Open Access Article
Open Access ArticleMultifunctional self-assembled peptide nanoparticles for multimodal imaging-guided enhanced theranostic applications against glioblastoma multiforme†
Syed Faheem Askari
Rizvi
 *ab,
Azam
Ali
a,
Munir
Ahmad
b,
Shuai
Mu
a and
Haixia
Zhang
*ab,
Azam
Ali
a,
Munir
Ahmad
b,
Shuai
Mu
a and
Haixia
Zhang
 *a
*a
aCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou-730000, Gansu Province, P. R. China, +86-931-8912058. E-mail: zhanghx@lzu.edu.cn; syed2018@lzu.edu.cn; Fax: +86-931-8912582
bDepartment of Nuclear Medicine, Institute of Nuclear Medicine and Oncology (INMOL), Lahore-54000, Punjab, Pakistan
First published on 15th September 2021
Abstract
The synthesis of self-assembled peptide nanoparticles using a facile one-pot synthesis approach is gaining increasing attention, allowing therapy in combination with diagnosis. Their drawback is limited diagnostic potential, which can be improved after necessary modifications and efficacious functionalization. Herein, a cyclic heptapeptide having the Arg-Gly-Asp-Lys-Leu-Ala-Lys sequence was modified by conjugation of the ε-amino group of the terminal lysine residue with diethylenetriamine pentaacetic acid (DTPA) as a bifunctional chelating agent (BFC) for radiolabeling with a γ-emitting radionuclide (99mTc, half-life 6.01 h; energy 140 keV). Further, the free amino group of the middle lysine residue was successfully conjugated with near-infrared fluorescence (NIRF) dye Cyanine5.5 N-succinimidyl ester (Ex/Em = 670/701 nm) by a co-assembly method to form newly designed novel NIRF dye conjugated self-assembled peptide–DTPA (Cy5.5@SAPD) nanoparticles. The fluorescent nanoparticle formation was confirmed by using a fluorescence spectrophotometer (Ex/Em = 650/701 nm), and the transmission electron microscope (TEM) images showed a size of ∼ 40 nm with a lattice fringe distance of 0.294 nm. Cytotoxicity and confocal laser scanning microscopy (CLSM) studies showed that these nanoparticles possess a high affinity for the αvβ3-integrin receptor overexpressed on brain tumor glioblastoma with an EC50 = 20 μM. Moreover, these nanoparticles were observed to have potential to internalize into U87MG cells more prominently than HEK-293 cancer cells and induce apoptosis. The apoptosis assay showed 79.5% apoptotic cells after 24 h treatment of Cy5.5@SAPD nanoparticles. Additionally, these nanoparticles were also radiolabeled with 99mTc for the single photon emission computed tomography (SPECT) imaging study in tumor-bearing female Balb/c mice. The excellent imaging feature of Cy5.5@SAPD-99mTc nanoparticles as a multimodal (SPECT/NIRF) diagnostic probe, as well as noteworthy therapeutic potential was observed. The results suggested that our newly designed novel dual-targeting dual-imaging nanoparticles may serve as an admirable theranostic probe to treat brain tumor glioblastoma multiforme.
1 Introduction
The development of malignant cells in the circulatory system is progressively assisted by the growth of new blood vessels by utilizing oxygen and nutrients, ultimately increasing tumor angiogenesis. Self-regulation of angiogenesis causes the progression of diseases such as the proliferation of cancer cells, glioblastoma multiforme (GBM), myocardial infarction, and atherosclerosis.1 For the treatment of cancer, anti-angiogenic therapy in combination with anticancer therapy has emerged as a novel strategy to combat the growth of tumor cells by discontinuing their nutrient oxygen supply. Multidomain-targeting drug delivery serves as a guided missile to effectively and efficiently target the cancer cell vasculature. These targeted drugs have been developed using peptides/proteins, integrin-receptor ligands, antibodies, and aptamers possessing recognition domains and effector domains.2 There are many cognate receptors and pro-angiogenic factors involved to promote vessel formation in tumors such as the fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF).3 These growth factors up-regulate the expression of integrins including α1β1, α2β1, α4β1, α5β1, α9β1, and αvβ3-integrins on blood and lymphatic vessels. Several investigations implicate integrins as key regulators of tumor angiogenesis which also regulate endothelial cell survival and migration and mediate cell–cell adhesion and cell–extracellular matrix (ECM) events.4,5 Basic clinical studies reveal that angiogenesis can be blocked by inhibiting the angiogenic signaling pathways, ultimately resulting in tumor dormancy and metastasis.6,7Integrin-mediated signaling pathways play an important role in tissue development and homeostasis, while its deregulation causes multiple brain diseases.8 Interestingly, integrins are crucial glycoproteins essential for many physiological processes such as proliferation, cell migration, wound healing, hemostasis, bone remodeling, and oncogenic transformation.9,10 They are composed of nineteen α- and eight β-subunits,11,12 and among them, αvβ3-, αvβ5-, αvβ8-, α5β1-, and αIIbβ3-integrins have been extensively studied for their active role as an excellent candidate for cancer theranostics.13 More specifically, the αvβ3-integrin serves as a receptor for ECM proteins such as fibronectin, vitronectin, fibrinogen, collagen, laminin, and osteopontin with an exposed arginine-glycine-aspartic acid (RGD) sequence.14 It is expressed on epithelial and mature epithelial cells at low levels, while highly expressed on the surface of many tumors including carcinomas of breast and lungs, melanomas, osteosarcomas, and glioblastoma.15–17 Hence, the αvβ3-integrin is considered as a molecular target of interest for the early diagnosis of cancer and selective attachment and internalization of RGD-containing peptides and peptidomimetics for cancer therapy.18 The cyclic monomeric (RGDfV) and multimeric (Galacto-RGD) peptides have emerged in phase-III clinical trials for the diagnosis of glioblastoma and in phase-II trials for many other tumors.19 It is the first anti-angiogenic small molecule drug that specifically targets the αvβ3-, αvβ5-, and α5β1-integrins and is the first potent and superactive αvβ3-integrin receptor antagonist.
Over the last two decades, both linear and cyclic RGD peptide analogs have been discovered, radiolabeled (99mTc, 111In, 68Ga, and 18F), and evaluated as radiotracers for tumor diagnosis using single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging.20 Linear RGD peptides showed a lack of αvβ3-integrin specificity, low binding affinity, and high enzymatic degradation of the aspartic acid residue due to free rotation around a single bond. In contrast, cyclic RGD peptides were observed to be highly stable towards proteases and had increased affinity to the αvβ3-integrin receptor with reduced structural flexibility.21 The literature provides ample knowledge on the development of hybridized RGD conjugated anticancer peptide analogs including RGD-111In-DTPA-octreotate,22,2318F-bombesin-RGD,2499mTc-RGD-bombesin,2564Cu-RGD2-PG12-bombesin heterodimers,26 and 5FU-loaded SF-cRGDfK-Ce6 (ref. 27) for targeted drug delivery and improved diagnostic as well as therapeutic efficacy.
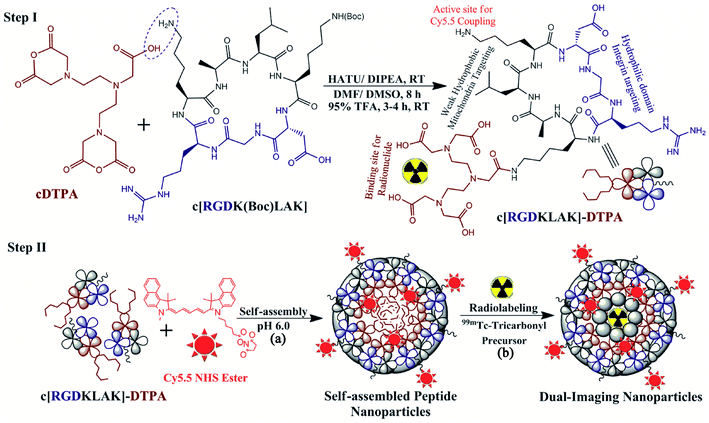
In the light of the above knowledge, we hypothesized that designing a short head-to-tail cyclic heptapeptide sequence using the RGD (αvβ3-integrin receptor binding) motif along with the KLAK (mitochondria targeting) motif without the addition of unnecessary linking amino acids can improve integrin targeting and mitochondrial damaging potential for therapeutic applications. Secondly, the modification of this cyclic heptapeptide by incorporating a NIRF dye for optical imaging as well as a BFC for radiolabeling with β/γ-emitting radionuclides for the SPECT imaging study resulted in self-assembled peptide nanoparticles that could be effective as a novel dual-imaging agent for SPECT/NIRF diagnosis.
To the best of our knowledge, we are the first who have designed this novel head-to-tail cyclic RGD-KLAK heptapeptide sequence.2 To evaluate the validation of our hypothesis, first we modified the ε-amino group of the terminal lysine residue with diethylenetriamine pentaacetic acid (DTPA; as the BFC). Secondly, this peptide–DTPA complex was intrinsically converted to self-assembled peptide nanoparticles via co-assembly with Cy5.5 (as the NIRF-probe), which covalently conjugated with the free amino group of the intermediate lysine residue and introduced optical imaging features. Further aim was to investigate the effectiveness and efficacy of this newly developed novel NIRF-dye conjugated self-assembled peptide nanoparticles radiolabeled with 99mTc (Cy5.5@SAPD-99mTc) for in vitro and in vivo diagnosis of glioblastoma multiforme (GBM) as well as therapeutic potential as a multimodal-imaging (SPECT/NIRF) and dual-targeting probe.
2 Materials and methods
2.1. Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to isolate the NIRF-dye coupled self-assembled cyclic peptide–DTPA (Cy5.5@SAPD) nanoparticles, followed by washing with deionized (DI) water twice to remove the acidic water. Next, the mixture was sonicated for 2 h to obtain dispersed SAPD nanoparticles in DI water and they were characterized by fluorescence spectroscopy, dynamic light scattering (DLS), and transmission electron microscopy (TEM).
000 rpm to isolate the NIRF-dye coupled self-assembled cyclic peptide–DTPA (Cy5.5@SAPD) nanoparticles, followed by washing with deionized (DI) water twice to remove the acidic water. Next, the mixture was sonicated for 2 h to obtain dispersed SAPD nanoparticles in DI water and they were characterized by fluorescence spectroscopy, dynamic light scattering (DLS), and transmission electron microscopy (TEM).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (95
HCl (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5% v/v) as the mobile phase (Rf for 99mTc-tricarbonyl = 0.85–0.95; Rf for Cy5.5@SAPD-99mTc(CO)3 = 0.55–0.75) as well as a radio-HPLC method. Approximately 98% pure 99mTc-tricarbonyl complex (∼185 MBq/0.5 mL saline) was filtered through a 0.22 μm Millipore membrane and added into Eppendorf tubes containing Cy5.5@SAPD nanoparticles (as the ligand; 20 μg/100 μL Na-PBS, pH 6.5). The reaction mixture was allowed to incubate for 20 min at 55 °C. After successful radiolabeling, the newly developed radiotracer (Cy5.5@SAPD-99mTc(CO)3 nanoparticles) was allowed to cool at room temperature, and the quality control analysis was performed using TLC-SG and ultra-centrifugation methods.
5% v/v) as the mobile phase (Rf for 99mTc-tricarbonyl = 0.85–0.95; Rf for Cy5.5@SAPD-99mTc(CO)3 = 0.55–0.75) as well as a radio-HPLC method. Approximately 98% pure 99mTc-tricarbonyl complex (∼185 MBq/0.5 mL saline) was filtered through a 0.22 μm Millipore membrane and added into Eppendorf tubes containing Cy5.5@SAPD nanoparticles (as the ligand; 20 μg/100 μL Na-PBS, pH 6.5). The reaction mixture was allowed to incubate for 20 min at 55 °C. After successful radiolabeling, the newly developed radiotracer (Cy5.5@SAPD-99mTc(CO)3 nanoparticles) was allowed to cool at room temperature, and the quality control analysis was performed using TLC-SG and ultra-centrifugation methods.
2.2. In vivo imaging, pharmacokinetics and therapeutic study
For the in vivo study, female Balb/c mice (5 weeks old, 16–18 g, 20 mice in total) were purchased from the National Institute of Health (NIH), Islamabad, Pakistan. All animal studies were carried out as per guidelines issued by the animal ethical committee of the National Institute of Health (NIH), Islamabad, Pakistan and National Regulation of China for Care and Use of Laboratory Animals (NRCCUL) of Lanzhou University, China. All the experiments performed in this study were approved by NIH and NRCCUL. Each mouse model bearing brain tumor glioblastoma and human embryonic kidney cancers was successfully established using U87MG and HEK-293 cells, respectively, using a concentration of 5 × 107 cells suspended in 100 μL PBS (pH 7.4). The cells were subcutaneously injected into the right flank area of each mouse and the mouse were kept at standard conditions till the tumor volume reached to 50–60 mm3. All mice were divided into two groups (n = 3 for each group) randomly to receive normal saline (control) and Cy5.5@SAPD-99mTc (treated). To observe the in vivo tumor accumulation and therapeutic potential, a single therapeutic dose of Cy5.5@SAPD in saline (30 mg per kg body weight; b.w) was injected intravenously via the tail vein of each group. The body weight and tumor size of each mouse were monitored after a couple of days. On the sixth day post-injection, mice were injected with 20 μg/200 μL of Cy5.5@SAPDN-99mTc(CO)3 and images were acquired using a SPECT camera. Later on, they were sacrificed after giving chloroform anesthesia; tumor mass, as well as other organs, were segregated and weighed and radioactivity was measured using a NaI(Tl) γ-scintillation counter. At the same time, the images were also acquired using an IN VIVO FX camera for optical imaging, and the segregated organs were also observed for the uptake of our newly designed nanoparticles by observing the fluorescence intensity in each organ.2.3. Statistical analysis
All experiments reported in this study were performed in triplicate and results are given as ±standard deviation (±SD) of ‘n’ independent measurements. Statistical significance was calculated using the Student's t-test. The significance level was assigned as p < 0.05.3 Results and discussion
3.1. Chemistry
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups; respectively, and a peak at 2109.7 cm−1 assigned to the bending vibrations of the –COOH group (Fig. S3†).
O groups; respectively, and a peak at 2109.7 cm−1 assigned to the bending vibrations of the –COOH group (Fig. S3†).
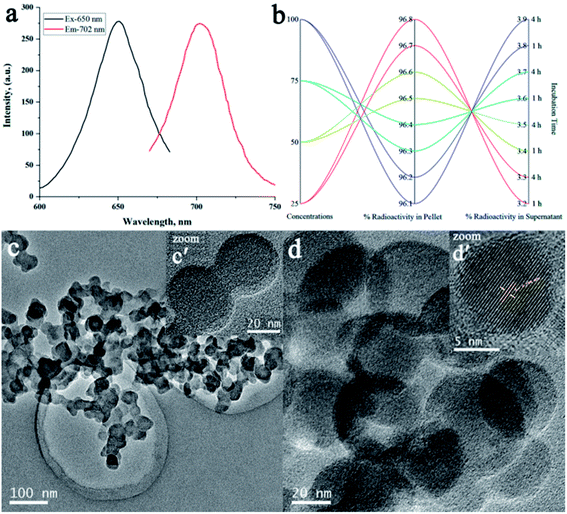
The radiosynthon introduced by Alberto et al. has been widely used for preferential radiolabeling of peptides/proteins to achieve relatively high specificity, while retaining the biological properties of bioactive molecules.36 The radiosynthon was successfully prepared with a high radiochemical purity of ≥97% showing a single high-intensity peak at retention time Rt = 4.727 min, as indicated by radio-HPLC analysis (Fig. S4†). The inset figure shows the TLC-SG results which showed that fac-[99mTc(CO)3(H2O)3]+ moves with the solvent front at Rf = 0.90, while free 99mTc remain at the point of spotting (Rf = 0.00). The electron donor nitrogen and oxygen groups of DTPA provide an easy platform for radiolabeling with 99mTc in the presence of NaBH4 as the reducing agent and an optimum pH value, as shown in Step-II(b) of Scheme 1. Consequently, the radiolabelled nanoparticles were observed to remain stable at room temperature over a 4 h incubation period and no change in the radiolabeling efficiency was seen by increasing the concentration of Cy5.5@SAPD-99mTc nanoparticles with a percent radiochemical purity of >96% (Fig. 1b), obtained using the ultracentrifugation technique. The same results were obtained from the TLC-SG/methanol technique, and the inset images presented in Fig. S4† show ∼97% yield at Rf = 0.65, while <3% impurities were found in saline.30
Additionally, transmission electron microscope (TEM) images presented in Fig. 1c and d show a clear spherical morphology of Cy5.5@SAPD and Cy5.5@SAPD-99mTc nanoparticles, respectively, with uniform dispersion in an aqueous medium. The size of Cy5.5@SAPD nanoparticles was observed in between 30–40 nm, as indicated in the inset image Fig. 1c′ acquired by using a high resolution-TEM, and for Cy5.5@SAPD-99mTc nanoparticles, the size reduced to 20–25 nm, as shown in the inset image Fig. 1d′. The clear consecutive bight and dark lattice fringes with an interplanar lattice fringe distance of 0.294 nm, as presented in Fig. 1d′, indicates a tight interface among all three-ingredient (i.e. SAPD, NIRF-dye, and radionuclide 99mTc) which could facilitate in balancing the surface charge as well as demonstrates the successful self-assembly of the cPD complex with Cy5.5 having crystallinity in novel designed nanoparticles.37 The dynamic light scattering (DLS) measurements revealed that the hydrodynamic size of Cy5.5@SAPD nanoparticles with a diameter of less than 100 ± 28 nm, as depicted in Fig. S5,† enabled the capabilities of novel designed nanoparticles in vitro and in vivo.38
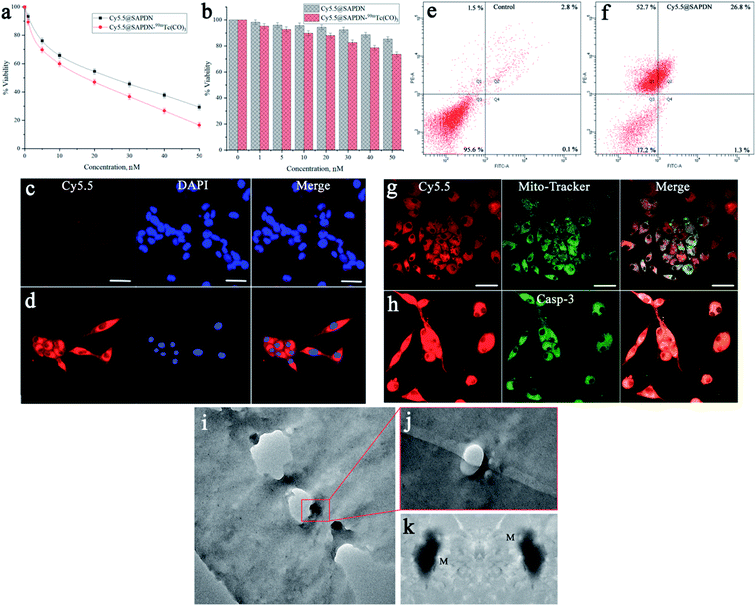
3.2. In vitro cell study
3.3. Pharmacokinetics, in vivo imaging, and therapeutic study
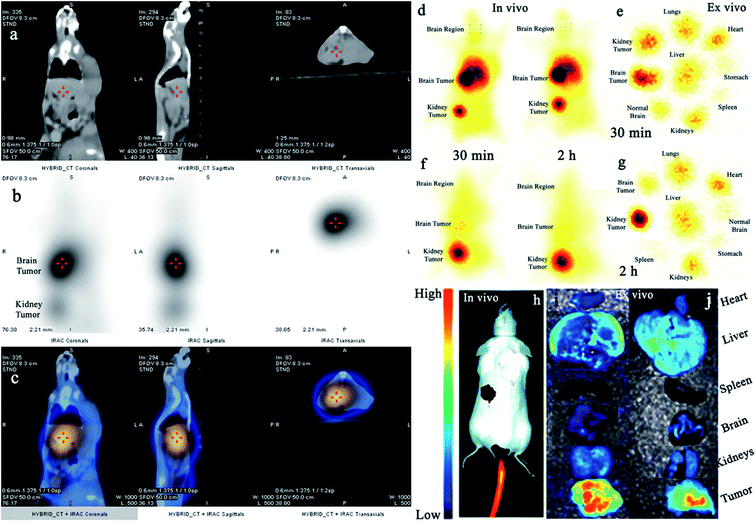
Additionally, we also investigated the dual-imaging potential of Cy5.5@SAPD-99mTc nanoparticles in brain tumor glioblastoma (U87MG cells) and human embryonic kidney (HEK-293) tumor-induced (5 × 107 cells per mice subcutaneously) female Balb/c mouse models. Firstly, the nanoparticles with a concentration of 20 μg/200 μL (74 MBq) saline were injected via the tail vein, and images were acquired using a dynamic SPECT/CT camera (NM/CT 670 Pro Discovery) as well as a fluorescence imaging camera (IN VIVO FX Pro Carestream). The images presented in Fig. 3a–c showed CT, SPECT, and SPECT/CT images acquired after 30 min post-injection (p.i) with coronal, sagittal, and transaxial directions, indicating the occurrence of brain tumor glioblastoma and the kidney tumor (as indicated in CT images) with excellent accumulation of novel Cy5.5@SAPD-99mTc nanoparticles (SPECT images), as shown in the tumor-to-background contrast at the left side of Balb/c mice (presented in SPECT/CT images).46 The results presented in Fig. 3d–g show planar SPCET images acquired at 30 min p.i (Fig. 3d left) and 2 h p.i (right) before therapeutic dose treatment, showing high internalization of the radiotracer in brain tumor glioblastoma with 4.7 ± 0.8% ID g−1 as compared to the kidney tumor (2.1 ± 0.9% ID g−1) with a target-to-nontarget ratio (T/NT) of 2.24 ± 0.6 at 30 min p.i. The results of the planar SPECT imaging study are comparable with those of the biodistribution study, as presented in the bar graph in Fig. S6 (see the ESI†).After therapeutic dose (30 mg per kg b.w) treatment of Cy5.5@SAPD within a very short period of time (say nearly one week), the planar SPECT imaging study was performed again. Fig. 3f clearly showed much less or negligible accumulation of the radiotracer at the site of a brain tumor while prominent uptake can be seen at the kidney tumor site; this is because of the binding potential of the RGD motif to the αvβ1-integrin overexpressed on HEK-293 cells.9 A sharp decrease in the brain tumor size indicates the excellent therapeutic potential of Cy5.5@SAPD nanoparticles for GBM. Moreover, Fig. 3e shows an ex vivo image presenting the pharmacokinetics of Cy5.5@SAPD-99mTc nanoparticles before and (Fig. 3g) after therapeutic dose treatment. The main accumulation of the radiotracer was found in the brain, liver, lungs, and kidneys and nonspecific uptake was observed in the heart, stomach, and spleen. Furthermore, the brain tumor-bearing female Balb/c mice models were subjected to fluorescence camera imaging, and the results presented in Fig. 3h showed in vivo and those in Fig. 3i and j showed ex vivo images after 30 min and 2 h p.i; respectively. The in vivo live imaging study showed the accumulation of Cy5.5@SAPD nanoparticles at the site of brain glioma tumors. The tumor uptake was observed to be highly prominent than normal brain tissues, and other body organs pointed out the efficacy, specificity, and effectiveness of our newly designed novel Cy5.5@SAPD nanoparticles as compared to those of previously reported nanoparticles.47–50
4 Conclusion
In the light of the above-mentioned results, we presented the successful development of a dual-targeting peptide sequence consisting of the RGD motif for targeting the αvβ3-integrin and KLAK pro-apoptotic motif for targeting the mitochondria, which ultimately resulted in inducing cancer cell apoptosis by the activation of the caspase-3 enzyme. This dual-targeting peptide probe was further modified with DTPA. Further, the cyclic peptide–DTPA complex was self-assembled into peptide nanoparticles via co-assembly with NIRF-dye Cy5.5 to form uniform spherical shaped nanoparticles, followed by radiolabeling with 99mTc for molecular imaging studies as a novel dual-imaging probe. Consequently, these novel dual-imaging and dual-targeting self-assembled cyclic peptide nanoparticles were successfully designed to possess improved diagnostic and therapeutic capabilities with enhanced specificity and efficiency for GBM. The in vitro cytotoxicity assay, apoptosis assay, and CLSM imaging studies illustrated that these newly synthesized Cy5.5@SAPD nanoparticles have potential to internalize specifically and efficiently into U87MG brain tumor cells as compared to HEK-293 kidney tumor cells for early diagnosis of GBM. SPECT/NIRF diagnostic studies in tumor-bearing female Balb/c mouse models showed the excellent potential of our novel designed Cy5.5@SAPD-99mTc nanoparticles to diagnose brain tumor more prominently. The therapeutic dose treatment showed effective depletion in tumor tissues, indicating the remarkable therapeutic effectiveness within one-week treatment. These outcomes suggested that our novel theranostic nanoparticles (Cy5.5@SAPD-99mTc) may serve efficiently, and specifically as a potential SPECT/NIRF nanoprobe for future pre-clinical and clinical studies on brain tumor glioblastoma multiforme.Funding
None of the author(s) received funding from any institution for the execution of this research work and/or publication of the article.Author contributions
S. F. A. Rizvi: conceptualization, methodology, validation, investigation, visualization, writing – original draft, writing – review & editing. A. Ali: formal analysis, visualization. M. Ahmad: resources, software, data curation. S. Mu: validation, formal analysis. H. Zhang: validation, writing – review & editing, supervision, project administration.Conflicts of interest
All authors declare no potential conflict of interest in any way.Acknowledgements
The authors are cordially thankful to the Director of the Institute of Nuclear Medicine and Oncology, Lahore, Pakistan for providing hot-lab facilities and the necessary radioisotopes and SPECT/CT camera facility for animal study.References
- H. Shi, R. T. K. Kwok, J. Liu, B. Xing, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 17972 CrossRef CAS PubMed.
- S. F. A. Rizvi, S. Mu, Y. Wang, S. Li and H. Zhang, Biomed. Pharmacother., 2020, 127, 110179 CrossRef CAS PubMed.
- R. Lugano, M. Ramachandran and A. Dimberg, Cell. Mol. Life Sci., 2020, 77, 1745 CrossRef CAS PubMed.
- C. J. Avraamides, B. Garmy-Susini and J. A. Varner, Nat. Rev. Canc., 2008, 8, 604 CrossRef CAS PubMed.
- S. Hirakawa, S. Kodama, R. Kunstfeld, K. Kajiya, L. F. Brown and M. Detmar, J. Exp. Med., 2005, 201, 1089 CrossRef CAS PubMed.
- E. Y. Lin and J. W. Pollard, Cancer Res., 2007, 67, 5064 CrossRef CAS PubMed.
- N. Ferrara and R. S. Kerbel, Nature, 2005, 438, 967 CrossRef CAS PubMed.
- A. Ellert-Miklaszewska, K. Poleszak, M. Pasierbinska and B. Kaminska, Int. J. Mol. Sci., 2020, 21, 888 CrossRef CAS PubMed.
- J. Schnittert, R. Bansal, G. Storm and J. Prakash, Adv. Drug Delivery Rev., 2018, 129, 37 CrossRef CAS PubMed.
- K. K. Ganguly, S. Pal, S. Moulik and A. Chatterjee, Cell Adhes. Migrat., 2013, 7, 251 CrossRef PubMed.
- F. Danhier, A. Le Breton and V. Préat, Mol. Pharm., 2012, 9, 2961 CrossRef CAS PubMed.
- Y. Zheng, S. Ji, A. Czerwinski, F. Valenzuela, M. Pennington and S. Liu, Bioconjugate Chem., 2014, 25, 1925 CrossRef CAS PubMed.
- H. M. Sheldrake and L. H. Patterson, J. Med. Chem., 2014, 57, 6301 CrossRef CAS PubMed.
- E. J. Park, P. K. Myint, A. Ito, M. G. Appiah, S. Darkwah, E. Kawamoto and M. Shimaoka, Front. Cell Dev. Biol., 2020, 8, 588066 CrossRef PubMed.
- K. Y. Yeung, A. Dickinson, J. F. Donoghue, G. Polekhina, S. J. White, D. K. Grammatopoulos, M. McKenzie, T. G. Johns and J. C. St John, Acta Neuropathol. Commun., 2014, 2, 1 CrossRef PubMed.
- L. Malric, S. Monferran, J. Gilhodes, S. Boyrie, P. Dahan, N. Skuli, J. Sesen, T. Filleron, A. Kowalski-Chauvel, E. Cohen-Jonathan Moyal, C. Toulas and A. Lemarié, Oncotarget, 2017, 8, 86947 CrossRef PubMed.
- J. Wan, A. A. Guo, I. Chowdhury, S. Guo, J. Hibbert, G. Wang and M. Liu, Front. Oncol., 2019, 9, 1413 CrossRef PubMed.
- C. Mas-Moruno, F. Rechenmacher and H. Kessler, Anticancer Agents Med. Chem., 2010, 10, 753 CrossRef CAS PubMed.
- M. Jamous, U. Haberkorn and W. Mier, Molecules, 2013, 18, 3379 CrossRef CAS PubMed.
- S. Ji, A. Czerwinski, Y. Zhou, G. Shao, F. Valenzuela, P. Sowiński, S. Chauhan, M. Pennington and S. Liu, Mol. Pharm., 2013, 10, 3304 CrossRef CAS PubMed.
- A. L. Tornesello, L. Buonaguro, M. L. Tornesello and F. M. Buonaguro, Molecules, 2017, 22, 1282 CrossRef PubMed.
- A. Capello, E. P. Krenning, B. F. Bernard, W. A. Breeman, M. P. van Hagen and M. de Jong, J. Nucl. Med., 2004, 45, 1716 CAS.
- L. J. Hofland, A. Capello, E. P. Krenning, M. de Jong and M. P. van Hagen, J. Nucl. Med., 2005, 46, 191 Search PubMed.
- Y. Yan, K. Chen, M. Yang, X. Sun, S. Liu and X. Chen, Amino Acids, 2011, 41, 439 CrossRef CAS PubMed.
- Z. Liu, J. Huang, C. Dong, L. Cui, X. Jin, B. Jia, Z. Zhu, F. Li and F. Wang, Mol. Pharm., 2012, 9, 1409 CrossRef CAS PubMed.
- E. Lucente, H. Liu, Y. Liu, X. Hu, E. Lacivita, M. Leopoldo and Z. Cheng, Bioconjugate Chem., 2018, 29, 1595 CrossRef CAS PubMed.
- B. Mao, C. Liu, W. Zheng, X. Li, R. Ge, H. Shen, X. Guo, Q. Lian, X. Shen and C. Li, Biomaterials, 2018, 161, 306 CrossRef CAS PubMed.
- J. Shi, Y. S. Kim, S. Chakraborty, Y. Zhou, F. Wang and S. Liu, Amino Acids, 2011, 41, 1059 CrossRef CAS PubMed.
- P. Katyal, M. Meleties and J. K. Montclare, ACS Biomater. Sci. Eng., 2019, 5, 4132 CrossRef CAS PubMed.
- R. H. Gaonkar, R. Baishya, B. Paul, S. Dewanjee, S. Ganguly, M. C. Debnath and S. Ganguly, MedChemComm, 2018, 9, 812 RSC.
- Z. Liu, G. Niu, F. Wang and X. Chen, Eur. J. Nucl. Med. Mol. Imag., 2009, 36, 1483 CrossRef CAS PubMed.
- Z. Nie, N. Luo, J. Liu, X. Zeng, Y. Zhang and D. Su, Nanoscale Res. Lett., 2020, 15, 81 CrossRef CAS PubMed.
- L. Sun, Z. Fan, Y. Wang, Y. Huang, M. Schmidt and M. Zhang, Soft Matter, 2015, 11, 3822 RSC.
- Q. Chen, X. Wang, C. Wang, L. Feng, Y. Li and Z. Liu, ACS Nano, 2015, 9, 5223 CrossRef CAS PubMed.
- Z. Cheng, Y. Wu, Z. Xiong, S. S. Gambhir and X. Chen, Bioconjugate Chem., 2005, 16, 1433 CrossRef CAS PubMed.
- R. Alberto, R. Schibli, A. Egli, A. P. Schubiger, U. Abram and T. A. Kaden, J. Am. Chem. Soc., 1998, 120, 7987 CrossRef CAS.
- C. Song, Y. Wang and N. L. Rosi, Angew. Chem., Int. Ed., 2013, 52, 3993 CrossRef CAS PubMed.
- P.-P. Yang, K. Zhang, P.-P. He, Y. Fan, X. J. Gao, X. Gao, Z.-M. Chen, D.-Y. Hou, Y. Li, Y. Yi, D.-B. Cheng, J.-P. Zhang, L. Shi, X.-Z. Zhang, L. Wang and H. Wang, Sci. Adv., 2020, 6, 4107 CrossRef PubMed.
- S. F. Askari Rizvi and H. Zhang, Eur. J. Med. Chem., 2021, 221, 113538 CrossRef CAS PubMed.
- S. Dufort, L. Sancey, A. Hurbin, S. Foillard, D. Boturyn, P. Dumy and J.-L. Coll, J. Drug Target., 2011, 19, 582 CrossRef CAS PubMed.
- J. Lopez and S. W. G. Tait, Br. J. Cancer, 2015, 112, 957 CrossRef CAS PubMed.
- P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035 RSC.
- X. Zhang, Q. Sun, Z. Huang, L. Huang and Y. Xiao, J. Mater. Chem. B, 2019, 7, 2749 RSC.
- W. Tang, W. Fan, J. Lau, L. Deng, Z. Shen and X. Chen, Chem. Soc. Rev., 2019, 48, 2967 RSC.
- A. Ayo and P. Laakkonen, Pharmaceutics, 2021, 13, 481 CrossRef CAS PubMed.
- Z.-Q. Zhao, Y. Yang, W. Fang and S. Liu, Nucl. Med. Biol., 2016, 43, 661 CrossRef CAS PubMed.
- S. Goel, C. G. England, F. Chen and W. Cai, Adv. Drug Deliv. Rev., 2017, 113, 157 CrossRef CAS PubMed.
- J. Key and J. F. Leary, Int. J. Nanomedicine, 2014, 9, 711 Search PubMed.
- C. Dong, S. Yang, J. Shi, H. Zhao, L. Zhong, Z. Liu, B. Jia and F. Wang, Sci. Rep., 2016, 6, 18905 CrossRef CAS PubMed.
- C. Rangger, A. Helbok, J. Sosabowski, C. Kremser, G. Koehler, R. Prassl, F. Andreae, I. J. Virgolini, E. von Guggenberg and C. Decristoforo, Int. J. Nanomedicine, 2013, 8, 4659 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00597a |
| This journal is © The Royal Society of Chemistry 2021 |