tool for the detection Hg2+ colorimetrically with a detection limit of 0.03 mM.
Further stimuli-responsive polymeric sensors 76a and 76b were developed for the detection of Hg2+ in a colorimetric way with pH-tunable sensitivity.138 Sensor 76a was developed by the protection of the aldehyde group of the azo-polymer with ethanethiol and 76b was developed by the protection of the aldehyde of the azo-polymer with 3-marcaptopropionic acid. These azo-polymer derivatives can detect Hg2+ in a HEPES buffer solution of pH 7.4 in a colorimetric fashion. But, interestingly, 76a detected Hg2+ in ∼60 minutes and 76b in ∼15 minutes at pH = 7.4. So the deprotection of dithioacetal promoted by Hg2+ was 4 times faster in the case of 76b compared to 76a due to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Hg interaction, which further triggered the C⋯S–Hg cleavage which leads to aldehyde formation. But in the case of pH = 11, 76b showed a ∼60 minute reaction time in the presence of excess of Hg2+ ions, which suggested the pH-tunable sensitivity of the sensors. Ultimately the pH-dependent deprotection of dithioacetal was the main mechanism (Fig. 39) for the colorimetric detection ability of both sensors (76a and 76b).
O⋯Hg interaction, which further triggered the C⋯S–Hg cleavage which leads to aldehyde formation. But in the case of pH = 11, 76b showed a ∼60 minute reaction time in the presence of excess of Hg2+ ions, which suggested the pH-tunable sensitivity of the sensors. Ultimately the pH-dependent deprotection of dithioacetal was the main mechanism (Fig. 39) for the colorimetric detection ability of both sensors (76a and 76b).
A set of sensors 77a and 77b were developed as PEGylated BODIPY polymers.139 The presence of a dithia-dioxa-aza cyclopentadecane ligand to the BODIPY core triggered the ICT process and both sensors were non-emissive. Due to the presence of PEG units, 77a and 77b can be used for the detection of Hg2+ ions in pure water (Fig. 40). In the presence of Hg2+, a drastic change in color and enhanced fluorescence were observed in both 77a and 77b. A yellow emission with a pink color to the naked eye for 77a and red emission with blue color to the naked eye for 77b were observed in the presence of Hg2+. Complexation of Hg2+ with the dithia-dioxa-aza cyclopentadecane ligand leads to the color and emission change. Due to the intense and bright emissive nature of 77a and 77b, they can be used at the cellular level to detect Hg2+ ions. The LOD value for 77a is 8.1 ppb and that for 77b is 129.3 ppb.
A tryptophan-dithiocarbamate-based fluorogenic polymeric probe 78140 was developed for the selective detection and removal of Hg2+ ions. In pure aqueous medium, 78 was able to detect Hg2+ by an enhancement in fluorescence signal at 366 nm due to the inhibition of the PET process. Due to the presence of a dithiocarbamate unit, it can bind the Hg2+ ion and stop the PET process to give the fluorescence signal (Fig. 41). With a detection limit of 1.5 nM, sensor 78 is able to detect Hg2+ at the cellular level. Another water-soluble copolymer 79141 was developed with a tryptophan unit and a pyridine segment attached through an imine bond. Due to the complexation of Hg2+ through the N atoms of the imine bond and pyridine units, quenching of fluorescence was observed (Fig. 41). This quenched fluorescence of 79 was used to detect Hg2+ ions at the cellular level with an LOD value of 7.41 nM.
Another BODIPY-based thiosemicarbazone moiety containing polymeric sensor 80a142a was developed for detecting Hg2+ ions in a pure aqueous (HEPES buffer solution, pH = 7.4) medium. Upon the addition of Hg2+, a solution of 80a showed yellow emission from fluorescence-off mode. The binding of Hg2+ ions with two thiosemicarbazone groups of nearby polymeric chains stopped the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and resulted in a yellow fluorescence (Fig. 42). Complexation of Hg2+ with 80a resulted in a precipitate to remove Hg2+ successfully from the contaminated water source. Highly selective polymeric sensor 80a has a detection limit of 0.36 μM and the capability to remove mercury.
N isomerization and resulted in a yellow fluorescence (Fig. 42). Complexation of Hg2+ with 80a resulted in a precipitate to remove Hg2+ successfully from the contaminated water source. Highly selective polymeric sensor 80a has a detection limit of 0.36 μM and the capability to remove mercury.
For the first time a chitosan thiomer, 80b142b has been synthesized successfully and applied for the colorimetric detection of Hg2+ in aqueous solution. As Hg2+ has strong affinity towards thiol groups, 80b turned out to be an excellent binding material for it. Due to the formation of an S–Hg–S bond (Fig. 42), a drastic change in colour is observed from colorless to yellow to light brown to brown. This polymeric material has also been applied for removal of Hg2+ ions due to strong binding. Polymer 80b has been developed as an excellent sensor material with an ultra-low LOD of 0.465 ppb.
In this section, we have discussed polymeric sensors for the detection of Hg2+ ions. In recent years polymeric sensors have been developed to treat the analyte in pure water, which can be achieved by the synthesis of functionalized polymers with hydrophilic and hydrophobic segments. A summary of the information about polymeric sensors (73–80b) is given in Table 4.
| Compound | Medium | LOD | Type of sensing | Biological study |
|---|---|---|---|---|
| 73 | THF | 1 × 10−6 M | Turn-on fluorescence | NA |
| 74 | Pure water | 1.1 × 10−6 M | Turn-on fluorescence | NA |
| 75 | Water | 0.03 × 10−3 M | Colorimetric | NA |
| 76a and 76b | Pure water | NA | Colorimetric | NA |
| 77a | Pure water | 8.1 ppb | Turn-on fluorescence and colorimetric | Done |
| 77b | Pure water | 129.3 ppb | Turn-on fluorescence and colorimetric | Done |
| 78 | Pure water | 1.5 × 10−9 M | Turn-on fluorescence | Done |
| 79 | Pure water | 7.41 × 10−9 M | Turn-off fluorescence | Done |
| 80a | HEPES buffer | 0.37 × 10−6 M | Turn-on fluorescence | NA |
| 80b | Aqueous medium | 0.465 ppb | Colorimetric | NA |
We have now discussed the development and implementation of different types of detection systems based on heteroatom-containing ligand systems, reaction-based systems, and polymeric systems, for sensing Hg2+ ion in various sources.
3. Fluorescent and colorimetric detection systems for As(III)
The most toxic form of arsenic is the +3-oxidation state. In groundwater, arsenic exists as arsenite (As(III)) and arsenate (As(V)), though the arsenite form is more toxic than As(V). Except for conventional methods, rapid detection techniques using color change or emission change are very rare in number. In this section, we are going to discuss some of the chemical sensors which have been used to detect As(III) in a colorimetric way or a fluorometric way.A combination of boron trifluoride diethyletherate ((C2H5)2OBF3) and curcumin produced sensor 81143 for the selective detection of As(III) colorimetrically. A solution of 81 in 60% ethanol showed an orange color, but upon addition of As(III), it turned blue. This redshift in absorbance spectra was observed due to the deprotonation of the hydroxyl groups by As(III) to promote the delocalization of the negative charges towards the acceptor part (Fig. 43). This prominent change in color in the presence of As(III) can be used for the selective detection of As(III) with an LOD of 0.26 μM. Amberlite XAD-2 resin coated with 81 was used for the detection of As(III) in a solid phase with an LOD of 3 × 10−5 M.
Two isatin-appended Schiff bases 82a and 82b144 were developed for the colorimetric detection of As(III). DMSO solutions of 82a and 82b showed a brown color but upon adding AsO2−, they turned blue with absorption maxima of 600 nm and 624 nm, respectively. The formation of a charge-transfer complex between As(III) and 82a/82b resulted in the changed colors. For both sensors As(III) was bound through C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N segments, as depicted in Fig. 43. In a colorimetric fashion, 82a and 82b can be used as selective sensors for As(III) detection with detection limits of 0.26 ppm and 0.56 ppm.
N segments, as depicted in Fig. 43. In a colorimetric fashion, 82a and 82b can be used as selective sensors for As(III) detection with detection limits of 0.26 ppm and 0.56 ppm.
Sensor 83,145 a combination of 2,6-diformyl-p-cresol and 4-aminoantipyrine was developed for the selective detection of arsenite in colorimetric and fluorometric fashion. A HEPES buffer (1 mM, pH 7.4; water/DMSO (v/v), 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of 83 showed no color and no emission due to the strong PET effect originating from the hydroxyl group. Addition of AsO33− ions to the solution of 83 produced a light-yellow greenish color and green emission. This drastic and prominent change in color and emission was the result of the binding of arsenite with 83, which disturbed the PET process and triggered the CHEF process via intermolecular hydrogen bonding (Fig. 44). Its turn-on fluorescence feature was used effectively for intracellular tracking of arsenite. The dual-mode sensing process and excellent LOD value of 4.12 made 83 a potential tool in the field of rapid and effective arsenite detection.
1) solution of 83 showed no color and no emission due to the strong PET effect originating from the hydroxyl group. Addition of AsO33− ions to the solution of 83 produced a light-yellow greenish color and green emission. This drastic and prominent change in color and emission was the result of the binding of arsenite with 83, which disturbed the PET process and triggered the CHEF process via intermolecular hydrogen bonding (Fig. 44). Its turn-on fluorescence feature was used effectively for intracellular tracking of arsenite. The dual-mode sensing process and excellent LOD value of 4.12 made 83 a potential tool in the field of rapid and effective arsenite detection.
Condensation between rhodamine 6G hydrazide and 5-methyl salicylaldehyde produced sensor 84.146 In acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES buffer (4
HEPES buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) solution, 84 did not show any color or emission due to the spirolactam ring-closed form. After the addition of As(III), a solution of 84 turned pink in color with an intense yellow emission. Spirolactam ring-opening followed by strong 1
1, v/v, pH 7.4) solution, 84 did not show any color or emission due to the spirolactam ring-closed form. After the addition of As(III), a solution of 84 turned pink in color with an intense yellow emission. Spirolactam ring-opening followed by strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between As(III) and 84 resulted in the observed color and emission change (Fig. 45). Due it turn-on fluorescence, 84 was also used for detecting As(III) at the cellular level. Dual-channel sensor 84 emerged as an excellent material for the selective detection of As(III) with an LOD of 0.164 ppb.
1 binding between As(III) and 84 resulted in the observed color and emission change (Fig. 45). Due it turn-on fluorescence, 84 was also used for detecting As(III) at the cellular level. Dual-channel sensor 84 emerged as an excellent material for the selective detection of As(III) with an LOD of 0.164 ppb.
An aggregation-induced emission-based sensor 85 was developed using tetraphenylethene (TPE) as a fluorescent moiety and cysteine as a binding ligand for As(III).147 An aqueous solution (THF–water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) of 85 (Fig. 45) was nonfluorescent, but upon addition of As(III) an intense blue fluorescence was observed and it gradually became more intense with an increasing concentration of As(III). Binding of As(III) with the –SH group of cysteine of 85 through the formation of As–S bond promoted the π–π stacking of TPE units to produce the intense blue emission which is typical of the AIE process. Sensor 85 has a detection limit of 0.5 ppb, which is much lower than the WHO limit.
99, v/v) of 85 (Fig. 45) was nonfluorescent, but upon addition of As(III) an intense blue fluorescence was observed and it gradually became more intense with an increasing concentration of As(III). Binding of As(III) with the –SH group of cysteine of 85 through the formation of As–S bond promoted the π–π stacking of TPE units to produce the intense blue emission which is typical of the AIE process. Sensor 85 has a detection limit of 0.5 ppb, which is much lower than the WHO limit.
An oxime-based fluorogenic probe 86148 was applied for the detection of arsenite with turn-on fluorescence. In a pure aqueous medium of pH 7.24, 86 did not show any prominent fluorescence signal, but upon the introduction of AsO2− a strong blue emission was observed. This turn-on fluorescence signal helped to detect AsO2− in pure aqueous medium with a detection limit of 1.32 μM. The output fluorescence signal was the result of H-bonding interaction between AsO2− and 86 (Fig. 46). This H-bonding interaction leads to the formation of different nano/microstructures in pure aqueous solution. Sensor 86 was successfully utilized for cell imaging in the presence of AsO2− ions. This sensor suffered from a selectivity issue, as it showed similar fluorescence properties in the presence of arsenate as well. Along with the sensitivity issue, the LOD value of 86 towards AsO2− is not up to the mark compared to the WHO level.
A colorimetric probe 87 was designed as a benzothiazole Schiff base for the highly sensitive and selective detection of As3+ ions.149 Sensor 87 showed a light yellow color in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium. After the addition of As3+ ions, a solution of 87 turned brownish orange. Due to strong 1
1) medium. After the addition of As3+ ions, a solution of 87 turned brownish orange. Due to strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between 87 and As3+ (Fig. 46), a strong change in color was observed. But unfortunately, the same characteristics were observed when 87 was treated with As5+ with the same absorbance spectrum. Though the drastic change in color was helpful for recognizing As3+ ions, the selectivity issue was a drawback for this system. It can be used for detecting arsenic (As3+/As5+) in water but cannot be used for the selective detection of As3+. Sensor 87 suffered from selectivity issues, but the detection limit was excellent with an LOD value of 7.2 ppb for As3+ ions.
1 binding between 87 and As3+ (Fig. 46), a strong change in color was observed. But unfortunately, the same characteristics were observed when 87 was treated with As5+ with the same absorbance spectrum. Though the drastic change in color was helpful for recognizing As3+ ions, the selectivity issue was a drawback for this system. It can be used for detecting arsenic (As3+/As5+) in water but cannot be used for the selective detection of As3+. Sensor 87 suffered from selectivity issues, but the detection limit was excellent with an LOD value of 7.2 ppb for As3+ ions.
A chromogenic–fluorescence probe 88150 was applied for the detection of As3+ along with Cr3+, Fe3+, Al3+, Ga3+, and In3+ ions. In a water–MeCN (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) solution, in the presence of As3+, the color of 88 changed to violet from colorless along with a quenching of fluorescence. For all other trivalent cations as well, the same observation was found. It was hypothesized that cation-induced dehydration formed a highly conjugated cationic molecule and that led to the color change (Fig. 47). Sensor 88 has LODs of 10.4 μM (UV-vis) and 10.7 μM (fluorescence). Though the color change is drastic in the case of 88, it suffered from low selectivity towards As3+ and a low detection limit. Due to these issues, 88 cannot be used to separately detect As3+ ions. Similarly, another sensor 89 was developed by the combination of 2-hydroxy-1-naphthaldehyde and thiosemicarbazide.151 With no color or emission in DMF–water (9
5, v/v) solution, in the presence of As3+, the color of 88 changed to violet from colorless along with a quenching of fluorescence. For all other trivalent cations as well, the same observation was found. It was hypothesized that cation-induced dehydration formed a highly conjugated cationic molecule and that led to the color change (Fig. 47). Sensor 88 has LODs of 10.4 μM (UV-vis) and 10.7 μM (fluorescence). Though the color change is drastic in the case of 88, it suffered from low selectivity towards As3+ and a low detection limit. Due to these issues, 88 cannot be used to separately detect As3+ ions. Similarly, another sensor 89 was developed by the combination of 2-hydroxy-1-naphthaldehyde and thiosemicarbazide.151 With no color or emission in DMF–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, HEPES, pH = 7.2), 89 changed its color to yellow with a unique emission at 495 nm upon addition of AsO2− ions. A strong hydrogen bonding interaction between AsO2− and the –OH and –NH groups of 89 leads to a color and emission change (Fig. 47). Eventually, 89 could be a useful sensor with an LOD value of 66 nM but it suffers from the selectivity issue as it can also detect CN− ions with the same spectroscopic properties.
1, v/v, HEPES, pH = 7.2), 89 changed its color to yellow with a unique emission at 495 nm upon addition of AsO2− ions. A strong hydrogen bonding interaction between AsO2− and the –OH and –NH groups of 89 leads to a color and emission change (Fig. 47). Eventually, 89 could be a useful sensor with an LOD value of 66 nM but it suffers from the selectivity issue as it can also detect CN− ions with the same spectroscopic properties.
Another hydrazine-based thiocarbamide chemosensor 90 was applied for the colorimetric and fluorometric detection of AsO33− ions.152 Sensor 90 detected AsO33− ions in MeCN–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.2) by changing the color from colorless to yellow and non-fluorescent to yellow fluorescence. Here also the H-bonding interaction between the anion, the –NH group of thiocarbamide segment, and the Ar–OH group was the reason behind the change in color as well as in fluorescence (Fig. 48). The dual-sensing properties of 90 with an excellent detection limit of 15 nM meant it emerged as a good tool for detecting As(III) in real applications as it was able to detect the analyte in contaminated real samples. It can also detect phosphate ions with the same color and emission change as AsO33−, so there might be interference.
1, v/v, pH = 7.2) by changing the color from colorless to yellow and non-fluorescent to yellow fluorescence. Here also the H-bonding interaction between the anion, the –NH group of thiocarbamide segment, and the Ar–OH group was the reason behind the change in color as well as in fluorescence (Fig. 48). The dual-sensing properties of 90 with an excellent detection limit of 15 nM meant it emerged as a good tool for detecting As(III) in real applications as it was able to detect the analyte in contaminated real samples. It can also detect phosphate ions with the same color and emission change as AsO33−, so there might be interference.
Another fluorogenic probe 91 was designed by the combination of pyrene and calix[4]arene for detecting As3+.153 Due to the presence of an amide group in the system, it can be a catalysis binding site as well as helping the CHEF process. In the presence of As3+ ions, the fluorescence intensity of 91 decreased, as a result of binding between the sensor and analyte for activation of the PET process (Fig. 48). This turn-off fluorescence of 91 was used to analyze real samples contaminated by As3+, as the sensor has an LOD value of 11.53 nM for As3+. Besides the excellent sensitivity of 91 towards As3+, selectivity was a problem as the sensor was able to detect Nd3+ and Br− by turn-on and turn-off fluorescence, respectively, under the same experimental conditions.
A colorimetric sensor 92 was developed with condensation between 2,4-dinitrophenyl hydrazine and 2,4-dihydroxy benzaldehyde.154 Due to the presence of 2,4-dinitrophenyl hydrazine, it can act as an H-bonding formation segment with some unique chromophoric features. Sensor 92 (Fig. 49) showed different colors in water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and water–MeCN (9
1) and water–MeCN (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures. The color of 92 in water–DMSO solution changed to purple from orange in the presence of As3+ but in the case of a water–MeCN solution, it changed from yellow to red. The presence of Ar–OH and –NH groups in the sensor was attributed to the strong bonding of As3+ by H-bonding interaction which resulted in a drastic and intense color change. So, sensor 92 can serve as a good naked-eye sensor with high selectivity and an LOD value of 0.35 × 10−6 M for As3+ ions.
1) mixtures. The color of 92 in water–DMSO solution changed to purple from orange in the presence of As3+ but in the case of a water–MeCN solution, it changed from yellow to red. The presence of Ar–OH and –NH groups in the sensor was attributed to the strong bonding of As3+ by H-bonding interaction which resulted in a drastic and intense color change. So, sensor 92 can serve as a good naked-eye sensor with high selectivity and an LOD value of 0.35 × 10−6 M for As3+ ions.
 | ||
| Fig. 49 The chemical structures of 92, 93, and 94, and the proposed sensing mechanisms of 93 and 94. | ||
Two coumarin-based sensors 93155 and 94156 were designed as coumarin-appended benzothiazolines for the detection of As(III) in organic media. Both sensors were able to detect As(III) in THF medium by turn-on fluorescence. It was expected that As(III) coordination with the Schiff-base thiolate form of the sensors followed by the formation of benzothiazole was the reason behind the turning on of emission in both cases (Fig. 49). Both sensors were able to detect As(III) with high selectivity in pure THF medium. Sensor 94 was able to sense inorganic As(III) with an LOD value of 0.14 ppb. Similarly, sensor 93 was also extremely sensitive towards As(III) with a detection limit of 0.24 ppb. But both sensors were able to sense As(III) in an organic medium, which could be a drawback for them.
A norbornene-derived monomer 95a and its homopolymer 95b were developed for detecting As(III), where rhodamine B was used as the signaling unit.157 Both the monomer and polymer solution in methanol–water solution remained colorless as well as non-emissive due to the spirolactam ring-closed form of the rhodamine unit. In the presence of KIO3 and HCl, the solution of 95a turned pink in color and reddish in emission due to the opening of the spirolactam ring. After the addition of As(III) solution, the pH changed from 1.34 to 4.23, and the color of the solution changed to brown, and the emission changed to greenish. In the presence of KIO3 and HCl, As(III) oxidized to As(V) and generated I2 in the solution. This I2 further reacted with the double bond of the norbornene unit, and as a result, the color and fluorescence were changed (Fig. 50). A similar mechanism was followed by polymer 95b. The oxidation process was further supported by cyclic voltammetry which suggested that two-electron oxidation took place during the sensing process. Paper strips coated with polymer 95b were successfully used for the “in-field” detection of As(III) in water sources. So it is clear that 95a and 95b are unique for their selective detection of As(III) in both colorimetric and fluorometric processes in a novel mechanistic pathway. Sensor 95a has a detection limit of 200 nM for As(III) ions.
We have discussed reported chemical sensors for the detection of As(III) in different mediums with different mechanisms. Most of the sensors preferably interact with As(III) to produce a new signal for the identification of the analyte. All the information about sensors 81–95b is given in Table 5.
| Compound | Medium | LOD | Type of sensing | Biological study |
|---|---|---|---|---|
| 81 | 60% ethanol | 3 × 10−5 M | Colorimetric | NA |
| 82a | DMSO | 0.26 ppm | Colorimetric | NA |
| 82b | DMSO | 0.56 ppm | Colorimetric | NA |
| 83 | Water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) 1, v/v, pH = 7.4) |
4.12 ppb | Colorimetric and turn-on fluorescence | NA |
| 84 | MeCN–HEPES (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.4) 1, v/v, pH = 7.4) |
0.164 ppb | Colorimetric and turn-on fluorescence | Done |
| 85 | THF–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) 99, v/v) |
0.5 ppb | Turn-on fluorescence | NA |
| 86 | Pure aq. medium | 1.32 × 10−6 M | Turn-on fluorescence | Done |
| 87 | DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
7.2 ppb | Colorimetric | NA |
| 88 | Water–MeCN (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) 5, v/v) |
10.4 × 10−6 M (UV), 10.7 × 10−6 M (FL) | Colorimetric and turn-on fluorescence | NA |
| 89 | DMF–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, HEPES, pH = 7.2) 1, v/v, HEPES, pH = 7.2) |
66 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
| 90 | MeCN–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.2) 1, v/v, pH = 7.2) |
15 × 10−9 M | Turn-on fluorescence and colorimetric | NA |
| 91 | MeCN–PBS (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, pH = 7.2) 2, v/v, pH = 7.2) |
11.53 × 10−9 M | Turn-off fluorescence | NA |
| 92 | Water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
0.35 × 10−6 M | Colorimetric | NA |
| 93 | THF | 0.24 ppb | Turn-on fluorescence | NA |
| 94 | THF | 0.14 ppb | Turn-on fluorescence | NA |
| 95a | MeOH–water | 200 × 10−9 M | Colorimetric and turn-on fluorescence | NA |
4. Conclusions and perspectives
Due to the toxic extent of Hg2+ and As(III) in humans and in different biological systems, these ions have received extensive interest from researchers with the aim of creating detection tools. The initial section of our review covered reported colorimetric and fluorescence sensors for Hg2+ developed after 2015. Recognition of Hg2+ ions was achieved successfully by ligand-based systems and rhodamine systems; many of the systems suffered from selectivity issues, but most of them are reversible. This reversible nature can be used for the reuse of the sensor for detection purposes, which is a great advantage. These selectivity issues have been overcome by the development of reaction-based sensor systems where Hg2+-mediated new molecule formation leads to output signals. Unfortunately, most of the small molecule sensors we have discussed here suffer from low solubility in water. Despite the solubility issues, many of these have excellent sensitivity, with LOD values lower than the permissible concentration of Hg2+ set by the EPA/WHO. However, solubility issues have been taken care of by introducing polymeric materials, where different hydrophilic segments were used to increase the water solubility of the sensors. Despite the water solubility of the polymeric materials, the sensitivity of these sensors is not up to the mark compared to small molecular sensors. Many of the discussed sensors were able to successfully track Hg2+ ions in biological systems with a distinct emission change, which is a new development in the field of mercury sensors. Apart from Hg2+, some of the sensors discussed here can detect CH3Hg+ in both environmental samples and biological systems, which is an impressive development and opens up a new area for the design and development of new sensors. On the other hand, the development of As(III) ion sensors is still very rare due to their complicated chemical behavior. We have tried to cover most of the available chemical sensors for As(III) in this review. Most of the sensors for As(III) are based on H-bonding interactions, which leads to a color change or fluorescence change. Unfortunately, most of the discussed sensors suffer from interference from other trivalent metal ions or anions with the same output signal. This interference is harsh in the case of the distinct identification of As(III), either in cationic form or in anionic form. Apart from the selectivity viewpoint, most of the sensors have a detection limit way below the WHO/EPA guidelines.For both toxic ions, different new methods have been developed in the last decade with new strategies, new applications, etc. We believe that in the case of Hg2+ ion detection, polymeric sensors can evolve as an excellent system due to their many advantages, such as signal amplification (as many repeating units are present), water solubility, and the stability of the probes. The detection limits should be improved for better implementation of “in-field” applications of polymeric materials. On the other hand, in the area of As(III) detection, there is a huge chance for improvement with respect to the design and development of sensors. For a more useful toolkit for As(III) detection, highly selective and sensitive sensors are in high demand to date. More and more development of polymeric sensors for As(III) detection is needed for device fabrication to provide a permanent solution for societies that are greatly affected by arsenic poisoning.
List of abbreviations
| LED | Light-emitting diode |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| THF | Tetrahydrofuran |
| UV-vis | Ultraviolet-visible |
| LCST | Lower critical solution temperature |
| HEPES | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| PBS | Phosphate-buffered saline |
| BODIPY | Boron-dipyrromethene |
| NBD | 7-Nitrobenz-2-oxa-1,3-diazole |
| NIR | Near-infrared |
| ICT | Intramolecular charge transfer |
| PET | Photoinduced electron transfer |
| FRET | Förster resonance energy transfer |
| ESIPT | Excited-state intramolecular proton transfer |
| AIEE | Aggregation induced enhanced emission |
| AIE | Aggregation induced emission |
| CHEF | Chelation-enhanced fluorescence |
| TICT | Twisted intramolecular charge transfer |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
T. S. thanks IISER Kolkata for a research fellowship. R. S. thanks DST, DBT, IISER Kolkata for funding. T. S. and R. S. thank IISER Kolkata for infrastructure support.References
- G. Banfalvi, Cellular Effects of Heavy Metals, Springer, Netherlands, London, New York, 2011 Search PubMed.
- N. Herawati, S. Suzuki, K. Hayashi, I. F. Rivai and H. Koyoma, Cadmium, copper and zinc levels in rice and soil of Japan, Indonesia and China by soil type, Bull. Environ. Contam. Toxicol., 2000, 64, 33–39 CrossRef CAS.
- S. Sarkar and R. Shunmugam, Unusual redshift of the sensor while detecting the presence of Cd2+ in aqueous environment, ACS Appl. Mater. Interfaces, 2013, 5, 7379–7383 CrossRef CAS.
- W. Salomons, U. Forstner and P. Mader, Heavy Metals: Problems and Solutions, Springer-Verlag, Berlin, Germany, 1995 Search PubMed.
- S. Bhattacharya, V. N. Rao, S. Sarkar and R. Shunmugam, Unusual emission from norbornene derived phosphonate molecule-a sensor for FeIII in aqueous environment, Nanoscale, 2012, 4, 6962–6966 RSC.
- World Health Organization, Guidelines for drinking-water quality, Geneva, 3rd edn, 2004, vol. 1, p. 188 Search PubMed.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Heavy metal toxicity and the environment, Molecular, clinical and environmental toxicology, Springer, Basel, 2012, pp. 133–164 Search PubMed.
- (a) J. C. Saha, A. K. Dikshit, M. Bandyopadhyay and K. C. Saha, A review of arsenic poisoning and its effects on human health, Crit. Rev. Environ. Sci. Technol., 1999, 29, 281–313 CrossRef CAS; (b) K. Christen, Environ. Sci. Technol, 2001, 35, 184–185 CrossRef.
- S. Kapaj, H. Peterson, K. Liber and P. Bhattacharya, Human Health Effects from Chronic Arsenic Poisoning – A Review, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 2399–2428 CrossRef CAS.
- K. S. M. Abdul, S. S. Jayasinghe, E. P. Chandana, C. Jayasumana and P. M. C. De Silva, Arsenic and human health effects: A review, Environ. Toxicol. Pharmacol., 2015, 40, 828–846 CrossRef.
- A. H. Smith, C. Hopenhayn-Rich, M. N. Bates, H. M. Goeden, I. Hertz-Picciotto, H. M. Duggan, R. Wood, M. J. Kosnett and M. T. Smith, Cancer risks from arsenic in drinking water, Environ. Health Perspect., 1992, 97, 259–267 CrossRef CAS.
- W. R. Cullen and K. J. Reimer, Arsenic speciation in the environment, Chem. Rev., 1989, 89, 713–764 CrossRef CAS.
- N. Bhandari, R. J. Reeder and D. R. Strongin, Photoinduced oxidation of arsenite to arsenate in the presence of goethite, Environ. Sci. Technol., 2012, 46, 8044–8051 CrossRef CAS.
- United States Environmental Protection Agency Arsenic and Clarifications to Compliance and New Source Monitoring Rule: A Quick Reference Guide, EPA 816-F-01-004, US EPA, Wasington, D.C., 2001.
- B. Weiss, Why methylmercury remains a conundrum 50 years after Minamata, Toxicol. Sci, 2007, 97, 223–225 CrossRef CAS.
- L. Amin-Zaki, S. Elhassani, M. A. Majeed, T. W. Clarkson, R. A. Doherty and M. Greenwood, Intra-uterine methylmercury poisoning in Iraq, Problems of Birth Defects, Springer, Dordrecht, 1974, pp. 233–241 Search PubMed.
- F. Di Natale, A. Lancia, A. Molino, M. Di Natale, D. Karatza and D. Musmarra, Capture of mercury ions by natural and industrial materials, J. Hazard. Mater., 2006, 132, 220–225 CrossRef CAS.
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Marine biogeochemical cycling of mercury, Chem. Rev., 2007, 107, 641 CrossRef CAS.
- T. W. Clarkson, L. Magos and G. J. Myers, The toxicology of mercury-current exposures and clinical manifestations, N. Engl. J. Med., 2003, 349, 1731–1737 CrossRef CAS.
- R. Von Burg, Inorganic mercury, J. Appl. Toxicol., 1995, 15, 483–493 CrossRef CAS.
- R. Shunmugam, G. J. Gabriel, C. E. Smith, K. A. Aamer and G. N. Tew, A highly selective colorimetric aqueous sensor for mercury, Chem. – Eur. J., 2008, 14, 3904–3907 CrossRef CAS.
- J. Chen, J. Pan and S. Chen, A Naked-Eye Colorimetric Sensor for Hg2+ Monitoring with Cascade Signal Amplification Based on Target Induced Conjunction of Split DNAzyme Fragments, Chem. Commun., 2017, 53, 10224–10227 RSC.
- V. Iyengar and J. Woittiez, Trace elements in human clinical specimens: evaluation of literature data to identify reference values, Clin. Chem., 1988, 34, 474–481 CrossRef CAS.
- A. T. Townsend, K. A. Miller, S. McLean and S. Aldous, The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS, J. Anal. At. Spectrom., 1998, 13, 1213–1219 RSC.
- X. C. Le, M. Ma and N. A. Wong, Speciation of arsenic compounds using high-performance liquid chromatography at elevated temperature and selective hydride generation atomic fluorescence detection, Anal. Chem., 1996, 68, 4501–4506 CrossRef CAS.
- C. B’Hymer and J. A. Caruso, Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry, J. Chromatogr. A, 2004, 1045, 1–13 CrossRef.
- K. C. Thompson and R. J. Reynolds, Atomic Absorption. Fluorescence and Flame Emission Spectroscopy, Charles Griftin, London, 2nd edn, 1978, vol. 51, pp. 175–176 Search PubMed.
- J. L. Gomez-Ariza, D. Sánchez-Rodas, I. Giráldez and E. Morales, A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples, Talanta, 2000, 51, 257–268 CrossRef CAS.
- T. Nakazato and H. Tao, A high-efficiency photooxidation reactor for speciation of organic arsenicals by liquid chromatography – hydride generation – ICPMS, Anal. Chem., 2006, 78, 1665–1672 CrossRef CAS.
- M. Mulvihill, A. Tao, K. Benjauthrit, J. Arnold and P. Yang, Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water, Angew. Chem., 2008, 120, 6556–6560 CrossRef.
- H. M. Anawar, Arsenic Speciation in Environmental Samples by Hydride Generation and Electrothermal Atomic Absorption Spectrometry, Talanta, 2012, 88, 30–42 CrossRef CAS.
- W. Holak, Determination of Arsenic by Cathodic Stripping Voltammetry with a Hanging Mercury Drop Electrode, Anal. Chem., 1980, 52, 2189–2192 CrossRef CAS.
- S. S. Hassan, A. R. Sirajuddin, T. G. Solangi, M. S. Kazi, Y. Kalhoro, Z. Junejo, A. Tagar and N. H. Kalwar, Nafion Stabilized Ibuprofen-Gold Nanostructures Modified Screen Printed Electrode as Arsenic(III) Sensor, J. Electroanal. Chem., 2012, 682, 77–82 CrossRef CAS.
- Y. Zhou, Z. Xu and J. Yoon, Fluorescent and colorimetric chemosensors for detection of nucleotides, FAD and NADH: highlighted research during 2004–2010, Chem. Soc. Rev., 2011, 40, 2222–2235 RSC.
- D. T. Quang and J. S. Kim, Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Fluorescent and colorimetric probes for detection of thiols, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions), Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- E. M. Nolan and S. J. Lippard, Tools and tactics for the optical detection of mercuric ion, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- G. Chen, Z. Guo, G. Zeng and L. Tang, Fluorescent and colorimetric sensors for environmental mercury detection, Analyst, 2015, 140, 5400–5443 RSC.
- M. B. Gumpu, S. Sethuraman, U. M. Krishnan and J. B. B. Rayappan, A review on detection of heavy metal ions in water–an electrochemical approach, Sens. Actuators, B, 2015, 213, 515–533 CrossRef CAS.
- M. Mulvihill, A. Tao, K. Benjauthrit, J. Arnold and P. Yang, Surface-enhanced Raman spectroscopy for trace arsenic detection in contaminated water, Angew. Chem., Int. Ed., 2008, 47, 6456–6460 CrossRef CAS.
- J. Hao, M. J. Han, S. Han, X. Meng, T. L. Su and Q. K. Wang, SERS detection of arsenic in water: A review, J. Environ. Sci., 2015, 36, 152–162 CrossRef CAS.
- E. Priyadarshini and N. Pradhan, Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review, Sens. Actuators, B, 2017, 238, 888–902 CrossRef CAS.
- M. Li, H. Gou, I. Al-Ogaidi and N. Wu, Nanostructured sensors for detection of heavy metals: a review, ACS Sustainable Chem. Eng., 2013, 1, 713–723 CrossRef CAS.
- L. Farzin, M. Shamsipur and S. Sheibani, A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals, Talanta, 2017, 174, 619–627 CrossRef CAS.
- A. Maity, A. Sil, S. Nad and S. K. Patra, A highly selective, sensitive and reusable BODIPY based ‘OFF/ON'fluorescence chemosensor for the detection of Hg2+ Ions, Sens. Actuators, B, 2018, 255, 299–308 CrossRef CAS.
- C. B. Bai, P. Xu, J. Zhang, R. Qiao, M. Y. Chen, M. Y. Mei, B. Wei, C. Wang, L. Zhang and S. S. Chen, Long-Wavelength Fluorescent Chemosensors for Hg2+ based on Pyrene, ACS Omega, 2019, 4, 14621–14625 CrossRef CAS.
- A. Kumar, R. Ananthakrishnan, G. Jana, P. K. Chattaraj, S. Nayak and S. K. Ghosh, An Intramolecular Charge Transfer Induced Fluorescent Chemosensor for Selective Detection of Mercury (II) and its Self-Turn-On Inside Live Cells at Physiological pH, ChemistrySelect, 2019, 4, 4810–4819 CrossRef CAS.
- B. Muzey and A. Naseem, An AIEE active 1, 8-naphthalimide-sulfamethizole probe for ratiometric fluorescent detection of Hg2+ ions in aqueous media, J. Photochem. Photobiol., A, 2020, 391, 112354 CrossRef CAS.
- Y. Yuan, X. Chen, Q. Chen, G. Jiang, H. Wang and J. Wang, New switch on fluorescent probe with AIE characteristics for selective and reversible detection of mercury ion in aqueous solution, Anal. Biochem., 2019, 585, 113403 CrossRef CAS.
- T. P. Yoon and E. N. Jacobsen, Privileged chiral catalysts, Science, 2003, 299, 1691–1693 CrossRef CAS.
- A. Dalla Cort, P. De Bernardin, G. Forte and F. Y. Mihan, Metal–salophen-based receptors for anions, Chem. Soc. Rev., 2010, 39, 3863–3874 RSC.
- K. Liu, Y. Zhang, T. Zhou, X. Liu, B. Chen, X. Wang, X. Zhao, J. Huo, Y. Wang and B. Zhu, Sequential recognition of Hg2+ and I− based on a novel BODIPY-salen sensor, Sens. Actuators, B, 2017, 253, 1194–1198 CrossRef CAS.
- N. Dey, J. Kulhanek, F. Bures and S. Bhattacharya, J. Org. Chem., 2019, 84, 1787–1796 CrossRef CAS.
- Y. Li, W. Shi, J. Ma, X. Wang, X. Kong, Y. Zhang, L. Feng, Y. Hui and Z. Xie, A novel optical probe for Hg2+ in aqueous media based on mono-thiosemicarbazone Schiff base, J. Photochem. Photobiol., A, 2017, 338, 1–7 CrossRef CAS.
- A. Kumar and N. Ahmed, Indirect Approach for CN–Detection: Development of “Naked-Eye” Hg2+-Induced Turn-Off Fluorescence and Turn-On Cyanide Sensing by the Hg2+ Displacement Approach, Ind. Eng. Chem. Res., 2017, 56, 6358–6368 CrossRef.
- K. M. Vengaian, C. D. Britto, K. Sekar, G. Sivaraman and S. Singaravadivel, Phenothiazine-diaminomalenonitrile based Colorimetric and Fluorescence “Turn-off-on” Sensing of Hg2+ and S2−, Sens. Actuators, B, 2016, 235, 232–240 CrossRef CAS.
- L. N. Neupane, P. K. Mehta, J. U. Kwon, S. H. Park and K. H. Lee, Selective red-emission detection for mercuric ions in aqueous solution and cells using a fluorescent probe based on an unnatural peptide receptor, Org. Biomol. Chem., 2019, 17, 3590–3598 RSC.
- M. L. Presti, R. Martínez-Máñez, J. V. Ros-Lis, R. M. Batista, S. P. Costa, M. M. M. Raposo and F. Sancenón, A dual channel sulphur-containing a macrocycle functionalised BODIPY probe for the detection of Hg (II) in a mixed aqueous solution, New J. Chem., 2018, 42, 7863–7868 RSC.
- A. K. Jha, S. Umar, R. K. Arya, D. Datta and A. Goel, Pyrano [3, 2-c] julolidin-2-ones: a novel class of fluorescent probes for ratiometric detection and imaging of Hg 2+ in live cancer cells, J. Mater. Chem. B, 2016, 4, 4934–4940 RSC.
- R. X. Zhang, P. F. Li, W. J. Zhang, N. Li and N. Zhao, A highly sensitive fluorescent sensor with aggregation-induced emission characteristics for the detection of iodide and mercury ions in aqueous solution, J. Mater. Chem. C, 2016, 4, 10479–10485 RSC.
- A. Tang, Z. Chen, D. Deng, G. Liu, Y. Tu and S. Pu, Aggregation-induced emission enhancement (AIEE)-active tetraphenylethene (TPE)-based chemosensor for Hg2+ with solvatochromism and cell imaging characteristics, RSC Adv., 2019, 9, 11865–11869 RSC.
- S. Gharami, K. Aich, P. Ghosh, L. Patra, N. Murmu and T. K. Mondal, A fluorescent “ON–OFF–ON” switch for the selective and sequential detection of Hg2+ and I− with applications in imaging using human AGS gastric cancer cells, Dalton Trans., 2020, 49, 187–195 RSC.
- Y. F. Xiao, L. Yu and B. Quan, A highly selective and sensitive fluorescein-based chemodosimeter for Hg2+ ions in aqueous media, Anal. Chim. Acta, 2007, 584, 95–100 CrossRef.
- K. M. K. Swamy, K. K. Soo, L. N. Ha, K. S. M. Shantha, K. S. Jong and Y. Juyoung, Fluorescent sensing of pyrophosphate and ATP in 100% aqueous solution using a fluorescein derivative and Mn2+, Tetrahedron Lett., 2007, 48, 8683–8686 CrossRef CAS.
- K. J. Hee, P. E. Ji, C. G. Myung, A. Sangdoo and C. K. Suk, Selective chromogenic and fluorogenic signalling of Hg2+ ions using a fluorescein-coumarin conjugate, Dyes Pigm., 2010, 84, 54–58 CrossRef.
- P. Piyanuch, S. Watpathomsub, V. S. Lee, H. A. Nienaber and N. Wanichacheva, Highly sensitive and selective Hg2+-chemosensor based on dithia-cyclic fluorescein for optical and visual-eye detections in aqueous buffer solution, Sens. Actuators, B, 2016, 224, 201–208 CrossRef CAS.
- R. V. Rathod, S. Bera, P. Maity and D. Mondal, Mechanochemical Synthesis of a Fluorescein-Based Sensor for the Selective Detection and Removal of Hg2+ Ions in Industrial Effluents, ACS Omega, 2020, 5, 4982–4990 CrossRef CAS.
- H. Mohammad, A. S. M. Islam, C. Prodhan and M. Ali, A fluorescein-based chemosensor for “turn-on” detection of Hg2+ and the resultant complex as a fluorescent sensor for S2− in semi-aqueous medium with cell-imaging application: experimental and computational studies, New J. Chem., 2019, 43, 5297–5307 RSC.
- N. Kaur, G. Jindal and S. Kumar, Cascade recognition of Hg2+ and cysteine using a naphthalene based ESIPT sensor and its application in a set/reset memorized device, New J. Chem., 2019, 43, 436–443 RSC.
- J. Wang, Q. Rao, H. Wang, Q. Zhang, G. Liu, Z. Wu, J. Yu, X. Zhu, Y. Tian and H. Zhou, A terpyridine-based test strip for the detection of Hg2+ in various water samples and drinks, Anal. Methods, 2019, 11, 227–231 RSC.
- P. G. Sutariya, H. Soni, S. A. Gandhi and A. Pandya, Luminescent behavior of pyrene-allied calix [4] arene for the highly pH-selective recognition and determination of Zn2+, Hg2+ and I− via the CHEF-PET mechanism: computational experiment and paper-based device, New J. Chem., 2019, 43, 9855–9864 RSC.
- T. Anand and S. K. Sahoo, Cost-effective approach to detect Cu (II) and Hg (II) by integrating a smartphone with the colorimetric response from a NBD-benzimidazole based dyad, Phys. Chem. Chem. Phys., 2019, 21, 11839–11845 RSC.
- X. Cao, Y. Li, Y. Yu, S. Fu, A. Gao and X. Chang, Nanoscale, 2019, 11, 10911–10920 RSC.
- D. Liu, H. Zhu, J. Shi, X. Deng, T. Zhang, Y. Zhao, P. Qi, G. Yang and H. He, A 1, 8-naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Hg2+ in aqueous solution and living cells, Anal. Methods, 2019, 11, 3150–3154 RSC.
- Y. Zhang, C. Zhang, Y. Wu, B. Zhao, L. Wang and B. Song, RSC Adv., 2019, 9, 23382–23389 RSC.
- S. Srivastava, N. Thakur, A. Singh, P. Shukla, V. K. Maikhuri, N. Garg, A. Prasad and R. Pandey, Development of a fused imidazo [1,2-a] pyridine based fluorescent probe for Fe3+ and Hg2+ in aqueous media and HeLa cells, RSC Adv., 2019, 9, 29856–29863 RSC.
- S. Sahana, G. Mishra, S. Sivakumar and P. K. Bharadwaj, A 2-(2′-hydroxyphenyl) benzothiazole (HBT)–quinoline conjugate: a highly specific fluorescent probe for Hg2+ based on ESIPT and its application in bioimaging, Dalton Trans., 2015, 44, 20139–20146 RSC.
- F. Bu, B. Zhao, W. Kan, L. Ding, T. Liu, L. Wang, B. Song, W. Wang and Q. Deng, An ESIPT characteristic “turn-on” fluorescence sensor for Hg2+ with large Stokes shift and sequential “turn-off” detection of S2− as well as the application in living cells, J. Photochem. Photobiol., A, 2020, 387, 112165 CrossRef CAS.
- L. Zong, C. Wang, Y. Song, Y. Xie, P. Zhang, Q. Peng, Q. Li and Z. Li, A dual-function probe based on naphthalene diimide for fluorescent recognition of Hg2+ and colorimetric detection of Cu2+, Sens. Actuators, B, 2017, 252, 1105–1111 CrossRef CAS.
- L. Zong, Y. Xie, Q. Li and Z. Li, A new red fluorescent probe for Hg2+ based on naphthalene diimide and its application in living cells, reversibility on strip papers, Sens. Actuators, B, 2017, 238, 735–743 CrossRef CAS.
- M. D. Gholami, S. Manzhos, P. Sonar, G. A. Ayoko and E. L. Izake, Dual chemosensor for the rapid detection of mercury (ii) pollution and biothiols, Analyst, 2019, 144, 4908–4916 RSC.
- P. Wang, Y. An and J. Wu, Highly sensitive turn-on detection of mercury (II) in aqueous solutions and live cells with a chemosensor based on tyrosine, Spectrochim. Acta, Part A, 2020, 230, 118004 CrossRef CAS.
- J. C. Qin, J. Yan, B. D. Wang and Z. Y. Yang, Rhodamine–naphthalene conjugate as a novel ratiometric fluorescent probe for recognition of Al3+, Tetrahedron Lett., 2016, 57, 1935–1939 CrossRef CAS.
- K. Wechakorn, K. Suksen, P. Piyachaturawat and P. Kongsaeree, Rhodamine-based fluorescent and colorimetric sensor for zinc and its application in bioimaging, Sens. Actuators, B, 2016, 228, 270–277 CrossRef CAS.
- M. Devi, A. Dhir and C. P. Pradeep, A sandwich-type zinc complex from a rhodamine dye based ligand: a potential fluorescent chemosensor for acetate in human blood plasma and a molecular logic gate with INHIBIT function, New J. Chem., 2016, 40, 1269–1277 RSC.
- Y. Wang, H. Q. Chang, W. N. Wu, X. L. Zhao, Y. Yang, Z. Q. Xu, Z. H. Xu and L. Jia, Rhodamine-2-thioxoquinazolin-4-one conjugate: a highly sensitiveandselective chemosensor for Fe3 + ions and crystal structures of its Ag(I) and Hg(II) complexes, Sens. Actuators, B, 2017, 239, 60–68 CrossRef CAS.
- Y. L. Lin, R. Sung and K. Sung, Bis(rhodamine)-based polyether type of turn-on fluorescent sensors: selectively sensing Fe(III), Tetrahedron, 2016, 72, 5744–5748 CrossRef CAS.
- J. Kuchlyan, S. Basak, D. Dutta, A. K. Das, D. Mal and N. Sarkar, A new rhodamine derived fluorescent sensor: Detection of Hg2+ at cellular level, Chem. Phys. Lett., 2017, 673, 84–88 CrossRef CAS.
- S. Hazra, C. Bodhak, S. Chowdhury, D. Sanyal, S. Mandal, K. Chattopadhyay and A. Pramanik, A novel tryptamine-appended rhodamine-based chemosensor for selective detection of Hg2+ present in aqueous medium and its biological applications, Anal. Bioanal. Chem., 2019, 411, 1143–1157 CrossRef CAS.
- Y. Wang, H. Ding, Z. Zhu, C. Fan, Y. Tu, G. Liu and S. Pu, Selective rhodamine–based probe for detecting Hg2+ and its application as test strips and cell staining, J. Photochem. Photobiol., A, 2020, 390, 112302 CrossRef CAS.
- Y. Fang, X. Li, J.-Y. Li, G.-Y. Wang, Y. Zhou, N.-Z. Xu, Y. Hu and C. Yao, Sens. Actuators, B, 2018, 255, 1182–1190 CrossRef CAS.
- G. Singh, S. I. Reja, V. Bhalla, D. Kaur, P. Kaur, S. Arora and M. Kumar, Hexaphenylbenzene appended AIEE active FRET based fluorescent probe for selective imaging of Hg2+ ions in MCF-7 cell lines, Sens. Actuators, B, 2017, 249, 311–320 CrossRef CAS.
- M. She, S. Wu, Z. Wang, S. Ma, Z. Yang, B. Yin, P. Liu, S. Zhang and J. Li, Exploration of congeneric Hg (II)-mediated chemosensors driven by S-Hg affinity, and their application in living system, Sens. Actuators, B, 2017, 247, 129–138 CrossRef CAS.
- P. Venkatesan, N. Thirumalivasan and S. P. Wu, A Rhodamine-based chemosensor with diphenylselenium for highly selective fluorescence turn-on detection of Hg2+in vitro and in vivo, RSC Adv., 2017, 7, 21733–21739 RSC.
- G. Yang, X. Meng, S. Fang, H. Duan, L. Wang and Z. Wang, A highly selective colorimetric fluorescent probe for detection of Hg2+ and its application on test strips, RSC Adv., 2019, 9, 8529–8536 RSC.
- M. Hong, Y. Chen, Y. Zhang and D. Xu, A novel rhodamine-based Hg2+ sensor with a simple structure and fine performance, Analyst, 2019, 144, 7351–7358 RSC.
- M. Ozdemir, A rhodamine-based colorimetric and fluorescent probe for dual sensing of Cu2+ and Hg2+ ions, J. Photochem. Photobiol., A, 2016, 318, 7–13 CrossRef CAS.
- S. Erdemir, M. Yuksekogul, S. Karakurt and O. Kocyigit, Dual-channel fluorescent probe based on bisphenol A-rhodamine for Zn2+ and Hg2+ through different signaling mechanisms and its bioimaging studies, Sens. Actuators, B, 2017, 241, 230–238 CrossRef CAS.
- D. Cheng, W. Zhao, H. Yang, Z. Huang, X. Liu and A. Han, Detection of Hg2+ by a FRET ratiometric fluorescent probe based on a novel BODIPY-RhB system, Tetrahedron Lett., 2016, 57, 2655–2659 CrossRef CAS.
- M. Hong, S. Lu, F. Lv and D. Xu, A novel facilely prepared rhodamine-based Hg2+ fluorescent probe with three thiourea receptors, Dyes Pigm., 2016, 127, 94–99 CrossRef CAS.
- C. Kan, X. Shao, F. Song, J. Xu, J. Zhu and L. Du, Bioimaging of a fluorescence rhodamine-based probe for reversible detection of Hg (II) and its application in real water environment, Microchem. J., 2019, 150, 104142 CrossRef CAS.
- B. Gu, L. Huang, W. Su, X. Duan, H. Li and S. Yao, A benzothiazole-based fluorescent probe for distinguishing and bioimaging of Hg2+ and Cu2+, Anal. Chim. Acta, 2017, 954, 97 CrossRef CAS.
- L. Lan, Q. Niu and T. Li, A highly selective colorimetric and ratiometric fluorescent probe for instantaneous sensing of Hg2+ in water, soil and seafood and its application on test strips, Anal. Chim. Acta, 2018, 1023, 105 CrossRef CAS.
- D. Xu, L. Tang, M. Tian, P. He and X. Yan, A benzothizole-based fluorescent probe for Hg2+ recognition utilizing ESIPT coupled AIE characteristics, Tetrahedron Lett., 2017, 58, 3654–3657 CrossRef CAS.
- T. Leng, Y. Ma and G. Chen, A novel ratiometric fluorescence and colorimetric probe with a large Stokes shift for Hg2+ sensing, J. Photochem. Photobiol., A, 2018, 353, 143–149 CrossRef CAS.
- L. Huang, Z. Yang, Z. Zhou, Y. Li, S. Tang, W. Xiao, M. Hu, C. Peng, Y. Chen, B. Gu and H. Li, A dual colorimetric and near-infrared fluorescent turn-on probe for Hg2+ detection and its applications, Dyes Pigm., 2019, 163, 118–125 CrossRef CAS.
- Y. Zhou, X. He, H. Chen, Y. Wang, S. Xiao, N. Zhang, D. Li and K. Zheng, An ESIPT/ICT modulation based ratiometric fluorescent probe for sensitive and selective sensing Hg2+, Sens. Actuators, B, 2017, 247, 626–631 CrossRef CAS.
- (a) Y. Y. Liu, S. Naha, N. Thirumalaivasan, S. Velmathi and S. P. Wu, A novel nanomolar highly selective fluorescent probe for imaging mercury (II) in living cells and zebrafish, Sens. Actuators, B, 2018, 277, 673–678 CrossRef CAS; (b) M. Tian, C. Wang, Q. Ma, Y. Bai, J. Sun and C. Ding, A Highly Selective Fluorescent Probe for Hg2+ Based on a 1, 8-Naphthalimide Derivative, ACS Omega, 2020, 5, 18176–18184 CrossRef CAS; (c) Z. Wang, Y. Zhang, J. Yin, Y. Yang, H. Luo, J. Song, X. Xu and S. Wang, A novel camphor-based “turn-on” fluorescent probe with high specificity and sensitivity for sensing mercury (II) in aqueous medium and its bioimaging application, ACS Sustainable Chem. Eng., 2020, 8, 12348–12359 CrossRef CAS.
- J. Shanmugapriya, S. Singaravadivel, G. Sivaraman and D. Chellappa, Anthracene-based highly selective and sensitive fluorescent “turn-on” chemodosimeter for Hg2+, ACS Omega, 2018, 3, 12341–12348 CrossRef.
- S. L. Pan, K. Li, L. L. Li, M. Y. Li, L. Shi, Y. H. Liu and X. Q. Yu, A reaction-based ratiometric fluorescent sensor for the detection of Hg (ii) ions in both cells and bacteria, Chem. Commun., 2018, 54, 4955–4958 RSC.
- C. Wu, J. Wang, J. Shen, C. Bi and H. Zhou, Coumarin-based Hg2+ fluorescent probe: Synthesis and turn-on fluorescence detection in neat aqueous solution, Sens. Actuators, B, 2017, 243, 678–683 CrossRef CAS.
- M. Dong, J. Tang, Y. Lv, Y. Liu, J. Wang, T. Wang, J. Bian and C. Li, A dual–function fluorescent probe for Hg(II) and Cu(II) ions with two mutually independent sensing pathways and its logic gate behavior, Spectrochim. Acta, Part A, 2020, 226, 117645 CrossRef CAS.
- Y. Chen, C. Yang, Z. Yu, B. Chen and Y. Han, A highly sensitive hemicyanine-based fluorescent chemodosimeter for mercury ions in aqueous solution and living cells, RSC Adv., 2015, 5, 82531–82534 RSC.
- A. Gomathi and P. Viswanathamurthi, Near-infrared fluorogenic switches for the detection of Hg (II) ions: applications in real samples and living cells, Anal. Methods, 2019, 11, 2769–2777 RSC.
- B. Gu, L. Huang, N. Mi, P. Yin, Y. Zhang, X. Tu, X. Luo, S. Luo and S. Yao, An ESIPT-based fluorescent probe for highly selective and ratiometric detection of mercury(II) in solution and in cells, Analyst, 2015, 140, 2778–2784 RSC.
- C. Zhang, H. Zhang, M. Li, Y. Zhou, G. Zhang, L. Shi, Q. Yao, S. Shuang and C. Dong, A turn-on reactive fluorescent probe for Hg2+ in 100% aqueous solution, Talanta, 2019, 197, 218–224 CrossRef CAS.
- Y. Gao, C. Zhang, S. Peng and H. Chen, A fluorescent and colorimetric probe enables simultaneous differential detection of Hg2+ and Cu2+ by two different mechanisms, Sens. Actuators, B, 2017, 238, 455–461 CrossRef CAS.
- Y. Jiao, X. Liu, L. Zhou, H. He, P. Zhou and C. Duan, A Schiff-base dual emission ratiometric fluorescent chemosensor for Hg2+ ions and its application in cellular imaging, Sens. Actuators, B, 2017, 247, 950–956 CrossRef CAS.
- Y. Li, S. Qi, C. Xia, Y. Xu, G. Duan and Y. Ge, A FRET ratiometric fluorescent probe for detection of Hg2+ based on an imidazo [1, 2-a] pyridine-rhodamine system, Anal. Chim. Acta, 2019, 1077, 243–248 CrossRef CAS.
- Y. Ge, A. Liu, R. Ji, S. Shen and X. Cao, Detection of Hg2+ by a FRET ratiometric fluorescent probe based on a novel pyrido [1,2-a] benzimidazole-rhodamine system, Sens. Actuators, B, 2017, 251, 410–415 CrossRef CAS.
- A. Tantipanjaporn, S. Prabpai, K. Suksen and P. Kongsaeree, A thiourea-appended rhodamine chemodosimeter for mercury(II) and its bioimaging application, Spectrochim. Acta, Part A, 2018, 192, 101–107 CrossRef CAS.
- (a) C. G. Chen, N. Vijay, N. Thirumalaivasan, S. Velmathi and S. P. Wu, Coumarin-based Hg2+ fluorescent probe: Fluorescence turn-on detection for Hg2+ bioimaging in living cells and zebrafish, Spectrochim. Acta, Part A, 2019, 219, 135–140 CrossRef CAS; (b) G. Singh, P. Raj, H. Singh and N. Singh, Colorimetric detection and ratiometric quantification of mercury(II) using azophenol dye:‘dip & read’based handheld prototype device development, J. Mater. Chem. C, 2018, 6, 12728–12738 RSC.
- A. Malek, K. Bera, S. Biswas, G. Perumal, A. K. Das, M. Doble, T. Thomas and E. Prasad, Development of a next-generation fluorescent turn-on sensor to simultaneously detect and detoxify mercury in living samples, Anal. Chem., 2019, 91, 3533–3538 CrossRef CAS.
- A. Picard-Lafond, D. Lariviere and D. Boudreau, Revealing the hydrolysis mechanism of a Hg2+-reactive fluorescein probe: Novel insights on thionocarbonated Dyes, ACS Omega, 2020, 5, 701–711 CrossRef CAS.
- J. Xu, Z. Xu, Z. Wang, C. Liu, B. Zhu, X. Wang, K. Wang, J. Wang and G. Sang, A carbonothioate-based highly selective fluorescent probe with a large Stokes shift for detection of Hg2+, Luminescence, 2018, 33, 219–224 CrossRef CAS.
- D. Zhang, J. Liu, H. Yin, H. Wang, S. Li, M. Wang, M. Li, L. Zhou and J. Zhang, A Turn on ESIPT Probe for Rapid and Ratiometric Fluorogenic Detection of Hg2+ and its Application in Live-Cell Imaging, J. Fluoresc., 2016, 26, 1367–1372 CrossRef CAS.
- X. Duan, Z. Li, F. He and S. Wang, A sensitive and homogeneous SNP detection using cationic conjugated polymers, J. Am. Chem. Soc., 2007, 129, 4154–4155 CrossRef CAS.
- B. Liu and G. C. Bazan, Homogeneous fluorescence-based DNA detection with water-soluble conjugated polymers, Chem. Mater., 2004, 16, 4467–4476 CrossRef CAS.
- X. Cheng, Q. Li, J. Qin and Z. Li, A new approach to design ratiometric fluorescent probe for mercury (II) based on the Hg2+-promoted deprotection of thioacetals, ACS Appl. Mater. Interfaces, 2010, 2, 1066–1072 CrossRef CAS.
- S. Sarkar and R. Shunmugam, Polynorbornene derived 8-hydroxyquinoline paper strips for ultrasensitive chemical nerve agent surrogate sensing, Chem. Commun., 2014, 50, 8511–8513 RSC.
- S. Sarkar, A. Mondal, A. K. Tiwari and R. Shunmugam, Unique emission from norbornene derived terpyridine-a selective chemodosimeter for G-type nerve agent surrogates, Chem. Commun., 2012, 48, 4223–4225 RSC.
- S. Bhattacharya and R. Shunmugam, Unique norbornene based triazole molecule for selective Fe (II) sensing, RSC Adv., 2015, 5, 74973–74976 RSC.
- J. Ding, H. Li, Y. Xie, Q. Peng, Q. Li and Z. Li, Reaction-based conjugated polymer fluorescent probe for mercury(II): good sensing performance with “turn-on” signal output, Polym. Chem., 2017, 8, 2221–2226 RSC.
- U. Haldar and H. I. Lee, BODIPY-derived multi-channel polymeric chemosensor with pH-tunable sensitivity: selective colorimetric and fluorimetric detection of Hg2+ and HSO4− in aqueous media, Polym. Chem., 2018, 9, 4882–4890 RSC.
- H. J. Kim and H. I. Lee, Thermo-tunable colorimetric detection of mercury (II) ions driven by the temperature-dependent assembly and disassembly of a block copolymer, Polym. Chem., 2019, 10, 4017–4024 RSC.
- A. Balamurugan and H. I. Lee, Water-soluble polymeric probes for the selective sensing of mercury ion: pH-driven controllable detection sensitivity and time, Macromolecules, 2015, 48, 1048–1054 CrossRef CAS.
- B. Wu, L. Xu, S. Wang, Y. Wang and W. Zhang, A PEGylated colorimetric and turn-on fluorescent sensor based on BODIPY for Hg (II) detection in water, Polym. Chem., 2015, 6, 4279–4289 RSC.
- N. Choudhury, B. Saha, B. Ruidas and P. De, Dual-action polymeric probe: turn-on sensing and removal of Hg2+; chemosensor for HSO4−, ACS Appl. Polym. Mater., 2019, 1, 461–471 CrossRef CAS.
- N. Choudhury, B. Ruidas, B. Saha, K. Srikanth, C. D. Mukhopadhyay and P. De, Multifunctional tryptophan-based fluorescent polymeric probes for sensing, bioimaging and removal of Cu2+ and Hg2+ ions, Polym. Chem., 2020, 11, 2015–2026 RSC.
- (a) U. Haldar and H. I. Lee, BODIPY-derived polymeric chemosensor appended with thiosemicarbazone units for the simultaneous detection and separation of Hg (II) ions in pure aqueous media, ACS Appl. Mater. Interfaces, 2019, 11, 13685–13693 CrossRef CAS; (b) K. Chauhan, P. Singh and R. K. Singhal, New chitosan–thiomer: an efficient colorimetric sensor and effective sorbent for mercury at ultralow concentration, ACS Appl. Mater. Interfaces, 2015, 7, 26069–26078 CrossRef CAS.
- S. Sirawatcharin, A. Saithongdee, A. Chaicham, B. Tomapatanaget, A. Imyim and N. Praphairaksit, Naked-eye and colorimetric detection of arsenic (III) using difluoroboron-curcumin in aqueous and resin bead support systems, Anal. Sci., 2014, 30, 1129–1134 CrossRef CAS.
- T. G. Akshay Krishna, V. Tekuri, M. Mohan and D. R. Trivedi, Selective colorimetric chemosensor for the detection of Hg2+ and arsenite ions using Isatin based Schiff's bases; DFT Studies and Applications in test strips, Sens. Actuators, B, 2019, 284, 271–280 CrossRef CAS.
- S. Lohar, S. Pal, B. Sen, M. Mukherjee, S. Banerjee and P. Chattopadhyay, Selective and sensitive turn-on chemosensor for arsenite ion at the ppb level in aqueous media applicable in cell staining, Anal. Chem., 2014, 86, 11357–11361 CrossRef CAS.
- S. Paul, S. Bhuyan, S. K. Mukhopadhyay, N. C. Murmu and P. Banerjee, Sensitive and selective in vitro recognition of biologically toxic As (III) by rhodamine based chemoreceptor, ACS Sustainable Chem. Eng., 2019, 7, 13687–13697 CrossRef CAS.
- M. Baglan and S. Atılgan, Selective and sensitive turn-on fluorescent sensing of arsenite based on cysteine fused tetraphenylethene with AIE characteristics in aqueous media, Chem. Commun., 2013, 49, 5325–5327 RSC.
- A. S. M. Islam, R. Alam, A. Katarkar, K. Chaudhuri and M. Ali, Di-oxime based selective fluorescent probe for arsenate and arsenite ions in a purely aqueous medium with living cell imaging applications and H-bonding induced microstructure formation, Analyst, 2015, 140, 2979–2983 RSC.
- K. Chauhan, P. Singh, B. Kumari and R. K. Singhal, Synthesis of new benzothiazole Schiff base as selective and sensitive colorimetric sensor for arsenic on-site detection at ppb level, Anal. Methods, 2017, 9, 1779–1785 RSC.
- M. L. Presti, S. El Sayed, R. Martínez-Máñez, A. M. Costero, S. Gil, M. Parra and F. Sancenon, Selective chromo-fluorogenic detection of trivalent cations in aqueous environments using a dehydration reaction, New J. Chem., 2016, 40, 9042–9045 RSC.
- N. Yadav and A. K. Singh, Dual anion colorimetric and fluorometric sensing of arsenite and cyanide ions, RSC Adv., 2016, 6, 100136–100144 RSC.
- R. Purkait, S. Maity and C. Sinha, A hydrazine-based thiocarbamide probe for colorimetric and turn-on fluorometric detection of PO43− and AsO33− in semi-aqueous medium, New J. Chem., 2018, 42, 6236–6246 RSC.
- P. G. Sutariya, H. Soni, S. A. Gandhi and A. Pandya, Single-step fluorescence recognition of As3+, Nd3+ and Br− using pyrene-linked calix [4] arene: application to real samples, computational modelling and paper-based device, New J. Chem., 2019, 43, 737–747 RSC.
- A. Deepa and V. Padmini, Highly Efficient Colorimetric Sensor for Selective and Sensitive Detection of Arsenite Ion (III) in Aqueous Medium, J. Fluoresc., 2019, 29, 813–818 CrossRef CAS.
- V. C. Ezeh and T. C. Harrop, A sensitive and selective fluorescence sensor for the detection of arsenic (III) in organic media, Inorg. Chem., 2012, 51, 1213–1215 CrossRef CAS.
- V. C. Ezeh and T. C. Harrop, Synthesis and properties of arsenic (III)-reactive coumarin-appended benzothiazolines: a new approach for Inorganic arsenic detection, Inorg. Chem., 2013, 52, 2323–2334 CrossRef CAS.
- S. Bhattacharya, S. Sarkar and R. Shunmugam, Unique norbornene polymer based “in-field” sensor for As (III), J. Mater. Chem. A, 2013, 1, 8398–8405 RSC.
| This journal is © The Royal Society of Chemistry 2021 |