Open Access Article
Open Access ArticleReduced graphene oxide integrated poly(ionic liquid) functionalized nano-fibrillated cellulose composite paper with improved toughness, ductility and hydrophobicity†
Rama K.
Layek
 *ab,
Vijay Singh
Parihar
*ab,
Vijay Singh
Parihar
 c,
Jukka
Seppälä
d,
Alexander
Efimov
c,
Jukka
Seppälä
d,
Alexander
Efimov
 e,
Sarianna
Palola
e,
Sarianna
Palola
 a,
Mikko
Kanerva
a,
Shambhavee
Annurakshita
a,
Mikko
Kanerva
a,
Shambhavee
Annurakshita
 f,
Minna
Kellomäki
f,
Minna
Kellomäki
 c and
Essi
Sarlin
a
c and
Essi
Sarlin
a
aTampere University, Engineering Material Science, P.O. Box 589, 33101 Tampere, Finland. E-mail: rama.layek@tuni.fi; Tel: +35850 4478355
bDepartment of Separation Science, School of Engineering Science, LUT University, Mukkulankatu 19, 15210 Lahti, Finland. E-mail: rama.layek@lut.fi
cBiomaterials and Tissue Engineering Group, BioMediTech, Faculty of Medicine and Health Technology, Tampere University, 33720 Tampere, Finland
dPolymer Technology, School of Chemical Engineering, Aalto University, Finland
eFaculty of Engineering and Natural Science, Tampere University, 33720 Tampere, Finland
fPhotonics Laboratory, Tampere University, 33720 Tampere, Finland
First published on 15th January 2021
Abstract
Reduced graphene oxide (rGO) integrated nano-fibrillated cellulose (NFC) grafted poly(ionic liquid) (PIL) composite paper with enhanced hydrophobicity, ductility and toughness was fabricated by vacuum filtration of an aqueous dispersion of rGO/NFC-graft-PIL (NFC-g-PIL). The NFC-g-PIL was synthesized by growing PIL chains from the NFC surface using free radical polymerization of a pyridinium acrylate-based ionic liquid monomer.
Nano-fibrillated cellulose (NFC) is an extremely interesting material class due to its environmental greenness, renewable resources, abundant feedstock, lightweight, high aspect ratio, optical transparency, and excellent strength and stiffness.1,2 NFC has several oxygen-containing functional groups on the surface that can bind the nanofibrils together via H-bonding interaction to produce strong paper-like structures2,3 that are very promising for the replacement of the fossil fuel-derived synthetic plastics in the near future. Recently, NFC based composite papers have been attractive to replace many petroleum-derived polymers for various demanding applications3,4 due to their excellent properties.
Graphene, a multifunctional nanomaterial with a 2-D nanostructure has outstanding physical, and mechanical properties.5,6 Hence, NFC composites with graphene are gaining significant interest for various demanding applications, such as structural films, flexible electronic device packaging films, and other hybrid materials due to their promising mechanical, gas barrier and electrical properties.7,8 In this regard, a few studies are focusing on the design of graphene/NFC composites to bring new functionalities such as higher stiffness and mechanical strength, improved thermal stability and hydrophobicity, good electrical and thermal conductivity, and gas barrier properties to boost their applicability in demanding areas to replace the petroleum-derived advanced polymer composites.7–9 Graphene oxide (GO)/NFC composite papers show a remarkable improvement of mechanical properties10 due to the interfacial interaction between the hydrophilic oxygen-containing functional groups of GO and NFC. These hydrophilic oxygen-containing functional groups may absorb moisture from the environment,10 which reduces the performance of graphene/NFC composite films. The in situ reduced graphene oxide (rGO)/NFC composites and graphene nanoplates/NFC composites obtained by a vacuum-assisted assembly process display enhancement of multifunctionality9 with a decreased ductility due to nano integration and decrease of molecular mobility of the NFC chain. Hence, the majority of the graphene/nanocellulose composite films are either hydrophilic in nature, or less ductile due to the formation of a stiff and brittle composite. These are very serious issues and must be overcome before full utilization of the outstanding functionalities of graphene/NFC composite papers.
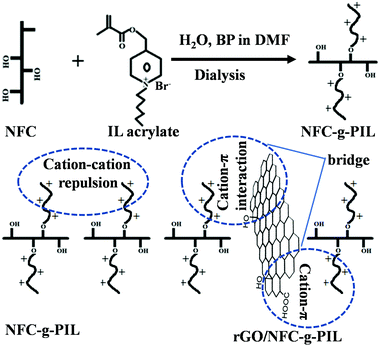
These issues of the graphene/NFC composites can be overcome by generating additional functionalities such as hydrophobicity, ductility and toughness in graphene/NFC composite films via tailoring the chemical functionalities and interfacial interaction between NFC and graphene or derivatives of graphene. It has been already reported that an ionic liquid (IL) containing hydrophobic moiety anchored on the surface of the NFC fibrils can enhance the hydrophobicity of the cellulosic derivatives.11 Besides this, IL also enhances the processability, physical properties, mechanical properties and ductility of the cellulosic derivatives by reducing the interactions between the cellulose fibrils.11 Recently, the scope of IL-based polymers is gaining significant attention over ILs due to their ability to improve the processability and mechanical properties within the polymeric structure over the IL monomer.12,13 Polyionic liquid (PIL) contains IL moieties in each repeating unit as it is synthesized by polymerizing the IL monomers, and can introduce new functionalities via tailoring the chemical functionalities of the PIL. Though there are numerous procedures reported to tailor the multifunctionalities of graphene/NFC composites, to the best of our knowledge, there is no report of simultaneous tailoring of the ductility, toughness, and hydrophobicity. In this communication, for the first time, we are reporting the synthesis of a novel NFC-graft-PIL (NFC-g-PIL) containing hydrophobic pyridyl hexyl chains and its free-standing composite paper with hydrophobic rGO. The novelty of the preparation rGO/NFC-g-PIL paper is in its ductility and hydrophobicity. The ductility of the rGO/NFC-g-PIL paper is related to elongation at break. The obtained rGO/NFC-g-PIL paper shows higher hydrophobicity and ductility, along with significant enhancement of mechanical strength with respect to NFC paper.
NFC-g-PIL was synthesized by growing PIL from NFC (Scheme 1) by free radical polymerization of 1-hexyl-4-(methacryloyloxy-methyl) pyridinium bromide ionic liquid (IL acrylate) using benzoyl peroxide (BP) as an initiator.14 GO was synthesized by Hummers' method15a and rGO was prepared by reducing GO with hydrazine hydrate in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF
1 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O dispersion.15b The free-standing rGO/NFC and rGO/NFC-g-PIL composite papers were prepared by a vacuum-assisted assembly procedure (details in the ESI,† Experimental section). The required amount of rGO dispersion (1 wt% with respect NFC) was mixed with 500 mL of aqueous dispersion of NFC (1.4 mg mL−1) and NFC-g-PIL (1.4 mg mL−1) and sonicated for 30 minutes to obtain uniform rGO/NFC and rGO/NFC-g-PIL solutions. Finally, the rGO/NFC and rGO/NFC-g-PIL composite films were obtained by vacuum filtration followed by compression at 70 °C and named rGO1/NFC and rGO1/NFC-g-PIL (number 1 represent wt% of rGO w.r.t. NFC and NFC-g-PIL). Pure NFC and NFC-g-PIL films were also obtained from the 1.4 mg mL−1 aqueous NFC and NFC-g-PIL dispersions, respectively, without using any rGO for comparison. The detailed synthesis procedure of NFC-g-PIL and fabrication of NFC, NFC-g-PIL paper, rGO1/NFC and rGO1/NFC-g-PIL composite papers and their digital photographs (ESI,† Fig. S1) are presented in the ESI.†
H2O dispersion.15b The free-standing rGO/NFC and rGO/NFC-g-PIL composite papers were prepared by a vacuum-assisted assembly procedure (details in the ESI,† Experimental section). The required amount of rGO dispersion (1 wt% with respect NFC) was mixed with 500 mL of aqueous dispersion of NFC (1.4 mg mL−1) and NFC-g-PIL (1.4 mg mL−1) and sonicated for 30 minutes to obtain uniform rGO/NFC and rGO/NFC-g-PIL solutions. Finally, the rGO/NFC and rGO/NFC-g-PIL composite films were obtained by vacuum filtration followed by compression at 70 °C and named rGO1/NFC and rGO1/NFC-g-PIL (number 1 represent wt% of rGO w.r.t. NFC and NFC-g-PIL). Pure NFC and NFC-g-PIL films were also obtained from the 1.4 mg mL−1 aqueous NFC and NFC-g-PIL dispersions, respectively, without using any rGO for comparison. The detailed synthesis procedure of NFC-g-PIL and fabrication of NFC, NFC-g-PIL paper, rGO1/NFC and rGO1/NFC-g-PIL composite papers and their digital photographs (ESI,† Fig. S1) are presented in the ESI.†
The 1H and 13C NMR spectra of pyridinium IL and pyridinium IL acrylate are presented in the ESI,† Fig. S2–S5 respectively. The detail interpretation of NMR (1H and 13C) spectra is presented in the Experimental section of the ESI,† and it confirms the synthesis of pyridinium IL and pyridinium IL acrylate. The grafting of PIL from the NFC surface was confirmed by comparing solid state 1H-NMR spectra of pure NFC with NFC-g-PIL, where the distinguished aromatic pyridine ring protons and aliphatic hexyl protons of the repeating unit of pyridinium IL peaks clearly appear in the spectra (ESI,† Fig. S6 and S8). The grafting of PIL was further supported by comparing solid-state cross-polarization magic angle spinning Carbon-13 NMR (CP/MAS 13C-NMR) of pure NFC with NFC-g-PIL in the ESI,† Fig. S7 and S9.
NFC is a hydrophilic biopolymer that contains several hydroxyl groups on the surface and hence, the sonication of NFC in an aqueous medium produces a stable aqueous suspension. The vacuum filtration of these aqueous suspensions produces a free-standing NFC film through the H-bonding interaction and NFC-g-PIL film through the H-bonding and electrostatic interactions. The sonication of rGO dispersion with an aqueous suspension of NFC and NFC-g-PIL produced homogeneous aqueous suspensions of rGO/NFC and rGO/NFC-g-PIL. The vacuum-assisted composite formation assembly of rGO/NFC is governed by the H-bonding and hydrophobic interaction; whereas the composite formation assembly of rGO/NFC-g-PIL by H-bonding, hydrophobic and cation–π interactions.16
In order to investigate the interfacial interaction between rGO and NFC-g-PIL; the Fourier transform infrared spectroscopy (FTIR) studies of the NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composite papers were carried out and the recorded spectra are shown in Fig. 1(A). The NFC exhibits a typical intense and broad peak at 3310 cm−1 for the stretching vibrations of inter and intramolecular H-bonded –OH groups and the C–H stretching vibration peak in the region of 3000–2800 cm−1.17 This H-bonded –OH stretching peak of NFC is shifted in NFC-g-PIL from 3310 cm−1 to 3325 cm−1 and becomes less broad in NFC-g-PIL. This indicates that interfibrillar H-bonding interaction between the hydroxyl groups is reduced in NFC-g-PIL due to the grafting of PIL from the NFC surface. Besides this, NFC-g-PIL also shows a characteristic peak of polymethyl IL acrylate at 1738 cm−1 for the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of ester groups.6 The C–H stretching vibration peak in the region of 3000–2800 cm−1 in NFC-g-PIL increases due to the presence of the hexyl chain in the pyridinium ring and methyl group in polymethyl IL acrylate. rGO shows a characteristic peak at ∼1718 cm−1, ∼3426 cm−1 and ∼1620 cm−1 for the C
O stretching vibration of ester groups.6 The C–H stretching vibration peak in the region of 3000–2800 cm−1 in NFC-g-PIL increases due to the presence of the hexyl chain in the pyridinium ring and methyl group in polymethyl IL acrylate. rGO shows a characteristic peak at ∼1718 cm−1, ∼3426 cm−1 and ∼1620 cm−1 for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the –COOH group, stretching vibration of –OH groups and skeletal vibration of the unoxidized graphitic domain.6,17 In the rGO/NFC composites the –OH stretching vibration peak appeared in the same position as for pure NFC. The –OH stretching vibration band in rGO/NFC-g-PIL is also shifted from 3325 cm−1 to 3320 cm−1 due to the H-bonding and ionic interaction. The NFC-g-PIL displays a typical peak at 1547 cm−1 for N+–C bond of PIL. In rGO/NFC-g-PIL composites, this N+–C stretching vibration band is shifted from 1547 cm−1 to 1535 cm−1 and this may be due to the cation–π interaction between the cationic PIL of NFC-g-PIL and π-cloud of an aromatic rGO ring (Scheme 1).16 These results demonstrate that rGO sheets bridge inter-fibrillated NFC-g-PIL via H-bonding, ionic and cationic–π interactions.
O stretching vibration of the –COOH group, stretching vibration of –OH groups and skeletal vibration of the unoxidized graphitic domain.6,17 In the rGO/NFC composites the –OH stretching vibration peak appeared in the same position as for pure NFC. The –OH stretching vibration band in rGO/NFC-g-PIL is also shifted from 3325 cm−1 to 3320 cm−1 due to the H-bonding and ionic interaction. The NFC-g-PIL displays a typical peak at 1547 cm−1 for N+–C bond of PIL. In rGO/NFC-g-PIL composites, this N+–C stretching vibration band is shifted from 1547 cm−1 to 1535 cm−1 and this may be due to the cation–π interaction between the cationic PIL of NFC-g-PIL and π-cloud of an aromatic rGO ring (Scheme 1).16 These results demonstrate that rGO sheets bridge inter-fibrillated NFC-g-PIL via H-bonding, ionic and cationic–π interactions.
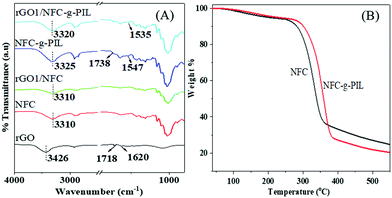 | ||
| Fig. 1 (A) FTIR spectra of rGO, NFC, rGO1/NFC, NFC-g-PIL and rGO1/NFC-g-PIL; and (B) TGA of NFC and NFC-g-PIL. | ||
The TGA thermograms of NFC, and NFC-g-PIL are displayed in Fig. 1(B). The NFC shows a very small amount of weight loss below 100 °C due to the removal of associated water molecules and a sharp weight loss in the temperature range of 330 and 550 °C due to dehydration and decomposition of the NFC moiety.18 The NFC-g-PIL film shows a relatively higher weight loss compared to the pure NFC film in this temperature range. This may be due to the degradation of the PIL moiety of NFC-g-PIL along with the dehydration and decomposition of the NFC moiety in the same temperature range.13
To investigate the morphology and height profile of the individual NFC and NFC-g-PIL fibers, the AFM image and the corresponding height profile of NFC and NFC-g-PIL are presented in the ESI,† Fig. S10(A) and (B). The AFM image and the corresponding height profile of pure NFC show that most of the NFC fibrils have a diameter of less than 10 nm, but very few thicker NFC fibrils with aggregated bundle structures are observed. The grafting of PIL from the NFC surface does not alter the fibrillar structure of NFC; however, both the image and height profile of NFC-g-PIL show somewhat higher thickness than that of pure NFC. The increase of thickness of NFC-g-PIL fibers compared to NFC fibers proved that the PIL chain has been grafted from the NFC surface. The grafting is also evident from the solid state 1H and 13C NMR, and FTIR spectroscopy results. The AFM image (Fig. 2(A)) of rGO was taken by casting a drop of dilute H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF(9
DMF(9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution on a mica substrate. These results show a sheet-like structure of rGO; and the corresponding height profile (Fig. 2(B)) shows an rGO sheet height of ≈0.85 nm, which indicates that the rGO sheets remain highly exfoliated in the dilute H2O
1) solution on a mica substrate. These results show a sheet-like structure of rGO; and the corresponding height profile (Fig. 2(B)) shows an rGO sheet height of ≈0.85 nm, which indicates that the rGO sheets remain highly exfoliated in the dilute H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF(9
DMF(9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution.15b In order to investigate the cross-sectional morphology and structure of the materials, scanning electron microscopy (FESEM) images of cryo fractured cross-sections of NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composites are shown in Fig. S2 and the ESI,† Fig. S11. Fig. 2(C) and (D) show the morphology of the assembled fiber of NFC and NFC-g-PIL respectively. These assembled fiber formations were observed due to the stacking of NFC and NFC-g-PIL fibrils together during the vacuum filtration process. From Fig. 2(E), it is noticeable that the exfoliated rGO sheets are well dispersed throughout the NFC-g-PIL matrix due to strong H-bonding, electrostatic and cation–π interactions.16 The rGO sheets also display good dispersion throughout the NFC matrix in rGO1/NFC composites (ESI,† Fig. S11) due to the hydrophobic interaction.4
1) solution.15b In order to investigate the cross-sectional morphology and structure of the materials, scanning electron microscopy (FESEM) images of cryo fractured cross-sections of NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composites are shown in Fig. S2 and the ESI,† Fig. S11. Fig. 2(C) and (D) show the morphology of the assembled fiber of NFC and NFC-g-PIL respectively. These assembled fiber formations were observed due to the stacking of NFC and NFC-g-PIL fibrils together during the vacuum filtration process. From Fig. 2(E), it is noticeable that the exfoliated rGO sheets are well dispersed throughout the NFC-g-PIL matrix due to strong H-bonding, electrostatic and cation–π interactions.16 The rGO sheets also display good dispersion throughout the NFC matrix in rGO1/NFC composites (ESI,† Fig. S11) due to the hydrophobic interaction.4
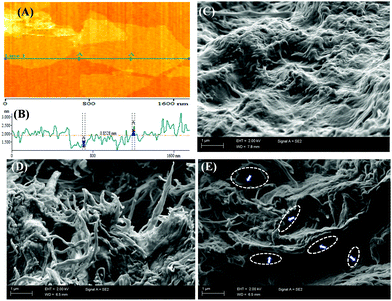 | ||
| Fig. 2 (A) AFM image of rGO and (B) corresponding height profile; FESEM image of the cross-section of (C) NFC; (D) NFC-g-PIL and (E) the rGO1/NFC-g-PIL composite. | ||
To investigate the hydrophobicity of the rGO1/NFC-g-PIL, the water droplet contact angle results of the NFC, NFC-g-PIL, rGO 1/NFC and rGO1/NFC-g-PIL are shown in Fig. 3(A) and ESI,† Table S1. Pure NFC shows a contact angle of 49 ± 2° indicating that it is hydrophilic in nature. Due to the grafting of PIL from the NFC surface, the water contact angle values increased from 49 ± 2° to 65 ± 1.5° for NFC-g-PIL. The rGO1/NFC and rGO1/NFC-g-PIL composites display contact angles of 66 ± 1° and 75 ± 1°. It is reported that graphene sheets have outstanding hydrophobicity19 and hence, successful integration of rGO improved the hydrophobicity of rGO1/NFC and rGO1/NFC-g-PIL composites in this study.
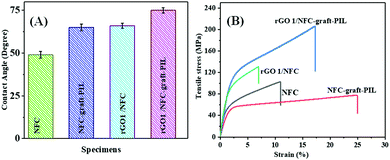 | ||
| Fig. 3 (A) The contact angle and (B) the stress–strain curve of NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composites. | ||
To examine the mechanical behaviour of the composite films, the stress–strain curves of the NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composites are presented in Fig. 3(B). Due to the presence of the strong H-bonding interaction between NFC nanofibers, the pure NFC film shows an excellent tensile strength of 105 ± 5 MPa, Young's modulus of 6.1 ± 0.30 GPa, and toughness of 9.21 MJ m−3 with an elongation at break of 12 ± 1.5%. For the NFC-g-PIL film, the tensile strength decreases from 105 ± 5 MPa to 80 ± 4.3 MPa and the Young's modulus from 6.1 ± 0.30 GPa to 3.5 ± 0.20 GPa. However, the elongation at break and toughness of the NFC-g-PIL film increase from 12 ± 1.5% to 25% and 9.21 MJ m−3 to 15.9 MJ m−3 respectively. The covalently grafted cationic PIL remains anchored over the NFC surface via electrostatic interaction and possibly weakens the interfibrillar interactions due to electrostatic repulsion between the cationic PIL of the NFC-g-PIL compared to pure NFC causing the decrease of tensile strength and Young's modulus but with a significant enhancement of ductility and toughness. It is reported that, with the introduction of multifunctional fillers, the ductility of the NFC composite films decreased significantly and produced a very brittle composite film with respect to pure NFC.9,20 The rGO1/NFC composite shows a tensile strength of 135 ± 3 MPa, Young's modulus of 11 ± 0.40 GPa, toughness of 6.70 MJ m−3 and elongation at break of 6 ± 1.5%. Hence, the nano-integration of rGO into the NFC structure makes the material more brittle and decreases its ductility significantly. However, in rGO1/NFC-g-PIL composites, the rGO sheets interact with NFC-g-PIL chains by multiple level molecular interactions, such as cation–π interaction and H-bonding interaction, and bridge the NFC-g-PIL molecules together. This leads to the formation of a ductile composite with a significant improvement in the tensile strength (200 ± 4 MPa) and Young's modulus (5.2 ± 0.30 GPa). It is reported in the literature that toughness values of NFC and NFC based composite papers can vary from 6.62–51 MJ m−3 depending on several factors such as the source, and origin of the NFC fibers, processing of the NFC fibers and methods of fabrication of the NFC and NFC based composite film.21 Our pure NFC paper shows a toughness of 9.21 MJ m−3, whereas the obtained NFC-g-PIL film and rGO/NFC-g-PIL film show a considerable toughness of 15.9 MJ m−3 and 25.3 MJ m−3 respectively with a significant enhancement of elongation at break of 50% and 108% compared to pure NFC films. It also signifies that our NFC-g-PIL film and rGO/NFC-g-PIL film have very good ductility. The interfibril connection of NFC-g-PIL with rGO by noncovalent cation–π interaction and H-bonding interaction may offer a chance of sliding the NFC-g-PIL over the rGO surface during mechanical elongation and this may lead to the significant enhancement of mechanical properties of rGO/NFC-g-PIL compared to pure NFC and NFC-g-PIL film.
In conclusion, we have successfully synthesized NFC-g-PIL by simple free radical polymerization of a pyridinium acrylate-based IL monomer from the NFC surface. This NFC-g-PIL shows good dispersion in an aqueous medium. For the first time, we used this NFC-g-PIL for the fabrication of rGO/NFC-g-PIL composite paper by a vacuum-assisted assembly procedure. rGO acts as a bridge in binding the NFC-g-PIL fibrils by cation–π interaction and it governs the fabrication of rGO1/NFC-g-PIL composite paper. Our free-standing, rGO/NFC-g-PIL composite paper exhibits a substantial improvement of mechanical strength, toughness, and ductility along with hydrophobicity compared to pure NFC film. Therefore, this highly flexible rGO integrated NFC-g-PIL composite film may provide a new direction for the development of multifunctional hydrophobic NFC composites with enhanced sustainability and boost the future of biocomposite based bioeconomy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Academy of Finland prostdoctoral project (project no-326453) and Center of Excellence in Body-On-Chip Research (project no. 312409).References
- D. Trache, M. H. Hussin, M. K. Mohamad Haafiz and V. K. Thakur, Nanoscale, 2017, 9, 1763 RSC.
- N. Lavoine and L. Bergstrom, J. Mater. Chem. A, 2017, 5, 16105 RSC.
- M. Henriksson, L. A. Berglund, P. Isaksson, T. Lindstrom and T. Nishio, Biomacromolecules, 2008, 9, 1579 CrossRef CAS PubMed.
- J. Malho, P. Laaksonen, A. Walther, O. Ikkala and M. B. Linder, Biomacromolecules, 2012, 13, 1093 CrossRef CAS PubMed.
- R. K. Layek, K. R. Ramakrishnan, E. Sarlin, O. Orell, M. Kanerva, J. Vuorinen and M. Honkanen, J. Mater. Chem. A, 2018, 6, 13203 RSC.
- R. K. Layek and A. K. Nandi, Polymer, 2013, 54, 5087 CrossRef CAS.
- Q. Jiang, C. Kacica, T. Soundappan, K. Liu, S. Tadepalli and P. B. S. Singamaneni, J. Mater. Chem. A, 2017, 5, 13976 RSC.
- J. Duan, S. Gong, Y. Gao, X. Xie, L. Jiang and Q. Cheng, ACS Appl. Mater. Interfaces, 2016, 8, 10545 CrossRef CAS PubMed.
- L. N. Dang and J. Seppala, Cellulose, 2015, 22, 1799 CrossRef CAS.
- A. Kafy, A. Akther, M. I. R. Shishir, H. C. Kim, Y. Yun and J. Kim, Sens. Actuators, A, 2016, 247, 221 CrossRef CAS.
- M. Shimizu, T. Saito, H. Fukuzumi and A. Isogai, Biomacromolecules, 2014, 15, 4320 CrossRef CAS PubMed.
- J. Yuan and M. Antonietti, Polymer, 2011, 52, 1469 CrossRef CAS.
- K. Grygiel, B. Wicklein, Q. Zhao, M. Eder, T. Pettersson, L. Bergstrom, M. Antoniettia and J. Yuan, Chem. Commun., 2014, 50, 12486 RSC.
- D. Roy, M. Semsarilar, J. T. Guthriea and S. Perrier, Chem. Soc. Rev., 2009, 38, 2046 RSC.
- (a) W. S. Hummers, Jr. and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef; (b) S. Park, J. An, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, ACS Nano, 2010, 4, 3845 CrossRef PubMed.
- H. Gao, S. Zhang, F. Lu, H. Jia and L. Zheng, Colloid Polym. Sci., 2012, 290, 1785 CrossRef CAS.
- R. K. Layek, Md. E. Uddin, N. H. Kim, A. K. T. Lau and J. H. Lee, Composites, Part B, 2017, 128, 155 CrossRef CAS.
- D. Zheng, Y. Zhang, Y. Guo and J. Yue, Polymers, 2019, 11, 1130 CrossRef PubMed.
- Y. Bai, AIP Conf. Proc., 2017, 1794, 02008 Search PubMed.
- Z. Shen and J. Feng, ACS Appl. Mater. Interfaces, 2018, 10, 24193 CrossRef CAS PubMed.
- (a) P. Dhar, J. Phiri, G. R. Szilvay, A. Westerholm-Parvinen, T. Maloneya and P. Laaksonen, J. Mater. Chem. A, 2020, 8, 656 RSC; (b) H. Dua, M. Parita, M. Wub, X. Cheb, Y. Wang, M. Zhang, R. Wang, X. Zhang, Z. Jiang and B. Li, J. Hazard. Mater., 2020, 400, 123106 CrossRef PubMed; (c) M. Henriksson, L. A. Berglund, P. Isaksson, T. Lindstrom and T. Nishino, Biomacromolecules, 2008, 9, 1579 CrossRef CAS PubMed; (d) Y. Zhou, C. Chen, S. Zhu, C. Sui, C. Wang, Y. Kuang, U. Ray, D. Liu, A. Brozena, U. H. Leiste, N. Quispe, H. Guo, A. Vellore, H. A. Bruck, A. Martini, B. Foster, J. Lou, T. Li and L. Hu, Mater. Today, 2019, 30, 17 CrossRef CAS; (e) F. Chen, W. Xiang, D. Sawada, L. Bai, M. Hummel, H. Sixta and T. Budtova, ACS Nano, 2020, 14, 11150 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the synthesis procedure and 1H and 13C NMR analysis of pyridinium IL, pyridinium IL acrylate, and NFC-g-PIL; fabrication of NFC, NFC-g-PIL, rGO1/NFC and rGO1/NFC-g-PIL composite papers and their digital photographs, contact angles, 1H and 13C NMR spectra of pyridinium IL and pyridinium IL acrylate, solid state 1H and CP/MAS 13C-NMR spectra of NFC and NFC-g-PIL, AFM images and height profiles of NFC and NFC-g-PIL, and cross-sectional FESEM image of rGO1/NFC composite paper. See DOI: 10.1039/d0ma00874e |
| This journal is © The Royal Society of Chemistry 2021 |