Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A nanoporous CeO2 nanowire array by acid etching preparation: an efficient electrocatalyst for ambient N2 reduction†
Yuyao
Ji
 and
Xingquan
Liu
and
Xingquan
Liu
 *
*
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu 610054, China. E-mail: lxquan@uestc.eu.cn
First published on 11th May 2021
Abstract
It is highly attractive but still remains a key challenge to develop earth-abundant electrocatalysts for efficient NH3 electrosynthesis via the N2 reduction reaction (NRR). In this work, a nanoporous CeO2 nanowire array on a Ti mesh (np-CeO2/TM) was derived from MnO2–CeO2/TM by acid etching of MnO2 that acts as a pore-forming agent. In 0.1 M HCl, this catalyst achieves a high faradaic efficiency of 4.7% with a NH3 yield of 38.6 μg h−1 mg−1cat. at −0.3 V vs. reversible hydrogen electrode, outperforming most reported Ce-based NRR electrocatalysts under ambient conditions. It also demonstrates high electrochemical stability and excellent selectivity for NH3 generation. The acid preparation strategy is highly valuable for future design of active NRR catalysts with desired compositions in various electrocatalysis fields.
As an important industrial chemical, NH3 has attracted much attention as a potential energy carrier and a fertilizer precursor.1,2 With the increase of the population and the decrease of fossil fuels, the large demand for NH3 has become an urgent social problem, which promotes the in-depth study of artificial NH3 production technology. Due to the need for hydrogen input and energy consumption from fossil fuels, the traditional industry for producing ammonia (350–550 °C and 150–350 atm) is an energy intensive procedure: the Haber–Bosch process results in a great deal of carbon dioxide.3 Therefore, there is a tough importunity for the development of facile and sustainable alternative strategies for NH3 production.
As a kind of nitrogen reduction reaction (NRR) that can synthesize NH3 at room temperature via using only a high efficiency electrocatalyst,4,5 the electrocatalytic NRR plays a significant role in attracting the attention of researchers.6–9 Recently, considerable attention has been focused on exploring non-noble-free NRR electrocatalysts.10–23 Porous noble metals are displayed to be effectual electrocatalysts for electrochemical storage and energy conversion,24–26 which need to be investigated for the NRR. Instead of homogeneous metal surface, the coordinatively unsaturated active sites on phosphide surface might be beneficial for the bonding of nitrogen-related intermediates, is worth discussing in the NRR. Cerium(IV) oxide (CeO2) has benefits of desirable electronic/ionic conductivity, and the cerium ion group plays a role as an intermediate in catalytic reaction and adsorption of gas, and is exposed.27 Both element doping28 and interface engineering29 are verified productively to improve the NRR ability of catalysts. Porous nanostructures have the apparent advantage of high surface-area,30 providing good benefit to improve the electrocatalytic NRR catalysis. It is thus trusted that constructing porous Ce-based catalysts is a good strategy to enhance the NRR activity of transition metal catalysts.
Herein, we report our finding that CeO2 nanowires are a splendid catalyst for NH3 synthesis under ambient conditions. The key idea is to selectively generate NP-CeO2 nanowires with different corrosion stability, using oxalic acid on MnO2 and CeO2. CeO2 achieves a high FE (4.7%) and NH3 yield (38.6 μg h−1 mg−1cat.) at −0.3 V vs. reversible hydrogen electrode (RHE), which are notably higher than those for the MnO2–CeO2 precursor (NH3 yield: 14.3 μg h−1 mg−1cat., and FE: 1.6%) and most reported Ce-based NRR electrocatalysts under the conditions of 0.1 M HCl.
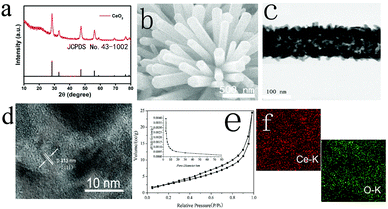
X-ray diffraction (XRD) results for CeO2 (scratched down from TM) are shown in Fig. 1a. CeO2 shows six peaks at 28.5°, 33.9°, 47.8°, 56.2°, 58.5°, and 69.1° indexed to the (111), (200), (220), (311), (222), and (400) facets of CeO2 (JCPDS No. 43-1002), proposing the effective etching of MnO2. As it is shown in the SEM image, MnO2–CeO2 nanowire arrays are anchored on TM (Fig. S1, ESI†), indicating that the construction of np-CeO2/TM maintains the nanowire array feature (Fig. 1b). The transmission electron microscopy (TEM) image of etched np-CeO2 is shown in Fig. 1e, which expresses a truth that the high-resolution TEM (HRTEM) supports interplanar distance of 0.313 nm corresponding to the (111) plane of CeO2 (Fig. 1c).
The Brunauer–Emmett–Teller (BET) pore-size distribution curves of np-CeO2 (Fig. 1e) exhibit an extensive peak centering at 8.6 nm, associated excellently with the TEM data. Meanwhile, the energy-dispersive X-ray (EDX) elemental mapping images of CeO2 clearly show that Ce and O elements are evenly distributed on the surface. All these measurements absolutely prove the convincing formation of MnO2–CeO2 resulting in high surface area nanoporous CeO2 nanowires under the condition of etching via acid.
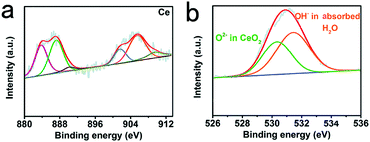
X-ray photoelectron spectroscopy (XPS) was used to investigate the elemental composition and chemical valence states of porous CeO2. As shown in Fig. 2b, high-resolution Ce 1s spectra (Fig. 2a) display binding energies of about 882.6 and 901.2 eV matching to Ce 3d5/2 and Ce 3d3/2, accordingly.31 For O 1s, we can attribute it to three characteristic peaks. The two peaks at 530.1 and 531.7 eV correspond well to the ordered lattice oxygen ions of CeO2, and the oxygen vacancy. For the peak at 533.3 eV, it can be defined to the absorbed hydroxyl on the surfaces of the CeO2 from water molecules.32,33 The difference of peak area at 531.2 eV indicated that the oxygen vacancy of CeO2 increased significantly during hydrogen reduction after acid treatment.34,35
Conventional NRR is a conventional hydrogenation reduction after N2 bubbling at the cathode surface, where H+ could convert the electrolyte to product NH3 by reacting with CeO2/N2. For our experiment, the NRR tests were conducted in a two-chamber cell separately at ambient conditions, which is partitioned by a Nafion membrane (115). For our research, the NH3 obtained at the cathode is formed by the interaction of N2 and H+ by avoiding oxidation of the produced NH3 at the anode, by avoiding passing through the spaced cell. At a moderate temperature and atmospheric pressure, the voltage was corrected by means of a reversible hydrogen electrode (RHE). The NH3 and N2H4 produced by electrocatalytic reaction were determined via the indophenol blue method,36 as well as by the Watt and Chrisp method.37 The electrolyte was colored with indophenol indicator after 2 h electrocatalytic NRR reaction at constant potentials for collecting UV-Vis absorption spectra (Fig. S2 and S3, ESI†).
Np-CeO2/GCE (0.3 mg cm−2) demonstrates exceptional selectivity without N2H4-production (Fig. S4, ESI†). Fig. 3b exhibits average NH3 yields, and FEs at different potentials. In the study of the effect of load on catalytic activity, it was found that when the load was 0.3 mg, the best NRR activity was shown (Fig. S5, ESI†). The optimum NRR rate is fixed at −0.3 V vs. RHE, causing an average yield of 38.6 μg h−1 mg−1cat NH3, and 4.7% FE. As a catalyst with good performance, it has a great advantage over most reported NRR catalysts, including Au nanorods (6.042 μg h−1 mg−1, 4%),38 Cu3P-rGO (26.38 μg h−1 mg−1cat., 1.9%),39 γ-Fe2O3 (0.212 μg h−1 mg−1cat., 1.9%),40 and N-doped nanocarbon (27.2 μg L−1 h−1, 1.42%).41 Detailed comparison is presented in Table S1 (ESI†). Fig. 3a displays that the yield increases with the increase of potential. In view of the surface competitive adsorption between N2 and H, the catalyst performance is significantly reduced when the voltage transcends −0.3 V. For comparison, we provide hydrogen yield rates for hydrogen evolution reactions (Fig. S5, ESI†). By comparing the pH test paper of the electrolyte solution before and after electrolysis (Fig. S6, ESI†), it can be concluded that the pH hardly changed in the experiment, which shows that the whole system has not transformed through the reaction. In Fig. 3b, np-CeO2/GCE exposits a speedier NRR rate than MnO2–CeO2/GCE (14.3 μg h−1 mg−1cat.), demonstrating that the element N plays an important role in NRR. Meanwhile, in the whole process, the weak signal value expressed by the blank GCE is completely offset. To confirm that the sensed NH3 is produced through NRR of np-CeO2/GCE, a series of control experiments is conducted (experimental conditions: Ar for carrier gas, −0.3 V vs. RHE for open-circuit potential and 20 h for electrochemical reaction). Moreover, in 0.1 M HCl, we tested the NRR performance of the nanoporous CeO2 nanowires deposited on carbon paper, and it also shows the greatest NH3 yield of 34.6 μg h−1 mg−1cat. and a high FE of 4.6% (Fig. S7, ESI†). For comparison purposes, the NH3 yield and FE of the MnO2–CeO2 are shown in Fig. S8 (ESI†), and this result also demonstrates that np-CeO2 has better NRR performance. Meanwhile, in 0.1 M H2SO4, our catalyst achieves a high FE of 4.61% along with a NH3 yield of 36.9 μg h−1 mg−1cat. at −0.3 V vs. RHE, and it shows almost no changes when measured in 0.1 M HCl and in 0.1 M H2SO4 (Fig. S9, ESI†).
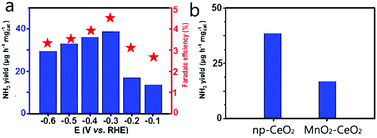 | ||
| Fig. 3 (a) NH3 yields and FEs at each given potential. (b) NH3 yields with different catalysts at −0.3 V vs. RHE under ambient conditions. | ||
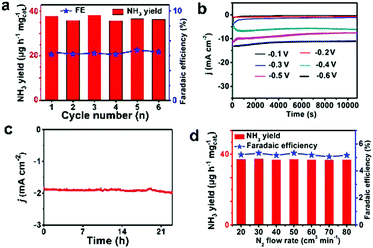
Stability is an additional significant parameter to estimate the catalyst behavior. Np-CeO2/TM has insignificant changes in NH3 yield and FE through recycling experiments for 6 times (Fig. 4a). Fig. 4b displays the long-term electrolysis at a set of potentials, which indicates good stability of np-CeO2/TM. Moreover, a slight change occurred after the NRR reaction at −0.3 V for 24 h (Fig. 4c). The XRD (Fig. S10, ESI†) and XPS (Fig. S11, ESI†) show almost no changes before and after the long test, and they also demonstrate high electrochemical stability. The FE for np-CeO2 demonstrates slight loss compared to the initial one after long-term testing. Based on the experimental data, it can be concluded that np-CeO2 is exceptionally stable and durable for the NRR under ambient reaction conditions. The influence of N2 flow rate on electrocatalytic N2 reduction was examined concurrently. What is shown in Fig. 4d is that there is inapparent fluctuation in FEs and NH3 yields following a series of N2 flow-rates, suggesting that the rate of reduction is impartial to the gas–solid interface. What is more, N2 is transported toward the cathodic catalyst surface within the N2 of the electrolyte. In addition, since the speed of electrocatalytic reaction is independent of N2 concentration, it can be concluded that the diffusion of N2 is not the decisive step of the reaction.
In summary, np-CeO2 nanowire is proven as an efficient and selective electrocatalyst for NH3 electrosynthesis from N2 and water in acidic media. The np-CeO2 nanowires attain a NH3 yield of 38.6 μg h−1 mg−1cat. and an FE of 4.7% at a potential of −0.3 V. Besides, what is surprising is that np-CeO2 possesses appealing selectivity and long-term stability for electro-hydrogenation under ambient conditions. This investigation is not only the first demonstration of applying np-CeO2 for efficient and stable NRR electrocatalysis, but would expose a stimulating new path to the advancement of transition metal nitrides as attractive low-cost NRR catalyst materials for implementations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21575050).Notes and references
- R. Schlögl, Catalytic synthesis of ammonia-a “never-ending story”?, Angew. Chem., Int. Ed., 2003, 42, 2004–2008 CrossRef PubMed.
- W. Gu, Y. Guo, Q. Li, Y. Tian and K. Chu, Lithium iron oxide (LiFeO2) for electroreduction of dinitrogen to ammonia, ACS Appl. Mater. Interfaces, 2020, 12, 37258–37264 CrossRef CAS PubMed.
- I. Dybkjaer, In Ammonia: catalysis and manufacture, ed. A. Nielsen, Springer, Heidelberg, 1995, 199–308 Search PubMed.
- S. Li, Y. Wang, J. Liang, T. Xu, D. Ma, Q. Liu, T. Li, S. Xu, G. Chen, A. M. Asiri, Y. Luo, Q. Wu and X. Sun, TiB2 thin film enabled efficient NH3 electrosynthesis at ambient conditions, Mater. Today Phys., 2021, 18, 100396 CrossRef.
- S. Gao, Y. Zhu, Y. Chen, M. Tian, Y. Yang, T. Jiang and Z. Wang, Self-power electroreduction of N2 into NH3 by 3D printed triboelectric nanogenerators, Mater. Today, 2019, 28, 17–24 CrossRef CAS.
- Y. Ji, L. Li, W. Cheng, Y. Xiao, C. Li and X. Liu, A CeP nanoparticle-reduced graphene oxide hybrid: an efficient electrocatalyst for the NH3 synthesis under ambient conditions., Inorg. Chem. Front., 2021, 8, 2103–2106 RSC.
- T. Xu, B. Ma, J. Liang, L. Yue, Q. Liu, T. Li, H. Zhao, Y. Luo, S. Lu and X. Sun, Recent progress in metal-free electrocatalysts toward ambient N2 reduction reaction., Acta Phys.-Chim. Sin., 2021, 37, 2009043 Search PubMed.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Ambient ammonia synthesis via palladium-catalyzed electro hydrogenation of dinitrogen at low overpotential, Nat. Commun., 2018, 9, 1795 CrossRef PubMed.
- K. Chu, Y. Liu, Y. Li, Y. Guo and Y. Tian, Two-dimensional (2D)/2D interface engineering of a MoS2/C3N4 heterostructure for promoted electrocatalytic nitrogen fixation, ACS Appl. Mater. Interfaces, 2020, 12, 7081–7090 CrossRef CAS PubMed.
- S. Chen, S. Perathoner, C. Ampelli, C. Mebrahtu, D. Su and G. Centi, Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst, Angew. Chem., Int. Ed., 2017, 56, 2699–2703 CrossRef CAS PubMed.
- T. Wang, S. Li, B. He, X. Zhu, Y. Luo, Q. Liu, T. Li, S. Lu, C. Ye, A. M. Asiri and X. Sun, Commercial indium-tin oxide glass: a catalyst electrode for efficient N2 reduction at ambient conditions, Chin. J. Catal., 2021, 42, 1024–1029 CrossRef CAS.
- C. Li, D. Ma, S. Mou, Y. Luo, B. Ma, S. Lu, G. Cui, Q. Li, Q. Liu and X. Sun, Porous LaFeO3 nanofiber with oxygen vacancies as an efficient electrocatalyst for N2 conversion to NH3 under ambient conditions, J. Energy Chem., 2020, 50, 402–408 CrossRef.
- C. Lv, Y. Qian, C. Yan, Y. Ding, Y. Liu, G. Chen and G. Yu, Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions, Angew. Chem., Int. Ed., 2018, 57, 10246–10250 CrossRef CAS PubMed.
- B. Ma, J. Liang, T. Li, Q. Liu, Y. Luo, S. Lu, A. M. Asiri, D. Ma and X. Sun, Iron-group electrocatalysts for ambient nitrogen reduction reaction in aqueous media, Nano Res., 2021, 14, 555–569 CrossRef CAS.
- S. Mukherjee, D. A. Cullen, S. Karakalos, K. Liu, H. Zhang, S. Zhao, H. Xu, K. L. More, G. Wang and G. Wu, Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes, Nano Energy, 2018, 48, 217–226 CrossRef CAS.
- T. Wang, Q. Liu, T. Li, S. Lu, G. Chen, X. Shi, A. M. Asiri, Y. Luo, D. Ma and X. Sun, Magnetron sputtered Mo3Si thin film: an efficient electrocatalyst for N2 reduction at ambient conditions, J. Mater. Chem. A, 2021, 9, 884–888 RSC.
- Y. Liu, M. Han, Q. Xiong, S. Zhang, C. Zhao, W. Gong, G. Wang, H. Zhang and H. Zhao, Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li-S interactions on MoS2 electrocatalyst, Adv. Energy Mater., 2019, 9, 1803935 CrossRef.
- Q. Li, Y. Guo, Y. Tian, W. Liu and K. Chu, Activating VS2 basal planes for enhanced NRR electrocatalysis: the synergistic role of S-vacancies and B dopants, J. Mater. Chem. A, 2020, 8, 16195–16202 RSC.
- K. Chu, J. Wang, Y. Liu, Q. Li and Y. Guo, Mo-doped SnS2 with enriched S-vacancies for highly efficient electrocatalytic N2 reduction: the critical role of the Mo–Sn–Sn trimer, J. Mater. Chem. A, 2020, 8, 7117–7124 RSC.
- H. Jin, L. Li, X. Liu, C. Tang, W. Xu, S. Chen, L. Song, Y. Zheng and S. Qiao, Nitrogen vacancies on 2D layered W2N3: a stable and efficient active site for nitrogen reduction reaction., Adv. Mater., 2019, 31, 1902709 CrossRef PubMed.
- H. Cheng, L. Ding, G. Chen, L. Zhang, J. Xue and H. Wang, Nitrogen reduction reaction: molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient condition, Adv. Mater., 2018, 30, 1803694 CrossRef PubMed.
- P. Wei, Q. Geng, A. I. Channa, X. Tong, Y. Luo, S. Lu, G. Chen, S. Gao, Z. Wang and X. Sun, Electrocatalytic N2 reduction to NH3 with high faradaic efficiency enabled by vanadium phosphide nanoparticle on V foil, Nano Res., 2020, 13, 2967–2972 CrossRef CAS.
- F. Wang, X. Lv, X. Zhu, J. Du, S. Lu, A. A. Alshehri, K. A. Alzahrani, B. Zheng and X. Sun, Bi nanodendrites for efficient electrocatalytic N2 fixation to NH3 under ambient conditions, Chem. Commun., 2020, 56, 2107–2110 RSC.
- O. D. Velev, P. M. Tessier, A. M. Lenhoff and E. W. Kaler, Materials: a class of porous metallic nanostructures, Nature, 1999, 401, 548 CrossRef CAS.
- M. Chen, Y. Zhang, L. Xing, Y. Qiu, S. Yang and W. Li, Nanoprobes: activatable photoacoustic nanoprobes for in vivo ratiometric imaging of peroxynitrite, Adv. Mater., 2017, 29, 1607015 CrossRef PubMed.
- V. Malgras, H. Ataee-Esfahani, H. Wang, B. Jiang, C. Li, K. C. W. Wu, J. H. Kim and Y. Yamauchi, ChemInform abstract: nanoarchitectures for mesoporous metals, Adv. Mater., 2016, 28, 993–1010 CrossRef CAS PubMed.
- Y. Ji, J. Liu, S. Hao, Y. Xiao, L. Li and X. Liu, Full water splitting by a nanoporous CeO2 nanowire array under alkaline conditions, Inorg. Chem. Front., 2020, 7, 2533–2537 RSC.
- T. Wu, X. Li, X. Zhu, S. Mou, Y. Luo, X. Shi, A. M. Asiri, Y. Zhang, B. Zheng, H. Zhao and X. Sun, P-doped graphene toward enhanced electrocatalytic N2 reduction, Chem. Commun., 2020, 56, 1831–1834 RSC.
- W. Guo, Z. Liang, J. Zhao, B. Zhu, K. Cai, R. Zou and Q. Xu, Recent advances in graphene quantum dots: synthesis, properties, and applications, Small Methods, 2018, 2, 1800204 CrossRef.
- X. Zhu, S. Mou, Q. Peng, Q. Liu, Y. Luo, G. Chen, S. Gao and X. Sun, Aqueous electrocatalytic N2 reduction for ambient NH3 synthesis: recent advances in catalysts developing and performances boosting, J. Mater. Chem. A, 2020, 8, 1545–1556 RSC.
- K. Chu, Y. Cheng, Q. Li, Y. Liu and Y. Tian, Fe-doping induced morphological changes oxygen vacancies and Ce3+–Ce3+ pairs in CeO2 for promoting electrocatalytic nitrogen fixation, J. Mater. Chem. A, 2020, 8, 5865–5873 RSC.
- K. Chu, Q. Li, Y. Cheng and Y. Liu, Efficient electrocatalytic nitrogen fixation on FeMoO4 nanorods, ACS Appl. Mater. Interfaces, 2020, 12, 11789–11796 CrossRef CAS PubMed.
- K. Chu, Y. Liu, Y. Cheng and Q. Li, Synergistic boron-dopants and boron-induced oxygen vacancies in MnO2 nanosheets to promote electrocatalytic nitrogen reduction, J. Mater. Chem. A, 2020, 8, 5200–5208 RSC.
- G. Zhu, J. Zhu, W. Jiang, Z. Zhang, J. Wang, Y. Zhuang and Q. Zhang, Semimetallic MoP2: an active and stable hydrogen evolution electrocatalyst over the whole pH range, Nanoscale, 2016, 8, 8500–8504 RSC.
- Z. Geng, X. Kong, W. Chen, H. Su, Y. Liu, F. Cai, G. Wang and J. Zeng, Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO, Angew. Chem., Int. Ed., 2018, 57, 6054–6059 CrossRef CAS PubMed.
- D. Zhu, L. Zhang, R. E. Ruther and R. J. Hamers, Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction, Nat. Mater., 2013, 12, 836–841 CrossRef CAS PubMed.
- G. W. Watt and J. D. Chrisp, Photoinactivation and carbethoxylation of leucine aminopeptidase, Anal. Chem., 2012, 24, 2006–2008 CrossRef.
- D. Bao, Q. Zhang, F. Meng, H. Zhong, M. Shi, Y. Zhang, J. Yan, Q. Jiang and X. Zhang, Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed.
- R. Zhao, Q. Geng, L. Chang, P. Wei, Y. Luo, X. Shi, A. M. Asiri, S. Lu, Z. Wang and X. Sun, Cu3P nanoparticles-reduced graphene oxide hybrid: an efficient electrocatalyst to realize N2-to-NH3 conversion under ambient conditions, Chem. Commun., 2020, 56, 9328–9331 RSC.
- J. Kong, A. Lim, C. Yoon, J. H. Jang, H. C. Ham, J. Han, S. Nam, D. Kim, Y.-E. Sung, J. Choi and H. S. Park, Electrochemical synthesis of NH3 at low temperature and atmospheric pressure using a γ-Fe2O3 catalyst, ACS Sustainable Chem. Eng., 2017, 5, 10986–10995 CrossRef CAS.
- Y. Liu, Y. Su, X. Quan, X. Fan, S. Chen, H. Yu, H. Zhao, Y. Zhang and J. Zhao, Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon, ACS Catal., 2018, 8, 1186–1191 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00243k |
| This journal is © The Royal Society of Chemistry 2021 |