Asymmetric β,γ′-regioselective [4 + 3] and [4 + 2] annulations of α-vinylenals via cascade iminium ion-dienamine catalysis†
Yang
Gao
a,
Xue
Song
a,
Ru-Jie
Yan
a,
Wei
Du
a and
Ying-Chun
Chen
 *ab
*ab
aKey Laboratory of Drug-Targeting and Drug Delivery System of the Ministry of Education and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy, Sichuan University, Chengdu 610041, China. E-mail: ycchen@scu.edu.cn
bCollege of Pharmacy, Third Military Medical University, Chongqing 400038, China
First published on 22nd October 2020
Abstract
Introduction of an α-vinyl group into enal substrates can prohibit the traditional [3 + 2] cycloaddition with N-2,2,2-trifluoroethyl isatin imines catalysed by a chiral secondary amine, but give β,γ′-regioselective [4 + 3] annulation products via cascade iminium ion-dienamine catalysis. A spectrum of CF3-containing spirooxindoles incorporating an azepane motif were constructed with good to excellent enantioselectivities. In addition, asymmetric [4 + 2] annulations between α-vinylenals and α,α-dicyanoalkenes were disclosed through a similar catalytic strategy, generally affording complex tricyclic frameworks with outstanding enantioselectivities.
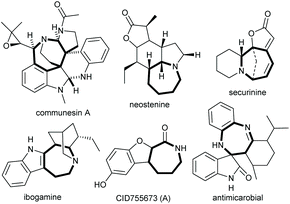
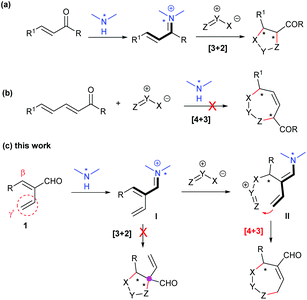
The seven-membered N-heterocycles, as a type of medium-sized ring motif, are ubiquitous in natural products and bioactive compounds, as exemplified in Fig. 1.1 Thus, the construction of such architectures, especially in an asymmetric catalytic manner, has attracted much attention.2 The enantioselective [4 + 3] annulation reaction from readily available starting materials, particularly classic 1,3-dipoles or in situ formed zwitterionic species, represents one of the most straightforward strategies to access these N-heterocycles,3 but faces the challenging transannular interactions and entropic effects during the ring formation process.4 With the development of asymmetric catalysis, a diversity of enantioenriched seven-membered rings have been synthesized via the catalysis of transition metals,5 phosphines,6 N-heterocyclic carbenes,7 Brønsted acids,8 or even via cooperative catalysis;9 however, similar reactions catalysed by chiral amines have been rarely explored.
Actually, asymmetric [3 + 2] dipolar cycloaddition reactions between α,β-unsaturated carbonyl compounds and 1,3-dipoles via iminium ion activation have been well documented, usually in a concerted manner (Scheme 1a).10 Nevertheless, the related [4 + 3] annulation reaction could not be possible by employing dienals or dienones with an extended double bond as substrates due to the more favourable [3 + 2] pathway (Scheme 1b).11 With the continuing interest in developing asymmetric [4 + 3] annulation reactions to construct seven-membered N-heterocycles,9c,12 we designed enals 1 with an α-vinyl group,13 which could be activated as iminium ions I in the presence of amine catalysts. It was envisaged that the classical [3 + 2] cycloaddition with 1,3-diploes might be prevented by the α-substituent because of the formation of a sterically hindered quaternary centre; in contrast, the β,γ′-regioselective [4 + 3] annulation pathway would be accomplished via cascade iminium ion-dienamine catalysis,14 finally affording the expected seven-membered N-heterocycles, as proposed in Scheme 1c.
 | ||
| Scheme 1 Amine-catalysed cycloaddition or annulation reaction of unsaturated carbonyl compounds with 1,3-dipoles. | ||
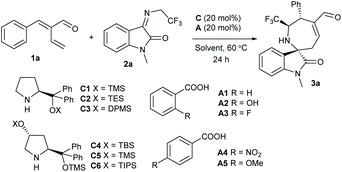
Based on the above considerations, we explored the reactions between readily available α-vinylenal 1a and several types of 1,3-dipole substrates.15 Although most previously formed 1,3-dipoles generally showed inert reactivity, fortunately, N-2,2,2-trifluoroethylisatin imine 2a,16 which can easily isomerize to active azomethine ylide-type species, exhibited good reactivity with 1a in DCE at 60 °C, under the catalysis of chiral secondary amine catalyst C1 and benzoic acid A1. The desired β,γ′-regioselective [4 + 3] product 3a, proceeding in a stepwise Michael addition and Mannich reaction, was isolated in a good yield with exclusive diastereoselectivity and high enantioselectivity (Table 1, entry 1). It should be noted that the α,β-regioselective cycloadduct was not detected.17 Subsequently, more catalytic parameters were screened to improve the enantiocontrol. Using bulkier amines C2 and C3 resulted in a dramatic decrease of yield (entries 2 and 3), and amines C4–C6 derived from L-hydroxyproline provided better enantioselectivity (entries 4–6). Nevertheless, the assemblies of amine C4 and other acid additives generally delivered inferior results (entries 7–10). Performing the reaction at lower temperature afforded slightly increased data (entry 11), but poor conversion was observed at 40 °C (entry 12). A few solvents were evaluated but no better results were obtained (entries 13–16). Finally, we conducted the asymmetric reaction on a larger scale, and comparable data were obtained (entry 17).
| Entry | C | A | Solvent | Yieldb (%) | eec (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Unless noted otherwise, reactions were performed with enal 1a (0.12 mmol, 1.2 equiv.), isatin imine 2a (0.1 mmol, 1.0 equiv.), amine C (0.02 mmol, 20 mol%) and acid A (0.02 mmol, 20 mol%) in solvent (0.2 mL) at 60 °C for 24 h.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase; dr >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.
d At 50 °C.
e At 40 °C.
f On a 1.0 mmol scale. 1 by 1H NMR analysis.
d At 50 °C.
e At 40 °C.
f On a 1.0 mmol scale.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | C1 | A1 | DCE | 80 | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | C2 | A1 | DCE | 65 | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | C3 | A1 | DCE | 40 | 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | C4 | A1 | DCE | 70 | 91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | C5 | A1 | DCE | 70 | 88 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | C6 | A1 | DCE | 62 | 87 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | C4 | A2 | DCE | 65 | 83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | C4 | A3 | DCE | 60 | 90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | C4 | A4 | DCE | 45 | 80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | C4 | A5 | DCE | 68 | 90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | C4 | A1 | DCE | 75 | 92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12e | C4 | A1 | DCE | <10 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13d | C4 | A1 | CHCl3 | 80 | 88 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14d | C4 | A1 | THF | 60 | 84 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15d | C4 | A1 | Toluene | 70 | 88 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16d | C4 | A1 | CH3CN | 72 | 90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17d,f | C4 | A1 | DCE | 70 | 90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
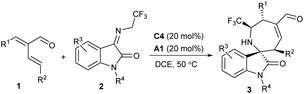
Consequently, we explored the substrate scope and limitations of this [4 + 3] annulation reaction. The results are summarised in Table 2. First, an array of α-vinylenals 1 with diverse β-aryl or heteroaryl groups were explored in the reactions with N-2,2,2-trifluoroethylisatin imine 2a. In general, high to excellent enantioselectivities were obtained for the tested reactions (Table 2, entries 2–8), whereas slightly reduced yields were isolated for the substrates with electron-withdrawing ones (entries 2 and 3). Unfortunately, the enals 1 with β-alkyl substituents showed poor reactivity.15 High yield and enantioselectivity were produced for an enal 1i bearing an α-styryl group (entry 9), but the desired annulation products were not formed by using enals 1 having a γ′-alkyl group.15 On the other hand, a wide range of N-2,2,2-trifluoroethylisatin imines 2 with either electron-withdrawing or electron-donating groups on the phenyl ring could be well tolerated in the reactions with enal 1a, and the corresponding products 3j−3p were delivered in moderate to good yields with good to excellent enantioselectivities. Furthermore, the isatin imine derivatives 2 with an N-MOM or N-Bn group were also compatible (entries 17 and 18).
| Entry | R1, R2 | R3, R4 | Yieldb (%) | eec (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Unless noted otherwise, reactions were performed with enal 1 (0.12 mmol, 1.2 equiv.), isatin imine 2 (0.1 mmol, 1.0 equiv.), amine C4 (0.02 mmol, 20 mol%) and acid A1 (0.02 mmol, 20 mol%) in DCE (0.2 mL) at 50 °C for 12–24 h.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase; dr >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.
d The absolute configuration of enantiopure 3b was determined by X-ray analysis. The other products were assigned by analogy. 1 by 1H NMR analysis.
d The absolute configuration of enantiopure 3b was determined by X-ray analysis. The other products were assigned by analogy.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Ph, H | H, Me | 3a, 75 | 92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 3-BrC6H4, H | H, Me | 3b, 65 | 87d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 4-BrC6H4, H | H, Me | 3c, 60 | 88 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 4-MeC6H4, H | H, Me | 3d, 80 | 91 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 3-MeOC6H4, H | H, Me | 3e, 80 | 92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 4-MeOC6H4, H | H, Me | 3f, 73 | 86 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 2-Naphthyl, H | H, Me | 3g, 82 | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 2-Thienyl, H | H, Me | 3h, 80 | 88 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Ph, Ph | H, Me | 3i, 90 | 92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Ph, H | 5-MeO, Me | 3j, 75 | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Ph, H | 5-Me, Me | 3k, 75 | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Ph, H | 6-Cl, Me | 3l, 68 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Ph, H | 7-F, Me | 3m, 60 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Ph, H | 7-Cl, Me | 3n, 60 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Ph, H | 7-Me, Me | 3o, 75 | 80 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Ph, H | 7-MeO, Me | 3p, 78 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Ph, H | H, Bn | 3q, 78 | 93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Ph, H | H, MOM | 3r, 80 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
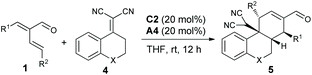
The application of α-vinylenals 1 as successful 4C partners via cascade iminium ion-dienamine catalysis inspired us to explore their potential in other types of annulation reactions. Actually, six-membered carbocyclic compounds are widespread in nature, which could be efficiently constructed via concerted or stepwise [4 + 2] reactions.18 Electron-deficient α,α-dicyanoalkenes have been applied as bifunctional vinylogous Michael donors and Michael acceptors in [4 + 2] annulations with α,β-unsaturated ketones via aminocatalysis.19 As a result, we investigated the reaction between enal 1a and α,α-dicyanoalkene 4a (Table 3, X = S). To our gratification, the expected [4 + 2] annulation proceeded smoothly in THF at ambient temperature, and product 5a was obtained in an excellent yield with an outstanding ee value under the catalysis of amine C2 and acid A4 (Table 3, entry 1).15 A range of α-vinyl enals 1 with diverse β-aryl groups could be well tolerated in the reactions with substrate 4a, and the corresponding tricyclic frameworks 5b−5k were constructed in moderate to high yields with excellent enantioselectivities (entries 2–11). Nevertheless, a significantly reduced ee value was obtained by using enal 1i (entry 12). In addition, α,α-dicyanoalkene 4b derived from chroman-4-one exhibited much lower reactivity, whereas the desired adduct 5m was furnished in remarkable enantiocontrol (entry 13).
| Entry | X | R1, R2 | Yieldb (%) | eec (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Unless noted otherwise, reactions were performed with enal 1 (0.12 mmol, 1.2 equiv.), α,α-dicyanoalkene 4 (0.1 mmol, 1.0 equiv.), amine C3 (0.02 mmol, 20 mol%) and acid A4 (0.02 mmol, 20 mol%) in THF (0.2 mL) at rt for 12 h.
b Yield of the isolated product.
c Determined by HPLC analysis on a chiral stationary phase; dr >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.
d The absolute configuration of enantiopure 5g was determined by X-ray analysis. The other products were assigned by analogy. 1 by 1H NMR analysis.
d The absolute configuration of enantiopure 5g was determined by X-ray analysis. The other products were assigned by analogy.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | S | Ph, H | 5a, 92 | 98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | S | 3-ClC6H4, H | 5b, 75 | 94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | S | 4-ClC6H4, H | 5c, 92 | 98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | S | 4-BrC6H4, H | 5d, 86 | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | S | 4-NO2C6H4, H | 5e, 65 | 92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | S | 4-MeC6H4, H | 5f, 89 | 99 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | S | 2-MeOC6H4, H | 5g, 70 | 98d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | S | 3-MeOC6H4, H | 5h, 90 | 97 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | S | 4-MeOC6H4, H | 5i, 90 | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | S | 2-Naphthyl, H | 5j, 76 | 98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | S | 2-Thienyl, H | 5k, 95 | 99 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | S | Ph, Ph | 5l, 70 | 55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | O | Ph, H | 5m, 48 | 93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
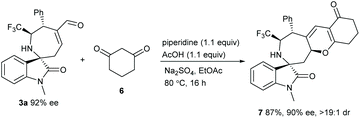
In addition, the remaining α,β-unsaturated aldehyde moiety of cycloadduct 3a could participate in a [3 + 3] annulation reaction with cyclohexane-1,3-dione 6,20 and the product 7 with higher molecular complexity was obtained in a high yield (Scheme 2).
In summary, we designed α-vinyl α,β-unsaturated aldehydes, which could be applied as 4C synthons in β,γ′-regioselective [4 + 3] annulations with N-2,2,2-trifluoroethylisatin imines via cascade iminium ion-dienamine catalysis, rather than the traditional α,β-regioselective [3 + 2] dipolar cycloadditions. A series of spirooxindoles incorporating an azepane motif were efficiently constructed with good to excellent enantioselectivities. These α-vinylenals could be further utilised in [4 + 2] annulations with bifunctional α,α-dicyanoalkenes via the same catalytic strategy, affording highly enantioenriched tricyclic architectures. More results in regard to these synthons will be reported in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 21772126 and 21961132004).Notes and references
- For selected examples, see: (a) M. M. Pompeo, J. H. Cheah and M. Movassaghi, J. Am. Chem. Soc., 2019, 141, 14411 CrossRef CAS; (b) H.-S. Chung, P.-M. Hon, G. Lin, P. P.-H. But and H. Dong, Planta Med., 2003, 69, 914 CrossRef CAS; (c) J. A. Beutler and A. N. Brubaker, Drugs Future, 1987, 12, 957 Search PubMed; (d) G. K. Jana and S. Sinha, Tetrahedron Lett., 2010, 51, 1994 CrossRef CAS; (e) E. T. Marquez, J. S. Smith, S. Guha, R. Kui, T. Waldron, O. Rey and E. Rozengurt, Biochem. Biophys. Res. Commun., 2010, 391, 63 CrossRef; (f) S. Shiotani, T. Kometani, K. Mitsuhasi, T. Nozawa, A. Kurobe and O. Futsukaichi, J. Med. Chem., 1976, 19, 803 CrossRef CAS; (g) J. Schimer, P. Cígler, J. Veselý, K. G. Šašková, M. Lepšík, J. Brynda, P. Řezáčová, M. Kožíšek, I. Císařová, H. Oberwinkler, H.-G. Kraeusslich and J. Konvalinka, J. Med. Chem., 2012, 55, 10130 CrossRef CAS.
- For [5 + 2] annulations, see: (a) H. Pellissier, Adv. Synth. Catal., 2011, 353, 189 CrossRef CAS; (b) K. E. O. Ylijoki and J. M. Stryker, Chem. Rev., 2013, 113, 2244 CrossRef CAS; (c) H. Pellissier, Adv. Synth. Catal., 2018, 360, 1551 CrossRef CAS; for [3 + 2 + 2] annulations, see: (d) L. Cui, L. Ye and L. Zhang, Chem. Commun., 2010, 46, 3351 RSC; (e) M.-B. Zhou, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 4196 CrossRef CAS; (f) T. Li, F. Xu, X. Li, C. Wang and B. Wan, Angew. Chem., Int. Ed., 2016, 55, 2861 CrossRef CAS; for [6 + 1] annulation examples, see: (g) M. Yoshimatsu, M. Tanaka, Y. Fujimura, Y. Ito, Y. Goto, Y. Kobayashi, H. Wasada, N. Hatae, G. Tanabe and O. Muraoka, J. Org. Chem., 2015, 80, 9480 CrossRef CAS.
- For selected reviews, see: (a) C. S. Jeffrey, K. L. Barnes, J. A. Eickhoff and C. R. Carson, J. Am. Chem. Soc., 2011, 133, 7688 CrossRef CAS; (b) J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784 RSC; (c) L. L. Anderson, Asian J. Org. Chem., 2016, 5, 9 CrossRef CAS; (d) S.-I. Murahashi and Y. Imada, Chem. Rev., 2019, 119, 4686 CrossRef.
- C. Galli and L. Mandolini, Eur. J. Org. Chem., 2000, 3117 CrossRef CAS.
- For selected examples, see: (a) R. Shintani, M. Murakami, T. Tsuji, H. Tanno and T. Hayashi, Org. Lett., 2009, 11, 5642 CrossRef CAS; (b) B. D. Schwartz, J. R. Denton, Y. Lian, H. M. L. Davies and C. M. Williams, J. Am. Chem. Soc., 2009, 131, 8329 CrossRef CAS; (c) I. Alonso, H. Faustino, F. López and J. L. Mascareñas, Angew. Chem., Int. Ed., 2011, 50, 11496 CrossRef CAS; (d) J.-J. Feng, T.-Y. Lin, H.-H. Wu and J.-L. Zhang, J. Am. Chem. Soc., 2015, 137, 3787 CrossRef CAS; (e) H. Xu, J.-L. Hu, L.-J. Wang, S.-H. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006 CrossRef CAS; (f) C.-Z. Zhu, J.-J. Feng and J.-L. Zhang, Angew. Chem., Int. Ed., 2017, 56, 1351 CrossRef CAS; (g) C.-R. Xu, K.-X. Wang, D.-W. Li, L.-L. Lin and X.-M. Feng, Angew. Chem., Int. Ed., 2019, 58, 18438 CrossRef CAS; (h) B. M. Trost and Z.-J. Zuo, Angew. Chem., 2019, 59, 1243 CrossRef.
- (a) C.-H. Yuan, L.-J. Zhou, M.-R. Xia, Z.-H. Sun, D.-Q. Wang and H.-C. Guo, Org. Lett., 2016, 18, 5644 CrossRef CAS; (b) K. Kumar, R. Kapoor, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 2023 CrossRef CAS; (c) S.-Q. Zheng and X.-Y. Lu, Org. Lett., 2009, 11, 3978 CrossRef CAS; (d) C.-F. Jing, R.-S. Na, B. Wang, H.-L. Liu, L. Zhang, J. Liu, M. Wang, J.-C. Zhong, O. Kwon and H.-C. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS; (e) J. Zheng, Y. Huang and Z.-M. Li, Org. Lett., 2013, 15, 5758 CrossRef CAS; (f) J.-L. Chen and Y. Huang, Org. Lett., 2017, 19, 5609 CrossRef CAS.
- (a) H. Lv, W.-Q. Jia, L.-H. Sun and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 8607 CrossRef CAS; (b) M. Wang, Z.-J. Huang, J.-F. Xu and Y. R. Chi, J. Am. Chem. Soc., 2014, 136, 1214 CrossRef CAS; (c) Z.-Q. Liang, Z.-H. Gao, W.-Q. Jia and S. Ye, Chem. – Eur. J., 2015, 21, 1868 CrossRef CAS; (d) S.-Y. Zhu, Y.-Z. Zhang, W. Wang and X.-P. Hui, Org. Lett., 2017, 19, 5380 CrossRef CAS; (e) W.-J. Li, H.-J. Yuan, Z.-T. Liu, Z.-Y. Zhang, Y.-Y. Cheng and P.-F. Li, Adv. Synth. Catal., 2018, 360, 2460 CrossRef CAS; (f) C. Fang, J. Cao, K.-W. Sun, J.-D. Zhu, T. Lu and D. Du, Chem. – Eur. J., 2018, 24, 2103 CrossRef CAS.
- (a) Y. Wang, F. Shi, X.-X. Yao, M. Sun, L. Dong and S.-J. Tu, Chem. – Eur. J., 2014, 20, 15047 CrossRef CAS; (b) S. M. Banik, A. Levina, A. M. Hyde and E. N. Jacobsen, Science, 2017, 358, 761 CrossRef CAS; (c) L. Villar, U. Uria, J. I. Martínez, L. Prieto, E. Reyes, L. Carrillo and J. L. Vicario, Angew. Chem., Int. Ed., 2017, 56, 10535 CrossRef CAS; (d) C. Gelis, G. Levitre, J. Merad, P. Retailleau, L. Neuville and G. Masson, Angew. Chem., Int. Ed., 2018, 57, 12121 CrossRef CAS; (e) M. Sun, C. Ma, S.-J. Zhou, S.-F. Lou, J. Xiao, Y.-C. Jiao and F. Shi, Angew. Chem., Int. Ed., 2019, 58, 8703 CrossRef CAS.
- (a) C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2016, 138, 7840 CrossRef CAS; (b) C. Guo, D. Janssen-Müller, M. Fleige, A. Lerchen, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2017, 139, 4443 CrossRef CAS; (c) Z.-C. Chen, Z. Chen, Z.-H. Yang, L. Guo, W. Du and Y.-C. Chen, Angew. Chem., Int. Ed., 2019, 58, 15021 CrossRef CAS.
- For selected reviews, see: (a) H. Pellissier, Tetrahedron, 2007, 63, 3235 CrossRef CAS; (b) J. Adrio and J. C. Carretero, Chem. Commun., 2014, 50, 12434 RSC; (c) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS; (d) C. Nájera, J. M. Sansano and M. Yus, Org. Biomol. Chem., 2015, 13, 8596 RSC.
- P. H. Poulsen, S. Vergura, A. Monleón, D. K. B. Jørgensen and K. A. Jørgensen, J. Am. Chem. Soc., 2016, 138, 6412 CrossRef CAS.
- (a) R.-J. Yan, B.-X. Liu, B.-X. Xiao, W. Du and Y.-C. Chen, Org. Lett., 2020, 22, 4240 CrossRef CAS; (b) G. Zhan, M.-L. Shi, Q. He, W. Du and Y.-C. Chen, Org. Lett., 2015, 17, 4750 CrossRef CAS.
- For selected examples of related dendralen-type substrates, see: (a) A. D. Payne, G. Bojase, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2009, 48, 4836 CrossRef CAS; (b) S. V. Pronin and R. A. Shenvi, J. Am. Chem. Soc., 2012, 134, 19604 CrossRef CAS; (c) N. J. Green, A. L. Lawrence, G. Bojase, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2013, 52, 8333 CrossRef CAS; (d) S.-M. Tan, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn, Angew. Chem., Int. Ed., 2016, 55, 3081 CrossRef CAS; (e) J. George and M. S. Sherburn, J. Org. Chem., 2019, 84, 14712 CrossRef CAS.
- For selected reviews, see: (a) P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2018, 47, 6138 CrossRef; (b) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4704 CrossRef; (c) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS.
- For more details, see the ESI.†.
- For selected examples, see: (a) Q.-T. Sun, X.-Y. Li, J.-H. Su, L. Zhao, M.-X. Ma, Y.-Y. Zhu, Y.-Y. Zhao, R.-R. Zhu, W.-J. Yan, K.-R. Wang and R. Wang, Adv. Synth. Catal., 2015, 357, 3187 CrossRef CAS; (b) Y. You, W.-Y. Lu, Z.-H. Wang, Y.-Z. Chen, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2018, 20, 4453 CrossRef CAS; (c) H. Ryu, J. Seo and H. M. Ko, J. Org. Chem., 2018, 83, 14102 CrossRef CAS; (d) H.-W. Zhao, L.-R. Wang, J.-M. Guo, W.-Q. Ding, X.-Q. Song, H.-H. Wu, Z. Tang, X.-Z. Fan and X.-F. Bi, Adv. Synth. Catal., 2019, 361, 4761 CrossRef CAS; (e) X.-Y. Wu, Y.-N. Gao and M. Shi, Eur. J. Org. Chem., 2019, 1620 CrossRef CAS; (f) B. Niu, X.-Y. Wu, Y. Wei and M. Shi, Org. Lett., 2019, 21, 4859 CrossRef CAS.
- (a) M.-X. Ma, Y.-Y. Zhu, Q.-T. Sun, X.-Y. Li, J.-H. Su, L. Zhao, Y.-Y. Zhao, S. Qiu, W.-J. Yan, K.-R. Wang and R. Wang, Chem. Commun., 2015, 51, 8789 RSC; (b) X.-Y. Gao, R.-J. Yan, B.-X. Xiao, W. Du, Ł. Albrecht and Y.-C. Chen, Org. Lett., 2019, 21, 9628 CrossRef CAS.
- For selected reviews, see: (a) X.-X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS; (b) D.-J. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743 CrossRef CAS; (c) L. Klier, F. Tur, P. H. Poulsen and K. A. Jørgensen, Chem. Soc. Rev., 2017, 46, 1080 RSC.
- (a) J.-W. Xie, W. Chen, R. Li, M. Zeng, W. Du, L. Yue, Y.-C. Chen, Y. Wu, J. Zhu and J.-G. Deng, Angew. Chem., Int. Ed., 2007, 46, 389 CrossRef CAS; (b) T.-R. Kang, J.-W. Xie, W. Du, X. Feng and Y.-C. Chen, Org. Biomol. Chem., 2008, 6, 2673 RSC.
- (a) A. V. Kurdyumov and R. P. Hsung, J. Am. Chem. Soc., 2006, 128, 6272 CrossRef CAS; (b) W. Xiao, X. Yin, Z. Zhou, W. Du and Y.-C. Chen, Org. Lett., 2016, 18, 116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data for new compounds, NMR, HRMS spectra and HPLC chromatograms, and CIF files of enantiopure products 3b and 5g (CCDC 2036410 and 2036411). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ob02068k |
| This journal is © The Royal Society of Chemistry 2021 |