Open Access Article
Open Access ArticleGlycine betaine grafted nanocellulose as an effective and bio-based cationic nanocellulose flocculant for wastewater treatment and microalgal harvesting†
Jonas
Blockx
 ab,
An
Verfaillie
ab,
Olivier
Deschaume
c,
Carmen
Bartic
ab,
An
Verfaillie
ab,
Olivier
Deschaume
c,
Carmen
Bartic
 c,
Koenraad
Muylaert
*b and
Wim
Thielemans
c,
Koenraad
Muylaert
*b and
Wim
Thielemans
 *a
*a
aSustainable Materials Laboratory, Department of Chemical Engineering, KU Leuven, Campus Kulak Kortrijk, Etienne Sabbelaan 53 box 7659, 8500, Kortrijk, Belgium. E-mail: Wim.Thielemans@kuleuven.be
bLaboratory for Aquatic Biology, KU Leuven, Campus Kulak Kortrijk, Etienne Sabbelaan 53 box 7659, 8500, Kortrijk, Belgium. E-mail: Koenraad.Muylaert@kuleuven.be
cSoft Matter and Biophysics Unit, Department of Physics and Astronomy, KU Leuven, Celestijnenlaan 200 D, 3001, Leuven, Belgium
First published on 14th June 2021
Abstract
Flocculation is a widely used technology in industry including for wastewater treatment and microalgae harvesting. To increase the sustainability of wastewater treatment, and to avoid contamination of the harvested microalgal biomass, there is a need for bio-based flocculants to replace synthetic polymer flocculants or metal salt coagulants. We developed the first cellulose nanocrystalline flocculant with a grafted cationic point charge, i.e. glycine betaine (i.e. N,N,N-trimethylglycine) grafted cellulose nanocrystals (CNCs) effective for the flocculation of kaolin (a model system for wastewater treatment), the freshwater microalgae Chlorella vulgaris, and the marine microalgae Nannochloropsis oculata. We successfully grafted glycine betaine onto CNCs using a one-pot reaction using a tosyl chloride activated esterification reaction with a degree of substitution ranging from 0.078 ± 0.003 to 0.152 ± 0.002. The degree of substitution is controlled by the reaction conditions. Flocculation of kaolin (0.5 g L−1) required a dose of 2 mg L−1, a comparable dose to commercial polyacrylamide-based flocculants. Flocculation was also successful for freshwater as well as marine microalgae (biomass concentration about 300 mg L−1 dry matter), although the flocculation efficiency of the latter remained below 80%. The dose to induce flocculation (DS = 0.152 ± 0.002) was 20 mg L−1 for the freshwater Chlorella vulgaris and 46 mg L−1 for the marine Nannochloropsis oculata, comparable to other bio-based flocculants such as chitosan or TanFloc.
Introduction
Flocculation is an important process in both the microalgal industry and the wastewater treatment industry.1,2 Flocculation is mostly induced by addition of metal salts or synthetic polymers.1 Polymer flocculants are especially attractive because they form large flocs with a low dose.3 In the wastewater industry, there is a growing demand to replace synthetic polymers by biobased alternatives to reduce environmental impact and toxicity.4 Examples of biobased flocculants are chitosan,3,5–9 cationic starch,10,11 poly-γ-glutamic acid,12 tannins13 and modified cellulose.14–17 In microalgae harvesting, it is of paramount importance to use bio-based flocculants as the flocculant ends up in and therefore contaminates the harvested biomass. A general overview of the most important types of flocculants and their main advantages and disadvantages are listed in Table 1.| Flocculant type | Main advantages | Main disadvantages | References |
|---|---|---|---|
| Metal salts | Low cost | High dose requirement | 18 and 19 |
| Suitable for high ionic strength media | Biomass contamination | ||
| Synthetic polymers | Low dose requirement | Biomass contamination | 20 and 21 |
| Unsuitable for high ionic strength media | |||
| Bio-based polymers | Low dose requirement | Unsuitable for high ionic strength media | 3, 5–13 and 22 |
| No biomass contamination | |||
| Existing CNC-based flocculants | Low dose requirement | Synthetic grafts | 14–17 and 23 |
| Suitable for high ionic strength media | |||
| Control of reaction flocculant properties |
Cellulose is an abundantly available, biodegradable and renewable resource that is an attractive feedstock for the production of novel bio-based chemicals.24 The crystalline parts, extracted from native cellulose as cellulose nanocrystals (CNCs), have excellent mechanical properties, a high aspect ratio, a low density (1.6 g cm−3) and high surface reactivity due to available OH-groups.24–26 Surface modification of the hydroxyl groups on the CNCs can be used to introduce new surface functionalities that bestow new properties leading to new and different applications.25,26 Several studies have been using modified CNCs as flocculants in wastewater treatment, both with anionic and cationic charges.15–17,27–30 Some studies used anionically modified CNCs as a coagulant aid in combination with metal salt coagulants to improve the flocculation process.27,28,31,32 Other studies used cationically modified CNCs for the removal of negatively charged particles from wastewater, which avoids the use of metal coagulants.28,29,33–35 Few papers also report on the flocculation of microalgae with cationic CNCs. Vandamme et al.15 and Blockx et al.17 used fixed positive charges for flocculation while, Eyley et al.16 and Ge et al.14 used pH-responsive flocculants. Recently we also reported the successful flocculation of marine microalgae using cationic CNCs.36 Cationic modified hairy CNCs were also reported to flocculate microalgae.37
The flocculation mechanism of microalgae with CNC-based flocculants was attributed to an electrostatic patch mechanism, where the flocculant forms electrostatic patches on the surface of the microalgae.17 The higher the charge density on the flocculant, the more efficient the flocculation, indicating the importance of electrostatic interactions. This was also confirmed by atomic force microscopy in an earlier study.38 Moreover, the ability to control the degree of substitution (DS) on the microalgae allows for extra control over the flocculation behavior.39,77 The crystalline structure of the CNCs is critical in enabling the patch mechanism, as the rigidity of the crystal does not allow the flocculant to bend and conform itself to direct all its positive charges to the microalgal surface. Adsorption of cationic CNCs to the microalgal surface thus results in the creation of positive patches where the CNCs are adsorbed next to negative areas without CNCs. The crystalline nature of CNCs has the added advantage that coiling cannot happen in high ionic strength media, allowing the flocculation of marine microalgae, while this is troublesome for many other conventional and bio-based flocculants.36 The flocculation mechanism, rigid conformation and ability to change and tune the amount of surface charges make CNC-based flocculants an interesting alternative for flocculation as it can be utilized universally and under widely varying conditions.
One of the main drivers for the use of CNC-based flocculants is the biological origin of the cellulose.28 However, when modifying the CNCs with cationic charges, the added functional group is often not bio-based and could even render the CNCs toxic.40 Examples of reported synthetic cationic grafts are poly(4-vinylpyridine), polyacrylamide derivatives, polyethylenimine, and pyridinium- and imidazolium-derived groups.16,33–35,41–43 With this study, we wanted to create cationic CNC-based flocculants where the graft is a bio-based molecule. N,N,N-trimethylglycine, often referred to as glycine betaine or historically even simply as betaine, was chosen for this purpose. It is a naturally occurring amino-acid derivative first discovered in sugar beets (Beta vulgaris) but found in several different foods.44,45 On average, people consume roughly 1 g of glycine betaine every day through a regular diet.44 Glycine betaine and its derivatives have been used in many applications ranging from agriculture to medicine.46–48 The chemical properties with a quaternary ammonium group on the one hand and carboxylic acid on the other also found its application in the chemical research fields including polymers, dyes, determination of polarity, ionic liquids, etc.49–51 The presence of the carboxylic acid of betaine makes it a potential candidate for esterification with the hydroxyl groups on the surface of CNCs. Similar esterification reactions on the surface of CNCs have been carried out for a wide variety of grafting agents, as reviewed by Eyley and Thielemans.25 Successful grafting of glycine betaine would also result in cationic CNCs with a permanent point charge, which, to our knowledge, would be the first such CNC-based flocculant and would allow us to compare it to other reported flocculants. We used our previously reported esterification procedure using tosyl chloride in pyridine to graft N,N,N-trimethylglycine to CNCs as it enables us to exert control over the degree of substitution and allows direct one-on-one comparison with other reported CNC-based flocculants prepared using the same procedure. Success in this proof of concept and comparison study is a driver to develop a more sustainable grafting route that allows for the same level of control over the degree of substitution as our current route, and we are working on this.
The newly-made flocculant was tested on two model systems: kaolin and microalgae. Kaolinite (Al2Si2O5(OH)4), the main component of kaolin, is used in several industries such as ceramics production and as a filler in the paper industry.52–54 It is a common clay particle with an overall negative surface charge, which makes it stable in suspension in water, while it is critical to remove these particles from suspension to avoid water pollution. Kaolin is therefore a representative model of clay particles in wastewater treatment. Microalgae are a promising new feedstock for biomass, but harvesting the biomass is challenging.55 Flocculation is considered to be a promising approach to reduce the cost and energy demand of the dewatering of microalgal cultures, and therefore we tested our newly developed cationic CNCs on their ability to flocculate both a freshwater and a marine microalgae species.56
Materials and methods
Materials
Cotton wool (German Pharmacopeia grade, used as the cellulose source for CNC production) and KBr (>99%, IR spectroscopy grade) were obtained from Chem-lab Analytical. Pyridine (99.5%, extra dry) was purchased from Acros Organics NV. Dichloromethane (GPR Rectapur), HCl (37%), and ethanol (99.8%, absolute) were purchased from VWR International. Sodium hydroxide (≥98%, reagent grade, in pellets) and kaolin were purchased from Sigma-Aldrich BVBA. p-Toluenesulfonyl chloride (98%) and N,N,N-trimethyl glycine hydrochloride (glycine betaine HCl, 99%) were purchased from Alfa Aesar. 2,5-Bis(5-tert-butyl-2-benzo-oxazol-2-yl)thiophene (BBOT) EA standard was purchased from Elemental Microanalysis (UK). Homarsel sea salt was bought from Zoutman Roeselare (Roeselare, Belgium). All products were used as received.Synthesis of cellulose nanocrystals (CNCs)
The synthesis of the CNCs was carried out as previously described by Blockx et al.17 Cotton wool (25 g) was added to a heated aqueous solution of HCl (0.5 L, 4 M, 80 °C) and subsequently mechanically stirred for 4 h. The hydrolysis was quenched by pouring the reaction mixture in 2 L of demineralised water. The obtained suspension was washed several times by centrifugation (8500 rpm or 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g, 4 °C, 10 min) of the suspension, removing the supernatant and redispersing in fresh demineralised water until the pH was >4. The product was then dialysed against running deionized water for 72 h, further purified with Amberlite MB-6113 mixed bed ion-exchange resin (50 g) for 24 h prior to sonication (with a Branson Sonifier 250 tip sonicator operated at a frequency of 20.000 kHz for 5 min, 2 s ON and 1 s OFF at 25% power), and freeze-drying for 48 h at −56 °C after freezing the suspension in liquid nitrogen. The dried CNCs were further purified by soxhlet extraction with ethanol to remove any adsorbed organic impurities for 48 h and dried in vacuo at 40 °C overnight.57 This procedure was performed 6 times and the different batches were collected and mixed before being used as starting material for modifications.
000 g, 4 °C, 10 min) of the suspension, removing the supernatant and redispersing in fresh demineralised water until the pH was >4. The product was then dialysed against running deionized water for 72 h, further purified with Amberlite MB-6113 mixed bed ion-exchange resin (50 g) for 24 h prior to sonication (with a Branson Sonifier 250 tip sonicator operated at a frequency of 20.000 kHz for 5 min, 2 s ON and 1 s OFF at 25% power), and freeze-drying for 48 h at −56 °C after freezing the suspension in liquid nitrogen. The dried CNCs were further purified by soxhlet extraction with ethanol to remove any adsorbed organic impurities for 48 h and dried in vacuo at 40 °C overnight.57 This procedure was performed 6 times and the different batches were collected and mixed before being used as starting material for modifications.
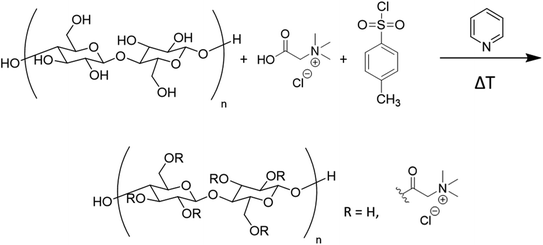
Synthesis of betainium grafted CNCs ([Cl][Bet]-g-CNCs)
Betainium grafted CNCs were synthesised using a one-pot reaction as shown in Fig. 1. The reaction was performed five times under different reaction conditions (time, temperature) and using different molar ratios of the reagents as shown in Table 2 with the aim to obtain different degrees of substitution (DS). It was shown before for similar esterification reactions that controlling these reaction conditions enabled control over the DS.17,58 The CNCs, p-toluenesulfonyl chloride, and glycine betaine HCl were suspended in dry pyridine (200 mL) under argon atmosphere. The suspension was stirred for the indicated time at the indicated temperature (Table 2). Dichloromethane (DCM) was then added to the reaction mixture to stop the reaction and the suspension was filtered through a soxhlet extraction thimble. The reaction products were washed by soxhlet extraction, first with DCM (24 h), then with ethanol (48 h). After drying in vacuo (24 h, 40 °C) a white to light brown solid powder was obtained. The betainium grafted CNCs ([Cl][Bet]-g-CNCs) will be further referred to as bet-g-CNCs throughout this work.| CNCs (g) | p-Toluenesulfonyl-chloride (g, mmol) | Glycine betaine HCl (g, mmol) | Temperature (°C) | Reaction time (h) | DS |
|---|---|---|---|---|---|
| 5.0 | 5.3 g (27.6 mmol) | 4.3 g (27.9 mmol) | 80 | 16 | 0.152 ± 0.002 |
| 5.0 | 5.2 g (27.2 mmol) | 4.2 g (27.6 mmol) | 25 | 16 | 0.107 ± 0.002 |
| 5.0 | 5.3 g (27.7 mmol) | 4.3 g (27.8 mmol) | 70 | 16 | 0.091 ± 0.004 |
| 5.0 | 2.6 g (13.5 mmol) | 2.3 g (14.9 mmol) | 70 | 16 | 0.080 ± 0.003 |
| 5.0 | 5.3 g (27.6 mmol) | 4.3 g (27.9 mmol) | 80 | 48 | 0.078 ± 0.003 |
Characterisation of the CNCs
The characterisation of the unmodified and modified CNCs was done according to a method developed in our laboratory.39,77 A Bruker ALPHA FT-IR spectrophotometer was used to assess the success of the modification of the CNCs. Transparent pellets of dry unmodified and modified CNCs (1.5 mg) mixed with potassium bromide (200 mg) were used to measure the transmission spectra between 4000 and 400 cm−1 as the sum of 16 scans.ζ-Potential measurements were performed to verify the presence of positive charges on the CNCs. A suspension of unmodified and modified CNCs was prepared at a concentration of 0.01 wt% in MilliQ water and sonicated (Agar Scientific bath sonicator, 60 W, 40 kHz, for 5 min). The suspensions were measured on a Brookhaven NanoBrook Omni instrument in phase analysis light scattering mode. Equilibration was allowed before measurement of 30 cycles. The reported values are an average of three samples, three measurements each.
The degree of substitution (DS) was determined by combining the results of elemental analysis (EA) corrected by the water content obtained with thermogravimetric analysis (TGA) as described by Eyley and Thielemans.25,42 Briefly, a Thermo Scientific FLASH 2000 elemental analyser was used to obtain the mass fractions of C, H, N and S of approximately 1 mg of dried CNCs. 2,5-Bis(5-tert-butyl-2-benzo-oxazol-2-yl)thiophene (BBOT) was chosen as the calibration standard and 10 mg of vanadium(V) allowed for an accurate sulphur determination. The measurements were performed in triplicate. These values were corrected for the water content, taken as the mass loss at 120 °C during TGA measurements on a Netzsch TG 209 F3 tarsus. Approximately 5 mg sample was heated in platinum crucibles to 600 °C with a ramp rate of 10 °C min−1. The chemical knowledge of the modified CNCs, where hydroxyl groups are replaced by betainium grafts, is used in combination with the experimental (EA and TGA) results. The difference between the expected chemical empirical formula of the modified anhydroglucose unit (AGU) of the CNCs and the experimental elemental values was minimized, resulting in the best fitting DS.
X-ray photoelectron spectroscopy (XPS) was performed to assess the purity of the samples. The measurements were done on a Kratos Axis Supra photoelectron spectrometer with a monochromated Al- Kα (hν = 1486.7 eV, 5 mA) X-ray source and hybrid (magnetic/electrostatic) optics. The hemispherical analyser was used for survey spectra at a pass energy of 160 eV and for high resolution spectra at a pass energy of 20 eV. An electron flood gun within the field of the magnetic lens was used to acquire spectra under charge neutralisation conditions. Data processing of the spectra was performed in CasaXPS using the aliphatic carbon peak for correction and fixing it at 285.0 EV. For integration, the Tougaard 2-parameter backgrounds were used and LA(α, m) (Voigtian function with Lorentian exponent α and Gaussian line width m) lineshapes for fitting high resolution spectra. For the oxygen 1s spectra LF(α, β, w, m, n) (Lorentian exponents α = β, tail dampening w, Gaussian line width m and n the amount of times the Gaussian is applied) asymmetric lineshapes were used to fit the data. Kratos Analytical (Manchester, UK) supplied empirical relative sensitivity factors for use in the quantification.
For atomic force microscopy characterisation, betaine-modified CNCs were dispersed in ultrapure water to a concentration of 0.1 wt%, and the resulting suspensions were horn-sonicated for 15 s. The suspensions were incubated for 1 min on silicon substrates freshly cleaned with piranha solution, before rinsing with ultrapure water and drying in a flow of nitrogen gas. An Agilent 5500 AFM system with MSNL-F cantilevers (f = 110 = 120 kHz, k = 0.6 N m−1, average tip radius of 2–12 nm) was used for topographical imaging in intermittent contact mode. The AFM topography images were levelled and line-corrected using Gwyddion,59 a free and open-source SPM (scanning probe microscopy) data visualization and analysis program. CNC size analysis was performed on 5 × 5 μm images (at least 300 particles) using ImageJ, to measure CNC length and height.60 The values of CNC length were corrected for an AFM tip radius of c.a. 14 nm, measured as the difference between fibre width and fibre height.
Kaolin flocculation experiments
Kaolin is often used as a model system to evaluate the performance of flocculants that are to be used for wastewater treatment.61 Bet-g-CNCs were suspended in stock suspensions of 5 g L−1 in MQ water by sonication (Agar Scientific bath sonicator, 60 W, 40 kHz, 10 min) until a stable suspension was obtained. A stock suspension of kaolin particles was prepared by suspending 0.6 g L−1 kaolin in demineralised water and adjusted to pH = 6 by addition of HCl or NaOH (0.5 M) followed by stirring for 1 h. Aliquots of 50 mL were taken to undergo jar test flocculation experiments. The suspension was stirred fast (550 rpm) while adding flocculant to obtain a specific flocculant concentration (0, 1, 2, 5, 7, 10, 15, 20, 30 and 50 mg L−1). The suspension was then stirred for 20 min at 200 rpm to promote floc formation before flocs were allowed to settle for 30 min. The residual turbidity of the suspension was then evaluated by measuring the optical density (at a wavelength of 750 nm, OD750 nm) of the supernatant (ODf) at around 3 cm below the surface with a UV-Vis spectrometer (Genesys 10 s, Thermo Fisher Scientific). The residual turbidity was compared with the initial turbidity of the kaolin suspension (ODi) and the difference between this value and the ODf calculated according to eqn (1) was a measure for the flocculation efficiency. It should be noted that this indicator is a measure of the separation of kaolin by flocculation followed by sedimentation.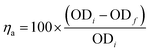 | (1) |
To assess the dependence of the flocculation on pH, a flocculation experiment was carried out at various pH levels. pH values tested were 3.88, 4.94, 5.96, 6.52, 7.11, 7.52, 8.04, 8.52, 8.92 and 9.92, using a flocculant dose of 15 mg L−1.
The ζ-potential of kaolin particles was measured on a filtered (0.2 μm, to remove dust particles) kaolin suspension (0.01 wt%). The suspensions were measured on a Brookhaven NanoBrook Omni instrument in phase analysis light scattering mode. Equilibration was allowed before measurement of 30 cycles. The reported values are an average of three measurements.
Cultivation of microalgae
To assess the potential to use the bet-g-CNCs for microalgae biomass, we used two microalgae species as model systems: the freshwater microalgae species Chlorella vulgaris 211-11b (SAG) and the marine microalgae species Nannochloropsis oculata 38.38 (SAG). We used a freshwater as well as a marine species because it is known that the performance of many flocculants is strongly influenced by the ionic strength of the medium (e.g. Bilanovic and Shelef,62 Roselet et al.63). Both species were cultivated in Wright's Cryptophyte medium in demineralised water, with an additional 30 g L−1 of synthetic sea salt added for Nannochloropsis.64 The algae were cultivated in bubble column photobioreactors (30 L) aerated with filtered (0.2 μm) air (5 L min−1) for mixing and CO2 supply. A pH-stat system was used to control the pH between 8 and 8.5 with CO2 to acidify the culture. Late exponential phase cultures (days 6–8) were used for Chlorella experiments (biomass concentration ± 0.28 g L−1) while the early stationary phase (10–12 days) cultures were used for Nannochlorospis (biomass concentration ± 0.31 g L−1).Flocculation experiments of microalgae
Flocculation experiments with microalgae generally followed the same procedure as the kaolin experiments. The algal cultures were brought to a standardized optical density (ODi) of around 0.7 (corresponding to a biomass concentration of 0.30 g L−1) by diluting the culture with fresh medium if necessary and adjusted to a pH of 6 ± 0.1 by adding 0.5 M of HCl or NaOH. Jar test experiments were performed on 50 mL of culture where the bet-g-CNC flocculant (from the 5 g L−1 stock solution, preparation vide supra) was added while stirring at 550 rpm. The final concentrations for the added CNC flocculant were 0, 10, 20, 40, 50, 60, 75, 100, 150, and 200 mg L−1. The algae were allowed to flocculate at low stirring speed (200 rpm) for 20 min before letting the suspension rest for 30 min to settle. The flocculation efficiency was calculated similarly to the flocculation efficiency of the kaolin suspensions according to eqn (1). In this case the flocculation efficiency also indicates the efficiency of flocculation followed by sedimentation. The ODf was determined as the optical density of the supernatant after settling of the suspension.Statistical analysis
The dose–response curve for each bet-g-CNC flocculant was described by a sigmoidal model in accordance with Lama et al.65 and Blockx et al.17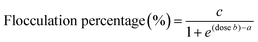 | (2) |
Parameter c equals the maximum flocculation percentage that can be achieved. The ratio a/b indicates the inflection point and equals the dose needed to achieve a flocculation efficiency of 50%. It is generally used as a measure for the minimum bet-g-CNCs dose needed to induce flocculation.17,65 The effect of the degree of substitution (DS) on the dose needed to induce flocculation (a/b) as well as on the maximum flocculation percentage (c), was tested using a general linear model in R (version 3.4.1).
Results and discussion
Characterisation of the bet-g-CNCs
The unmodified CNCs and the bet-g-CNCs were fully characterised (see methods for full description of techniques) and the results were combined to obtain a complete characterization of the products according to a method developed in our laboratory.39,77 The unmodified CNCs were prepared by acid hydrolysis of native cellulose fibres with HCl. The unmodified CNCs were used as template for the one-pot modification with betainium. All analytical results can be found in the ESI Fig. 1 through 11 and Table 1 through 8.†FTIR results confirmed the success of the modification, as was evident from the appearance of two bands around 1756 cm−1 and 1281 cm−1 indicative of the formation of a carbonyl ester bond by reaction of the carboxylic acid from the glycine betaine HCl with the cellulose hydroxyl groups. The presence of positive charges was confirmed by ζ-potential and gave an initial comparison of the charge strength between the different modifications of bet-g-CNCs. While the unmodified CNCs carried a slightly negative ζ-potential, the modified CNCs clearly showed a positive ζ-potential, indicating positive charges on the surface of the CNCs, (ESI Fig. 3†).
TGA was used to determine the water content (the mass loss taken at 120 °C). This value is required for the correct determination of the elemental content, measured by elemental analysis (EA). The results for both TGA and EA can be found in ESI Table 2.† The degree of substitution (DS) can subsequently be calculated from the water corrected results obtained from EA. For the bet-g-CNCs, the higher the nitrogen (and carbon) content within a sample, compared to the unmodified CNCs, the higher the calculated DS of the sample (see Table 2 and ESI Table 2†). The different calculated DS can be found in Table 2. In previous studies, performed within the authors' research group, modifying CNCs with cationic groups with different DS could be well controlled by changing these reaction parameters.17,58 When comparing the results obtained for the modification of CNCs with glycine betaine as shown in Table 2, it can be seen that the different reaction conditions did not result in substantial changes in DS. This is the most clearly visible for the modification at 80 °C for 48 h: it was expected that the larger amount of reactants (compared to CNCs), high temperature, and long reaction time would result in the highest DS, yet it has the lowest DS of all modifications. The sample with DS = 0.107 ± 0.002 (molar ratio of the products = 1, 25 °C, 16 h reaction) was expected to have a lower DS compared to the samples modified at high temperature, as was earlier shown in Blockx et al. (2019) for pyridinium and methylimidazolium modifications of CNCs.17 Despite the generally low DS, differences in DS were reflected in differences in zeta potential (ESI Fig. 3†) as well as in the flocculation performance of the bet-g-CNCs (see below).
XPS measurements of the different samples confirm the FTIR, EA and TGA results discussed before. The chloride anion is the only counterion present, but when zooming in on the chorine 2p peak ranging between 195.45 and 206.60 eV shows apart from the counterion also a second chemical environment: partial chlorination of the cellulose by the p-toluene sulfonyl chloride (observed between 199.35 and 203.35 eV). This partial chlorination has been observed before when similar esterification reactions with p-toluene sulfonyl chloride were performed.15,17
Atomic force microscopy (AFM) was used for visualisation and size analysis of the different bet-g-CNCs. The figures and size distributions can be found in the ESI (SI Fig. 12 through 21†) The unmodified CNCs were not analysed with AFM as they did not stick on the unmodified silicon substrate, while the grafted CNCs did. This is an extra argument for the successful modification of the CNCs with betaine. The bet-g-CNCs display similar size distributions and crystal morphologies independently of DS. The lengths of the bet-g-CNCs varied between 89 ± 100 nm and 104 ± 118 nm for the samples with a DS = 0.078 ± 0.003 and 0.008 ± 0.003 respectively.
Successful surface modification of CNCs with betaine was obtained. Compared to previous studies, where the surface modification was performed with the use of a benzoic acid graft, this work achieved an aliphatic graft, which has been shown previously to be non-trivial.15,17,25,66 The benzoic acid linker could be omitted, resulting in cationic CNCs which are the first example of a fully bio-based product, with abundantly available and low-cost grafted molecules. The synthetic route presented in this work cannot be considered sustainable at this point due to the use of pyridine and tosyl chloride, but we choose it as it enables us to control the degree of substitution and we wanted to first establish the performance of these new flocculants in wastewater treatment and algal harvesting before embarking on the development of a new synthesis route.
Evaluation of the bet-g-CNCs for kaolin flocculation
The five different DS of the bet-g-CNCs were tested in kaolin flocculation experiments to evaluate their potential for use in wastewater treatment. The results of the experiments can be found in (Fig. 2). The different samples of bet-g-CNCs were able to remove the kaolin particles with high (>90%) efficiency for very low doses. The lowest dose of 2 mg L−1 was required for bet-g-CNCs with a DS = 0.152 ± 0.002, and a dose of 7 mg L−1 was required for bet-g-CNCs with a DS = 0.078 ± 0.003. The higher the DS of the flocculant the lower the flocculant dose that was required to fully remove kaolin from suspension, in accordance with the expectations. There is a higher charge density for samples with a higher DS and since the flocculation of kaolin is believed to take place by electrostatic interactions, a lower dose should be required when using flocculants with a higher opposite charge.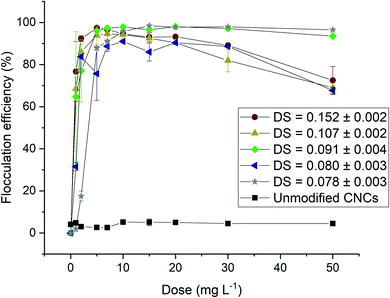 | ||
| Fig. 2 Flocculation efficiency of kaolin with unmodified CNCs and CNCs functionalized with glycine betaine (DS between 0.078 ± 0.003 and 0.152 ± 0.002) at pH = 6. | ||
The bet-g-CNC flocculation efficiency was not affected by changes in pH due to a fixed cationic charge on the quaternary ammonium group. This was confirmed by a flocculation test with different flocculation pH and constant flocculant dose (15 mg L−1, ESI Fig. 23†).
The efficiency of particle removal with bet-g-CNCs is in accordance with or more efficient than reported biopolymer-based wastewater treatment for kaolin model systems.34,53,54,67,68 Cationic starch54 required >5 mg g−1 to remove the kaolin suspensions (compared to 4 mg g−1 reported in this study). Chitosan also effectively removed kaolin from suspension (dose: 2.5 mg g−1), but required a settling time of 22 h. Finally cationic lignin68 required 6 mg g−1 to remove 96.4% of the kaolin from suspension. Cellulose-based flocculants are proven effective when considering coagulation–flocculation, where an inorganic coagulant is needed before the cellulose based flocculant is added.69–71 The synthesis of negatively-charged CNCs is often straightforward and could therefore be considered easier than the synthesis of bet-g-CNCs.69 However, the amount of material needed, both the coagulant and the cellulose-based flocculant, is often significantly higher than what is reported in this study: 30 mg L−1 of the coagulant CaCl2 and 40 mg L−1 of the flocculant aid is not uncommon.70,71 Direct flocculation of kaolin with cationic CNC-based materials has also been reported on several occasions.34,72,73 Microcrystalline cellulose, grafted with acrylamide was reported to require a 3.2 mg g−1 dose to remove >98% of the kaolin from suspension.73 Polyacrylamide grafted CNCs were used to efficiently flocculate kaolin where they report 400 mg g−1 as optimal flocculant dose requirement.34 Cationized dialdehyde celluloses (CDACs) were also successfully used, but no exact concentration of kaolin was reported.72 The flocculation dose for some of these studies is within the same order of magnitude as for the bet-g-CNCs, but these studies make use of polyacrylamide grafts, which is preferably avoided when looking for a biobased product.34,73
Evaluation of the bet-g-CNCs for flocculation of microalgae
Flocculation of microalgae with cationically modified CNCs has recently successfully been carried out in several studies.15–17,66 The functional groups with which the flocculants were modified in these papers were not bio-based: Vandamme et al.15 and Blockx et al.17 used pyridinium-grafted CNCs, with bromomethylbenzoic acid as a reagent to chemically link pyridinium to the CNCs. Eyley et al.16 and Blockx et al.17 used imidazolium-based functional groups linked to the CNCs with the same linker molecule as for the pyridinium grafts and Ge et al.66 made use of 1-(3-aminopropyl)imidazole (APIm). All of these molecules that are part of the final flocculant are synthetic molecules. On the other hand, the bet-g-CNCs are entirely bio-based since glycine betaine is a molecule of biological origin and there is no need for a linker molecule.In Fig. 3, the flocculation efficiency of the bet-g-CNCs with different DS is shown for the freshwater microalgae Chlorella vulgaris. A high flocculation efficiency (>84%) was achieved for all tested DS. An increase in DS correlated with an increase in flocculation efficiency. For a DS = 0.152 ± 0.002 a flocculation efficiency of 95% was achieved at a flocculant dose of 40 mg L−1.
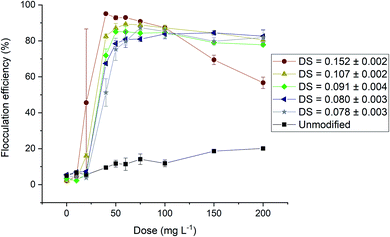 | ||
| Fig. 3 Flocculation efficiency of Chlorella v. with unmodified CNCs and bet-g-CNCs (DS between 0.078 ± 0.003 and 0.152 ± 0.002) at pH = 6. | ||
The dose–response curves for all bet-g-CNC flocculants were modelled as sigmoidal functions in line with standard practice. There was no significant difference between the sigmoidal models and the datapoints according to a chi square test (p > 0.097) (ESI Table 9†). The high correlation (>0.99) between the predicted values and measured data shows the model can be used to predict dose–response curves for flocculation. The only exception was bet-g-CNC with DS = 0.152 ± 0.002, where a deviation from the sigmoidal model (correlation = 0.88) is due to a decrease in flocculation efficiency at high doses. The calculated values for a, b and c of the sigmoidal models were compared for all bet-g-CNCs. Parameter c equals the theoretical maximum flocculation percentage and calculated values are presented in SI Fig. 22.† The ratio a/b corresponds to the inflection point of the sigmoidal curve, which is commonly taken as the dose needed to induce flocculation. The lower this ratio, the lower the dose needed to induce flocculation. The values for the inflection point were used to evaluate the effect of DS on flocculation based on a general linear regression. An increase in DS significantly reduced the dose needed to achieve a flocculation efficiency of 50% (p < 0.0003, adjusted R2 = 0.97, Fig. 4). These observations are in line with the results for the PYR-and MIM-functionalised CNCs earlier reported by our group.17
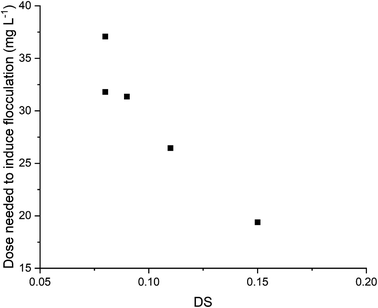 | ||
| Fig. 4 Degree of substitution (DS) of bet-g-CNCs vs. the dose needed to induce flocculation, based on the inflection point of the modeled sigmoidal curves. | ||
To compare with other types of positive charges (as methylimidazolium or pyridinium) reported earlier (Blockx et al. 2019),17 the effect of the type of charge (methylimidazolium, pyridinium or betainium) on the minimum dose needed to induce flocculation was compared between modified CNCs with a similar DS. The minimum dose was recalculated to mmol AGU L−1 to correct for the difference in molecular weight between the grafted positive charges. For PYR-modified CNCs (DS = 0.13) and MIM-modified CNCs (DS = 0.10), the reported minimum dose to induce flocculation in this previous study17 after correction for the AGU, were 0.22 mmol L−1 and 0.25 mmol L−1 respectively. This dose is higher compared to bet-g-CNCs (DS = 0.107 ± 0.002), which only required 0.15 mmol L−1 to induce flocculation.
Many polymer flocculants perform poorly in seawater because the high ionic strength of seawater results in shielding of charged groups and this results in coiling of the polymer.20 It was shown recently that CNC-based flocculants bearing pyridinium or methylimidazolium groups are efficient in flocculating marine microalgae.36 It was concluded that the rigidity of the crystals avoids the coiling of the CNC-based flocculants. For bet-g-CNCs prepared in this work, the flocculation of Nannochloropsis oculata was indeed successful as can be seen in Fig. 5. The maximum flocculation efficiency, however, was lower when compared to the experiments with the freshwater microalgae Chlorella vulgaris (<80% for all different DS for all applied doses). When comparing the flocculation efficiency at an applied dose of 50 mg L−1, the flocculation efficiency for fresh water microalgae ranges between 78.5 ± 1.7% (for DS = 0.078 ± 0.003) and 92.9 ± 1.1% (for DS = 0.152 ± 0.002), while this was much lower for salt water microalgae ranging between 32.5 ± 3.6% (for DS = 0.080 ± 0.003) and 59.3 ± 4.8% (for DS = 0.078 ± 0.003).
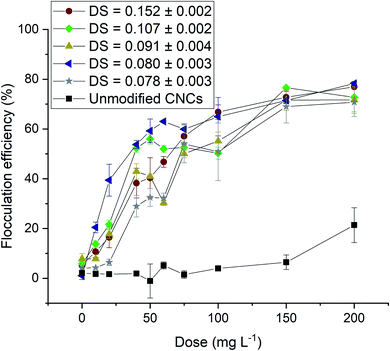 | ||
| Fig. 5 Flocculation efficiency of Nannochloropsis o. with unmodified CNCs and bet-g-CNCs (DS between 0.078 ± 0.003 and 0.152 ± 0.002) at pH = 6. | ||
It should be noted that the flocculation efficiency in our experiments reflects flocculation combined with subsequent gravity sedimentation. We noted in our experiments that the bet-g-CNCs induced the formation of flocs in Nannochloropsis oculata suspensions, but a proportion of the flocs failed to settle. This is in line with the results for the flocculation with PYR- or MIM-modified CNCs reported earlier by Verfaillie et al.36 In this study, the small flocs that remained in suspension could be easily removed by centrifugation at low centrifugal forces or by screening using a mesh with a coarse mesh size (20 μm), without clogging the filter.
Overall, the flocculation results for bet-g-CNCs are similar to the results of PYR- and MIM-modified CNCs, if one compares CNCs with similar DS. The maximum DS achieved in this study is lower than the theoretically maximally obtainable DS. However, the bet-g-CNCs have a point charge and there is no need to have a linker between the CNCs and the cationic group, which makes it cheaper and easier to obtain cationically modified CNCs. A much higher dose was needed to induce flocculation (ratio a/b, calculated using the model in eqn (2)) of microalgae suspensions when compared to the kaolin suspensions. On a per weight basis, the dose was 1.7 mg g−1 for kaolin, 69 mg g−1 for the freshwater microalgae Chlorella and 148.4 mg g−1 for the marine microalgae Nannochloropsis for bet-g-CNCs with a DS = 0.152 ± 0.002. This is in accordance with flocculant requirements found in literature; generally, a higher dose is required for the efficient flocculation of microalgae, compared to kaolin flocculation. Several explanations could be given for this difference: the colloids (algal cells or kaolin particles) have very different sizes and shapes; algae are an order of magnitude larger in diameter than kaolin particles. Also, the charge density of the particles is different, kaolin is less colloidally stable than microalgae as is indicated by their ζ-potential values respectively −25.4 ± 3.1 mV for kaolin and −51.3 ± 0.4 mV for Chlorella vulgaris. The suspension is also very different: in the algal cultures, ions (nutrients and sea salt) and organic molecules (generally referred to as algal organic matter (AOM)) are present which interfere with the flocculation mechanism.63,74
Table 3 compares the bet-g-CNCs synthesised in this study with common flocculants for both fresh water microalgae and marine microalgae. Flocculation with metal salts and synthetic polymers results in contamination of the biomass and are therefore not ideal candidates albeit they have a good flocculation efficiency (synthetic polymers) or have a low cost (metal salts).63,65,75 When comparing biobased polymers, the CNC-based flocculants are only slightly less efficient compared to chitosan for freshwater microalgae, but, due to their rigidity and consequently resistance to coiling, the CNC-based flocculants are superior for flocculating marine microalgae. Considering the lower DS obtained for the bet-g-CNCs, they could potentially prove more efficient compared to PYR-or MIM-modified CNCs.
| Flocculant type | Algal species | Flocculation efficiency (%) | Dose (mg g−1 algae) | Remarks | References | |
|---|---|---|---|---|---|---|
| Fresh water microalgae | ||||||
| Metal salts | Ferric chloride | C. zofingiensis | >90% | >1000 | 75 | |
| Synthetic polymers | Flopam FO 4800SH | C. vulgaris | >90% | 66 | 63 | |
| Bio-based polymers | Chitosan | C. vulgaris | >90% | 36 | 17 | |
| PYR-g-CNCs (DS = 0.34) | C. vulgaris | >90% | 72 | 17 | ||
| Bet-g-CNCs (DS = 0.154 ± 0.002) | C. vulgaris | 50% | 69 | Flocculation efficiency of 95% was obtained | Current study | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Marine microalgae | ||||||
| Metal salts | Ferric chloride | N. oculata | >90% | 30 | Species specific, higher dose requirement for other species | 65 |
| Synthetic polymers | Flopam FO 4800SH | N. oculata | >80% | 66 | 63 | |
| Bio-based polymers | Chitosan | N. oculata | >80% | 300 | 9 | |
| PYR-g-CNCs (DS = 0.34) | N. oculata | >75% | 83 | 36 | ||
| Bet-g-CNCs (DS = 0.154 ± 0.002) | N. oculata | 50% | 148 | Flocculation efficiency of 75% was obtained | Current study | |
Further research however is required to optimize the reaction and the flocculant. A higher DS could still be achieved without loss of the CNC crystallinity, and several of the reaction products, such as pyridine, tosyl chloride and DCM, are to be avoided. Avoiding these products and increasing the reaction DS would benefit the environmental impact and potentially reduce the production cost of the flocculant making it more industrially relevant. However, bet-g-CNCs are the first example of a cationic CNC-based flocculant with a permanent cationic point charge, while in previous studies, the (methyl)imidazolium and pyridinium grafts have a cationic charge on an aromatic ring.15–17,23,66 These new bet-g-CNCs are the first CNC-based flocculant with proven flocculation efficacy for microalgae harvesting and in the field of wastewater treatment. Furthermore, these fully bio-based cationic CNCs could have other potential applications that could be further investigated, for example in electrostatically stabilised gels or composites together with anionic polymers or in electromagnetic functional composites for electronic devices.76
Conclusion
Cationic CNCs with betainium functional groups were successfully synthesised using a one-pot esterification reaction. The final bio-based product had a maximum DS = 0.152 ± 0.002 and the cationic moiety could be directly grafted to the CNC surface, making the reaction more atom efficient. The cationically modified CNCs were successfully used for waste-water treatment, with kaolin as model suspended particle species and for the flocculation of both fresh and marine water microalgae. The new flocculant has a fixed biobased positive charge, which makes it perform independently of the pH of the medium. The rigidity of the cellulose nanocrystals has the advantage that the flocculant can also be used to flocculate species in media with different ionic strengths as it does not coil under these conditions. Improvements can be made by gaining more understanding of the reaction properties to obtain a higher DS or by making the synthesis more environmentally friendly by using other solvents.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank Jolien Joye for the great help with the experimental work during her internship supervised by J.B, and Dr Samuel Eyley from the Sustainable Materials Lab, KU Leuven campus Kulak Kortrijk for the X-ray Photoelectron Spectroscopy data acquisition. The authors also want to acknowledge financial support for this work from Research Foundation Flanders (grant G.0608.16N), KU Leuven (grant C14/18/061), and from the EU Interreg France-Wallonie-Vlaanderen program through the ALPO project. W. T. further acknowledges the Provincie West-Vlaanderen for his Chair in Advance Materials, Research Foundation Flanders for his Odysseus fellowship (grant G.0C60.13N), and the European Union's European Fund for Regional Development, Flanders Innovation & Entrepreneurship, and the Province of West-Flanders for financial support in the Accelerate3 project (Interreg Vlaanderen-Nederland program).References
- E. Molina Grima, E. H. Belarbi, F. G. Acién Fernández, A. Robles Medina and Y. Chisti, Recovery of Microalgal Biomass and Metabolites: Process Options and Economics, Biotechnol. Adv., 2003, 20(7–8), 491–515, DOI:10.1016/S0734-9750(02)00050-2.
- F. Fu and Q. Wang, Removal of Heavy Metal Ions from Wastewaters: A Review, J. Environ. Manage., 2011, 92(3), 407–418, DOI:10.1016/j.jenvman.2010.11.011.
- F. Renault, B. Sancey, P. Badot and G. Crini, Chitosan for Coagulation/Flocculation Processes – An Eco-Friendly Approach, Eur. Polym. J., 2009, 45(5), 1337–1348, DOI:10.1016/j.eurpolymj.2008.12.027.
- M. R. Granados, F. G. Acién, C. Gómez, J. M. Fernández-Sevilla and E. Molina Grima, Evaluation of Flocculants for the Recovery of Freshwater Microalgae, Bioresour. Technol., 2012, 118, 102–110, DOI:10.1016/j.biortech.2012.05.018.
- R. Divakaran and V. N. S. Pillai, Flocculation of Algae Using Chitosan, J. Appl. Phycol., 2002, 14(5), 419–422, DOI:10.1023/A:1022137023257.
- Y. Xu, S. Purton and F. Baganz, Chitosan Flocculation to Aid the Harvesting of the Microalga Chlorella Sorokiniana, Bioresour. Technol., 2013, 129, 296–301, DOI:10.1016/j.biortech.2012.11.068.
- M. S. Farid, A. Shariati, A. Badakhshan and B. Anvaripour, Using Nano-Chitosan for Harvesting Microalga Nannochloropsis Sp, Bioresour. Technol., 2013, 131, 555–559, DOI:10.1016/j.biortech.2013.01.058.
- A. L. Ahmad, N. H. Mat Yasin, C. J. C. Derek and J. K. Lim, Optimization of Microalgae Coagulation Process Using Chitosan, Chem. Eng. J., 2011, 173(3), 879–882, DOI:10.1016/j.cej.2011.07.070.
- J. Blockx, A. Verfaillie, W. Thielemans and K. Muylaert, Unravelling the Mechanism of Chitosan-Driven Flocculation of Microalgae in Seawater as a Function of PH, ACS Sustain. Chem. Eng., 2018, 6(9), 11273–11279, DOI:10.1021/acssuschemeng.7b04802.
- D. Vandamme, I. Foubert, B. Meesschaert and K. Muylaert, Flocculation of Microalgae Using Cationic Starch, J. Appl. Phycol., 2010, 22(4), 525–530, DOI:10.1007/s10811-009-9488-8.
- C. Banerjee, P. Gupta, S. Mishra, G. Sen, P. Shukla and R. Bandopadhyay, Study of Polyacrylamide Grafted Starch Based Algal Flocculation towards Applications in Algal Biomass Harvesting, Int. J. Biol. Macromol., 2012, 51(4), 456–461, DOI:10.1016/j.ijbiomac.2012.06.011.
- H. Zheng, Z. Gao, J. Yin, X. Tang, X. Ji and H. Huang, Harvesting of Microalgae by Flocculation with Poly (γ-Glutamic Acid), Bioresour. Technol., 2012, 112, 212–220, DOI:10.1016/j.biortech.2012.02.086.
- R. Gutiérrez, F. Passos, I. Ferrer, E. Uggetti and J. García, Harvesting Microalgae from Wastewater Treatment Systems with Natural Flocculants: Effect on Biomass Settling and Biogas Production, Algal Res., 2015, 9, 204–211, DOI:10.1016/j.algal.2015.03.010.
- S. Ge, P. Champagne, H.-D. Wang, P. G. Jessop and M. F. Cunningham, Microalgae Recovery from Water for Biofuel Production Using CO2-Switchable Crystalline Nanocellulose, Environ. Sci. Technol., 2016, 50(14), 7896–7903 Search PubMed.
- D. Vandamme, S. Eyley, G. Van den Mooter, K. Muylaert and W. Thielemans, Highly Charged Cellulose-Based Nanocrystals as Flocculants for Harvesting Chlorella Vulgaris, Bioresour. Technol., 2015, 194, 270–275, DOI:10.1016/j.biortech.2015.07.039.
- S. Eyley, D. Vandamme, S. Lama, G. Van den Mooter, K. Muylaert and W. Thielemans, CO 2 Controlled Flocculation of Microalgae Using PH Responsive Cellulose Nanocrystals, Nanoscale, 2015, 14413–14421, 10.1039/C5NR03853G.
- J. Blockx, A. Verfaillie, S. Eyley, O. Deschaume, C. Bartic, K. Muylaert and W. Thielemans, Cationic Cellulose Nanocrystals for Flocculation of Microalgae: Effect of Degree of Substitution and Crystallinity, ACS Appl. Nano Mater., 2019, 2(6), 3394–3403, DOI:10.1021/acsanm.9b00315.
- M. L. Gerardo, S. Van Den Hende, H. Vervaeren, T. Coward and S. C. Skill, Harvesting of Microalgae within a Biorefinery Approach: A Review of the Developments and Case Studies from Pilot-Plants, Algal Res., 2015, 11, 248–262, DOI:10.1016/j.algal.2015.06.019.
- V. M. Rwehumbiza, R. Harrison and L. Thomsen, Alum-Induced Flocculation of Preconcentrated Nannochloropsis Salina: Residual Aluminium in the Biomass, FAMEs and Its Effects on Microalgae Growth upon Media Recycling, Chem. Eng. J., 2012, 200–202, 168–175, DOI:10.1016/j.cej.2012.06.008.
- F. Roselet, D. Vandamme, M. Roselet, K. Muylaert and P. C. Abreu, Screening of Commercial Natural and Synthetic Cationic Polymers for Flocculation of Freshwater and Marine Microalgae and Effects of Molecular Weight and Charge Density, Algal Res., 2015, 10, 183–188, DOI:10.1016/j.algal.2015.05.008.
- B. Bolto and J. Gregory, Organic Polyelectrolytes in Water Treatment, Water Res., 2007, 41(11), 2301–2324, DOI:10.1016/j.watres.2007.03.012.
- N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde and A. Hoadley, Dewatering of Microalgal Cultures: A Major Bottleneck to Alge-Based Fuels, J. Renewable Sustainable Energy, 2010, 2(1), 012701, DOI:10.1063/1.3294480.
- A. Verfaillie, J. Blockx, R. Praveenkumar, W. Thielemans and K. Muylaert, Harvesting of Marine Microalgae Using Cationic Cellulose Nanocrystals, Carbohydr. Polym., 2020, 116165, DOI:10.1016/j.carbpol.2020.116165.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites, Chem. Soc. Rev., 2011, 40(7), 3941–3994, 10.1039/C0CS00108B.
- S. S. Eyley and W. Thielemans, Surface Modification of Cellulose Nanocrystals, Nanoscale, 2014, 6(14), 7764–7779, 10.1039/c4nr01756k.
- J. Tang, J. Sisler, N. Grishkewich and K. C. Tam, Functionalization of Cellulose Nanocrystals for Advanced Applications, J. Colloid Interface Sci., 2017, 494, 397–409, DOI:10.1016/j.jcis.2017.01.077.
- R. Khiari, S. Dridi-Dhaouadi, C. Aguir and M. F. Mhenni, Experimental Evaluation of Eco-Friendly Flocculants Prepared from Date Palm Rachis, J. Environ. Sci., 2010, 22(10), 1539–1543, DOI:10.1016/S1001-0742(09)60286-2.
- C. S. Lee, J. Robinson and M. F. Chong, A Review on Application of Flocculants in Wastewater Treatment, Process Saf. Environ. Prot., 2014, 92(6), 489–508, DOI:10.1016/j.psep.2014.04.010.
- H. Kono, Cationic Flocculants Derived from Native Cellulose: Preparation, Biodegradability, and Removal of Dyes in Aqueous Solution, Resour.-Effic. Technol., 2017, 3(1), 55–63, DOI:10.1016/j.reffit.2016.11.015.
- B. Yuan, L. Li, V. Murugadoss, S. Vupputuri, J. Wang, N. Alikhani and Z. Guo, Nanocellulose-Based Composite Materials for Wastewater Treatment and Waste-Oil Remediation, ES Food Agrofor., 2020, 41–52, DOI:10.30919/esfaf0004.
- L. Jin, W. Li, Q. Xu and Q. Sun, Amino-Functionalized Nanocrystalline Cellulose as an Adsorbent for Anionic Dyes, Cellulose, 2015, 22(4), 2443–2456, DOI:10.1007/s10570-015-0649-4.
- T. Suopajärvi, H. Liimatainen, O. Hormi and J. Niinimäki, Coagulation-Flocculation Treatment of Municipal Wastewater Based on Anionized Nanocelluloses, Chem. Eng. J., 2013, 231, 59–67, DOI:10.1016/j.cej.2013.07.010.
- K. H. M. Kan, J. Li, K. Wijesekera and E. D. Cranston, Polymer-Grafted Cellulose Nanocrystals as pH Responsive Reversible Flocculants, Biomacromolecules, 2013, 14(9), 3130–3139, DOI:10.1021/bm400752k.
- T. Liu, E. Ding and F. Xue, Polyacrylamide and Poly(N,N-Dimethylacrylamide) Grafted Cellulose Nanocrystals as Efficient Flocculants for Kaolin Suspension, Int. J. Biol. Macromol., 2017, 103, 1107–1112, DOI:10.1016/j.ijbiomac.2017.05.098.
- L. W. Zhang, J. R. Hua, W. J. Zhu, L. Liu, X. L. Du, R. J. Meng and J. M. Yao, Flocculation Performance of Hyperbranched Polyethylenimine-Grafted Cellulose in Wastewater Treatment, ACS Sustain. Chem. Eng., 2018, 6(2), 1592–1601, DOI:10.1021/acssuschemeng.7b02343.
- A. Verfaillie, J. Blockx, R. Praveenkumar, W. Thielemans and K. Muylaert, Harvesting of Marine Microalgae Using Cationic Cellulose Nanocrystals, Carbohydr. Polym., 2020, 240, 116165, DOI:10.1016/j.carbpol.2020.116165.
- P. Lopez-Exposito, C. Campano, T. G. M. van de Ven, C. Negro and A. Blanco, Microalgae Harvesting with the Novel Flocculant Hairy Cationic Nanocrystalline Cellulose, Colloids Surf. B Biointerfaces, 2019, 178(March), 329–336, DOI:10.1016/j.colsurfb.2019.03.018.
- I. Demir, J. Blockx, E. Dague, P. Guiraud, W. Thielemans, K. Muylaert and C. Formosa-Dague, Nanoscale Evidence Unravels Microalgae Flocculation Mechanism Induced by Chitosan, ACS Appl. Bio Mater., 2020, 3(12), 8446–8459, DOI:10.1021/acsabm.0c00772.
- S. Eyley, C. Schütz and W. Thielemans, Surface Chemistry and Characterization of Cellulose Nanocrystals, Cellulose Science and Technology, John Wiley & Sons, Inc., 2018, pp. 223–252 Search PubMed.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Updating and Further Expanding GSK's Solvent Sustainability Guide, Green Chem., 2016, 18(13), 3879–3890, 10.1039/c6gc00611f.
- L. Jasmani, S. Eyley, R. Wallbridge and W. Thielemans, A Facile One-Pot Route to Cationic Cellulose Nanocrystals, Nanoscale, 2013, 5, 10207–10211, 10.1039/c3nr03456a.
- L. Jasmani, S. Eyley, C. Schütz, H. Van Gorp, S. De Feyter and W. Thielemans, One-Pot Functionalization of Cellulose Nanocrystals with Various Cationic Groups, Cellulose, 2016, 23(6), 3569–3576, DOI:10.1007/s10570-016-1052-5.
- S. Qiu, L. Wang, P. Champagne, G. Cao and Z. Chen, Effects of Crystalline Nanocellulose on Wastewater-Cultivated Microalgal Separation and Biomass Composition, Appl. Energy, 2019, 239(July 2018), 207–217, DOI:10.1016/j.apenergy.2019.01.212.
- S. A. S. Craig, Betaine in Human Nutrition, Am. J. Clin. Nutr., 2004, 80(3), 539–549, DOI:10.1093/ajcn/80.3.539.
- S. H. Zeisel, M.-H. Mar, J. C. Howe and J. M. Holden, Concentrations of Choline-Containing Compounds and Betaine in Common Foods, J. Nutr., 2003, 133(5), 1302–1307, DOI:10.1093/jn/133.5.1302.
- T. H. H. Chen and N. Murata, Glycinebetaine Protects Plants against Abiotic Stress: Mechanisms and Biotechnological Applications, Plant, Cell Environ., 2011, 34(1), 1–20, DOI:10.1111/j.1365-3040.2010.02232.x.
- M. Ralser, R. Querfurth, H. J. Warnatz, H. Lehrach, M. L. Yaspo and S. Krobitsch, An Efficient and Economic Enhancer Mix for PCR, Biochem. Biophys. Res. Commun., 2006, 347(3), 747–751, DOI:10.1016/j.bbrc.2006.06.151.
- J. Yang, X. Zhang, Y. H. Ma, G. Gao, X. Chen, H. R. Jia, Y. H. Li, Z. Chen and F. G. Wu, Carbon Dot-Based Platform for Simultaneous Bacterial Distinguishment and Antibacterial Applications, ACS Appl. Mater. Interfaces, 2016, 8(47), 32170–32181, DOI:10.1021/acsami.6b10398.
- S. Kudaibergenov, W. Jaeger and A. Laschewsky, Polymeric Betaines: Synthesis, Characterization, and Application, Adv. Polym. Sci., 2006, 201(1), 157–224, DOI:10.1007/12_078.
- C. Reichardt, Polarity of Ionic Liquids Determined Empirically by Means of Solvatochromic Pyridinium N-Phenolate Betaine Dyes, Green Chem., 2005, 7(5), 339–351, 10.1039/b500106b.
- T. Vander Hoogerstraete, J. Blockx, H. Decoster and K. Binnemans, Selective Single-Step Separation of a Mixture of Three Metal Ions by a Triphasic Ionic-Liquid-Water-Ionic-Liquid Solvent Extraction System, Chem. - Eur. J., 2015, 21(33), 11757–11766, DOI:10.1002/chem.201500825.
- J. Konta, Clay and Man: Clay Raw Materials in the Service of Man, Appl. Clay Sci., 1995, 10(4), 275–335, DOI:10.1016/0169-1317(95)00029-4.
- L. Jin, Y. Wei, Q. Xu, W. Yao and Z. Cheng, Cellulose Nanofibers Prepared from TEMPO-Oxidation of Kraft Pulp and Its Flocculation Effect on Kaolin Clay, J. Appl. Polym. Sci., 2014, 131(12), 1–8, DOI:10.1002/app.40450.
- S. Bratskaya, S. Schwarz, T. Liebert and T. Heinze, Starch Derivatives of High Degree of Functionalization: 10. Flocculation of Kaolin Dispersions, Colloids Surf., A, 2005, 254(1–3), 75–80, DOI:10.1016/j.colsurfa.2004.11.030.
- M. Vanthoor-Koopmans, R. H. Wijffels, M. J. Barbosa and M. H. M. Eppink, Biorefinery of Microalgae for Food and Fuel, Bioresour. Technol., 2013, 135, 142–149, DOI:10.1016/j.biortech.2012.10.135.
- D. Vandamme, I. Foubert and K. Muylaert, Flocculation as a Low-Cost Method for Harvesting Microalgae for Bulk Biomass Production, Trends Biotechnol., 2013, 31(4), 233–239, DOI:10.1016/j.tibtech.2012.12.005.
- M. Labet and W. Thielemans, Improving the Reproducibility of Chemical Reactions on the Surface of Cellulose Nanocrystals: ROP of ε-Caprolactone as a Case Study, Cellulose, 2011, 18(3), 607–617, DOI:10.1007/s10570-011-9527-x.
- S. Lombardo and W. Thielemans, Thermodynamics of the Interactions of Positively Charged Cellulose Nanocrystals with Molecules Bearing Different Amounts of Carboxylate Anions, Phys. Chem. Chem. Phys., 2018, 20, 17637–17647 Search PubMed.
- D. Necas and P. Plapetek, Gwyddion: An Open-Source Software for SPM Data Analysis, Cent. Eur. J. Phys., 2012, 10(1), 181–188, DOI:10.2478/s11534-011-0096-2.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, HISTORICAL Commentary NIH Image to ImageJ: 25 Years of Image Analysis, Nat. Methods, 2012, 9(7), 671–675, DOI:10.1038/nmeth.2089.
- W. X. Gong, S. G. Wang, X. F. Sun, X. W. Liu, Q. Y. Yue and B. Y. Gao, Bioflocculant Production by Culture of Serratia Ficaria and Its Application in Wastewater Treatment, Bioresour. Technol., 2008, 99(11), 4668–4674, DOI:10.1016/j.biortech.2007.09.077.
- D. Bilanovic, G. Shelef and A. Sukenik, Flocculation of Microalgae with Cationic Polymers - Effects of Medium Salinity, Biomass, 1988, 17(1), 65–76, DOI:10.1016/0144-4565(88)90071-6.
- F. Roselet, D. Vandamme, M. Roselet, K. Muylaert and P. C. Abreu, Effects of PH, Salinity, Biomass Concentration, and Algal Organic Matter on Flocculant Efficiency of Synthetic Versus Natural Polymers for Harvesting Microalgae Biomass, BioEnergy Res., 2017, 10(2), 427–437, DOI:10.1007/s12155-016-9806-3.
- R. R. L. Guillard and C. J. Lorenzen, YELLOW-GREEN ALGAE WITH CHLOROPHYLLIDE C 2, J. Phycol., 1972, 8(1), 10–14, DOI:10.1111/j.1529-8817.1972.tb03995.x.
- S. Lama, K. Muylaert, T. B. Karki, I. Foubert, R. K. Henderson and D. Vandamme, Flocculation Properties of Several Microalgae and a Cyanobacterium Species during Ferric Chloride, Chitosan and Alkaline Flocculation, Bioresour. Technol., 2016, 220, 464–470, DOI:10.1016/j.biortech.2016.08.080.
- S. Ge, P. Champagne, H. Wang, P. G. Jessop and M. F. Cunningham, Microalgae Recovery from Water for Biofuel Production Using CO2-Switchable Crystalline Nanocellulose, Environ. Sci. Technol., 2016, 50(14), 7896–7903, DOI:10.1021/acs.est.6b00732.
- E. Guibal, M. Van Vooren, B. A. Dempsey and J. Roussy, A Review of the Use of Chitosan for the Removal of Particulate and Dissolved Contaminants, Sep. Sci. Technol., 2006, 41(11), 2487–2514, DOI:10.1080/01496390600742807.
- Z. Liu, X. Lu, J. Xie, B. Feng and Q. Han, Synthesis of a Novel Tunable Lignin-Based Star Copolymer and Its Flocculation Performance in the Treatment of Kaolin Suspension, Sep. Purif. Technol., 2019, 210(February 2018), 355–363, DOI:10.1016/j.seppur.2018.08.025.
- M. Nourani, M. Baghdadi, M. Javan and G. N. Bidhendi, Production of a Biodegradable Flocculant from Cotton and Evaluation of Its Performance in Coagulation-Flocculation of Kaolin Clay Suspension: Optimization through Response Surface Methodology (RSM), J. Environ. Chem. Eng., 2016, 4(2), 1996–2003, DOI:10.1016/j.jece.2016.03.028.
- H. Y. Yu, D. Z. Zhang, F. F. Lu and J. Yao, New Approach for Single-Step Extraction of Carboxylated Cellulose Nanocrystals for Their Use As Adsorbents and Flocculants, ACS Sustain. Chem. Eng., 2016, 4(5), 2632–2643, DOI:10.1021/acssuschemeng.6b00126.
- H. Zhu, Y. Zhang, X. Yang, H. Liu, L. Shao, X. Zhang and J. Yao, One-Step Green Synthesis of Non-Hazardous Dicarboxyl Cellulose Flocculant and Its Flocculation Activity Evaluation, J. Hazard. Mater., 2015, 296, 1–8, DOI:10.1016/j.jhazmat.2015.04.029.
- H. Liimatainen, J. Sirviö, O. Sundman, M. Visanko, O. Hormi and J. Niinimäki, Flocculation Performance of a Cationic Biopolymer Derived from a Cellulosic Source in Mild Aqueous Solution, Bioresour. Technol., 2011, 102(20), 9626–9632, DOI:10.1016/j.biortech.2011.07.099.
- X. Yu, X. Huang, C. Bai and X. Xiong, Modification of Microcrystalline Cellulose with Acrylamide under Microwave Irradiation and Its Application as Flocculant, Environ. Sci. Pollut. Res., 2019, 32859–32865, DOI:10.1007/s11356-019-06317-1.
- D. Vandamme, I. Foubert, I. Fraeye and K. Muylaert, Influence of Organic Matter Generated by Chlorella Vulgaris on Five Different Modes of Flocculation, Bioresour. Technol., 2012, 124, 508–511, DOI:10.1016/j.biortech.2012.08.121.
- N. B. Wyatt, L. M. Gloe, P. V. Brady, J. C. Hewson, A. M. Grillet, M. G. Hankins and P. I. Pohl, Critical Conditions for Ferric Chloride-Induced Flocculation of Freshwater Algae, Biotechnol. Bioeng., 2012, 109(2), 493–501, DOI:10.1002/bit.23319.
- P. Xie, Y. Liu, M. Feng, M. Niu, C. Liu, N. Wu, K. Sui, R. R. Patil, D. Pan, Z. Guo and R. Fan, Hierarchically Porous Co/C Nanocomposites for Ultralight High-Performance Microwave Absorption, Adv. Compos. Hybrid Mater., 2021, 4(1), 173–185, DOI:10.1007/s42114-020-00202-z.
- S. Eyley, C. Schütz and W. Thielemans, Surface Chemistry and Characterization of Cellulose Nanocrystals, Cellulose Science and Technology, John Wiley & Sons, Inc., 2018, pp. 223–252 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00102g |
| This journal is © The Royal Society of Chemistry 2021 |