 Open Access Article
Open Access ArticleAdvances in implant surface modifications to improve osseointegration
Guang
Zhu
ab,
Guocheng
Wang
 *a and
Jiao Jiao
Li
*a and
Jiao Jiao
Li
 *bc
*bc
aResearch Center for Human Tissues & Organs Degeneration, Shenzhen Institute of Advanced Technology, Chinese Academy of Science, Shenzhen, Guangdong, 518055, China. E-mail: gc.wang@siat.ac.cn; Tel: +86-755-86392561
bKolling Institute, Faculty of Medicine and Health, University of Sydney, St Leonards, NSW 2065, Australia
cSchool of Biomedical Engineering, Faculty of Engineering and IT, University of Technology Sydney, NSW 2007, Australia. E-mail: jiaojiao.li@uts.edu.au; Tel: +61-2-95149232
First published on 10th September 2021
Abstract
Metallic biomaterials are widely used in implants to strengthen, repair, or replace damaged bone tissue, and their material characteristics have direct influences on short- and long-term implant performance. Of these, titanium and its alloys are the most widely applied due to their superior corrosion resistance, biocompatibility, and mechanical properties, such as in joint replacements, dental implants, and spinal fusion cages. However, Ti and Ti alloys are bioinert materials that have difficulty in binding directly to bone tissue after implantation, due to a lack of osteoconductive and osteoinductive properties. Bacterial adhesion and colonisation at the implantation site may also lead to infection-associated complications. The surface of the titanium implant directly interfaces with blood, cells, and tissues in vivo, and the surface properties can have profound influences on protein- and cell-based interactions that then promote or impede osseointegration. Therefore, the material surface morphology, chemistry and antibacterial function are key parameters in implant design, and contribute to determining the long-term success of the implant. In this review, we systematically present the latest advances in surface modification techniques for orthopaedic implants, including mechanical, physical, chemical, and biological modification. We also analyse and compare different surface modification approaches, including drug loading, metallic element doping, and bionic coatings, as well as topographical modifications such as nanotubes, nanopores and nanowires. Finally, we present a critical analysis and future perspectives on the use of surface modifications to improve the osseointegration and antibacterial properties of orthopaedic implants.
1. Introduction
In recent decades, the worldwide demand for orthopaedic implants has continued to increase at an alarming rate. For Australia alone, the incidence rate of primary total hip arthroplasty (THA) is predicted to increase by 66% between 2013 and 2046 according to a conservative projection model.1 In the US, the total number of hip and knee replacements has reached 7 million cases per year.2 Revision surgeries due to peri-implant diseases or implant failure also increase the demand for orthopaedic implants. According to a cross-sectional analysis, one-quarter of patients and one-sixth of implants will develop peri-implantitis after 11 years.3 A statement from the third EAO consensus conference 2012 stated that approximately one-fifth of patients will suffer from peri-implantitis within 5 years of implant placement.4 To meet this significant demand, a wide range of biomaterials have been used and are being developed for orthopaedic implants, including metallic, ceramic, and polymeric materials.5 Metallic biomaterials have the advantages of high strength and toughness, easy processing, and good biocompatibility. Ceramic biomaterials have relatively poor mechanical properties, but they have stable chemical properties and are biocompatible or bioactive, leading to their primary use as implant coatings. Polymeric biomaterials generally have the advantages of biological aging resistance and high purity, but their mechanical properties are often insufficient for hard tissue replacement and the majority are biologically inert.Among the clinically applied metallic biomaterials, titanium (Ti) and its alloys, such as Ti6Al4V, are the most popular candidates for bone implants due to their excellent mechanical properties (high mechanical strength, low density, immunity to corrosion) and superior biocompatibility, compared to conventional materials such as stainless steel 316L and cobalt–chromium (CoCr) alloys.6,7 However, the direct use of Ti and its alloys as implant materials is subject to several limitations. Firstly, their inherent bioinertness leads to a high rate of implant failure as they lack the ability to form chemical bonds with surrounding tissues.8,9 Ti forms only a physical bond with bone tissue, the stability of which is much lower than chemical osseous bonding, leading to a higher risk of implant loosening or failure during long-term use.10 Secondly, Ti lacks natural antimicrobial properties, easily allowing bacterial colonisation during the early stages of implantation.11 Following colonisation, the bacteria can grow to form a biofilm that interferes with the function of antibiotics and leads to subsequent infection.11 The infection rate of implants is also influenced by the surgical site and procedure. For example, the chance of infection is 2–30% for transcutaneous fracture fixation pins, and 13% for bone supplementation.12 The situation is further complicated by drug resistance in common pathogens such as Escherichia coli and Staphylococcus aureus, and antibiotic-resistant infections are increasing globally at an alarming rate.13 Antibiotic-resistant bacteria are estimated to cause at least 2 million infections and 23,000 deaths per year in the US, incurring a cost of $55–70 billion.14 Moreover, patients with infections associated with orthopaedic implants are reported to have worse clinical outcomes when the infection is caused by antibiotic-resistant compared to antibiotic-sensitive bacteria.14 Thirdly, the corrosion resistance of Ti and its alloys still needs further improvement, since ion release due to surface corrosion can lead to toxicity and bone loss.10,15 Although a titanium oxide film is easily generated on the surface of Ti implants, which is relatively stable, ion release can still occur after this thin film is eroded off. For instance, corrosion in Ti6Al4V implants can lead to the release of Al and V, where Al is linked to brain dementia, and V is harmful to the surrounding cells and can cause metal sensitisation.16,17
To address the limitations of Ti and its alloys as implant materials, improvements in biomedical performance (such as interactions with bone-related cells) can be introduced by modifying their surface roughness and morphology, as well as chemical properties.18 Osseointegration refers to the strong bond formed between an implant and osseous tissue, a concept first described by Branemark in the 1980s.19 The ability of an implant to achieve osseointegration to a large extent determines its long-term success in the body. Since the implant surface is in direct contact with cells, tissues and blood in vivo, material surface properties play a significant role in osseointegration and hence implant performance. Therefore, surface modification of metal implants is an effective approach to improve bone cell adhesion, proliferation, and differentiation when fast healing is needed.20 The purpose of surface modification is to simultaneously maintain the excellent mechanical properties and biocompatibility of the implant, while also optimising its antibacterial properties, corrosion resistance, and bioactivity. As such, surface modification is a primary and one of the most rapidly advancing areas in the development and optimisation of Ti and its alloys for biomedical applications.
The surface modification of materials can focus on several aspects, including the topographical structure, chemical composition, surface energy, hydrophilicity and hydrophobicity, and phosphate forming ability of materials.21,22 The most common strategy is to design and prepare a bioactive coating to enhance implant osseointegration and associated biological processes. Various bioactive implant coatings have been applied, such as hydroxyapatite (HA), other bio-ceramics, and bioactive oxides.23–25 Among the existing bioactive oxide coatings, such as TiO2, ZrO2, Al2O3, CuO, ZnO and some other oxides, titanium dioxide has been widely used for modifying the surface of Ti alloy implants due to its excellent chemical stability, biocompatibility, and strong binding with the Ti alloy substrate.25–27 Moreover, TiO2 has a bactericidal effect due to its photocatalytic properties, indicating potential applications as an antibacterial implant coating.28 Other interesting structures have been developed such as TiO2 nanotubes, which may be applied for drug delivery, anti-inflammatory functions, and osteogenesis.29 Interestingly, by adjusting the nanotube diameter (15, 60, or 120 nm) on the Ti surface, the blood clotting characteristics of the implant can be modulated to improve its immune response, rendering the implant anti-thrombotic and improving its ability to encourage osseointegration and bone regeneration.30
Other interesting developments in the area of surface modification for bone implants include designs that functionalise the surface with various abilities to promote osteogenesis, such as angiogenesis, production of reactive oxygen species (ROS), macrophage regulation, and antimicrobial effects.31–33 Ti alloy surfaces with composite functions have been designed to facilitate implant osseointegration by responding to different biological environments.34,35 Furthermore, the performance of Ti implant surfaces in patients with specific disease conditions, such as type II diabetes, and their improvement strategies have been studied.36
In this review, we present the latest developments in the surface modification of Ti and Ti alloy implants (Fig. 1), through research articles published mostly within the last five years. We have classified various surface modification approaches into four broad categories: mechanical modification, physical modification, chemical modification, and biological modification. Some modification methods combined features across categories, and were classified according to their primary concept. We acknowledge other recently published impactful reviews on the topic of surface modification for biomaterial implants, which have provided insightful perspectives into surface modification of Ti and its alloys without a specific focus on their biomedical applications,37 or discussed surface modification to promote osseointegration without focusing on metal implant materials.38,39 Other reviews have focused on specific aspects of surface modification in Ti and Ti alloys for encouraging bone growth, such as the surface corrosion properties,40,41 biological resemblance to bone,42 and antibacterial function.43 Our review provides a comprehensive overview of the latest advances in surface modification methods of Ti and Ti alloy implants to improve their osseointegration, covering different mechanisms of modification and an extensive sample of the most recent studies.
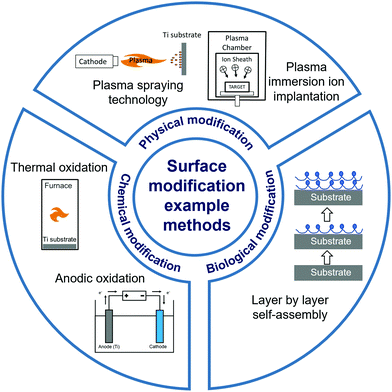 | ||
| Fig. 1 Schematic overview of the categories and examples of surface modification methods discussed in this review. | ||
2. Surface modification methods
2.1 Mechanical modification methods
Mechanical modification mainly involves relatively basic treatment methods such as grinding, polishing, sandblasting, and vacuum annealing.41 These methods are used to make the biomaterial surface smooth or rough, thereby modifying its properties including biological adhesion, surface hydrophilicity, bone tissue affinity, electrical potential energy, and surface tension. Mechanical modification can also be used to remove surface contamination and increase the binding strength of the substrate for subsequent treatment. For instance, Bacchelli et al.44 compared the bone integration ability of Ti implants that were zirconia sandblasted (Zr–SL), with control, titanium oxide plasma sprayed (TPS), and alumina sandblasted (Al–SL) implants in sheep at 2, 4 and 12 weeks after insertion. The Zr–SL and Al–SL implants achieved similar performance, which promoted better peri-implant new bone formation compared to control and TPS. Barranco et al.45 prepared and characterised polished, finely blasted and coarsely blasted Ti6Al4V surfaces. The blasting process was found to alter the surface morphology and chemical properties, leading to an increase in the capacitance values of the blasted samples, but did not change their corrosion behaviour. Günay-Bulutsuz et al.46 produced ultrafine-grain (UG) commercial purity (CP) Ti from 99.5% pure Ti Grade 4 by equal angular channel pressing. Sandblasting the sample surfaces was found to produce a more favourable grain size and surface roughness, which promoted the attachment and proliferation of human gingival fibroblast cells. Recently, Lowe et al.47 studied the biocompatibility of UG and coarse-grain (CG) CP Ti with or without polishing treatment, and concluded that the UG and polished Ti could enhance the proliferation of MC3T3-E1 pre-osteoblastic cells. The average grain boundary length per surface-attached cell was also identified as a new biophysical parameter that was correlated with cell proliferation. Shi et al.48 designed a hydrothermal sterilisation (HS) method by placing Ti samples in pure water at 121 °C for 20 minutes, followed by storing them in the same water until utilisation, which would reduce hydrocarbon contamination on Ti surfaces encountered during traditional sterilisation and storage. This method was found to produce super-hydrophilicity on the sandpaper-polished Ti surface, which improved the initial osteoblast response compared with autoclaving.Mechanical modification is often used as a pre-treatment strategy to improve the outcomes of subsequent surface modification methods. Miao et al.49 systematically evaluated the effects of Ti surface nanotopography on the behaviour of a range of human cells as well as bacteria, and suggested that changes in nanotopography could promote bone and soft tissue healing. A smooth Ti surface (Smooth) was first prepared by polishing, and then used to fabricate nano-rough (Nano) and micro-rough (Micro) surfaces by alkali-hydrothermal treatment and sandblasting and acid etching (SLA), respectively. The different surfaces influenced the osteogenic activity of MG-63 cells (Nano = Micro > Smooth), and the attachment ability of human gingival epithelial cells (Nano > Smooth > Micro) and human gingival fibroblasts (Nano = Micro > Smooth). Moreover, changes in nanotopography did not affect macrophage polarisation (Nano = Micro = Smooth), but did affect Streptococcus mutans attachment (Nano < Smooth < Micro). Wang et al.50 pre-treated Ti6Al4V flakes with sandpaper of different meshes, followed by treatment in three groups: grinding, sandblasting and etching, or nanosecond laser (Fig. 2). When tested using rat bone marrow-derived mesenchymal stem cells (BMSCs), it was found that cell proliferation was not affected by surface roughness alone, since cells tended to exhibit higher proliferation on flatter surfaces with a larger surface area. The spread of the cell cytoskeleton was suggested to be influenced by the surface energy barrier, where samples with micro-groove arrays produced by nanosecond laser treatment induced cells to form a cytoskeleton with higher aspect ratio.
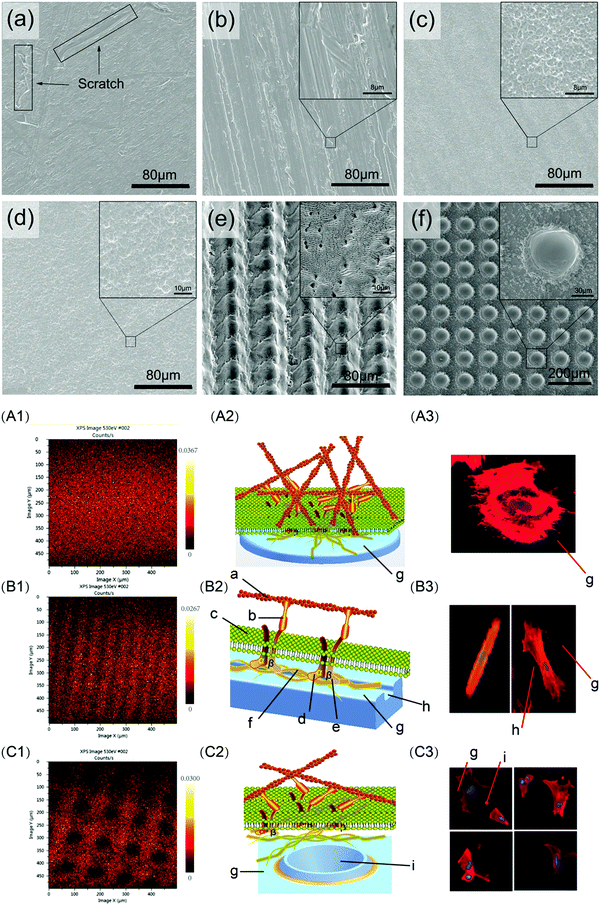 | ||
| Fig. 2 Different surface morphologies (top panel) produced by grinding (a and b), blasting and etching (c and d), and nanosecond laser (e and f), inducing differences in focal adhesion formation and cytoskeletal morphology of cells (bottom panel; A, B and C correspond to surfaces a, e and f respectively from the top panel).50 Reprinted with permission. Copyright 2020, Elsevier. | ||
2.2 Physical modification methods
Physical modification methods typically involve no or only a minor chemical reaction during the whole surface modification process, and they include plasma spraying technology (PST), plasma immersion ion implantation (PIII), and laser cladding. Physical modification is typically used for the dry transformation of passive inert implants into smart implant surfaces that actively instruct the physiological environment towards bone tissue regeneration.21Hydroxyapatite (HA) coatings, which are commonly used in clinical practice to improve osseointegration, are formed by spraying HA particles onto the implant surface at high temperature and cooling them rapidly.52 However, the HA coating has some disadvantages, a primary one being that the coating binds easily to bone tissue but has a low bonding strength to the metal alloy substrate. When sprayed at high temperatures, HA is prone to structural changes or even degradation, and its coefficient of thermal expansion (CTE) differs greatly from that of Ti and Ti alloys. These factors affect the bonding strength of the coating, which may lead to poor adhesion or delamination at the interface between the coating and the substrate, and even implant failure due to cracking and peeling of the coating.53
To better meet the requirements of clinical application, Fomin et al.54 investigated induction pre-heating (from 200 to 1000 °C) to improve the structural and mechanical properties of HA coatings on Ti implants using PST. Pre-heating the sample in the temperature range of 400–600 °C produced uniform nanostructures on the surface. The modified HA coatings showed high hardness (0.9–1.2 GPa) and elastic modulus (7–16 GPa). However, the bonding force between the coating and the substrate was not tested. To improve the coating–substrate bonding force, post-heat treatment of the HA coating can be applied. As an example, Ullah et al.55 synthesised (Sr, Zn)-HA powders by combining heat treatment with the hydrothermal method, and used PST to prepare Zn- and Sr-doped HA coatings on a Ti6Al4V substrate. Zn ions were used to provide antibacterial properties, and Sr ions were included to reduce the cytotoxicity of Zn. Post-heat treatment was performed on the Zn- and Sr-doped HA coatings at 500 and 600 °C for 3 hours. The results indicated that the (Sr, Zn)-HA coating post-treated at 500 °C had excellent mechanical properties, biocompatibility, and antibacterial properties. The coating post-treated at 600 °C showed cytotoxicity to MC3T3-E1 cells which limited its biomedical applications, but had remarkable mechanical and antibacterial properties. To reduce the mismatch in CTE between the metal alloy and the HA coating, Ke et al.56 fabricated a gradient HA layer using laser engineered net shaping (LENSTM) between the Ti6Al4V substrate and the PST HA coating (Fig. 3A). It was found that this middle layer should have a small wt% of HA to reduce the CTE mismatch between the overlying coating and the underlying substrate, and increase the bonding force of the coating. The middle layer did not affect the surface roughness of the overlying coating. In addition, MgO and Ag2O were introduced into the overlying PST HA coating. Ag2O showed sustained antimicrobial activity against E. coli and S. aureus, while MgO had no significant effects on promoting bone formation. Other approaches for improving HA coating performance include producing composite coatings. Lahiri et al.57 formed a HA–carbon nanotube (CNT) composite powder by spray drying, and plasma sprayed HA–4 wt % CNT powder on a Ti6Al4V substrate to prepare the coating. This HA–CNT composite coating showed superior mechanical (higher wear resistance and less debris production) and biological (higher osteoblast activity and less cytotoxicity) properties compared to HA coating.
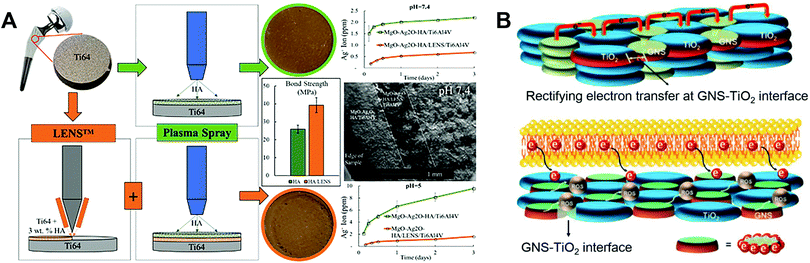 | ||
| Fig. 3 Schematic illustration of (A) the fabrication process for a gradient HA layer by combining LENSTM and PST,56 and (B) electron transfer mechanism leading to ROS generation in an antibacterial GNS–TiO2 coating fabricated using PST.31 Reprinted and adapted with permission. Copyright 2019 & 2020, Elsevier. | ||
A drug-loaded coating can be applied to HA-coated Ti implants to further improve osseointegration. As an example, Sarkar and Bose58 designed a top coating loaded with curcumin and vitamin K2, which was applied on HA-coated Ti6Al4V implants. The dual drug solution containing dissolved curcumin and vitamin K2 was added on the top surface of implants overnight in the dark to form a drug coating above the HA coating. The dual drug loaded samples reduced the in vitro proliferation of attached osteosarcoma cells, as well as the survival rate of unattached cells by 92–95% compared to the control group. In a rat distal femur model, the drug loaded implant promoted osseointegration after 5 days of implantation compared to the control group. Further studies to evaluate long-term osseointegration and bone repair in large animal models are warranted.
Sr has been widely employed in bone tissue engineering due to its ability to promote osteogenesis.59 Xu et al.60 produced TiO2 coatings using PST with different SrO-doping percentages, and found that the 20% SrO–TiO2 coating showed the best ability to promote the proliferation and differentiation of rat BMSCs. The Sr ions showed a continuous release behaviour as modulated by their Sr configuration, either as interstitial atoms in solid solution (TiySr2−2yO2) or as strontium titanate (SrTiO3). Zhang et al.61 prepared Sr-hardystonite (Sr-HT) ceramic (Ca2ZnSi2O7) coatings on Ti6Al4V implants by PST, with hybrid micro-/nano-scale structures. Both the coating–implant and coating–bone tissue adhesive bond strength were found to be improved. In vitro and in vivo experiments indicated the bioactivity of the coating and its ability to promote rapid bone formation in a canine model. The hybrid micro-/nano-scale structures promoted the adhesion of canine BMSCs, while bioactive ion release from the Sr-HT coating regulated BMSC differentiation. Strontium zinc silicate (SZnS, Sr2ZnSi2O7) has a strong mineralisation ability and can be applied in coatings and scaffolds.62,63 Wang et al.62 produced a SZnS coating on Ti alloy samples using PST, and applied a modified autoclave sterilisation process whereby samples were immersed in a buffered solution containing 4.2 mM HCO32−, to form biomorphic strontium carbonate (SrCO3) on the coating surface. The subcellular topographical features of SrCO3 biomorphs were found to prevent the burst release of ions, promote osteoblast adhesion and collagen production, and upregulate their alkaline phosphatase (ALP) activity. SZnS preferentially releases Sr ions, leaving behind a silica-rich gel on the ceramic surface which results in an alkaline pH in the local area, thereby providing a growth condition for the bimorphic crystals.63 The nanostructured mineralised surfaces were found to promote osteogenic differentiation and induce increased new bone formation in a rabbit model compared to non-mineralised surfaces.
The role of reactive oxygen species (ROS) in modulating biological activity has been increasingly studied and applied in biomaterial design.64 Yang et al.31 designed an antibacterial graphene nanosheet (GNS)–TiO2 coating based on an electron transfer mechanism, which was fabricated on Ti alloy samples using PST. The combination of unpaired π electrons of GNS and Ti atoms increased the electrical conductivity of the TiO2 surface. A bactericidal effect resulted from enhanced electron transfer from the bacterial cell membrane to the coating surface, and enrichment of electrons at the interface between the bacterial cell and the GNS–TiO2 coating. Specifically, an “electron sink” is created at the GNS and TiO2 interfaces, which can attract and store electrons extruded from bacterial cells due to Schottky barrier effects. Within the coating, the holes between TiO2 are surrounded by electrons, leading to ROS generation from oxidation reactions at the interface between bacteria and TiO2, resulting in a strong bactericidal effect (Fig. 3B).
A number of studies have implanted silver into Ti substrates as an inorganic antibacterial agent to combat periprosthetic infection. The bactericidal effect of Ag nanoparticles (NPs) doped in Ti surfaces is found to be independent of the release of silver ions.66,67 Qin et al.66 immobilised Ag NPs on the surface of Ti implants by PIII to endow the implants with antibacterial activity. The Ag NPs showed no obvious cytotoxicity, reduced biofilm formation of Staphylococcus epidermidis by inhibiting bacterial adhesion and icaAD gene transcription, and were effective against several cycles of bacterial attack in vitro. The coating also showed antibacterial effects in a rat tibia osteomyelitis model. The mechanism of antibacterial activity was found to be not related to silver release. Wang et al.67 combined Ag NPs as an inorganic bactericide with vancomycin as an organic antibacterial agent to design a surface with both contact-killing and release-killing antimicrobial capabilities, as well as cell-assisting functions (Fig. 4). The Ag NPs were embedded in TiO2 nanotubes by anodic oxidation (AO) and PIII, and vancomycin was then incorporated into the nanotubes by vacuum extraction and lyophilisation. The hybrid surface showed antibacterial and anti-biofilm properties against mobile and attached methicillin-resistant S. aureus (MRSA) ST239 in vitro and in a rabbit model of periprosthetic joint infection, without appreciable silver ion release.
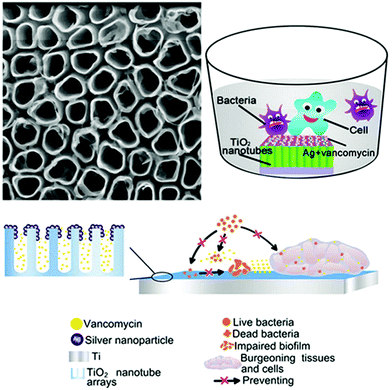 | ||
| Fig. 4 A TiO2 nanotube coating with embedded Ag NPs as an inorganic bactericide and vancomycin as an organic antibacterial agent, fabricated using AO and PIII.67 Reprinted with permission. Copyright 2016, American Chemical Society. | ||
Wang et al.68 studied the antibacterial behaviour and the underlying mechanism of Ag-NPs embedded in a Ti substrate by PIII, and found that antibacterial function relied on the conductivity of the substrate. This study showed that electron transfer between Ag-NPs and Ti or between the Ag-NP doped Ti surface and bacteria could produce ROS and induce oxidative stress. Intracellular and extracellular oxidative stress can induce physiological changes in bacterial cells and lead to cell death. Cao et al.69 doped size-tunable Ag NPs into a plasma sprayed titanium oxide coating (TOC) by PIII. Large Ag NPs (5–25 nm) were found to induce enhanced oxidation reactions on TOC compared to small NPs (∼4 nm), resulting in improved antibacterial activity of the TOC against S. aureus and E. coli. The mechanism by which extracellular electron transfer stimulates the bactericidal effect of Ag doped TOC in the dark was also investigated. The Ag NPs induced “bacterial charging” by storing extracellular electrons transferred from bacterial membranes, inducing valence-band hole (h+) accumulation and leading to oxidation reactions in the dark on the TOC surface. Zhao et al.70 co-doped Mg and Ag into Ti substrates by PIII, utilising the synergistic effect of multiple functional elements to improve both antibacterial and osteogenic properties. The Mg/Ag-doped group showed the best osteogenic activity compared to Mg-doped and Ag-doped groups. The mechanism is thought to be related to the galvanic effects between Mg and Ag NPs, which promoted Mg release but reduced silver release.
CaO–SiO2 coatings on Ti implants prepared by laser cladding, containing different phases including CaTiO3, α-Ca2(SiO4), SiO2, TiO2 and CaO, can promote osteogenic activity in vivo after implantation. Li et al.71 added Na2O and ZnO into the CaO–SiO2 precursor to prepare bioceramic coatings by laser cladding on a Ti substrate (Fig. 5). At temperatures above 1200 °C, CaTiO3 was easily formed in the CaO–SiO2 coating in comparison to CaSiO3. The effect of Na2O and ZnO on the microstructure and properties of CaO–SiO2 coatings was studied. The addition of Na2O was found to have little effect on the coating, but ZnO refined the microstructure of the coating and reduced its wear resistance and average hardness. The same researchers also explored the use of MgO to improve CaO–SiO2 coatings.72 The CaO–SiO2–MgO coating system produced by laser cladding showed a refined surface microstructure, and slightly lower average hardness compared with the CaO–SiO2 control. An increase in MgO content led to a decrease in wear resistance. In vitro and in vivo tests indicated good biocompatibility of the coating, which did not cause haemolysis reactions or toxicity to cells and living animals. The modified CaO–SiO2 coatings in these studies were shown to induce apatite formation, but no further in vitro or in vivo experiments were performed to test their ability in promoting osseointegration.
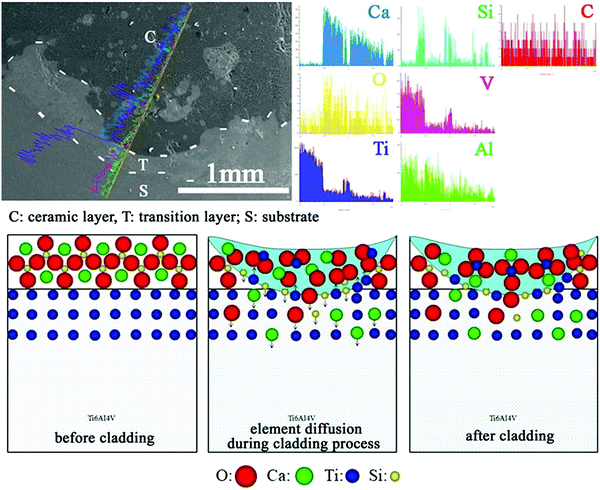 | ||
| Fig. 5 Schematic illustration of the cross-sectional morphology and line analyses of CaO–SiO2 coatings formed by laser cladding, and the mechanism of surface formation.71 Reprinted with permission. Copyright 2021, Elsevier. | ||
2.3 Chemical modification methods
Chemical modification methods usually involve a violent chemical reaction, which for a Ti substrate occurs at the interface of the metal surface and the imposed media (solution or gas phase). The reaction is often accompanied by luminescence, heating, redox reaction, and so on. Chemical modification methods including sandblasting and acid etching, thermal oxidation, hydrothermal treatment, anodic oxidation, and micro arc oxidation have been widely employed for preparing surfaces with special topographical structures and/or complex compositions.37Raines et al.73 prepared SLA and modified SLA (modSLA; SLA without exposure to air to retain high surface free energy) surfaces, which were cultured with primary human osteoblasts, MG63 cells, and MG63 cells silenced for α2 integrin. Analysis of secreted levels of vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor (FGF-2), epidermal growth factor (EGF), and angiopoietin-1 (Ang-1) by these cells indicated that the secretion of angiogenic growth factors by osteoblastic cells was modulated by Ti surface micro-topography and surface energy, which was at least partially mediated by α2β1 integrin signalling. Boyan et al.74 used SLA or plasma spraying to produce microrough Ti surfaces with an average roughness of 4–7 μm, and demonstrated through an in vitro model that microscale roughness could create a microenvironment for osteoblasts to form new bone. Gittens et al.75 combined SLA with thermal oxidation to prepare surfaces with micro-/sub-micro-scale roughness and nanoscale features. The micro-/sub-micro-scale surface roughness was found to facilitate osteoblast adhesion, while the nanoscale features promoted osteoblast proliferation and differentiation. Interestingly, greatly enhanced osteoblast differentiation was seen on surfaces with both microscale roughness and a high density of nanoscale features, indicating a possible synergistic effect due to mimicking the layered complexity of bone. On sample surfaces without microscale roughness, the nanoscale features alone did not induce appreciable increases in osteoblast differentiation markers. In another study, Zhang et al.76 modified Ti surfaces using SLA followed by further alkali or hydrogen peroxide treatment, and then heat treatment. Both types of surfaces promoted the osteogenic activity of MC3T3-E1 cells, and also showed good osseointegration in a canine mandibular defect model.
SLA-treated Ti surfaces have also been studied in the context of certain diseases or conditions. For instance, Lee et al.36 produced hydrophilic nanostructured Ti surfaces by modifying with SLA and then storing in the absence of air to induce spontaneous formation of surface features (Fig. 6). This surface was found to promote osseous healing under type II diabetic conditions. When implanted into bone defects in a diabetic rat model, Ti implants with hydrophilic nanostructured surfaces promoted osteogenesis, together with the expression of anti-inflammatory proteins including AnxA1, S100-A8 and S100-A9. Surfaces cultured with macrophages in vitro reduced pro-inflammatory cytokine secretion and promoted M2 phenotype differentiation. As a strategy for combating infection, Liu et al.77 prepared a Ti-Cu alloy and modified the surface by SLA treatment. Compared to a mechanically polished surface, the SLA-treated surface was found to release more Cu ions, possess better antibacterial properties against S. aureus, and improve the osteogenic activity of MC3T3-E1 cells.
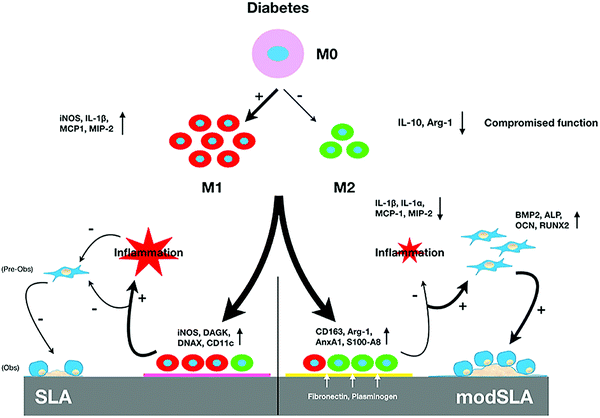 | ||
| Fig. 6 Schematic illustration of the mechanism by which modified hydrophilic nanostructured surfaces produced using SLA can modulate inflammation and promote osseous healing under type II diabetic conditions.36 Reprinted with permission. Copyright 2021, Elsevier. | ||
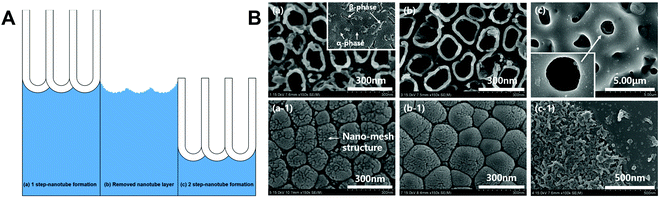
Scarano et al.78 conducted oxidation treatment on Ti implants at 802 °C for 1 min in the air, where the temperature was chosen as it caused an increase in crystalline anatase, and the short time was chosen to improve the mechanical properties. Compared to control implants without oxidation treatment, the treated implants showed better bioactivity and bone–implant contact in a rabbit femoral defect model. Wang et al.79 designed a thermal oxidation method to produce micro-/nano-scale features on the modified Ti surface. The Ti substrate was thermally treated at 450 °C for 6 hours, which enhanced the osteogenic differentiation of rat BMSCs and promoted in vivo osseointegration in the rabbit femur. Longhitano et al.80,81 tested the effects of different thermal oxidation temperatures (from 650 to 1050 °C) on Ti6Al4V samples made by additive manufacturing (Fig. 7A). The main differences in surface microstructure between different heating temperatures were found to be the nucleation and growth of α and β phases. Jenkins et al.82 prepared TiO2 nanopillars on Ti6Al4V samples through a thermal oxidation reaction at 850 °C in acetone vapour. Interestingly, contrary to previous studies speculating that the antimicrobial properties of a nanopillar surface morphology are based on mechanical damage, this study showed that nanopillars caused deformation and penetration of the cell envelope in Gram-positive and Gram-negative bacteria (Fig. 7B). They could inhibit bacterial cell division and trigger production of ROS, but did not rupture or lyse the bacteria. These results suggested that the antibacterial activity of Ti surface nanopillars might be mediated by oxidative stress rather than inducing bacterial lysis and death.
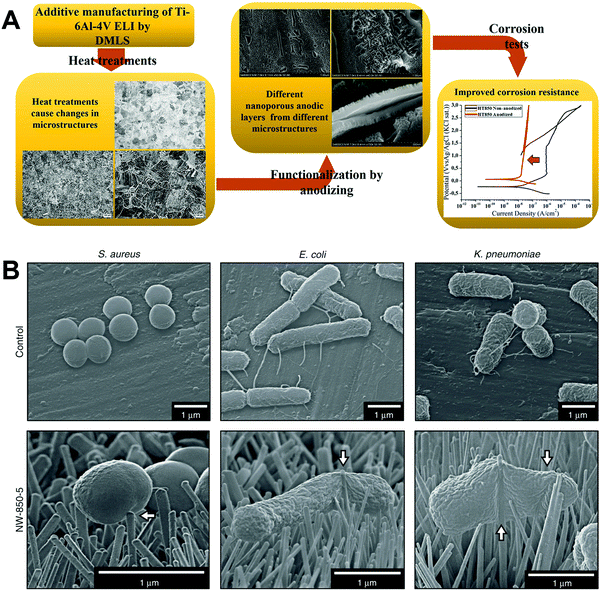 | ||
| Fig. 7 (A) Use of thermal oxidation to fabricate surface microstructures on Ti6Al4V samples made by additive manufacturing (direct metal laser sintering, DMLS).81 (B) Morphology of different bacteria on TiO2 nanopillars compared to flat Ti6Al4V surfaces.82 White arrows = nanopillar-induced envelope deformation. Reprinted with permission. (A) Copyright 2018, Elsevier; (B) Copyright 2020, Springer Nature. | ||
Heat treatment can be used as a pre- or post-modification method for other technologies to improve the effect of surface modification. For instance, Gu et al.83 used a thermal treatment of 160 °C for 2 hours, which enhanced the adhesive bond strength of graphene on a Ti substrate without affecting the favourable ability of the graphene coating to induce osteogenic activity in human bone marrow and adipose tissue derived stem cells. To improve the stability of plasma sprayed HA coatings, Bose et al.84 applied a thermal treatment on sprayed Ti6Al4V samples at 800 °C for 10 min. The treatment increased coating crystallinity from 64 to 75% and adhesion strength from 26 to 31 MPa, while decreasing the HA dissolution rate. This method could be useful for reducing the chance of HA coating delamination from the metal implant due to poor interfacial bonding.
| Reactants | Hydrothermal reaction conditions | Outcomes | Ref. |
|---|---|---|---|
| 40 mL of 0.02 M Sr(OH)2 solution in a 60 mL Teflon-lined autoclave | 200 °C, 1 h or 3 h | Sr ions were doped into the TiO2 nanotube layer. Anodisation voltage of 10 V and treatment time of 3 h showed the best biocompatibility and osteogenic properties | 85 |
| 10 mL of 0.005 M Sr(OH)2 and saturated Ca(OH)2 solution in a 40 mL Teflon-lined autoclave | 200 °C, 2 h | Co-doping of Sr and Ca into the TiO2 nanotube layer accelerated extracellular matrix mineralisation | 86 |
m(Sr(NO3)2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(Na3PO4) = 10 m(Na3PO4) = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
150 °C, 24 h | Sr-incorporated bioactive apatite coating | 87 |
| 5 wt% H3PO4, 8 wt% H2O2 | 0.15 MPa pressure, 120 °C, 24 h | Micro/nanograss Ti surface | 88 |
| 2 wt% H3PO4, 14 wt% H2O2 | Micro/nanoclump Ti surface | ||
| 9 wt% H3PO4, 7 wt% H2O2 | Micro/nanorod Ti surface | ||
m(H3PO4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(H2O2) = 1 m(H2O2) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
220 °C, 24 h | Step 1: prepared Ti surface with nanofibrous biomimetic topography | 89 |
| Modified Ti, 0.2 g mL−1 CaCl2 | 120 °C, 8 h | Step 2: introduced Ca into the modified Ti surface | |
| Ca(OH)2 aqueous solution | 0.5 MPa pressure, 200 °C, 5 h | Ca was introduced into the Ti surface to induce apatite formation | 90 |
| 100 mL of 1 M KOH | 150 °C, treatment times: 0.5, 1, 2, 3, 4, 5, 6, 24 and 60 h | All samples annealed at 450 °C for 4 h after treatment. Bactericidal nanosheet pattern was prepared on the Ti surface | 91 and 92 |
| 75 mL of 1 M NaOH | 200 °C, 4 h | All samples annealed at 300 °C for 1 h. Porous surfaces formed | 93 |
| 50 mL of 5 M NaOH | 60 °C, 24 h | ||
| 75 mL of 1 M NaOH | 200 °C, 2.5 h | After annealing, samples were immersed in 50 mL of 0.6 M HCl solution for 1 h and annealed at 600 °C for 2 h. Granular surfaces formed |
A number of studies have used hydrothermal treatment to dope Sr in orthopaedic implant coatings, since Sr can promote bone formation and inhibit bone resorption.85–87 Dang et al.85 used electrochemical anodisation and hydrothermal treatment to produce Sr-doped Ti nanotubes on Ti implant surfaces. The surface prepared using an anodisation voltage of 10 V and a hydrothermal treatment time of 3 hours showed the best ability to support in vivo bone formation. In an orthotopic rat model, implants with this surface achieved the best osseointegration and bone–implant contact ratio compared to other surfaces. The ability of the surface to induce rapid in vivo bone formation was thought to be due to the synergistic effect of surface micro-/nano-morphology and element release from the coating. Zhang et al.86 synthesised a Ti surface with Sr and Ca co-doped TiO2 nanotubes (M-SrCaNT) by applying a modified anodisation and hydrothermal treatment. In this study, half of the Sr in Sr-incorporated TiO2 nanotubes (M-SrNT) was replaced by Ca to obtain M-SrCaNT, which did not change the surface micro-/nano-structure. M-SrCaNT showed better ability to promote osteogenic activities (gene expression, extracellular matrix synthesis and mineralisation) in MC3T3-E1 cells than M-SrNT and unmodified TiO2 nanotubes. However, in vivo experiments were not performed to verify the ability of the coating to support osseointegration. Geng et al.87 prepared a snail-inspired Sr-doped HA coating on Ti by electrochemical corrosion, ultrasonic treatment, and hydrothermal treatment. In vitro and in vivo experiments showed that this coating effectively promoted the proliferation and differentiation of osteoblasts while inhibiting the formation of osteoclasts, as shown through the expression of relevant genes (ITG α5β1, Col-I, OCN, RANKL). In a rabbit model, the biomimetically structured surface with pore spaces was beneficial for bone formation and allowed implant–bone mechanical interlocking. The bioactive HA composition was thought to promote bone formation, while the doped Sr ions inhibited bone resorption.
Phosphate has gained an increasing number of biomedical applications in recent years, and its formation on Ti surfaces by hydrothermal treatment has been explored. Titanium phosphate (TiP) coatings with a variety of morphologies have been prepared and studied. For example, Jiang et al.88 produced three hybrid coatings containing TiP and TiO2, named micro/nanoclump Ti (P–C–Ti), micro/nanograss Ti (P–G–Ti), and micro/nanorod Ti (P–R–Ti) (Fig. 8A). The preparation method mainly involved a reaction between Ti, H2O2, and H3PO4. Ti4+ ions were produced as shown in eqn (1) and (2):
| H2O2 + 2H+ + 2e− → 2H2O | (1) |
| Ti + 2H2O2 + 4H+ → Ti4+ + 4e− | (2) |
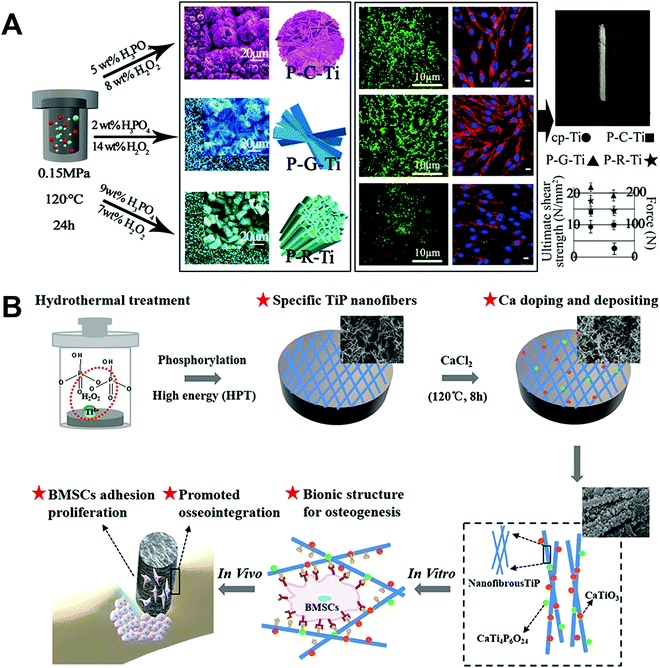 | ||
| Fig. 8 Use of hydrothermal treatment on Ti implants to fabricate (A) three unique micro-/nano-structured surface coatings involving TiP,88 and (B) bioinspired nanofibrous Ca-doped TiP coating.89 Reprinted with permission. Copyright 2018 & 2020, American Chemical Society. | ||
Further reaction could form Ti(O2)(OH)n−2(4−n)+ (pH < 1) or Ti2O5(OH)x2−x (pH > 1, x = 1–6). Then, the deposition of amorphous or low crystallinity TiP was produced by a reaction between the oxidised substrate and phosphate or hydrogen phosphate ions. During the treatment process, H3PO4/H2O2 concentrations were adjusted to control the coating morphology and composition (see Table 1). In vitro and in vivo experiments indicated that P–G–Ti allowed the best protein adsorption and rat BMSC viability, adhesion, and differentiation, as well as produced the highest tissue–implant interfacial bonding strength in rat tibia.
Cai et al.89 fabricated a bioinspired nanofibrous Ca-doped TiP coating on Ti by a two-step hydrothermal treatment method (Fig. 8B). In step one, the Ti sample was immersed in an aqueous solution (m(H3PO4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m(H2O2) = 1
m(H2O2) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) at 220 °C for 24 hours to form TiP. In step two, 0.2 g mL−1 CaCl2 solutions were applied to dope Ca into the Ti surface at 120 °C for 8 h. The main reaction process is shown in eqn (3), (4) and (5):
9) at 220 °C for 24 hours to form TiP. In step two, 0.2 g mL−1 CaCl2 solutions were applied to dope Ca into the Ti surface at 120 °C for 8 h. The main reaction process is shown in eqn (3), (4) and (5):
| CaCl2 → Ca2+ + 2Cl− | (3) |
| Ca2+ + H2O2 + TiO2 → CaTiO3 + H2O | (4) |
| Ca2+ + 2Ti(HPO4)2 + TiH2P2O8 → CaTiO3P6O24 + H2O | (5) |
In vitro tests using rat BMSCs and in vivo assessment in the rat tibia showed an improved ability of the Ca-doped TiP coating to encourage osteogenic behaviour and induce osseointegration in the early implantation state together with higher interfacial bonding strength, compared to the control TiP coating. In another coating containing Ca, Ansar et al.90 introduced Ca into the Ti surface using Ca(OH)2 through a one-step hydrothermal treatment method. CaO and CaTiO3 were detected in the coating, where the reaction for CaTiO3 formation is shown in eqn (6) and (7):
| Ca(OH)2 (s) → Ca2+ + 2OH− | (6) |
| Ca2+ + Ti(OH) → CaTiO3 (s) + 2H+ + H2O | (7) |
This is a simple, quick, and low-cost method for preparing a bioactive coating on Ti that has the ability to induce apatite formation, but the coating has no special morphology.
The optimisation of antimicrobial properties is crucial for implant surface modification, and a number of studies have used hydrothermal treatment for this purpose. Wandiyanto et al.91 prepared a Ti surface coating with sharp-edged (about 10 nm) TiO2 nanosheets to confer bactericidal properties. The nanosheet surface with different edge lengths was made by adjusting the hydrothermal treatment time, from 0.5 to 60 hours. The surface produced by 6 hours of treatment showed the best antibacterial performance, eliminating 90 ± 9% of S. aureus and ∼100% of P. aeruginosa cells contacting the coating. However, biocompatibility and bone integration experiments were not performed in this study. Following on from this, Clainche et al.92 tested two types of antibacterial coatings that combined both osteogenic and antibiotic activity, comparing hydrothermal treatment with plasma etching as the fabrication methods. The coating produced by hydrothermal treatment has been described by Wandiyanto et al.91 to contain sharp nanosheet protrusions to kill bacteria by cutting the cell membrane, while the other coating produced by plasma etching has been described by Linklater et al.94 to contain a microscale two-layer hierarchical topography to both reduce bacterial attachment and rupture bacteria that contact the surface. In vitro experiments showed that both coatings could selectively destroy bacterial cells, since they promoted the adhesion and differentiation of human adipose-derived stem cells (ASCs) and supported their osteogenesis. Further in vivo testing is necessary to confirm these observations.
Thrombosis is one of the reasons for bone implant failure. To address this problem, Manivasagam and Popat93 prepared Ti and Ti6Al4V surfaces with different morphologies using hydrothermal treatment, by adjusting the solution concentration, reaction temperature, and treatment time. A porous surface prepared on Ti was found to be the most haemocompatible compared to all other surfaces, which was produced by immersing the sample in 50 mL of 5 M NaOH solution, hydrothermal treatment at 60 °C for 24 hours, and annealing at 300 °C for 1 hour. The porous surface could modulate fibrinogen adsorption, cell adhesion, platelet activation, and whole blood clotting in vitro. This may lead to increased blood compatibility, reduced thrombosis and improved osteogenesis in vivo, although animal experiments were not performed in this study.
Microwave hydrothermal treatment (MWHT) is a method for producing nanoscale structures on alloy surfaces, where the reaction rate of organic and inorganic synthesis in hydrothermal treatment is increased by microwave heating. Compared with traditional hydrothermal treatment technology, MWHT is characterised by low temperature and high speed. Cheng et al.95 examined several MWHT surfaces prepared on two substrates (microrough and smooth Ti surfaces) in three solutions (distilled water, aqueous H2O2 solutions (1–2.5 M), and aqueous NH4OH solutions (1–2.5 M)) at 200 °C for 1 hour. Microrough Ti surfaces subjected to MWHT with distilled water or NH4OH solutions produced anatase titania nano-protuberances with average diameters of 23–60 nm, while MWHT with H2O2 solutions produced rutile-bearing titania nano-protuberances with average diameters of 22–51 nm. Interestingly, while these nanoscale modifications improved the hydrophilicity of the microrough Ti surface, they had no significant effects on osteogenic differentiation by MG63 cells and normal human osteoblasts compared to microrough Ti controls not treated by MWHT.
| Electrolyte composition | Work potential (V) | Time (min) | Thickness (μm) | Average roughness (nm) | Water contact angle (°) | Nanotube inner diameter (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| 0.5 wt% NH4F, 5 vol% H2O, ethylene glycol (EG) | 0 | 60 | 0 | 10.67 | 79.2 ± 0.2 | — | 100 |
| 20 | 0.21 | 29.36 | 122.0 ± 0.1 | — | |||
| 40 | 1.90 | 38.75 | 142.4 ± 0.1 | — | |||
| 60 | 1.81 | 56.35 | 130.7 ± 0.1 | — | |||
| 80 | 1.81 | 132.11 | 142.1 ± 0.1 | — | |||
| 100 | 1.92 | 111.97 | 103.8 ± 1.0 | — | |||
| 1 M lactic acid, 0.1 M NH4F, 1.5 wt% H2O, EG | 160 | 15 | 79 | — | — | — | 101 |
| 0.5 wt% NH4F, 1 wt% H2O, EG | 60 | 10 | 1.30 ± 0.09 | — | 44.6 ± 1 | 100 | 102 |
| 20 | — | 43.1 ± 1 | 75 | ||||
| 30 | — | 61.8 ± 1 | 65 | ||||
| 40 | — | 40.8 ± 1 | 70 | ||||
| 50 | — | 29.4 ± 1 | 75 | ||||
| 60 | — | 15.9 ± 1 | 50 | ||||
| 0.25 wt% NH4F, 2 wt% H2O, EG | 15 | 60 | — | 15.62 ± 1.85 | 39.2 ± 4.4 | 40–50 | 103 |
| 0.5 wt% NH4F, 5 vol% CH3OH, 5 vol% H2O, EG | 10 | 60 | 0.045 | — | — | 30 | 85 |
| 40 | 40 | 0.04 | — | — | 80 |
In 1999, Zwilling et al.104 first prepared a layer of TiO2 nanotubes on a Ti substrate in chromic acid electrolytes containing hydrogen fluoride at low voltage, which was approximately 500 nm thick. At the time, the prepared TiO2 nanotubes did not have a tightly organised structure and the sidewall was not uniform. Moreover, it was found that the thickness of the TiO2 nanotube layer did not increase with treatment time, possibly due to an equilibrium state of oxide growth and chemical dissolution. Since then, the use of AO to prepare TiO2 nanotubes has attracted extensive attention.29,104 Durdu et al.100 recently prepared a well-organised hydrophobic TiO2 nanotube coating with high crystallinity as well as homogeneous morphology and elemental distribution, by combining the AO method at different voltages with post-heat treatment. The post-heat treatment was found to not affect the morphological changes in the coating. Ti samples with the TiO2 nanotube coated surface produced at 20 V showed better mechanical properties when compared with uncoated controls and other coatings. Increases in the voltage value resulted in higher coating hydrophilicity, as well as changes in morphology from well-ordered to random organisation of nanotubes. In a study by Alijani et al.,101 an accurate optimisation of AO conditions was first successfully realised at room temperature to produce HAR nanotubes. The surface could be produced in a short time without the traditional high temperature requirement, by adding lactic acid in the electrolyte to prevent dielectric breakdown and enable anodisation at higher potentials. This method produced a high growth rate for the nanotube layer of up to 5.266 μm min−1. Torres-Avila et al.102 tested the impact of different oxidation times (10 to 60 min) on TiO2 nanotubes produced on Ti6Al4V using AO at a constant work potential of 60 V. The oxidation time was found to significantly affect the inner diameter of nanotubes, which was directly correlated with cell proliferation. Nanotubes with an inner diameter of 60 nm allowed the highest proliferation of a human osteoblast cell line.
AO can be applied to modulate surface charge, which is important since electrical stimulation can promote osteogenesis through biological interactions, such as with naturally charged biomolecules. This has been explored for ceramic biomaterials such as hydroxyapatite.105 A convergence of surface chemistry, surface charge, topography, and hydrophilicity is necessary for designing and modifying the biomaterial surface to target specific applications. For instance, Bandyopadhyay et al.106 used an ethylene glycol-based electrolyte containing 1 vol% HF to produce TiO2 nanotubes on Ti surfaces by AO, at a potential difference of 40 V for 1 hour followed by charge storage using electrothermal polarisation. These electrically polarised TiO2 nanotube surfaces enhanced the proliferation and osteogenic differentiation of human foetal osteoblasts by improving the surface hydrophilicity and bioactivity compared to untreated implants, and also promoted early-stage osseointegration in a rat distal femur model. Another recent application of AO was in exploring the responses of ASCs to nanostructured surfaces. As an example, Cowden et al.107 fabricated TiO2 nanotube surfaces by AO, and found that the ASCs increased their proliferation and osteogenic differentiation on the modified surfaces in a manner that was dependent on the size of nanotubes, suggesting a possible application in modulating cell responses directly through orthopaedic implant coatings.
Under certain AO conditions, nanowires can form on top of TiO2 nanotubes, and their formation is mainly affected by voltage and treatment time. Hsu et al.108 proposed a ‘strings of through holes’ model to explain the mechanism of nanowire formation, consisting of four stages: (1) dissolving the nanotube wall near the upper end, causing thinning of the tube wall thickness; (2) forming strings of holes in the top section of the nanotube wall; (3) splitting into nanowires; and (4) folding and further thinning of nanowires. AO can also be an effective technique for inducing HA growth on treated surfaces. Nguyen et al.109 used an electrolyte containing calcium acetate, calcium glycerophosphate hydrate, and different concentrations of Na2SO4 to incorporate Ca and P into the oxide film of Ti6Al4V by AO. These Ca-containing films were bioactive and dissolved when immersed in simulated body fluid (SBF), enhancing the degree of supersaturation of the SBF solution and inducing the growth of HA on the sample surface. Lim and Cloe110 developed another method for inducing bioactive apatite formation on an AO-treated Ti alloy, through a two-step loop process. The first step forms TiO2 nanotubes on the Ti6Al4V surface using AO, and the second step removes the nanotube layer by ultrasonic degradation, followed by plasma electrolytic oxidation (PEO) treatment in an electrolyte containing bioactive ions (Ca, P ± Sr, Mn, Mg, Zn, Si) to form bioactive apatite (Fig. 9A). This process was looped several times. As the number of loops increased, the diameter and size of nanotubes increased while the distance between nanotubes decreased, forming a mesh-shaped surface with increased surface roughness and bonding strength. After the third loop, the treated surface showed bioactive apatite formation with anatase and hydroxyapatite phases (Fig. 9B).
 | ||
| Fig. 9 (A) A two-step loop process to form bioactive apatite on the surface of Ti6Al4V and (B) SEM images of the surface morphology after loops one (left) and three (middle, right; the right images depict PEO in an electrolyte containing all bioactive ions (Ca, P, Sr, Mn, Mg, Zn, Si)).110 Reprinted with permission. Copyright 2019, Elsevier. | ||
AO treatment can be additionally used to optimise the surface of Ti6Al4V implants produced by additive manufacturing techniques, such as selective laser melting and electron beam melting. These manufacturing methods for titanium implants are being increasingly explored as they couple good biocompatibility, corrosion resistance and strength with high implant customisability.111 However, to be practically useful for biomedical applications, certain shortcomings need to be overcome such as high porosity and poor surface finish. Methods such as electrically assisted ultrasonic nanocrystal surface modification (EA-UNSM)112 may be useful for this purpose. Alternatively, as reported by Ren et al.,103 a layered micro/nano structure can be produced on 3D-printed Ti6Al4V implant surfaces by combining ultrasonic acid etching with AO. This surface promoted the proliferation and osteogenic differentiation of MC3T3-E1 cells by enhancing the surface hydrophilicity and bioactivity compared with the untreated implant, and also increased bone formation and osseointegration in a rat femoral defect model.
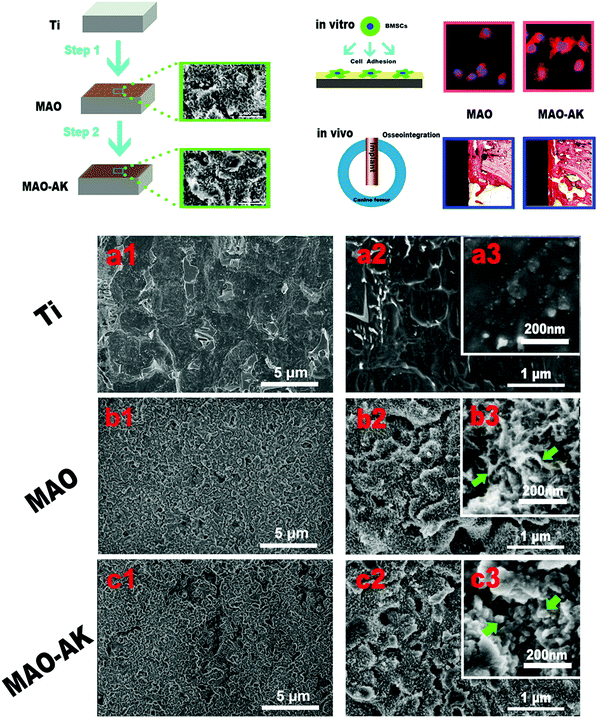 | ||
| Fig. 10 Changes in the nano-morphology of Ti samples, treated or not with MAO and post-modified or not with electrochemical treatment (MAO-AK).114 Green arrows: b3 = petal-like nanostructures; c3 = granular nanostructures. Reprinted with permission. Copyright 2016, American Chemical Society. | ||
Metal ions such as Al, V, Co, Cr and Mo in implant materials can have a negative effect on cell viability, and such effects are often closely related to their concentration.115 A possible approach for increasing the biological activity of the Ti6Al4V alloy is to inhibit the release of Al and V from the implant. Guan et al.17 presented a method to form a porous oxide coating on Ti6Al4V through MAO that reduces the Al and V content of the coating, by using a Na2B4O7 electrolyte added with KOH. It was found that an increased concentration of KOH in the electrolyte led to better removal of Al and V. The modified MAO coating showed superhydrophilicity and excellent cytocompatibility with MC3T3 cells.
To further improve the performance of MAO coatings on Ti substrates, some elements that support bone formation or have antibacterial properties have been incorporated into the coating.34,116–119 Approaches to include bioactive elements such as Si, Sr, and Mg have been effective, but antibacterial elements such as Zn, Cu, and Ag cannot be easily fixed on the implant surface using MAO, which does not allow the coating to confer a lasting antibacterial effect. Zhao et al.116 used MAO to prepare TiO2 microporous coatings with Si uniformly distributed on the coating surface, and found that Si doping did not change the surface morphology, roughness, or phase composition. The Si-incorporated surface increased in vitro osteoblast adhesion, spreading, proliferation, and differentiation, as well as early stage in vivo osseointegration in a rabbit model. Antibacterial properties were not tested in this study. Li et al.117 used MAO to prepare porous TiO2 coatings doped with Ca and Sr, by adding Cao and SrO in tetraborate electrolytes. The resulting coatings were superhydrophilic and highly biocompatible, and allowed easy release of Ca and Sr into aqueous solution. When tested using human BMSCs, the coating doped with Ca and Sr showed significantly higher cell proliferation compared to that doped with only Ca or Sr. Another group doped different concentrations of Mg into TiO2 coatings by MAO, and showed that the electrolyte with 2 g L−1 magnesium acetate produced coatings with the best ability to encourage rat BMSC adhesion, proliferation, and osteogenic differentiation.120
Strategies to incorporate antibacterial elements include a two-step method for preparing an Ag-doped TiO2 surface, described by Durdu et al.118 This method first used thermal evaporation-physical vapor deposition (TE-PVD) to deposit a thin Ag layer on the Ti surface, followed by coating the surface using MAO in sodium silicate and potassium hydroxide. Energy dispersive spectroscopy (EDS) analysis indicated that Ag was released from the inner Ag layer through the surface pores, and was localised only around the region of the pores. Compared to surfaces treated only with MAO, the Ag-doped MAO surface significantly reduced the active colony ratio of E. coli and S. aureus (both by >50%), as well as increased surface wettability and in vitro apatite formation. However, cytotoxicity or biocompatibility tests were not reported. Ye et al.119 used MAO to produce Zn-doped TiO2 coatings with excellent bactericidal activity and osseointegration ability. The coating had a bilayer structure, where the amorphous outer layer consisted of Ti, O, and Zn with Zn doped in the form of weakened Zn–O bonds, and the inner layer was partially crystallised with nanocrystalline TiO2 and Zn2TiO4 with an embedded amorphous matrix. The weakened Zn–O bonds were found to be the key factor controlling Zn2+ release and ROS production. When produced at higher voltage (530 V compared to 300 or 400 V), enhanced ROS formation on the MAO coating led to bacterial cell wall breakage, coupled with enhanced Zn2+ release and extracellular H2O2 formation causing increased intracellular ROS levels, which acted in parallel to result in bacterial death. However, coatings with excessive Zn concentration were seen to induce excessive ROS levels and lead to bone cell apoptosis. The coating produced at 400 V showed the best balance of performance, which promoted osseointegration in rat tibiae that were uninfected or infected with S. aureus.
HA is a popular implant coating material due to its high biocompatibility and stable chemical properties, and its incorporation onto Ti implants using MAO has been explored. Studies have reported that an MAO coating decorated with HA nanostructures could provide a suitable microenvironment for promoting bone formation, immunomodulation and angiogenesis, which was termed “osteo-immunomodulation”.32,33 In the first study, Bai et al.32 prepared a microporous HA-modified TiO2 coating using MAO, followed by annealing at different temperatures to tune the physical and chemical properties of the coating. Annealing at 650 °C was found to produce the most favourable coating properties, including a hybrid micro–nano morphology, superhydrophilicity, and HA NPs with high crystallinity. In vitro tests using osteoblasts and endothelial cells and in vivo implantation in a rabbit model demonstrated the ability of this coating to promote bone formation and angiogenesis, as well as modulate the immune response in macrophages. In the second study, Bai et al.33 prepared two types of microporous surfaces which were respectively decorated with HA NPs and nanorods. MAO was used followed by steam-hydrothermal treatment (SHT), whereby the nanostructure of HA was altered by adjusting the SHT processing time. In vitro and in vivo experiments indicated that MAO surfaces decorated with HA NPs were conducive to osteo-immunomodulation, bone formation, and angiogenesis, while those decorated with HA nanorods did not show the same effects. The key signalling pathways involved were thought to be related to TGF-β, OPG/RANKL, and VEGF. In another approach to form a HA-doped coating on a Ti substrate using MAO, Qaid et al.121 synthesised phase pure HA from calcined eggshells, and added different concentrations of this into a tri-sodium orthophosphate (Na3PO4·12H2O) based electrolyte solution in a one-step MAO process. The MAO coating produced using a 1.5 g L−1 concentration of eggshell-derived HA was found to give the best adhesive strength and corrosion resistance. Tests for biocompatibility or biological activity of the coating were not performed.
2.4 Biological modification methods
Biological modification methods involve fixing specific bioactive molecules such as proteins, peptides, and enzymes on the implant surface. After implantation in the body, the biologically modified implant surface induces the adsorption of different proteins from the internal environment to form a protein layer. These bioactive layers can provide active sites for a variety of biological responses involving cellular receptors, leading to downstream effects such as cell adhesion, migration, proliferation, differentiation, and apoptosis, which can contribute to tissue formation, osseointegration, and other biological processes. Compared to physical and chemical modification methods, biological surface modification is more direct and efficient. However, the preservation time of bioactive molecules on the implant surface is typically limited by a set of environmental conditions including air, temperature, humidity, light, and pH, which increase the product cost and restrict the large-scale manufacturing and use of implants with biologically modified surfaces.Implant coatings with antioxidant properties may help with bone healing after implant surgery by reducing oxidative stress from excess ROS production. Chen et al.124 constructed a multilayer coating with antioxidant activity on a Ti substrate, consisting of chitosan–catechol, gelatin, and HA nanofibres. This coating effectively protected osteoblasts from oxidative damage, by regulating gene expression related to cell adhesion molecules (integrin αv, β3, CDH11, CDH2), as well as by regulating cell adhesion and anti-apoptotic proteins (p-MYPT1, p-FAK, p-Akt, Bcl-2). The coating also promoted in vitro osteoblast differentiation and early bone healing in vivo in a rabbit model. Building on this work, the same group used the multilayer coating as a platform to investigate the coupling between osteogenesis and angiogenesis during bone healing.125 The coating was found to mediate in vitro communication between co-cultured rat ASCs and human umbilical vein endothelial cells (HUVECs) through cell–matrix interactions that enhanced their paracrine effects (Fig. 11A). When grown in co-culture in the presence of the multilayer coating, both ASCs and HUVECs increased their transmigration, as well as gene or protein expression of angiogenic factors such as SDF-1 and VEGF, compared to cells grown on tissue culture plastic or uncoated Ti. There was some evidence of bone formation when Ti samples with the multilayer coating were implanted into rats subcutaneously or in the femur, but the process of in vivo osseointegration requires further investigation.
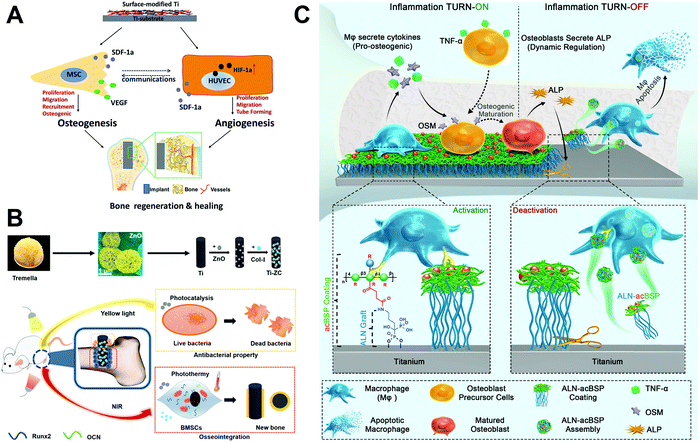 | ||
| Fig. 11 Schematic illustration of (A) a multilayer biological coating on a Ti substrate produced by LBL that mediates coupling of osteogenesis and angiogenesis to promote bone healing,125 (B) a tremella-like coating that could respond to yellow light and near infrared light to provide antibacterial and osteogenic functions,126 and (C) a bio-responsive, “bridge-burning” coating to turn on and turn off macrophage activity on implants to provide osteo-immunomodulation and help bone formation.34 Reprinted with permission. (A) Copyright 2018, Elsevier; (B) Copyright 2020, American Chemical Society; (C) Copyright 2020, John Wiley and Sons. | ||
The concept of “osteo-immunomodulation” has gained increasing understanding and recognition of its importance in bone healing, with emerging strategies aimed at biomaterial modifications that modulate the host immune environment to provide anti-inflammatory and anti-infective properties.130
Recent studies have focused on producing new coatings to regulate macrophage activity on implant surfaces. Wang et al.34 designed a bio-responsive, endogenously triggered, “bridge-burning” coating to regulate macrophage activity on the Ti surface, containing acetyl glucomannan (acBSP) as a macrophage-activating glycan covalently crosslinked by alendronate (ALN) as a macrophage-killing bisphosphonate (Fig. 11C). The specific “bridge-burning” process used by the coating to regulate biological response is divided into two steps: (1) After implantation, the acBSP activates host macrophages to secrete pro-osteogenic cytokines, “turning on” to promote bone differentiation. (2) An increasing number of mature bone cells release ALP, cleaving the ALN–acBSP complexes from the implant surface which are preferentially internalised by inflammatory macrophages. This “turning off” induces macrophage apoptosis to suppress inflammation and promote healing. When tested in a rat osteoporosis model, the coated implant significantly enhanced bone–implant integration (88.4% higher contact ratio) compared to uncoated Ti. In another study, Shi et al.35 used a biologically derived component as an implant coating. Zymosan, a fungal component, was covalently grafted onto Ti implants to target and regulate macrophage activity. This polysaccharide coating could activate host macrophages through toll-like receptors (TLR) to produce osteogenic and angiogenic factors. In vivo testing in the rat femur indicated that a medium dose of zymosan in the coating (0.1 μg mm−2) could significantly improve osseointegration, without inducing severe inflammation as occurred with the high dose group.
Dopamine is the most abundant catecholamine neurotransmitter in the brain, which regulates various physiological functions of the central nervous system. Polydopamine (PDA) is a mussel-derived biomimetic material that forms by self-polymerisation from dopamine in a weakly alkaline environment. In 2007, inspired by the composition of adhesive proteins in mussels, Lee et al.128 first reported that PDA could be coated on almost any solid material surface. Since then, coatings containing PDA have been widely explored and applied in various biomaterial modifications. PDA has several advantages as a biological coating material: (1) PDA can enhance the biocompatibility and hydrophilicity of the implant surface to promote adhesion and spreading of diverse cell types, including chondrocytes, endothelial cells, and BMSCs, even on superhydrophobic surfaces.131–133 (2) PDA can enhance the osteogenic differentiation of mesenchymal stem cells (MSCs), by increasing their expression levels of osteogenic genes134 or by promoting their calcium mineralisation through simulating the biological environment of the bone extracellular matrix.135 Recently, Wang et al.129 fabricated PDA coatings on polyetheretherketone (PEEK), Ti6Al4V and HA as representative polymer, metal and ceramic biomaterials, by immersing samples in dopamine solution at 37 °C for 24 hours. The PDA coating was found to significantly improve the hydrophilicity of the material surface, and directly enhance the adhesion, proliferation and osteogenic differentiation of rat BMSCs through FAK and p38 signalling pathways. An in vivo study in the rat femur using coated and uncoated PEEK as representative implant materials confirmed that the PDA coating significantly enhanced osseointegration and accelerated new bone formation.
3. Perspectives and outlook
Traditional Ti and Ti alloy implants have a low probability of long-term clinical success and may have a short service life. Secondary or even multiple surgical revisions may be required due to implant failure, particularly in younger patients.136 Surface modification technology is evolving as an interdisciplinary field and a primary means for improving Ti implant osseointegration. Modification strategies could be inspired by a variety of bone-related structures and processes, including the inorganic and organic phases of bone, and its immune system and vasculature.42 The outcomes of modification could involve improvements in surface hydrophilicity, antibacterial activity, antithrombotic property, corrosion resistance, durability, biocompatibility, and even bioactivity. In this review, we introduced a range of surface modification methods for Ti implants from the recent literature, categorised them according to the primary mechanism for coating formation, and critically analysed their coating effects and applications. This knowledge synthesis is important for providing inspiration for further research to translate as well as extend the applications of surface modified implant materials in biomedical applications.Each type of surface modification method discussed in this review has a different set of characteristics, which are associated with certain advantages and disadvantages as summarised in Table 3. It can be seen that some factors are present for all modification methods that may limit their ability to be adapted for large-scale manufacturing or clinical application. Research is being undertaken in this field to develop new coating techniques that may avoid or reduce the limitations of existing approaches. For instance, Song et al.137 recently developed an alkalinity-activated solid-state dewetting technique (AAD), producing quasi-periodic nanoscale pimple-like TiO2−x structures on the Ti6Al4V surface that cannot be formed through existing surface treatments. The modified surface showed strong interfacial effects including increased implant surface energy and hydrophilicity, and anti-inflammatory and pro-regenerative functions by modulating the cross-talk between osteoblasts and macrophages. The coated implants demonstrated a strong ability to induce osseointegration and osteo-immunomodulation in a rat tibial defect model.
| Method | Advantages | Disadvantages |
|---|---|---|
| PST54–56 | – Good quality coating | – Molten particles may produce oxidation during spraying, forming uneven particles which damage the coating quality |
| – Simple spraying operation | – Coatings have a certain porosity and may be prone to delamination | |
| – Wide selection of materials | – Poor bond strength of coating particles to the substrate may lead to dissemination of wear particles, causing histopathological response and possible implant failure | |
| – High production efficiency | ||
| – Low cost | ||
| PIII65,70 | – Good biocompatibility | – Limited in achieving large-scale industrial applications |
| – Versatile and less hazardous process due to low temperature | – Chance of implanting undesired impurities in the plasma | |
| – Thin injection layer does not affect material properties | – May be difficult to achieve uniform ion implantation over a large area, or monitor precise dosage | |
| Laser cladding71,72,138 | – Can prepare coatings with a fine grained and compact structure | – This technology has been rarely applied in clinical practice due to its short history of development, currently mostly applied in laboratory settings |
| – High bonding strength of the coating to the substrate | – Expensive equipment and complex process may limit its large-scale application | |
| – Wide selection of the cladding layer and substrates | ||
| – Use of laser is clean and pollution-free, and easy to realise automation | ||
| SLA139,140 | – Can obtain surface micropores of different sizes | – Little control over surface structure |
| – Technology is economical, convenient, and mature | – Produces an irregular surface with great fluctuations in roughness values | |
| Thermal oxidation78,80,81,141 | – Coating can improve the mechanical properties of the substrate | – Oxide debonding occurs at high temperatures or oxidation times |
| – Can be used to enhance the bonding strength between coating and substrate | – Cracks can occur due to thermal stress and associated forces | |
| – A simple, convenient, and cost-effective method | – Low compactness of the surface | |
| – Process has poor control | ||
| Hydrothermal treatment142–144 | – Can be used to dope various elements into the surface | – Lack of in-depth research on factors influencing crystal nucleation and growth |
| – Crystalline surface structure can be directly obtained without high temperature sintering | – High cost due to requirements for high temperature and pressure conditions | |
| – High bonding strength between coating and substrate | – Poor reproducibility and difficulty in realising mass production | |
| AO102,145 | – Can be used to prepare ordered TiO2 tube structures or oxide films on material surface with good mechanical properties and corrosion resistance | – Process produces substances that are harmful to the body and the environment |
| – Effective and simple electrochemical process with low cost | – Change in processing conditions has a great influence on the result of surface modification, requiring strict control of conditions including temperature, current density, voltage, and electrolyte composition, hence making large-scale production more difficult | |
| – Coating has high surface energy, which may easily attract contaminants | ||
| MAO31,116 | – Can be used to produce porous coatings with high hardness and corrosion resistance, that are firmly adherent on the substrate | – Power supply consumes a large amount of energy |
| – Can be used for in situ coating formation | – Lack of in-depth understanding of the ion film-forming reaction in the electrolyte, as well as factors influencing gradient film design | |
| – Ability of the technology to allow rapid large-scale preparation of stable implant coatings has not been confirmed | ||
| LBL124,125 | – Widely used technique for preparing multilayered biological coatings | – Process is time-consuming and has low production efficiency, often requiring hours to days to make a batch of samples depending on the number of layers |
| – Applicable to a wide range of substrates and provides controllable coating thickness | – Repetitive deposition limits the number of coating layers and hence coating thickness, which may not be suitable for certain applications | |
| – Simple preparation process and low preparation temperature | – Repetitive deposition may cause cross-contamination of different stock materials used for coating fabrication | |
| – Strong bonding force between coating and substrate | ||
Presently, it is challenging to combine the desirable characteristics of wear resistance, corrosion resistance, biocompatibility or bioactivity, and antibacterial activity in biomedical implants solely through surface modification technology. A primary future goal of this research area is to develop new multilayer composite coatings for biomedical implants with a combination of desirable properties, which can serve stably for a long period of time in the biological environment, by integrating surface modification with other technologies. As described earlier in this review, the combination of modification methods, such as thermal oxidation and AO, with additive manufacturing may provide new strategies for overcoming technological barriers.103,111,112 Some of the early work on surface modification using additive manufacturing led to the development of porous Ti146,147 or Ta148,149 coatings with 3D interconnected porosity reaching 70%. With the assistance of computer-aided design (CAD) and surface optimisation technology, 3D-printed bone implants can now be fabricated which simultaneously possess gradient porous structures and functional surfaces, thereby greatly increasing their ability for osseointegration.150,151 As an example, Mitra et al.152 successfully fabricated Ti–tantalum (Ta) alloy implants with multiscale structural variations by 3D printing. With combined micro-porosity and nanoscale surface features, the Ti–Ta alloys showed superior biological performance compared with commercially pure Ti, and had great potential to be developed further as part of the next generation of implant materials. Reflecting the current status of research, this review focused on surface modifications in the two-dimensional plane, while modifications of three-dimensional Ti scaffold surfaces have been rarely reported. In this emerging area, Rifai et al.153 prepared a polycrystalline diamond coating (PCD) on three-dimensional Ti6Al4V scaffold implants by additive manufacturing and chemical vapour deposition. This implant was found to significantly resist bacterial attachment and improve osseointegration, providing new inspiration for patient-specific implant design.
In addition to the modification methods discussed in this review, micro-printing techniques that involve a combination of physical and chemical mechanisms can be used to produce micro-patterns on implant surfaces, constructing an ordered microenvironment for cell growth. By combining geometric micro-patterns with controllable size and chemical action, it is possible to achieve greater control over cell behaviour and produce specific cell morphologies and structures, such as spheroids.154 Using the same principles, the generation of precisely controlled surface micro-patterns could enable implants to perform advanced functions. For instance, Gilabert-Chirivella et al.155 etched surface microgrooves on a Ti implant using a modified 3D printer, and fixed chitosan to the bottom of the grooves without the need for covalent bonding, endowing the implant with antibacterial properties and with the ability to support preferential pre-osteoblast adhesion. Other techniques, including lithography, soft etching, electrochemical micromachining, and laser etching, can be used to produce fine micro-patterns at smaller length scales. Among these, micro-contact printing is a low cost technique that provides high reliability and versatility, and has been widely applied in constructing cell culture platforms, biosensing platforms, and devices for other biological applications,156 and it could be a useful technique to consider for implant coatings.
Recent research has seen an emergence of studies that combine physical, chemical and biological modification approaches to endow implant surfaces with complex biological functions. For instance, Li et al.157 prepared a micro-nanostructured HA coating on Ti by MAO, and then loaded chitosan as an antibacterial agent through dip-coating to improve the surface biological and antibacterial properties. Drug loading has also been applied to Ti surfaces modified using various techniques to provide antibacterial function, involving mostly broad-spectrum antibiotics such as gentamicin, cephalothin, simvastatin, vancomycin, and tobramycin.158–162 Nevertheless, implant surfaces with a biological component still face a number of challenges in their translation to clinical applications, including considerations around implant stability, reliability, disinfection, and storage. Further research is required to optimise such implant designs and gain a better understanding of the coating mechanisms and effects, active ingredients, toxicity limits, and possible biological interactions. As an example, multifunctional PDA-based coatings are now being increasingly studied to address infection and aseptic loosening of orthopaedic implants.163
Surface modification of Ti and its alloys can greatly improve the osseointegration and antibacterial properties of biomedical implants, bearing great significance to the orthopaedics field and the health and well-being of patients. A variety of modification techniques can contribute to modulating the implant–bone interface and lead to better outcomes of osseointegration and/or osteogenesis.164 Methods such as PST, SLA, and AO so far remain the most popular surface modification strategies since they do not require much expensive, customised, or highly specialised equipment, are controllable and reproducible, and allow efficient production for scale-up manufacturing.165 For new implant coating techniques to be considered for clinical translation, their performance needs to be first verified in large animal models with clinically relevant skeletal defects and similar mechanisms and time frames of bone healing as humans. This, together with the costs of industrial upscaling and the cost-effectiveness of the coating method itself, constitutes a rate-limiting step for the majority of techniques in development. For these reasons, only a small number of new coating technologies have reached clinical trials and commercialisation, while most have remained in the preclinical phase.165 Other studies have focused on developing and optimising the process of new techniques, such as more refined nanolithography, rather than undertaking extensive in vitro and/or in vivo investigations to understand the biological effects of the coating, or the mechanisms driving cell and tissue responses to the coating.166–168 Looking into the future, it is of great scientific significance and practical value to study and combine various surface modification approaches, as well as to conduct physiologically relevant testing, for developing the next generation of orthopaedic implant coatings with the combined functions of inducing successful bone healing while preventing adverse reactions at the implant site.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support of the National Key R&D Program of China (2021YFE0113000), the National Natural Science Foundation of China (NSFC; 51872318), Shenzhen Science and Technology Research Funding (JCYJ20210324120012034), the National Health and Medical Research Council (NHMRC, Australia; GNT1120249), and the Australian Research Council Training Centre for Innovative BioEngineering (IC170100022).References
- M. C. S. Inacio, S. E. Graves, N. L. Pratt, E. E. Roughead and S. Nemes, Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: A conservative local model with international implications, Clin. Orthop. Relat. Res., 2017, 475, 2130–2137 CrossRef PubMed.
- H. M. Kremers, D. R. Larson, C. S. Crowson, W. K. Kremers, R. E. Washington, C. A. Steiner, W. A. Jiranek and D. J. Berry, Prevalence of total hip and knee replacement in the United States, J. Bone Jt. Surg., Am. Vol., 2015, 97, 1386 CrossRef.
- D. M. Daubert, B. F. Weinstein, S. Bordin, B. G. Leroux and T. F. Flemmig, Prevalence and predictive factors for peri-implant disease and implant failure: A cross-sectional analysis, J. Periodontol., 2015, 86, 337–347 CrossRef PubMed.
- B. Klinge and J. Meyle, Peri-implant tissue destruction. The Third EAO Consensus Conference 2012, Clin. Oral Implants Res., 2012, 23, 108–110 CrossRef PubMed.
- D. Duraccio, F. Mussano and M. G. Faga, Biomaterials for dental implants: current and future trends, J. Mater. Sci., 2015, 50, 4779–4812 CrossRef CAS.
- Y. F. Ding, R. W. Li, M. Nakai, T. Majumdar, D. H. Zhang, M. Niinomi, N. Birbilis, P. N. Smith and X. B. Chen, Osteoanabolic implant materials for orthopedic treatment, Adv. Healthcare Mater., 2016, 5, 1740–1752 CrossRef CAS.
- M. Niinomi, Metallic biomaterials, J. Artif. Organs, 2008, 11, 105–110 CrossRef CAS PubMed.
- W. S. W. Harun, R. I. M. Asri, J. Alias, F. H. Zulkifli, K. Kadirgama, S. A. C. Ghani and J. H. M. Shariffuddin, A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials, Ceram. Int., 2018, 44, 1250–1268 CrossRef CAS.
- M. Iafisco, I. Foltran, S. Sabbatini, G. Tosi and N. Roveri, Electrospun nanostructured fibers of collagen-biomimetic apatite on titanium alloy, Bioinorg. Chem. Appl., 2012, 2012, 123953 Search PubMed.
- D. D. Bosshardt, V. Chappuis and D. Buser, Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions, Periodontology, 2017, 73, 22–40 CrossRef PubMed.
- Y. Zheng, J. Li, X. Liu and J. Sun, Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface, Int. J. Nanomed., 2012, 7, 875–884 CAS.
- N. J. Hickok and I. M. Shapiro, Immobilized antibiotics to prevent orthopaedic implant infections, Adv. Drug Delivery Rev., 2012, 64, 1165–1176 CrossRef CAS PubMed.
- T. Monteiro, M. Wysocka, E. Tellez, O. Monteiro, L. Spencer, E. Veiga, S. Monteiro, C. de Pina, D. Gonçalves, S. de Pina, A. Ludgero-Correia, J. Moreno, T. Conceição, M. Aires-de-Sousa, H. de Lencastre, L. J. Gray, M. Pareek, D. R. Jenkins, S. Beleza, M. R. Oggioni and I. I. Araujo, A five-year retrospective study shows increasing rates of antimicrobial drug resistance in Cabo Verde for both Staphylococcus aureus and Escherichia coli, J. Glob. Antimicrob. Resist., 2020, 22, 483–487 CrossRef.
- B. Li and T. J. Webster, Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections, J. Orthop. Res., 2018, 36, 22–32 Search PubMed.
- S. A. Alves, R. Bayón, V. S. de Viteri, M. P. Garcia, A. Igartua, M. H. Fernandes and L. A. Rocha, Tribocorrosion Behavior of Calcium- and Phosphorous-Enriched Titanium Oxide Films and Study of Osteoblast Interactions for Dental Implants, J. Bio- Tribo-Corros., 2015, 1, 1–21 CrossRef.
- N. S. Manam, W. S. W. Harun, D. N. A. Shri, S. A. C. Ghani, T. Kurniawan, M. H. Ismail and M. H. I. Ibrahim, Study of corrosion in biocompatible metals for implants: A review, J. Alloys Compd., 2017, 701, 698–715 CrossRef CAS.
- S. Guan, M. Qi, C. Wang, S. Wang and W. Wang, Enhanced cytocompatibility of Ti6Al4V alloy through selective removal of Al and V from the hierarchical micro-arc oxidation coating, Appl. Surf. Sci., 2021, 541, 148547 CrossRef CAS.
- E. M. Lotz, M. B. Berger, Z. Schwartz and B. D. Boyan, Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties, Acta Biomater., 2018, 68, 296–307 CrossRef CAS PubMed.
- P.-I. Branemark, Osseointegration and its experimental background, J. Prosthet. Dent., 1983, 50, 399–410 CrossRef CAS.
- S. Migita and K. Araki, Effect of nanometer scale surface roughness of titanium for osteoblast function, AIMS Bioeng., 2017, 4, 162–170 Search PubMed.
- R. Rasouli, A. Barhoum and H. Uludag, A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance, Biomater. Sci., 2018, 6, 1312–1338 RSC.
- G. Wang, S. Moya, Z. Lu, D. Gregurec and H. Zreiqat, Enhancing orthopedic implant bioactivity: refining the nanotopography, Nanomedicine, 2015, 10, 1327–1341 CrossRef CAS PubMed.
- J. T. B. Ratnayake, M. Mucalo and G. J. Dias, Substituted hydroxyapatites for bone regeneration: A review of current trends, J. Biomed. Mater. Res., Part B, 2017, 105, 1285–1299 CrossRef CAS PubMed.
- M. Al-Amin, A. M. Abdul Rani, A. A. Abdu Aliyu, M. G. Bryant, M. Danish and A. Ahmad, Bio-ceramic coatings adhesion and roughness of biomaterials through PM-EDM: a comprehensive review, Mater. Manuf. Processes, 2020, 35, 1157–1180 CrossRef CAS.
- M. Khokhlova, M. Dykas, V. Krishnan-Kutty, A. Patra, T. Venkatesan and W. Prellier, Oxide thin films as bioactive coatings, J. Phys.: Condens. Matter, 2019, 31, 033001 CrossRef CAS.
- X. Liu and C. Ding, Plasma sprayed wollastonite/TiO2 composite coatings on titanium alloys, Biomaterials, 2002, 23, 4065–4077 CrossRef CAS PubMed.
- X. Zhao, G. Wang, H. Zheng, Z. Lu, X. Cheng and H. Zreiqat, Refining nanotopographical features on bone implant surfaces by altering surface chemical compositions, RSC Adv., 2014, 4, 54226–54234 RSC.
- S. Park, J. Park, J. Heo, B. Y. Hong and J. Hong, Growth behaviors and biocidal properties of titanium dioxide films depending on nucleation duration in liquid phase deposition, Appl. Surf. Sci., 2017, 425, 547–552 CrossRef CAS.
- K. Lee, A. Mazare and P. Schmuki, One-dimensional titanium dioxide nanomaterials: nanotubes, Chem. Rev., 2014, 114, 9385–9454 CrossRef CAS.
- L. Bai, Y. Zhao, P. Chen, X. Zhang, X. Huang, Z. Du, R. Crawford, X. Yao, B. Tang, R. Hang and Y. Xiao, Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration, Small, 2021, 17, e2006287 CrossRef PubMed.
- M. Yang, H. Liu, C. Qiu, I. Iatsunskyi, E. Coy, S. Moya, Z. Wang, W. Wu, X. Zhao and G. Wang, Electron transfer correlated antibacterial activity of biocompatible graphene Nanosheets-TiO2 coatings, Carbon, 2020, 166, 350–360 CrossRef CAS.
- L. Bai, Z. Du, J. Du, W. Yao, J. Zhang, Z. Weng, S. Liu, Y. Zhao, Y. Liu, X. Zhang, X. Huang, X. Yao, R. Crawford, R. Hang, D. Huang, B. Tang and Y. Xiao, A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration, Biomaterials, 2018, 162, 154–169 CrossRef CAS PubMed.
- L. Bai, Y. Liu, Z. Du, Z. Weng, W. Yao, X. Zhang, X. Huang, X. Yao, R. Crawford, R. Hang, D. Huang, B. Tang and Y. Xiao, Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration, Acta Biomater., 2018, 76, 344–358 CrossRef CAS PubMed.
- Z. Wang, Y. Niu, X. Tian, N. Yu, X. Yin, Z. Xing, Y. Li, L. Dong and C. Wang, Switching on and off macrophages by a “bridge-burning” coating improves bone-implant integration under osteoporosis, Adv. Funct. Mater., 2020, 31, 2007408 CrossRef.
- Y. Shi, L. Wang, Y. Niu, N. Yu, P. Xing, L. Dong and C. Wang, Fungal component coating enhances titanium implant-bone integration, Adv. Funct. Mater., 2018, 28, 1804483 CrossRef.
- R. S. B. Lee, S. M. Hamlet, H. J. Moon and S. Ivanovski, Re-establishment of macrophage homeostasis by titanium surface modification in type II diabetes promotes osseous healing, Biomaterials, 2021, 267, 120464 CrossRef CAS PubMed.
- L.-C. Zhang, L.-Y. Chen and L. Wang, Surface modification of titanium and titanium alloys: Technologies, developments, and future interests, Adv. Eng. Mater., 2020, 22, 1901258 CrossRef CAS.
- S. Devgan and S. S. Sidhu, Evolution of surface modification trends in bone related biomaterials: A review, Mater. Chem. Phys., 2019, 233, 68–78 CrossRef CAS.
- Y. Liu, B. Rath, M. Tingart and J. Eschweiler, Role of implants surface modification in osseointegration: A systematic review, J. Biomed. Mater. Res., Part A, 2020, 108, 470–484 CrossRef CAS PubMed.
- R. I. M. Asri, W. S. W. Harun, M. Samykano, N. A. C. Lah, S. A. C. Ghani, F. Tarlochan and M. R. Raza, Corrosion and surface modification on biocompatible metals: A review, Mater. Sci. Eng., C, 2017, 77, 1261–1274 CrossRef CAS PubMed.
- Y. Sasikumar, I. Karuppusamy and R. Nallaiyan, Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: A review, J. Bio- Tribo-Corros., 2019, 5, 36 CrossRef.
- C. Hu, D. Ashok, D. R. Nisbet and V. Gautam, Bioinspired surface modification of orthopedic implants for bone tissue engineering, Biomaterials, 2019, 219, 119366 CrossRef CAS PubMed.
- H. Chouirfa, H. Bouloussa, V. Migonney and C. Falentin-Daudré, Review of titanium surface modification techniques and coatings for antibacterial applications, Acta Biomater., 2019, 83, 37–54 CrossRef CAS PubMed.
- B. Bacchelli, G. Giavaresi, M. Franchi, D. Martini, V. De Pasquale, A. Trirè, M. Fini, R. Giardino and A. Ruggeri, Influence of a zirconia sandblasting treated surface on peri-implant bone healing: An experimental study in sheep, Acta Biomater., 2009, 5, 2246–2257 CrossRef CAS.
- V. Barranco, M. L. Escudero and M. C. García-Alonso, 3D, chemical and electrochemical characterization of blasted TI6Al4V surfaces: Its influence on the corrosion behaviour, Electrochim. Acta, 2007, 52, 4374–4384 CrossRef CAS.
- A. Günay-Bulutsuz, Ö. Berrak, H. A. Yeprem, E. D. Arisan and M. E. Yurci, Biological responses of ultrafine grained pure titanium and their sand blasted surfaces, Mater. Sci. Eng., C, 2018, 91, 382–388 CrossRef.
- T. C. Lowe, R. A. Reiss, P. E. Illescas, C. F. Davis, M. C. Connick and J. A. Sena, Effect of surface grain boundary density on preosteoblast proliferation on titanium, Mater. Res. Lett., 2020, 8, 239–246 CrossRef CAS.
- X. Shi, L. Xu, Q. Wang, Sunarso and L. Xu, Hydrothermal sterilization improves initial osteoblast responses on sandpaper-polished titanium, Materials, 2017, 10, 812 CrossRef PubMed.
- X. Miao, D. Wang, L. Xu, J. Wang, D. Zeng, S. Lin, C. Huang, X. Liu and X. Jiang, The response of human osteoblasts, epithelial cells, fibroblasts, macrophages and oral bacteria to nanostructured titanium surfaces: a systematic study, Int. J. Nanomed., 2017, 12, 1415–1430 CrossRef CAS PubMed.
- Y. Wang, Z. Yu, K. Li and J. Hu, Effects of surface properties of titanium alloys modified by grinding, sandblasting and acidizing and nanosecond laser on cell proliferation and cytoskeleton, Appl. Surf. Sci., 2020, 501, 144279 CrossRef CAS.
- L. Łatka, L. Pawłowski, M. Winnicki, P. Sokołowski, A. Małachowska and S. Kozerski, Review of functionally graded thermal sprayed coatings, Appl. Sci., 2020, 10, 5153 CrossRef.
- I. Ratha, P. Datta, V. K. Balla, S. K. Nandi and B. Kundu, Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review, Ceram. Int., 2021, 47, 4426–4445 CrossRef CAS.
- K. A. Khor, Y. W. Gu, D. Pan and P. Cheang, Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti–6Al–4V composite coatings, Biomaterials, 2004, 25, 4009–4017 CrossRef CAS.
- A. Fomin, M. Fomina, V. Koshuro, I. Rodionov, A. Zakharevich and A. Skaptsov, Structure and mechanical properties of hydroxyapatite coatings produced on titanium using plasma spraying with induction preheating, Ceram. Int., 2017, 43, 11189–11196 CrossRef CAS.
- I. Ullah, M. A. Siddiqui, H. Liu, S. K. Kolawole, J. Zhang, S. Zhang, L. Ren and K. Yang, Mechanical, biological, and antibacterial characteristics of plasma-sprayed (Sr,Zn) substituted hydroxyapatite coating, ACS Biomater. Sci. Eng., 2020, 6, 1355–1366 CrossRef CAS PubMed.
- D. Ke, A. A. Vu, A. Bandyopadhyay and S. Bose, Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants, Acta Biomater., 2019, 84, 414–423 CrossRef CAS PubMed.
- D. Lahiri, A. P. Benaduce, F. Rouzaud, J. Solomon, A. K. Keshri, L. Kos and A. Agarwal, Wear behavior and in vitro cytotoxicity of wear debris generated from hydroxyapatite-carbon nanotube composite coating, J. Biomed. Mater. Res., Part A, 2011, 96, 1–12 CrossRef PubMed.
- N. Sarkar and S. Bose, Controlled delivery of curcumin and vitamin K2 from hydroxyapatite-coated titanium implant for enhanced in vitro chemoprevention, osteogenesis, and in vivo osseointegration, ACS Appl. Mater. Interfaces, 2020, 12, 13644–13656 CrossRef CAS.
- D. Cheng, Q. Liang, Y. Li, J. Fan, G. Wang, H. Pan and C. Ruan, Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone, Colloids Surf., B, 2018, 162, 279–287 CrossRef CAS.
- Z. Xu, H. Lu, J. Lu, C. Lv, X. Zhao and G. Wang, Enhanced osteogenic activity of Ti alloy implants by modulating strontium configuration in their surface oxide layers, RSC Adv., 2018, 8, 3051–3060 RSC.
- W. Zhang, G. Wang, Y. Liu, X. Zhao, D. Zou, C. Zhu, Y. Jin, Q. Huang, J. Sun, X. Liu, X. Jiang and H. Zreiqat, The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration, Biomaterials, 2013, 34, 3184–3195 CrossRef CAS PubMed.
- G. Wang, X. Zhao, M. Möller and S. E. Moya, Interfacial reaction-driven formation of silica carbonate biomorphs with subcellular topographical features and their biological activity, ACS Appl. Mater. Interfaces, 2015, 7, 23412–23417 CrossRef CAS.
- Z. Xu, J. Long, N. Zhang, H. Cao, W. Tang, K. Shi, X. Wang, S. Moya, L. Duan, H. Pan, Y. Lai, D. Wang and G. Wang, Strong mineralization ability of strontium zinc silicate: Formation of a continuous biomorphic mineralized layer with enhanced osteogenic activity, Colloids Surf., B, 2019, 176, 420–430 CrossRef CAS PubMed.
- A. Abdal Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha, G. M. Yang, H. Y. Choi and S. G. Cho, The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles, Int. J. Mol. Sci., 2017, 18, 120 CrossRef PubMed.
- X. Zhao, L. You, T. Wang, X. Zhang, Z. Li, L. Ding, J. Li, C. Xiao, F. Han and B. Li, Enhanced osseointegration of titanium implants by surface modification with silicon-doped titania nanotubes, Int. J. Nanomed., 2020, 15, 8583–8594 CrossRef PubMed.
- H. Qin, H. Cao, Y. Zhao, C. Zhu, T. Cheng, Q. Wang, X. Peng, M. Cheng, J. Wang, G. Jin, Y. Jiang, X. Zhang, X. Liu and P. K. Chu, In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium, Biomaterials, 2014, 35, 9114–9125 CrossRef PubMed.
- J. Wang, J. Li, S. Qian, G. Guo, Q. Wang, J. Tang, H. Shen, X. Liu, X. Zhang and P. K. Chu, Antibacterial Surface Design of Titanium-Based Biomaterials for Enhanced Bacteria-Killing and Cell-Assisting Functions Against Periprosthetic Joint Infection, ACS Appl. Mater. Interfaces, 2016, 8, 11162–11178 CrossRef PubMed.
- G. Wang, W. Jin, A. M. Qasim, A. Gao, X. Peng, W. Li, H. Feng and P. K. Chu, Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species, Biomaterials, 2017, 124, 25–34 CrossRef PubMed.
- H. Cao, Y. Qiao, X. Liu, T. Lu, T. Cui, F. Meng and P. K. Chu, Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better, Acta Biomater., 2013, 9, 5100–5110 CrossRef CAS PubMed.
- Y. Zhao, H. Cao, H. Qin, T. Cheng, S. Qian, M. Cheng, X. Peng, J. Wang, Y. Zhang, G. Jin, X. Zhang, X. Liu and P. K. Chu, Balancing the osteogenic and antibacterial properties of titanium by codoping of Mg and Ag: An in vitro and in vivo study, ACS Appl. Mater. Interfaces, 2015, 7, 17826–17836 CrossRef CAS PubMed.
- H. C. Li, D. G. Wang, C. Hu, J. H. Dou, H. J. Yu and C. Z. Chen, Effect of Na2O and ZnO on the microstructure and properties of laser cladding derived CaO-SiO2 ceramic coatings on titanium alloys, J. Colloid Interface Sci., 2021, 592, 498–508 CrossRef CAS PubMed.
- H. C. Li, D. G. Wang, C. Hu, J. H. Dou, H. J. Yu, C. Z. Chen and G. C. Gu, Microstructure, mechanical and biological properties of laser cladding derived CaO–SiO2–MgO system ceramic coatings on titanium alloys, Appl. Surf. Sci., 2021, 548, 149296 CrossRef CAS.
- A. L. Raines, R. Olivares-Navarrete, M. Wieland, D. L. Cochran, Z. Schwartz and B. D. Boyan, Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy, Biomaterials, 2010, 31, 4909–4917 CrossRef CAS PubMed.
- B. D. Boyan, S. Lossdorfer, L. Wang, G. Zhao, C. H. Lohmann, D. L. Cochran and Z. Schwartz, Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies, Eur. Cells Mater., 2003, 6, 22–27 CrossRef CAS PubMed.
- R. A. Gittens, T. McLachlan, R. Olivares-Navarrete, Y. Cai, S. Berner, R. Tannenbaum, Z. Schwartz, K. H. Sandhage and B. D. Boyan, The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation, Biomaterials, 2011, 32, 3395–3403 CrossRef CAS PubMed.
- E. W. Zhang, Y. B. Wang, K. G. Shuai, F. Gao, Y. J. Bai, Y. Cheng, X. L. Xiong, Y. F. Zheng and S. C. Wei, In vitro and in vivo evaluation of SLA titanium surfaces with further alkali or hydrogen peroxide and heat treatment, Biomed. Mater., 2011, 6, 025001 CrossRef CAS PubMed.
- H. Liu, R. Liu, I. Ullah, S. Zhang, Z. Sun, L. Ren and K. Yang, Rough surface of copper-bearing titanium alloy with multifunctions of osteogenic ability and antibacterial activity, J. Mater. Sci. Technol., 2020, 48, 130–139 CrossRef.
- A. Scarano, E. Crocetta, A. Quaranta and F. Lorusso, Influence of the thermal treatment to address a better osseointegration of Ti6Al4V dental implants: Histological and histomorphometrical study in a rabbit model, BioMed Res. Int., 2018, 2349698 Search PubMed.
- G. Wang, J. Li, K. Lv, W. Zhang, X. Ding, G. Yang, X. Liu and X. Jiang, Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration, Sci. Rep., 2016, 6, 31769 CrossRef CAS PubMed.
- G. A. Longhitano, M. A. Larosa, A. L. Jardini, C. A. D. C. Zavaglia and M. C. F. Ierardi, Correlation between microstructures and mechanical properties under tensile and compression tests of heat-treated Ti-6Al–4 V ELI alloy produced by additive manufacturing for biomedical applications, J. Mater. Process. Technol., 2018, 252, 202–210 CrossRef CAS.
- G. A. Longhitano, M. A. Arenas, A. Conde, M. A. Larosa, A. L. Jardini, C. A. D. C. Zavaglia and J. J. Damborenea, Heat treatments effects on functionalization and corrosion behavior of Ti-6Al-4V ELI alloy made by additive manufacturing, J. Alloys Compd., 2018, 765, 961–968 CrossRef CAS.
- J. Jenkins, J. Mantell, C. Neal, A. Gholinia, P. Verkade, A. H. Nobbs and B. Su, Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress, Nat. Commun., 2020, 11, 1626 CrossRef CAS PubMed.
- M. Gu, L. Lv, F. Du, T. Niu, T. Chen, D. Xia, S. Wang, X. Zhao, J. Liu, Y. Liu, C. Xiong and Y. Zhou, Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates, Sci. Rep., 2018, 8, 8141 CrossRef PubMed.
- S. Bose, D. Ke, A. A. Vu, A. Bandyopadhyay and S. B. Goodman, Thermal oxide layer enhances crystallinity and mechanical properties for plasma-sprayed hydroxyapatite biomedical coatings, ACS Appl. Mater. Interfaces, 2020, 12, 33465–33472 CrossRef PubMed.
- Y. Dang, L. Zhang, W. Song, B. Chang, T. Han, Y. Zhang and L. Zhao, In vivo osseointegration of Ti implants with a strontium-containing nanotubular coating, Int. J. Nanomed., 2016, 11, 1003–1011 Search PubMed.
- Y. Zhang, K. Wang, Y. Song, E. Feng, K. Dong, Y. Han and T. Lu, Ca substitution of Sr in Sr-doped TiO2 nanotube film on Ti surface for enhanced osteogenic activity, Appl. Surf. Sci., 2020, 528, 147055 CrossRef.
- Z. Geng, X. Li, L. Ji, Z. Li, S. Zhu, Z. Cui, J. Wang, J. Cui, X. Yang and C. Liu, A novel snail-inspired bionic design of titanium with strontium-substituted hydroxyapatite coating for promoting osseointegration, J. Mater. Sci. Technol., 2021, 79, 35–45 CrossRef.
- N. Jiang, Z. Guo, D. Sun, Y. Li, Y. Yang, C. Chen, L. Zhang and S. Zhu, Promoting osseointegration of Ti Implants through micro/nanoscaled hierarchical Ti phosphate/Ti oxide hybrid coating, ACS Nano, 2018, 12, 7883–7891 CrossRef PubMed.
- B. Cai, P. Tan, N. Jiang, Z. Guo, B. Ay, S. Li, Y. Hou, Y. Li, Y. You, L. Zhang and S. Zhu, Bioinspired fabrication of calcium-doped TiP coating with nanofibrous microstructure to accelerate osseointegration, Bioconjugate Chem., 2020, 31, 1641–1650 CrossRef PubMed.
- E. B. Ansar, K. Ravikumar, S. Suresh Babu, F. B. Fernandez, M. Komath, B. Basu and P. R. Harikrishna, Varma, Inducing apatite pre-layer on titanium surface through hydrothermal processing for osseointegration, Mater. Sci. Eng., C, 2019, 105, 110019 CrossRef PubMed.
- J. V. Wandiyanto, T. Tamanna, D. P. Linklater, V. K. Truong, M. Al Kobaisi, V. A. Baulin, S. Joudkazis, H. Thissen, R. J. Crawford and E. P. Ivanova, Tunable morphological changes of asymmetric titanium nanosheets with bactericidal properties, J. Colloid Interface Sci., 2020, 560, 572–580 CrossRef PubMed.
- T. L. Clainche, D. Linklater, S. Wong, P. Le, S. Juodkazis, X. L. Guevel, J. L. Coll, E. P. Ivanova and V. Martel-Frachet, Mechano-bactericidal titanium surfaces for bone tissue engineering, ACS Appl. Mater. Interfaces, 2020, 12, 48272–48283 CrossRef PubMed.
- V. K. Manivasagam and K. C. Popat, In Vitro Investigation of Hemocompatibility of Hydrothermally Treated Titanium and Titanium Alloy Surfaces, ACS Omega, 2020, 5, 8108–8120 CrossRef PubMed.
- D. P. Linklater, S. Juodkazis, R. J. Crawford and E. P. Ivanova, Mechanical inactivation of Staphylococcus aureus and Pseudomonas aeruginosa by titanium substrata with hierarchical surface structures, Materialia, 2019, 5, 100197 CrossRef.
- A. Cheng, W. B. Goodwin, B. M. deGlee, R. A. Gittens, J. P. Vernon, S. L. Hyzy, Z. Schwartz, K. H. Sandhage and B. D. Boyan, Surface modification of bulk titanium substrates for biomedical applications via low-temperature microwave hydrothermal oxidation, J. Biomed. Mater. Res., Part A, 2018, 106, 782–796 CrossRef PubMed.
- X. Nie, S. Yin, W. Duan, Z. Zhao, L. Li and Z. Zhang, Recent Progress in Anodic Oxidation of TiO2 Nanotubes and Enhanced Photocatalytic Performance: A Short Review, NANO, 2021, 16, 2130002 CrossRef.
- K. Das, A. Bandyopadhyay and S. Bose, Biocompatibility and in situ growth of TiO2 nanotubes on Ti using different electrolyte chemistry, J. Am. Ceram. Soc., 2008, 91, 2808–2814 CrossRef.
- B. C. Ward and T. J. Webster, Increased functions of osteoblasts on nanophase metals, Mater. Sci. Eng., C, 2007, 27, 575–578 CrossRef.
- T. J. Webster and J. U. Ejiofor, Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo, Biomaterials, 2004, 25, 4731–4739 CrossRef PubMed.
- S. Durdu, G. Cihan, E. Yalcin and A. Altinkok, Characterization and mechanical properties of TiO2 nanotubes formed on titanium by anodic oxidation, Ceram. Int., 2021, 47, 10972–10979 CrossRef.
- M. Alijani, H. Sopha, S. Ng and J. M. Macak, High aspect ratio TiO2 nanotube layers obtained in a very short anodization time, Electrochim. Acta, 2021, 376, 138080 CrossRef.
- I. P. Torres-Avila, I. I. Padilla-Martínez, N. Pérez-Hernández, A. E. Bañuelos-Hernández, J. C. Velázquez, J. L. Castrejón-Flores and E. Hernández-Sánchez, Surface modification of the Ti-6Al-4V alloy by anodic oxidation and its effect on osteoarticular cell proliferation, Coatings, 2020, 10, 491 CrossRef.
- B. Ren, Y. Wan, C. Liu, H. Wang, M. Yu, X. Zhang and Y. Huang, Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study, Mater. Sci. Eng., C, 2021, 118, 111505 CrossRef PubMed.
- V. Zwilling, E. Darque-Ceretti, A. Boutry-Forveille, D. David, M.-Y. Perrin and M. Aucouturier, Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy, Surf. Interface Anal., 1999, 27, 629–637 CrossRef.
- S. Bodhak, S. Bose and A. Bandyopadhyay, Electrically polarized HAp-coated Ti: In vitro bone cell–material interactions, Acta Biomater., 2010, 6, 641–651 CrossRef PubMed.
- A. Bandyopadhyay, A. Shivaram, I. Mitra and S. Bose, Electrically polarized TiO2 nanotubes on Ti implants to enhance early-stage osseointegration, Acta Biomater., 2019, 96, 686–693 CrossRef PubMed.
- K. Cowden, M. F. Dias-Netipanyj and K. C. Popat, Effects of titania nanotube surfaces on osteogenic differentiation of human adipose-derived stem cells, Nanomedicine, 2019, 17, 380–390 CrossRef CAS PubMed.
- M. Y. Hsu, H. L. Hsu and J. J. J. O. T. E. S. Leu, TiO2 nanowires on anodic TiO2 nanotube arrays (TNWs/TNAs): Formation mechanism and photocatalytic performance, J. Electrochem. Soc., 2012, 159, H722 CrossRef CAS.
- V.-T. Nguyen, T.-C. Cheng, T.-H. Fang and M.-H. Li, The fabrication and characteristics of hydroxyapatite film grown on titanium alloy Ti-6Al-4V by anodic treatment, J. Mater. Res. Technol., 2020, 9, 4817–4825 CrossRef CAS.
- S.-G. Lim and H.-C. Choe, Bioactive apatite formation on PEO-treated Ti-6Al-4V alloy after 3rd anodic titanium oxidation, Appl. Surf. Sci., 2019, 484, 365–373 CrossRef CAS.
- L. Facchini, E. Magalini, P. Robotti, A. Molinari, S. H. Ges and K. J. R. P. J. Wissenbach, Ductility of a Ti-6Al-4V alloy produced by selective laser melting of prealloyed powders, Rapid Prototyp. J., 2010, 16, 450–459 CrossRef.
- H. Zhang, J. Zhao, J. Liu, H. Qin, Z. Ren, G. L. Doll, Y. Dong and C. Ye, The effects of electrically-assisted ultrasonic nanocrystal surface modification on 3D-printed Ti-6Al-4V alloy, Addit. Manuf., 2018, 22, 60–68 CAS.
- J. Wang, Y. Pan, R. Feng, H. Cui, B. Gong, L. Zhang, Z. Gao, X. Cui, H. Zhang and Z. Jia, Effect of electrolyte composition on the microstructure and bio-corrosion behavior of micro-arc oxidized coatings on biomedical Ti6Al4V alloy, J. Mater. Res. Technol., 2020, 9, 1477–1490 CrossRef CAS.
- G. Li, H. Cao, W. Zhang, X. Ding, G. Yang, Y. Qiao, X. Liu and X. Jiang, Enhanced osseointegration of hierarchical micro/nanotopographic titanium fabricated by microarc oxidation and electrochemical treatment, ACS Appl. Mater. Interfaces, 2016, 8, 3840–3852 CrossRef CAS PubMed.
- G. O. Alrabeah, P. Brett, J. C. Knowles and H. Petridis, The effect of metal ions released from different dental implant-abutment couples on osteoblast function and secretion of bone resorbing mediators, J. Dent., 2017, 66, 91–101 CrossRef CAS PubMed.
- Q. M. Zhao, X. K. Li, S. Guo, N. Wang, W. W. Liu, L. Shi and Z. Guo, Osteogenic activity of a titanium surface modified with silicon-doped titanium dioxide, Mater. Sci. Eng., C, 2020, 110, 110682 CrossRef CAS PubMed.
- Y. Li, W. Wang, F. Yu, D. Wang, S. Guan, Y. Li and M. Qi, Characterization and cytocompatibility of hierarchical porous TiO2 coatings incorporated with calcium and strontium by one-step micro-arc oxidation, Mater. Sci. Eng., C, 2020, 109, 110610 CrossRef CAS PubMed.
- S. Durdu, S. L. Aktug, K. Korkmaz, E. Yalcin and S. Aktas, Fabrication, characterization and in vitro properties of silver-incorporated TiO2 coatings on titanium by thermal evaporation and micro-arc oxidation, Surf. Coat. Technol., 2018, 352, 600–608 CrossRef CAS.
- J. Ye, B. Li, M. Li, Y. Zheng, S. Wu and Y. Han, ROS induced bactericidal activity of amorphous Zn-doped titanium oxide coatings and enhanced osseointegration in bacteria-infected rat tibias, Acta Biomater., 2020, 107, 313–324 CrossRef CAS PubMed.
- X. Li, M. Wang, W. Zhang, Y. Bai, Y. Liu, J. Meng and L. Zhang, A magnesium-incorporated nanoporous titanium coating for rapid osseointegration, Int. J. Nanomed., 2020, 15, 6593–6603 CrossRef CAS PubMed.
- T. H. Qaid, S. Ramesh, F. Yusof, W. J. Basirun, Y. C. Ching, H. Chandran, S. Ramesh and S. Krishnasamy, Micro-arc oxidation of bioceramic coatings containing eggshell-derived hydroxyapatite on titanium substrate, Ceram. Int., 2019, 45, 18371–18381 CrossRef CAS.
- D. Gregurec, G. Wang, R. H. Pires, M. Kosutic, T. Ludtke, M. Delcea and S. E. Moya, Bioinspired titanium coatings: self-assembly of collagen-alginate films for enhanced osseointegration, J. Mater. Chem. B, 2016, 4, 1978–1986 RSC.
- Y. He, K. Xu, K. Li, Z. Yuan, Y. Ding, M. Chen, C. Lin, B. Tao, X. Li, G. Zhang, P. Liu and K. Cai, Improved osteointegration by SEW2871-encapsulated multilayers on micro-structured titanium via macrophages recruitment and immunomodulation, Appl. Mater. Today, 2020, 20, 100673 CrossRef.
- W. Chen, X. Shen, Y. Hu, K. Xu, Q. Ran, Y. Yu, L. Dai, Z. Yuan, L. Huang, T. Shen and K. Cai, Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis, Biomaterials, 2017, 114, 82–96 CrossRef CAS PubMed.
- W. Chen, K. Xu, B. Tao, L. Dai, Y. Yu, C. Mu, X. Shen, Y. Hu, Y. He and K. Cai, Multilayered coating of titanium implants promotes coupled osteogenesis and angiogenesis in vitro and in vivo, Acta Biomater., 2018, 74, 489–504 CrossRef CAS PubMed.
- S. Zhao, Y. Xu, W. Xu, Z. Weng, F. Cao, X. Wan, T. Cui, Y. Yu, L. Liao and X. Wang, Tremella-like ZnO@Col-I-decorated titanium surfaces with dual-light-defined broad-spectrum antibacterial and triple osteogenic properties, ACS Appl. Mater. Interfaces, 2020, 12, 30044–30051 CrossRef CAS PubMed.
- J. Chen, G. Hu, T. Li, Y. Chen, M. Gao, Q. Li, L. Hao, Y. Jia, L. Wang and Y. Wang, Fusion peptide engineered “statically-versatile” titanium implant simultaneously enhancing anti-infection, vascularization and osseointegration, Biomaterials, 2021, 264, 120446 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Mussel-inspired surface chemistry for multifunctional coatings, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- H. Wang, C. Lin, X. Zhang, K. Lin, X. Wang and S. G. Shen, Mussel-inspired polydopamine coating: A general strategy to enhance osteogenic differentiation and osseointegration for diverse implants, ACS Appl. Mater. Interfaces, 2019, 11, 7615–7625 CrossRef CAS PubMed.
- S. Amin Yavari, S. M. Castenmiller, J. A. G. van Strijp and M. Croes, Combating implant infections: Shifting focus from bacteria to host, Adv. Mater., 2020, 32, 2002962 CrossRef CAS PubMed.
- S. H. Ku and C. B. Park, Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering, Biomaterials, 2010, 31, 9431–9437 CrossRef CAS PubMed.
- W.-B. Tsai, W.-T. Chen, H.-W. Chien, W.-H. Kuo and M.-J. Wang, Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering, Acta Biomater., 2011, 7, 4187–4194 CrossRef CAS PubMed.
- H. Xu, G. Zhang, K. Xu, L. Wang, L. Yu, M. M. Q. Xing and X. Qiu, Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction, Mater. Sci. Eng., C, 2018, 90, 379–386 CrossRef CAS PubMed.
- N. G. Rim, S. J. Kim, Y. M. Shin, I. Jun, D. W. Lim, J. H. Park and H. Shin, Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells, Colloids Surf., B, 2012, 91, 189–197 CrossRef CAS PubMed.
- E. Ko, J. S. Lee, H. Kim, S. Y. Yang, D. Yang, K. Yang, J. Lee, J. Shin, H. S. Yang, W. Ryu and S.-W. Cho, Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells, ACS Appl. Mater. Interfaces, 2018, 10, 7614–7625 CrossRef CAS PubMed.
- S. Bose, N. Sarkar and D. Banerjee, Natural medicine delivery from biomedical devices to treat bone disorders: A review, Acta Biomater, 2021, 126, 63–91 CrossRef CAS PubMed.
- X. Song, F. Liu, C. Qiu, E. Coy, H. Liu, W. Aperador, K. Załęski, J. J. Li, W. Song, Z. Lu, H. Pan, L. Kong and G. Wang, Nanosurfacing Ti alloy by weak alkalinity-activated solid-state dewetting (AAD) and its biointerfacial enhancement effect, Mater. Horiz., 2021, 8, 912–924 RSC.
- Z. Zhao, J. Chen, S. Guo, H. Tan, X. Lin and W. Huang, Influence of α/β interface phase on the tensile properties of laser cladding deposited Ti–6Al–4V titanium alloy, J. Mater. Sci. Technol., 2017, 33, 675–681 CrossRef CAS.
- J. Alayan, C. Vaquette, S. Saifzadeh, D. Hutmacher and S. Ivanovski, Comparison of early osseointegration of SLA® and SLActive® implants in maxillary sinus augmentation: a pilot study, Clin. Oral. Implants Res., 2017, 28, 1325–1333 CrossRef PubMed.
- J. Zhang, J. Liu, C. Wang, F. Chen, X. Wang and K. Lin, A comparative study of the osteogenic performance between the hierarchical micro/submicro-textured 3D-printed Ti6Al4V surface and the SLA surface, Bioact. Mater., 2020, 5, 9–16 CrossRef PubMed.
- A. H. A. Qamheya, V. Arısan, Z. Mutlu, M. Karabaglı, M. Soluk Tekkeşin, K. Kara, A. Erol and S. Ersanlı, Thermal oxidation and hydrofluoric acid treatment on the sandblasted implant surface: A histologic histomorphometric and biomechanical study, Clin. Oral. Implants Res., 2018, 29, 741–755 CrossRef PubMed.
- M. Zuldesmi, A. Waki, K. Kuroda and M. Okido, Hydrothermal treatment of titanium alloys for the enhancement of osteoconductivity, Mater. Sci. Eng., C, 2015, 49, 430–435 CrossRef CAS PubMed.
- J. Vishnu, V. K. Manivasagam, V. Gopal, C. Bartomeu Garcia, P. Hameed, G. Manivasagam and T. J. Webster, Hydrothermal treatment of etched titanium: A potential surface nano-modification technique for enhanced biocompatibility, Nanomedicine, 2019, 20, 102016 CrossRef CAS PubMed.
- X. Bai, X. Shi, L. Xu, F. Huang, C. Zheng, L. Xu, B. Li and Q. Wang, Effects of hydrothermal treatment on physicochemical and anticorrosion properties of titanium nitride coating on pure titanium, Appl. Surf. Sci., 2020, 507, 145030 CrossRef CAS.
- D. Khudhair, A. Bhatti, Y. Li, H. A. Hamedani, H. Garmestani, P. Hodgson and S. Nahavandi, Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations, Mater. Sci. Eng., C, 2016, 59, 1125–1142 CrossRef CAS PubMed.
- B. V. Krishna, S. Bose and A. Bandyopadhyay, Low stiffness porous Ti structures for load-bearing implants, Acta Biomater., 2007, 3, 997–1006 CrossRef CAS PubMed.
- W. Xue, B. V. Krishna, A. Bandyopadhyay and S. Bose, Processing and biocompatibility evaluation of laser processed porous titanium, Acta Biomater., 2007, 3, 1007–1018 CrossRef CAS PubMed.
- V. K. Balla, S. Banerjee, S. Bose and A. Bandyopadhyay, Direct laser processing of a tantalum coating on titanium for bone replacement structures, Acta Biomater., 2010, 6, 2329–2334 CrossRef CAS PubMed.
- V. K. Balla, S. Bodhak, S. Bose and A. Bandyopadhyay, Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties, Acta Biomater., 2010, 6, 3349–3359 CrossRef CAS PubMed.
- S. Bose, S. F. Robertson and A. Bandyopadhyay, Surface modification of biomaterials and biomedical devices using additive manufacturing, Acta Biomater., 2018, 66, 6–22 CrossRef CAS PubMed.
- Q. Wang, P. Zhou, S. Liu, S. Attarilar, R. L.-W. Ma, Y. Zhong and L. Wang, Multi-scale surface treatments of titanium implants for rapid osseointegration: A review, Nanomaterials, 2020, 10, 1244 CrossRef CAS PubMed.
- I. Mitra, S. Bose, W. S. Dernell, N. Dasgupta, C. Eckstrand, J. Herrick, M. J. Yaszemski, S. B. Goodman and A. Bandyopadhyay, 3D Printing in alloy design to improve biocompatibility in metallic implants, Mater. Today, 2021, 45, 20–34 CrossRef CAS PubMed.
- A. Rifai, N. Tran, D. W. Lau, A. Elbourne, H. Zhan, A. D. Stacey, E. L. H. Mayes, A. Sarker, E. P. Ivanova, R. J. Crawford, P. A. Tran, B. C. Gibson, A. D. Greentree, E. Pirogova and K. Fox, Polycrystalline diamond coating of additively manufactured titanium for biomedical applications, ACS Appl. Mater. Interfaces, 2018, 10, 8474–8484 CrossRef CAS PubMed.
- H. Byun, Y. Bin Lee, E. M. Kim and H. Shin, Fabrication of size-controllable human mesenchymal stromal cell spheroids from micro-scaled cell sheets, Biofabrication, 2019, 11, 035025 CrossRef CAS PubMed.
- E. Gilabert-Chirivella, R. Pérez-Feito, C. Ribeiro, S. Ribeiro, D. M. Correia, M. L. González-Martín, J. M. Manero, S. Lanceros-Méndez, G. G. Ferrer and J. L. Gómez-Ribelles, Chitosan patterning on titanium implants, Prog. Org. Coat., 2017, 111, 23–28 CrossRef CAS.
- S. Qiu, J. Ji, W. Sun, J. Pei, J. He, Y. Li, J. J. Li and G. Wang, Recent advances in surface manipulation using micro-contact printing for biomedical applications, Smart Materials in Medicine, 2021, 2, 65–73 CrossRef.
- B. Li, X. Xia, M. Guo, Y. Jiang, Y. Li, Z. Zhang, S. Liu, H. Li, C. Liang and H. Wang, Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium, Sci. Rep., 2019, 9, 14052 CrossRef PubMed.
- D. Liu, C. He, Z. Liu and W. Xu, Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits, Int. J. Nanomed., 2017, 12, 5461–5471 CrossRef CAS PubMed.
- M. Lilja, C. Lindahl, W. Xia, H. Engqvist and M. Strømme, The Effect of Si-Doping on the Release of Antibiotic from Hydroxyapatite Coatings, J. Biomater. Nanobiotechnol., 2013, 4, 237–241 CrossRef.
- Z. Du, J. Chen, F. Yan and Y. Xiao, Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats, Clin. Oral. Implants Res., 2009, 20, 145–150 CrossRef PubMed.
- C. G. Park, M. Park, B. H. Kim, S. H. Lee, J. Y. Park, H. H. Park, K. Lee, H.-K. Seok and Y. B. Choy, Pattern-coated titanium bone fixation plate for dual delivery of vancomycin and alendronate, Macromol. Res., 2017, 25, 756–762 CrossRef CAS.
- O. Janson, J. Sörensen, M. Strømme, H. Engqvist, P. Procter and K. Welch, Evaluation of an alkali-treated and hydroxyapatite-coated orthopedic implant loaded with tobramycin, J. Biomater. Appl., 2019, 34, 088532821986796 CrossRef PubMed.
- L. Jia, F. Han, H. Wang, C. Zhu, Q. Guo, J. Li, Z. Zhao, Q. Zhang, X. Zhu and B. Li, Polydopamine-assisted surface modification for orthopaedic implants, J. Orthop. Translat., 2019, 17, 82–95 CrossRef PubMed.
- T. Stich, F. Alagboso, T. Křenek, T. Kovářík, V. Alt and D. Docheva, Implant-bone-interface: reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo, Bioeng. Transl. Med., 2021, e10239 Search PubMed.
- A. Civantos, E. Martínez-Campos, V. Ramos, C. Elvira, A. Gallardo and A. Abarrategi, Titanium coatings and surface modifications: Toward clinically useful bioactive implants, ACS Biomater. Sci. Eng., 2017, 3, 1245–1261 CrossRef CAS PubMed.
- G. Liu, M. Hirtz, H. Fuchs and Z. Zheng, Development of Dip-Pen Nanolithography (DPN) and Its Derivatives, Small, 2019, 15, 1900564 CrossRef PubMed.
- K. T. M. Tran and T. D. Nguyen, Lithography-based methods to manufacture biomaterials at small scales, J. Sci.: Adv. Mater. Devices, 2017, 2, 1–14 Search PubMed.
- C. Liao, A. Wuethrich and M. Trau, A material odyssey for 3D nano/microstructures: two photon polymerization based nanolithography in bioapplications, Appl. Mater. Today, 2020, 19, 100635 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |
