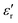Open Access Article
Open Access ArticleWater-dispersible and ferroelectric PEGylated barium titanate nanoparticles†
M.
Taheri
abc,
B.
Zanca
a,
M.
Dolgos
 a,
S.
Bryant
c and
S.
Trudel
a,
S.
Bryant
c and
S.
Trudel
 *ab
*ab
aDept. of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, Canada. E-mail: trudels@ucalgary.ca; maryam.taheri2@ucalgary.ca
bInstitute for Quantum Science and Technology, University of Calgary, 2500 University Drive NW, Calgary, AB, Canada
cDept. of Chemical and Petroleum Engineering, University of Calgary, 2500 University Drive NW, Calgary, AB, Canada
First published on 15th June 2021
Abstract
Dispersions of ferroelectric nanoparticles in aqueous medium can find promising applications in electro-optical, medical, and smart fluid technologies. In this report, we show the development of highly dispersed nano-sized ferroelectric barium titanate (BaTiO3) powders with high dielectric constant prepared using a simple, one-step low temperature solution method. The surface of these tetragonal-structured nanoparticles were modified with poly(ethylene glycol) as a stabilizer and dispersant. The crystal structure, morphology and dielectric constant of samples are discussed in detail. The colloidal stability and surface behavior of these PEGylated barium titanate nanoparticles are studied by means of ζ-potential and dynamic light scattering measurements. We show changing the reaction conditions allows to tune the nanoparticle size. This research promotes a pathway to develop advanced ferroelectric nanomaterials with engineered properties in a simple way.
1 Introduction
Perovskite-type barium titanate (BaTiO3) with four temperature-dependent crystalline forms (i.e. cubic, tetragonal, orthorhombic, and rhombohedral) is the most widely used ferroelectric (FE) material with applications in the electrical and electronic industries, mainly as multilayer ceramic capacitors, piezoelectric sensors and dielectrics.1 The tetragonal phase (space group P4/mmm, see Fig. S1, ESI†) supports a permanent electric polarization and a high dielectric constant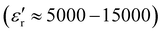 up to the FE Curie–Weiss temperature (≈130 °C); at higher temperatures it converts to a fully symmetric cubic structure and becomes paraelectric. In this symmetric phase, BaTiO3 shows a temporary polarization under an application of electric field. It is well known that the tetragonal phase of BaTiO3 switches to the cubic non-ferroelectric phase at room temperature below a critical size.2 Reported critical sizes vary from as small as a few nm3,4 to ca. 100 nm.5
up to the FE Curie–Weiss temperature (≈130 °C); at higher temperatures it converts to a fully symmetric cubic structure and becomes paraelectric. In this symmetric phase, BaTiO3 shows a temporary polarization under an application of electric field. It is well known that the tetragonal phase of BaTiO3 switches to the cubic non-ferroelectric phase at room temperature below a critical size.2 Reported critical sizes vary from as small as a few nm3,4 to ca. 100 nm.5
Stable FE nanoparticle (NP) suspensions are required for various proposed applications including drug delivery, imaging, solution-based ceramics and coatings fabrication, and pigments.6–8 Recent efforts have focused on the dispersion of ferroelectric BaTiO3 nanopowders in either aqueous or organic media. Polymer surface stabilization is an approach conducive to this goal. Various polymers or polyelectrolytes, including ammonium salts of poly(acrylic acid) (PAA),6 poly-L-lysine,8 and poly(vinylpyrrolidone)9,10 have been used. Pang et al. developed amphiphilic BaTiO3 NPs using unimolecular star-like poly(acrylic acid)-block-polystyrene diblock copolymers.11 Similarly, Jiang et al. synthesized amphiphilic poly(vinylidene fluoride)(PVDF)- BaTiO3 nanocomposites using a PAA-block-PVDF building block, where the PAA block was used a scaffold for BaTiO3 growth, yielding tunable nanoparticle sizes and PVDF coating thickness.12
Since these ceramic particles boast superior ferroelectric/piezoelectric/pyroelectric properties, are competitively low-cost, non-toxic and bio-compatible, they present great potential for use in electrorheological fluids and biomedical imaging applications.8,13 Several synthesis methods for the preparation of BaTiO3 nanoparticles have been proposed, including high temperature solid-state reaction,14 sol–gel,15 co-precipitation,16 and hydrothermal17 approaches. However, most of these methods are not conducive to making uniform well-dispersed BaTiO3 nanoparticles. Bare BaTiO3 particles are not thermodynamically stable in water/organic solvents, specifically in acidic aqueous dispersions where Ba2+ ions are leached out from the surface of BaTiO3 molecules. Therefore adding surfactants, polymers or polyelectrolytes is needed to make them dispersible in the liquid phase.18
We chose poly(ethylene glycol) (PEG) to stabilize aqueous-dispersed BaTiO3 nanoparticles based on its high solubility in water, bio-compatibility and eco-friendliness, and its well-documented ability to solubilize metal and metal oxide nanoparticles.19–22 Direct synthesis in PEG through a one-pot synthesis makes upscaling of BaTiO3 production feasible.20
Although PEG has been widely used in the synthesis of nanoparticles to promote stability control and nanoparticle size,22 its use to disperse BaTiO3 nanoparticles is limited.23–25 Moreover, modification with PEG is suggested to improve the dielectric properties of ceramic particles.2,23,26 In addition to improving NP stability in solution, PEG-modified metal oxides were used to form high-quality ferroelectric thin films such as strontium-27 and lead-doped28 BaTiO3.
In this work, we present the preparation and characterization of PEGylated BaTiO3 nanoparticles prepared using a low-temperature one-pot synthesis. We synthesise tetragonal BaTiO3-PEG core–shell nanoparticles in the 40–70 nm diameter range, with high water dispersibility and large dielectric constant. We show how we can modify the synthetic parameters to control the particle size, and their corresponding stability in water. Furthermore, we report the temperature-dependence dielectric constant of a selected sample. This method is fast, simple, and cost-effective, attributes that are necessary for large-scale industrial deployment.
2 Experimental methods
2.1 Nanoparticle synthesis
PEGylated BaTiO3 nanoparticles were prepared using a one-step protocol. All reactions were carried out under stirring and inert nitrogen atmosphere while the temperature was monitored and controlled with a digital PID controller. All syntheses are fully detailed in the ESI.† In a representative synthesis to prepare sample BT-1, we mixed powdered Ba(acac)2·xH2O (355 mg, 1 mmol) and (O-i-Pr)2Ti(acac)2 solution (273 mg, 1 mmol) in 3 mL of PEG400 in a round-bottom flask under nitrogen atmosphere, and let the solution stir for 30 min. We then added aqueous KOH (6 mL, 1.5 M) to the mixture to adjust the pH of the solution to ca. 14, which was found to be crucial for the nucleation of BaTiO3 particles.29 Immediately after, we increased the reaction temperature to reflux (ca. 100 ± 5 °C), and allowed the reaction to reflux for 2 h. The color of the solution gradually changed from orange/brown to white. After 2 h of reflux, we added 6 mL of distilled water to the mixture and maintained it at 100 ± 5 °C for an additional 2 h. We then opened the system to air and let the reaction cool down to room temperature. White precipitates were collected by washing and centrifugation (6000 rpm for 10 min) two times with ethanol, followed by formic acid (1 M). Carbonate impurities were removed by washing the product with diluted (0.5 w/w%) acetic acid. Finally, the nanoparticles were dried at 60 °C in a vacuum oven overnight.In order to study the effect of reaction conditions on particle size and dispersibility, we modified the ratio of the metal precursors (Ti and Ba molar ratio), KOH and PEG for samples BT-1 to BT-5 as summarized in Table 1. We prepared sample BT-0 under similar conditions to BT-1, without adding PEG, to provide a reference sample with no surface modification.
| Sample | [Ba]/[Ti] | relative [PEG] | [KOH] (M) | D XRD (nm) | D SEM (nm) | D TEM (nm) |
|---|---|---|---|---|---|---|
| BT-1 | 1 | 1 | 1.5 | 39.8 ± 2.1 | 69.9 ± 9.5 | 57.6 ± 10.3 |
| BT-2 | 2 | 1 | 1.5 | 32.3 ± 1.6 | 47.9 ± 7.7 | 40.4 ± 7.9 |
| BT-3 | 1 | 2 | 1.5 | 31.2 ± 1.8 | 43.9 ± 6.3 | 40.2 ± 5.4 |
| BT-4 | 1 | 1 | 1.6 | 33.6 ± 2.2 | 39.8± 6.4 | 41.8 ± 6.3 |
| BT-5 | 1 | 1 | 1.7 | 29.9 ± 2.4 | 43.4 ± 7.2 | 37.4± 4.8 |
2.2 Physical characterization
The density ρexp of the pellet was measured with a Mettler Toledo analytical balance equipped with an Archimedes kit (with the accuracy of ± 0.01 g cm−3). The relative density ρr of the pellet was calculated as ρr = 100% (ρexp·ρtheo−1), where ρtheo is the theoretical crystallographic density obtained from XRD pattern refinements. To perform dielectric constant measurement, the pellet was polished on all sides using 800, 1200, and 4000 grit silicon-carbide polishing films (Stuers). Then, silver electrodes were painted on both sides of the pellet using a silver paint (SPI Supplies) and sintered at 550 °C for 15 min with a heating/cooling ramp rate of 5 °C min−1. The dielectric properties were measured with a Solartron 1260 and 1296 coupled with a Probostat high-temperature sample stage inserted into a vertical tube furnace (Carbolite-Gero, VTF). The sample was heated/cooled at a rate of 1 °C min−1 from room temperature (≈20–22 °C) to 200 °C. Data was collected on both the heating and cooling cycles, every 5 °C until near the transition temperature (TC ≈ 115), then every 1 °C until after TC, then every 10 °C. The tested frequencies ranged from 1 Hz to 100 kHz with 5 points per decade.
3 Results and discussion
3.1 Structural characterization and size control
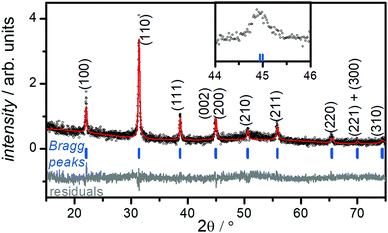
PXRD of the collected powders prior to the washing step indicated the major product of the reaction is BaTiO3. However, the presence of undesired BaCO3 was detected (Fig. S2a, ESI†). This impurity is easily removed by washing the powders with diluted acetic acid (0.5% w/w), which is known to dissolve BaCO3.34 After washing, all PXRD patterns show a single-phase product consisting of only BaTiO3 (see Fig. S2b, ESI†).The size of the crystalline BaTiO3 cores (DXRD) were estimated from Scherrer's formula31 through fitting of the (110) peak (see Fig. S3 and eqn (S1), (S2), ESI†). A size of ∼40 nm is determined for BT-1; DXRD decreases from this reference point through increasing concentration of Ba+2, PEG content, or KOH(aq) molarity (see Table 1).
Fig. 1 shows the room-temperature PXRD pattern of sample BT-1. The observation of two closely related (002) and (200) reflections near 2θ ≈ 45° suggests the formation of a tetragonal structure with the space group P4/mmm (Fig. 1, inset), whereas a single (200) peak would be observed for cubic BaTiO3 (space group: Pm3m). Rietveld refinement of the XRD pattern gives lattice parameters of a = b = 4.03 Å and c = 4.05 Å for sample BT-1, which are slightly larger than the lattice parameters of bulk BaTiO3 (a = b = 3.99 Å and c = 4.02 Å) and in good agreement with published values of 4.03 to 4.05 Å in BaTiO3 nanoparticles.35,36 The c/a ratio of 1.005 suggests the structure is moderately tetragonal, while strong tetragonality value is reported to be about 1.010.37Table 2 summarizes the refined lattice parameters and selected bond distances. The data shows that the lattice parameters of samples with smaller particle sizes slightly increase, indicating smaller tetragonality value. Similarly, internal bond distances increase with this lattice expansion. Smaller nanoparticles can have larger unit cell, owing to various factors including defects, surface tension or symmetry reduction. A lattice expansion concurrent with size reduction has frequently been reported for BaTiO3 nanoparticles; this trend converts the tetragonal phase to the cubic phase structure in very small particles.38,39
| Sample | a = b (Å) | c (Å) | c/a | d Ba–Ti (Å) | d Ti–O1 (Å) | d Ti–O2 (Å) |
|---|---|---|---|---|---|---|
| BT-1 | 4.03(1) | 4.05(2) | 1.005 | 3.49(8) | 2.02(6) | 2.01(6) |
| BT-2 | 4.05(7) | 4.07(5) | 1.004 | 3.51(9) | 2.03(8) | 2.02(9) |
| BT-3 | 4.04(9) | 4.06(4) | 1.004 | 3.51(1) | 2.03(2) | 2.02(5) |
| BT-4 | 4.04(5) | 4.06(4) | 1.004 | 3.50(8) | 2.03(2) | 2.02(3) |
| BT-5 | 4.04(6) | 4.06(5) | 1.004 | 3.50(9) | 2.03(3) | 2.02(2) |
It is well known that the presence of a tetragonal phase in BaTiO3 can be detected with Raman spectroscopy.40 To confirm the crystal structure of sample, we measured the Raman spectrum for sample BT-1, which is shown in Fig. S4 (ESI†). Bands are observed at around 250 and 520 cm−1 (assigned to the transverse optical modes of A1 symmetry), around 720 cm−1, and a tetragonal characteristic peak at around 305 cm−1 (assigned to a B1 mode).40 The presence of the latter supports the tetragonal structure formation, in agreement with the XRD results presented above.
The morphology of PEGylated BaTiO3 nanoparticles was analyzed using SEM and TEM. As can be seen in the SEM micrographs (Fig. S5, ESI†), BT-1 particles are uniformly quasi-spherical with rough surfaces ascribed to the PEG adsorbed on the nanoparticles' surfaces. The average particle size decreases from ≈ 70 nm for BT-1 to ≈40–48 nm in samples BT-2 to BT-5, with no change in the shape of the particles. The formation of BaTiO3-PEG core–shell structures in all samples was observed in the TEM micrographs (Fig. 2) with an average shell thickness of ca. 5–7 nm in the dry state. Lattice fringes can be observed in HRTEM images of BT-1 (Fig. 2b and Fig. S6, ESI†), indicating the nanoparticles are single-crystal. The polymer layer is seen to be uniform around the NPs in Fig. S6a (ESI†). The average particle size observed through TEM are in good agreement with data obtained from SEM. All average particle sizes are compiled in Table 1. The differences between DXRD and DSEM or DTEM are consistent with the presence of a 5–7-nm PEG shell.
 | ||
| Fig. 2 TEM micrographs of samples BT-1 (a and b), BT-3 (c), and BT-5 (d), showing core–shell structures, where the lighter shell is the PEG polymer coating. | ||
According to the literature, a dissolution–precipitation mechanism is responsible for BaTiO3 nanoparticle formation in this work, involving a reaction between Ti(OH)x4−x hydroxytitanium complexes and barium ions, resulting in the precipitation of BaTiO3 particles.29,41 Taking BT-1 as a reference point, a higher concentration of Ba2+ ions in BT-2 decreases the average particle size. This is ascribed to an increase of the nucleation rate; more seeds being present implies the precursor is redistributed on more particles, leading to an average lower size. Similarly, increasing the amount of PEG yields smaller particles. The average particle size with no PEG present (BT-0) was obtained ≈ 110 nm; it decreased to ≈ 60 nm in BT-1 and further to ≈ 40 nm for BT-3. We infer the polymer adsorbed on the surface of growing BaTiO3 particles hinders grain growth, resulting in the smaller nanoparticles. A similar behavior has been seen in poly(vinylpyrrolidone)-coated BaTiO3 nanoparticles synthesized by Li et al.,29 where the particle size decreased upon adding more poly(vinylpyrrolidone). Finally, higher KOH concentration also accelerates nucleation rate, creating smaller particles (see samples BT-1, BT-4, and BT-5).
3.2 Nanoparticle coating and water dispersibility
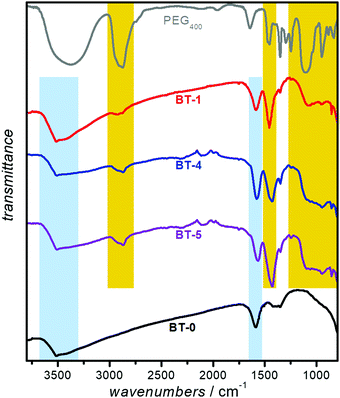
The presence of the polymer on the nanoparticles' surface was confirmed by ATR-FTIR spectroscopy. Fig. 3 represents the FTIR spectra of PEGylated- BaTiO3 nanoparticles (BT-1, BT-4 and BT-5), as well as neat PEG400. Several peaks are attributed to the presence of PEG: the C-H out-of-plane bending vibrations (near 950 cm−1); in-plane C-H and O-H as well as C-O-C stretching vibrations (features between 990 to 1250 cm−1); C-H bending vibrations (near 1450 cm−1), and CH symmetric and asymmetric stretches (bands around 2900 and 2850 cm−1), respectively.20 As expected, the bands assigned to PEG400 are present in the PEGylated samples (highlighted in yellow), while they are absent in the spectrum of the bare BT-0 nanoparticles. We suggest the PEG is bound to the NP surface through hydrogen bonding between the PEG and surface-terminating hydroxyl groups.22 The broad O-H stretching modes of surface-adsorbed water observed around 3500 cm−1 and the deformation mode of absorbed H2O molecules, assigned to the bending vibration around 1600 cm−1 were features that have been reported in BaTiO3 FTIR spectrum42 which are highlighted in blue in all BaTiO3 samples.DLS and ζ-potential measurements is a combination of techniques to determine the size distribution of small particles in suspension, and their surface charge. As suspended nanoparticles undergo Brownian motion, they scatter an incident laser beam in all directions; the scattering intensity fluctuates over time due to constructive and destructive interference. An analysis of this scattered light yields the diffusion coefficient of the scattering species; the hydrodynamic diameter is calculated through the Stokes–Einstein equation. The particles aggregation in the dispersion can be recorded by DLS measurements of a sample over time whether the hydrodynamic diameter of the particle increases or not.43,44 The ζ-potential, which depends on the surface charge, is a useful parameter to determine the stability of nanoparticles in a suspension. Nanoparticles with ζ-potential magnitude of 20 to 30 mV typically show the highest degrees of stability.44
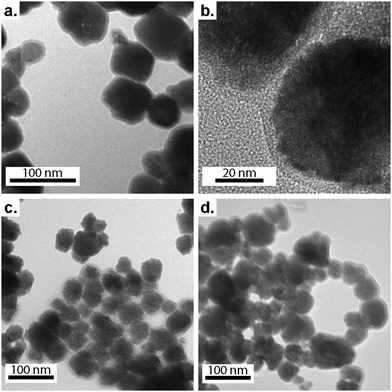
Fig. S7a (ESI†) illustrates the images of 100 ppm dispersion of BaTiO3 nanoparticles (in DI water, pH ≈ 7) after 10 min sonication with an ultrasonic homogenizer (≈4 kJ energy); Fig. S7b (ESI†) shows the same dispersions 24 h later, with no disturbing. This visual test shows that the BT-0 dispersion is more whitish-milky with phase separation, and that the particles clearly precipitate after 24 h (Fig. S7b, ESI†), highlighting the importance of the PEG coating for colloidal stability. The large hydrodynamic size (≈300 nm, see Fig. 4) indicates agglomeration and is the likely reason for the low stability of these uncoated nanoparticles in the dispersion.
 | ||
| Fig. 4 (a) ζ-potential and hydrodynamic diameter of all BaTiO3 samples, and (b) time variation of ζ-potentials and hydrodynamic diameter of BT-1 nanoparticles over 24 h, indicating stability. | ||
We examined the nanoparticle's ζ-potential and hydrodynamic diameters (DDLS) (Fig. 4) to better assess their stability in water. All particles show zeta potentials in −31 to −22 mV range; this is indicative of stable nanoparticles in this medium.44 Sample BT-1 shows the highest ζ-potential among all samples (-30.6 ± 0.5 mV). Their hydrodynamic diameters is around 150–250 nm; the trend generally follows the trends observed in Table 1, although the sizes are larger as they represent the hydrodynamic diameter, not only the inorganic core size. A polymer shell of 10-nm thickness was observed in the TEM images; we however note this is in a dry state. Upon immersion in water, multi-fold swelling of the PEG shell may be expected.45 Combined will low-level aggregation (2–3 NPs per agglomerate), this adequately explains the increased hydrodynamic sizes obesevred. Aggregation is likely occurring in sample BT-5, which has a ζ-potential at the limit of colloidal stability, and a larger hydrodynamic diameter.
In order to better understand these nanoparticles' stability, the ζ-potential and hydrodynamic diameter of 100 ppm BT-1 nanoparticles in DI water measured over a 24 h period (Fig. 4b). The measured ζ-potentials show only a slight variation, oscillating around an average value of −30 ± 0.5 mV, indicating good stability for dispersion of these nanoparticles. Moreover, the negative surface charge of particles due to the electrostatic interaction of surface-modified particles in the aqueous medium might explain the agglomeration delay and enhanced particles’ distribution in the solvent. The DDLS shows a similar behavior – albeit with more dispersion – showing an essentially stable diameter of ∼225 nm. In general non-ionic PEG polymer acts as a dispersant and prevents agglomeration by creating steric repulsion between particles covered with a hydrated layer in DI water;19 therefore, nanoparticles coated with PEG have smaller hydrodynamic particle sizes.
3.3 Ferroelectric properties
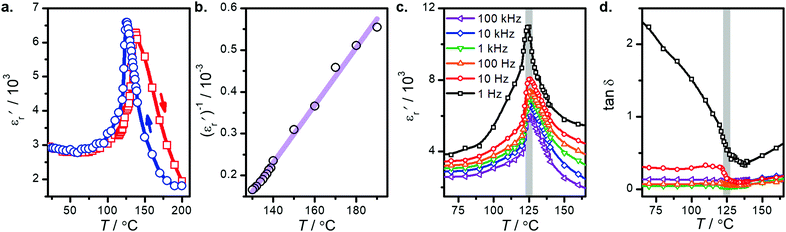
The relative dielectric constant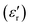 of BT-1 pellet is measured as a function of temperature (Fig. 5); these measurements are conducted on a pellet with a relative density of 86.7% at a frequency of 10 kHz during heating and cooling cycles. The observed dielectric behaviour is typical of ferroelectric materials,
of BT-1 pellet is measured as a function of temperature (Fig. 5); these measurements are conducted on a pellet with a relative density of 86.7% at a frequency of 10 kHz during heating and cooling cycles. The observed dielectric behaviour is typical of ferroelectric materials, 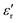 increases with temperature to reach the maximum at the ferroelectric-to-paraelectric (FE-PE) transition temperature (TC), and then decreases. The observed TC of ≈ 130 °C is in close agreement with the reported phase transition for BaTiO3 ceramics.1 The
increases with temperature to reach the maximum at the ferroelectric-to-paraelectric (FE-PE) transition temperature (TC), and then decreases. The observed TC of ≈ 130 °C is in close agreement with the reported phase transition for BaTiO3 ceramics.1 The 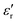 value of ≈2900 at room temperature and
value of ≈2900 at room temperature and 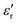 (max) of 6500 at the TC indicate that BT-1 is a strong ferroelectric compound, owing to its tetragonal structure. Above this transition temperature (paraelectric region),
(max) of 6500 at the TC indicate that BT-1 is a strong ferroelectric compound, owing to its tetragonal structure. Above this transition temperature (paraelectric region), 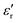 follows the Curie–Weiss law,
follows the Curie–Weiss law,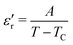 | (1) |
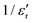 versus (T − TC) (see Fig. 5b). The determined Curie–Weiss constant of A ≈ 14.7 × 104 is in the same range of reported values in literature.3 The obtained heating and cooling cycles show a thermal hysteresis at TC (difference between TC (heating) and TC (cooling)), indicating that the ferroelectric-paraelectric anomaly is first-order.46,47 The transition temperature detected on heating (TC = 135 °C) is usually larger than the transition temperature observed on cooling (TC = 125 °C). The thermal hysteresis of 10 °C at the FE-PE transition temperature of BaTiO3 has already been reported by Baskaran et al.,46 although the hysteresis is often reported to be less than 5 °C.3,47
versus (T − TC) (see Fig. 5b). The determined Curie–Weiss constant of A ≈ 14.7 × 104 is in the same range of reported values in literature.3 The obtained heating and cooling cycles show a thermal hysteresis at TC (difference between TC (heating) and TC (cooling)), indicating that the ferroelectric-paraelectric anomaly is first-order.46,47 The transition temperature detected on heating (TC = 135 °C) is usually larger than the transition temperature observed on cooling (TC = 125 °C). The thermal hysteresis of 10 °C at the FE-PE transition temperature of BaTiO3 has already been reported by Baskaran et al.,46 although the hysteresis is often reported to be less than 5 °C.3,47
The temperature dependence of 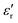 and loss tangent (tan
and loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) during cooling cycle, at different frequencies, are shown in Fig. 5c-d. We observe that
δ) during cooling cycle, at different frequencies, are shown in Fig. 5c-d. We observe that 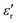 and tanδ decrease, and that the Curie–Weiss transition temperature slightly shifts to a lower temperature with increasing frequency. The highest value of
and tanδ decrease, and that the Curie–Weiss transition temperature slightly shifts to a lower temperature with increasing frequency. The highest value of 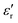 at 1 Hz is 11000 at TC = 126 °C. The lowest value of 5950 at the transition temperature is also recorded for the frequency of 100 kHz.
at 1 Hz is 11000 at TC = 126 °C. The lowest value of 5950 at the transition temperature is also recorded for the frequency of 100 kHz.
When preparing pellets, there is always a concern as to how the pellet sintering and pressing affects the crystal sizes, and hence the resultant properties. We attempted to minimize such effects by doing a two-step sintering process previously shown to yield high-density pellets, with minimal effect on the NP size.33 In order to understand the effect of heating on the crystal structure of particles, the lattice parameters and crystallite size of ground pellet of BT-1 were obtained via PXRD analysis (Fig. S8, ESI†). Results show that crystallite sizes slightly increased from 39.8 ± 2.1 nm to 42.6 ± 3.9 nm; in doing so, the tetragonality was enhanced to c/a = 1.008. According to Wada et al., large values of dielectric constant in nanoparticles can be attributed to the existence of uniform grains, easier domain walls movement, surface effect and also higher local space charge field due to the large surface area.48 In our case, uniform grains and enhanced tetragonality due to the two-step sintering technique creates this large FE response.
4 Conclusions
In summary, easily water-dispersed BaTiO3-PEG core–shell particles with an average particle size of 60 nm and a tetragonality value of 1.005 were prepared using a simple and fast low-temperature synthesis method. The impact of experimental conditions on particle size and crystal structure were studied, confirming lattice expansion and tetragonality reduction in smaller particles. Surface bonding between BaTiO3 particles and PEG molecules were studied using FT-IR. The measured ζ-potential of ≈ −30 mV is indicative of good stability and redispersibility of these surface-modified particles. Furthermore, the large values of dielectric constant (≈6000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) at the transition temperature of TC = 125–135 °C confirm the strong ferroelectricity in this sample. These findings provide a simple solution to nanoparticle aggregation, thereby improving the stability of ferroelectric dispersion.
000) at the transition temperature of TC = 125–135 °C confirm the strong ferroelectricity in this sample. These findings provide a simple solution to nanoparticle aggregation, thereby improving the stability of ferroelectric dispersion.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery Grant, RTI, and PDF), the Canada Excellence Research Chair (CERC) program, and the University of Calgary's Global Research Initiative in Sustainable Low Carbon Unconventional Resources funded by the Canada First Research Excellence Fund (CFREF) for supporting this research. We thank Dr Berton for performing thermal analysis and 4D LABS (Simon Fraser University) for HRTEM imaging.Notes and references
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rodel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed.
- B. Jiang, J. Iocozzia, L. Zhao, H. Zhang, Y. Harn, Y. Chen and Z. Lin, Chem. Soc. Rev., 2019, 48, 1194–1228 RSC.
- Z. Zhao, V. Buscaglia, M. Viviani, M. T. Buscaglia, L. Mitoseriu, A. Testino, M. Nygren, M. Johnsson and P. Nanni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 024107 CrossRef.
- C. Xiao, Z. Chi, W. Zhang, F. Li, S. Feng, C. Q. Jin, X. Wang, X. Deng and L. Li, J. Phys. Chem. Solids, 2007, 68, 311–314 CrossRef CAS.
- K. Uchino, E. Sadanaga and T. Hirose, J. Am. Ceram. Soc., 1989, 72, 1555 CrossRef CAS.
- Z. G. Shen, J. F. Chen, H. K. Zou and J. Yun, J. Colloid Interface Sci., 2004, 275, 158–164 CrossRef CAS PubMed.
- A. Hirata, Y. Nitta and M. Kawabata, J. Ceram. Soc. Jpn., 1994, 102, 1168–1172 CrossRef.
- G. Ciofani, S. Danti, S. Moscato, L. Albertazzi, D. D’Alessandro, D. Dinucci, F. Chiellini, A. Petrini and M. Menciassi, Colloids Surf., B, 2010, 76, 535–543 CrossRef CAS PubMed.
- J. Li, K. Inukai, A. Tsuruta, Y. Takahashi and W. Shin, J. Asian. Ceram. Soc., 2017, 5, 444–451 CrossRef.
- C. Hai, K. Inukai, Y. Takahashi, N. Izu, T. Akamatsu, T. Itoh and W. Shin, Mater. Res. Bull., 2014, 57, 103–109 CrossRef CAS.
- X. Pang, Y. He, B. Jiang, J. Iocozzia, L. Zhao, H. Guo, J. Liu, M. Akinc, N. Bowler, X. Tan and Z. Lin, Nanoscale, 2013, 5, 8695–8702 RSC.
- B. Jiang, X. Pang, B. Li and Z. Lin, J. Am. Chem. Soc., 2015, 137, 11760–11767 CrossRef CAS PubMed.
- X. Wang, T. Karaki, K. Koyanagi and T. Fujii, Jpn. J. Appl. Phys., 2020, 59, SCCB05 CrossRef CAS.
- L. S. Simon-Seveyrat, A. Hajjaji, Y. Emziane, B. Guiffard and D. Guyomar, Ceram. Int., 2007, 33, 35–40 CrossRef CAS.
- G. Panomsuwan and H. Manuspiya, J. Appl. Phys. A, 2018, 124(10), 1–8 CAS.
- S. K. Lee, T. J. Park, G. J. Choi, K. K. Koo and S. W. Kim, Mater. Chem. Phys., 2003, 82, 742–749 CrossRef CAS.
- S. G. Kwon, B. H. Park, K. Choi, E. S. Choi, S. Nam, J. W. Kim and J. H. Kim, J. Eur. Ceram. Soc., 2006, 26, 1401–1404 CrossRef CAS.
- S. S. Tripathy and A. M. Raichur, J. Exp. Nanosci., 2011, 6, 127–137 CrossRef CAS.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- A. Banerjee, B. Blasiak, E. Pasquier, B. Tomanek and S. Trudel, RSC Adv., 2017, 7, 38125 RSC.
- J. S. Suk, Q. Xu, N. Kim, J. Hanes and L. M. Ensign, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed.
- A. S. Karakoti, S. Das, S. Thevuthasan and S. Seal, Angew. Chem., Int. Ed., 2011, 50, 1980–1994 CrossRef CAS.
- S. Moharana, M. K. Mishra, B. Behera and R. N. Mahalinga, Polym. Sci., 2017, 59, 405–415 CAS.
- J. G. Kim, W. P. Tai, K. J. Lee and W. S. Cho, Ceram. Int., 2004, 30, 2223–2227 CrossRef CAS.
- J. Čulić-Viskota, W. P. Dempsey, S. E. Fraser and P. Pantazis, Nat. Protoc., 2012, 7(9), 1618–1633 CrossRef.
- J. Zhu, W. Li, X. Huo and Y. Zhu, J. Phys. D: Appl. Phys., 2015, 48, 355301 CrossRef.
- R. B. Upadhyay, S. Annam, M. R. Patel, A. K. Sharma, P. Mevada and U. S. Joshi, Ferroelectr., Lett. Sect., 2016, 43, 25–33 CrossRef CAS.
- S. W. Boland, PhD thesis, California Institute of Technology, 2005.
- J. Li, K. Inukai, Y. Takahashi and W. Shin, J. Asian. Ceram. Soc., 2016, 4, 394–402 CrossRef.
- T. Roisnel and J. Rodriguez-Carvajal, in Proceedings of the Seventh European Powder Diffraction Conference, ed. R. Delhez and E.J. Mittenmeijer, 2000, pp. 118–123 Search PubMed.
- D. M. Smilgies, J. Appl. Crystallogr., 2009, 42, 1030–1034 CrossRef CAS PubMed.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS PubMed.
- M. Taheri, B. Zanca, S. J. Villegas, M. Dolgos, S. Bryant and S. Trudel, 2020 Joint Conference of the IEEE International Frequency Control Symposium and International Symposium on Applications of Ferroelectrics (IFCS-ISAF), 2020, pp. 1–4.
- Y. Gao, V. V. Shvartsman, A. Elsukova and D. C. Lupascu, J. Mater. Chem., 2012, 22, 17573–17583 RSC.
- S. Yoon, S. Baikw, M. G. Kim, N. Shin and I. Kim, Am. Ceram. Soc., 2007, 90, 311–314 CrossRef CAS.
- O. G. Grendal, A. B. Blichfeld, S. L. Skjærvø, W. V. Beek, S. M. Selbach, T. Grande and M. A. Einarsrud, Crystals, 2018, 8, 253 CrossRef.
- M. B. Smith, K. Page, T. Siegrist, P. L. Redmond, E. C. Walter, R. Seshadri, L. E. Brus and M. L. Steigerwald, J. Am. Chem. Soc., 2008, 130, 6955–6963 CrossRef CAS PubMed.
- P. M. Diehm, P. Agoston and K. Albe, Chem. Phys. Chem., 2012, 13, 2443–2454 CrossRef CAS PubMed.
- S. Tsunekawa, K. Ishikawa, Z. Q. Li, Y. Kawazoe and A. Kasuya, Phys. Rev. Lett., 2000, 85, 3440 CrossRef CAS PubMed.
- H. Hayashi, T. Nakamura and T. Ebina, J. Phys. Chem. Solids, 2013, 74(7), 957–962 CrossRef CAS.
- H. Xu and L. Gao, Mater. Lett., 2002, 57, 490–494 CrossRef CAS.
- A. Aala, T. R. Hammad, M. Zawrah, I. K. Battisha and A. B. Abou Hammad, Acta Phys. Pol., A, 2014, 126, 1318–1321 CrossRef.
- J. Stetefeld, S. McKenna and T. Patel, Biophys. Rev., 2016, 8, 409–427 CrossRef CAS PubMed.
- S. Bhattacharjee, J. Controlled Release, 2016, 235, 337–351 CrossRef CAS PubMed.
- A. V. Salvekar, W. M. Huang, R. Xiao, Y. S. Wong, S. S. Venkatraman, K. H. Tay and Z. X. Shen, Acc. Chem. Res., 2017, 50, 141–150 CrossRef CAS.
- N. Baskaran, A. Ghule, C. Bhongale, R. Murugan and H. Chang, J. Appl. Phys., 2002, 91, 10038 CrossRef CAS.
- D. Ricinschi, V. Tura, L. Mitoseriu and M. Okuyama, J. Phys.: Condens. Matter, 1999, 11, 1601–1613 CrossRef CAS.
- S. Wada, T. Hoshina, K. Takizawa, M. Ohishi, H. Yasuno, H. Kakemoto, T. Tsurumi, C. Moriyoshi and Y. Kuroiwa, J. Korean Phys. Soc., 2007, 51(2), 878–881 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, X-ray diffraction results, scanning electron micrographs, HRTEM of sample BT-1. See DOI: 10.1039/d1ma00317h |
| This journal is © The Royal Society of Chemistry 2021 |