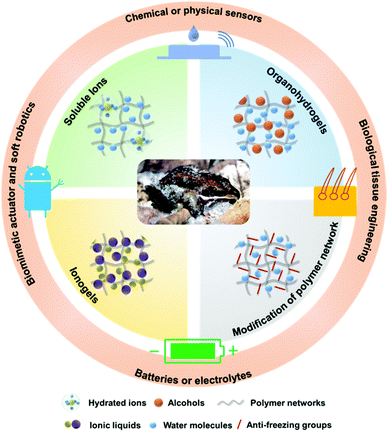
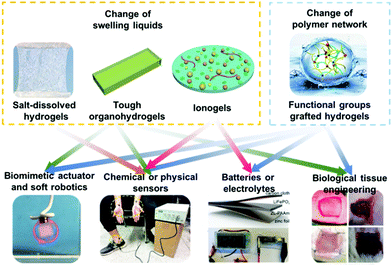
Biomimetic anti-freezing polymeric hydrogels: keeping soft-wet materials active in cold environments
Yukun
Jian†
ab,
Stephan
Handschuh-Wang†
c,
Jiawei
Zhang
 *ab,
Wei
Lu
*ab,
Wei
Lu
 ab,
Xuechang
Zhou
ab,
Xuechang
Zhou
 *c and
Tao
Chen
*c and
Tao
Chen
 *ab
*ab
aKey Laboratory of Marine Materials and Related Technologies, Zhejiang Key Laboratory of Marine Materials and Protective Technologies, Ningbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, Ningbo, 315201, China. E-mail: zhangjiawei@nimte.ac.cn; tao.chen@nimte.ac.cn
bCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, 518055, China
cUniversity of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, China. E-mail: xczhou@szu.edu.cn
First published on 3rd September 2020
Abstract
As one of the most outstanding materials, the analysis of the structure and function of hydrogels has been extensively carried out to tailor and adapt them to various fields of application. The high water content, which is beneficial for plenty of applications in the biomedical setting, prevents the adoption of hydrogels in flexible electronics and sensors in real life applications, because hydrogels lose their excellent properties, including conductivity, transparency, flexibility, etc., upon freezing at sub-zero temperatures. Therefore, depressing the liquid–solid phase transition temperature is a powerful means to expand the application scope of hydrogels, and will benefit the chemical engineering and materials science communities. This review summarizes the recent research progress of anti-freezing hydrogels. At first, approaches for the generation of anti-freezing (hydro)gels are introduced and their anti-freezing mechanisms and performances are briefly discussed. These approaches are either based on addition of salts, alcohols (cryoprotectants and organohydrogels), and ionic liquids (ionogels), modification of the polymer network or a combination of several techniques. Then, a concise overview of applications leveraged by the widened temperature resistance is provided and future research areas and developments are envisaged.
1. Introduction
Unusual structures often bring about extraordinary properties. As a cross-linked three-dimensional polymer network, hydrogels can absorb and retain large amounts of water. This unique feature in conjunction with the exceptional structure has fascinated researchers for decades. For example, although hydrogels contain up to 90% water, they have a specific shape and elasticity, which are properties attributed to solids. The coexistence of toughness and elasticity enables the utilization of hydrogels as mechanical structural materials,1–3 and has great impact for applications in the fields of grippers, valves, etc.4–8 In addition, the large amount of water in hydrogels facilitates preservation and transport of various substances, such as nutrients and waste metabolites, similar to the in vivo counterpart. Therefore, hydrogels are potent candidates for applications in the fields of wound dressing,9–13 tissue regeneration,14–19 and drug delivery.20–25 What is more, hydrogels can also be outfitted with various functionalities, including conductivity, (reversible) stimuli-responsiveness, adhesion, self-healing and so on. These functionalities can be well-integrated into hydrogels for the preparation of strain26–30 and chemical31,32 sensors and hydrogel electrolytes.33–35 Despite the ample fascinating properties, hydrogels inevitably face the obvious disadvantage of poor resistance to low temperatures due to the high water content, resulting in the loss of their functionality at sub-zero temperatures. In order to ensure the stable operation of a device based on a hydrogel at sub-zero temperatures, robust strategies for freezing resistant functional hydrogels are urgently needed.Biological organisms, i.e. cold-blooded animals (ectotherms), insects and plants, face challenges related to freezing of water located in cells at sub-zero temperatures. The generation of ice crystals is often fatal for cells. But after tens of thousands of years of evolution, living organisms have established methods to either mitigate the effect of ice crystallization or to prevent the cell fluid from freezing. For example, peeper frogs (Pseudacris crucifer) and many other frogs can endure subzero temperatures, i.e. 3–5 days at around −3 to −6 °C.36–38 Growth of ice crystals is impeded by accumulation of urea in their tissue, which is typically considered an osmotic defense against bodywater loss.39
Furthermore, the organs are protected against freezing by high concentrations of accumulated glucose (i.e. 280 mM in the liver of wood frogs). Glucose is excreted into the bodily fluids from the liver, where it is synthesized from glycogen by glycogen phosphorylase.40 Another common cryoprotectant in frogs is glycerol. The combination of these cryoprotectants can avoid freezing at low temperatures, while it attenuates the consequences of freezing for the cells. Copious other adaptations to sub-zero temperatures have been developed by other organisms. For example, biomacromolecules, including ice-binding proteins (IBPs), anti-freezing proteins (AFPs), etc., are used to prevent freezing.41–44
Inspired by the freezing resistance of biological organisms, many researchers have recently reported synthesis strategies for biomimetic anti-freezing hydrogels. Based on these recent developments, this review systematically summarizes the synthesis strategies that endow hydrogels with softness and flexibility in cold environments, including the introduction of salts, alcohols and ionic liquids, the modification of polymer networks, etc., briefly introducing the advantages and disadvantages of each strategy and showcasing intriguing applications of the thus obtained gels. In addition, the relationship between the synthesis strategy and the resulting structural characteristics of the hydrogel is discussed. Finally, we propose future development directions of anti-freezing hydrogels. We envisage that this review endows researchers with a readily accessible means to rapidly become acquainted with freezing-resistant gels, relevant synthesis strategies and design concepts for advanced functionality, and expect that the review bestows new inspiration on the audience and drives the research community to expand the scope of applications of hydrogels (Fig. 1).
2. Synthesis strategies for biomimetic anti-freezing hydrogels
Freezing of a solvent is related to its liquid–solid phase transition. Water, the most common liquid for biomedical applications and solvent for hydrogels, features a melting point of 0 °C; however, supercooling via homogeneous nucleation can be observed down to −35 to −38 °C for ultra-pure water by avoiding nuclei as well as external perturbations (heterogeneous nucleation).45 In conventional experiments, nuclei (i.e. defects in surfaces, small particles, etc.) are present and consequently the (ice) nucleation of water starts via a heterogeneous nucleation process.46 Water entrapped in hydrogels deviates from “normal” water as it features, instead of one phase transition, several phase transitions. Some studies have suggested that the water existing in hydrogels is in one of the following three states: “free water”, “weakly bound water” (also termed “intermediate water”) and “unfrozen water”, which feature distinct mobility and freezing temperatures.47–49 Free water has almost no interactions with the polymer networks, which is almost equivalent to freely flowing water outside and will crystallize at about 0 °C. Weakly bound water has little interactions with hydrophilic groups, which will freeze slightly below 0 °C. Finally, some water molecules have very strong interactions with the gel network, the so-called “strongly bound water” or “unfrozen water”, which maintains its mobility and thus its liquid state down −100 °C. Generally, the amount of free water is high compared to the intermediate and the bound water and thus only minute freezing depression by the gel matrix itself is observable. However, by grafting of certain molecules to the network, the freezing resistance can be improved, as detailed in Section 2.3. This provides theoretical guidance for the control of the freezing temperature of hydrogels: by increasing the interaction between water molecules and the polymer network, the amount of free water can be reduced. Alternatively, the composition of free water can be altered to depress its freezing temperature, i.e. by addition of ions or displacement with cryo-resistant liquids. In regard to exchange of water with more cryo-resistant liquids, the exchange rate of the water molecules is related to the state of the water. Free water is exchanged rapidly and intermediate water is exchanged slowly, while it is very difficult to displace the strongly bound water.Similar to copious adaptations developed by living organisms, the freezing point of water and hydrogels can be adjusted (depressed) by several biomimetic strategies. In this section, we introduce the core methods for anti-freezing gels, and their advantages and disadvantages, and state the limitations of these methods.
2.1 Introduction of ions in solvents
The nature of water is yet more complicated in reality than in theory and the facile Blagden's law is only valid for very dilute solutions. It was suggested, i.e. by Tekenaka et al., that at freezing temperature depressions greater than ΔT ≥ 3 °C the concentration of the solute is so high that the experimental freezing temperatures deviate substantially from the theoretical simple relation based on concentration.58 Typically, at higher concentrations than the Debye–Hückel domain, the concentration range where the freeze-point depression is linearly dependent on the solute molality, strong electrolyte solutions (i.e. NaCl, NaBr and LiCl) show an upward curvature, implying either a dissociation of more than 100% of the solute or less bulk solvent available. In fact, the missing bulk water is tied up by hydrating the solute (ions, specifically cations, and also liquid solutes, i.e. ethylene glycol, glycerol, etc.). Therefore, the average number of hydrating water molecules, the so-called hydration number (h), has to be considered, and h × C, where C denotes the concentration of the solute, has to be subtracted from the total number of moles of bulk water (Mw ≈ 55.51 mol L−1).59 The consideration of the bound water yields a linear relationship in the following thermodynamic relationship (eqn (1)) between the freezing point depression and the mole fraction (x) of the solute, for most solutes even up to the eutectic point.60
1/Tf − 1/T0 = −kc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 − x) ln(1 − x) | (1) |
In this equation, Tf and T0 denote the freezing temperature of the solution in the presence of x mol solute and the freezing temperature of the pure solvent, respectively. The slope, kc, is the cryoscopic constant kc = R/ΔHfus. R and ΔHfus denote the universal gas constant and heat of fusion, respectively. The linearity can be ascribed to the maintenance of the hydration number in a broad concentration range. However, at high solute concentrations, often nearly all molecules are tied up as hydration water. For example, HCl with a hydration number of 7 shows linearity up to 6.43 M HCl, at which 81% of the water is “bound” by the solute. As the solution “runs out” of bulk (free) water, a decrease in h is expected due to a dynamic equilibrium. The lower amount of free water at high solute concentrations is also suggested to render the formation of sizable ice nuclei challenging and thus reduces the likelihood of freezing. Finally, it should be noted that the hydrogel network, as discussed earlier, also affects the freezing of water by tightly binding it.
Inspired by the use of salts in anti-icing applications, Vlassak and co-workers have synthesized a series of polyacrylamide-alginate double network hydrogels, and immersed them in different concentrations of CaCl2 solutions.52 The synthesis by soaking is schematically depicted in Fig. 2a, where anions and cations diffuse into the hydrogel, endowing it with freeze-resistance. As shown in Fig. 2b, the concentration of CaCl2 affects the performance of hydrogels at subzero temperatures. Without CaCl2, the hydrogel freezes rapidly at −15 °C, becomes opaque due to the aggregation of ice crystals and easily tears under tensile stress. In contrast, the 30 wt% CaCl2 hydrogel behaves like a regular hydrogel at this low temperature and maintains its stretchability and transparency, while the 10 wt% CaCl2 hydrogel becomes a slurry gel, comprising mixed phases of ice crystals and unfrozen salt solution, at this temperature. Though the slurry gel is opaque, similar to the pristine hydrogel at this temperature, it maintains its stretchability.
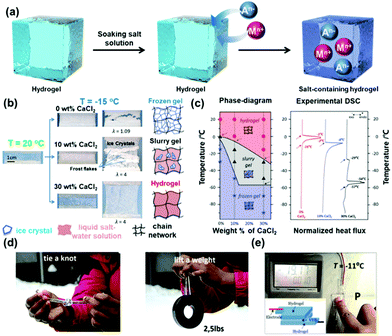 | ||
| Fig. 2 (a) The preparation of anti-freezing hydrogels by soaking in salt solution. An− and Mn+ denote anions and cations, respectively. (b) Freezing and mechanical (strain) resistance of a polyacrylamide-alginate hydrogel with different wt% of added CaCl2 at −15 °C. (c) Phase diagram depicting the unfrozen, slurry gel and fully frozen state of the hydrogel and DSC curves of the hydrogel with different wt% of added CaCl2. (d) Digital photos demonstrate the good stretchability of the gel in snowing conditions. (e) A touch sensor is fabricated by a sandwich structure with two layers of hydrogel and a layer of dielectric elastomer. Reproduced with permission from ref. 52. Copyright 2018 Wiley-VCH. | ||
A systematical DSC study unravelled that the hydrogels with different CaCl2 contents always exhibited a slurry gel region, the location dependent on the CaCl2 content, as shown in Fig. 2c. The slurry gel not only has the highest toughness, which was ascribed to crack pinning, crack deflection and energy dissipation via micro-cavitation, but also maintains conductivity at low temperatures because of free ions. The depicted phase diagram may be employed as a theoretical guideline for the design of anti-freezing hydrogels with controlled freezing resistance. Interestingly, a high salt concentration significantly reduces the nominal stress at room temperature, but has no significant effect on the maximum elongation. Below 0 °C, slurry gels and frozen gels exhibit relatively low fracture toughness. This may be due to the uneven growth of cracks caused by the distribution of ice crystals, facilitating the formation of large cracks at low temperatures. Due to its flexibility (see Fig. 2d) and ion conductivity at subzero temperatures, this gel is an interesting candidate for (touch) sensors.
Similarly, other ions can depress the freezing point of hydrogels. Yao and co-workers have integrated ions (ZnCl2/CaCl2) and glycerol into cellulose hydrogel networks, endowing these hydrogels with great freezing tolerance.53 Here, the cellulose provides plenty of hydroxyl groups for coordinating with metal ions, thereby improving the mechanical properties of the hydrogel, and glycerol or water was added to improve the coagulation rate of cellulose. Furthermore, the salts ZnCl2 and CaCl2 not only promote the dissolution of cellulose, but also endow the hydrogel with anti-freezing ability, as shown in Fig. 3a. Gel-water can be cooled to as low as −70 °C without indication of freezing and addition of glycerol can extend the minimum freezing temperature to −100 °C. Similarly, the presence of free ions enables conductivity of the hydrogel at sub-zero temperatures, affording adoption of this hydrogel as a conductive material or sensor even at low temperatures.
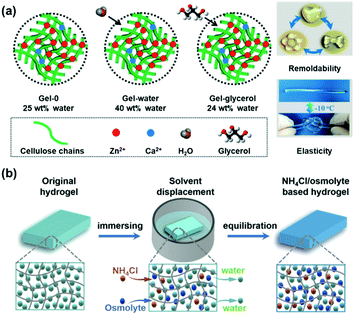 | ||
| Fig. 3 (a) Components of three cellulose hydrogels (gel-0, gel-water and gel-glycerol) and reversible remoldability and elasticity at low temperatures of cellulose gel-glycerol enabled by addition of ZnCl2 and CaCl2. Reproduced with permission from ref. 53. Copyright 2019 Wiley-VCH. (b) The preparation process of the NH4Cl/osmolyte-based hydrogel via solvent displacement. Reproduced with permission from ref. 54. Copyright 2019 Wiley-VCH. | ||
Besides inorganic salts, amphoteric organic ions or ionic liquids can also improve the freezing resistance of (hydro-)gels. Zhang and co-workers reported a one-pot solvent displacement method to replace water with zwitterionic osmolytes54 (Fig. 3b). For example, betaine or proline demonstrated a high effectivity to depress the freezing point of water with constants of about 10.9 and 9.3 °C kg mol−1, respectively. The hydrogel outfitted with zwitterionic osmolytes remained flexible (twistable and stretchable) in extreme cold environments (i.e. −40 °C). In addition, the gel is endowed with excellent electrical conductivity due to the mobility of the zwitterionic osmolytes. The combination of these intriguing properties opens the door for application as flexible electric devices.
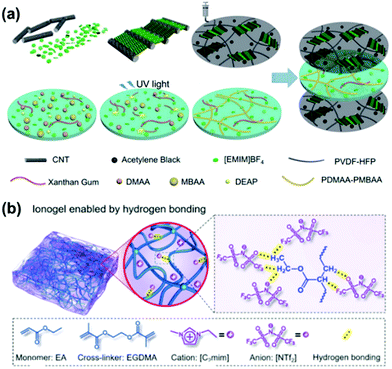 | ||
| Fig. 4 (a) Preparation method of carbon nanotube and ionogel-based supercapacitors, carbon nanotubes and an ionic liquid are mixed into the gel to prepare the ionogel. Reproduced with permission from ref. 63. Copyright 2019 Royal Society of Chemistry. (b) Schematic illustration of the hydrogen bonding between [NTf2] anions and the PEA matrix. Reproduced with permission from ref. 64. Copyright 2020 Royal Society of Chemistry. | ||
Wang and co-workers obtained an ionogel by free radical polymerization based on the precursors (N,N′-methylenebisacrylamide (MBAA, cross-linker), N,N-dimethylacrylamide (DMAA, monomer) and 2,2-diethoxyacetophenone (DEAP, photo-initiator)) in EMIMBF4 (1-ethyl-3-methylimidazolium tetrafluoroborate, ionic liquid).63 The ionogel has excellent elasticity and fatigue resistance upon repeated mechanical stresses. After 300 cycles of tensile testing (60% tensile strain), the appearance and mechanical performance of the ionogel are maintained. The ionic liquids endowed the ionogel with excellent electric conductivity and freezing resistance, while it maintains a reasonable capacitance performance down to −40 °C.
Liu et al. recently prepared a poly(ethyl acrylate)-based elastomer using an ethyl acrylate (EA) monomer and ethylene glycol dimethacrylate (EGDMA) crosslinker, which swelled with the ionic liquid [C2mim][NTf2], resulting in an ionogel.64 Amongst others, the as-prepared ionogel features excellent transparency, ionic conductivity and stretchability. Due to the non-volatility and chemical stability of ionic liquids, ionogels maintain their functionality in extreme environments, including a vacuum, high humidity and in a wide temperature range (here 100 °C and −70 °C). In addition, the gel is equipped with good adhesion – the ionogel could be attached to a metal plate and, after being stored in air for one year, the appearance of the ionogel was preserved and no chemical corrosion of the metal was detected. Due to the strong interaction between the ionic liquid [C2mim][NTf2] and the PEA-based elastomer, this hydrophobic ionic gel exhibits ultrahigh stretchability (up to 5000%) and super-toughness (4.7 kJ m−2). It should be noted that the polymer network type and the ionic liquid type (and mixtures) can be changed as needed. Therefore, this method can be universally applied for the preparation of various functional anti-freezing gels, including luminescent, stimuli-responsive and self-healing materials, as well as for various temperature regimes. Ionogels have the advantages of both ionic liquids and hydrogels, and great progress has been made in the development of ionogel-based soft robots, flexible wearable electronics and energy storage devices.
2.2 Freeze point depression by alcohols (as a solute)
The addition of alcohol to water depresses the freezing point of the resulting solution (Fig. 5). Similar to the mechanism of freeze point depression induced by salts as a solute, alcohols and other solvents induce a freeze point depression. Water/alcohol solutions reduce the freezing temperature by several means. First, the chemical potential of the solution is reduced due to the entropic effect discussed before. Second, the alcohols bind similarly to ions to water molecules and thus reduce the number of “free water” molecules in the solution, i.e. ethylene glycol: h = 1.8 and glycerol: h = 2.59 Third, the hydrophobic residues of the alcohols aggregate, thereby disturbing the three-dimensional H-bond network severely. Finally, nano-segregation into alcohol-rich and water-rich regions commences.69 At low concentrations of alcohol in the solution a percolated network of H-bonded water molecules exists. This percolated system is disturbed by addition of alcohol in the solution and nanodomains of aggregated alcohol. Near the eutectic point of the glycerol/water mixture both water and glycerol are percolated. In this case, it is suggested that water molecules are unable to form an extended water network, and water is suggested to be protected (from freezing) due to an extensive (high contact area between glycerol, which is disturbing the water network, and water) and encapsulating glycerol interface.70,71 In regard to organohydrogels, also the gel matrix affects the freezing of the incorporated solution by tightly binding molecules from the solution (see before: bound water). Still, sufficient evidence of a main mechanism of action is lacking and researchers are debating the exact mechanism of anti-freezing liquids in solution. In the literature related to anti-freezing gels it is therefore often suggested that hydrogen bonding between hydroxyl groups and water molecules reduces the interactions between water molecules (hydrogen bonds), thus preventing the formation of ice crystals (Fig. 6).72–83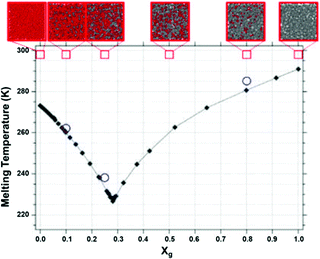 | ||
| Fig. 5 The equilibrium melting temperature of aqueous glycerol as a function of glycerol mole fraction (Xg). Red squares show the concentration of the glycerol–water mixture. Reproduced with permission from ref. 71. Copyright 2020 American Chemical Society. | ||
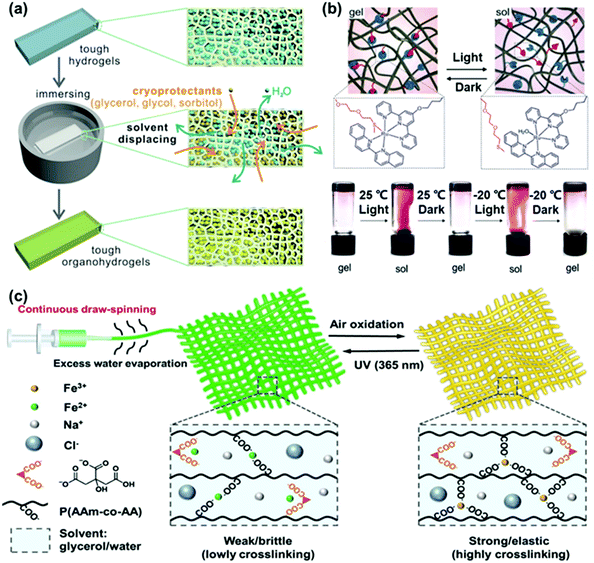 | ||
| Fig. 6 (a) The preparation method of tough organohydrogels by solvent displacement with cryoprotectants. Reproduced with permission from ref. 72. Copyright 2018 Wiley-VCH. (b) Mechanism of the photo-responsive organohydrogel and photographs of the light-triggered sol–gel transition at 25 °C and −20 °C. Reproduced with permission from ref. 74. Copyright 2020 Wiley-VCH. (c) Schematic illustration of the fabrication method of a robust and conductive P(AAm-co-PAA)/Fe(III) hydrogel microfiber net via draw-spinning and the mechanism of tuning the mechanical properties of the hydrogel microfiber net through reversible redox reactions. Reproduced with permission from ref. 75. Copyright 2020 Wiley-VCH. | ||
Strictly speaking, the gels discussed in this section are water–organic liquid hybrids, rather than a pure hydrogel. In the literature these gels are often termed organohydrogels. A very simple method to obtain anti-freezing (organo-)hydrogels was introduced by Zhou and co-workers, who employed cryoprotectants as anti-freezing agents. A Ca-alginate/polyacrylamide hydrogel was facilely immersed in glycerol, glycol, sorbitol or mixtures of these. During immersion, the water was displaced by the organic solvent.72 For example, after solvent-displacement, the organohydrogels can remain soft even at −45 °C. It is noteworthy that the anti-freezing performance can be tuned by the immersion time, varying the ratio of solvents or choosing other cryoprotectants. Benefitting from the low vapor pressure of cryoprotectants, the organohydrogels can be stored in dry air or even in a vacuum for a long time without losing their mass (water or cryoprotectant). This approach is further promoted by the fact that many different solvents can be integrated via the solvent displacement technique, and this technique can also be transferred to other solutes (aqueous salt solutions and ionic liquids). The interaction between alcohol molecules and polymer networks is slightly higher than that of water molecules. Therefore, the mechanical properties of the water–alcohol hybrid gel are slightly better than those of the pure hydrogel.
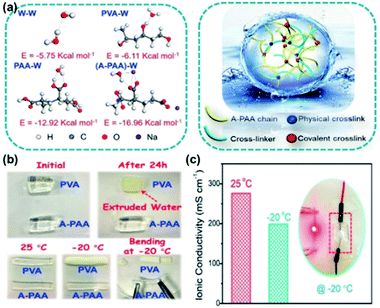 | ||
| Fig. 7 (a) Molecular model for simulating interactions between water molecules and different terminal groups. (b) Digital photos showing the comparison of water retention and anti-freezing properties of KOH-filled A-PAA and pure PVA hydrogels. (c) Ionic conductivity of the KOH-filled A-PAA hydrogel at 25 °C and −20 °C. Reproduced with permission from ref. 84. Copyright 2020 Wiley-VCH. | ||
In contrast to this post-processing (soaking) technique, the organic solvent or solvent mixtures can be added prior to gelation of the gel. For example, Wang et al. prepared an organohydrogel by gelation of poly vinyl alcohol (PVA) in a binary (glycerol/water) solvent, endowing the obtained gel with enhanced mechanical properties, such as tensile strength, elongation and great freezing tolerance.73 The PVA–glycerol hydrogel will not completely freeze even at −78.5 °C, and still maintains a certain elasticity.
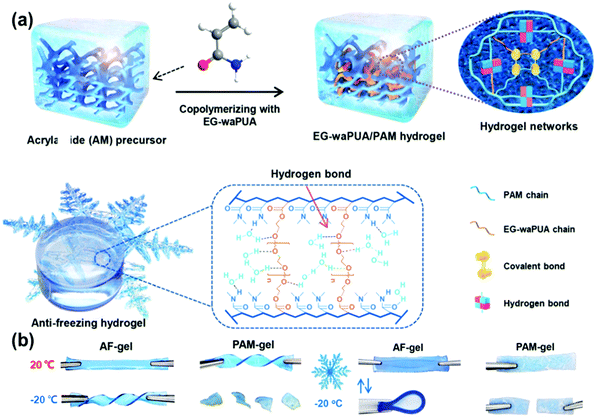 | ||
| Fig. 8 (a) Interactions of covalent bonds and hydrogen bonds in the EG-waPUA/PAM hydrogel. (b) Digital photos of the AF-gel and PAM-gel at −20 °C under twisting, bending and compression. Reproduced with permission from ref. 85. Copyright 2019 Royal Society of Chemistry. | ||
Yet, due to their certain chemical inertness and low freezing temperature, polyol cryoprotectants, such as glycerol, endow hydrogels with excellent anti-freezing properties while not affecting the functional components inside the hydrogel. For example, Wu and co-workers have reported metallopolymer organohydrogels that are photo-responsive even at −20 °C.74 The addition of an organic solvent instead of an ion into the photo-responsive hydrogels is advantageous as the organic solvent does not compete for coordination with the photosensitive group (Ru-thioether), while salts (ions) would compete with it. Upon light irradiation the Ru-coordination bonds will collapse, while they would re-form when the light is turned off. The crosslinking densities can be controlled by such reversible ligand coordination, leading to the change of the network structure and properties, further used for sol–gel transition, self-healing of surface damage and information storage and encryption. With the help of glycerol, the application of these functions can be extended to −20 °C while almost maintaining the performance of the gel at room temperature.
Hydrogel-based fiber nets often lack mechanical and long-term stability. By introducing organic solvents, the properties of (organohydro)gels can be tuned to the envisaged application. For example, Wu and co-workers made use of iron-citrate redox chemistry-based hydrogel microfibers and robust fiber nets.75 The process of oxidation from Fe2+ to Fe3+ is like a curing process, which enhances the mechanical properties of the gel after spinning. Glycerol was introduced into the gel not only to enhance the spinnability, but also to improve the freezing tolerance of the hydrogel microfibers. The hydrogel microfibers maintained their great elasticity even at subzero temperatures and will not freeze until −61 °C. In particular, because of the redox components and movable ions, hydrogel microfibers are sensitive to UV light and humidity. When switching between 90% and 11% humidity, the change of the resistance ratio of hydrogel microfibers can be as high as 50%, which signifies their great potential as a humidity sensor.
2.3 Modification of the polymer network
Although alcohol–water mixtures, ionic liquids or other solutes (ions) in the solvents are very effective for the construction of freezing resistant (hydro-)gels, the disadvantages of simple physical mixing are also obvious: the solutes may escape the (hydro-)gel network, which affects the freezing tolerance of the hydrogel. To solve this issue, “solutes” may be immobilized in the hydrogel matrix. Mikhalovsky et al. have shown that the unfrozen water content was mainly affected by the chemical structure of the network, because the unfrozen water comprises mainly water molecules that are tightly bound to the polymer network.48 For polymer networks with similar main chains but different side chains, i.e. more hydrophilic or hydrophobic, the freezing resistance of the water molecules is different. This is because water molecules form strong hydrogen bonds with hydrophilic groups (strong interactions, strong freezing point depression), and weaker hydrogen bonds with hydrophobic groups (weak interactions). It has to be noted that similar considerations for the case of ions and organic solutes should be taken into account. The addition of anchored solutes is energetically favorable and reduces the chemical potential by increasing the entropy of the system. Moreover, the grafted molecules will bind some water as hydration water, typically 1–6 for smaller hydrophilic small organic molecules.60 Furthermore, the grafted residues and the bound water may disrupt the structure of water, thus impeding the generation of ice nuclei,69 resulting in a sub-zero freezing temperature of the hydrogel if a particular group on the polymer chain has a strong interaction with water molecules (energetically more favorable, far greater than the interactions between water molecules).Chen and co-workers published density functional theory calculation results which revealed that the interaction energy between polarized terminal groups and water molecules is much greater than that between water molecules, and such a strong interaction may be able to disrupt the formation of ice crystals (Fig. 7).84 Based on this theory, the authors synthesized an alkalified polyacrylic acid (A-PAA) hydrogel by free-radical polymerization, equipped with copious carboxyl groups on the polymer chain. The experimental results show that the freezing point of the A-PAA hydrogel containing 10% KOH solution is −25 °C. One could assume that this freezing point depression is purely related to the concentration of the solute. Yet, the freezing point of a PVA hydrogel containing the same content of KOH is only −13 °C, indicating that the polarized terminal groups further depress the freezing temperature of the hydrogel. In light of the superior conductivity and strong low-temperature tolerance of the A-PAA hydrogel, the hydrogel was employed for the assembly of a zinc–air battery that can be utilized at subzero temperatures. Still, a drop of bitterness is the fact that ions from the A-PAA hydrogel may diffuse out (i.e. K+ and OH−) upon contact with other solvents.
In contrast, Zhi and co-workers utilized the interactions between hydroxyl groups and water molecules to depress the freezing temperature of gels, thus circumventing this issue. To achieve this goal, they synthesized a series of ethylene glycol-based waterborne anionic polyurethane acrylates (EG-waPUA) (Fig. 8).85 The hydroxyl group of EG is anchored to the polymer network by chemical bonds, and the strong hydrogen bonding interactions between EG, water molecules and the PAAm matrix hinder the formation of ice crystals. Using a zinc-containing EG-waPUA hydrogel as an electrolyte, the ionic conductivity exhibited great stability over a wide temperature range as it only decreased by 10% at −20 °C compared to that at room temperature. Moreover, this hydrogel has excellent mechanical properties and can adapt to various mechanical deformations, including bending and compression. The combination of freezing tolerance and excellent mechanical properties means that this gel has great potential in elastic and flexible batteries and wearable electronic devices in cold environments. As the polar groups are chemically bound to the hydrogel network, the polar groups are unlikely to diffuse out, thus solving the disadvantage of common solute induced freeze temperature depression systems.
2.4 Other methods for preparing anti-freezing hydrogels
Instead of purely relying on the capacity of a solute to depress the freezing temperature, Wang and co-workers prepared a crystal-type composite gel with an anti-freezing property by a dissolution–recrystallization method.86 They first dissolved excess sodium acetate (NaAc) in a pre-gel solution at high temperature to obtain a supersaturated solution. After the polymerization was completed, the supersaturated salt was induced to crystallize rapidly, forming a crystal-type composite gel. The solubility of NaAc is highly dependent on temperature, as shown in Fig. 9a. Upon an increase of temperature, the NaAc crystals in the gel dissolve while the dissolution process absorbs heat, which is equivalent to slowing down the temperature increase. Upon a decline of the external temperature, the crystallization of NaAc has a heating effect. This means that the composite gel can be used normally at low or high temperatures, as it benefits from its dissolution–crystallization change in (heat) energy. The NaAc crystal containing hydrogel exhibits a greater modulus (up to 474.24 MPa) and higher operating voltage (2.0 V) than the pure hydrogel (without crystals), and these properties are conducive to the preparation of a stable and safe conductive material. Therefore, supercapacitors using this gel as an electrolyte can work in extreme environments (−40 °C to 80 °C).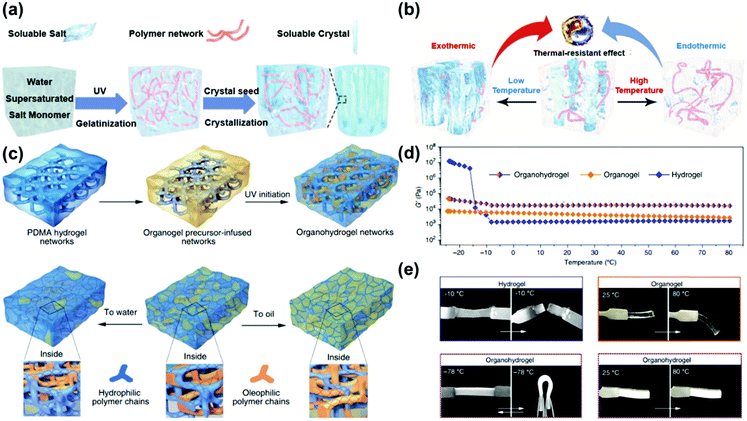 | ||
| Fig. 9 (a) Synthesis of a NaAC supersaturated crystal-type composite gel. (b) Schematic illustrating the temperature resistance of the crystal-type composite gel. Reproduced with permission from ref. 86. Copyright 2019 Wiley-VCH. (c) The preparation of an organohydrogel with an interpenetrating network and solvent adsorption in different solvents. (d) Storage modulus of the hydrogel, organogel and organohydrogel during a temperature sweep in the range of −20 °C to 80 °C. (e) Hydrogels will break, while organogels can be bent at low temperatures; organogels become flexible at 80 °C, while organohydrogels remain rigid at 80 °C. Reproduced with permission from ref. 87. Copyright 2017 Nature Publishing Group. | ||
In Section 2.3, we introduced a method to reduce the freezing point by increasing the interaction between water molecules and the (hydrophilic) hydrogel network. Similarly, if water molecules are dispersed in small areas by physical methods and their free movement is restricted, the aggregation of small ice crystals can be hindered. This is commonly regarded as confinement. However, in typical confinement the amount of water in the gel is very limited. In this regard, Liu and co-workers designed an organohydrogel obtained by in situ polymerization of an oleophilic polymer within a crosslinked hydrophilic matrix swollen with amphiphilic solvents.87 (Fig. 9c) Traditional hydrogels swell in water and shrink in organic solvents, but this organohydrogel can swell in both aqueous solutions and organic solvents. The results of DMA scanning show that the modulus of the organohydrogel at −30 °C will only slightly increase, signifying the excellent anti-freezing performance. Above 0 °C, the modulus of organogels is higher than that of pure hydrogels, which may be attributed to the interpenetrating hydrophilic/oleophilic network. The authors observed by polarizing microscopy that ordinary hydrogels have large and continuous ice crystal regions at low temperatures, but they are not observed in organohydrogels. The authors believe that this is due to the heterogeneous structure of the hydrophilic/oleophilic networks strongly inhibiting the further aggregation of tiny ice crystals, so that the organohydrogel maintains good elasticity.
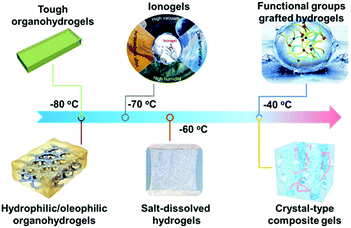 | ||
| Fig. 10 Freezing resistance performance of anti-freezing hydrogels enabled by different synthesis strategies. | ||
2.5 Discussion of different synthesis strategies
Obviously, the strategy employed for obtaining freezing resistance influences the properties of the resulting gels and thus determines the range of applications (Fig. 10). Therefore, we summarize the advantages and disadvantages of the strategies to facilitate the choice of the appropriate strategy and give reasonable guidelines on adequate applications based on the design principle of the gel. Introduction of a solute into hydrogels leads to a pronounced freezing temperature depression, which is dependent on the solute. For example, by addition of a salt, the hydrogel remained usable down to a temperature of −60 °C,52 while the temperature range was extended to −70 °C and −80 °C for ionogels and organohydrogels, respectively. Yet, salt addition typically affords only moderate freezing resistances to around −20 °C in hydrogels. However, salt addition features several advantages. First, soluble salts can be added either before gelation or after gelation via solvent exchange (soaking, post-processing), which renders this technique rather simple and versatile. Second, benefitting from the addition of movable ions, hydrogels obtain good electric conductivity, which allows them to be employed as flexible electronic devices. Third, most salts are considered to be biocompatible, enabling the application of these hydrogels for close contact with human skin (e-skin, etc.). However, these freeze resistant hydrogels inevitably suffer from evaporation of water under arid conditions, which leads to an increase in ion concentration, instability of the gel's conductivity and a loss of functionality (and also a loss of flexibility) in long-term applications due to desiccation. Granted, the evaporation of water can be impeded by coating the hydrogel with an elastomer which features high barrier properties toward water vapor diffusion.88,89 Yet, this complicates the synthesis procedure and alters the mechanical properties substantially. Long-term stable gels can be generated by the introduction of ionic liquids or organic solvents as a solute.The introduction of water-miscible alcohols (organic liquids) has the advantages of easy synthesis and ready transfer of this approach to copious hydrogels. Furthermore, the organic liquids improve not only the freezing resistance but also the evaporation resistance due to low vapor pressure.81,94 Yet, water–alcohol mixed solutions usually exhibit low conductivity due to the low number of movable ions, as alcohols are usually uncharged and possess certain chemical inertness. It is important to note that this issue can be overcome by the addition of ions or usage of an aqueous salt solution mixed with an alcohol,75,83,100 nano/micro particles endowed with conductivity, such as graphene sheets,76 carbon nanotubes,99etc. or the utilization of conductive polymers77,80,82 for the preparation of the hydrogel network.
Similar to organo(hydro)gels, ionogels feature high temperature resistance (low and high temperature). Furthermore, ionogels offer good conductivity which is rather stable over a wide temperature regime. Yet, due to their inherent toxicity and the fact that the used ionic liquids are miscible with water, they cannot be employed in applications where contact with living organisms or the environment cannot be excluded. In this context, one common issue of anti-freezing gels made via addition of movable solute molecules (salts, alcohols and ionic liquids) is the tendency of them to diffuse out of the gel when in contact with water-rich solvents, which would degrade the functionality of the gel and in some cases also strain the environment (i.e. ionogels). This diffusion is related to the concentration gradient of the solute between the environment and the gel, and might be avoided by diffusion barriers similar to the elastomers stated before. Another common issue of freezing resistant gels is the fact that addition of the solute may change the mechanical properties (either detrimental or favorable) via cross-linking (ions) and so on.
An intriguing strategy to overcome the diffusion of the “solute” (cryoprotectant) was recently reported, which is based on grafting functional components to the polymer network. Though only moderate freezing resistance was obtained via this technique, i.e. −20 °C for a 24 wt% EG-waPUA gel, this is a sustainable approach for realizing long-term stable devices. In this regard, the resistance towards drying and possibilities to improve the freezing resistance should be investigated. Notably, an increase in the grafting density of the anti-freezing component (solute) improves the anti-freezing ability, while degrading the mechanical performance of the gel. Therefore, a balance between freezing resistance and mechanical performance needs to be established. As this technique is still in its infancy, only a few functional groups with good freeze resistance performance are known thus far, which limits the current applicability. Finally, the grafting of the functional groups necessitates fixing the functional groups during or after synthesis via coupling chemistry, rendering the synthesis more complicated than anti-freezing gels with a mobile solute.
3. Promising applications of anti-freezing hydrogels
3.1 Biomimetic actuators and soft robotics
An actuator is a device that transforms various external stimuli into deformation. Benefiting from the great biocompatibility, hydrogel actuators have attracted ample attention in the fields of biomimetic grippers and artificial muscles.90–92 However, freezing at sub-zero temperatures limits their widespread employment. Here, solutes introduced into gels can endow them with a certain freezing resistance.Sun and co-workers have demonstrated a novel method to construct an ultra-tough, self-healing glycerol-hydrogel by introducing functionalized boron nitride nanosheets (f-BNNSs) into a poly(acrylamide-co-maleic anhydride) hydrogel.93 The introduction of such nanosheets can not only enhance the mechanical properties of the gel, but also gives rise to self-healing ability of the gel. After polymerization, the solvent was displaced by a glycerol–water mixture. With the help of glycerol, the gel showed excellent environmental resistance (minimum −45 °C), while the hydrogel part presumably does not have high temperature resistance. Taking advantage of the different dehydration resistances of the P(AM-co-MAH)/f-BNNS hydrogel and P(AM-co-MAH)/f-BNNS glycerol-hydrogel, bilayer hybrid-based versatile actuators could be assembled. When placed in a dry environment, the hydrogel layer will lose water rapidly and shrink, while the content of the solvent in the organohydrogel layer remains virtually unaltered, resulting in complex deformation of the entire assembly. Importantly, the deformation is reversed by addition of water. Fig. 11b demonstrates the application of the hydrogel-based actuator as a gripper, which was employed to move an object. The gripping action was actuated by changes in the humidity (and temperature) of the environment. Similarly, the deformation of the gel upon humidity was employed as an actuator.
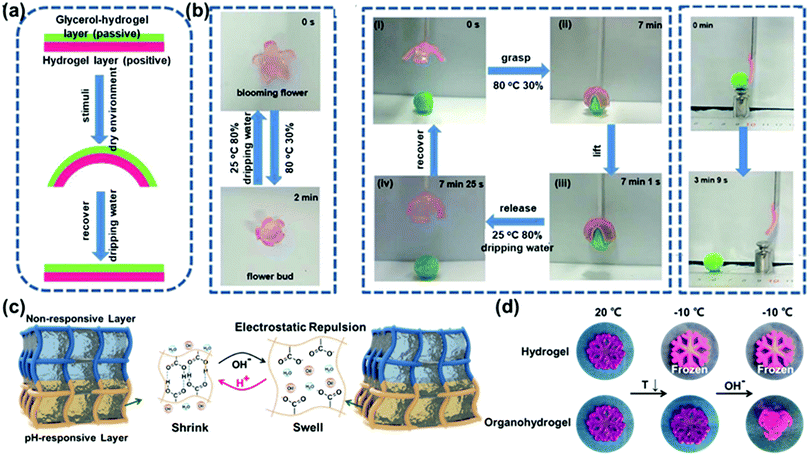 | ||
| Fig. 11 (a) Schematic of the deformation principle of the actuator based on the hydrogel/organohydrogel bilayer. (b) Application of the bilayer gel as a flower bud, gripper and actuator. Reproduced with permission from ref. 93. Copyright 2019 Royal Society of Chemistry. (c) Schematic of the deformation principle of the PAAm/PAA bilayer hydrogel. (d) Comparison of the deformation of the hydrogel and organohydrogel snowflake at low temperature. Reproduced with permission from ref. 94. Copyright 2019 American Association for the Advancement of Science. | ||
In contrast to the bilayer gel discussed before without (or with low) freezing resistance (one layer), in our group a bilayer organohydrogel, comprising a pH responsive polyacrylic acid (PAA) gel and a non-responsive polyacrylamide (PAAm) layer, was endowed with freezing resistance due to employment of a mixed solvent (water/glycerol) and its application as an actuator was investigated.94 The deprotonation of PAA in alkaline solution enables the formation of COO− and swelling of it. Therefore, the bilayer organohydrogels bend toward the PAAm layer (the PAAm layer is located at the concave side). Importantly, the bending is reversible. In acidic solution, the bilayer reverts to its original shape due to protonation of the COO− moieties. Here, the introduction of glycerol protects the gel from freezing, enabling implementation of the organohydrogel actuator down to temperatures of −20 °C, as shown in Fig. 11d. Through pre-designing the shape of the organohydrogel, a weight-lifting robot, an artificial valve and a robotic arm have been constructed, which provide new ideas for the development of soft robots that function in extremely cold environments. The pH-sensitive actuator can be used for the transportation of objects and to detect pH changes in an aqueous environment. Moreover, KI was introduced into the organohydrogel, providing the organohydrogel with conductivity and granting it potential for wearable electronic sensors.
Yang et al. synthesized a hydro/organo Janus gel actuator through direct one-step interfacial polymerization.95 For the synthesis, the hydrophilic monomer poly(ethylene glycol) methyl ether methacrylate (PEGMA) and the hydrophobic monomer polypropylene glycol monobutyl ether acrylate (PPGMA) were dissolved in water and low polarity organic solvents, respectively. The two liquids separated into two layers in the container. After the polymerization, a monolithic asymmetric gel was prepared. Due to the different hydrophilicity of the two sides of the gel, their swelling ability with solvents of different polarities varies greatly and this can be exploited for actuation. In polar solvents (such as water), the hydrophilic side swells significantly, causing the entire gel actuator to bend toward the hydrophobic side. Interestingly, the Janus gel actuator maintains good elasticity and swelling capacity at −20 °C. Due to the low water content, the hydrophobic side exhibits a good anti-freezing performance while the hydrophilic part also shows a surprising freezing tolerance. This is because the hydrogen bonds between water molecules and PEGMA anchor the water molecules firmly in the polymer network (compare Section 2.3), effectively preventing the formation of ice crystals. The Janus actuator designed by the authors was used as an indicator with excellent sensitivity for solution polarity, which has rarely been reported before.
3.2 Chemical or physical sensors
Hydrogels are promising materials for sensing applications, especially in the field of biosensors due to their excellent biocompatibility. Hydrogel-based sensors detect events by changes of the swelling properties in response to environmental changes, including physical changes, such as temperature, pressure, force and electrical fields, and chemical changes, such as ionic strength and pH, and biological responses. As the stimuli-responsive unit can be tailored according to researchers’ needs, this enables detection of specific parameters with high sensitivity. Therefore, in the past decade, hydrogels have been widely used in the fields of detection and diagnosis. Furthermore, some nano-particles, such as graphene and carbon nanotubes, can be introduced to prepare nanocomposite hydrogels. Compared with nanocomposite elastomers, nanocomposite hydrogels contain a lot of water, similar to biological tissue, and can provide a channel for material transmission and detect changes in liquid environments. However, the application of hydrogel sensors is limited to temperatures at or near room temperature due to freezing at sub-zero temperatures.96–98 To fully access the potential of hydrogel-based sensors, the working temperature range needs to be expanded.To advance this field, Lu and co-workers developed an adhesive and conductive hydrogel with extreme temperature tolerance.99 In the synthesis process, polydopamine-grafted carbon nanotubes were mixed into the pre-gel solution to endow the hydrogel (PAM-PAA) with great conductivity and adhesion. A glycerol/water mixture was applied as a solvent to provide extreme temperature tolerance (−20 to 60 °C) and water-lock in capacity. The excellent adhesion and conductivity of this gel function jointly in gel-based electrodes that can be directly attached to human skin and feed back electrical signals of human movements or breathing via a strain sensor mechanism. As shown in Fig. 12b, due to the anti-freezing and water retention properties provided by glycerol, the gel-based sensor can be directly exposed to dry air and work in cold environments.
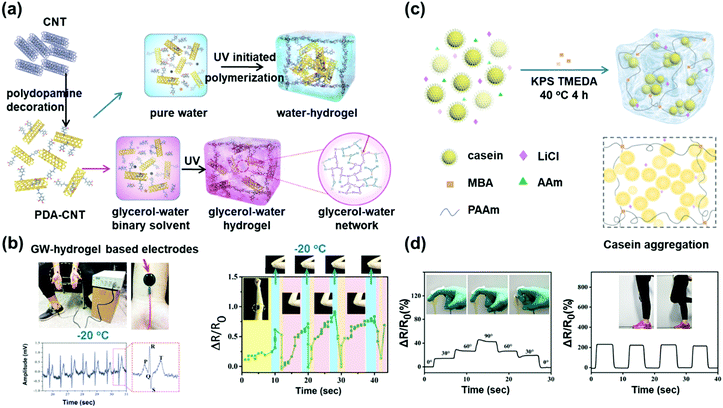 | ||
| Fig. 12 (a) Preparation method of the anti-freezing gel containing dopamine grafted carbon nanotubes based on a water–glycerol binary solvent. (b) Electrical signals of the GW-hydrogel-based sensor for the detection of the pulse and bending of a joint at −20 °C. Reproduced with permission from ref. 99. Copyright 2017 Wiley-VCH. (c) Preparation method of the PAAm/casein hydrogels. (d) The real-time detection of human motions. The hydrogel sensors were directly attached to the finger and knee. Reproduced with permission from ref. 100. Copyright 2019 Royal Society of Chemistry. | ||
In contrast to the addition of modified nanotubes and introduction of an organic solvent, addition of ions to the hydrogel not only affords good anti-freezing performance, but also electrical conductivity. Gao and co-workers have developed a wearable strain sensor on the basis of addition of casein and LiCl into a traditional PAAm hydrogel.100 Casein is a hydrophobic macromolecule and the interpenetrating network formed by casein and PAAm polymer chains enhances its mechanical properties. Furthermore, casein interacts with various substrates because of its active groups, endowing the hydrogel with excellent adhesion performance. LiCl provides the hydrogel with good electrical conductivity and freezing resistance down to −21 °C. Based on these functions, the authors successfully designed a freezing resistant sensor that can adhere to the human body and monitor speaking or joint motion (Fig. 12d). In the context of freezing resistant hydrogels, it is important to point out that this research field is still young and progress from simple strain sensors (mechanical deformation sensors) to more elaborate sensors for sensing gases, small molecules and so on is anticipated to be established in the foreseeable future.
3.3 Batteries or electrolytes
With the vigorous development of wearable electronic devices, portable, deformable and stable energy supply equipment is urgently needed. In this regard, hydrogels exhibit the favorable characteristics of adjustable mechanical properties, environmental friendliness, easy modifiability and so on. Conductive hydrogels containing a large amount of electrolyte solution can carry out rapid and effective electrochemical reactions, and have been widely employed in foldable supercapacitors or batteries.101–103 However, at low temperatures, ordinary hydrogel-based batteries will inevitably freeze, losing elasticity and ion migration. Therefore, it is of great significance to develop aqueous batteries that can be used at low temperatures. To tackle the aforementioned issue, Zhu and co-workers introduced high concentrations of ZnSO4 and LiCl in a conventional PAAm hydrogel (Fig. 13).104 Highly hydrated cations not only endow the gel with anti-freezing ability, but also improve the reversibility during cycling of the zinc anode in the hydrogel.Experimental results showed that batteries using this zinc-lithium PAAm (ZL-PAAm) hydrogel as the electrolyte achieved a capacity retention of 98% upon cooling from room temperature to −20 °C. Moreover, a capacity retention of nearly 100% after 500 cycles at −20 °C with a Coulombic efficiency of >99.5% was achieved, thus solving the limitation of aqueous hydrogel-based electrolytes for batteries at low temperatures.
3.4 Other applications
Freezing resistant hydrogels are not limited to soft electronics, soft robotics, soft sensors and soft energy storage devices, but may find applications in various other fields, such as oil/water separation, wound dressings and so on due to their exquisite biocompatibility and environmental compatibility.For example, He and co-workers synthesized a superhydrophilic and underwater superoleophobic anti-freezing (3-aminopropyl) triethoxysilane-functionalized silica/polyvinyl alcohol (APTES-SiO2/PVA) organohydrogel.105 An ethylene glycol/water binary solvent was selected as an anti-freezing agent while APTES–SiO2 nanoparticles were incorporated into the gel network to construct a tough surface. After coating the gel on a stainless-steel mesh, an oil/water separation mesh was obtained. The introduction of ethylene glycol (EG) is crucial to achieve oil/water separation in various complex environments, such as subzero temperature and seawater. Experimental results show that for oily wastewater, the separation efficiency of the organohydrogel-coated mesh at −20 °C (98.8%) is almost the same as that at room temperature (99.5%), while the hydrogel-coated mesh lost the ability to separate water and oil after storing at −10 °C (Fig. 14).
Traditionally, as-made hydrogels have been widely employed for wound dressings and drug delivery systems as they feature great shape adaptability, biocompatibility and the ability to deliver drugs and nutrients, while they can be endowed with self-adhesion and so on. Wound dressing materials need to be used in a variety of unusual conditions. In this regard, it is necessary to develop extreme temperature-resistant hydrogel dressings to avoid delay of wound treatment. Fei and co-workers reported a one-pot method to synthesize a freezing and drying resistant antibacterial hydrogel dressing.106 Polyvinyl pyrrolidone (PVP), acrylamide (AM), 1-vinyl-3-butylimidazolium (VBIMBr), polyethylene glycol dimethacrylate (PEGDA), AIBN, and water were mixed and polymerization was completed at 70 °C. The positively charged VBIMBr segments were uniformly dispersed in the polymer network. The uniformly distributed cations effectively prevent water molecules or small ice crystals from forming large-scale crystals, as detailed in Section 3.3. The hydrogel dressing demonstrates excellent tissue adhesiveness, can adhere to finger skin and deforms following the bending of the finger. More importantly, the authors proved the bactericidal activity of the dressing, which is related to the electrostatic action of the poly(ionic liquid) and the polar groups of VBIMBr, affording rupturing of the bacterial cell membrane, eventually killing the bacteria. Furthermore, wound closure and histopathological experiments show that the gel promotes the wound healing effectively. Therefore, this hydrogel-based wound dressing is anticipated to exhibit great potential in clinical applications.
In addition to being able to directly sense external stimuli, human skin can also convert external stimuli into neurons and retain them, even if the stimuli disappear. Generally, bionic electronic skin is unable to remember external stimuli. To tackle this issue, Wu and co-workers introduced a composite gel comprising polydopamine-functionalized gold nanoparticles (Au@PDA), polyacrylamide (PAAm) and a glycerol/water mixture as a solvent.107 Due to the strong interaction between glycerol and water, the gel can maintain good stretching and self-healing ability at −15 °C. Most importantly, a resistance memory function has been realized, which is based on the negative differential resistance (NDR) of the gel and trap-enhanced tunneling. Moreover, the authors showed that this effect can be employed for the generation of a resistance memory device based on this anti-freezing gel, by which a write–read–erase cycle could be demonstrated.
3.5 Correlation of the synthesis strategy and performance in a specific application
Different synthesis strategies will lead to different structural characteristics, affecting the performance in specific applications. Therefore, we briefly give guidelines on adequate applications based on the design and synthesis principle of the gel. In Fig. 15, a preliminary connection between different synthesis strategies and corresponding preferable applications is established. Based on the intrinsic ion conductivity of freezing resistant gels made by salt addition, they are good candidates for flexible conductors, electrodes and sensors. Since evaporation of water causes changes in ion concentration and instability of conductivity, anti-freezing hydrogels containing large amounts of salts are not suitable for batteries or supercapacitors that require high accuracy and long-term stability. Furthermore, though evaporation-driven actuators have been described before, typically, the unstable water content is detrimental to the precise control of the deformation of (hydro)gel actuators. Thus, translation of published gel actuators based on salt addition for industrial applications is rather unlikely. It should be noted that for long-term stability, a diffusion barrier can be employed, which alters the mechanical properties of the gel, affording difficulties in application of these gels in actuators and so on, and complicates the synthesis strategy substantially.108–110 In contrast, organohydrogels are excellent candidates for actuators, biomedical applications and also sensors due to their long-term stability, temperature resistance and controllable mechanical performance, while ionogels are more suitable for applications in (flexible) electronics, such as conductors, batteries and supercapacitators. However, in regard to ionogels, the reader should be aware that most ionic liquids are rather harmful to the environment and biological organisms.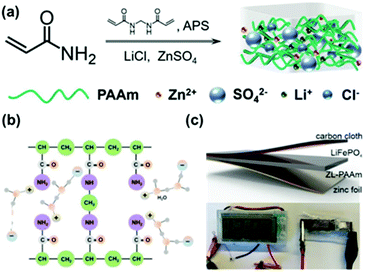 | ||
| Fig. 13 (a) Preparation method of the anti-freezing hydrogel electrolyte. (b) Chemical structure of the ZL-PAAm hydrogel. (c) Scheme of the flexible hydrogel-based battery assembly and its optical image taken during powering a digital clock. Reproduced with permission from ref. 104. Copyright 2019 Wiley-VCH. | ||
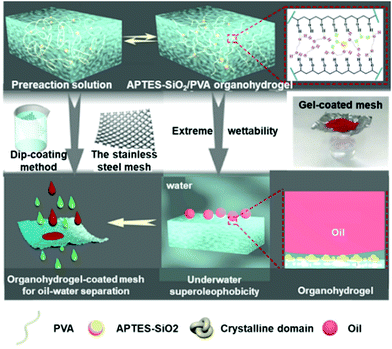 | ||
| Fig. 14 Preparation method and the oil–water separation mechanism of the anti-freezing underwater superoleophobic organohydrogel. Reproduced with permission from ref. 105. Copyright 2019 Elsevier B.V. | ||
A drawback of the methods discussed up to now is the fact that diffusion of the solute is commencing in typical aqueous environments. While diffusion may be exploited for localized patterning (i.e. enhanced mechanical properties) with other ions and so on,111,112 it is typically unwanted as it means that these gels lack long-term stability in underwater and biomedical applications.
A promising solution for diffusion induced instability of the performance of gels is the modification of the polymer network instead of introduction of highly mobile ions or solvent molecules, as this strategy can be universally applied, i.e. in biomedical settings, energy storage devices, electrodes, sensors and actuators. As the solute is in this case chemically bound to the polymer network, the diffusion induced instabilities in performance, which arise from the concentration gradient of the solute, are prevented. Of course, depending on the application, other requirements, such as biocompatibility for biomedical applications, need to be met. Still, up to now, the freezing-resistance is rather low and the long-term stability of these gels is not fully analyzed (i.e. in arid environments). However, these gels may leverage the diffusion resistance of their grafted solute in a solvent to obtain long-term stability without necessitating evaporation resistance.
It should be noted that anti-freezing gels based on immobilized solutes are just in their infancy. Therefore, we anticipate that these gels, once matured, will account for considerable advances in anti-freezing gels and their applications. Considering that anti-freezing gels are usually employed at sub-zero temperatures or near freezing temperature, they are not designed for direct contact with living organisms at constant (elevated) temperature. We anticipate that potential applications lie in the fields of wearable sensors and batteries (used in cold environments), since research on gel-based sensors and batteries has been carried out extensively, and there are practical needs for them in cold conditions.
3.6 Performance at low temperatures
The impact of low temperatures is highly dependent on the envisioned applications. Actuators suffer from lower diffusion rates of molecules and thus the actuation speed is much lower than that at room temperature.94 In contrast, field controlled anti-freezing actuators should not be subject to strong degradation in performance under the premise that the mechanical properties remain fairly similar, yet no related work has been reported thus far.Several applications, such as batteries, supercapacitators and sensors, are reliant on the conductivity of the gel. The conductivity can be obtained by employing a conductive polymer scaffold, by overcoming the percolation threshold of an added conductive filler and by endowing the gel with ion conductivity. Anti-freezing gels (salt induced freezing resistant gels and ionogels) obtain their conductivity typically due to added ions (ion mobility). Yet, the ion mobility is hampered at low temperatures, which can be observed in their conductivity. For example, an ionogel made of N-methyl-N-propylpiperidinium bis(fluorosulfonyl)imide (PIP13FSI) and N-butyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide (PYR14FSI) showed reliable conductivity between −40 and 60 °C, which is similar to the conduction in the ionic liquid. However, the conductivity was substantially reduced at temperatures below −20 °C. A supercapacitator based on this gel at −20 °C maintained 75% of its capacitance at room temperature, while it lost a further 50% during cooling from −20 to −40 °C.113 The low performance at −40 °C and below was ascribed to crystallization of the ionic liquid in micropores, limiting the movement of the ions. Similarly, the conductivity of an aqueous salt solution is significantly lower at subzero temperatures than that at room temperature. For example, Rong et al. synthesized an organohydrogel consisting of a binary mixture of water/ethylene glycol and added LiCl for ion conductivity. The performance of the supercapacitator was maintained down to −40 °C (a capacitance retention of 70%, with a capacitance retention of 85% at −20 °C). Significantly, a flexible aqueous zinc-ion battery based on a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte showed excellent performance down to −35 °C. However, the discharge capacity and current density were somewhat lower due to the lower ionic conductivity of the gel compared to the liquid electrolyte. For example, the discharge capacity of the gel is 225 mA h g−1 at 25 °C, lower than that of the liquid electrolyte (250 mA h g−1). The discharge capacity dwindles for the gel via 210 mA h g−1 (0 °C) and 175 mA h g−1 (−20 °C) to 125 mA h g−1 at −35 °C, which is significantly lower than the liquid electrolyte at room temperature. However, it should be considered that a battery made with the liquid electrolyte does not show any discharge capacity at and near the freezing temperature (of water).114
However, the performance of sensors mainly depends on their stability and sensitivity. For example, Morelle et al. showed that the performance of a capacitance touch sensor can be maintained at subzero temperatures.52 Furthermore, Qin et al. employed an organohydrogel based electronic sensor comprising gelatin (gel) and a citrate water/glycerol binary solvent. The resulting organohydrogel maintains a good strain response upon cooling down to −30 °C115 and could be employed as a strain sensor at subzero temperatures.
It should be noted that direct comparisons between the performance of a device at room temperature and subzero temperature and comparisons between anti-freezing gels and normal hydrogels are scarce. Often the exploited property (conductivity) is only compared with the literature, while no comparison of the performance in a device is given. We think that this should be improved upon in the future. Notably, many properties, such as flexibility, transparency, mechanical properties (to a certain degree) and so on can be maintained at subzero temperatures.
4. Summary and outlook
In this review, we systematically summarized the methods for preparing anti-freezing hydrogels, including the introduction of solutes (i.e. salts, alcohols and ionic liquids) and modification of polymer networks (introduction of immobilized solutes), and we listed common applications of anti-freezing hydrogels, such as actuators, sensors, batteries etc.A current hot research topic is anti-freezing and frost-protected surfaces.116–118 Anti-freezing hydrogels may play a prominent role in this field due to the adaptive nature of these gels and their dynamic (diffusion of molecules) interface.119,120 Though the diffusion of the molecules is beneficial in a deicing setting, the diffusion of molecules from “just” physically mixed solutes out of the gel upon contact with aqueous solution due to osmotic pressure may be detrimental to long-term usage. Furthermore, the diffused solutes may pollute the environment or affect the performance (anti-freezing, conductivity, etc.) of the gels. Therefore, research should focus on devising advanced techniques for solute retention. Interesting strategies include the coating of the gels with elastomers or other impermeable materials, such as hydrophobic organogels,121 and anchoring the solute to the gel matrix, and should thus be advanced in the future. Moreover, research should focus on unravelling the anti-freezing mechanism of anti-freezing induced by solutes grafted to the gel matrix. Though the mechanism should be similar to the anti-freezing mechanism of solutes like polyols, there should be distinct differences due to the immobilized solutes. For example, the immobilization of the solute is expected to impede the aggregation of solute molecules and phase segregation into water-rich and alcohol-rich nanodomains.
In addition, existing anti-freezing hydrogel synthesis strategies require the addition of a considerable amount of solute, which may affect the performance (i.e. mechanical) of the hydrogel. Whether the use of very small amounts of additives could achieve a considerable anti-freezing effect is a very fascinating topic, which should be investigated in the future together with research on the mechanism of icing and anti-freezing. On the other hand, by modifying the polymer network or constructing a dual network organohydrogel to fix the anti-freezing functional components (solute), the leakage of the anti-freezing component can be avoided, which is an alternative way to solve the trouble caused by the freely movable anti-freezing agents. Furthermore, more effort should be devoted to the development of new synthetic strategies for anti-freezing gels as well as to application of these gels outside of the anti-freezing setting. For example, Chen et al. have demonstrated that hygroscopic organogels are an excellent material for atmospheric water harvesting.122
At present, the functionality of freezing resistant hydrogels remains relatively simple. A gel system usually only has a single function, such as only mechanical sensing or stimuli-responsive actuation. In the future, we envision that hydrogels will act smart and autonomously according to changes in the environment. For example, freezing resistant gels perceive changes in the concentration of specific substances (sensing, diagnostics) in the environment and autonomously release drugs (therapy) in so-called theranostic applications. In this regard, it is important to point out that anti-freezing is a complex field of study at the interface of physical chemistry, chemical engineering and so on, while the problem of freezing is a general problem/effect nearly every person faces on a day-to-day basis. We anticipate that this review will inspire researchers in different fields to further and strengthen scientific collaboration and facilitate the transition of hydrogel application from laboratory experiments to real-life applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21922303, 51873223, 51773215, 21774138), Youth Innovation Promotion Association of Chinese Academy of Sciences (2017337, 2019297), the Shenzhen Science and Technology Foundation (SGLH20180622151607182), Key Research Program of Frontier Science, Chinese Academy of Sciences (QYZDB-SSW-SLH036) and K. C. Wong Education Foundation (GJTD-2019-13).References
- X. Dai, Y. Zhang, L. Gao, T. Bai, W. Wang, Y. Cui and W. Liu, Adv. Mater., 2015, 27, 3566 CrossRef CAS.
- T. L. Sun, T. Kurokawa, S. Kuroda, A. B. Ihsan, T. Akasaki, K. Sato, M. A. Haque, T. Nakajima and J. P. Gong, Nat. Mater., 2013, 12, 932 CrossRef CAS.
- J. Y. Sun, X. Zhao, W. R. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. G. Suo, Nature, 2012, 489, 133 CrossRef CAS.
- C. X. Ma, X. X. Le, X. L. Tang, J. He, P. Xiao, J. Zheng, H. Xiao, W. Lu, J. W. Zhang, Y. J. Huang and T. Chen, Adv. Funct. Mater., 2016, 26, 8670 CrossRef CAS.
- C. Yao, Z. Liu, C. Yang, W. Wang, X. J. Ju, R. Xie and L. Y. Chu, Adv. Funct. Mater., 2015, 25, 2980 CrossRef CAS.
- Y. Osada, H. Okuzaki and H. Hori, Nature, 1992, 355, 242 CrossRef CAS.
- M. Bassil, M. Ibrahim and M. El Tahchi, Soft Matter, 2011, 7, 4833 RSC.
- J. J. Wu, Y. T. Lin and J. Z. Sun, J. Mater. Chem., 2012, 22, 17449 RSC.
- N. Golafshan, R. Rezahasani, M. Esfahani and S. N. Khorasani, Carbohydr. Polym., 2017, 176, 392 CrossRef CAS.
- W. J. Yang, X. Tao, T. T. Zhao, L. X. Weng, E. T. Kang and L. H. Wang, Polym. Chem., 2015, 6, 7027 RSC.
- Z. N. Cao, X. G. Luo, H. Zhang, Z. Fu, Z. Shen, N. Cai, Y. N. Xue and F. Q. Yu, Cellulose, 2016, 23, 1349 CrossRef CAS.
- L. Y. Wang, J. Z. Jiang, W. X. Hua, X. P. Song, S. Chen and X. Z. Qiu, Adv. Funct. Mater., 2016, 26, 4293 CrossRef CAS.
- T. Dvir, B. P. Timko, M. D. Brigham, S. R. Naik, S. S. Karajanagi, O. Levy, H. W. Jin, K. K. Parker, R. Langer and D. S. Kohane, Nat. Nanotechnol., 2011, 6, 720 CrossRef CAS.
- H. Onoe, T. Okitsu, A. Itou, M. Kato-Negishi, R. Gojo, D. Kiriya, K. Sato, S. Miura, S. Iwanaga, K. K. Shigetomi, Y. T. Matsunaga, Y. Shimoyama and S. Takeuchi, Nat. Mater., 2013, 12, 584 CrossRef CAS.
- G. D. Prestwich and J. A. Burdick, Adv. Mater., 2011, 23, H41 CrossRef.
- R. A. Marklein and J. A. Burdick, Adv. Mater., 2010, 22, 175 CrossRef CAS.
- F. T. Moutos, L. E. Freed and F. Guilak, Nat. Mater., 2007, 6, 162 CrossRef CAS.
- H. C. Ding, B. Q. Li, Z. L. Liu, G. Liu, S. Z. Pu, Y. J. Feng, D. C. Jia and Y. Zhou, Adv. Healthcare Mater., 2020, 2000454 CrossRef CAS.
- R. Jin, L. S. M. Teixeira, P. J. Dijkstra, M. Karperien, Z. Y. Zhong and J. Feijen, Biomaterials, 2009, 30, 2544 CrossRef CAS.
- M. Wu, J. S. Chen, W. J. Huang, B. Yan, Q. Y. Peng, J. F. Liu, L. Y. Chen and H. B. Zeng, Biomacromolecules, 2020, 21, 2409 CrossRef CAS.
- P. Gupta, K. Vermani and S. Garg, Drug Discovery Today, 2002, 7, 569 CrossRef CAS.
- A. K. Bajkai, S. K. Shukla, S. Bhanu and S. Kankane, Prog. Polym. Sci., 2008, 33, 1088 CrossRef.
- S. C. T. Moorcroft, L. Roach, D. G. Jayne, Z. Y. Ong and S. D. Evans, ACS Appl. Mater. Interfaces, 2020, 12, 24544 CrossRef CAS.
- M. Guvendiren, H. D. Lu and J. A. Burdick, Soft Matter, 2012, 8, 260 RSC.
- K. Y. Lee and S. H. Yuk, Prog. Polym. Sci., 2007, 32, 669 CrossRef CAS.
- H. Yang, D. P. Qi, Z. Y. Liu, T. Wang, J. C. Yu and X. D. Chen, Adv. Mater., 2016, 28, 9175 CrossRef CAS.
- Y. Y. Wang, Q. Z. Xu, T. J. Chen, M. Li, B. Feng, J. Weng, K. Duan, W. Z. Peng and J. X. Wang, J. Mater. Chem. B, 2019, 7, 3044 RSC.
- P. B. Wan, X. M. Wen, C. Z. Sun, B. K. Chandran, H. Zhang, X. M. Sun and X. D. Chen, Small, 2015, 11, 5409 CrossRef CAS.
- Z. Y. Lei, Q. K. Wang, S. T. Sun, W. C. Zhu and P. Y. Wu, Adv. Mater., 2017, 29, 1700321 CrossRef.
- J. J. Guo, X. Y. Liu, N. Jiang, A. K. Yetisen, H. Yuk, C. X. Yang, X. H. Zhao and S. K. Yun, Adv. Mater., 2016, 28, 10244 CrossRef CAS.
- M. Sun, R. B. Bai, X. Y. Yang, J. Q. Sun, M. Qin, Z. G. Suo and X. M. He, Adv. Mater., 2018, 30, 1804916 CrossRef.
- M. J. Yin, M. Yao, S. R. Gao, A. P. Zhang, H. Y. Tam and P. K. Wai, Adv. Mater., 2016, 28, 1394 CrossRef CAS.
- H. C. Gao, F. Xiao, C. B. Ching and H. W. Duan, ACS Appl. Mater. Interfaces, 2012, 4, 2801 CrossRef CAS.
- J. Y. Nan, G. T. Zhang, T. Y. Zhu, Z. K. Wang, L. J. Wang, H. S. Wang, F. X. Chu, C. P. Wang and C. B. Tang, Adv. Sci., 2020, 2000587 CrossRef CAS.
- Y. X. Xu, Z. Y. Lin, X. Q. Huang, Y. Wang, Y. Huang and X. F. Duan, Adv. Mater., 2013, 25, 5779 CrossRef CAS.
- K. B. Storey and J. M. Storey, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 1986, 83, 613 CrossRef CAS.
- K. B. Storey and J. M. Storey, Can. J. Zool., 1986, 64, 49 CrossRef CAS.
- K. B. Storey and J. M. Storey, Copeia, 1987, 3, 720 CrossRef.
- J. P. Costanzo and R. E. Lee, J. Exp. Biol., 2005, 208, 4079 CrossRef.
- K. B. Storey, J. Bischof and B. Rubinsky, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 1992, 263, R185 CrossRef CAS.
- S. P. Graether, M. J. Kuiper, S. M. Gagne, V. K. Walker, Z. C. Jia, B. D. Sykes and P. L. Davies, Nature, 2000, 406, 325 CrossRef CAS.
- F. Sicheri and D. Yang, Nature, 1995, 375, 427 CrossRef CAS.
- J. A. Raymond and A. L. Devries, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 14739 CrossRef.
- J. Krasensky and C. Jonak, J. Exp. Bot., 2012, 63, 1593 CrossRef CAS.
- B. Riechers, F. Wittbracht, A. Hutten and T. Koop, Phys. Chem. Chem. Phys., 2013, 15, 5873 RSC.
- H. Desnos, A. Baudot, M. Teixeira, G. Louis, L. Commin, S. Buff and P. Bruyère, Thermochim. Acta, 2018, 667, 193 CrossRef CAS.
- S. Cerveny, J. Colmenero and A. Alegria, Macromolecules, 2005, 38, 7056 CrossRef CAS.
- V. Gunko, I. Savina and S. Mikhalovsky, Gels, 2017, 3, 37 CrossRef.
- S. Elgersma and L. Unsworth, Materials, 2019, 12, 832 CrossRef CAS.
- H. F. Gibbard and A. F. Gossmann, J. Solution Chem., 1974, 3, 385 CrossRef CAS.
- M. M. Conde, M. Rovere and P. Gallo, J. Mol. Liq., 2018, 261, 513 CrossRef CAS.
- X. P. Morelle, W. R. Illeperuma, K. Tian, R. B. Bai, Z. G. Suo and J. J. Vlassak, Adv. Mater., 2018, 30, 1801541 CrossRef.
- X. F. Zhang, X. F. Ma, T. Hou, K. C. Guo, J. Y. Yin, Z. G. Wang, L. Shu, M. He and J. F. Yao, Angew. Chem., Int. Ed., 2019, 58, 7366 CrossRef CAS.
- X. J. Sui, H. S. Guo, P. G. Chen, Y. N. Zhu, C. Y. Wen, Y. H. Gao, J. Yang, X. Y. Zhang and L. Zhang, Adv. Funct. Mater., 2020, 30, 1907986 CrossRef CAS.
- G. Ge, W. Yuan, W. Zhao, Y. Lu, Y. Z. Zhang, W. J. Wang, P. Chen, W. Huang, W. L. Si and X. C. Dong, J. Mater. Chem. A, 2019, 7, 5949 RSC.
- J. S. Kim and A. Yethiraj, J. Chem. Phys., 2008, 129, 124504 CrossRef.
- S. Bauerecker, P. Ulbig, V. Buch, L. Vrbka and P. Jung-wirth, J. Phys. Chem., 2008, 112, 7631 CrossRef CAS.
- N. Takenaka, A. Ueda, T. Daimon, H. Bandow, T. Dohmaru and Y. Maeda, J. Phys. Chem., 1996, 100, 13874 CrossRef CAS.
- A. A. Zavitsas, J. Phys. Chem. B, 2001, 105, 7805 CrossRef CAS.
- A. A. Zavitsas, Chem. – Eur. J., 2010, 16, 5942 CrossRef CAS.
- M. Armand, F. Endres, D. R. Macfarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621 CrossRef CAS.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- C. Y. Yin, X. H. Liu, J. J. Wei, R. Tian, J. Zhou, M. Z. Ouyang, H. Z. Wang, S. J. Cooper, B. Wu and Q. G. Wang, J. Mater. Chem. A, 2019, 7, 8826 RSC.
- Z. Q. Cao, H. L. Liu and L. Jiang, Mater. Horiz., 2020, 7, 912–918 RSC.
- Y. Wang, Y. Chen, J. Gao, H. G. Yoon, L. Jin, M. Forsyth, T. J. Dingemans and L. A. Madsen, Adv. Mater., 2016, 28, 2571 CrossRef CAS.
- Y. Ding, J. Zhang, L. Chang, X. Zhang, H. Liu and L. Jiang, Adv. Mater., 2017, 1704253 CrossRef.
- L. Sun, S. Chen, Y. Guo, J. Song, L. Zhang, L. Xiao, Q. Guan and Z. You, Nano Energy, 2019, 63, 103847 CrossRef CAS.
- Y. Ren, J. Guo, Z. Liu, Z. Sun, Y. Wu, L. Liu and F. Yan, Sci. Adv., 2019, 5, eaax0648 CrossRef CAS.
- S. Daschakraborty, J. Chem. Phys., 2018, 148, 134501 CrossRef.
- W. Zhu, C. Zhang, Y. D. Zhu, R. An, X. H. Lu, Y. J. Shi and S. Y. Jiang, J. Mol. Liq., 2019, 291, 111238 CrossRef CAS.
- J. J. Towey, A. K. Soper and L. Dougan, J. Phys. Chem. B, 2016, 120, 4439 CrossRef CAS.
- F. Chen, D. Zhou, J. H. Wang, T. Z. Li, X. H. Zhou, T. S. Gan, S. Handschuh-Wang and X. C. Zhou, Angew. Chem., Int. Ed., 2018, 57, 6568 CrossRef CAS.
- S. J. Shi, X. Peng, T. Q. Liu, Y. N. Chen, C. C. He and H. L. Wang, Polymer, 2017, 111, 168 CrossRef CAS.
- J. H. Liu, C. M. Xie, A. Kretzschmann, K. Koynow, H. J. Butt and S. Wu, Adv. Mater., 2020, 1908324 CrossRef CAS.
- M. Ju, B. H. Wu, S. T. Sun and P. Y. Wu, Adv. Funct. Mater., 2020, 1910387 CrossRef CAS.
- S. Xia, S. X. Song, Y. Li and G. H. Gao, J. Mater. Chem. C, 2019, 7, 11303 RSC.
- Q. H. Wang, X. F. Pan, C. M. Lin, D. Z. Lin, Y. H. Ni, L. H. Chen, L. L. Huang, S. L. Gao and X. J. Ma, Chem. Eng. J., 2019, 370, 1039 CrossRef CAS.
- K. X. Yang, Y. N. Wang, Y. Z. You, H. Y. Yang and X. Hao, Chem. Eng. J., 2020, 382, 122926 CrossRef CAS.
- H. Liu, W. Zhao, G. H. Gao and X. Y. Ren, Mater. Today Commun., 2019, 21, 100609 CrossRef CAS.
- C. X. Hu, Y. L. Zhang, X. D. Wang, L. Xing, L. Y. Shi and R. Ran, ACS Appl. Mater. Interfaces, 2018, 10, 44000 CrossRef CAS.
- S. S. Xue, Y. P. Wu, M. L. Guo, Y. M. Xia, D. Liu, H. W. Zhou and W. W. Lei, Soft Matter, 2019, 15, 3680 RSC.
- Q. F. Rong, W. W. Lei, L. Chen, Y. A. Yin, J. J. Zhou and M. J. Liu, Angew. Chem., Int. Ed., 2017, 56, 14159 CrossRef CAS.
- Q. F. Rong, W. W. Lei, J. Huang and M. J. Liu, Adv. Energy Mater., 2018, 8, 1801967 CrossRef.
- Z. X. Pei, Z. W. Yuan, C. J. Wang, S. L. Zhao, J. Y. Fei, L. Wei, J. S. Shen, C. Wang, R. J. Qi, Z. W. Liu and Y. Chen, Angew. Chem., Int. Ed., 2020, 59, 4793 CrossRef CAS.
- F. N. Mo, G. J. Liang, Q. Q. Meng, Z. X. Liu, H. F. Li, J. Fan and C. Y. Zhi, Energy Environ. Sci., 2019, 12, 706 RSC.
- J. J. Wei, G. M. Wei, Y. H. Shang, J. Zhou, C. Wu and Q. G. Wang, Adv. Mater., 2019, 31, 1900248 CrossRef.
- H. N. Gao, Z. G. Zhao, Y. D. Cai, J. J. Zhou, W. D. Hua, L. Chen, L. Wang, J. Q. Zhang, D. Han, M. J. Liu and L. Jiang, Nat. Commun., 2016, 8, 15911 CrossRef.
- H. Yuk, T. Zhang, G. A. Parada, X. Liu and X. Zhao, Nat. Commun., 2016, 7, 12028 CrossRef.
- T. Liu, M. Liu, S. Dou, J. Sun, Z. Cong, C. Jiang, C. Du, X. Pu, W. Hu and Z. L. Wang, ACS Nano, 2018, 12, 2818–2826 CrossRef CAS.
- S. J. Jeon, A. W. Hauser and R. C. Hayward, Acc. Chem. Res., 2017, 50, 161 CrossRef CAS.
- X. X. Le, W. Lu, J. W. Zhang and T. Chen, Adv. Sci., 2018, 1801584 Search PubMed.
- X. Li, X. Cai, Y. Gao and M. J. Serpe, J. Mater. Chem. B, 2017, 5, 2804 RSC.
- M. L. Guo, Y. P. Wu, S. S. Xue, Y. M. Xia, X. Yang, Y. Dzenis, Z. Y. Li, W. W. Lei, A. T. Smith and L. Y. Sun, J. Mater. Chem. A, 2019, 7, 25969 RSC.
- Y. K. Jian, B. Y. Wu, X. X. Le, Y. Liang, Y. C. Zhang, D. C. Zhang, L. Zhang, W. Lu, J. W. Zhang and T. Chen, Research, 2019, 2384347 CAS.
- C. Fang, K. X. Yang, Q. Zhou, K. Peng and H. Y. Yang, RSC Adv., 2018, 8, 35094 RSC.
- D. Buenger, F. Topuz and J. Groll, Prog. Polym. Sci., 2012, 37, 1678 CrossRef CAS.
- A. Richter, G. Paschew, S. Klatt, J. Lienig, K. F. Arndt and H. P. Adler, Sensors, 2008, 8, 561 CrossRef CAS.
- M. Qin, M. Sun, R. Bai, Y. Mao, X. Qian, D. Sikka, Y. Zhao, H. J. Qi, Z. Suo and X. He, Adv. Mater., 2018, 30, e1800468 CrossRef.
- L. Han, K. Z. Liu, M. H. Wang, K. F. Wang, L. M. Fang, H. T. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 CrossRef.
- L. Guan, S. Yan, X. Liu, X. Y. Li and G. H. Gao, J. Mater. Chem. B, 2019, 7, 5230 RSC.
- W. Zhang, P. Feng, J. Chen, Z. M. Sun and B. X. Zhao, Prog. Polym. Sci., 2019, 88, 220 CrossRef CAS.
- Z. F. Wang, H. F. Li, Z. J. Tang, Z. X. Liu, Z. H. Ruan, L. T. Ma, Q. Yang, D. H. Wang and C. Y. Zhi, Adv. Funct. Mater., 2018, 28, 1804560 CrossRef.
- E. Armelin, M. P. Madrigal, C. Aleman and D. D. Diaz, J. Mater. Chem. A, 2016, 4, 8952 RSC.
- M. S. Zhu, X. J. Wang, H. M. Tang, J. W. Wang, Q. Hao, L. X. Liu, Y. Li, K. Zhang and O. G. Schmidt, Adv. Funct. Mater., 2020, 30, 1907218 CrossRef CAS.
- Y. Liu, J. Y. Yin, Y. B. Fu, P. P. Zhao, Y. H. Zhang, B. Q. He and P. X. He, Chem. Eng. J., 2020, 382, 122925 CrossRef CAS.
- K. Wang, J. H. Wang, L. Li, L. Q. Xu, N. Feng, Y. Wang, X. Fei, J. Tian and Y. Li, Chem. Eng. J., 2019, 372, 216 CrossRef CAS.
- Y. M. Xia, Y. P. Wu, T. Yu, S. S. Xue, M. L. Guo, J. L. Li and Z. Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 21117 CrossRef CAS.
- T. Y. Zhao, G. Y. Wang, D. Z. Hao, L. Chen, K. S. Liu and M. J. Liu, Adv. Funct. Mater., 2018, 1800793 CrossRef.
- W. K. Xie, J. J. Duan, H. Wang, J. Li, R. Liu, B. Y. Yu, S. Y. Liu and J. Zhou, J. Mater. Chem. A, 2018, 6, 24114 RSC.
- C. Tondera, T. F. Akbar, A. K. Thomas, W. L. Lin, C. Werner, V. Busskamp, Y. X. Zhang and I. R. Minev, Small, 2019, 1901406 CrossRef.
- X. Peng, T. Q. Liu, Q. Zhang, C. Shang, Q. W. Bai and H. L. Wang, Adv. Funct. Mater., 2017, 1701962 CrossRef.
- X. X. Le, W. Lu, J. He, M. J. Serpe, J. W. Zhang and T. Chen, Sci. China Mater., 2019, 62, 831 CrossRef CAS.
- L. Negrea, B. Daffos, V. Turq, P. L. Taberna and P. Simon, Electrochim. Acta, 2016, 206, 490 CrossRef.
- M. F. Chen, W. J. Zhou, A. R. Wang, A. X. Huang, J. Z. Chen, J. L. Xu and C. P. Wong, J. Mater. Chem. A, 2020, 8, 6828 RSC.
- Z. H. Qin, X. Sun, H. T. Zhang, Q. Y. Yu, X. Y. Wang, S. S. He, F. L. Yao and J. J. Li, J. Mater. Chem. A, 2020, 8, 4447 RSC.
- Y. K. Jin, C. Y. Wu, Y. L. Yang, J. G. Wu, Z. Y. He and J. J. Wang, ACS Nano, 2020, 14, 5000 CrossRef CAS.
- Z. Y. He, C. Y. Wu, M. T. Hua, S. W. Wu, D. Wu, X. Y. Zhu, J. J. Wang and X. M. He, Matter, 2020, 2, 723 CrossRef.
- Z. Y. He, K. Liu and J. J. Wang, Acc. Chem. Res., 2018, 51, 1082 CrossRef CAS.
- Y. Z. Zhuo, S. B. Xiao, V. Hakonsen, J. Y. He and Z. L. Zhang, ACS Mater. Lett., 2020, 2, 616 CrossRef CAS.
- Y. F. Ru, R. C. Fang, Z. D. Gu, L. Jiang and M. J. Liu, Angew. Chem., Int. Ed., 2020, 59, 11876 CrossRef CAS.
- M. T. I. Mredha, H. H. Le, J. X. Cui and I. Jeon, Adv. Sci., 2020, 7, 1903145 CrossRef CAS.
- F. Ni, N. X. Qiu, P. Xiao, C. Zhang, Y. K. Jian, Y. Liang, W. P. Xie, L. K. Yan and T. Chen, Angew. Chem., Int. Ed., 2020, 59, 2 CrossRef.
Footnote |
| † Equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |