Open Access Article
Open Access ArticleSynthesis of ultrafine polymer nanofibers†
Hongyu
Meng
 *abc and
Xuezhi
Qiao
*abc and
Xuezhi
Qiao
 c
c
aCAS Center for Excellence in Nanoscience, Beijing Key Laboratory of Micro-Nano Energy and Sensor, Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing, 100083, China. E-mail: menghongyu@binn.cas.cn
bSchool of Nanoscience and Technology, University of Chinese Academy of Sciences, Beijing 100049, China
cBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Polymer Physics and Chemistry, Key Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing 100190, China
First published on 8th October 2021
Abstract
The preparation of nanofibers with finer diameters is of great importance to meet the needs of nanomaterials in fabricating nano-devices. The diameter of polydivinylbenzene nanofibers can be reduced from 200 nm to 20–50 nm by adding a very small amount of oleic acid-capped Fe3O4 nanoparticles during the polymerization of divinylbenzene. Through ultrasonic disruption and magnetic separation of the nanofiber composites, we can obtain purified ultrafine nanofibers without Fe3O4 nanoparticles. The ultrafine nanofibers have widespread potential applications in nanomaterials and nano-devices with smaller requirements.
One-dimensional (1D) nanomaterials have aroused great interest in both fundamental research and practical applications.1 For example, carbon nanofibers and nanotubes have demonstrated potential applications in microelectronics,2,3 imaging and therapeutics,4 and as adsorbents for organic solvents5 and heavy metal ions;6 in addition, polyaniline nanofibers can be used as conductive nano-devices.7 In order to meet the needs of nanomaterials in fabricating small devices, preparation of nanofibers with finer diameters is of great importance.
We have previously reported a method for the large-scale synthesis of crosslinked polydivinylbenzene (PDVB) nanofibers via template-free rapid cationic polymerization.8 The initiator boron trifluoride diethyl etherate (BFEE) is dispersed in n-hexane as immiscible nano-droplets under ultrasonication. When the monomer divinylbenzene (DVB) is introduced, the cationic polymerization of DVB occurs on the surface of the BFEE droplets, forming cross-linked PDVB. Eventually the PDVB nanofibers, with a diameter of about 200 nm (Fig. 2a and b), were formed upon removal of the droplets. Compared with carbon nanofibers and polyaniline nanofibers, PDVB nanofibers have abundant reactive groups8 and their morphology and monomer component is variable,9,10 which has great advantages in their further modification for use in nanomaterials and nano-devices.11 Furthermore, compared with other methods of preparing nanofibers, including electro-spinning12,13 and the template method,14,15 our method has the advantages of low cost, high efficiency and ease of large-scale preparation.
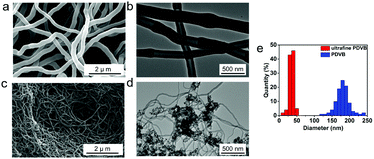
Herein, we develop an approach to prepare ultrafine PDVB nanofibers with a diameter of 20–50 nm. In the presence of oleic acid-capped Fe3O4 nanoparticles (NPs) (Fig. S1, ESI†), ultrafine PDVB nanofiber composites are achieved during polymerization. Emulsification of the oleic acid-capped Fe3O4 NPs and the extrusion force of the internal initiator droplets caused by polymerization facilitate the dispersion of BFEE nano-droplets into smaller droplets. The diameter of the obtained PDVB nanofibers is 50 nm (Fig. 1a). Through ultrasonic disruption and magnetic separation (Fig. 1b), we can obtain pure ultrafine PDVB nanofibers from the composites.
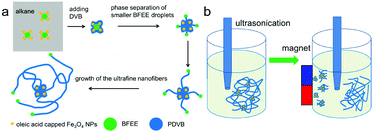 | ||
| Fig. 1 (a) Schematic of the formation and growth of ultrafine PDVB nanofiber composites; and (b) schematic of the ultrasonic disruption and magnetic separation of ultrafine PDVB nanofibers. | ||
We were inspired by the “template-free method”16,17 and “nano-seeding”18–20 for the synthesis of ultrafine PDVB nanofibers. A very small amount of oleic acid-capped Fe3O4 NPs (0.05 wt%) is added in n-hexane, followed by the addition of the initiator BFEE and the monomer DVB. The PDVB ultrafine nanofiber composites are precipitated and the diameter of the new PDVB nanofibers is below 50 nm (Fig. 2c–e). In comparison, the diameter of our previous nanofibers was 150–200 nm (Fig. 2a and b).8
We investigated the effect of different amounts of oleic acid-capped Fe3O4 NPs on the diameter and morphology of the PDVB nanofibers using SEM (Fig. 3) and TEM (Fig S2, ESI†). In the presence of a smaller concentration of Fe3O4 NPs (0.01 wt%; 0.02 wt%), we can obtain blended PDVB nanofibers with a diameter of about 200 nm (coarse) and below 50 nm (ultrafine) (Fig. 3a and b). With an increase in the Fe3O4 NP content, the proportion of ultrafine PDVB nanofibers gradually increases. When the Fe3O4 NP content is 0.05 wt%, the diameter of all of the PDVB nanofibers is under 50 nm (Fig. 2c). With a further increase in the Fe3O4 NP content, the product obtained is ultrafine PDVB nanofibers mixed with irregular protrusions (Fig. 3c). The proportion of irregular protrusions increases as the amount of Fe3O4 NP is increased. When the Fe3O4 NP content reaches 0.2 wt%, the products become irregular protrusions. The diameter of these protrusions is about 30 nm and the protrusions are easily agglomerated to form coral-like structures (Fig. 3d). In addition, in the case of a low Fe3O4 NP content (below 0.02 wt%), there are also irregular protruding structure exists, although their content is low (Fig S3, ESI†).
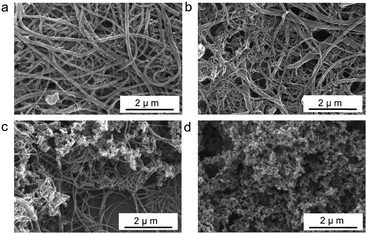 | ||
| Fig. 3 SEM images of PDVB nanofibers synthesized at different concentration of Fe3O4 NPs: (a) 0.01 wt%; (b) 0.02 wt%; (c) 0.1 wt%; and (d) 0.2 wt%. DVB and BFEE are fixed at 2 wt% and 0.05 wt%. | ||
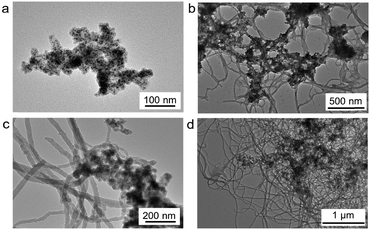
As the reaction progresses, the morphology of the composites changes (Fig. 4 and Fig. S4, ESI†), and we explain the mechanism of the evolution of the ultrafine nanofiber composite as follows (Fig. 1a). The oleic acid on the surface of the Fe3O4 NPs acts as an emulsifier for the BFEE and the n-hexane, so that the Fe3O4 NPs tend to distribute on the surface of the BFEE droplets and the BFEE droplets are dispersed into the smaller BFEE droplets. After adding DVB, the cationic polymerization of the monomer initially starts on the surface of the BFEE droplets, thereby forming cationic living polymer chains of PDVB. When the cationic living polymer chains meet the oleic acid-capped Fe3O4 NPs, they reacted with the reactive double bonds of the oleic acid.21 The oleic acid-capped Fe3O4 NPs are encapsulated into PDVB forming Fe3O4 NP/PDVB core–shell composite nanoparticles. The nanoscale composites are further polymerized into aggregates and dendrites (Fig. 4a). PDVB further polymerizes and squeezes the BFEE inside, causing the phase separation of BFEE, and forming smaller BFEE nanoscale droplets (Fig. 1a). Ultrafine PDVB nanofibers are grown from surface of the aggregates (Fig. 4b and c). With the movement of the BFEE nanoscale droplets, the ultrafine PDVB nanofibers gradually grow longer and are interwoven (Fig. 4d).
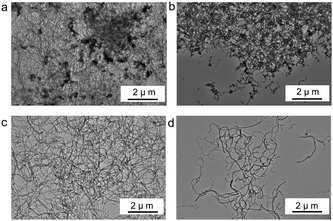
We can separate pure ultrafine PDVB nanofibers from the magnetic dendrites using the method of ultrasonic disruption and magnetic separation (Fig. 1b). We disperse the magnetic PDVB nanofiber composites (Fig. 5a) into ethanol and break them using an ultrasonic cell homogenizer for more than 20 minutes. Part of the ultrafine nanofibers can be broken and separated from the irregular magnetic dendrites that encapsulate the Fe3O4 NPs. When we use the magnet to attract the composites during the entire ultrasonic process, the Fe3O4 NPs-enriched dendrites part is attracted more closely to the magnet (Fig. 5b), so we can obtain a higher proportion of ultrafine PDVB nanofibers at the farthest position from the magnet (Fig. 5c). The length of separated nanofibers is about 10–15 μm, but the proportion of magnetic composite in the separated nanofibers is significantly reduced. We can obtain purer ultrafine PDVB nanofibers when extending the time close to 40 minutes of ultrasonic disruption, but the length of the separated ultrafine PDVB nanofibers is broken to lengths below 5 μm (Fig. 5d). Furthermore, the oleic acid on the Fe3O4 NPs acts as an emulsifier and plays an important role in the further dispersion of the BFEE nano-droplets; however, only using surfactant (such as oleic acid or its derivatives) during the synthesis, it is difficult to obtain pure ultrafine nanofibers,21 even by means of separation.
Furthermore, there are a few reports on the use of template-free polymerization to synthesize polymer nanofibers successfully, except for polyaniline,18 polypyrrole22 and PDVB. The polymerization of polyaniline and polypyrrole nanofibers is an oxidation reaction, while the polymerization of PDVB nanofibers is a cationic polymerization. Compared with polyaniline and polypyrrole nanofibers, PDVB nanofibers have abundant reactive groups, which are a great advantage for further modification if they are to be used in nanomaterials and nano-devices. For a polymer to be synthesized using a template-free method of forming nanofibers, the combination of suitable solvents, initiators, and dopants or surfactants is the key; also in addition, there are still other unknown factors that remain to be discovered. Preparing polymer nanofibers using a template-free method has the advantages of low cost, high efficiency and ease of large-scale preparation. The template-free synthesis method for nanofibers of other materials needs to be developed.
Conclusions
In conclusion, we have successfully reduced the diameter of PDVB nanofibers from 200 nm to less than 50 nm by adding very small amounts of oleic acid-capped Fe3O4 NPs during the polymerization of DVB. And through ultrasonic disruption and magnetic separation of the ultrafine PDVB nanofiber composites, we can obtain purified ultrafine PDVB nanofibers without the Fe3O4 NPs. Moreover, the ultrafine PDVB nanofibers can be produced on a large scale with a high concentration more than 10 wt% of DVB. Abundant the reactive groups of PVDF nanofibers provide the great advantage of further modification. The ultrafine PDVB nanofibers have great potential applications in nanomaterials and nanodevices with small size requirements.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51833005, T2125003 and 61875015).Notes and references
- Y. Zhao, H. Fu, A. Peng, Y. Ma, D. Xiao and J. Yao, Adv. Mater., 2008, 20, 2859–2876 CrossRef CAS.
- M. F. De Volder, S. H. Baughman, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872–1876 CrossRef CAS PubMed.
- K. Kostarelos, A. Bianco and M. Prato, Nat. Nanotechnol., 2009, 4, 627–633 CrossRef CAS PubMed.
- H. Bi, Z. Yin, X. Cao, X. Xie, C. Tan, X. Huang, B. Chen, F. Chen, Q. Yang, X. Bu, X. Lu, L. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- M. H. Dehghani, M. M. Taher, A. K. Bajpai, B. Heibati, I. Tyagi, M. Asif, S. Agarwal and V. Gupta, Chem. Eng. J., 2015, 279, 344–352 CrossRef CAS.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- W. Ni, F. Liang, J. Liu, X. Qu, C. Zhang, J. Li, Q. Wang and Z. Yang, Chem. Commun., 2011, 47, 4727–4729 RSC.
- D. Lv, W. Ni, F. Liang, Q. Wang, X. Qu and Z. Yang, Chin. J. Polym. Sci., 2015, 33, 1344–1350 CrossRef CAS.
- S. Cui, X. Ji, F. Liang and Z. Yang, Chin. Chem. Lett., 2015, 26, 942–945 CrossRef CAS.
- Z. Liu, Q. Wang, F. Liang, X. Qu, C. Zhang, J. Li and Z. Yang, Polymer, 2013, 54, 4948–4954 CrossRef CAS.
- D. Li and Y. Xia, Adv. Mater., 2004, 16, 1151–1170 CrossRef CAS.
- Y. Zhao, X. Cao and L. Jiang, J. Am. Chem. Soc., 2007, 129, 764–765 CrossRef CAS PubMed.
- C. R. Martin, Acc. Chem. Res., 1995, 28, 61–68 CrossRef CAS.
- Q. Zhang, C. Deng, F. Soyekwo, Q. Liu and A. Zhu, Adv. Funct. Mater., 2016, 26, 792–800 CrossRef CAS.
- X. Zhang and S. K. Manohar, Chem. Commun., 2004, 2360–2361 RSC.
- H. Ding, J. Shen, M. Wan and Z. Chen, Macromol. Chem. Phys., 2008, 209, 864–871 CrossRef CAS.
- Z. Wei, M. Wan, T. Lin and L. Dai, Adv. Mater., 2003, 15, 136–139 CrossRef CAS.
- Z. Niu, J. Liu, A. Lee, M. Bruckman, D. Zhao, G. Koley and Q. Wang, Nano Lett., 2007, 7, 3729–3733 CrossRef CAS PubMed.
- X. Zhang, W. Goux and S. Manohar, J. Am. Chem. Soc., 2004, 126, 4502–4503 CrossRef CAS PubMed.
- D. Lv, L. Sheng, J. Wan, J. Dong, H. Ouyang, H. Jiao and J. Liu, Polym. Chem., 2019, 10, 331–335 RSC.
- H. D. Tran, K. Shin, W. G. Hong, J. M. D’Arcy, R. W. Kojima, B. H. Weiller and R. B. Kaner, Macromol. Rapid Commun., 2007, 28, 2289–2293 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00693b |
| This journal is © The Royal Society of Chemistry 2021 |