Open Access Article
Open Access ArticleModulated synthesis of thiol-functionalized fcu and hcp UiO-66(Zr) for the removal of silver(I) ions from water†
Bastian
Moll
 ,
Tim
Müller
,
Carsten
Schlüsener
,
Alexa
Schmitz
,
Philipp
Brandt
,
Tim
Müller
,
Carsten
Schlüsener
,
Alexa
Schmitz
,
Philipp
Brandt
 ,
Secil
Öztürk
and
Christoph
Janiak
,
Secil
Öztürk
and
Christoph
Janiak
 *
*
Institut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität, D-40204 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de
First published on 16th December 2020
Abstract
In recent years there has been a developing interest in the capture of Ag(I) ions from wastewater. In this work we present the synthesis and characterization of two modified thiol-containing UiO-66 metal–organic frameworks (MOFs) capable of removing Ag(I) from aqueous solution. Mercaptoacetic acid (HMAc) was used as a modifier and incorporated as mercaptoacetate into the MOFs. The modulation of the UiO-66 synthesis with HMAc represents an easy and low-cost method for the introduction of thiol groups into the UiO-66 framework, while preserving the general UiO-66 properties like porosity and stability. The usage of very high equivalents of HMAc in relation to ZrCl4 led to reproducible formation of the rare hexagonal close packed (hcp) topology of UiO-66 instead of its common face-centered cubic (fcu) packing. The materials showed a maximum uptake for Ag(I) from aqueous solutions of over 84 mg g−1 for the fcu structure and 36 mg g−1 for the hcp structure. The uptake follows a Langmuir type behavior for both topologies. Uptake kinetics indicates a pseudo second-order rate law for the initial uptake before diffusion control takes over.
Introduction
Metal–organic frameworks (MOF) are potentially porous materials built by organic ligands connected to metal nodes.1,2 Zirconium(IV)-based MOFs are widely studied3 and show relatively high chemical4 and hydrothermal stability.5 They are promising materials for applications in catalysis,6 gas storage/separation7 and for drug delivery.8 Functionalized zirconium MOFs (Zr-MOFs), especially UiO-66,9,10 a relatively water-stable MOF with the organic linker benzene-1,4-di-carboxylate (BDC2−),4 showed promising potential for wastewater treatment as it is capable of removing heavy metal ions of mercury11,12 and chromium13 or other impurities.14–17,24 In addition UiO-66 is regarded as non-toxic, which is of great importance for the purification of water.18–20Thiol-modified Zr-MOFs should be useful for the uptake or removal of soft Lewis-acid metal cations like Hg2+ and Ag+ from water. To this day few thiol modifications of UiO-66 have been reported in the literature and were synthesized from thiol-functionalized linkers12,13,21 through postsynthetic linker exchange or linker modification.12 Yee et al. described the synthesis with 2,5-dimercapto-1,4-benzenedicarboxylic acid (H2DMBD), yielding UiO-66 type MOFs, which they tested for the removal of Hg2+. Their MOF had incorporated 15 wt% sulfur. However, they observed very high amounts of disulfide groups via thiol oxidation. The BET surface area was 513 m2 g−1, which is around half the BET surface area of unmodified UiO-66.21 H2DMBD, as the obvious thiol-functionalized common linker terephthalate, would guarantee a high sulfur content, but is not commercially available and has to be synthesized in a complex 3-step organic synthesis under protective gas.21–23
Yang et al. reported two UiO-type MOFs with the organic linkers mercaptosuccinic acid (MSA) and dimercaptosuccinic acid (DMSA) forming zirconium fumarate analogs Zr-MSA and Zr-DMSA with ∼12 wt% sulfur for Zr-MSA and surface areas of 513 m2 g−1 and 275 m2 g−1, respectively.13 Just recently Ding et al. postsynthetically introduced rhodanine into UiO-66 (UiO-66-Rd) and amino-functionalized UiO-66-NH2 (UiO-66-NH2-Rd). The functionalized MOFs showed BET surface areas of 518–606 m2 g−1, sulfur contents of less than 5 wt%, and achieved high uptake of silver from aqueous solution but at the expense of a decrease of crystallinity in PXRD and BET surface area.24
Silver ions (Ag+) and colloidal silver (silver nanoparticles Ag-NP) are known for their microbiological toxicity25,26 The toxicity of silver nanoparticles seems to be associated with their release of silver ions into the surrounding media.27,28 This antimicrobial effect led to numerous applications of silver ions or nanoparticles in consumer goods,29–31 and in medical applications.27,32 The massive amount of silver used for such applications leads to an increasing release of silver nanoparticles and silver ions into wastewater29,33 and a drastically increased exposure of living organisms and the environment to silver(I) ions and silver nanoparticles. The effects of silver on the human organism are still a widely researched topic. In vitro studies showed the accumulation of silver nanoparticles in human tissue and organs.34 Several in vitro studies concentrate on the toxicity of silver sources on cell lines.35,36 Therefore strategies for the removal of silver sources become more and more important. Until now several materials have been evaluated for the removal of silver, including mercapto-functionalized polymers,37,38 coffee grounds39 or inorganic materials like layered hydroxides40 and mercapto-functionalized nanosized TiO2.41 In 2016 Conde-González et al. demonstrated the promising properties of MOFs for silver removal by using the MOF HKUST-1 to adsorb silver nanoparticles from water.42
We note that in the literature the terms “filtration” or “adsorption” are often used for the metal-thiolate binding in porous materials. Adsorption refers to physisorption, while metal-thiolate binding is chemisorption. To avoid ambiguity, we will use the terms “uptake” or “removal” here. The thiophilic character of silver and mercury metal cations is well-known in the literature and can be explained by the HSAB concept (HSAB = hard and soft acids and bases) where interactions of soft Lewis bases (like thiol or thiolate) and soft Lewis acids are preferred.43
It is well established that the addition of monocarboxylic acid modulators, like benzoic acid or acetic acid,3 can enhance the formation of defects in UiO-MOFs,44,45 which in turn can improve porosity,46 stability47 and even catalytic activity.6a In this work we used mercaptoacetic acid (HMAc, Scheme 1) to modulate the synthesis of UiO-66, opening the potential of an easy and low-cost method for the introduction of thiol groups into the UiO-66 framework, while preserving general UiO-66 properties like porosity and stability. The mercaptoacetic acid modulator should be incorporated as mercaptoacetate (MAc−) into the framework to enhance the uptake capacities of UiO-66 in the removal of silver ions.
When using high amounts of HMAc (100 eq.) versus ZrCl4 we identified a change in the structure from the usual UiO-66 face-centered cubic (fcu)48 structure to a hexagonal close packed (hcp) structure, which was rarely described in the literature after the first observation in 2018.49 To the best of our knowledge we herein present the first functionalized structure of a hcp UiO-66 MOF. We tested two of the synthesized MOFs for the removal capacities of silver(I) from aqueous solution.
Experimental
Materials and methods
Zirconium(IV) chloride (ZrCl4, purity ≥98%) and potassium cyanide (KCN, purity ≥97%) were obtained from Alfa Aesar, benzene-1,4-dicarboxylic acid (H2BDC, purity 98%) was obtained from Sigma Aldrich and mercaptoacetic acid (HMAc, purity 98%) was obtained from PanReac AppliChem. All solvents were of analysis grade. All chemicals and solvents were used without further purification. AAS standard solution for silver (AgNO3, with Ag(I) concentration = 1000 mg L−1, stabilized by HNO3) was obtained from Sigma Aldrich. Ultrapure water was produced with a Merck Millipore Synergy© system.Synthesis
All materials were synthesized using a protocol for the synthesis of acetic acid modulated UiO-66,3 by employing mercaptoacetic acid instead of acetic acid (Scheme 2).In a typical synthesis, 1 mmol of ZrCl4 (229 mg) and 1 mmol (170 mg) of terephthalic acid (benzene-1,4-dicarboxylic acid, H2BDC) were mixed with 25 mL of N,N-dimethylformamide (DMF) and 5 mL of water in a Pyrex bottle with a screw-cap. Mercaptoacetic acid (10–100 eq. in relation to ZrCl4) was added and dissolved under ultrasonification for 10 minutes (for used weights and exact molar ratios see Table S1, ESI†). Afterwards the bottle was placed in an oven and heated to 120 °C for 24 h (ramp time 3 h for heating and 3 h for cooling). The solid product was separated by centrifugation, decanting the supernatant, and washing three times with 35 mL of methanol (MeOH) for 24 h each. Finally, the colorless solid was centrifuged and dried at 60 °C under a vacuum. Obtained yields were between 234 and 249 mg. Products are denoted as UiO-66-MAc-X, with X = 10, 30, 50 or 100 of used equivalents of HMAc versus ZrCl4 in the synthesis. Importantly, the amount of incorporated MAc− is only between 0.37 and 0.80 eq. However, in the literature it is common to name the samples according to the equivalents of modulator used during the synthesis in relation to the metal salt.3
Results and discussion
Synthesis and characterization
The synthesis of UiO-66-MAc-X from ZrCl4 and terephthalic acid with different equivalents of mercaptoacetic acid as the modulator yielded colorless solids after drying (Fig. S1, ESI†).3 The products form small primary particles, which are highly agglomerated (Fig. S2–S5, ESI†), possibly due to the formation of interparticle S–S disulfide bonds on the MOF surface through air oxidation of the thiol groups. The designation of the products as UiO-66-MAc-X with X = 10, 30, 50 or 100 used equivalents of HMAc in the synthesis followed the general principle of modulated UiOs used in the literature, where the synthesis equivalents of the modulator are indicated, even if the actually incorporated amount is much lower. In our work here, the amount of MAc− incorporated is only between 0.31 and 0.80 eq. in relation to the organic linker. However, a large excess of modulator can have additional, for example morphological, effects. When Shearer et al. investigated the influence of different modulators in the synthesis of UiO-66, the amount of incorporated modulator was only between 0.1 and 0.8 eq. in relation to the organic linker. Yet, an increased amount of modulator (6 to 100 eq.) during the synthesis led to the additional effect of a stepwise phase change from the fcu phase of UiO-66 to a primitive cubic reo phase.3 Zhao et al. synthesized nano-sized MIL-101(Cr) through the addition of up to 17 eq. of acetic acid (vs. Cr and H2BDC).50 With increasing equivalents of benzoic acid as modulator (from 3 to 10 eq.) under otherwise similar hydrothermal synthetic conditions. Instead of nanocrystals of MIL-101(Cr) the microparticulated MIL-88B(Cr) product was formed.51 In 2019 Yang et al. had briefly noted the idea of using mercaptoacetic acid as a modulator for the synthesis of a thiol-modified UiO-66 but concentrated on the use of thiol-modified mercaptosuccinate and dimercaptosuccinate linkers for the removal of chromates.13The powder X-ray diffraction (PXRD) patterns of UiO-66-MAc-X match the simulated pattern of fcu UiO-66 for X = 10, 30, and 50 (Fig. 1).52 The broadening of the reflexes can be explained through the formation of very small crystallites (Fig. S2–S5, ESI†).
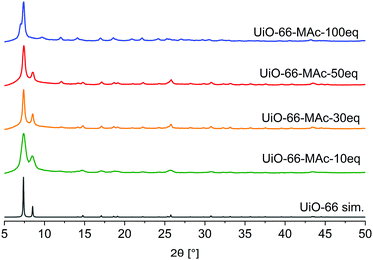 | ||
| Fig. 1 PXRD patterns of UiO-66-MAc with different HMAc equivalents used in the synthesis and simulated diffractogram of UiO-66 (from CIF CCDC 837796).52 | ||
When conducting the synthesis in the presence of 100 eq. HMAc there was a reproducible change in the powder pattern indicating the presence of a different phase (see UiO-66-MAc-100 eq., Fig. 1). A powder pattern with higher resolution and measured to lower angles of 2θ < 5° (Fig. 2) was in good accordance with the simulated PXRD of the hexagonal close packed (hcp) phase of UiO-66, first reported in 2018 by Ermer et al.49
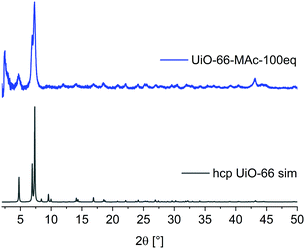 | ||
| Fig. 2 PXRD pattern of UiO-66-MAc-100 eq. and simulated diffractogram of hcp UiO-66 (from CIF CSD-Refcode: KINGUM).49 | ||
A brief structural description of fcu and hcp UiO-66 is provided in Section S7, ESI. †hcp UiO-66 was, so far, obtained by either using a terephthalate-containing ionic liquid or poly(ethylene terephthalate) as linker or by using large amounts of hydrochloric acid or acetic acid modulators.49,53–55 To the best of our knowledge there is no functionalized hcp UiO-66 known. PXRD patterns showed the transition to the hcp structure starting with the use of ∼80 eq. HMAc in relation to ZrCl4 (for details see Section S14, ESI†). The product showed hcp reflexes in the PXRD pattern but with poor crystallinity compared to the 100 eq. UiO-66-MAc. This may be due to the formation of both fcu and hcp phases albeit in small and not well-developed crystallites. The use of 90 eq. HMAc yielded the hcp structure of UiO-66. The amount of incorporated MAc− in UiO-66-MAc was determined by digestion 1H NMR, (details in Section S3, ESI†), and by TGA (details in Section S4, ESI†). All samples were dried before the measurements to ensure a minimal presence of residual solvent. From digestion 1H-NMR and from TGA analysis the molar BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− ratios are directly obtained (Table 1). Elemental CHNS analysis (Table S4, ESI†) shows the presence of significant amounts of sulfur but does not provide the possibility to derive a sum formula or linker to modulator ratio because of the undefinable solvent residues. With the sum formulae derived from NMR and TGA, the found wt% sulfur matches the calculated values reasonably well.
MAc− ratios are directly obtained (Table 1). Elemental CHNS analysis (Table S4, ESI†) shows the presence of significant amounts of sulfur but does not provide the possibility to derive a sum formula or linker to modulator ratio because of the undefinable solvent residues. With the sum formulae derived from NMR and TGA, the found wt% sulfur matches the calculated values reasonably well.
| UiO-66-MAc- | BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− 1H-NMRa MAc− 1H-NMRa |
BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− TGAb MAc− TGAb |
BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− from defect calc.c MAc− from defect calc.c |
BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− average MAc− average |
Sum formulad |
|---|---|---|---|---|---|
| a Detailed calculation presented in Section S3, ESI. b Molar ratios directly determined from weight loss steps. Further details in Section S4, ESI. c Sum formula calculated from linker-defect calculations presented in Table S5, ESI. For further details see Section S4, ESI. d Sum formula calculated from the average linker to modulator ratios under the assumption that one BDC2− linker in ideal fcu UiO-66 with the formula [Zr6O4(OH)4(BDC)6] and in ideal hcp UiO-66 with the formula [(Zr6O4(OH)4)2(OH)6(BDC)9] is replaced by two MAc− molecules. | |||||
| 10 eq. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.30 0.30 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.29 0.29 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.31 0.31 |
Zr6O4(OH)4(BDC)4.58(MAc)1.42 |
| 30 eq. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.62 0.62 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.55 0.55 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56 0.56 |
Zr6O4(OH)4(BDC)3.85(MAc)2.15 |
| 50 eq. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.77 0.77 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85 0.85 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.79 0.79 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.80 0.80 |
Zr6O4(OH)4(BDC)3.33(MAc)2.67 |
| 100 eq. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.64 0.64 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
Method not suitable | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.57 0.57 |
[(Zr6O4(OH)4)2(OH)6](BDC)5.73(MAc)3.27 |
In the ideal UiO-66 formula of [Zr6O4(OH)4(BDC)6] the molar Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC ratio is 1
BDC ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, UiO-66 is subject to linker defects, which are the basis for MAc incorporation.
1. However, UiO-66 is subject to linker defects, which are the basis for MAc incorporation.
The number of linker defects can be calculated from TGA analysis (details Section S4, ESI†) by a procedure from Shearer et al.3 This method is, however, not suitable for the hcp-structured UiO-66-MAc-100 eq. because the decomposition does not lead to pure ZrO2.49 The comparison of the sulfur contents derived from the linker defect analysis and from CHNS analysis is presented in Section S4 and Table S5, ESI.† When the BDC2− linker defects are assumed to be replaced by two MAc− equivalents a BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− ratio is obtained.
MAc− ratio is obtained.
The three BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− ratios from NMR, TGA and linker-defect analysis agree within experimental error and the average BDC2−
MAc− ratios from NMR, TGA and linker-defect analysis agree within experimental error and the average BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MAc− ratio was taken as the basis for a sum formula (Table 1). In addition, SEM-EDX with elemental mapping allowed us to estimate the Zr:S ratio (Section S2, ESI†). The wt% sulfur from elemental CHNS analysis (Table S5, ESI†) as well as the Zr- and S-EDX mappings show a uniform and concomitant distribution of Zr and S over the entire particles (Section S2, ESI†). The amount of introduced modulator MAc− increased with the amount of used modulator during the synthesis for fcu structured UiO-66-MAc-10 eq. to -50 eq. At 100 eq. modulator the UiO-66 phase changed from fcu to hcp.
MAc− ratio was taken as the basis for a sum formula (Table 1). In addition, SEM-EDX with elemental mapping allowed us to estimate the Zr:S ratio (Section S2, ESI†). The wt% sulfur from elemental CHNS analysis (Table S5, ESI†) as well as the Zr- and S-EDX mappings show a uniform and concomitant distribution of Zr and S over the entire particles (Section S2, ESI†). The amount of introduced modulator MAc− increased with the amount of used modulator during the synthesis for fcu structured UiO-66-MAc-10 eq. to -50 eq. At 100 eq. modulator the UiO-66 phase changed from fcu to hcp.
At the same time a lower incorporated modulator content was found for the hcp structured UiO-66-MAc-100 eq. This correlates with a decreased Zr-BDC-linker ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 for the hcp phase compared to 1
0.75 for the hcp phase compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the fcu structure (see structural information for fcu and hcp UiO-66 in Section S7, ESI†).
1 for the fcu structure (see structural information for fcu and hcp UiO-66 in Section S7, ESI†).
The FT-IR spectra of UiO-66-MAc showed no visible vibrations which can be assigned to the MAc− modulator (Section S5, ESI†). Slight differences in the intensities of the –CO2 linker vibrations between fcu structured UiO-66 and the hcp phase can be explained by the differences between the SBUs.
However, –SH and C–S vibrations are Raman active and in general show stronger vibration bands compared to the intensities in IR-spectroscopy. In the Raman spectra (Section S5, ESI†) for the 50 eq. and 100 eq. modulated UiOs, signals at 2580 cm−1 can be assigned to –SH vibrations (Section S5, ESI†) and were reproducibly visible after enhancing the laser power up to 700 mW and with longer measurements.
The strong agglomeration of primary particles may be due to the formation of interparticle S–S disulfide bonds on the MOF surface after thiol oxidation through oxygen from air. Yee et al. reported the formation of disulfide bonds in their 2,5-dimercapto-1,4-benzenedicarboxylate UiO-MOF.21 There were, however, no characteristic S–S vibrational signals visible in IR- or Raman-spectroscopy in UiO-66-MAc-X (Section S5, ESI†), which indicates that the amount of such S–S bonds, if any, will be very low.
Nitrogen sorption measurements at 77 K of the 10 to 50 eq. fcu materials showed a Type IV isotherm with H2 hysteresis (Fig. 3), indicating that the samples are micro- to mesoporous.56 The mesoporosity can be caused by the linker defects and/or by the aggregation of the nano-sized MOF crystallites, forming meso- (and macroporous) cavities.57 This aggregation could also be induced by S–S bonds between the crystallites. H2 hysteresis can be attributed to pore-blocking/percolation in a narrow (H2a) or wider (H2b) range of pore necks or to cavitation-induced evaporation. The hysteresis decreased with increasing amounts of modulator. For hcp UiO-66-100 eq. the sorption isotherm is a combination of Type I (for the microporosity at low p/p0) and Type II (at high p/p0, given by macroporous adsorbents) as the adsorbed multilayer appears to increase without limit when p/p0 = 1. There is only a small hysteresis loop for the hcp sample.
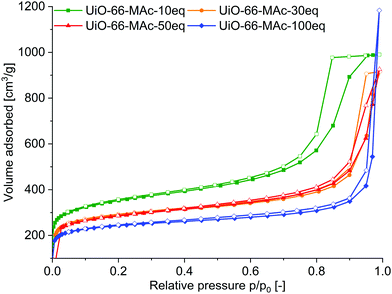 | ||
| Fig. 3 N2 sorption isotherms of UiO-66-MAc-10 eq., 30 eq., 50 eq. and 100 eq. at 77 K. Filled symbols: adsorption; empty symbols: desorption. | ||
The total BET surface area decreased with increasing modulator content (Table 2). BET surface areas of the MAc−-modulated fcu MOFs with 1290–1070 m2 g−1 compare well with the surface areas of unmodulated UiO-66 of 1100 m2 g−1,52 and modulated UiO-66 ranging from 700 up to 1600 m2 g−1.3,52,58 Also, the BET surface area of hcp UiO-66-MAc-100 eq. is in good accordance with the literature values.49,53–55
| Material | S BET [m2 g−1] | A Int [m2 g−1] | A Ext [m2 g−1] | V total [cm3 g−1] | Pore sizese [Å] |
|---|---|---|---|---|---|
| a Multipoint BET between p/p0 = 0.01 and 0.05. b Internal micropore surface area determined from t-plot and the V–t method. c External surface area, i.e., the surface area from meso- and macropores, determined from the t-plot and V–t method. d Total pore volume at p/p0 ≈ 0.8. e Pore size distribution for UiO-66-10 eq. and -30 eq. determined from nitrogen sorption, for -50 eq. and -100 eq. by Ar sorption, see Section S6, ESI. f Depending on the used modulator.3,52,58–62 g Values for AInt and AExt are from our measurements.49,53–55 | |||||
| Lit. fcu UiO-66f | 700–1700 | 790–1600 | 98–101- | 0.3–0.6 | 7, 11 |
| 10 eq. | 1289 | 830 | 459 | 0.85 | 11, 29, 54, 89, 106 |
| 30 eq. | 1253 | 896 | 357 | 0.65 | 14, 29, 50, 110 |
| 50 eq. | 1072 | 825 | 247 | 0.39 | 7, 10, 27, 48, 103 |
| Lit. hcp UiO-66g | 700–900 | 708–920 | 204 | 0.32–0.45 | 7, 10 |
| 100 eq. | 912 | 708 | 204 | 0.35 | 7, 11, 26, 100, 154 |
The total surface area was differentiated into the internal micropore surface area (AInt) and the external surface area (AExt) by the t-plot and the V–t-method (Table 2). The external surface area refers to all non-microporous areas and includes the surface area originating from meso- and macropores, i.e. pores with diameters larger than 2 nm. Interestingly, AInt with 825–896 m2 g−1 did not change much for the fcu UiOs when considering typical error margins or ±20 to 50 m2 g−1. Yet, the smaller the amount of MAc modulator the higher the external surface area (AExt) or the lower the ratio of AInt to AExt. The increased meso/macroporosity for smaller modulator amounts in the fcu UiOs is evident from the enhanced hysteresis when going from 50 eq. over 30 eq. to the 10 eq. sample (Fig. 3). The total pore volume Vtotal corresponds to the literature values of small sized fcu UiO-66 particles,59 which range from 0.3 to 0.6 cm3 g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 and to the hcp UiO-66, for which Vtotal values of 0.32 and 0.45 cm3 g−1 were found.53–55
60 and to the hcp UiO-66, for which Vtotal values of 0.32 and 0.45 cm3 g−1 were found.53–55
Pore size distribution (using the QSDFT method) and total pore volumes were calculated from nitrogen or argon (Ar) physisorption isotherms (Table 2 and Fig. S36 to S41, ESI†).
Pore size distribution by Ar sorption isotherms showed micropores of the same size as the triangular pore window (∼6 Å), the tetrahedral pore (∼8 Å) and the octahedral cage of fcu UiO-66 (at around 11 Å).61 In defect-rich UiO-type structures the tetrahedral pore size is often increased from 8 Å to 10–12 Å, which is consistent with the presented data.62 Also, for the hcp UiO the micropores of 7 and 10 Å are in good accordance with the literature values.53 For all samples, additional mesopores above 2 nm (Table 2, Fig. S37, S39–S41, ESI†) can be due to linker defects or formed through agglomeration of small MOF particles by dithiol-bonds (see the SEM images in Fig. S4 and S5, ESI†).
Removal of silver(I)–ions from aqueous solutions
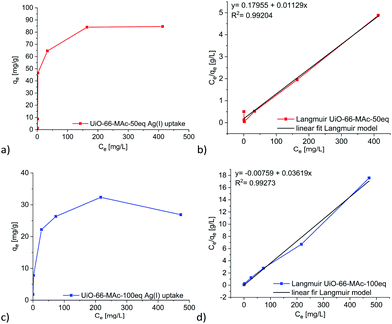
The above-determined UiO pore sizes should allow hydrated silver(I) ions to fit inside the pores of the thiol-functionalized MOFs for sorption from aqueous solutions. The literature states the diameter of a hydrated AgNO3 ion pair in aqueous solution as 6.3 Å and for hydrated Ag+ in solution as 4.8 Å. Both values refer to the distance from silver to the oxygen atoms of water in the first hydration shell.63 Stability tests were carried out at pH 4, 7 and 10 with UiO-66-MAc-50 eq. and UiO-66-MAc-100 eq. to determine the potential pH range for the silver uptake experiments. Powder X-ray diffractograms show that UiO-66-MAc-50 eq. (Fig. S45, ESI†) is stable in water at pH 7 but loses crystallinity at pH 4 and decomposes at pH 10. The literature reported that UiO-66 is stable under acidic conditions up to pH 1 for extended periods,5 while decomposing under basic conditions. For hcp UiO-66-MAc-100 eq. (Fig. S46, ESI†) the results show preservation of the structure at pH 7 and 4, whereas at basic conditions (pH 10) the material loses crystallinity. Ermer et al.49 has shown stability for hcp UiO-66 from pH 0 to pH 12 over a period of three days. The lower pH-stability of the thiol-modified UiO-66 MOFs is reasoned by the high number of defects. Hence, the silver uptake experiments and the kinetic studies were carried out at pH 7, at which both MOFs tended to be stable for at least 24 h.Removal of silver(I) from water was tested with UiO-66-MAc-50 eq., because of its high amount of incorporated sulfur-containing modulator. Additionally, hcp UiO-66-MAc-100 eq. was also tested for the removal of silver to check how the different structures influence the behavior for silver uptake. The maximum Ag(I) uptake for UiO-66-MAc-30 eq. was lower than that for UiO-66-MAc-50 eq. but slightly higher than that for UiO-66-MAc-100 eq., which we refer to as the lower and similar to slightly higher sulfur content of UiO-66-MAc-30 eq. compared to those of UiO-66-MAc-50 eq. and UiO-66-MAc-100 eq. (for the silver(I) uptake of UiO-66-MAc-30 eq. see Section S12, ESI†). Both UiO-66-MAc-materials exhibit a steep uptake of silver ions with increasing Ag-concentration, akin to a Type I isotherm. A plateau is reached at a concentration of 164 mg L−1 with an Ag+ uptake of 84 mg g−1 for UiO-66-MAc-50 eq., and at a concentration of 216 mg L−1 with an Ag+ uptake of 32 mg g−1 for UiO-66-MAc-100 eq. (Fig. 4). Both isotherms could be best fitted by a Langmuir adsorption isotherm model (Fig. 4b, details in Section S9, ESI†). Therefore, it corresponds well to previous reported uptake behaviors for the capture of Ag(I) and other heavy metal ions like Hg(II) with MOFs.11,13,24
The uptake depends on the incorporated sulfur amount, which is around 1.5 times higher in UiO-66-MAc-50 eq. over -100 eq. as determined in the CHNS analysis.
If the uptake of silver takes place at the thiol group of the MAc modulator then 38 mol% of the sulfur atoms in UiO-66-MAc-50 eq. become occupied by silver and 21 mol% in UiO-66-MAc-100 eq. SEM-EDX mappings after the uptake experiments for 12 h in 500 mg L−1 silver solution showed Ag to be evenly distributed over the MOF particles together with zirconium and sulfur (Fig. S53 and S54, ESI†). There are no significant silver EDX signals observed outside of the MOF particles, so it is verified that the decrease of silver concentration is solely due to the uptake in the MOF.
Porous materials like MOFs and COFs (covalent-organic frameworks) and also clay-type composite materials show a wide distribution in silver ion uptake capability between 8 and 450 mg g−1. Most materials follow Langmuir-type adsorption models and the uptake can be described by chemisorption. For example, Ding et al. were able to reach a maximum uptake of 163 mg g−1 for their rhodanine (rd) post-functionalized UiO-66-NH2-rd,24 where each rhodanine moiety contains two accessible sulfur atoms. Conde-González et al. achieved a silver nanoparticle uptake of around 80 mg g−1 with HKUST-1.42 Other materials show uptake between 8 mg g−1 for expanded perlite, which is a volcanic glass,64 35 mg g−1 for Fe3O4@SiO2@TiO2-IIP65 (IIP = ion imprinted polymer), nearly 227 mg g−1 for a magnetite, thiourea and glutaraldehyde-modified chitosan resin66 and up to 450 mg g−1 for a MgAl-layered double hydroxide67 (a literature overview is provided in Table S11 in Section S15, ESI†).
Ding et al. observed a comparatively small Ag+ uptake of 15 to 20 mg g−1 at a silver equilibrium concentration of 500 mg L−1 in pure UiO-66, which increases gradually with rising concentration.24 While UiO-66-MAc-50 eq. reached the maximum uptake at a concentration of 200 mg L−1 Ag+, pure UiO-66 reached the maximum of 20 mg g−1 at ∼500 mg L−1.24 Therefore, the incorporation of thiol-groups into the framework enhances the sorbent–sorptive interactions, which is the affinity for silver, resulting in pore filling at very low concentrations. Ding et al. noticed the same effect for their rhodanine-modified UiO-66 derivates.24 The introduced MAc modulator enhances the maximum specific uptake up to 4 times for UiO-66-MAc-50 eq. and around 1.5 times for hcp UiO-66-MAc-100 eq. compared to unfunctionalized UiO-66.
The kinetic silver uptake curves for UiO-66-MAc-50 eq. and -100 eq. (Fig. 5) were fitted to a pseudo second-order model until 30 minutes, when a change to pseudo first-order seemed to occur (Fig. 5 and Fig. S48, equations in Section S10, ESI†). Both models are often described for the removal of heavy metals from aqueous solutions with solid sorbents, including MOFs.11,13,24,68
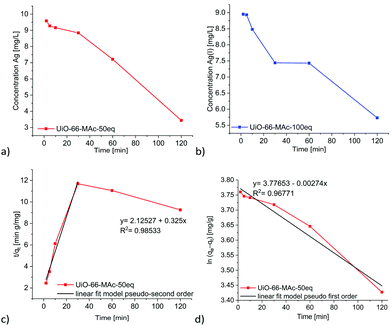 | ||
| Fig. 5 Time-dependent removal of Ag(I) by UiO-66-MAc-50 eq. (a) and UiO-66-MAc-100 eq. (b) from a starting Ag+ concentration of 10 mg L−1. Linear fit for pseudo second-order kinetics (c) and for pseudo first-order kinetics (d) for the uptake in UiO-66-MAc-50 eq. See Fig. S48, ESI† for the linear fits for the uptake in UiO-66-MAc-100 eq. | ||
We suggest that for the second-order kinetics both the silver and thiol concentrations determine the chemisorption of silver on easily accessible thiol groups, for example, at the outside of the particles and near the pore mouths. The chemisorption is then followed by the thiol groups inside the MOF pores, which is most likely diffusion controlled.69,70
X-ray photoelectron spectra show the oxidation state of silver in UiO-66-MAc-50 eq. and UiO-66-MAc-100 eq. as a mixture of predominantly Ag(0) and to a minor extent of Ag(I), indicating largely the reduction of silver(I) to silver(0) through the oxidation of thiol groups and for the remaining Ag(I) the formation of R–S–Ag bonds (for XPS spectra and detailed analysis see Section S13, ESI†). At the same time, the crystallinity of the UiO samples decreases in the presence of increasing silver concentration concomitant with the uptake (for PXRD and N2-sorption see Section S13, ESI†). After the silver(I) uptake experiments nitrogen-sorption isotherms showed a significant loss in BET surface area (Fig. S51, ESI†) consistent with the intended pore filling. It was not possible to restore the BET surface area by regeneration through silver removal with KCN (Fig. S58, ESI†) This behavior was also reported by Ding et al. for their rhodanine-UiO-66-NH2.24 A possible regeneration of the Ag@UiO-66 samples was investigated through the silver extraction with potassium cyanide (KCN),71 by formation of the dicyanoargentate(I) complex [Ag(CN)2]−. SEM-EDX verified the successful extraction of silver with KCN as only background noise was detected for silver at the MOF particles, yet the previous crystallinity of the MOF samples was not reinstated according to PXRD (see Section S13, ESI†).
Conclusion
We were able to reproducibly synthesize UiO-66 with the usage of Mac− as a modulator, yielding a highly porous, microcrystalline UiO-66 with evenly distributed modulator as seen in the SEM-EDX mappings. The amount of incorporated modulator was thoroughly determined by several analytical techniques, whereas in the literature often only the defect characterization is used to quantify the amount of modulator.UiO-66-MAc MOFs showed high sulfur contents for a modulated UiO-MOF with very high BET surface areas in the range of typical UiO-66 BET surface areas. The PXRD data for the products that were synthesized with 10, 30 and 50 eq. HMAc exhibited the typical fcu topology associated with UiO-66, whereas the synthesis with 100 eq. HMAc reproducingly yielded hcp UiO-66. To the best of our knowledge, this is the first functionalized hcp UiO-66.
The thiol-group was still intact after the synthesis as seen in the FT-Raman results. The presented synthesis route therefore offers a fast and cost-efficient possibility for the in-situ functionalization of UiO-66 with thiol groups. Mercaptoacetic acid as a modulator represents an affordable and easy way of introducing thiol groups into UiO-MOFs yielding high incorporated sulfur amounts of up to 7 wt%, while keeping the expected BET surface area of UiO-66.
The incorporation of Mac− modulator ligands with free thiol groups led to enhanced uptake of Ag+ from aqueous solutions when compared to non-functionalized UiO-66, with a maximum uptake of up to 84 mg g−1 for UiO-66-MAc-50 eq. and of 32 mg g−1 for UiO-66-MAc-100 eq. The uptake isotherms followed the Langmuir model and an initial pseudo second-order kinetics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr T.-O. Knedel for his introduction to the atomic absorption spectrometer. We also thank Mrs Birgit Tommes for IR measurements.Notes and references
- C. Janiak and J. K. Vieth, New J. Chem., 2010, 34, 2366–2388 RSC.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. Grant Glover, Y. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 RSC.
- K. Leus, T. Bogaerts, J. De Decker, H. Depauw, K. Hendrickx, H. Vrielinck, V. Van Speybroeck and P. Van Der Voort, Microporous Mesoporous Mater., 2016, 226, 110–116 CrossRef CAS.
- (a) F. Vermoortele, B. Bueken, G. Le Bars, B. Van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. Van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS; (b) R. Limvorapitux, H. Chen, M. L. Mendonca, M. Liu, R. Q. Snurr and S. T. Nguyen, Catal. Sci. Technol., 2019, 9, 327–335 RSC.
- (a) S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynsa, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC; (b) M. Waqas Anjum, F. Vermoortele, A. Laeeq Khan, B. Bueken, D. E. De Vos and I. F. J. Vankelecom, ACS Appl. Mater. Interfaces, 2015, 7, 25193–25201 CrossRef.
- (a) S. Rojas, I. Colinet, D. Cunha, T. Hidalgo, F. Salles, C. Serre, N. Guillou and P. Horcajada, ACS Omega, 2018, 3, 2994–3003 CrossRef CAS; (b) M. Nasrabadi, M. A. Ghasemzadeh and M. R. Z. Monfared, New J. Chem., 2019, 43, 16033–16040 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef.
- G. C. Shearer, J. G. Vitillo, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 7190–7193 CrossRef CAS.
- L. Huang, M. He, B. Chen and B. Hu, J. Mater. Chem. A, 2015, 3, 11587–11595 RSC.
- P. Yang, Y. Shu, Q. Zhuang, Y. Li and J. Gu, Chem. Commun., 2019, 55, 12972–12975 RSC.
- P. Yang, Y. Shu, Y. Li and J. Gu, Langmuir, 2019, 35, 16226–16233 CrossRef CAS.
- C. Wang, X. Liu, J. P. Chen and K. Li, Sci. Rep., 2015, 5, 16613 CrossRef CAS.
- Y. Fang, L. Zhang, Q. Zhao, X. Wang and X. Jia, Chem. Pap., 2019, 73, 1401–1411 CrossRef CAS.
- M. R. Azhar, H. R. Abid, V. Periasamy, H. Sun, M. O. Tade and S. Wang, J. Colloid Interface Sci., 2017, 500, 88–95 CrossRef CAS.
- S. Zaboon, H. R. Abid, Z. Yao, R. Gubner, S. Wang and A. Barifcani, J. Colloid Interface Sci., 2018, 523, 75–85 CrossRef CAS.
- C. Orellana-Tavra, R. J. Marshall, E. F. Baxter, I. Abánades Lázaro, A. Tao, A. K. Cheetham, R. S. Forgan and D. Fairen-Jimenez, J. Mater. Chem. B, 2016, 4, 7697–7707 RSC.
- H.-X. Zhao, Q. Zou, S.-K. Sun, C. Yu, X. Zhang, R.-J. Li and Y.-Y. Fu, Chem. Sci., 2016, 7, 5294–5301 RSC.
- H. Molavi, M. Zamani, M. Aghajanzadeh, H. K. Manjili, H. Danafar and A. Shojaei, Appl. Organomet. Chem., 2018, 32, e4221 CrossRef.
- K.-K. Yee, N. Reimer, J. Liu, S.-Y. Cheng, S.-M. Yiu, J. Weber, N. Stock and Z. Xu, J. Am. Chem. Soc., 2013, 135, 7795–7798 CrossRef CAS.
- L. Vial, R. F. Ludlow, J. Leclaire, R. Pérez-Fernández and S. Otto, J. Am. Chem. Soc., 2006, 128, 10253–10257 CrossRef CAS.
- M.-Q. Li, Y.-L. Wong, T.-S. Lum, K. S.-Y. Leung, P. K. S. Lam and Z. Xu, J. Mater. Chem. A, 2018, 6, 14566–14570 RSC.
- L. Ding, P. Shao, Y. Luo, X. Yin, S. Yu, L. Fang, L. Yang, J. Yang and X. Luo, Chem. Eng. J., 2020, 382, 123009 CrossRef CAS.
- J. C. McGreer, R. C. Playle, C. M. Wood and F. Galvez, Environ. Sci. Technol., 2000, 34, 4199–4207 CrossRef.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS.
- S. Kittler, C. Greulich, J. Diendorf, M. Köller and M. Epple, Chem. Mater., 2010, 22, 4548–4554 CrossRef CAS.
- A. Panáček, L. Kvítek, R. Prucek, M. Kolář, R. Večeřová, N. Pizúrovúá, V. K. Sharma, T. Nevěčná and R. Zbořil, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS.
- J. Farkas, H. Peter, P. Christian, J. A. G. Urrea, M. Hassellöv, J. Tuoriniemi, S. Gustafsson, E. Olsson, K. Hylland and K. V. Thomas, Environ. Int., 2011, 37, 1057–1062 CrossRef CAS.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed., 2013, 52, 1636–1653 CrossRef CAS.
- D. Roe, B. Karandikar, N. Bonn-Savage, B. Gibbins and J.-B. Roullet, J. Antimicrob. Chemoth., 2008, 61, 869–876 CrossRef CAS.
- R. Kaegi, A. Voegelin, C. Ort, B. Sinnet, B. Thalmann, J. Krismer, H. Hagendorfer, M. Elumelu and E. Mueller, Water Res., 2013, 47, 3866–3877 CrossRef CAS.
- S. Takenaka, E. Karg, C. Roth, H. Schulz, A. Ziesenis, U. Heinzmann, P. Schramel and J. Heyder, Environ. Health Perspect., 2001, 109, 547–551 CAS.
- P. V. AshaRani, G. Low Kah Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS.
- H. Bouwmeester, J. Poortman, R. J. Peters, E. Wijma, E. Kramer, S. Makama, K. Puspitaninganindita, H. J. P. Marvin, A. A. C. M. Peijnenburg and P. J. M. Hendriksen, ACS Nano, 2011, 5, 4091–4103 CrossRef CAS.
- S. Wang, H. Li, X. Chen, M. Yang and Y. Qi, J. Environ. Sci., 2012, 24, 2166–2172 CrossRef CAS.
- B. Zhang, S. Wang, L. Fu, J. Zhao, L. Zhang and J. Peng, Water, Air, Soil Pollut., 2018, 229, 199 CrossRef.
- C. Jeon, Korean J. Chem. Eng., 2017, 34, 384–391 CrossRef CAS.
- L. Ma, Q. Wang, S. M. Islam, Y. Liu, S. Ma and M. G. Kanatzidis, J. Am. Chem. Soc., 2016, 138, 2858–2866 CrossRef CAS.
- N. Pourreza, S. Rastegarzadeh and A. Larki, J. Ind. Eng. Chem., 2014, 20, 127–132 CrossRef CAS.
- J. E. Conde-González, E. M. Peña-Méndez, S. Rybáková, J. Pasán, C. Ruiz-Pérez and J. Havel, Chemosphere, 2016, 160, 659–666 CrossRef.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- R. Thür, N. Van Velthoven, V. Lemmens, M. Bastin, S. Smolders, D. D. Vos and I. F. J. Vankelecom, ACS Appl. Mater. Interfaces, 2019, 11, 44792–44801 CrossRef.
- S. Øien, D. Wragg, H. Reinsch, S. Svelle, S. Bordiga, C. Lamberti and K. P. Lillerud, Cryst. Growth Des., 2014, 14, 5370–5372 CrossRef.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS.
- C. G. Piscopo, A. Polyzoidis, M. Schwarzer and S. Loebecke, Microporous Mesoporous Mater., 2015, 208, 30–35 CrossRef CAS.
- M. J. Cliffe, W. Wan, X. Zou, P. A. Chater, A. K. Kleppe, M. G. Tucker, H. Wilhelm, N. P. Funnell, F.-X. Coudert and A. L. Goodwin, Nat. Commun., 2014, 5, 4176 CrossRef CAS.
- M. Ermer, J. Mehler, M. Kriesten, Y. S. Avadhut, P. S. Schulz and M. Hartmann, Dalton Trans., 2018, 47, 14426–14430 RSC.
- T. Zhao, L. Yang, P. Feng, I. Gruber, C. Janiak and Y.-J. Liu, Inorg. Chim. Acta, 2018, 471, 440–445 CrossRef CAS.
- L. Yang, T. Zhao, I. Boldog, C. Janiak, X.-Y. Yang, Q. Li, Y.-J. Zhou, Y. Xia, D.-W. Lai and Y.-J. Liu, Dalton Trans., 2019, 48, 989–996 RSC.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- L. Zhou, S. Wang, Y. Chen and C. Serre, Microporous Mesoporous Mater., 2019, 290, 109674 CrossRef CAS.
- C. A. Clark, K. N. Heck, C. D. Powell and S. Wong, ACS Sustainable Chem. Eng., 2019, 7, 6619–6628 CrossRef CAS.
- X. Chen, Y. Lyu, Z. Wang, X. Qiao, B. C. Gates and D. Yang, ACS Catal., 2020, 10, 2906–2914 CrossRef CAS.
- M. Thommes, K. Kaneko, A. Neimark, J. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CAS.
- S. Tai, W. Zhang, J. Zhang, G. Luo, Y. Jia, M. Deng and Y. Ling, Microporous Mesoporous Mater., 2016, 220, 148–154 CrossRef CAS.
- C. Atzori, G. C. Shearer, L. Maschio, B. Civalleri, F. Bonino, C. Lamberti, S. Svelle, K. P. Lillerud and S. Bordiga, J. Phys. Chem. C, 2017, 121, 9312–9324 CrossRef CAS.
- Y. Han, M. Liu, K. Li, Y. Zuo, Y. Wie, S. Xu, G. Zhang, C. Song, Z. Zhang and X. Guo, CrystEngComm, 2015, 17, 6434–6440 RSC.
- C. G. Piscopo, F. Trapani, A. Polyzoidis, M. Schwarzer, A. Pace and S. Loebbecke, New J. Chem., 2016, 40, 8220–8224 RSC.
- M. Kim and S. M. Cohen, CrystEngComm, 2012, 14, 4096–4104 RSC.
- M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC.
- Y. Marcus, Chem. Rev., 1988, 88, 1475–1498 CrossRef CAS.
- H. Ghassabzadeh, A. Mohadespour, M. Torab-Mostaedi, P. Zaheri, M. G. Maragheh and H. Taheri, J. Hazard. Mater., 2010, 177, 950–955 CrossRef CAS.
- X. Yin, J. Long, Y. Xi and X. Luo, ACS Sustainable Chem. Eng., 2017, 5, 2090–2097 CrossRef CAS.
- A. M. Donia, A. A. Atia and K. Z. Elwakeel, Hydrometallurgy, 2007, 87, 197–206 CrossRef CAS.
- L. Ma, Q. Wang, S. M. Islam, Y. Liu, S. Ma and M. G. Kanatzidis, J. Am. Chem. Soc., 2016, 138, 2858–2866 CrossRef CAS.
- F. Luo, K. L. Chen, L. L. Dang, W. N. Zhou, H. L. Lin, J. Q. Li, S. J. Liu and M. B. Luo, J. Mater. Chem. A, 2015, 3, 9616–9620 RSC.
- J. P. Simonin, Chem. Eng. J., 2016, 300, 254–263 CrossRef CAS.
- D. Robati, J. Nanostruct. Chem., 2013, 3, 55 CrossRef.
- T. S. Ho, Chem. Rev., 1975, 75, 1–20 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details, SEM-EDX, NMR, TGA, IR and Raman, Ar sorption, UiO structure description, stability tests, adsorption and kinetic modelling. See DOI: 10.1039/d0ma00555j |
| This journal is © The Royal Society of Chemistry 2021 |