Overcoming thermal quenching in upconversion nanoparticles
Yanze
Wang†
 ab,
Bing
Chen†
ab,
Bing
Chen†
 ab and
Feng
Wang
ab and
Feng
Wang
 *ab
*ab
aDepartment of Materials Science and Engineering, City University of Hong Kong, 83 Tat Chee Avenue, Hong Kong SAR, China. E-mail: fwang24@cityu.edu.hk
bCity University of Hong Kong Shenzhen Research Institute, Shenzhen 518057, China
First published on 15th January 2021
Abstract
Thermal quenching that is characterized by loss of light emission with increasing temperature is widely observed in luminescent materials including upconversion nanoparticles, causing problems in technological applications such as lighting, displays, and imaging. Because upconversion processes involve extensive intra-particle energy transfer that is temperature dependent, methods have been established to fight against thermal quenching in upconversion nanoparticles by engineering the energy transfer routes. In this minireview, we discuss the origin of thermal quenching and the role of energy transfer in thermal quenching. Accordingly, recent efforts in overcoming thermal quenching of upconversion are summarized.
1. Introduction
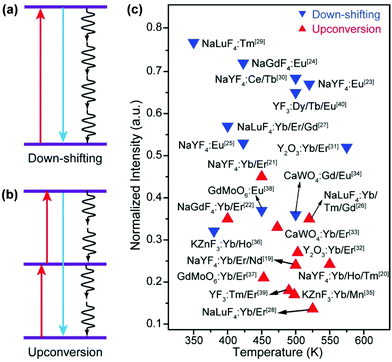
Luminescent materials have been playing important roles in a variety of modern technologies such as lasers, displays, and solar energy conversion.1 As a unique class of luminescent materials, lanthanide-doped upconversion nanoparticles (UCNPs) are particularly useful for biomedical and photonic applications due to their ability to generate visible and ultraviolet emissions by excitation of near-infrared light, which is less absorbed and scattered than the conventional ultraviolet excitation light.2–10 Due to their advantages of a large anti-Stokes frequency shift, high chemical stability, sharp emission peak, and low toxicity, UCNPs excel in many aspects than other types of optical materials such as quantum dots and organic dyes.11–15In practical applications, a luminescent component should display bright emission that is stable against variations in the environment such as temperature. However, most luminescent materials show attenuation of emission intensity with the increase in the temperature, which is well known as thermal quenching.16–18 Typically, thermal quenching in UCNPs is more complicated and severe than that in relevant Stokes shifting luminescent materials because the multiple excited states involved in the upconversion process are all subjected to thermal deactivation (Fig. 1).19–40 The problem has stimulated considerable research interest in combating thermal quenching in UCNPs.41
In this minireview, we focus on recent advances in the development of UCNPs that are resistant to thermal quenching. In section 2, we discuss various thermal quenching mechanisms and their influence on lanthanide-based upconversion. Accordingly, recent efforts in overcoming thermal quenching are reviewed in section 3.
2. The origin of thermal quenching
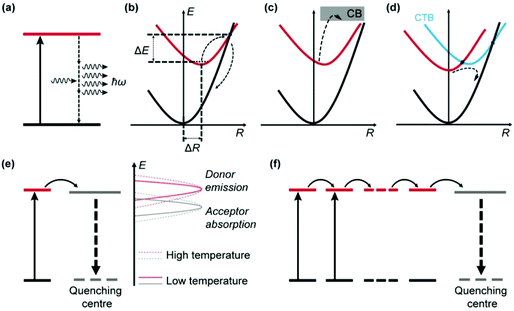
Thermal quenching of luminescence results from the increased dissipation of excitation energy at elevated temperatures, which can be due to the prevalence of nonradiative relaxations in individual luminescent centres and enhancement of interionic processes involving electron and energy transfer (Fig. 2).2.1 Thermal quenching in individual luminescent centres
Apart from emitting light, an excited luminescent centre has several possible deactivation channels, such as multiphonon relaxation, crossover and thermal ionization (Fig. 2a–c). In general, these nonradiative processes are enhanced with an increase in temperature due to the increased vibrational energy of the host lattice, thereby leading to increased loss of light emission.Multiphonon relaxation is a quenching process in which the energy gap (ΔEm) between the excited state and the next lower-lying state is bridged by simultaneously emitting phonons (Fig. 2a).42–45 The probability of multiphonon relaxation (Knr) is dependent on ΔEm and phonon energy (ħω) in the host lattice as described by eqn (1)46
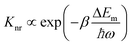 | (1) |
Crossover is a thermally activated process in which electrons at the excited energy level jump over the intersection between the excited state and the ground state parabola by overcoming an energy barrier (ΔE), followed by nonradiative relaxation to the ground state (Fig. 2b).48 The probability of crossover is strongly dependent on the polyhedral average bond length difference between the excited state and the ground state (ΔR). A large ΔR results in a small ΔE and thus a strong thermal quenching effect.
Thermal ionization involves the ejection of electrons from the localized excited state to the conduction band (CB) of the host material, as shown in Fig. 2c.49 After thermal stimulation, the ionized electrons often recombine nonradiatively with the photo-oxidized luminescent centre. Alternatively, the ionized electrons may be captured by traps in the host (e.g., substitutional impurity atoms, vacancies and anti-site defects), followed by various relaxation processes.50 In both cases, the light output from the original luminescent centre is reduced.
Electronic transitions in lanthanide dopant ions primarily occur within the 4f orbital that is shielded by the 5s and 5p orbitals, resulting in extremely small changes in the polyhedral average bond length. Therefore, direct thermal quenching in upconversion centres is mainly induced by multiphonon relaxation.
2.2 Thermal quenching due to interionic processes
In addition to intra-ionic transitions, a luminescent ion may lose its excitation energy to an adjacent optical centre by electron or energy transfer (Fig. 2d–f), which is typically enhanced by thermal energy and eventually leads to the quenching of emission.Optical quenching by electron transfer is illustrated in Fig. 2d for a system comprising two species A and B, which involves a ground state (A + B), an excited state (A* + B), and a charge transfer (CT) state (A+ + B−).51–53 The CT state bridges the excited state and ground state in a process that resembles crossover, leading to quenching of the emission. CT typically occurs between two optical centres with opposite redox tendencies (e.g., Eu3+ and Ce3+),54–56 which results in a low CT state energy favourable for the crossover to proceed.
The principle of energy transfer between two species was developed by Förster and Dexter.57,58 The probability of energy transfer (PET) largely depends on spectral overlap between the energy donor and acceptor pairs according to eqn (2).58,59
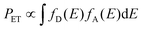 | (2) |
UCNPs typically comprise a large number of lanthanide dopant ions (e.g., Yb3+) that undergo extensive energy transfer interactions in the host lattice. Consequently, a lanthanide ion that is far from a quenching centre may be affected following multiple energy transfer steps (energy hopping) through the dopant sublattice (Fig. 2f).62,63 The energy hopping process exhibits a complex dependency on temperature and can be harnessed to manipulate the thermal quenching process.
3. Recent efforts in overcoming thermal quenching
Nonradiative relaxation processes in individual luminescent centres can hardly be alleviated as the temperature increases. Attempts to overcome thermal quenching are thus mainly based on the suppression of energy transfer to quenching sites at high temperatures. Because the luminescent centres (or activators) in UCNPs also receive energy from sensitizers (e.g., Yb3+ co-dopants), the effect of thermal quenching may alternatively be offset by thermal enhancement of the sensitization process.3.1 Thermal alleviation of energy dissipation
Nanoparticles are characterized by large surface-to-volume ratios and most quenching processes occur at the nanoparticle surface. In particular, surface absorbed water molecules induce significant quenching of upconversion emission by introducing high energy oscillation modes such as O–H vibration.64An increase in temperature typically induces desorption of water molecules and thus results in the alleviation of surface quenching, which offset thermal quenching and may eventually lead to enhancement of upconversion emission at elevated temperatures (Fig. 3a). The major origin of the energy loss in UCNPs is the strong interaction between heavily doped sensitizer ions (e.g., Yb3+) and high-energy oscillations of H2O molecules.65–68 In addition to multiphonon relaxation, the dopants may also be coupled to overtone transition of H2O molecules.69 Owing to the elimination of water-induced surface quenching, Jiang and co-workers observed a 12.6-fold enhancement of upconversion emission from NaGdF4:Yb/Ho (20/2%) nanoparticles under an Ar/H2O atmosphere as the temperature increased from 30 to 150 °C (Fig. 3b).70 The control experiment revealed that the luminescence was thermally quenched under a dry Ar atmosphere (Fig. 3c and d), which validated the role of water desorption for enhancing upconversion luminescence. The thermal enhancement process was reversible and can also be realized in NaGdF4:Yb/Er (or Tm) (20/2%) nanoparticles. A similar result was reported by Meijerink and co-workers, who observed a 3-fold thermal enhancement of integral upconversion emission of Er3+ ions in NaY(WO4)2:Yb/Er (49/1%) nanoparticles from 330 to 470 K under an ambient atmosphere.71 By contrast, thermal quenching of upconversion emission was observed in dry N2.
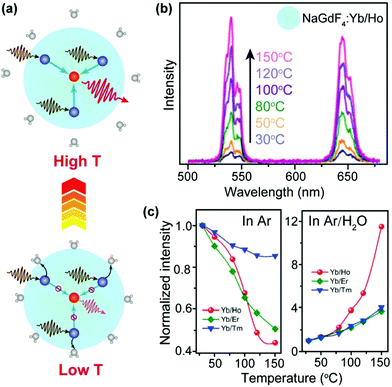 | ||
| Fig. 3 (a) Schematic diagram of temperature-dependent energy exchange interactions between sensitizer and activator ions as well as between sensitizer ions and surface H2O molecules in UCNPs. The navy and red spheres represent sensitizers and activators, respectively. (b) Emission spectra of NaGdF4:Yb/Ho (20/2%) nanoparticles in the temperature range of 30–150 °C. (c) Integral emission intensities of NaGdF4:Yb/Ho (or Er, Tm) (20/2%) nanoparticles as a function of temperature under Ar and Ar/H2O atmospheres, respectively. The intensities were normalized to those at 30 °C. Reproduced with permission from ref. 70. Copyright 2018, American Chemical Society. | ||
In a follow-up study, Jiang and co-workers reported wavelength-selective enhancement of upconversion in NaGdF4:Yb/Ce/Ho (20/30/2%) nanoparticles due to water desorption at elevated temperatures.72 Under 975 nm excitation, the green and red emission intensities at 450 K were about 1.7- and 10.2-fold stronger than those at 300 K, respectively. As a result, the emission colour clearly shifted from green to red with the increase in temperature, which was attributed to a thermally enhanced cross-relaxation between Ho3+ and Ce3+ ions. Notably, continuous laser irradiation was found to induce a heating effect, which can result in water desorption and thus modulation of upconversion emission.
Surface quenching may also affect dopant ions in the interior of the nanoparticles via energy migration.73–76 An increase in temperature normally causes the expansion of the host lattice, which hampers the long-distance energy migration process and consequently alleviates the quenching process (Fig. 4a). In an early example, Wang and co-workers described an anomalous temperature-dependence of upconversion luminescence in NaGdF4:Yb/Eu (20/10%) nanoparticles, which was related to energy migration through the sublattice of Yb3+ sensitizers.77 As a result of suppressed energy migration, a 16-fold enhancement of upconversion emission in Eu3+ ions (5D0 → 7F2 transition) was detected as the temperature increased from 303 to 423 K (Fig. 4b). In a recent study by our group, a thermal enhancement of upconversion emission was observed in oleate-capped NaYbF4:Er (2%)@NaLuF4:Yb (25%) UCNPs in the temperature range of 298–473 K, ascribed to lattice-expansion-induced interruption of energy migration among Yb3+ ions that were located in the core and active shell (Fig. 4c).78 When surface quenching effect was eliminated using an inert NaLuF4 shell, however, normal thermal quenching other than thermal enhancement of upconversion was observed in the same temperature range (Fig. 4d).
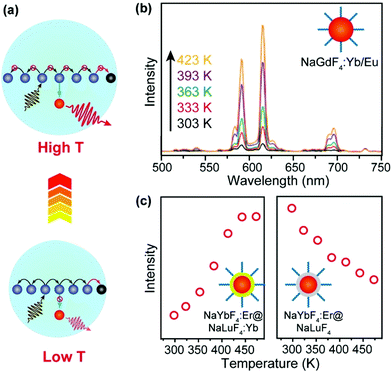 | ||
| Fig. 4 (a) Thermal suppression of energy migration to surface quenchers by lattice expansion. (b) Temperature-dependent upconversion emission spectra (excitation at 980 nm) of NaGdF4: Yb/Eu UCNPs with a size of 10 nm. Adapted with permission from ref. 77. Copyright 2017, Royal Society of Chemistry. (c) Emission intensities at 657 nm due to 4F9/2 → 4I15/2 transitions of Er3+ as a function of temperature in oleate-capped NaYbF4:Er (2%)@NaLuF4:Yb (25%) and NaYbF4:Er (2%)@NaLuF4 core–shell nanoparticles, respectively. Adapted with permission from ref. 78. Copyright 2019, American Chemical Society. | ||
It is clear from the above discussions that the thermally enhanced upconversion in this category is essentially an emission recovery process. If the UCNPs are initially protected against surface quenching in host materials with a core–inert shell structure or a large size, thermal quenching of upconversion luminescence is generally detected.79–83 Intriguingly, by integrating upconversion components displaying distinct thermal properties in a single nanoparticle or a nanoparticle mixture (Fig. 5a), novel systems can be constructed for applications such as thermometry84,85 and thermochromism.81,86–88
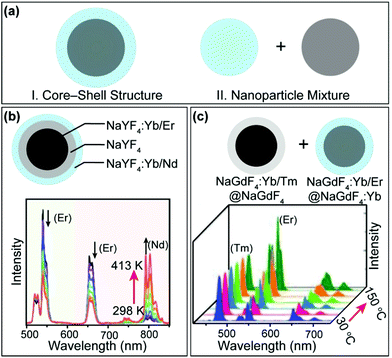 | ||
| Fig. 5 (a) Schematic illustration of integrating upconversion components displaying distinct temperature dependence of luminescence. (b) Emission spectra of NaYF4:Yb/Nd (20/6%)@NaYF4@NaYF4:Yb/Er (20/0.5%) nanorods in the temperature range of 298–413 K. Adapted with permission from ref. 89. Copyright 2019, American Chemical Society. (b) Emission spectra of NaGdF4:Yb/Er@NaGdF4 nanoparticles mixed with NaGdF4:Yb/Er@NaGdF4:Yb in the temperature range of 30–150 °C. Adapted with permission from ref. 90. Copyright 2019, Wiley-VCH. | ||
In a recent example, Mi et al. prepared a class of NaYF4:Yb/Er (20/2%)@NaYF4@NaYF4:Yb/Nd (60/4%) nanorods (Fig. 5b).89 It was reported that the Yb/Nd couple at the nanorod surface showed thermally enhanced emission, while the Yb/Er couple in the core of the nanorod exhibited thermally quenched luminescence. Based on the emission intensity ratio of Nd3+ and Er3+, sensitive nanothermometry (9.6%/K at 303 K) with a fast temperature response was demonstrated. In another development, Hu et al. constructed a class of thermochromic nanocomposite inks by mixing NaGdF4:Yb/Tm (20/1%)@NaGdF4 core–inert shell and NaGdF4:Yb/Er (20/1%)@NaGdF4:Yb (20%) core–active shell nanoparticles (Fig. 5c), which displayed thermally quenched and enhanced upconversion, respectively.90 The thermochromic nanocomposite inks are potentially useful for anticounterfeiting applications.
3.2 Thermal enhancement of energy collection
A majority of upconversion processes are based on energy transfer from the sensitizer (e.g., Yb3+) to activator (e.g., Er3+) ions. Such energy transfer processes can be enhanced with the increase in the temperature, which may offset thermal quenching in activator ions and even lead to enhancement of upconversion emissions at elevated temperatures.The energy transfer in UCNPs is usually a phonon-assisted process that is temperature dependent.90–93 Due to the quantum confinement effect, a cut-off of low-energy phonons in UCNPs was reported at low temperatures.94,95 Fortunately, an increase in temperature can mitigate the confinement effect, thereby promoting the energy transfer processes (Fig. 6a). In an early example, Jiang and co-workers reported thermally enhanced luminescence in small-sized NaYF4:Yb/Er (20/2%) UCNPs (24 nm), which was attributed to the activation of the phonon-assisted energy transfer process.79 The thermally activated phonons make up the energy mismatch between Yb3+ and Er3+, thereby facilitating the energy transfer process (Fig. 6b). Under 975 nm laser excitation, the luminescence intensity of Er3+ in the UCNPs increased 2-fold as the temperature increased from 298 to 358 K (Fig. 6c). In another study, Zhou et al. reported that as the temperature increases from 300 K to 453 K the oleate-capped NaYF4:Yb/Tm (49/1%) UCNPs would produce more surface phonons due to the [Yb⋯O] complexes, which made up the energy gap between Yb3+ sensitizers and Tm3+ activators, thereby resulting in about 2000-fold enhancement of the blue emission in Tm3+ (1G2 → 3F4).96 However, due to the extremely weak emission of the UCNPs at room temperature, the enhancement effect may be overvalued. On a separate note, Shi et al. suggested that the thermally enhanced upconversion in these small nanoparticles may also be partly attributed to water desorption,69 which was not examined in the original studies.
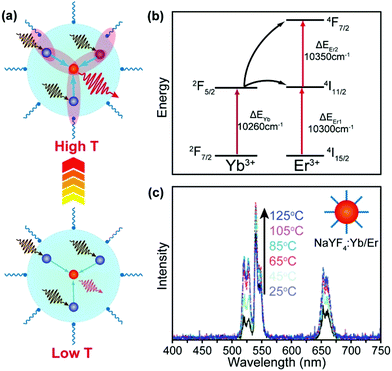 | ||
| Fig. 6 (a) Thermal activation of phonon-assisted energy transfer. (b) Mismatch of energy levels between Yb3+ and Er3+ ions, illustrating the need for phonon participation in the energy transfer process. (c) Temperature-dependent upconversion emission spectra of NaYF4:Yb/Er (20/2%) UCNPs (24 nm) in the temperature range of 25–125 °C. Reproduced with permission from ref. 79. Copyright 2014, American Chemical Society. | ||
Recently, the temperature dependence of upconversion luminescence has been assessed in host materials displaying anomalous thermal contraction characteristics.97–99 Owing to the negative thermal expansion (NTE), sensitizer–activator pairs are brought in close proximity at elevated temperatures, which favours the energy transfer process and thus promotes the upconversion emission (Fig. 7a).100 In a pioneering study by our group, a 29-fold enhancement of green emission and a 13-fold enhancement of overall emission in Yb2W3O12:Er (6%) microcrystals were achieved by increasing the temperature from 303 to 573 K (Fig. 7b).101 The mechanistic investigation confirmed that both light absorption by the Yb3+ sensitizer and energy transfer to Er3+ activators were enhanced with the increase in temperature, owing to lattice contraction and distortion. Subsequently, thermal enhancement of upconversion luminescence was also observed in other NTE materials such as Sc2Mo3O12:Yb/Ho (18/2%) microcrystals and ScF3:Yb/Er (18/2%)@ScF3 core–shell nanocrystals, demonstrating the versatility of the NTE effect for enhancing upconversion in a thermal field.102,103
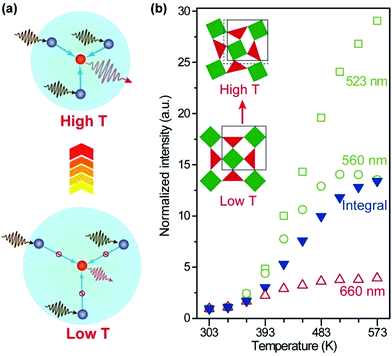 | ||
| Fig. 7 (a) Thermal promotion of energy delivery to activator ions by lattice contraction in negative thermal expansion crystals. (b) Normalized emission intensities of Yb2W3O12:Er (6%) nanocrystals as a function of temperature. Inset: schematic of the lattice contraction and distortion as the temperature increases. Adapted with permission from ref. 101. Copyright 2019, Wiley-VCH. | ||
It is worth noting that luminescent centres may gain energy from defect states in the host lattice at elevated temperatures, which also opposes thermal quenching. In a representative example, Kim et al. reported an electron loop (thermal ionization → defect trapping → detrapping) process in Na3Sc2(PO4)3:Eu (divalent, 0.7%) blue-emitting phosphors to combat thermal quenching.104 Na3Sc2(PO4)3 is a polymorphic material that undergoes cation disordering upon heating, leading to defect formation. The structural defects can trap thermally ionized electrons followed by energy transfer to the Eu2+ activators, thereby contributing to the recovery of light emission at elevated temperatures (Fig. 8). To date, such defect-assisted processes are primarily observed in down-shifting luminescent materials.105–109 Novel designs need to be adopted in order to take advantage of the structural defects in UCNPs.
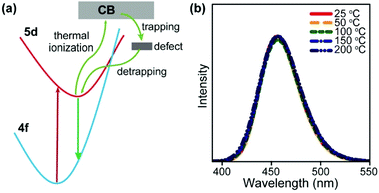 | ||
| Fig. 8 (a) A simplified model for the zero-thermal-quenching due to structural defects. (b) Temperature-dependent emission spectra of Na3Sc2(PO4)3:Eu (divalent, 0.7%) phosphors under 370 nm excitation in the temperature range of 25–200 °C. Reproduced with permission from ref. 104. Copyright 2017, Nature Publishing Group. | ||
4. Conclusions
In conclusion, we have summarized various nonradiative processes and addressed their temperature dependence. Accordingly, recent efforts for alleviating energy dissipation and enhancement of energy collection have been reviewed to combat thermal quenching in UCNPs. By the rational selection of host/dopant combination coupled with precise control of the nanoparticle size and surface coating, novel UCNPs have even been constructed to display thermally enhanced upconversion emission.Despite the achievements, the exact mechanism for these anti-thermal quenching phenomena is still a topic of debate though various explanations have been proposed by different research groups. Nanostructures often experience complex changes during heat treatment and may be subjected to chemical reactions even at moderate temperatures.110,111 Therefore, special attention should be paid to the temperature-dependent characterization of these nanomaterials in future mechanistic investigations. On a separate note, the established UCNPs that excel in thermal fields usually display poor upconversion performances. To achieve thermal enhancement of upconversion, or more precisely thermal recovery of radiative emission, lanthanide dopant ions are typically exposed to the nanoparticle surface, leading to significant surface quenching and thus low upconversion efficiency.
Considering the extensive investigations of upconversion in NaYF4 and its variants, future construction of UCNPs with high performance at both room and high temperatures may hinge on the development of new compositions and structures. One potential direction is to construct nonuniformly coated core–shell UCNPs through strain engineering,112–115 which modifies the crystal parameters of the host lattice and alters the crystal field around the luminescent dopants. In addition, the thermal response of anisotropic core–shell UCNPs is affected by multiple factors, thereby permitting the study of competitive relationships between thermal enhancement and quenching.
NTE hosts represent another important class of materials for future exploration. The NTE effect leads to thermal modulation of upconversion irrespective of the crystal surface status,101–103 which offers great flexibility for optimizing the luminescence efficiency. Given the large number of inorganic crystals that are found to show NTE characteristics, such as MgZrF6, CaZrF6 and CaHfF6,116–118 a family of new upconversion materials can be constructed to combat thermal quenching of luminescence.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21773200) and the Research Grants Council of Hong Kong (CityU 11204717 and 11205219).Notes and references
-
G. Blasse and B. Grabmaier, Luminescent Materials, Springer, Berlin, 1994 Search PubMed
.
- B. Chen, Y. Liu, Y. Xiao, X. Chen, Y. Li, M. Li, X. Qiao, X. Fan and F. Wang, J. Phys. Chem. Lett., 2016, 7, 4916–4921 CrossRef CAS
.
- W. Zheng, P. Huang, D. Tu, E. Ma, H. Zhu and X. Chen, Chem. Soc. Rev., 2015, 44, 1379–1415 RSC
.
- G. Chen, H. Ågren, T. Y. Ohulchanskyy and P. N. Prasad, Chem. Soc. Rev., 2015, 44, 1680–1713 RSC
.
- X. Ai, Z. Wang, H. Cheong, Y. Wang, R. Zhang, J. Lin, Y. Zheng, M. Gao and B. Xing, Nat. Commun., 2019, 10, 1087 CrossRef
.
- V. Marturano, J. Kozlowska, A. Bajek, M. Giamberini, V. Ambrogi, P. Cerruti, R. Garcia-Valls, J. M. Montornes and B. Tylkowski, Coord. Chem. Rev., 2019, 398, 213013 CrossRef CAS
.
- L. Sun, R. Wei, J. Feng and H. Zhang, Coord. Chem. Rev., 2018, 364, 10–32 CrossRef CAS
.
- J. C. Goldschmidt and S. Fischer, Adv. Opt. Mater., 2015, 3, 510–535 CrossRef CAS
.
- B. Chen, W. Kong, Y. Liu, Y. Lu, M. Li, X. Qiao, X. Fan and F. Wang, Angew. Chem., Int. Ed., 2017, 56, 10383–10387 CrossRef CAS
.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC
.
- G. Tessitore, S. L. Maurizio, T. Sabri and J. A. Capobianco, Angew. Chem., Int. Ed., 2019, 58, 9742–9751 CrossRef CAS
.
- B. Tian, A. Fernandez-Bravo, H. Najafiaghdam, N. A. Torquato, M. V. P. Altoe, A. Teitelboim, C. A. Tajon, Y. Tian, N. J. Borys, E. S. Barnard, M. Anwar, E. M. Chan, P. J. Schuck and B. E. Cohen, Nat. Commun., 2018, 9, 3082 CrossRef
.
- G. Jalani, V. Tam, F. Vetrone and M. Cerruti, J. Am. Chem. Soc., 2018, 140, 10923–10931 CrossRef CAS
.
- S. Fischer, R. D. Mehlenbacher, A. Lay, C. Siefe, A. P. Alivisatos and J. A. Dionne, Nano Lett., 2019, 19, 3878–3885 CrossRef CAS
.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS
.
- J. Ueda, P. Dorenbos, A. J. Bos, A. Meijerink and S. Tanabe, J. Phys. Chem. C, 2015, 119, 25003–25008 CrossRef CAS
.
- A. Taguchi, H. Nakagome and K. Takahei, J. Appl. Phys., 1991, 70, 5604–5607 CrossRef CAS
.
- J. Lambkin, D. Dunstan, K. Homewood, L. Howard and M. Emeny, Appl. Phys. Lett., 1990, 57, 1986–1988 CrossRef CAS
.
- B. Cao, Y. Bao, Y. Liu, J. Shang, Z. Zhang, Y. He, Z. Feng and B. Dong, Chem. Eng. J., 2020, 385, 123906 CrossRef CAS
.
- D. Li, W.-Y. Lai, X. Shen, Q. Shao and W. Huang, Mater. Chem. Front., 2019, 3, 791–795 RSC
.
- L. Li, F. Qin, Y. Zhou, Y. Zheng, H. Zhao and Z. Zhang, J. Lumin., 2019, 206, 335–341 CrossRef CAS
.
- T. Pang, W. Peng, M. Yang, J. Xie and W. Lu, J. Rare Earths, 2018, 36, 1136–1140 CrossRef CAS
.
- Q. Han, W. Gao, J. Qi, X. Zhao, J. Zhang, Y. Wang, S. Dong, W. Liu, A. Hao and J. Dong, J. Lumin., 2019, 212, 227–232 CrossRef CAS
.
- R. Luo, Q. Li, J. Wang, Z. Ning, Y. Zhao, M. Liu, X. Lai, C. Zhong, J. Bi and D. Gao, J. Alloys Compd., 2020, 828, 154375 CrossRef CAS
.
- P. Du and J. S. Yu, Curr. Appl. Phys., 2017, 17, 1662–1669 CrossRef
.
- K. Zheng, Z. Liu, C. Lv and W. Qin, J. Mater. Chem. C, 2013, 1, 5502–5507 RSC
.
- Z. Wang, P. Zhang, Q. Yuan, X. Xu, P. Lei, X. Liu, Y. Su, L. Dong, J. Feng and H. Zhang, Nanoscale, 2015, 7, 17861–17870 RSC
.
- K. Zheng, G. He, W. Song, X. Bi and W. Qin, J. Mater. Chem. C, 2015, 3, 11589–11594 RSC
.
- A. Yakovliev, T. Y. Ohulchanskyy, R. Ziniuk, T. Dias, X. Wang, H. Xu, G. Chen, J. Qu and A. S. Gomes, Part. Part. Syst. Charact., 2020, 37, 1900445 CrossRef CAS
.
- M. Ding, C. Lu, L. Chen and Z. Ji, J. Alloys Compd., 2018, 763, 85–93 CrossRef CAS
.
- X. Bai, H. Song, G. Pan, Y. Lei, T. Wang, X. Ren, S. Lu, B. Dong, Q. Dai and L. Fan, J. Phys. Chem. C, 2007, 111, 13611–13617 CrossRef CAS
.
- L. Li, F. Qin, L. Li, H. Gao and Z. Zhang, J. Mater. Chem. C, 2019, 7, 7378–7385 RSC
.
- W. Xu, Y. Hu, L. Zheng, Z. Zhang and W. Cao, J. Lumin., 2019, 215, 116617 CrossRef CAS
.
- K. W. Meert, V. A. Morozov, A. M. Abakumov, J. Hadermann, D. Poelman and P. F. Smet, Opt. Express, 2014, 22, A961–A972 CrossRef CAS
.
- E.-H. Song, S. Ding, M. Wu, S. Ye, F. Xiao, G.-P. Dong and Q.-Y. Zhang, J. Mater. Chem. C, 2013, 1, 4209–4215 RSC
.
- Z. Chen, E. Song, M. Wu, S. Ding, S. Ye and Q. Zhang, J. Alloys Compd., 2016, 667, 134–140 CrossRef CAS
.
- Y. Li, Z. Li, L. Guo, B. Yang and T. Li, J. Alloys Compd., 2020, 847, 156399 CrossRef CAS
.
- P. Du, Y. Guo, S. H. Lee and J. S. Yu, RSC Adv., 2017, 7, 3170–3178 RSC
.
- D. Chen, S. Liu, X. Li, S. Yuan and P. Huang, J. Eur. Ceram. Soc., 2017, 37, 4939–4945 CrossRef CAS
.
- H. Guan, Y. Sheng, Y. Song, C. Xu, X. Zhou, K. Zheng, Z. Shi and H. Zou, J. Phys. Chem. C, 2017, 121, 23080–23095 CrossRef CAS
.
- B. Chen and F. Wang, Trends Chem., 2020, 2, 427–439 CrossRef CAS
.
- M. Weber, Phys. Rev. B: Solid State, 1973, 8, 54 CrossRef CAS
.
- D. Yu, T. Yu, Y. Wang, Q. Zhang and A. Meijerink, Phys. Rev. Appl., 2020, 13, 024076 CrossRef CAS
.
- D. Yu, J. Ballato and R. E. Riman, J. Phys. Chem. C, 2016, 120, 9958–9964 CrossRef CAS
.
- E. C. Ximendes, A. F. Pereira, U. Rocha, W. F. Silva, D. Jaque and C. Jacinto, Nanoscale, 2019, 11, 8864–8869 RSC
.
- J. M. F. van Dijk and M. F. H. Schuurmans, J. Chem. Phys., 1983, 78, 5317–5323 CrossRef CAS
.
- L. A. Riseberg and H.-W. Moos, Phys. Rev., 1968, 174, 429 CrossRef CAS
.
- Y. Zhao, C. Riemersma, F. Pietra, R. Koole, C. de Mello Donegá and A. Meijerink, ACS Nano, 2012, 6, 9058–9067 CrossRef CAS
.
- M. Lax, J. Chem. Phys., 1952, 20, 1752–1760 CrossRef CAS
.
- J. Ueda, A. Meijerink, P. Dorenbos, A. J. Bos and S. Tanabe, Phys. Rev. B, 2017, 95, 014303 CrossRef
.
- A. D'Aléo, F. Pointillart, L. Ouahab, C. Andraud and O. Maury, Coord. Chem. Rev., 2012, 256, 1604–1620 CrossRef
.
- H. Hoefdraad, J. Inorg. Nucl. Chem., 1975, 37, 1917–1921 CrossRef CAS
.
- P. Dorenbos, J. Phys.: Condens. Matter, 2003, 15, 4797 CrossRef CAS
.
- J. J. Joos, L. Seijo and Z. Barandiarán, J. Phys. Chem. Lett., 2019, 10, 1581–1586 CrossRef CAS
.
- B. Chen, X. Qiao, D. Peng and X. Fan, J. Phys. Chem. C, 2014, 118, 30197–30201 CrossRef CAS
.
- F. Wang and X. Liu, Acc. Chem. Res., 2014, 47, 1378–1385 CrossRef CAS
.
- T. Forster, Ann. Phys., 1948, 2, 55–75 CrossRef CAS
.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef CAS
.
- B. Chen, Q. Su, W. Kong, Y. Wang, P. Shi and F. Wang, J. Mater. Chem. B, 2018, 6, 2924–2944 RSC
.
-
B. Henderson and G. F. Imbusch, Optical spectroscopy of inorganic solids, Oxford Science Publications, London, 2006 Search PubMed
.
- X. Chen, D. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318–1330 RSC
.
- T. Sun, Y. Li, W. L. Ho, Q. Zhu, X. Chen, L. Jin, H. Zhu, B. Huang, J. Lin, B. E. Little, S. T. Chu and F. Wang, Nat. Commun., 2019, 10, 1811 CrossRef
.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063–1070 CrossRef CAS
.
- R. Arppe, I. Hyppänen, N. Perälä, R. Peltomaa, M. Kaiser, C. Würth, S. Christ, U. Resch-Genger, M. Schäferling and T. Soukka, Nanoscale, 2015, 7, 11746–11757 RSC
.
- B. Chen and F. Wang, Acc. Chem. Res., 2019, 53, 358–367 CrossRef
.
- F. Wang, J. Wang and X. Liu, Angew. Chem., Int. Ed., 2010, 49, 7456–7460 CrossRef CAS
.
- B. Chen and F. Wang, Inorg. Chem. Front., 2020, 7, 1067–1081 RSC
.
- S. Guo, X. Xie, L. Huang and W. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 847–853 CrossRef CAS
.
- R. Shi, E. D. Martinez, C. D. S. Britesa and L. D. Carlos, Phys. Chem. Chem. Phys., 2021, 23, 20–42 RSC
.
- Y. Hu, Q. Shao, P. Zhang, Y. Dong, F. Fang and J. Jiang, J. Phys. Chem. C, 2018, 122, 26142–26152 CrossRef CAS
.
- Z. Wang, J. Christiansen, D. Wezendonk, X. Xie, M. A. Van Huis and A. Meijerink, Nanoscale, 2019, 11, 12188–12197 RSC
.
- Y. Hu, Q. Shao, X. Deng and J. Jiang, Nanophotonics, 2020, 9, 2879–2885 CrossRef CAS
.
- Q. Chen, X. Xie, B. Huang, L. Liang, S. Han, Z. Yi, Y. Wang, Y. Li, D. Fan and L. Huang, Angew. Chem., Int. Ed., 2017, 56, 7605–7609 CrossRef CAS
.
- Q. Su, S. Han, X. Xie, H. Zhu, H. Chen, C.-K. Chen, R.-S. Liu, X. Chen, F. Wang and X. Liu, J. Am. Chem. Soc., 2012, 134, 20849–20857 CrossRef CAS
.
- L. Liang, X. Qin, K. Zheng and X. Liu, Acc. Chem. Res., 2018, 52, 228–236 CrossRef
.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS
.
- X. Cui, Y. Cheng, H. Lin, F. Huang, Q. Wu and Y. Wang, Nanoscale, 2017, 9, 13794–13799 RSC
.
- B. Chen, W. Kong, N. Wang, G. Zhu and F. Wang, Chem. Mater., 2019, 31, 4779–4786 CrossRef CAS
.
- D. Li, Q. Shao, Y. Dong and J. Jiang, J. Phys. Chem. C, 2014, 118, 22807–22813 CrossRef CAS
.
- E. D. Martínez, C. D. Brites, L. D. Carlos, A. F. García–Flores, R. R. Urbano and C. Rettori, Adv. Funct. Mater., 2019, 29, 1807758 CrossRef
.
- Q. Shao, G. Zhang, L. Ouyang, Y. Hu, Y. Dong and J. Jiang, Nanoscale, 2017, 9, 12132–12141 RSC
.
- D. D. Li, Q. Y. Shao, Y. Dong, F. Fang and J. Q. Jiang, Part. Part. Syst. Charact., 2015, 32, 728–733 CrossRef CAS
.
- L. Lei, D. Chen, C. Li, F. Huang, J. Zhang and S. Xu, J. Mater. Chem. C, 2018, 6, 5427–5433 RSC
.
- C. D. Brites, E. D. Martínez, R. R. Urbano, C. Rettori and L. D. Carlos, Front. Chem., 2019, 7, 267 CrossRef CAS
.
- E. D. Martínez, R. R. Urbano and C. Rettori, ACS Appl. Nano Mater., 2019, 2, 6889–6897 CrossRef
.
- L. Lei, J. Xia, Y. Cheng, Y. Wang, G. Bai, H. Xia and S. Xu, J. Mater. Chem. C, 2018, 6, 11587–11592 RSC
.
- D. Baek, T. K. Lee, I. Jeon, S. H. Joo, S. Shin, J. Park, S. J. Kang, S. K. Kwak and J. Lee, Adv. Sci., 2020, 7, 2000104 CrossRef CAS
.
- Y. Hu, Q. Shao, X. Deng, D. Song, S. Han, Y. Dong and J. Jiang, J. Mater. Chem. C, 2019, 7, 11770–11775 RSC
.
- C. Mi, J. Zhou, F. Wang, G. Lin and D. Jin, Chem. Mater., 2019, 31, 9480–9487 CrossRef CAS
.
- Y. Hu, Q. Shao, X. Deng, S. Han, D. Song and J. Jiang, Adv. Mater. Technol., 2019, 4, 1800498 CrossRef
.
- J. Suyver, J. Grimm, K. Krämer and H.-U. Güdel, J. Lumin., 2005, 114, 53–59 CrossRef CAS
.
- G. Salley, R. Valiente and H. Guedel, J. Lumin., 2001, 94, 305–309 CrossRef
.
- X. Xu, W. Zhang, D. Yang, W. Lu, J. Qiu and S. F. Yu, Adv. Mater., 2016, 28, 8045–8050 CrossRef CAS
.
- X. Chen, H. Zhuang, G. Liu, S. Li and R. Niedbala, J. Appl. Phys., 2003, 94, 5559–5565 CrossRef CAS
.
- G. Liu, H. Zhuang and X. Chen, Nano Lett., 2002, 2, 535–539 CrossRef CAS
.
- J. Zhou, S. Wen, J. Liao, C. Clarke, S. A. Tawfik, W. Ren, C. Mi, F. Wang and D. Jin, Nat. Photonics, 2018, 12, 154 CrossRef CAS
.
- T. Varga, A. P. Wilkinson, C. Lind, W. A. Bassett and C.-S. Zha, J. Phys.: Condens. Matter, 2005, 17, 4271 CrossRef CAS
.
- W. Miller, C. Smith, D. Mackenzie and K. Evans, J. Mater. Sci., 2009, 44, 5441–5451 CrossRef CAS
.
- H. Liu, W. Sun, Z. Zhang, X. Zhang, Y. Zhou, J. Zhu and X. Zeng, Inorg. Chem. Front., 2019, 6, 1842–1850 RSC
.
- R. Zhu, K. Jia, Z. Bi, Y. Liu and Y. Lyu, J. Solid State Chem., 2020, 290, 121592 CrossRef CAS
.
- H. Zou, X. Yang, B. Chen, Y. Du, B. Ren, X. Sun, X. Qiao, Q. Zhang and F. Wang, Angew. Chem., Int. Ed., 2019, 58, 17255–17259 CrossRef CAS
.
- H. Zou, B. Chen, Y. Hu, Q. Zhang, X. Wang and F. Wang, J. Phys. Chem. Lett., 2020, 11, 3020–3024 CrossRef CAS
.
- B. Ren, B. Chen, J. Zhao, Y. Guo, X. Zhang, X. Chen, Y. Du, Z. Deng, G. Zhu and F. Wang, Chem. Mater., 2021, 33, 158–163 CrossRef CAS
.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, Nat. Mater., 2017, 16, 543–550 CrossRef CAS
.
- Z. Tang, G. Zhang and Y. Wang, ACS Photonics, 2018, 5, 3801–3813 CrossRef CAS
.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 CrossRef CAS
.
- J. Wen, Z. Guo, H. Guo, L. Ning, C.-K. Duan, Y. Huang, S. Zhan and M. Yin, Inorg. Chem., 2018, 57, 6142–6151 CrossRef CAS
.
- R. Shi, L. Ning, Z. Wang, J. Chen, T. K. Sham, Y. Huang, Z. Qi, C. Li, Q. Tang and H. Liang, Adv. Opt. Mater., 2019, 7, 1901187 CrossRef CAS
.
- Y. Chen, B. Yu, J. Gou and S. F. Liu, J. Mater. Chem. C, 2018, 6, 10687–10692 RSC
.
- L. Wang, X. Li, Z. Li, W. Chu, R. Li, K. Lin, H. Qian, Y. Wang, C. Wu, J. Li, D. Tu, Q. Zhang, L. Song, J. Jiang, X. Chen, Y. Xie and Y. Xiong, Adv. Mater., 2015, 27, 5528–5533 CrossRef CAS
.
- Z. Wang, X. Li, G. Zhang, Y. Luo and J. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 23309–23313 CrossRef CAS
.
- B. Chen, Y. Wang, Y. Guo, P. Shi and F. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 2327–2335 CrossRef CAS
.
- J. Zhao, X. Chen, B. Chen, X. Luo, T. Sun, W. Zhang, C. Wang, J. Lin, D. Su, X. Qiao and F. Wang, Adv. Funct. Mater., 2019, 29, 1903295 CrossRef CAS
.
- N. J. Johnson and F. C. van Veggel, ACS Nano, 2014, 8, 10517–10527 CrossRef CAS
.
- J. Zhao, B. Chen and F. Wang, Adv. Mater., 2020, 2004142 CrossRef CAS
.
- S. J. Baxter, B. R. Hester, B. R. Wright and A. P. Wilkinson, Chem. Mater., 2019, 31, 3440–3448 CrossRef CAS
.
- J. C. Hancock, K. W. Chapman, G. J. Halder, C. R. Morelock, B. S. Kaplan, L. C. Gallington, A. Bongiorno, C. Han, S. Zhou and A. P. Wilkinson, Chem. Mater., 2015, 27, 3912–3918 CrossRef CAS
.
-
D. J. Fisher, Negative Thermal Expansion Materials, Materials Research Forum LLC, Millersville, 2018 Search PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |