Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improved performance of solution processed OLEDs using N-annulated perylene diimide emitters with bulky side-chains†
Sergey V.
Dayneko
 ab,
Edward
Cieplechowicz
ab,
Edward
Cieplechowicz
 a,
Sachin Suresh
Bhojgude
a,
Jeffrey F.
Van Humbeck
a,
Sachin Suresh
Bhojgude
a,
Jeffrey F.
Van Humbeck
 a,
Majid
Pahlevani
*c and
Gregory C.
Welch
a,
Majid
Pahlevani
*c and
Gregory C.
Welch
 *a
*a
aDepartment of Chemistry, University of Calgary, 731Campus Place NW, Calgary, Alberta T2N 1N4, Canada. E-mail: gregory.welch@ucalgary.ca
bGenoptic LED Inc., 6000 72nd Avenue SE, Calgary, AB T2C 5C3, Canada
cDepartment of Electrical and Computer Engineering, Queen's University, 19 Union St., Kingston, ON K7L 3N6, Canada. E-mail: majid.pahlevani@queensu.ca
First published on 17th December 2020
Abstract
We have designed and synthesized three N-annulated perylene diimide (PDI) compounds containing sterically bulky alkyl-substituted benzyl moieties (PDI-X, where X = 1, 2, 3) with photoluminescence quantum yield (PLQY) in the solid-state >20%. The PDI molecules show PLQY of ∼0.8 in solution and ∼0.2 as neat films. Organic light-emitting diodes (OLEDs) have been fabricated using these new PDI molecules as light emitters with the active layer being solution processed from non-halogenated solvents. The OLED devices had brightness of ∼2000 cd m−2 and low turn-on voltage of ∼3 V, among the best for PDI based red colored OLEDs.
Perylene diimide (PDI) based materials have been actively explored in the context of fluorescence labels,1 organic semiconducting devices,2,3 and light harvesters.4,5 The PDI chromophore has key properties such as tunable photon absorption and emission in the visible region, high photothermal stability, and solution processability.6 The high photoluminescence quantum yield (PLQY) of light-emitting materials in the solid-state, excellent electron and hole transport capacity, good film-forming properties and colour purities are considered essential parameters for the development of high-performance organic light-emitting diodes (OLEDs).7 PDI materials with PLQY of close to 100% in solution8,9 have been reported but the PLQY in solid-state is generally low due to aggregation-caused quenching (ACQ).10
Efforts have been made to increase the PLQY of PDI materials in the solid-state. Zhang and coworkers synthesized PDI molecules with bulky di-tert-butylphenyl and trityl moieties enabling PLQY near 30% in neat films and an optical quantum efficiency of 54% for luminescent solar concentrators incorporating these molecules.4 Kozma and coworkers appended large naphthyl moieties to the PDI chromophore and were able to achieve PLQY ∼ 23% in neat films and developed OLEDs based on these materials with moderate external quantum efficiency (EQE) of 0.6%.11 The performance of OLEDs depends not only on the PLQY of the emitting layer but charge transport, radiative exciton formation efficiency, and light outcoupling efficiency,12 thus completely isolating the PDI chromophore is not a option. A way to increase PLQY without sacrificing electrical performance is to control the aggregation of the PDI units via the formation of dimers, trimers, tetramers.13 Such larger oligomers and/or starburst derivatives can reduce co-facial π–π stacking and limit ACQ.14
Recently, the Welch group has reported on a series of N-annulated PDI dimers with controlled aggregation tendencies15 and green solvent processability16 that have found utility in both organic solar cells17–20 and OLEDs.21,22 These compounds are readily made on scale, are highly soluble in common organic processing solvents, and exhibit a range of optoelectronic properties. N-Annulation destabilizes the frontier molecular orbital energy levels changing the PDI electronic structure23 while the pyrrolic N-position can be readily functionalized.24 We have shown that an N-annulated PDI dimer with bulky 2-ethylhexyl sidechains (tPDI2N-EH) can function as an effective light emitter in OLEDs21 and our hypothesis was that increasing the bulk of the side-chain would lead to greater PLQY in the film and subsequent increased OLED device performance.
Herein, we report the synthesis and characterisation of air stable red-light-emitting N-annulated PDI dimers substituted at pyrrolic N-atom position with 2,4,6-trimethylbenzyl, 2,4,6-trisopropylbenzyl, and 3,5-di-tert-butylbenzyl side-chains. The target structures tPDI2N-trimethylbenzyl (PDI-1), tPDI2N-trisopropylbenzyl (PDI-2), and tPDI2N-di-tert-butylbenzyl (PDI-3) are shown in Fig. 1. The benzyl side chains with sterically bulky alkyl substituents can lead to different intermolecular interactions as compared to alkyl side chains, with the possibility of different electronic influences on the dimeric PDI core. The PDI dimers are easily processed into uniform films from non-halogenated solvents. The UV-vis absorption and PL spectra of the PDI molecules in diluted solution were recorded and PLQYs were calculated. The absolute PLQY of PDI molecules dispersed into neat films were measured by an integrating sphere. Finally, OLED devices based on the emitting layer containing the PDI-X (X = 1, 2, 3) molecules were fabricated and tested in air. The OLED devices reported here show enhanced performance compared to previously reported PDI dimers.21,22 Complete synthesis and characterization are presented in the ESI.†
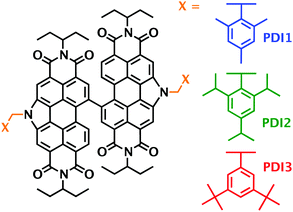 | ||
| Fig. 1 Chemical structures of the three new PDI molecules designed, synthesized, and used as light emitters in OLED devices. | ||
To investigate the influence of different substituted benzyl side chains on the photophysical properties of the PDI derivatives, the UV-vis absorption and emission spectra of PDI-1, PDI-2 and PDI-3 in o-xylene solution and as thin films were measured (Fig. 2 and Table 1). In the o-xylene solution, the absorption spectra of all PDI molecules are identical and demonstrate the two characteristic maxima of the PDI chromophore.25–27 All dimers have high PLQY of ∼0.7 in solution with similar PL spectra and emission maxima at ∼580 nm. The high PLQY of these compounds is attributed to the bulky-side chains which inhibits aggregation resulting in partial isolation of PDI dimer core.24,28,29 For films cast from o-xylene, the absorption spectra of all PDI molecules are slightly red-shifted and broadened compared to the solution indicating that the PDI dimers are weakly aggregated. All PDI molecules in the film have PLQY of 20–25% which is high for PDI-based light emitting materials.
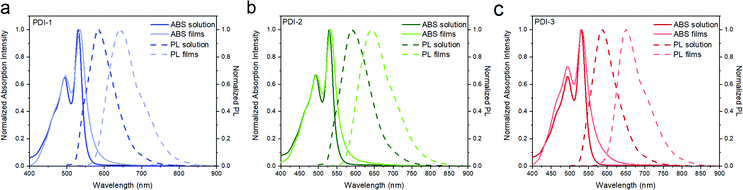 | ||
| Fig. 2 Absorption (ABS) and emission (PL) spectra of all PDI molecules in o-xylene solutions and neat films processed from o-xylene solutions. | ||
| Absorption (nm) | Emission (nm) | Stokes shiftc (cm-1) | PLQY (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Onsetb | 0–0 | 0–1 | Max | FWHMd | ||||
| a Solution and neat films of PDI 1–3 in o-xylene or spin-cast from o-xylene. b Absorption onset determined from the intersection of optical absorption and emission spectra. c Stokes Shift (Δν = νmax,PL − νmax,ABS).30 d FWHM: full width at half maximum of photoluminescence spectra. | ||||||||
| PDI-1 | Solutiona | 543 | 530 | 495 | 586 | 84 | 1803 | 76 |
| Filma | 564 | 535 | 496 | 640 | 97 | 3067 | 20 | |
| PDI-2 | Solutiona | 543 | 530 | 493 | 591 | 90 | 1947 | 65 |
| Filma | 584 | 532 | 494 | 642 | 101 | 3221 | 25 | |
| PDI-3 | Solutiona | 546 | 532 | 494 | 584 | 76 | 1674 | 74 |
| Filma | 572 | 532 | 494 | 650 | 83 | 3412 | 25 | |
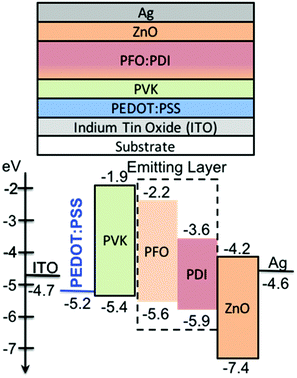
A multi-stack device structure was used to fabricated OLEDs31 (Fig. 3). The substrate was glass and indium tin oxide (ITO) was used as the anode. Poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonic acid) (PEDOT:PSS) and polyvinylcarbazole (PVK) were used as the hole injection and transport layers, respectively. Zinc oxide (ZnO) was used as an electronic transport layer with silver (Ag) as the top cathode. The PDI molecules were used individually in the emitting layer in combination with the polyfluorene polymer PFO (i.e. poly(9,9-di-n-octylfluorenyl-2,7-diyl)) to assist with hole transport.21
PEDOT:PSS films were formed by spin-casting aqueous suspension of PEDOT:PSS on top of cleaned glass/ITO and then annealed at 150 °C for 30 min in air. PVK was spin-coated from toluene on top of the PEDOT:PSS and annealed at 120 °C. Of note the PVK film is resistant to o-xylene solvent and thus allows for good formation of the emitting layer films on top.22 The emitter layer was spin-coated on top of the PVK film from o-xylene solutions of PFO:PDI molecules (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w ratio). ZnO nanoparticles in methanol were spin-coated on top and annealed at 60 °C for 30 min in air. The emitting layer films were found to show good tolerance to methanol solvent (Fig. S1 and S2, ESI†). The Ag top electrode deposited via thermal evaporation (Fig. 3).
9 w/w ratio). ZnO nanoparticles in methanol were spin-coated on top and annealed at 60 °C for 30 min in air. The emitting layer films were found to show good tolerance to methanol solvent (Fig. S1 and S2, ESI†). The Ag top electrode deposited via thermal evaporation (Fig. 3).
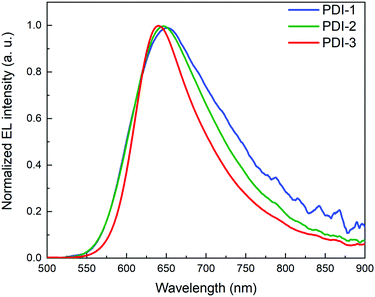
The device parameters of PDI-based OLEDs are summarized in Table 2. All PDI-based OLEDs had similar performance with a maximum external quantum efficiency (EQE) of ∼0.7%, luminous efficiency (LE) of 0.6 cd A−1, power efficiency (PE) of ∼0.3 lm W−1 and a maximum of luminous (Lmax) of 2400 cd m−2 and low turn-on voltage (Von) of ∼3 V. Notably, the electroluminescence (EL) spectra of all PDI molecules are similar with a deep red maximum at ∼650 nm with the CIE (international commission on illumination) coordinates of (x = 0.66; y = 0.33) (Fig. 4). The EQE is a critical parameter of a OLED device that describes the ratio between the number of emitted photons and injected charge carriers which has been expressed by equation EQE = γ·ηS/T·q·ηout,32–35 where γ is the charge carrier balance factor; ηS/T is the singlet–triplet factor; q is the effective quantum yield of the emitter material and ηout is the out-coupling efficiency of the emitted light. For theoretical evaluation of EQE the γ value is often assumed to be unity, ηS/T equal 0.25 for fluorescent emitters, q equals the PLQY of EL not considering the emitting dipole orientation factor and the Purcell effect;36ηout can be estimated as 1/(2n2) for isotropic emitters, where n is the refractive index of the organic EL.37 Here, the theoretical EQE is estimated at 1% based on a 20% PLQY and n ∼ 1.5.38 The OLED devices had a maximum EQE ranging from 0.4% to 0.7% for each different PDI molecule. Clearly by varying the sidechain of the PDI molecule, slight changes in OLED device performance are obtained. Compared to the literature these PDI based OLEDs have much improved performance.11,12
| Emittera | V on (V) | EQEmaxc (%) | LEmaxd (cd A−1) | PEmaxe (lm W−1) | L max (cd m−2) |
|---|---|---|---|---|---|
a Blened with PFO (ratio of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PFO 2 PFO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI).
b Turn on voltage was determined at the brightness of 1 cd m−2.
c EQE – external quantum efficiency.
d LE – luminous efficiency.
e PE – power efficiency.
f
L
max – maximum of luminance. PDI).
b Turn on voltage was determined at the brightness of 1 cd m−2.
c EQE – external quantum efficiency.
d LE – luminous efficiency.
e PE – power efficiency.
f
L
max – maximum of luminance.
|
|||||
| PDI-1 | 3.2 | 0.4 | 0.36 | 0.22 | 1800 |
| PDI-2 | 3.8 | 0.7 | 0.58 | 0.29 | 2145 |
| PDI-3 | 3.3 | 0.64 | 0.62 | 0.30 | 2400 |
| tPDI2N-EH21 | 2.6 | 0.06 | 0.05 | 0.03 | 400 |
Conclusion
A series of N-annulated perylene diimides with sterically bulky alkyl-substituted benzyl side chains were designed, synthesized, and used as emitters in OLED devices. These PDI dimers demonstrated high PLQY in the solution (∼80%) and as neat films (∼20%). The PDI molecules were used as emitting layers and solution processed from the non-halogenated solvent o-xylene. All OLED devices based on the PDI molecules showed good performance with EQE ∼ 0.7%, high brightness of 2400 cd m−2 with deep red maximum EL spectra of ∼650 nm, and CIE coordinates of (x = 0.66; y = 0.33). Future work is set to explore the utility of such molecules in complete roll-to-roll processed OLED devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All authors thank Alberta Innovates (grant # G2018000901) for funding. SVD would also like to thank the MITACS Accelerate and Elevate Post-Doctoral Programs and Geoptics LED Inc. for salary support.References
- K. Huth, M. Glaeske, K. Achazi, G. Gordeev, S. Kumar, R. Arenal, S. K. Sharma, M. Adeli, A. Setaro, S. Reich and R. Haag, Small, 2018, 14, 1800796 CrossRef.
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447–3507 CrossRef CAS.
- A. Wadsworth, M. Moser, A. Marks, M. S. Little, N. Gasparini, C. J. Brabec, D. Baran and I. McCulloch, Chem. Soc. Rev., 2019, 48, 1596–1625 RSC.
- B. Zhang, H. Soleimaninejad, D. J. Jones, J. M. White, K. P. Ghiggino, T. A. Smith and W. W. H. Wong, Chem. Mater., 2017, 29, 8395–8403 CrossRef CAS.
- C. Haines, M. Chen and K. P. Ghiggino, Sol. Energy Mater. Sol. Cells, 2012, 105, 287–292 CrossRef CAS.
- B. Lü, Y. Chen, P. Li, B. Wang, K. Müllen and M. Yin, Nat. Commun., 2019, 10, 767 CrossRef.
- B. Geffroy, P. le Roy and C. Prat, Polym. Int., 2006, 55, 572–582 CrossRef CAS.
- F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef.
- F. Würthner, Chem. Commun., 2004, 1564–1579 RSC.
- F. Zhang, Y. Ma, Y. Chi, H. Yu, Y. Li, T. Jiang, X. Wei and J. Shi, Sci. Rep., 2018, 8, 8208 CrossRef.
- E. Kozma, W. Mróz, F. Villafiorita-Monteleone, F. Galeotti, A. Andicsová-Eckstein, M. Catellani and C. Botta, RSC Adv., 2016, 6, 61175–61179 RSC.
- Y. Qin, G. Li, T. Qi and H. Huang, Mater. Chem. Front., 2020, 4, 1554–1568 RSC.
- M. Li, H. Yin and G.-Y. Sun, Appl. Mater. Today, 2020, 21, 100799 CrossRef.
- H. Hu, Y. Li, J. Zhang, Z. Peng, L. Ma, J. Xin, J. Huang, T. Ma, K. Jiang, G. Zhang, W. Ma, H. Ade and H. Yan, Adv. Energy Mater., 2018, 8, 1800234 CrossRef.
- F. Tintori, A. Laventure and G. C. Welch, Soft Matter, 2019, 15, 5138–5146 RSC.
- C. R. Harding, J. Cann, A. Laventure, M. Sadeghianlemraski, M. Abd-Ellah, K. R. Rao, B. S. Gelfand, H. Aziz, L. Kaake, C. Risko and G. C. Welch, Mater. Horiz., 2020, 7, 2959–2969 RSC.
- F. Tintori, A. Laventure, J. D. B. Koenig and G. C. Welch, J. Mater. Chem. C, 2020, 8, 13430–13438 RSC.
- S. V. Dayneko, A. D. Hendsbee, J. R. Cann, C. Cabanetos and G. C. Welch, New J. Chem., 2019, 43, 10442–10448 RSC.
- M. Nazari, M. Martell, T. A. Welsh, O. Melville, Z. Li, J. Cann, E. Cieplechowicz, Y. Zou, B. H. Lessard and G. C. Welch, Mater. Chem. Front., 2018, 2, 2272–2276 RSC.
- S. V. Dayneko, A. D. Hendsbee and G. C. Welch, Chem. Commun., 2017, 53, 1164–1167 RSC.
- S. V. Dayneko, M. Rahmati, M. Pahlevani and G. C. Welch, J. Mater. Chem. C, 2020, 8, 2314–2319 RSC.
- M. Rahmati, S. V. Dayneko, M. Pahlevani and G. C. Welch, ACS Appl. Electron. Mater., 2020, 2, 48–55 CrossRef CAS.
- M. Nazari, E. Cieplechowicz, T. A. Welsh and G. C. Welch, New J. Chem., 2019, 43, 5187–5195 RSC.
- S. V. Dayneko, A. D. Hendsbee and G. C. Welch, Small Methods, 2018, 2, 1800081 CrossRef.
- W. E. Ford, J. Photochem., 1986, 34, 43–54 CrossRef CAS.
- W. E. Ford and P. V. Kamat, J. Phys. Chem., 1987, 91, 6373–6380 CrossRef CAS.
- J. Cann, S. Dayneko, J.-P. Sun, A. D. Hendsbee, I. G. Hill and G. C. Welch, J. Mater. Chem. C, 2017, 5, 2074–2083 RSC.
- C. Huang, S. Barlow and S. R. Marder, J. Org. Chem., 2011, 76, 2386–2407 CrossRef CAS.
- A. Reisch and A. S. Klymchenko, Small, 2016, 12, 1968–1992 CrossRef CAS.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012 Search PubMed.
- S. B. Meier, D. Tordera, A. Pertegás, C. Roldán-Carmona, E. Ortí and H. J. Bolink, Mater. Today, 2014, 17, 217–223 CrossRef CAS.
- T. Tsutsui, E. Aminaka, C. P. Lin and D.-U. Kim, Philos. Trans. R. Soc. London, Ser. A, 1997, 355, 801–814 CrossRef CAS.
- S.-Y. Kim, W.-I. Jeong, C. Mayr, Y.-S. Park, K.-H. Kim, J.-H. Lee, C.-K. Moon, W. Brütting and J.-J. Kim, Adv. Funct. Mater., 2013, 23, 3896–3900 CrossRef CAS.
- B. Sim, C.-K. Moon, K.-H. Kim and J.-J. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 33010–33018 CrossRef CAS.
- W. Brütting, J. Frischeisen, T. D. Schmidt, B. J. Scholz and C. Mayr, Phys. Status Solidi, 2013, 210, 44–65 CrossRef.
- E. M. Purcell, H. C. Torrey and R. V. Pound, Phys. Rev., 1946, 69, 37–38 CrossRef CAS.
- N. C. Greenham, R. H. Friend and D. D. C. Bradley, Adv. Mater., 1994, 6, 491–494 CrossRef CAS.
- Z. Kişnişci, Ö. F. Yüksel and M. Kuş, Synth. Met., 2014, 194, 193–197 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00827c |
| This journal is © The Royal Society of Chemistry 2021 |