Open Access Article
Open Access ArticleNanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria
Seyedehhamideh
Razavi†
a,
Sajjad
Janfaza†
a,
Nishat
Tasnim
 a,
Deanna L.
Gibson
bc and
Mina
Hoorfar
a,
Deanna L.
Gibson
bc and
Mina
Hoorfar
 *a
*a
aSchool of Engineering, University of British Columbia, Kelowna, BC, Canada. E-mail: mina.hoorfar@ubc.ca
bDepartment of Biology, Faculty of Science, University of British Columbia, Kelowna, Canada
cDepartment of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, Canada
First published on 30th March 2021
Abstract
Probiotics are microorganisms that have beneficial health effects when administered in adequate dosages. The oral administration of probiotic bacteria is widely considered beneficial for both intestinal as well as systemic health but its clinical efficacy is conflicted in the literature. This may at least in part be due to the loss of viability during gastrointestinal passage resulting in poor intestinal delivery. Microencapsulation technology has been proposed as a successful strategy to address this problem by maintaining the viability of probiotics, thereby improving their efficacy following oral administration. More recently, nanomaterials have demonstrated significant promise as encapsulation materials to improve probiotic encapsulation. The integration of nanotechnology with microencapsulation techniques can improve the controlled delivery of viable probiotic bacteria to the gut. The current review aims at summarizing the types of nanomaterials used for the microencapsulation of probiotics and showing how they can achieve the delivery and controlled release of probiotics at the site of action.
1. Introduction
The World Health Organization defines probiotics as live microorganisms that confer various health benefits to the host when administered in adequate amounts at the desired target site in the digestive tract.1 Probiotics have been reported to offer protection from enteric infectious disease,2 enhance metabolism (including lactose intolerance,3 absorption of calcium,4 synthesis of vitamins and pre-digestion of proteins5), have anti-carcinogenic and anti-mutagenic activities,6 and reduce symptoms associated with anxiety and depression.7,8 Probiotic bacteria provide positive health responses based on three mechanisms: production of nutrients and cofactors, competition with pathogens for nutrients or adhesion sites, and stimulation of the host immune response.9 Given their immunomodulatory potential, probiotics have potential in the treatment of diseases such as inflammatory bowel disease (IBD) and cancer,10–12 and have been proposed to treat a multitude of immune and metabolic-driven diseases.13,14While the potential of the use of probiotics in the treatment or even prevention of gastrointestinal diseases is high, their clinical efficacy remains low due to conflicting clinical trial results for many diseases.15,16 This is in part due to the lack of viability of probiotics using traditional manufacturing and packaging methods.17 Several factors (such as pH, oxygen, and temperature) can significantly affect the viability and survival rate of probiotics during processing, storage, and passage through the gastrointestinal tract.18 Thus, probiotics must be able to survive the harsh gastric environment, remain metabolically active, and be released (in a controlled manner) in large enough quantities at the site of action in the lower gastrointestinal (GI) tract to confer beneficial health effects.19,20 Microencapsulation of probiotics has been proposed as an effective solution to improve the survival, resistance, and targeted release of sensitive microorganisms in the GI tract.21 It is a technology for entrapping small quantities of bioactive compounds and/or microorganisms in small polymer capsules. In essence, the goal of microencapsulation is to create a microenvironment that protects bacteria from exposure to exterior factors (such as low gastric pH) during digestion, and subsequently to reduce cell injury or cell death before their release at the target site.22 There are many reports on microencapsulation of probiotic bacteria resulting in highly enhanced viability of probiotics during storage and administration.23 The majority of these reports are based on the immobilization of probiotics into a polymer matrix, which retains its structure in the acidic environment of the stomach before swelling or dissolving in the intestine as a result of the pH change.24 With the advent of nanotechnology, innovative encapsulating materials (EMs) have recently been developed based on nanostructured compounds. These nanomaterials have shown improved properties for the encapsulation of probiotics. Due to their unique physical and chemical properties, nanostructured EMs demonstrate great promise for protection of microorganisms from the acidic conditions of the stomach and hence allow for the successful release of entrapped probiotic cells in the intestinal lumen with natural pH.
In this paper, we provide a critical review of nanomaterials in light of their suitability for probiotic microencapsulation. We provide an overview of nanostructured capsules used to protect probiotics from environmental and physiological factors (e.g., pH, oxygen, and temperature) and their advantages and limitations.
2. Microencapsulation of probiotics
Microencapsulation of probiotics has been widely utilized in the food and pharmaceutical industries. Microencapsulation results in the protection of probiotics by encapsulating them in small capsules. By building a functional barrier between the cells and environment, the capsule prevents probiotic bacteria from damage, leading to improved viability of probiotics. Encapsulated cells can reach the site of action without being adversely affected by environmental factors such as oxygen and pH.Exposure to a high oxygen environment is harmful to microaerophilic and anaerobic probiotics such as L. acidophilus (microaerophilic) and bifidobacteria (obligate anaerobic). It can result in cell lysis due to the formation of reactive oxygen species such as superoxides that can cause oxidative DNA damage to bacterial cells. It has been shown that the viability of free anaerobic probiotic cells (e.g., Bifidobacterium) upon exposure to oxygen decreases,25 while encapsulated cells survive for longer.26
Microencapsulation can also improve the survival of probiotic cells during gastrointestinal digestion by protecting them from the acidic environment of the stomach. It has been shown that a considerable number of probiotics (such as Lactobacillus rhamnosus and Bifidobacterium longum) are severely injured by the low pH of the stomach and high bile salt conditions of the intestine before entry into the colon.27
A key requirement for microencapsulation is the ability of the encapsulating material to release loaded probiotic cells from the microcapsules using environmental stimuli at the site of delivery. Encapsulated cells can be released by various mechanisms such as biodegradation, pH variation, mechanical rupture, and diffusion.9 In recent years, promising encapsulating materials have been developed for the targeted delivery of probiotics in the gastrointestinal tract with improved mucoadhesive properties. Mucoadhesion is one of the most important factors determining the ability of encapsulating materials to adhere to mucosal membranes and provide temporary retention in the GI tract, resulting in an increased residence time of probiotics, reduced administration frequency, improved bioavailability, and improved targeting of particular sites.28 The improvement of microcapsule mucoadhesion by the use of mucoadhesive encapsulating materials is, therefore, a great strategy for the delivery of probiotics to the gut. The low survival rate of probiotics due to undesirable reactions with external factors such as oxygen, temperature, and light during handling and storage is another challenge to be addressed. In this regard, microencapsulation has shown great potential in increasing the stability of probiotics.29,30
Despite the remarkable improvement in viability and delivery of probiotic cells by encapsulation using traditional materials and methods, there is still a need to develop new and innovative microcapsules for better protection of probiotics and improved function. Using nanostructures as EMs has been proposed as a promising approach to design an improved probiotic delivery system.
3. Nanostructured encapsulating materials
Nanostructured materials for the encapsulation of probiotics can be selected from a wide variety of natural polymers (e.g. carbohydrates, gums, and proteins) and synthetic materials.The composition of the EM affects the functional properties of the microcapsule,31 and each group of encapsulating materials has its pros and cons in terms of mechanical, chemical, physical, and biological properties.
Recently, many nanomaterials with different sizes, shapes, textures, and compositions have been investigated for the microencapsulation of probiotics. Because of their unique physical and chemical properties, nanostructured microcapsules have shown promising improvement in protecting probiotics from harsh environments. The employed nanomaterials (e.g., nanocellulose) are nontoxic and compatible with both probiotics and the body while providing maximum protection to probiotic bacteria. Some of them are capable of releasing loaded probiotics under certain conditions (e.g. pH).19 In the following subsections, we review these nanomaterials along with their applications in the microencapsulation of probiotics.
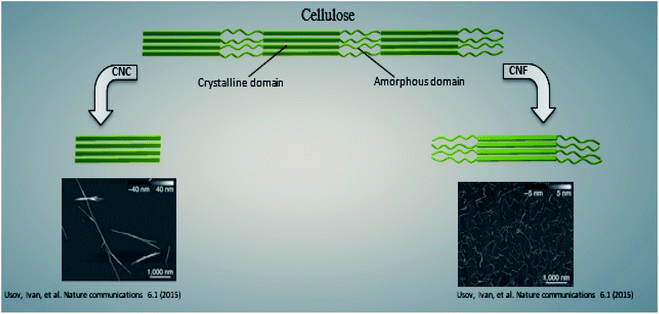
3.1. Nanocellulose
Cellulose is the most ubiquitous and abundant natural bio-macromolecule.32 The cellulose macromolecule consists of disordered (amorphous) regions and highly ordered (crystalline) regions.33 Nano-sized cellulose, also known as nanocellulose, has recently received much attention because of its remarkable features including low risk of toxicity, biocompatibility, and tunable surface properties.34–36 Nanocellulose is available in two forms: (1) cellulose nanocrystals (CNCs) and (2) cellulose nanofibers (CNFs), as shown in Fig. 1.37 The former is extracted from fibers after complete dissolution of the amorphous fractions of cellulose in acid media, while the latter results from mechanical treatment leading to a high degree of fibrillation, which yields highly interconnected fibrils. CNCs and CNFs with high surface areas can be modified by a wide variety of additives.36 Because of its physical and chemical properties, nanocellulose has attracted significant attention for applications in biomedical science and engineering.38 Diverse nanocellulose-based drug delivery systems for the controlled release of drugs have been developed recently using nanocellulose.34 More recently, it has been found that nanocellulose can improve the properties of probiotic delivery systems when used as the encapsulating material.39–41 CNC is a promising cellulosic nanomaterial for applications involving non-refrigerated long shelf-life pharmaceutical products.39 CNC improves the mechanical properties of the carrier. It has been shown that the presence of CNC in the formulation improves the compression strength of freeze-dried microbeads.39 Also, CNC as an encapsulating material decreases gastric fluid absorption39 and significantly improves the survival of probiotic bacteria in the gastrointestinal system.42CNF can be used as a filler for various biomaterials and has strong intermolecular bonds.43 It can form aqueous hydrogels for cell encapsulation, and the carboxyl groups in CNFs can be used to change the pore size of cellulose macrogels to improve their loading capacity.44,45 For instance, macrogels made with a high content of CNFs showed sustained release of Lactobacillus plantarum cells and delivered adequate viable cells to the desired region in the intestinal tract.45
Nanocellulose can also act as an agent of cryoprotection.40 Nanocellulose can be adsorbed on the surface of microorganisms and form a viscous layer that prevents the growth of ice crystals by increasing the viscosity of the solution and keeping the glassy structure of ice near the cells.40 For instance, the combination of nanocellulose produced from cotton lint with conventional cryoprotective agents has been shown to improve the cell viability of Lactobacillus plantarum during freeze-drying.40 Cellulose nanostructures can also be effective for the development of controlled release systems. Zhang et al.41 developed microspheres with pH-responsive properties based on the sodium alginate (SA)/cellulose nanofiber. The probiotic L. plantarum was encapsulated by extruding a mixture of sodium alginate and TEMPO-oxidized CNFs. The acidic environment causes the formation of hydrogen bonds between SA and CNFs, stabilizing the gel microspheres and providing better protection for the encapsulated bacteria. Their results presented successful protection of cells in the simulated gastric fluid (SGF) and targeted release in the neutral simulated intestinal fluid (SIF). In essence, the pH-responsive property of nanocellulose-based carriers is very important for intestine-targeted delivery of probiotics.
Nanocellulose can be obtained from different sources including plants and microorganisms.46,47 Among naturally occurring nanocellulose sources, bacterial nanocellulose (BNC) has recently received great interest in the field of biomedical materials. BNC is biosynthesized as a primary extracellular metabolite by several bacterial species, such as Komagataeibacter xylinus (K. xylinus), during a fermentation process that results in the secretion of high-quality cellulose ribbons of microfibrillar bundles.48 BNC is non-toxic49 and can be produced in various shapes, sizes, and surface structures depending on the manufacturing process.50,51 Compared to plant cellulose, BNC possesses higher purity, is devoid of other compounds present in plant pulp such as lignin and hemicellulose, and does not contain components of animal origin.52 BNC also has more stable mechanical properties compared to plant cellulose.53 BNC as a nanostructured biopolymer can improve probiotic protection due to its high crystallinity and available surface area.54 Khorasani et al.54 improved the survivability of Bacillus coagulans IBRC-M 10807 by encapsulating it into bionanocomposites composed of BNC, pectin, and Schizophyllum commune extract. Using the optimal bionanocomposite formulation (20% pectin with 80% BNC), the authors obtained a survival rate of 99.43% after microwave drying and 94.76% following sequential digestion under simulated gastrointestinal fluids. Moreover, BNC bionanocomposite encapsulation improved the viability of probiotic bacteria during long time storage at various temperatures.54
3.2. Chitosan nanoparticles
Chitosan (CS) is a polycationic natural polysaccharide derived from the alkaline deacetylation of chitin, a natural macromolecule forming the major constituent of arthropod exoskeletons and fungal cell walls. CS contains alternating units of (1 → 4) linked N-acetyl glucosamine and glucosamine units (Fig. 2a).55 The cationic properties of CS, resulting from its primary amino groups, present great potential (due to its biocompatibility, non-toxicity, and low cost) for a wide range of biomedical applications (including tissue engineering, drug delivery, and wound dressing56–58), as well as applications in the food and pharmaceutical industries.59 A variety of cells and molecules (including hydrophobic and hydrophilic molecules) can be encapsulated using CS.60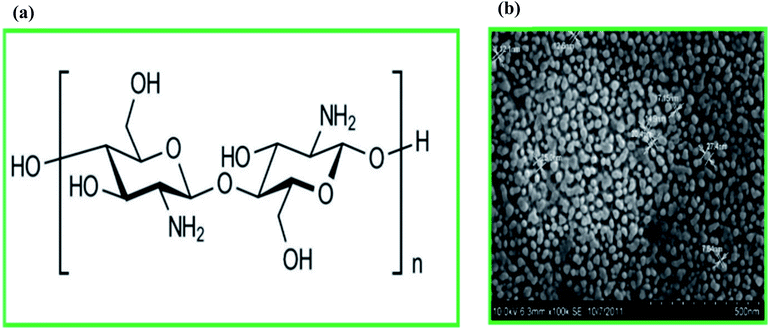 | ||
| Fig. 2 (a) Chemical structure of chitosan, and (b) the field emission scanning electron microscopy image of chitosan nanoparticles (the image was obtained with permission from Elsevier, copyright 2020).64 | ||
CS-based encapsulation of probiotic cells, in particular, has attracted a lot of attention recently, as CS protects encapsulated probiotics from the harsh conditions of the gastrointestinal tract and enhances its mucoadhesive properties.61 The mucoadhesive property of CS makes it an ideal candidate nanomaterial for developing controlled release drug-delivery systems. In essence, intestinal mucus is composed of negatively charged mucins. The structure of intestinal mucus offers many opportunities for the development of mucoadhesive delivery systems. Chitosan with a positive charge can form an electrostatic attraction with the sticky layer of mucus. Also, physical entanglements between chitosan and mucus components can enhance the adhesion of chitosan-based capsules to the gastric mucosa.62,63
More recently, chitosan nanoparticles (CSNPs) have been proposed for the micro- and nano-encapsulation of cells and molecules,64 respectively, including probiotic bacteria (Fig. 2b). Delivery systems based on CS-nanoparticles (CSNPs) have shown great mucoadhesive strength compared to bulk CS due to their significantly higher surface-to-volume ratio.28,58,65 There are various methods for the synthesis of CSNPs including ionotropic gelation, microemulsion, polyelectrolyte complexation, emulsification solvent diffusion, and the reverse micellar method.66 CSNPs also have excellent physicochemical, antimicrobial, and biological properties that make them a promising biopolymer for drug delivery applications.67 Several studies have shown the ability of CSNPs to improve the bioavailability and resistance of encapsulated drugs for effective GI delivery.68,69 CSNPs along with alginate have also been used to encapsulate probiotic Escherichia coli Nissle 1917 (EcN). Microencapsulated EcN was able to resist environmental stresses like low pH, high bile salt concentration, and high temperature. The survivability of encapsulated probiotic cells was significantly increased in an acidic environment (pH 1.5), high bile salt concentration (4%), and high temperature (70 °C) compared to non-encapsulated cells. Moreover, encapsulated EcN showed antimicrobial properties against Campylobacter jejuni, i.e., a leading bacterial pathogen responsible for gastroenteritis in humans. A reduction in the Campylobacter jejuni growth (by 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU) after exposure to microencapsulated EcN was observed.70 It has also been shown that the released bacteria have better contact with intestinal mucosa, which facilitates the successful colonization of probiotic cells in the target region. This is vital for pharmaceutical applications of probiotics and causes reduction of the required dose or frequency of administration.28,65 For instance, it has been reported that EcN encapsulated in CSNPs and alginate efficiently adhered to intestinal HT-29 cells reduce the invasion of the pathogenic microorganism C. jejuni.70
log CFU) after exposure to microencapsulated EcN was observed.70 It has also been shown that the released bacteria have better contact with intestinal mucosa, which facilitates the successful colonization of probiotic cells in the target region. This is vital for pharmaceutical applications of probiotics and causes reduction of the required dose or frequency of administration.28,65 For instance, it has been reported that EcN encapsulated in CSNPs and alginate efficiently adhered to intestinal HT-29 cells reduce the invasion of the pathogenic microorganism C. jejuni.70
3.3. Eudragit S100 nanoparticles
Eudragit®S100 [Eudragit S100] is a non-toxic anionic polymer synthesized from methacrylic acid and methacrylic acid methyl ester.71,72 The solubility of Eudragit®S100 is pH-dependent: it is insoluble in strongly acidic solutions, while it is slightly soluble in the region of the digestive tract with neutral to weakly alkaline pH.73,74Multi-walled microcapsules based on Eudragit S100 nanoparticles, alginate, and chitosan have been developed for improving the viability of the probiotic bacteria Lactobacillus acidophilus and Lactobacillus rhamnosus in simulated gastrointestinal conditions.75 In essence, the negatively charged Eudragit S100 nanoparticles attract positively charged chitosan in the first coating layer with ionic bonds to form the second layer. Since the chitosan layer is porous, coating it with Eudragit S100 nanoparticles covers the porous beads and forms an airtight bubble that protects the encapsulated core from adverse environments.75 Studies have shown that with Eudragit nanoparticles as the outer coating layer there is an increase in the strength of the encapsulated beads and an improvement in the viability of probiotic bacteria in comparison to single-coated beads.75
3.4. Magnesium oxide nanoparticles
Magnesium oxide (MgO) is a functional semiconductor with many applications ranging from pharmaceutics to optoelectronics.76 MgO is administered medically for the relief of cardiovascular diseases and stomach problems.77 Recently nanoscale MgO has attracted significant attention due to its high surface area, nontoxicity, high mechanical strength, thermal stability, and low cost.78 Magnesium oxide nanoparticles (MgO NPs) have been used in the industry for pharmaceuticals, toxic waste remediation, and toxic gas removal.79 More recently, MgO NPs have been used for the microencapsulation of probiotics. For instance, Yao et al.80 have encapsulated a model probiotic (Pediococcus pentosaceus Li05) in alginate–gelatin microbeads in the presence of MgO NPs. Probiotics encapsulated in MgO-loaded microcapsules have shown better viability (less than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU reduction after 40 min incubation) than free bacterial cells (5
log CFU reduction after 40 min incubation) than free bacterial cells (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU reduction in 10 min) in gastric fluids.80 There are two potential reasons for this cell survival improvement: MgO NPs fill pores inside the alginate–gelatin microgels, which may inhibit the oxygen exposure of probiotics, and MgO NPs neutralize hydrogen ions in the acidic environment, protecting probiotics in gastric fluids.
log CFU reduction in 10 min) in gastric fluids.80 There are two potential reasons for this cell survival improvement: MgO NPs fill pores inside the alginate–gelatin microgels, which may inhibit the oxygen exposure of probiotics, and MgO NPs neutralize hydrogen ions in the acidic environment, protecting probiotics in gastric fluids.
3.5. Starch nanoparticles
Starch is one of the most abundant biopolymers in nature produced by many plants and crops such as cereals.81,82 Starch consists of glucose residues linked by two types of bonds: α-1,4, and α-1,6 glucosidic linkages. Despite its simple chemistry, the starch granule is a complex semicrystalline structure (up to 100 μm long), containing linear amylose and highly branched amylopectin.83 Amylose has an amorphous structure comprising linear chains of α-1,4-linked glucose units.83 Amylopectin is a semicrystalline polysaccharide and consists solely of D-glucose residues covalently interconnected mostly by α-(1 → 4) glucosidic bonds, but also 4–5% α-(1 → 6) glucosidic bonds.84In recent years, the preparation and application of starch nanostructures have attracted significant research interest. Starch nanoparticles (SNPs) and nanocrystals (SNCs) are two nanostructures that have been widely used for biomedical applications, especially drug delivery applications.85,86 SNPs and SNCs are both nano-sized. SNCs refer to the crystalline portion of starch granules remaining after acid or enzymatic hydrolysis of their amorphous structure.87,88 SNCs have a crystal structure, while SNPs are amorphous. SNPs are generated from congealed starch, while starch crystallites develop as a result of the disruption of non-crystalline domains of semi-crystalline granules.89
Despite showing promising results for the delivery of bioactive compounds, SNPs may not be a great candidate for the microencapsulation of probiotics. Pediococcus acidilactici encapsulated using SNPs showed lower viability (1.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1) as compared to that encapsulated in native starch (2.65
log CFU g−1) as compared to that encapsulated in native starch (2.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1). In essence, SNPs cannot contain probiotic cells inside nanostructured microcapsules, and hence cells are released immediately in an environment with harsh conditions leading to cell death. Besides, cells are not protected against higher temperatures by SNPs; however, significantly better viability can be achieved at lower temperatures.90
log CFU g−1). In essence, SNPs cannot contain probiotic cells inside nanostructured microcapsules, and hence cells are released immediately in an environment with harsh conditions leading to cell death. Besides, cells are not protected against higher temperatures by SNPs; however, significantly better viability can be achieved at lower temperatures.90
Chemical modification of SNPs has been widely used to improve their properties via three important strategies namely chemical reactions with small molecules, the ‘grafting onto’ strategy (i.e., grafting of polymer chains in the presence of coupling agents), and the ‘grafting from’ strategy (i.e., polymer chain grafting by polymerization of monomers).91 The chemical modification of SNPs in combination with another encapsulating material may address their instability and improve their application in the microencapsulation of probiotics.
In contrast to SNPs, SNCs are reported92 to protect probiotics by encapsulation in alginate–starch nanocrystals under simulated gastrointestinal conditions and during long-life storage at 4 °C. This proves that alginate and starch nanocrystals are appropriate materials for probiotic encapsulation. As an example, Lactobacillus brevis encapsulated in alginate–nanocrystalline starch gel capsules showed a high survival rate of 94.97% in simulated gastrointestinal fluids. They also improved storage at 25 °C by up to two weeks. As a result, alginate–nanocrystalline starch is a promising material for probiotic preservation at room temperature (Table 1).
| Microencapsulating material | Probiotic | Storage conditions | Functionality | Freeze drying | Size | Microencapsulation technique | Ref. |
|---|---|---|---|---|---|---|---|
| Sodium alginate (SA)/cellulose nanofiber (CNF) | Lactobacillus plantarum CICC 6240 | — | — | Yes | A width of less than 50 nm and a length of less than 500 nm | Extrusion | 41 |
| Bacterial nanocellulose (BNC), pectin, and schizophyllum commune extract | Bacillus coagulans IBRC-M 10807 | At 25 °C, 4 °C, and −20 °C for 7, 15 and 30 days | Survival in the GI tract model | No | 50 nm | — | 54 |
| Whey protein isolate-crystalline nanocellulose–inulin | Lactobacillus rhamnosus ATCC 7469 | — | Survival in the GI tract model | Yes | — | — | 56 |
| Alginate–cellulose nanocrystal (CNC)–lecithin | Lactobacillus rhamnosus ATCC 9595 | At 4 °C and 25 °C up to 42 days | Survival in the GI tract model | Yes | — | Extrusion | 39 |
| Alginate and chitosan nanoparticles (CSNPs) | Escherichia coli Nissle 1917 (EcN) | At 4 °C | Survival in the GI tract model | Yes | — | Extrusion | 70 |
| Eudragit S100 nanoparticles, alginate, and chitosan | Lactobacillus acidophilus and Lactobacillus rhamnosus | — | Survival in the GI tract model | No | 100 nm | Extrusion | 75 |
| Alginate–gelatin–MgO NPs | Pediococcus pentosaceus Li05 | At 4 °C for 4 weeks | Survival in the GI tract model | Yes | — | Extrusion | 80 |
| Native starch and starch nanoparticles (SNPs) | Pediococcus acidolactici | — | Survival in the GI tract model | Yes | 271 nm | Extrusion | 90 |
| Starch nanocrystal–alginate | Lactobacillus brevis ST-69 | At 4 °C for 10 weeks | Survival in the GI tract model | NO | — | Emulsion technique | 92 |
4. Methods of nanostructured encapsulation
Several methods have been used for microencapsulation of probiotics, including chemical methods (such as interfacial polymerization), physical methods (such as spray drying), and physicochemical methods (such as coacervation and ionic gelation).93,94 Many of these microencapsulation methods can result in a significant decrease in probiotic cell viability because of the high temperature range or organic agents used in the encapsulation process.95 The principles of these methods have been comprehensively reviewed elsewhere.96,97 Here, we highlight two emerging and promising strategies for nanostructured encapsulation of probiotic cells, namely electrospinning98 and the layer-by-layer (LbL) method.994.1. Electrospinning
Electrospinning has demonstrated great potential for the encapsulation of bacterial cells by nanofibers that protect probiotic cells from harsh environmental conditions in the gut and enhance their survival (Fig. 3A). Electrospun nanofibers based on several polymers (such as polyvinyl alcohol (PVA),100 fructooligosaccharide (FOS)/PVA,98 gum arabic/pullulan,101 and alginate102) have demonstrated the ability to protect probiotic cells from harsh environments. As an example, the encapsulation of Lactobacillus plantarum by fructooligosaccharides developed by electrospinning was studied by Feng et al.98 Prebiotics are indigestible fibers that serve as substrates for probiotics (such as Bifidobacteria spp. and Lactobacilli) and selectively stimulate their growth and activity. The co-administration of probiotics and prebiotics (such as oligosaccharides and inulin) is enabled by nanostructured encapsulation techniques and has been proposed as a new strategy to improve probiotic survival, for example, by protecting them from acidity and bile salts,103 and promoting probiotic cell proliferation. It has also been reported that electrospun FOS/PVA nanofibers can significantly improve the survivability of encapsulated cells under moist heat treatment.98 Electrospun nanofibers may be composed of a single polymer or a combination of two or more polymers. Encapsulation by alginate-based electrospun nanofiber mats has been shown to protect probiotic bacteria from the harsh gastrointestinal environment. A viability rate of 64.1 to 70.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 has been achieved for Lactobacillus paracasei KS-199 encapsulated by alginate-based nanofibers in simulated gastrointestinal fluids.102 Electrospun nanofibers based on gum arabic/pullulan are another new type of material that can be used for the protection of probiotic cells. For instance, Lactobacillus-loaded electrospun nanofibres showed a probiotic survivability of 85.38–97.83% and retained viability during 28 day storage at 4 °C.101
CFU mL−1 has been achieved for Lactobacillus paracasei KS-199 encapsulated by alginate-based nanofibers in simulated gastrointestinal fluids.102 Electrospun nanofibers based on gum arabic/pullulan are another new type of material that can be used for the protection of probiotic cells. For instance, Lactobacillus-loaded electrospun nanofibres showed a probiotic survivability of 85.38–97.83% and retained viability during 28 day storage at 4 °C.101
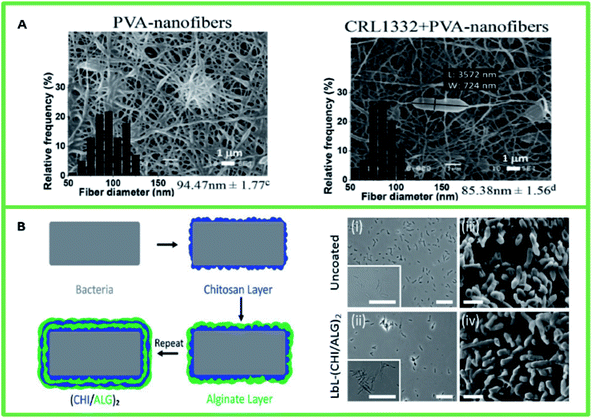 | ||
| Fig. 3 (A) Scanning electron microscopy of PVA nanofibers (left) and PVA nanofibers containing L. rhamnosus CRL1332 (right) obtained by electrospinning. Reproduced with permission from Elsevier, copyright 2021.100 (B) Layer-by-layer encapsulation of probiotic cells. (Left) Schematic LbL templating of chitosan and alginate on bacteria. (Right) Optical microscopy images of (i) uncoated bacteria and (ii) bacteria coated with CHI/ALG. SEM images of (iii) uncoated bacteria and (iv) bacteria coated with CHI/ALG. Reproduced with permission from Wiley, copyright 2021.99 | ||
4.2. Layer-by-layer method
The layer-by-layer (LbL) method is another promising approach to encapsulate and introduce specific probiotic species into the GI tract (Fig. 3B). This strategy is based on coating alternating layers of cationic (e.g., chitosan) and anionic (e.g., alginate) polymers on bacteria via electrostatic interaction.99 The LbL method enhances bacterial protection against acidic environments and bile salts, resulting in the proliferation of encapsulated probiotics on intestinal tissues. This method requires the minimum amount of polymer for complete encapsulation. The LbL technique can be utilized to encapsulate various types of probiotic cells and produce homogeneous nanocoatings with precise control of the structure.104Encapsulating probiotic cells using the LbL technique has been shown to improve bacterial protection in the gastrointestinal tract and promote microbial adhesion and growth at the targeted sites.99,105,106 For instance, Periya et al.105 have shown an enhancement in the survival of probiotic Lactobacillus acidophilus in adverse conditions encountered in the GI tract by encapsulation through LbL self-assembly of polyelectrolytes, chitosan, and carboxymethyl cellulose. The results showed that about 106 CFU/500 mg (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log%) of bacterial cells with nanocoatings survived when exposed to simulated gastric and intestinal fluids for 120 min; however, almost complete death of free cells is observed.105
log%) of bacterial cells with nanocoatings survived when exposed to simulated gastric and intestinal fluids for 120 min; however, almost complete death of free cells is observed.105
5. Summary and future perspectives
Probiotics as pharmaceutical and nutraceutical products are becoming more and more popular in the market. Microencapsulation of probiotics offers several advantages including (1) improving the survival of probiotic bacteria by protecting them against the harsh gastrointestinal environment and undesirable reactions with external factors (such as oxygen, temperature, and light during handling and storage); (2) promoting the effective attachment of probiotics to intestinal mucosal tissues; (3) ensuring the controlled and targeted release of the encapsulated materials with desired concentrations in the gastrointestinal tract; and (4) increasing the ability to incorporate desirable concentrations of probiotics (ranging from low to high levels).Various types of synthetic and natural materials have been traditionally used as EMs; however, there is still room for improvement. The use of nanomaterials in microencapsulation is an innovative approach that enables the manipulation of the physical and chemical properties of microcapsules to improve the delivery of active probiotics to the site of action.
Various types of nanostructures including cellulose, chitosan, MgO, starch, and PLGA nanoparticles have been employed as encapsulating materials to address two main challenges: (1) the viability of probiotics during processing, long-term storage, and passage through the gastrointestinal tract., and (2) controlled release of probiotics in the desired region of the intestinal tract.
The coating of microcapsules with certain materials, such as Eudragit S100 and MgO nanoparticles, has been shown to fill pores in the hydrogel structure of the beads and increase protection from harsh environments for probiotic bacteria. Other nanomaterials such as nanocellulose can be adsorbed on the surface of microorganisms to form a viscous layer that acts as a barrier against the growth of ice crystals near the cells, thereby protecting them during freeze-drying. Also, different types of nanofibers and nanocoatings (e.g., alginate electrospun nanofibers) have been used to improve the viability of probiotic cells during storage and gastrointestinal digestion. EMs can protect probiotic cells from the acidic pH of the stomach and release them in the gut. Moreover, the major challenge faced during the oral administration of probiotic bacteria is the rapid transit from the intestine in feces; this can be addressed by the use of some nanomaterials such as chitosan nanoparticles or nanocoatings, which have been shown to enhance mucoadhesion to the gut wall.
Future advances in nanotechnology and nanostructured microencapsulation techniques can lead to the development of improved probiotic products that can further our understanding of their health benefits.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Sharma, Importance of Probiotics in Cancer Prevention and Treatment, recent developments in applied microbiology and biochemistry, Elsevier, 2019, pp. 33–45 Search PubMed.
- M. S. R. Rajoka, H. Zhao, H. M. Mehwish, N. Li, Y. Lu, Z. Lian, D. Shao, M. Jin, Q. Li and L. Zhao, Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk, Food Res. Int., 2019, 123, 286–297 CrossRef PubMed.
- T. Ayichew, A. Belete, T. Alebachew, H. Tsehaye, H. Berhanu and A. Minwuyelet, Bacterial probiotics their importances and limitations: a review, J. Nutr. Health Sci., 2017, 4, 202 Search PubMed.
- M. R. Dubey and V. P. Patel, Probiotics: A Promising Tool for Calcium Absorption, Open Nutr. J., 2018, 12(1), 59–69 CrossRef CAS.
- L. Lazo-Pérez, Q. Ruiz, A. Elías, F. Herrera and I. Z. Rodríguez, Effect of a VITAFERT microbial additive on some bioproductive and health indicators in growing pigs, Cuban J. Agric. Sci., 2018, 51(3), 321–328 Search PubMed.
- F. Aboulfazli, A. B. Shori and A. S. Baba, Effects of the replacement of cow milk with vegetable milk on probiotics and nutritional profile of fermented ice cream, LWT–Food Sci. Technol., 2016, 70, 261–270 CrossRef CAS.
- M. Pirbaglou, J. Katz, R. J. de Souza, J. C. Stearns, M. Motamed and P. Ritvo, Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials, Nutr. Res., 2016, 36, 889–898 CrossRef CAS PubMed.
- S. Kamiya, H. Yonezawa and T. Osaki, Role of Probiotics in Eradication Therapy for Helicobacter pylori Infection, Helicobacter pylori in Human Diseases: Advances in Microbiology, Infectious Diseases and Public Health, 2019, 11, 243–255 Search PubMed.
- M. T. Cook, G. Tzortzis, D. Charalampopoulos and V. V. Khutoryanskiy, Microencapsulation of probiotics for gastrointestinal delivery, J. Controlled Release, 2012, 162, 56–67 CrossRef CAS PubMed.
- A. Tarasiuk and G. Eibl, Nutritional support and probiotics as potential treatment in IBD, Curr. Drug Targets, 2020, 21(14), 1417–1427 CrossRef CAS PubMed.
- S. Guandalini and N. Sansotta, Probiotics in the treatment of inflammatory bowel disease, Probiotics and Child Gastrointestinal Health, Springer, 2019, pp. 101–107 Search PubMed.
- D. Gibson, A. Godovannyi and S. Gill, Probiotic compositions and uses thereof, US pat., US20200069747A1, 2020, https://patents.google.com/patent/US20200069747A1/en Search PubMed.
- J. Aggarwal, G. Swami and M. Kumar, Probiotics and their effects on metabolic diseases: an update, J. Clin. Diagn. Res., 2013, 7, 173 CAS.
- K. J. Chua, W. C. Kwok, N. Aggarwal, T. Sun and M. W. Chang, Designer probiotics for the prevention and treatment of human diseases, Curr. Opin. Chem. Biol., 2017, 40, 8–16 CrossRef CAS PubMed.
- K. Jia, X. Tong, R. Wang and X. Song, The clinical effects of probiotics for inflammatory bowel disease: A meta-analysis, Medicine, 2018, 97(51), e13792 CrossRef PubMed.
- Z. Akram, S. S. Shafqat, S. Aati, O. Kujan and A. Fawzy, Clinical efficacy of probiotics in the treatment of gingivitis: A systematic review and meta-analysis, Aust. Dent. J., 2020, 65, 12–20 CrossRef CAS PubMed.
- J. Suez, N. Zmora, E. Segal and E. Elinav, The pros, cons, and many unknowns of probiotics, Nat. Med, 2019, 25, 716–729 CrossRef CAS PubMed.
- N. P. Shah, W. E. Lankaputhra, M. L. Britz and W. S. Kyle, Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage, Int. Dairy J., 1995, 5, 515–521 CrossRef.
- A. Geirnaert, A. Steyaert, V. Eeckhaut, B. Debruyne, J. B. Arends, F. Van Immerseel, N. Boon and T. Van de Wiele, Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions, Anaerobe, 2014, 30, 70–74 CrossRef CAS PubMed.
- S. Raghuwanshi, S. Misra, R. Sharma and P. Bisen, Probiotics: nutritional therapeutic tool, J. Probiotics Health, 2018, 6, 2 Search PubMed.
- D. Schell and C. Beermann, Fluidized bed microencapsulation of Lactobacillus reuteri with sweet whey and shellac for improved acid resistance and in-vitro gastro-intestinal survival, Food Res. Int., 2014, 62, 308–314 CrossRef CAS.
- M. Jantzen, A. Göpel and C. Beermann, Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey, J. Appl. Microbiol., 2013, 115, 1029–1036 CrossRef CAS PubMed.
- T. Petrović, V. Nedović, S. Dimitrijević-Branković, B. Bugarski and C. Lacroix, Protection of probiotic microorganisms by microencapsulation, Chem. Ind. Chem. Eng. Q., 2007, 13, 169–174 CrossRef.
- S. Chen, Y. Cao, L. R. Ferguson, Q. Shu and S. Garg, The effect of immobilization of probiotic Lactobacillus reuteri DPC16 in sub-100 μm microcapsule on food-borne pathogens, World J. Microbiol. Biotechnol., 2012, 28, 2447–2452 CrossRef PubMed.
- J.-B. Ahn, H.-J. Hwang and J.-H. Park, Physiological responses of oxygen-tolerant anaerobic Bifidobacterium longum under oxygen, J. Microbiol. Biotechnol., 2001, 11, 443–451 CAS.
- A. Talwalkar and K. Kailasapathy, Effect of microencapsulation on oxygen toxicity in probiotic bacteria, Aust. J. Dairy Technol., 2003, 58, 36 Search PubMed.
- W. Ding and N. P. Shah, An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage, J. Food Sci., 2009, 74, M53–M61 CrossRef CAS PubMed.
- S. Chen, Y. Cao, L. R. Ferguson, Q. Shu and S. Garg, Evaluation of mucoadhesive coatings of chitosan and thiolated chitosan for the colonic delivery of microencapsulated probiotic bacteria, J. Microencapsulation, 2013, 30, 103–115 CrossRef CAS PubMed.
- L. A. A. Lopes, R. d. S. F. Carvalho, N. S. S. Magalhães, M. S. Madruga, A. J. A. A. Athayde, I. A. Portela, C. E. Barão, T. C. Pimentel, M. Magnani and T. C. M. Stamford, Microencapsulation of Lactobacillus acidophilus La-05 and incorporation in vegan milks: Physicochemical characteristics and survival during storage, exposure to stress conditions, and simulated gastrointestinal digestion, Food Res. Int., 2020, 135, 109295 CrossRef PubMed.
- H. Liu, S. W. Cui, M. Chen, Y. Li, R. Liang, F. Xu and F. Zhong, Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review, Crit. Rev. Food Sci. Nutr., 2019, 59, 2863–2878 CrossRef PubMed.
- Y. Byun, Y. T. Kim, K. G. H. Desai and H. J. Park, Microencapsulation techniques for food flavour, Chem. Biol. Volatiles, 2010, 307–332 CAS.
- Q. Shi, D. Liu, Y. Wang, Y. Zhao, X. Yang and J. Huang, High-Performance Sodium-Ion Battery Anode via Rapid Microwave Carbonization of Natural Cellulose Nanofibers with Graphene Initiator, Small, 2019, 15, 1901724 CrossRef CAS PubMed.
- N.-M. Park, S. Choi, J. E. Oh and D. Y. Hwang, Facile extraction of cellulose nanocrystals, Carbohydr. Polym., 2019, 223, 115114 CrossRef PubMed.
- S. Salimi, R. Sotudeh-Gharebagh, R. Zarghami, S. Y. Chan and K. H. Yuen, Production of nanocellulose and its applications in drug delivery: A critical review, ACS Sustainable Chem. Eng., 2019, 7, 15800–15827 CrossRef CAS.
- H. Liu, B. Geng, Y. Chen and H. Wang, Review on the aerogel-type oil sorbents derived from nanocellulose, ACS Sustainable Chem. Eng., 2017, 5, 49–66 CrossRef CAS.
- L. Kian, N. Saba, M. Jawaid and M. Sultan, A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites, Int. J. Biol. Macromol., 2019, 121, 1314–1328 CrossRef CAS PubMed.
- F. Hoeng, A. Denneulin and J. Bras, Use of nanocellulose in printed electronics: a review, Nanoscale, 2016, 8, 13131–13154 RSC.
- S. Mondal, Preparation, properties and applications of nanocellulosic materials, Carbohydr. Polym., 2017, 163, 301–316 CrossRef CAS PubMed.
- T. Huq, C. Fraschini, A. Khan, B. Riedl, J. Bouchard and M. Lacroix, Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin, Carbohydr. Polym., 2017, 168, 61–69 CrossRef CAS PubMed.
- F. K. Nahr, R. R. Mokarram, M. A. Hejazi, B. Ghanbarzadeh, M. S. Khiyabani and K. Z. Benis, Optimization of the nanocellulose based cryoprotective medium to enhance the viability of freeze dried Lactobacillus plantarum using response surface methodology, LWT–Food Sci. Technol., 2015, 64, 326–332 CrossRef.
- H. Zhang, C. Yang, W. Zhou, Q. Luan, W. Li, Q. Deng, X. Dong, H. Tang and F. Huang, A pH-responsive gel macrosphere based on sodium alginate and cellulose nanofiber for potential intestinal delivery of probiotics, ACS Sustainable Chem. Eng., 2018, 6, 13924–13931 CrossRef CAS.
- O. Maleki, M. A. Khaledabad, S. Amiri, A. K. Asl and S. Makouie, Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate-crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability, LWT–Food Sci. Technol., 2020, 109224 CrossRef CAS.
- A. Amalraj, S. Gopi, S. Thomas and J. T. Haponiuk, Cellulose Nanomaterials in Biomedical, Food, and Nutraceutical Applications: A Review, Macromolecular Symposia, Wiley Online Library, 2018, p. 1800115 Search PubMed.
- R. Das and J. G. Fernandez, Cellulose nanofibers for encapsulation and pluripotency preservation in the early development of embryonic stem cells, Biomacromolecules, 2020, 21(12), 4814–4822 CrossRef CAS PubMed.
- Q. Luan, W. Zhou, H. Zhang, Y. Bao, M. Zheng, J. Shi, H. Tang and F. Huang, Cellulose-based composite macrogels from cellulose fiber and cellulose nanofiber as intestine delivery vehicles for probiotics, J. Agric. Food Chem., 2018, 66, 339–345 CrossRef CAS PubMed.
- M. Osorio, A. Cañas, R. Zuluaga, P. Gañán, I. Ortiz and C. Castro, Nanocellulose-Based Composites in Biomedical Applications, Adv. Green Compos., 2018, 369 CAS.
- M. M. Haafiz, A. Hassan, Z. Zakaria, I. Inuwa and M. Islam, Physicochemical characterization of cellulose nanowhiskers extracted from oil palm biomass microcrystalline cellulose, Mater. Lett., 2013, 113, 87–89 CrossRef.
- D. Klemm, E. D. Cranston, D. Fischer, M. Gama, S. A. Kedzior, D. Kralisch, F. Kramer, T. Kondo, T. Lindström and S. Nietzsche, Nanocellulose as a natural source for groundbreaking applications in materials science: Today's state, Mater. Today, 2018, 21, 720–748 CrossRef CAS.
- D. Klemm, D. Schumann, U. Udhardt and S. Marsch, Bacterial synthesized cellulose—artificial blood vessels for microsurgery, Prog. Polym. Sci., 2001, 26, 1561–1603 CrossRef CAS.
- M. Zaborowska, A. Bodin, H. Bäckdahl, J. Popp, A. Goldstein and P. Gatenholm, Microporous bacterial cellulose as a potential scaffold for bone regeneration, Acta Biomater., 2010, 6, 2540–2547 CrossRef CAS PubMed.
- S. Bottan, F. Robotti, P. Jayathissa, A. Hegglin, N. Bahamonde, J. A. Heredia-Guerrero, I. S. Bayer, A. Scarpellini, H. Merker and N. Lindenblatt, Surface-structured bacterial cellulose with guided assembly-based biolithography (GAB), ACS Nano, 2015, 9, 206–219 CrossRef CAS PubMed.
- A. F. Jozala, L. C. de Lencastre-Novaes, A. M. Lopes, V. de Carvalho Santos-Ebinuma, P. G. Mazzola, A. Pessoa Jr, D. Grotto, M. Gerenutti and M. V. Chaud, Bacterial nanocellulose production and application: a 10-year overview, Appl. Microbiol. Biotechnol., 2016, 100, 2063–2072 CrossRef CAS PubMed.
- L. M. Sampaio, J. Padrão, J. Faria, J. P. Silva, C. J. Silva, F. Dourado and A. Zille, Laccase immobilization on bacterial nanocellulose membranes: Antimicrobial, kinetic and stability properties, Carbohydr. Polym., 2016, 145, 1–12 CrossRef CAS PubMed.
- A. C. Khorasani and S. A. Shojaosadati, Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition, Int. J. Biol. Macromol., 2016, 83, 9–18 CrossRef CAS PubMed.
- M. E. Badawy and E. I. Rabea, A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection, Int. J. Carbohydr. Chem., 2011, 2011, 460381 Search PubMed.
- J. Liang, H. Yan, P. Puligundla, X. Gao, Y. Zhou and X. Wan, Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review, Food Hydrocolloids, 2017, 69, 286–292 CrossRef CAS.
- F. Ahmed, F. M. Soliman, M. A. Adly, H. A. Soliman, M. El-Matbouli and M. Saleh, Recent Progress in Biomedical Applications of Chitosan and its Nanocomposites in Aquaculture: A Review, Res. Vet. Sci., 2019, 126, 68–82 CrossRef CAS PubMed.
- M. A. Mohammed, J. Syeda, K. M. Wasan and E. K. Wasan, An overview of chitosan nanoparticles and its application in non-parenteral drug delivery, Pharmaceutics, 2017, 9, 53 CrossRef PubMed.
- L.-F. Călinoiu, B. E. Ştefănescu, I. D. Pop, L. Muntean and D. C. Vodnar, Chitosan coating applications in probiotic microencapsulation, Coatings, 2019, 9, 194 CrossRef.
- L.-M. Zhao, L.-E. Shi, Z.-L. Zhang, J.-M. Chen, D.-D. Shi, J. Yang and Z.-X. Tang, Preparation and application of chitosan nanoparticles and nanofibers, Braz. J. Chem. Eng., 2011, 28, 353–362 CrossRef CAS.
- W. Krasaekoopt, B. Bhandari and H. C. Deeth, Survival of probiotics encapsulated in chitosan-coated alginate beads in yoghurt from UHT-and conventionally treated milk during storage, LWT–Food Sci. Technol., 2006, 39, 177–183 CrossRef CAS.
- I. Bravo-Osuna, C. Vauthier, A. Farabollini, G. F. Palmieri and G. Ponchel, Mucoadhesion mechanism of chitosan and thiolated chitosan-poly (isobutyl cyanoacrylate) core-shell nanoparticles, Biomaterials, 2007, 28, 2233–2243 CrossRef CAS PubMed.
- Y. Kawashima, H. Yamamoto, H. Takeuchi and Y. Kuno, Mucoadhesive DL-lactide/glycolide copolymer nanospheres coated with chitosan to improve oral delivery of elcatonin, Pharm. Dev. Technol., 2000, 5, 77–85 CrossRef CAS PubMed.
- A. Shajahan, S. Shankar, A. Sathiyaseelan, K. S. Narayan, V. Narayanan, V. Kaviyarasan and S. Ignacimuthu, Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes, Int. J. Biol. Macromol., 2017, 104, 1449–1458 CrossRef CAS PubMed.
- N. A. Peppas and Y. Huang, Nanoscale technology of mucoadhesive interactions, Adv. Drug Delivery Rev., 2004, 56, 1675–1687 CrossRef CAS PubMed.
- S. Raghuwanshi, R. Agarwal, R. Raval and R. K. Gutti, Chitosan Nanoparticles and Their Applications in Drug Delivery, Hemostasis, and Stem Cell Research, Functional Bionanomaterials, Springer, 2020, pp. 129–143 Search PubMed.
- F. Assa, H. Jafarizadeh-Malmiri, H. Ajamein, H. Vaghari, N. Anarjan, O. Ahmadi and A. Berenjian, Chitosan magnetic nanoparticles for drug delivery systems, Crit. Rev. Biotechnol., 2017, 37, 492–509 CrossRef CAS PubMed.
- K. A. Janes, M. P. Fresneau, A. Marazuela, A. Fabra and M. A. J. Alonso, Chitosan nanoparticles as delivery systems for doxorubicin, J. Controlled Release, 2001, 73, 255–267 CrossRef CAS PubMed.
- B. Shi, Z. Shen, H. Zhang, J. Bi and S. Dai, Exploring N-imidazolyl-O-carboxymethyl chitosan for high performance gene delivery, Biomacromolecules, 2012, 13, 146–153 CrossRef CAS PubMed.
- A. Mawad, Y. A. Helmy, A.-G. Shalkami, D. Kathayat, G. Rajashekara and E. coli, Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro, Appl. Microbiol. Biotechnol., 2018, 102, 10675–10690 CrossRef CAS PubMed.
- R. C. Rowe, P. Sheskey and M. Quinn, Handbook of Pharmaceutical Excipients, Libros Digitales-Pharmaceutical Press, 2009 Search PubMed.
- D. Jain, D. Majumdar and A. Panda, Insulin loaded eudragit L100 microspheres for oral delivery: preliminary in vitro studies, J. Biomater. Appl., 2006, 21, 195–211 CrossRef CAS PubMed.
- L. A. Soares and E. Crema, Study of a delayed-release system for hard and soft capsules coated with eudragit® s100 acrylic polymers, Acta Sci., Health Sci., 2020, 42, e48422 CrossRef.
- D. Jain, A. K. Panda and D. K. Majumdar, Eudragit S100 entrapped insulin microspheres for oral delivery, AAPS PharmSciTech, 2005, 6, E100–E107 CrossRef PubMed.
- F. Ansari, H. Pourjafar, V. Jodat, J. Sahebi and A. Ataei, Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus, AMB Express, 2017, 7, 144 CrossRef PubMed.
- Z. Camtakan, S. Erenturk and S. Yusan, Magnesium oxide nanoparticles: preparation, characterization, and uranium sorption properties, Environ. Prog. Sustainable Energy, 2012, 31, 536–543 CrossRef CAS.
- S. Ge, G. Wang, Y. Shen, Q. Zhang, D. Jia, H. Wang, Q. Dong and T. Yin, Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro, IET Nanobiotechnol., 2011, 5, 36–40 CrossRef CAS PubMed.
- S. Shukla, G. Parashar, A. Mishra, P. Misra, B. Yadav, R. Shukla, L. Bali and G. Dubey, Nano-like magnesium oxide films and its significance in optical fiber humidity sensor, Sens. Actuators, B, 2004, 98, 5–11 CrossRef CAS.
- K. Imada, S. Sakai, H. Kajihara, S. Tanaka and S. Ito, Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease, Plant Pathol., 2016, 65, 551–560 CrossRef CAS.
- M. Yao, B. Li, H. Ye, W. Huang, Q. Luo, H. Xiao, D. J. McClements and L. Li, Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles, Food Hydrocolloids, 2018, 83, 246–252 CrossRef CAS.
- J. Kaur, G. Kaur, S. Sharma and K. Jeet, Cereal starch nanoparticles—A prospective food additive: A review, Crit. Rev. Food Sci. Nutr., 2018, 58, 1097–1107 CrossRef CAS PubMed.
- Á. L. Santana and M. A. A. Meireles, New starches are the trend for industry applications: a review, Food Publ. Health, 2014, 4, 229–241 CrossRef.
- A. Blennow, T. H. Nielsen, L. Baunsgaard, R. Mikkelsen and S. B. Engelsen, Starch phosphorylation: a new front line in starch research, Trends Plant Sci., 2002, 7, 445–450 CrossRef CAS.
- F. Zhu, H. Corke and E. Bertoft, Amylopectin internal molecular structure in relation to physical properties of sweetpotato starch, Carbohydr. Polym., 2011, 84, 907–918 CrossRef CAS.
- H.-Y. Kim, S. S. Park and S.-T. Lim, Preparation, characterization and utilization of starch nanoparticles, Colloids Surf., B, 2015, 126, 607–620 CrossRef CAS.
- M. A. OdeniyiA, O. A. OmotesoB, A. O. AdepojuB and K. T. JaiyeobaE, Starch nanoparticles in drug delivery: A review, Polim. Med., 2018, 48, 41–45 CrossRef PubMed.
- P. H. Campelo, A. S. Sant’Ana and M. T. P. S. Clerici, Starch nanoparticles: production methods, structure, and properties for food applications, Current Opinion in Food Science, 2020, 136–140 CrossRef.
- A. Dufresne, Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals, Molecules, 2010, 15, 4111–4128 CrossRef CAS PubMed.
- D. Le Corre, J. Bras and A. Dufresne, Starch nanoparticles: a review, Biomacromolecules, 2010, 11, 1139–1153 CrossRef CAS PubMed.
- M. Ahmad, A. Gani, F. Hamed and S. Maqsood, Comparative study on utilization of micro and nano sized starch particles for encapsulation of camel milk derived probiotics (Pediococcus acidolactici), LWT–Food Sci. Technol., 2019, 110, 231–238 CrossRef CAS.
- S. Kumari, B. S. Yadav and R. B. Yadav, Synthesis and modification approaches for starch nanoparticles for their emerging food industrial applications: A review, Food Res. Int., 2020, 128, 108765 CrossRef CAS PubMed.
- S. Thangrongthong, N. Puttarat, B. Ladda, T. Itthisoponkul, W. Pinket, K. Kasemwong and M. Taweechotipatr, Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch, Food Sci. Biotechnol., 2020, 29, 1475–1482 CrossRef CAS PubMed.
- G. Ozkan, P. Franco, I. De Marco, J. Xiao and E. Capanoglu, A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications, Food Chem., 2019, 272, 494–506 CrossRef CAS PubMed.
- K. S. Prakash, R. Chavan and V. Mishra, Microencapsulation of Probiotics and its Applications, Frontier Discoveries and Innovations in Interdisciplinary Microbiology, Springer, 2016, pp. 33–44 Search PubMed.
- A. Šipailienė and S. Petraitytė, Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms, Probiotics Antimicrob. Proteins, 2018, 10, 1–10 CrossRef PubMed.
- S. Benita, Microencapsulation: Methods and Industrial Applications, Crc Press, 2005 Search PubMed.
- A. Mortazavian, S. H. Razavi, M. R. Ehsani and S. Sohrabvandi, Principles and Methods of Microencapsulation of Probiotic Microorganisms, 2007 Search PubMed.
- K. Feng, M.-Y. Zhai, Y. Zhang, R. J. Linhardt, M.-H. Zong, L. Li and H. Wu, Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat, J. Agric. Food Chem., 2018, 66, 10890–10897 CrossRef CAS PubMed.
- A. C. Anselmo, K. J. McHugh, J. Webster, R. Langer and A. Jaklenec, Layer-by-layer encapsulation of probiotics for delivery to the microbiome, Adv. Mater., 2016, 28, 9486–9490 CrossRef CAS PubMed.
- J. A. Silva, P. R. De Gregorio, G. Rivero, G. A. Abraham and M. E. F. Nader-Macías, Immobilization of vaginal Lactobacillus in polymeric nanofibers for its incorporation in vaginal probiotic products, Eur. J. Pharm. Sci., 2021, 156, 105563 CrossRef CAS PubMed.
- J. Ma, C. Xu, H. Yu, Z. Feng, W. Yu, L. Gu, Z. Liu, L. Chen, Z. Jiang and J. Hou, Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology, Food Hydrocolloids, 2021, 111, 106381 CrossRef CAS.
- M. T. Yilmaz, O. Taylan, C. Y. Karakas and E. Dertli, An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir, Carbohydr. Polym., 2020, 244, 116447 CrossRef CAS PubMed.
- O. O. Adebola, O. Corcoran and W. A. Morgan, Synbiotics: the impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics, J. Funct. Foods, 2014, 10, 75–84 CrossRef CAS.
- T. Liu, Y. Wang, W. Zhong, B. Li, K. Mequanint, G. Luo and M. Xing, Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives, Adv. Healthcare Mater., 2019, 8, 1800939 CrossRef PubMed.
- A. J. Priya, S. Vijayalakshmi and A. M. Raichur, Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach, J. Agric. Food Chem., 2011, 59, 11838–11845 CrossRef CAS PubMed.
- M. Wang, J. Yang, M. Li, Y. Wang, H. Wu, L. Xiong and Q. Sun, Enhanced viability of layer-by-layer encapsulated Lactobacillus pentosus using chitosan and sodium phytate, Food Chem., 2019, 285, 260–265 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2021 |