Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oriented collagen films with high Young's modulus by self-assembly on micrometer grooved polydimethylsiloxane†
Miho
Aizawa
ab,
Hirona
Nakamura
ac,
Kohsuke
Matsumoto
ac,
Takahiro
Oguma
a and
Atsushi
Shishido
 *ac
*ac
aLaboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, Yokohama 226-8503, Japan. E-mail: ashishid@res.titech.ac.jp
bResearch Institute for Sustainable Chemistry, National Institute of Advanced Industrial Science and Technology, Tsukuba, Ibaraki 305-8565, Japan
cDepartment of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, Meguro, Tokyo 152-8552, Japan
First published on 14th September 2021
Abstract
We report on the fabrication of oriented collagen films by drying a dilute collagen solution on a polydimethylsiloxane (PDMS) substrate with a micrometer grooved surface. The resultant films show optical and mechanical anisotropy along the groove direction, and have a high mechanical strength with Young's modulus of 2.3 GPa. This process might enable collagen materials to be applied to anisotropic biocompatible substrates, large-area functional scaffolds, and soft robotic materials.
Collagen is the most abundant protein in mammals and has been continuously studied over the past several decades.1–3 One of the most useful approaches for maximizing the function of collagens is the orientation control of collagens4 due to their structural anisotropy ∼300 nm in length and ∼1.5 nm in diameter, i.e., for type I collagen.1 For example, cells grow along the oriented collagen fibers that act as a functional scaffold.5 In fact, it was reported that anisotropy of bone tissues was controllable by oriented collagen matrixes.6,7 In addition, oriented collagens have an advantage in mechanical strength. In vivo, the orientation of collagens plays a special role in the function of human organs; for example, bone osteons and tendons that have high mechanical strength.2 Also in vitro, Isobe et al. reported that an oriented collagen tube was transplanted as a blood vessel graft, taking advantage of its high mechanical strength.8 Several strategies to achieve the orientation of collagen molecules have been proposed, such as flow-induced alignment,5,7–12 elongation techniques,13 electrospinning,14,15 electrochemical alignment,16,17 nanopatterned surfaces,18 and magnetic alignment.19 However, most of these orientation methods are limited to fibrous and fibril scales, i.e., 50 nm to 500 nm in diameter. Thus, the difficulty in macroscopic orientation on a practical scale (such as several centimeters) prevents making full use of the advantages of orientation control. Indeed, the macroscopic tensile strength of the oriented collagen bundle (∼400 MPa)16 is much lower than that of collagen fibrils (∼2 GPa).20 Considering that the homogeneous collagen film without orientation control shows the same range of mechanical strength (i.e. 2.0 GPa),21 uniform collagen orientation control over a large area has the potential to provide a high-strength oriented film.
Here, we report formation of uniformly oriented collagen film over a large area, just by drying a dilute collagen solution on a polydimethylsiloxane (PDMS) substrate with a micrometer grooved surface (Fig. 1). The orientation occurs under certain conditions in vitro, due to the liquid-crystalline nature of collagen molecules.22–29 The liquid-crystallinity promotes a directional self-assembly process; liquid crystals recognize the direction of the groove in the surface of the PDMS substrate, and this macroscopically aligns collagens. We successfully observed that the fabricated collagen film exhibited optical and mechanical anisotropy depending on the orientation direction. Furthermore, a subsequent thermal cross-linking reaction made the collagen film insoluble in water, maintaining the mechanical anisotropy of the collagen films, which successfully achieved high mechanical strength with Young's modulus of 2.3 GPa. Hence, this process has great advantages to practical applications due to its simplicity, efficient orientation in a large area, high mechanical strength, and water resistance.
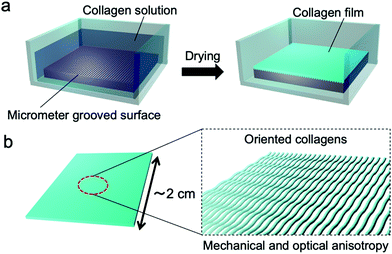 | ||
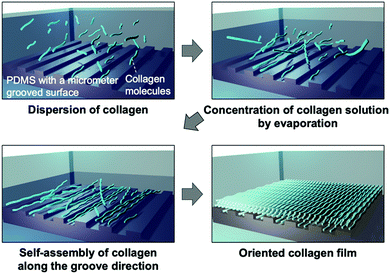
| Fig. 1 Schematic illustrations of (a) the fabrication process of the collagen film by drying on a micrometer grooved PDMS substrate and (b) the fabricated film with oriented collagens. | ||
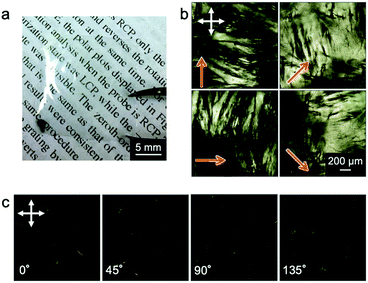
Hypothesizing that the concentration of a collagen solution induced liquid-crystallinity during the drying process and the resultant orientation of collagens, we designed an experiment to form a collagen film on a PDMS substrate with a micrometer groove in the surface, which promotes a directional self-assembly process (Fig. 1(a)). We used a pepsin solubilized porcine skin collagen type-I (Nitta Gelatin Inc.), which was purchased at stock concentrations of ∼5.0 mg mL−1 in dilute HCl (pH ∼ 3.1). The collagen solution was diluted with distilled water to a concentration of approximately 1.25 mg mL−1 to reduce the viscosity for easy handling. Collagen films were fabricated by drying the dilute collagen solution in a handmade glass box (size: 3 cm × 3 cm; height: 12.5 mm) in which a PDMS substrate (size: 2 cm × 2 cm; thickness: 1.25 mm) was adhered at the bottom. To direct self-assembly, we inscribed a micrometer groove in the PDMS substrate referring to a pervious study (Fig. S1, ESI†).30,31 The dilute collagen solution of 4 ml was cast in the glass box placed on a hotplate set at 38 °C. At this time, the solution temperature was 35 °C, which is below the denaturation temperature of collagen. After the solvent was dried, the collagen solution was cast again. This solution casting and drying process was repeated four times to obtain freestanding films with a sufficient film thickness. Fig. 2a shows a photograph of a collagen film (size: 2 cm × 2 cm; thickness: 20 μm) fabricated on the micrometer grooved PDMS substrate, exhibiting optical transparency. Observation of the collagen film by polarized optical microscopy (POM: Olympus BX50) revealed that the film has optical anisotropy (Fig. 2b). In contrast, a collagen film fabricated in a glass box with a flat PDMS substrate did not show optical anisotropy in any direction (Fig. 2c). These results imply that the emergence of the optical anisotropy in the collagen film was attributed to the presence of the micrometer grooves in the film formation process. Thus, we performed detailed analyses to investigate the effect of the orientation of collagen molecules on the optical anisotropic properties. From the observation of the film with a test plate (Olympus U-TP530), the optical axis of the anisotropy was found to be parallel to the groove direction, which was transferred from the surface of the PDMS substrate (Fig. S2, ESI†). This result is remarkable because it indicates that the collagen molecules oriented parallel to the groove direction.
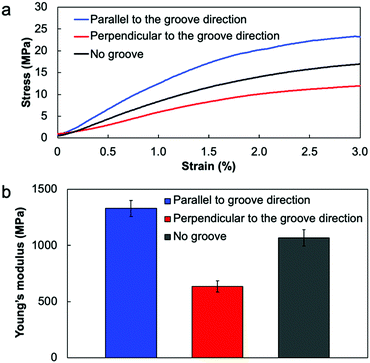
If the collagen molecules oriented in the film, there is a possibility to show the difference in the mechanical properties. To evaluate the mechanical anisotropy of the resultant collagen film, we carried out the tensile test by cutting the collagen films into rectangular shapes (15 mm length × 5 mm width) parallel or perpendicular to the groove direction. Fig. 3 shows the results of the tensile test for the collagen films. As a result, the Young's modulus calculated from the stress–strain curves showed a clear difference in the tensile directions; the Young's modulus parallel to the groove direction became higher than that in the perpendicular direction. These results indicate that the mechanical directionalities were linked with the orientation directions of collagens because the direction of this mechanical anisotropy was identical to the results of POM observation. In addition, the Young's modulus of the film fabricated on the flat PDMS substrate showed an intermediate value of Young's modulus, also supporting that the mechanical anisotropy was derived from the molecular orientation (Fig. 3b). It is important to note that the fabrication process of the collagen film enables not only fabricating the oriented collagen film but also providing the mechanical strength anisotropy.
The orientation of collagen molecules achieved in the present system can be rationalized by assuming that liquid-crystallinity of the collagen is the main factor. Collagen exhibits a lyotropic liquid crystalline state as the solution is concentrated, depending on the pH and the type of collagen.26,27 Indeed, it has been reported that the collagen solution shows liquid crystal phases such as nematic and cholesteric phases at concentrations above approximately 20 mg mL−1 under acidic conditions.5 As shown in a movie (ESI†), optical anisotropy was confirmed with the concentrated solution of approximately 60 mg mL−1, while no anisotropy was observed with the diluted collagen solution (1.25 mg mL−1), which clearly indicates that the liquid crystal phase appears during the drying process. Fig. 4 shows a schematic illustration of the proposed formation mechanism of the oriented collagen film based on the present system. Drying the solvent leads to concentration of collagen solution and increases the intermolecular interaction of collagen molecules, and then the collagen solution becomes liquid-crystalline. On the basis of the established theory, orientation of liquid crystals is affected by a surface grooved structure of a substrate.32,33 The liquid-crystalline collagens self-assembled along with the groove direction in the PDMS surface, resulting in the orientation of collagen molecules over a large area. By laminating the collagens by repeating the casting process, the collagens are accumulated, maintaining their orientation. We consider that the collagens are densely packed through the film thickness direction because the film shows optical anisotropy at any position in the thickness direction, and this brings the high mechanical strength.
 | ||
| Fig. 4 Schematic illustrations of the orientation mechanism of collagens on the micrometer grooved PDMS substrate. | ||
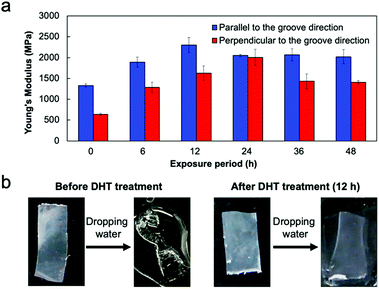
To provide a function of insolubility in water, which is an important characteristic for practical use of collagen films, we conducted a thermal cross-linking reaction. Dehydrothermal (DHT) treatment is known as a common method of cross-linking collagen molecules, which exposes collagens to raised temperature under a vacuum.34,35 This process removes water from the collagen molecules, resulting in the formation of intermolecular cross-links (amide bonds) through condensation reactions. The effect of DHT treatment on film properties was previously examined by Haugh et al.;34 the exposure period had an effect on the mechanical properties and cross-linking density. To conduct the thermal treatment for the fabricated collagen film, the film was wrapped in aluminum foil and placed in a vacuum oven at 140 °C (Fig. S3, ESI†). The exposure period was varied from 6 to 48 h. POM observation of the thermally treated film revealed that the optical anisotropy was stably maintained even after heating for 48 h (Fig. S4, ESI†). The tensile test revealed that Young's modulus showed an obvious dependence on the exposure period, keeping the relationship of the strength in parallel and perpendicular elongation directions (Fig. 5a). The highest Young's modulus of 2.3 GPa was obtained in the film heated for 12 h, in which Young's modulus increased by approximately 60% compared to the untreated collagen film. It must be noted that this value is 10% higher than that in the collagen film prepared under in vitro conditions.18 Hence, the fabrication of oriented films by self-assembly of liquid-crystalline collagens on the surface of a micrometer grooved PDMS substrate is a powerful process for preparing a film with high Young's modulus. In addition, we evaluated water solubility of the thermally cross-linked film. The untreated film was immediately swollen in water and finally dissolved; however, the cross-linked film thermally treated for 12 h kept its shape in water without swelling (Fig. 5b and ESI†). These results clearly indicate that the DHT treatment of the oriented collagen film brought about the cross-linking reaction, which leads to an increase in Young's modulus and water insolubility, maintaining its mechanical anisotropy.
Conclusions
In conclusion, we reported a facile fabrication process for the molecularly oriented collagen films over a large area (2 cm × 2 cm). The key strategy for this achievement is the directed self-assembly of liquid-crystalline collagen molecules during the drying process on the surface of the micrometer grooved PDMS substrate. The fabricated freestanding film showed optical and mechanical anisotropy due to the orientation of collagens. In addition, the thermal cross-linking reaction made the film water-insoluble and increased the mechanical strength, keeping its anisotropy of the strength in parallel and perpendicular elongation directions. To the best of our knowledge, this is the simplest example of fabricating oriented collagen film over a large area. This process is expected to widely open the use of the collagen materials for practical applications such as biocompatible substrates with optical and mechanical anisotropy, large-area functional scaffolds for accelerating the development of research in cell biology, and soft robotic materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in Aid for Scientific Research on Innovative Areas “Molecular Engine” (JSPS KAKENHI grant number JP18H05422). This work was supported by JST CREST grant number JPMJCR18I4, Japan. This work was supported by JSPS KAKENHI grant number JP20J14875. This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”. This work was performed under the Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices”.Notes and references
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS.
- D. J. S. Hulmes, J. Struct. Biol., 2002, 137, 2–10 CrossRef CAS.
- C. Sanchez, H. Arribart and M. M. Giraud-Guille, Nat. Mater., 2005, 4, 277–288 CrossRef CAS PubMed.
- A. Sorushanova, L. M. Delgado, Z. Wu, N. Shologu, A. Kshirsagar, R. Raghunath, A. M. Mullen, Y. Bayan, A. Pandit, M. Raghunath and D. I. Zeugolis, Adv. Mater., 2019, 31, 1801651 CrossRef PubMed.
- J. E. Kirkwood and G. G. Fuller, Langmuir, 2009, 25, 3200–3206 CrossRef CAS PubMed.
- A. Matsugaki, G. Aramoto, T. Ninomiya, H. Sawada, S. Hata and T. Nakano, Biomaterials, 2015, 37, 134–143 CrossRef CAS PubMed.
- A. Matsugaki, Y. Isobe, T. Saku and T. Nakano, J. Biomed. Mater. Res., Part A, 2015, 103, 489–499 CrossRef PubMed.
- Y. Isobe, T. Kosaka, G. Kuwahara, H. Mikami, T. Saku and S. Kodama, Materials, 2012, 5, 501–511 CrossRef CAS PubMed.
- N. Saeidi, E. A. Sander and J. W. Ruberti, Biomaterials, 2009, 30, 6581–6592 CrossRef CAS PubMed.
- O. F. A. Gutierrez and A. D. Rey, Langmuir, 2016, 32, 11799–11812 CrossRef PubMed.
- Y. Tanaka, K. Baba, T. J. Ducan, A. Kubota, T. Asahi, A. J. Quantock, M. Yamato, T. Okano and K. Mishida, Biomaterials, 2011, 32, 3358–3366 CrossRef CAS PubMed.
- B. Lanfer, U. Freudenberg, R. Zimmermann, D. Stamov, V. Körber and C. Werner, Biomaterials, 2008, 29, 3888–3895 CrossRef CAS PubMed.
- K. Poole, K. Khairy, J. Friedrichs, C. Franz, D. A. Cisneros, J. Howard and D. Mueller, J. Mol. Biol., 2005, 349, 380–386 CrossRef CAS PubMed.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3, 232–238 CrossRef CAS PubMed.
- T. J. Sill and H. A. von Recum, Biomaterials, 2008, 29, 1989–2006 CrossRef CAS PubMed.
- X. Cheng, U. A. Gurkan, C. J. Dehen, M. P. Tate, H. W. Hillhouse, G. J. Simpson and O. Akkus, Biomaterials, 2008, 29, 3278–3288 CrossRef CAS PubMed.
- J. A. Paten, S. M. Siadat, M. E. Susilo, E. N. Ismail, J. L. Stoner., J. P. Rothstein and J. W. Ruberti, ACS Nano, 2016, 10, 5027–5040 CrossRef CAS PubMed.
- F. A. Denis, A. Pallandre, B. Nysten, A. M. Jonas and C. C. Dupont-Gillain, Small, 2005, 10, 984–991 CrossRef PubMed.
- J. Torbet, M. Malbouyres, N. Builles, V. Justin, M. Roulet, O. Damour, A. Oldberg, F. Ruggiero and D. J. S. Hulmes, Biomaterials, 2007, 28, 4268–4276 CrossRef CAS PubMed.
- M. Minary-Jolandan and M.-F. Yu, Biomacromolecules, 2009, 10, 2565–2570 CrossRef CAS PubMed.
- S. Moreno, M. Baniasadi, S. Mohammed, I. Mejia, Y. Chen, M. A. Quevedo-Lopez, N. Kumar, S. Dimitrijevich and M. Minary-Jolandan, Adv. Electron. Mater., 2015, 1, 1500154 CrossRef.
- M.-M. Giraud-Guille, J. Mol. Biol., 1992, 224, 861–873 CrossRef CAS PubMed.
- L. Besseau and M.-M. Giraud-Guille, J. Mol. Biol., 1995, 251, 197–202 CrossRef CAS PubMed.
- M.-M. Giraud-Guille, L. Besseau and R. Martin, J. Biomech., 2003, 36, 1571–1579 CrossRef PubMed.
- G. Mosser, A. Anglo, C. Helary, Y. Bouligand and M.-M. Giraud-Guille, Matrix Biol., 2006, 25, 3–13 CrossRef CAS PubMed.
- F. Gobeaux, E. Belamie, G. Mosser, P. Davidson, P. Panine and M.-M. Giraud-Guille, Langmuir, 2007, 23, 6411–6417 CrossRef CAS PubMed.
- A. D. Rey, Soft Matter, 2010, 6, 3402–3429 RSC.
- P. De Sa Peixoto, A. Deniset-Besseau, M. Schmutz, A. Anglo, C. Illoul, M.-C. Schanne-Klein and G. Mosser, Soft Matter, 2013, 9, 11241–11248 RSC.
- S. Zhu, Q. Yuan, T. Yin, J. You, Z. Gu, S. Xiong and Y. Hu, J. Mater. Chem. B, 2018, 6, 2650–2676 RSC.
- N. Akamatsu, W. Tashiro, K. Saito, J. Mamiya, M. kinoshita, T. Ikeda, J. Takeya, S. Fujikawa, A. Priimagi and A. Shishido, Sci. Rep., 2014, 4, 5377 CrossRef CAS PubMed.
- R. Taguchi, N. Akamatsu, K. Kuwahara, K. Tokumitsu, Y. Kobayashi, M. Kishino, K. Yaegashi, J. Takeya and A. Shishido, Adv. Mater. Interfaces, 2021, 8, 2001662 CrossRef CAS.
- G. P. Bryan-Brown, C. V. Brown, I. C. Sage and V. C. Hui, Nature, 1998, 392, 365–367 CrossRef CAS.
- X. T. Li, A. Natansohn and P. Rochon, Appl. Phys. Lett., 1999, 74, 3791–3793 CrossRef CAS.
- M. G. Haugh, M. J. Jaasma and F. J. O’Brien, J. Biomed. Mater. Res., Part A, 2008, 89, 363–369 Search PubMed.
- K. Adamiak and A. Sionkowska, Int. J. Biol. Macromol., 2020, 161, 550–560 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00642h |
| This journal is © The Royal Society of Chemistry 2021 |