Open Access Article
Open Access ArticleVanadium doped CaTiO3 cuboids: role of vanadium in improving the photocatalytic activity†
Harsha
Bantawal
a,
U. Sandhya
Shenoy
 *b and
D. Krishna
Bhat
*b and
D. Krishna
Bhat
 *a
*a
aDepartment of Chemistry, National Institute of Technology Karnataka, Surathkal, Mangalore-575025, India. E-mail: denthajekb@gmail.com
bDepartment of Chemistry, College of Engineering and Technology, Srinivas University, Mukka, Mangalore-574146, India. E-mail: sandhyashenoy347@gmail.com
First published on 28th July 2021
Abstract
CaTiO3 has attracted enormous interest in the fields of photocatalytic dye degradation and water splitting owing to its low cost, excellent physicochemical stability and structural tunability. Herein, we have developed a simple one pot solvothermal approach which directs V into the Ti sites in the isovalent state during the synthesis of V doped CaTiO3 cuboids. The prediction of reduction in the band gap due to the formation of additional levels just beneath the conduction band edge by the first principles density functional electronic structure study is confirmed by the experimental results. The suppression of charge carrier recombination in 1.0 V leads to the highest photocatalytic activity in the degradation of methylene blue. The percentage degradation of 94.2 indicates its suitability as an excellent catalyst for photocatalytic water treatment.
1. Introduction
Environmental pollution and the energy crisis have become serious problems in the developed and developing countries in the world due to the expeditious growth of population and industrialization.1,2 Semiconductor materials, mainly oxides and chalcogenides have been found to be the most promising materials which can be used to solve the issue by using them in thermoelectric or photocatalytic applications.3–8 While thermoelectric materials help scavenge the waste heat and convert them to electricity without the release of harmful gases, the photocatalysis strategy tackles these issues by using sunlight as a green, low cost and renewable resource for generation of hydrogen and degradation of pollutants.9–12 Metal oxides and their composites have been utilized in energy and environmental remediation due to their ease of synthesis, excellent stability, photo-corrosion resistance and superior efficiency.4,13–15 Among the metal oxides, CaTiO3, is the first known perovskite material which has sparked research interest due to the above mentioned promising features for the decomposition of water and degradation of harmful organic pollutants.16–18 An efficient photocatalyst should have a suitable band gap which can effectively harvest the visible region of the solar spectrum, suppressed recombination rate of photoinduced charge carriers and excellent resistance towards photocorrosion.19–21 The band gap energy of CaTiO3 is about 3.2 eV, as a result, its photocatalytic activity is restricted to the UV portion of the solar spectrum only and hence various strategies like doping, construction of heterostructures and coupling with π-conjugated structures have been utilized in order to reduce the band gap.18,22–24 Among various strategies, doping foreign atoms in to the lattice of wide band gap photocatalysts has been found to be the promising method as there are three possible sites for doping (Ca, Ti and O). However, dopants are known to introduce bulk defects which can also act as recombination centres of photoinduced charges thus diminishing the photocatalytic efficiency of the material.25,26 Hence it is imperative to find dopants which can efficiently harvest the solar energy and also can suppress the recombination of photo induced charge carriers. Various dopants such as Ag, Cr, Cu, Er, Eu, Fe, La, N, Na, and Zr have been utilized to improve the photocatalytic efficiency of CaTiO3.24,27–34 In addition to this, co-doping has been utilized in order to passivate the unoccupied impurity states induced by mono-doped systems and thus suppress the recombination rate of photoinduced charges.24,27,28Recently we reported vanadium to be a promising dopant in SrTiO3 and BaTiO3 for photocatalytic degradation of MB and thermoelectric applications, and found that the site occupancy of the dopant decides the efficiency of the material.35–37 We know that vanadium is a transition element which can exhibit multiple oxidation states. Hence, substitutional doping requires it to be introduced with isovalent charge in order to avoid the formation of the so-called in-gap states acting as recombination centres due to the formation of oxygen vacancies.38,39 Hence, there is a dire need to develop a novel and eco-friendly synthetic technique which can avoid the formation of defect states. Herein, for the first time we have synthesized V doped CaTiO3 by a facile one pot solvothermal approach by avoiding high temperature calcination. The experimental results indicated the successful incorporation of V4+ into the lattice of CaTiO3 with a significant reduction in the band gap as predicted by density functional theory (DFT) thereby efficiently harvesting the visible light region of the solar spectrum. The photocatalytic activity of the material was tested using methylene blue (MB) dye as a model pollutant and the doped material was found to be highly efficient as compared to parent CaTiO3.
2. Methods
2.1 Preparation of V-doped CaTiO3
All the chemicals were purchased from Sigma-Aldrich and were used as received. Titanium(IV) isopropoxide (1.47 mL) was dissolved in 10 mL of 2-propanol. To this a calculated amount of vanadyl acetylacetonate was added and stirred for one hour. An appropriate amount of calcium nitrate tetrahydrate and 15 mL of 2 M KOH were added. The resultant mixture was sealed in an autoclave and kept in a hot air oven maintained at 180 °C for 24 hours. The resultant precipitates were washed thoroughly with acetic acid and water. The washed products were dried in an oven at 70 °C for 8 hours. The products obtained by using 0.25, 0.5, 1.0, 1.5 and 2.0 mol% of the V precursor were labelled as 0.25 V, 0.5 V, 1.0 V, 1.5 V and 2.0 V respectively. The samples were characterized using various techniques as given in the ESI†.2.2 Determination of photocatalytic activity
The photocatalytic efficiency was determined by taking MB dye as a model pollutant. The photocatalytic reactor was fitted with a high-pressure 250 W Hg vapour lamp which was used as a visible light source by using a filter. Prior to the photocatalytic studies, the photocatalyst (100 mg) was dispersed in 100 mL of MB solution (10 mg L−1) with the help of a sonicator for 5 minutes. The resultant solution was loaded into the reactor and then the visible light source is turned on. 5 mL of the reacted MB solution was sampled out periodically and centrifuged in order to remove the catalyst. The absorbance of the supernatant dye solution was measured using a UV-visible spectrometer at 664 nm. The percentage degradation of dye was calculated as per eqn (1).40| Degradation% = [(Co − C)/Co] × 100 | (1) |
2.3 Computational details
The electronic structure of pristine CaTiO3 and V doped CaTiO3 was simulated using an orthorhombic perovskite cell. The DFT calculations carried out using the Quantum ESPRESSO package used pseudopotentials with the Generalized Gradient Approximation (GGA) of Perdew, Burke and Ernzerhof (PBE) functional type.41,42 The electrons 4s2, 3d24s2, 2s22p4, and 3d34s2 of Ca, Ti, O and V, respectively were considered as the valence electrons by the pseudopotential. The plane wave basis representing the wave functions was terminated with an energy cutoff of 50 Ry and charge density cutoff of 400 Ry, respectively. The Brillouin zone was sampled using k point meshes of 8 × 8 × 8 and 16 × 16 × 16 for self-consistent and non-self-consistent field calculations, respectively. The electronic structure was determined along the high symmetry path of Γ–X–S–Y–Γ–Z–U–R–T–Z of the fully relaxed crystal.3. Results and discussion
3.1 XRD analysis
The XRD diffraction profiles of CaTiO3 and V-doped CaTiO3 can be well matched with the orthorhombic phase of CaTiO3 with JCPDS card number 42-0423 (Fig. 1a). There is no characteristic peak of vanadium oxide in the XRD profiles of V-doped CaTiO3, indicating the successful incorporation of V into the host lattice of CaTiO3. The shift in the 2θ values after V doping is negligible due to the similar radius of V4+ and Ti4+ ions. If V is doped in the Ca site, then an appreciable shift would have been observed as Ca2+ ions are larger in size compared to V4+ ions. The average crystallite sizes of the synthesized samples were calculated with the help of the Scherrer equation by using the diffraction angle and full width at half maximum values of the (121) crystal plane.43 The crystallite sizes were found to be 36.46 nm, 37.45 nm, 44.73 nm, 45.47 nm, 46.23 nm and 47.83 nm for CaTiO3, 0.25 V, 0.5 V, 1.0 V, 1.5 V and 2.0 V, respectively. The increase in the crystallite sizes with increase in doping points towards the increased crystallinity due to favoured directional growth with increase in the concentration of the V dopant.44,453.2 Morphological and surface area analysis
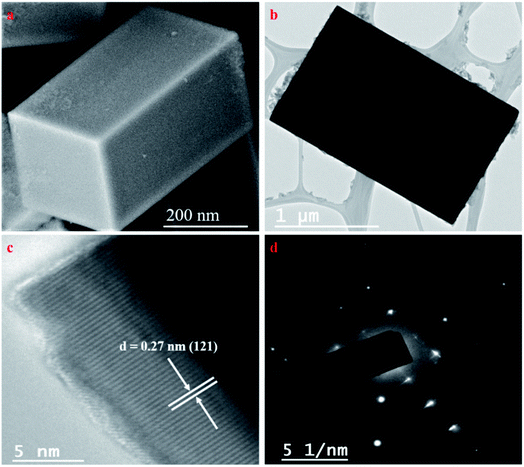
The morphology of the doped CaTiO3 was studied with the help of field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) analysis. From the FESEM image, we can observe that V doped CaTiO3 has cuboidal morphology (Fig. 2a). This fact is further supported by TEM analysis (Fig. 2b). V doping showed no change in the morphology of CaTiO3. High resolution transmission electron microscopy (HRTEM) analysis of 1.0 V revealed lattice fringes with 0.27 nm spacing, which could be indexed to the (121) plane of CaTiO3 (Fig. 2c). The selected area electron diffraction (SAED) pattern indicated the single crystalline nature of the material (Fig. 2d). Electron diffraction X-ray (EDX) analysis showed the presence of Ca, Ti, O and V which is in agreement with the obtained X-ray photoelectron spectroscopy (XPS) results (Fig. S1†). Further, elemental mapping of 1.0 V confirms the uniform distribution of atoms in the material (Fig. S2†).The textural properties of the undoped CaTiO3 and 1.0 V were investigated by BET analysis. The nitrogen adsorption–desorption isotherms indicated a type IV pattern with the hysteresis loops resembling type H3 (P/Po >0.4), revealing the presence of slit like pores (Fig. 3a and b).46,47 The BET surface area of 1.0 V was found to be 21.78 m2 g−1 which is higher in comparison to CaTiO3 (15.88 m2 g−1), which enables the efficient adsorption and degradation of the pollutants by enhancing the surface-active sites.48 The pore size distribution was determined using the Barrett–Joyner–Halenda (BJH) method.49 The BJH pore size distributions of the pure CaTiO3 and 1.0 V samples presented a narrow distribution ranging from 3 to 6.6 nm indicating the presence of mesopores (insets of Fig. 3a and b). The pore volume of 1.0 V was found to be 0.0270 cm3 g−1 which is slightly higher than that of CaTiO3 (0.0240 cm3 g−1), facilitating effective diffusion of molecules during the photocatalytic reaction.
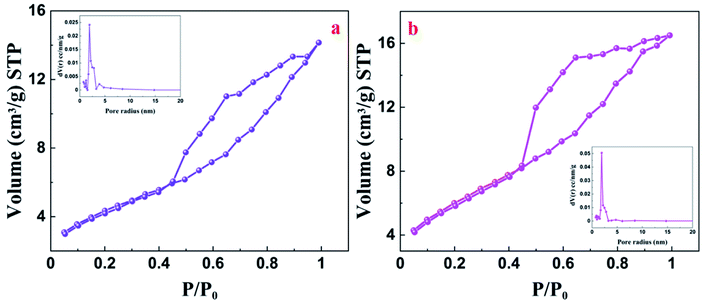 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms and BJH pore size distribution (inset) of (a) CaTiO3; (b) 1.0 V. | ||
3.3 XPS analysis
The XPS survey spectrum of CaTiO3 indicated the presence of Ca, Ti and O elements (Fig. S3†). However in the XPS spectrum of 1.0 V we see additional peaks due to V in addition to Ca, Ti and O. The double peaks at the binding energies of 346.7 eV and 350.3 eV could be attributed to Ca 2p3/2 and Ca 2p1/2 spin states of Ca2+, respectively in 1.0 V (Fig. 4a).50 The peaks at the binding energies of 458.8 eV and 464.6 eV could be ascribed to Ti 2p3/2 and Ti 2p1/2 states, respectively. These binding energy values confirmed the existence of the +4 oxidation state for Ti in the 1.0 V sample (Fig. 4b).50,51 Further, it is observed that the shift in the peaks for Ca 2p3/2 (346.2 eV in CaTiO3) and 2p1/2 (349.9 eV in CaTiO3) is less compared to Ti 2p3/2 (458.1 eV in CaTiO3) and Ti 2p1/2 (464.1 eV in CaTiO3) with V doping indicating that V is doped in the Ti site. The binding energies of 530.2 eV and 531.7 eV could be attributed to O2− in the lattice (OL) and the hydroxyl groups adsorbed on the surface (OOH), respectively in 1.0 V (Fig. 4c).35,52 However the same two peaks appear at 529.6 eV and 531.3 eV, respectively in CaTiO3. The double peaks at the binding energies of 515.9 eV and 523.3 eV could be ascribed to V 2p3/2 and V 2p1/2 states, respectively, indicating the oxidation state of V as +4 (Fig. 4d) and successful incorporation of V in CaTiO3.35,53–55 The maintenance of charge neutrality in the crystal thus avoids the formation of defect states and hence prevents recombination of charge carriers and enhances the carrier lifetime.3.4 Electronic structure analysis
CaTiO3, a perovskite material can occur in either cubic structure or orthorhombic structure. Since the experimental results indicated that the material was in the orthorhombic phase, the electronic structure and density of states (DOS) of CaTiO3 were simulated using the pristine orthorhombic structure containing 20 atoms (Fig. 5a). The estimated lattice parameters of the fully relaxed parent CaTiO3 were found to be a = 5.399 Å, b = 5.499 Å and c = 7.683 Å, respectively. Fig. 5b reveals a direct band gap of 2.397 eV at the Γ point. Such an underestimation of the band gap is typical of DFT calculations due to the presence of discontinuities in the derivative of energy with respect to the number of electrons.56,57 The contribution of the various atoms to the electronic structure was studied by projecting the atomic orbitals onto the DOS. Fig. 5c indicates that the O ‘p’ orbitals mainly contribute towards the valence band (VB) while the ‘d’ orbitals of Ti form the conduction band (CB) similar to the case of SrTiO3 and BaTiO3.36,37The site occupied by the dopant largely effects its electronic structure and properties.25,58 Since there are two possible cationic doping sites for V, both were simulated. Doping V in Ca sites led to a decrease in ‘a’ and ‘c’ lattice parameters to 5.371 Å and 7.671 Å, respectively while the ‘b’ parameter increased to 5.590 Å. It is observed that when V goes into the Ca site the band gap decreases to 2.501 eV at the Γ point (Fig. 6a). This is due to the ‘d’ states of V which pull the Ti ‘d’ states lower (Fig. 6b). Substitution of V in the Ti site decreased the cell volume by decreasing the lattice parameters to a = 5.383 Å, b = 5.475 Å and c = 7.652 Å, respectively. V in the Ti site leads to the formation of an energy band just beneath the CB decreasing the band gap at the Γ point to 1.832 eV. This band is formed due to the hybridization of ‘d’ states of V with ‘p’ states of O with a bandwidth of 1.029 eV. The continuous nature of the additional band with the conduction states leads to easy migration of charge carriers, effectively separating the electrons and holes avoiding the recombination.25 The results also indicate that directing V into the Ti site would be more beneficial for photocatalytic application due to the higher amount of decrease in the band gap.
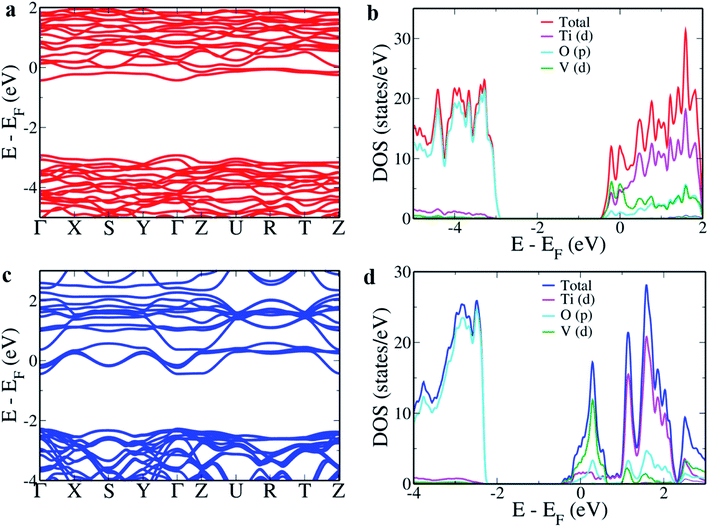 | ||
| Fig. 6 Electronic structure and pDOS of V doped CaTiO3 (a and b) V in the Ca site; (c and d) V in the Ti site. | ||
3.5 Optical absorbance analysis
As shown in Fig. 7a, the absorption edge of V-doped CaTiO3 was red shifted as compared to pristine CaTiO3. This could be attributed to the insertion of the t2g level of the V 3d orbital within the band gap as discussed in the previous section. The absorption data were derived using the Kubelka–Munk equation (eqn (2)).58,59 | (2) |
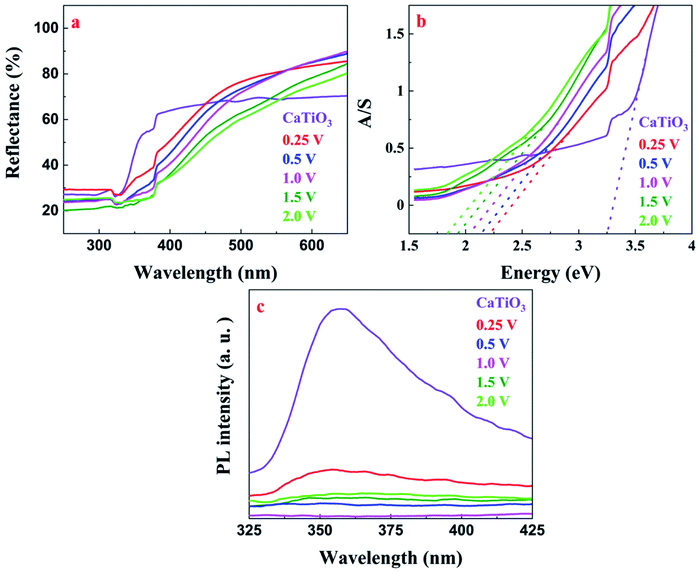 | ||
| Fig. 7 (a) UV-visible DR spectra; (b) electronic absorption spectra; (c) PL spectra of CaTiO3 and V-doped CaTiO3 samples. | ||
PL analysis was carried out in order to get more insight into defect chemistry and charge recombination behaviour of the synthesized materials.60 As shown in Fig. 7c, the V doped CaTiO3 sample exhibited inferior fluorescence intensity in comparison to pristine CaTiO3 which could be attributed to the incorporation of V in the isovalent state, thus suppressing the formation of the recombination centres. Among the different samples, 1.0 V showed the least PL intensity and as a result, it is expected to show the highest photocatalytic activity.
3.6 Photocatalytic activity
The photocatalytic activity of the synthesized catalysts was evaluated for the degradation of MB dye solution under visible light. Fig. 8a shows the photocatalytic degradation of MB as a function of irradiation time in the presence of CaTiO3 and V-doped CaTiO3 samples. The photocatalytic activity of V-doped CaTiO3 samples was found to be higher than that of CaTiO3 which could be attributed to the introduction of additional energy levels just beneath the CB effectively decreasing the band gap and reduction in the recombination of photoinduced charges due to efficient charge separation. Among the various V-doped samples, 1.0 V exhibited the highest photocatalytic activity, beyond which the photocatalytic activity decreases. This reduction in the photocatalytic activity can be attributed to the formation of recombination centres as confirmed by PL analysis. In addition to this, the 1.0 V sample exhibited higher surface area which enables the efficient adsorption and degradation of the pollutants during the photocatalytic reaction. It is observed that the adsorption capacity is almost three times higher for 1.0 V compared to CaTiO3 (Fig. S4†). Comparison of the photocatalytic activity of 1.0 V with the previously reported ones show that our material is highly efficient (Table S1†).24,25,30,32,35,50,51,61–65 | ||
| Fig. 8 (a) The photocatalytic degradation curves of MB; (b) the rate constants of the photocatalytic degradation of MB by the synthesized CaTiO3 and V doped CaTiO3. | ||
The degradation kinetics of MB by CaTiO3 and V-doped CaTiO3 is well fitted with the pseudo first-order rate equation given by (3).66
| −ln(C/Co) = kt | (3) |
Trapping experiments were carried out in order to assess the active species involved in the photocatalytic degradation by following the same procedure as that of photocatalytic activity evaluation in the presence of different radical scavenging agents such as benzoquinone (1 mM) as a superoxide anion radical (O2−˙) scavenger, potassium iodide (10 mM) as a hole (h+) scavenger and isopropyl alcohol (10 mM) as a hydroxyl radical (OH˙) scavenger.26 From Fig. 10 it can be seen that superoxide anion radicals are not the major active species as the corresponding scavenger benzoquinone did not deteriorate the photocatalytic activity much. This is also confirmed by the band edge positions as a decrease in the band gap with V doping brings the potential of the CB lower than the potential for the generation of superoxide anion radicals. Further, the addition of potassium iodide (hole scavenger) and isopropyl alcohol (OH˙ scavenger) reduced the photocatalytic activity significantly. As a result, holes followed by hydroxyl radicals are considered to be the active species for the effective degradation of the dye.
The mechanism of photocatalytic degradation of MB can be stated as follows: when V doped CaTiO3 is irradiated with an energy equal to or greater than its band gap, electrons from the VB get excited to the energy levels created by V just beneath the CB edge generating corresponding number of holes in the VB. These electrons react with oxygen to produce superoxide radicals (O2−˙). The holes in the VB either directly react with MB or react with surface hydroxyl groups to produce hydroxyl radicals (OH˙).25 The formed radicals are said to be active species for the effective degradation of MB to carbon dioxide and water (Fig. 11).
 | ||
| Fig. 11 Proposed mechanism for the photocatalytic degradation of MB under visible light irradiation. | ||
The thermodynamic parameters such as, activation energy (Ea), free energy of activation (ΔG#), enthalpy of activation (ΔH#) and entropy of activation (ΔS#) were computed by employing activation complex theory and the Eyring equation.35,51 From the values tabulated we observe that higher energy of activation is required for the photodegradation of MB without the catalyst, whereas a relatively lower energy of activation is needed in the presence of CaTiO3 and V-doped CaTiO3 catalysts (Table 1). This confirms that the catalyst alters the path of the reaction by lowering the activation energy barrier. The 1.0 V sample exhibited the lowest activation energy as compared to other samples. The endothermic and non-spontaneous nature of the reaction was indicated by the positive enthalpy and free energy change.
| Sample | E a (kJ mol−1) | ΔH# (kJ mol−1) | ΔS# (kJ mol−1) | ΔG# (kJ mol−1) |
|---|---|---|---|---|
| Without catalyst | 17.7 | 15.2 | −0.25 | 91.2 |
| CaTiO3 | 13.9 | 11.5 | −0.25 | 87.4 |
| 0.25 V | 12.0 | 9.5 | −0.25 | 85.5 |
| 0.5 V | 10.0 | 7.5 | −0.25 | 83.5 |
| 1.0 V | 9.4 | 6.9 | −0.25 | 82.8 |
| 1.5 V | 10.8 | 8.3 | −0.25 | 84.3 |
| 2.0 V | 11.4 | 8.9 | −0.25 | 84.9 |
4. Conclusions
V doped CaTiO3 was successfully synthesized by a simple one pot solvothermal approach by avoiding high temperature calcination. The present synthetic strategy involves incorporation of V4+ ions into the Ti sites of CaTiO3 with high surface area, as confirmed by XRD, XPS and BET results. First principles DFT calculations showed that the band gap of the doped samples decreased due to the formation of additional energy levels just beneath the conduction band. The PL results indicated the efficient charge separation due to the isovalent doping thereby boosting the photocatalytic efficiency. The superior photocatalytic activity and high stability of V doped CaTiO3 (1.0 V) for MB degradation reveal that the material can be a promising catalyst for photocatalytic water treatment.Conflicts of interest
There are no conflicts of interest to declare.References
- Q. Lei, S. Yang, D. Ding, J. Tan, J. Liu and R. Chen, Local-interaction-field-coupled semiconductor photocatalysis: recent progress and future challenges, J. Mater. Chem. A, 2021, 9, 2491–2525 RSC.
- D. K. Bhat and U. S. Shenoy, SnTe thermoelectrics: dual step approach for enhanced performance, J. Alloys Compd., 2020, 834, 155181 CrossRef CAS.
- X. Li, H. Zhao, J. Liang, Y. Luo, G. Chen, X. Shi, S. Lu, S. Gao, J. Hu, Q. Liu and X. Sun, A-site perovskite oxides: an emerging functional material for electrocatalysis and photocatalysis, J. Mater. Chem. A, 2021, 9, 6650–6670 RSC.
- U. S. Shenoy and D. K. Bhat, Electronic structure engineering of SrTiO3via rhodium doping: a DFT study, J. Phys. Chem. Solids, 2021, 148, 109708 CrossRef CAS.
- R. Shen, D. Ren, Y. Ding, Y. Guan, Y. H. Ng, P. Zhang and X. Li, Nanostructured CdS for efficient photocatalytic H2 evolution: a review, Sci. China Mater., 2020, 63, 2153–2188 CrossRef CAS.
- D. Ren, R. Shen, Z. Jiang, X. Lu and X. Li, Highly efficient visible-light photocatalytic H2 evolution over 2D–2D CdS/Cu7S4 layered heterojunctions, Chin. J. Catal., 2020, 41, 31–40 CrossRef CAS.
- R. Zhang, C. Wang, H. Chen, H. Zhao, J. Liu, Y. Li and B. Su, Cadmium sulfide inverse opal for photocatalytic hydrogen roduction, Acta Phys.-Chim. Sin., 2020, 36, 1803014 Search PubMed.
- D. K. Bhat and U. S. Shenoy, Resonance levels in GeTe thermoelectrics: zinc as a new multifaceted dopant, New J. Chem., 2020, 44, 17664–17670 RSC.
- Z. Wei, M. Xu, J. Liu, W. Guo, Z. Jiang and W. Shangguan, Simultaneous visible-light-induced hydrogen production enhancement and antibiotic wastewater degradation using MoS2@ZnxCd1-xS: solid-solution-assisted photocatalysis, Chin. J. Catal., 2020, 41, 103–113 CrossRef CAS.
- Z. Liang, R. Shen, Y. H. Ng, P. Zhang, Q. Xiang and X. Li, A review on 2D MoS2 cocatalysts in photocatalytic H2 production, J. Mater. Sci. Technol., 2020, 56, 89–121 CrossRef.
- U. S. Shenoy and D. K. Bhat, Bi and Zn co-doped SnTe thermoelectrics: interplay of resonance levels and heavy hole band dominance leading to enhanced performance and a record high room temperature ZT, J. Mater. Chem. C, 2020, 8, 2036–2042 RSC.
- D. K. Bhat and U. S. Shenoy, Mg/Ca doping ameliorates the thermoelectric properties of GeTe: influence of electronic structure engineering, J. Alloys Compd., 2020, 843, 155989 CrossRef CAS.
- R. Gusain, K. Gupta, P. Joshi and O. P. Khatri, Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: a comprehensive review, Adv. Colloid Interface Sci., 2019, 272, 102009 CrossRef CAS.
- M. M. J. Sadiq, U. S. Shenoy and D. K. Bhat, Enhanced photocatalytic performance of N-doped RGO-FeWO4/Fe3O4 ternary nanocomposite in environmental applications, Mater. Today Chem., 2017, 4, 133–141 CrossRef.
- P. Raizada, S. Sharma, A. Kumar, P. Singh, A. A. P. Khan and A. M. Asiri, Performance improvement strategies of CuWO4 photocatalyst for hydrogen generation and pollutant degradation, J. Environ. Chem. Eng., 2020, 8, 104230 CrossRef CAS.
- F. Dai, Y. Wang, R. Zhao, X. Zhou, J. Han and L. Wang, ZnIn2S4 modified CaTiO3 nanocubes with enhanced photocatalytic hydrogen performance, Int. J. Hydrogen Energy, 2020, 45, 28783–28791 CrossRef CAS.
- A. Kumar, V. Navakoteswara Rao, A. Kumar, M. Venkatakrishnan Shankar and V. Krishnan, Interplay between mesocrystals of CaTiO3 and edge sulfur atom enriched MoS2 on reduced graphene oxide nanosheets: enhanced photocatalytic performance under sunlight irradiation, ChemPhotoChem, 2020, 4, 427–444 CrossRef CAS.
- Y. Yan, H. Yang, Z. Yi, R. Li and T. Xian, Design of ternary CaTiO3/g-C3N4/AgBr Z-scheme heterostructured photocatalysts and their application for dye photodegradation, Solid State Sci., 2020, 100, 106102 CrossRef CAS.
- M. M. J. Sadiq, U. S. Shenoy and D. K. Bhat, Synthesis of BaWO4/NRGO–gC3N4 nanocomposites with excellent multifunctional catalytic performance via microwave approach, Front. Mater. Sci., 2018, 12, 247–263 CrossRef.
- S. Wageh, A. A. Al-Ghamdi, R. Jafer, X. Li and P. Zhang, A new heterojunction in photocatalysis: S-scheme heterojunction, Chin. J. Catal., 2021, 42, 667–669 CrossRef CAS.
- D. Cao, H. An, X. Yan, Y. Zhao, G. Yang and H. Mei, Fabrication of Z-scheme heterojunction of SiC/Pt/CdS nanorod for efficient photocatalytic H2 evolution, Acta Phys.-Chim. Sin., 2020, 36, 1901051–1901059 Search PubMed.
- E. Jiang, L. Yang, N. Song, X. Zhang, C. Liu and H. Dong, Multi-shelled hollow cube CaTiO3 decorated with Bi12O17Cl2 towards enhancing photocatalytic performance under the visible light, J. Colloid Interface Sci., 2020, 576, 21–33 CrossRef CAS PubMed.
- M. Shi, B. Rhimi, K. Zhang, J. Xu, D. W. Bahnemann and C. Wang, Visible light-driven novel Bi2Ti2O7/CaTiO3 composite photocatalyst with enhanced photocatalytic activity towards NO removal, Chemosphere, 2021, 275, 130083 CrossRef CAS PubMed.
- M. Chen, Q. Xiong, Z. Liu, K. Qiu and X. Xiao, Synthesis and photocatalytic activity of Na+ Co-doped CaTiO3: Eu3+ photocatalysts for methylene blue degradation, Ceram. Int., 2020, 46, 12111–12119 CrossRef CAS.
- U. S. Shenoy, H. Bantawal and D. K. Bhat, Band engineering of SrTiO3: effect of synthetic technique and site occupancy of doped rhodium, J. Phys. Chem. C, 2018, 122, 27567–27574 CrossRef CAS.
- H. Bantawal, M. Sethi, U. S. Shenoy and D. K. Bhat, Porous graphene wrapped SrTiO3 nanocomposite: Sr–C bond as an effective coadjutant for high performance photocatalytic degradation of methylene blue, ACS Appl. Nano Mater., 2019, 2, 6629–6636 CrossRef CAS.
- H. Zhang, G. Chen, X. He and J. Xu, Electronic structure and photocatalytic properties of Ag–La codoped CaTiO3, J. Alloys Compd., 2012, 516, 91–95 CrossRef CAS.
- R. Wang, S. Ni, G. Liu and X. Xu, Hollow CaTiO3 cubes modified by La/Cr Co-doping for efficient photocatalytic hydrogen production, Appl. Catal., B, 2018, 225, 139–147 CrossRef CAS.
- H. Zhang, G. Chen, Y. Li and Y. Teng, Electronic structure and photocatalytic properties of copper-doped CaTiO3, Int. J. Hydrogen Energy, 2010, 35, 2713–2716 CrossRef CAS.
- L. M. Lozano-Sánchez, S. Obregón, L. A. Díaz-Torres, S. W. Lee and V. Rodríguez-González, Visible and near-infrared light-driven photocatalytic activity of erbium-doped CaTiO3 system, J. Mol. Catal., 2015, 410, 19–25 CrossRef.
- B. G. Park, Photoluminescence of Eu3+-doped CaTiO3 perovskites and their photocatalytic properties with a metal ion loading, Chem. Phys. Lett., 2019, 722, 44–49 CrossRef CAS.
- H. Yang, C. Han and X. Xue, Photocatalytic activity of Fe-doped CaTiO3 under UV–visible light, J. Environ. Sci., 2014, 26, 1489–1495 CrossRef CAS.
- J. Wang, F. Han, Y. Rao, T. Hu, Y. Huang, J. J. Cao and S. C. Lee, Visible-light-driven nitrogen-doped carbon quantum dots/CaTiO3 composite catalyst with enhanced NO adsorption for NO removal, Ind. Eng. Chem. Res., 2018, 57, 10226–10233 CrossRef CAS.
- X. J. Huang, Y. A. N. Xin, H. Y. Wu, F. A. N. G. Ying, Y. H. Min, W. S. Li, S. Y. Wang and Z. J. Wu, Preparation of Zr-doped CaTiO3 with enhanced charge separation efficiency and photocatalytic activity, Trans. Nonferrous Met. Soc., 2016, 26, 464–471 CrossRef CAS.
- H. Bantawal, U. S. Shenoy and D. K. Bhat, Vanadium-doped SrTiO3 nanocubes: insight into role of vanadium in improving the photocatalytic activity, Appl. Surf. Sci., 2020, 513, 145858 CrossRef CAS.
- U. S. Shenoy and D. K. Bhat, Enhanced thermoelectric properties of vanadium doped SrTiO3: a resonant dopant approach, J. Alloys Compd., 2020, 832, 154958 CrossRef CAS.
- U. S. Shenoy and D. K. Bhat, Vanadium doped BaTiO3 as high performance thermoelectric material: role of electronic structure engineering, Mater. Today Chem., 2020, 18, 100384 CrossRef CAS.
- C. E. Choong, K. T. Wong, H. Kim, S. B. Jang, S. Y. Yoon, I. W. Nah, W. Kim, S. H. Kim, B. H. Jeon, Y. Yoon and M. Jang, Unexpected discovery of superoxide radical generation by oxygen vacancies containing biomass derived granular activated carbon, Water Res., 2020, 190, 116757 CrossRef.
- H. Bantawal, U. S. Shenoy and D. K. Bhat, Tuning the photocatalytic activity of SrTiO3 by varying the Sr/Ti Ratio: unusual effect of viscosity of the synthesis medium, J. Phys. Chem. C, 2018, 122, 20027–20033 CrossRef CAS.
- M. J. S. Mohamed, S. Shenoy and D. K. Bhat, Novel NRGO–CoWO4–Fe2O3 nanocomposite as an efficient catalyst for dye degradation and reduction of 4-nitrophenol, Mater. Chem. Phys., 2018, 208, 112–122 CrossRef CAS.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. L. Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, Quantum ESPRESSO: a modular and open-source software project for quantum simulations of materials, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- M. Sethi and D. K. Bhat, Facile solvothermal synthesis and high supercapacitor performance of NiCo2O4 nanorods, J. Alloys Compd., 2019, 781, 1013–1020 CrossRef CAS.
- B. Liu, X. Wang, G. Cai, L. Wen, Y. Song and X. Zhao, Low temperature fabrication of V-doped TiO2 nanoparticles, structure and photocatalytic studies, J. Hazard. Mater., 2009, 169, 1112–1118 CrossRef CAS PubMed.
- J. C. S. Wu and C. H. Chen, A visible-light response vanadium-doped titania nanocatalyst by sol–gel method, J. Photochem. Photobiol., A, 2004, 163, 509–515 CrossRef CAS.
- M. Sethi, U. S. Shenoy and D. K. Bhat, A porous graphene–NiFe2O4 nanocomposite with high electrochemical performance and high cycling stability for energy storage applications, Nanoscale Adv., 2020, 2, 4229–4241 RSC.
- D. Ren, Z. Liang, Y. H. Ng, P. Zhang, Q. Xiang and X. Li, Strongly coupled 2D–2D nanojunctions between P-doped Ni2S (Ni2SP) cocatalysts and CdS nanosheets for efficient photocatalytic H2 evolution, Chem. Eng. J., 2020, 390, 124496 CrossRef CAS.
- M. E. Malefane, Co3O4/Bi4O5I2/Bi5O7I C-scheme heterojunction for degradation of organic pollutants by light-emitting diode irradiation, ACS Omega, 2020, 5, 26829–26844 CrossRef CAS PubMed.
- M. Sethi, U. S. Shenoy and D. K. Bhat, Simple solvothermal synthesis of porous graphene-NiO nanocomposites with high cyclic stability for supercapacitor application, J. Alloys Compd., 2021, 854, 157190 CrossRef CAS.
- E. Jiang, N. Song, G. Che, C. Liu, H. Dong and L. Yang, Construction of a Z-scheme MoS2/CaTiO3 heterostructure by the morphology-controlled strategy towards enhancing photocatalytic activity, Chem. Eng. J., 2020, 399, 125721 CrossRef CAS.
- D. K. Bhat, H. Bantawal and U. S. Shenoy, Rhodium doping augments photocatalytic activity of barium titanate: effect of electronic structure engineering, Nanoscale Adv., 2020, 2, 5688–5698 RSC.
- J. Cai, A. Cao, J. Huang, W. Jin, J. Zhang, Z. Jiang and X. Li, Understanding oxygen vacancies in disorder-engineered surface and subsurface of CaTiO3 nanosheets on photocatalytic hydrogen evolution, Appl. Catal., B, 2020, 267, 118378 CrossRef CAS.
- I. Top, R. Binions, M. E. Warwick, C. W. Dunnill, M. Holdynski and I. Abrahams, VO2/TiO2 bilayer films for energy efficient windows with multifunctional properties, J. Mater. Chem. C, 2018, 6, 4485–4493 RSC.
- S. P. Vattikuti, A. K. R. Police, J. Shim and C. Byon, In situ fabrication of the Bi2O3–V2O5 hybrid embedded with graphitic carbon nitride nanosheets: oxygen vacancies mediated enhanced visible-light–driven photocatalytic degradation of organic pollutants and hydrogen evolution, Appl. Surf. Sci., 2018, 447, 740–756 CrossRef CAS.
- Y. Li, D. Gu, S. Xu, X. Zhou, K. Yuan and Y. Jiang, A monoclinic V1-xyTixRuyO2 thin film with enhanced thermal-sensitive performance, Nanoscale Res. Lett., 2020, 15, 1–10 CrossRef PubMed.
- D. K. Bhat and U. S. Shenoy, Zn: a versatile resonant dopant for SnTe thermoelectrics, Mater. Today Phys., 2019, 11, 100158 CrossRef.
- U. S. Shenoy and D. K. Bhat, Electronic structure modulation of Pb0.6Sn0.4Te via zinc doping and its effect on the thermoelectric properties, J. Alloys Compd., 2021, 872, 159681 CrossRef CAS.
- U. S. Shenoy and D. K. Bhat, Enhanced bulk thermoelectric performance of Pb0.6Sn0.4Te: effect of magnesium doping, J. Phys. Chem. C, 2017, 121, 20696–20703 CrossRef.
- L. Zhiming, L. Guoliang and H. Xinlin, Influence of surface defects and palladium deposition on the activity of CdS nanocrystals for photocatalytic hydrogen production, Acta Phys.-Chim. Sin., 2019, 35, 215–222 Search PubMed.
- R. Shen, Y. Ding, S. Li, P. Zhang, Q. Xiang, Y. H. Ng and X. Li, Constructing low-cost Ni3C/twin-crystal Zn0.5Cd0.5S heterojunction/homojunction nanohybrids for efficient photocatalytic H2 evolution, Chin. J. Catal., 2021, 42, 25–36 CrossRef CAS.
- C. Han, J. Liu, W. Yang, Q. Wu, H. Yang and X. Xue, Enhancement of photocatalytic activity of CaTiO3 through HNO3 acidification, J. Photochem. Photobiol., 2016, 322, 1–9 Search PubMed.
- A. Kumar, S. Kumar, A. Bahuguna, A. Kumar, V. Sharma and V. Krishnan, Recyclable, bifunctional composites of perovskite type N-CaTiO3 and reduced graphene oxide as an efficient adsorptive photocatalyst for environmental remediation, Mater. Chem. Front., 2017, 1, 2391–2404 RSC.
- Y. Yan, H. Yang, X. Zhao, R. Li and X. Wang, Enhanced photocatalytic activity of surface disorder-engineered CaTiO3, Mater. Res. Bull., 2018, 105, 286–290 CrossRef CAS.
- Y. Yan, H. Yang, Z. Yi, R. Li and X. Wang, Enhanced photocatalytic performance and mechanism of Au@CaTiO3 composites with Au nanoparticles assembled on CaTiO3 nanocuboids, Micromachines, 2019, 10, 254 CrossRef PubMed.
- X. Chen, X. He, X. Yang, Z. Wu and Y. Li, Construction of novel 2D/1D g–C3N4/CaTiO3 heterojunction with face-to-face contact for boosting photodegradation of triphenylmethane dyes under simulated sunlight, J. Taiwan Inst. Chem. Eng., 2020, 107, 98–109 CrossRef CAS.
- M. M. J. Sadiq, U. S. Shenoy and D. K. Bhat, NiWO4–ZnO–NRGO ternary nanocomposite as an efficient photocatalyst for degradation of methylene blue and reduction of 4-nitro phenol, J. Phys. Chem. Solids, 2017, 109, 124–133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00468a |
| This journal is © The Royal Society of Chemistry 2021 |