Two new seco-polycyclic polyprenylated acylphloroglucinol from Hypericum sampsonii†
Zi-Zhen
Zhang
abc,
Yan-Rong
Zeng
ac,
Ya-Nan
Li
ac,
Zhan-Xing
Hu
abc,
Lie-Jun
Huang
abc,
Wei
Gu
abc,
Xiao-Jiang
Hao
 *abcd and
Chun-Mao
Yuan
*abcd and
Chun-Mao
Yuan
 *abc
*abc
aState Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang 550014, People's Republic of China. E-mail: haoxj@mail.kib.ac.cn; yuanchunmao01@126.com; Fax: +86 851 83804649; Tel: +86 851 83804649
bSchool of Pharmaceutical Sciences, Guizhou Medical University, Guiyang 550025, China
cThe Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academy of Sciences, Guiyang 550014, China
dState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Boanty, Chinese Academy of Science, Kunming 650201, China
First published on 12th November 2020
Abstract
Hypsampsone A (1) and hyperhexanone F (2), two novel seco-polycyclic polyprenylated acylphloroglucinols, were isolated from Hypericum sampsonii. Hypsampsone A (1) features the first spirocyclic system fused with 5/6/5/5 tetracyclic skeleton. Hyperhexanone F (2) represents the second novel 1,2-seco-bicyclo[3.3.1]-PPAP skeleton. Their structures were established by extensive spectroscopic analysis, computer-assisted structure elucidation software, and calculated electronic circular dichroism spectra. A plausible biogenetic pathway of 1 was also proposed. Compounds 1 and 2 showed moderate multidrug resistance reversal activity to adriamycin (ADR) resistant cancer cell lines, HepG2/ADR and MCF-7/ADR, with the fold-reversals ranging from 16 to 38 at noncytotoxic concentration of 10 μM.
Introduction
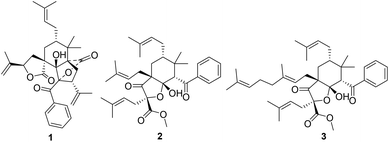
Polycyclic polyprenylated acylphloroglucinols (PPAPs), possessing a phloroglucinol core densely ornamented with prenyl, geranyl, or more highly substituted side chains, are mainly secondary metabolites of the Guttiferae family.1 More than 600 structurally complex PPAPs with different skeletons, such as adamantyl-type, homoadamantane-type, and spiro-type PPAPs, have been reported,2 some of which show a wide range of biological activities.3 Therefore, PPAPs have attracted continuing attention in natural product chemistry, synthetic chemistry, and pharmacology.Hypericum sampsoni is used as a traditional Chinese medicine to treat backache, burns, diarrhea, swelling, and cancer.4 Up to now, more than 100 natural PPAPs were isolated from this plant, which exhibited extensive biological activities including cytotoxic, antimicrobial, anti-inflammatory, and anti-HIV activities.4–9 In our systematic study for novel and bioactive PPAPs,10,11 two rare seco-PPAPs, hypsampsone A (1) and hyperhexanone F (2), along with a known analogue (3)5 (Fig. 1) were isolated from H. sampsoni. In this paper, we describe the structural elucidation, plausible biosynthesis pathway, and biological evaluation of these compounds.
Results and discussion
The molecular formula of hypsampsone A (1) was established as C33H40O6 by HRESIMS at m/z 533.2893 [M + H]+ (calcd for C33H41O6: 533.2898) with 14 degrees of unsaturation. The IR spectrum clearly exhibited absorption bands of hydroxyl (3328 cm−1), carbonyls (1791, 1731, and 1660 cm−1), and aromatic functionalities (1597 and 1447 cm−1). The 1H NMR spectrum of 1 (Table 1) showed the presence of a phenyl group and six singlet methyls. The 13C NMR data together with HSQC spectrum (Table 1) showed 33 carbon signals, comprising six methyls, six methylenes (two olefinic carbons), nine methines (six olefinic carbons), and 12 quaternary carbons (three carbonyls and four olefinic carbons). Apart from ten degrees of unsaturation accounted for by a characteristic prenyl, a benzoyl group, two pairs of terminal double bonds, and two ester carbonyls, the remaining four degrees of unsaturation indicated that compound 1 is a tetracyclic PPAPs.| Pos. | δ H (J in Hz) | δ C | Pos. | δ H (J in Hz) | δ C |
|---|---|---|---|---|---|
| 1 | 61.6 | 17α | 3.50 m | 33.3 | |
| 2α | 1.86 dd (14.4, 4.7) | 28.7 | 17β | 2.43 dd (12.5, 2.9) | |
| 2β | 2.33 dd (14.4, 10.7) | 18 | 5.25 d (9.4) | 79.1 | |
| 3 | 3.62 dd (10.7, 4.7) | 50.5 | 19 | 142.3 | |
| 4 | 98.5 | 20a | 5.11 s | 112.1 | |
| 5 | 90.4 | 20b | 4.99 s | ||
| 21 | 1.81 s | 18.5 | |||
| 6 | 46.4 | 22α | 2.11 dd (13.8, 5.6) | ||
| 7a | 1.75 dd (13.8, 2.5) | 34.7 | 22β | 1.70 overlap | 26.6 |
| 7b | 1.94 dd (13.8,12.0) | 23 | 4.99 overlap | 123.6 | |
| 8 | 1.51 m | 40.9 | 24 | 134 | |
| 9 | 34.5 | 25 | 1.55 s | 17.9 | |
| 10 | 173.9 | 26 | 1.71 s | 26.1 | |
| 11 | 181.6 | 27 | 198.9 | ||
| 12 | 1.21 s | 24.7 | 28 | 136.4 | |
| 13 | 1.32 s | 17.2 | 29/33 | 8.10 br d (7.8) | 130.4 |
| 14 | 141.1 | 30/32 | 7.42 t (7.8) | 128.5 | |
| 15a | 4.96 s | 114.8 | 31 | 7.55 t (7.8) | 133.9 |
| 15b | 4.81 s | 5-OH | 6.62 s | ||
| 16 | 1.64 s | 23.5 |
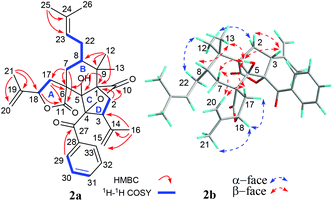
Four fragments with bold bonds were established from the 1H–1H COSY correlations of H2-7/H-8/H2-22/H-23, H2-17/H-18, H2-2/H-3, and H-29/H-30/H-31, as shown in Fig. 2a. A 11,18-γ-lactone ring (ring A) was established by HMBC correlations from H2-17 to C-6 and C-11, and from H-18 to C-11, which was further confirmed by the downfield shifted chemical shifts of C-18 (δC 79.0) and C-11 (δC 181.6). The HMBC correlations of 5-OH to C-1, C-5, and C-6, the gem-dimethyl (Me-12 and Me-13) to C-1, C-8, and C-9, H2-7 to C-5, C-6, C-11, and C-17 along with the above-mentioned segments not only assigned ring B but also implied a rare spirocyclic fusion of rings A and B, a 2-oxaspiro[4.5]decan-1-one ring system. Furthermore, the linkages of C-2, C-5, C-9, and C-10 via the quaternary carbon (C-1) were assigned from key HMBC correlations of H2-2 to C-1, C-5, C-9, and C-10. Two isopropenyl and a prenyl groups were assigned to C-3, C-18, and C-8, respectively from the HMBC correlations of Me-16 to C-3, C-14, and C-15, Me-21 to C-18, C-19, and C-20, and Me-25 to C-23 and C-24. However, there is no HMBC correlation of the quaternary carbon (C-4, δC 98.5). Luckily, with the help of the four “loose ends”, C-5 (δC 90.4), C-27 (δC 198.9), C-3 (δC 50.5), and a lactone carbonyl (C-10, δC 173.9), along with a non-connected oxidized characteristic quaternary carbon (C-4), the linkages of C-3, C-5, and C-27 through C-4 and the connection of C-4 and C-10 via an oxygen atom were assigned to account for last two degrees of unsaturation. Thus, a 2-oxabicyclo[2.2.1]heptan-3-one ring (rings C and D) was constructed. Finally, the planar structure of 1 was assigned.
Computer-assisted structure elucidation (CASE) is a widely used technology in recent years to elucidate complex natural products in recent years.12 In order to further confirm the structure of 1, CASE was used and 200 molecules could be generated and filtrated (ESI†) by inputting molecular formula and NMR data of 1 into the ACD/structure elucidator. Significantly, the top hit with the highest R2 and lowest standard deviation values by Neural Net (dN) and HOSE (dA) methods (Fig. 3), is the best-matched structure.13–15 Therefore, the planar structure of 1 was verified and consistent with previous elucidation as shown in Fig. 2.
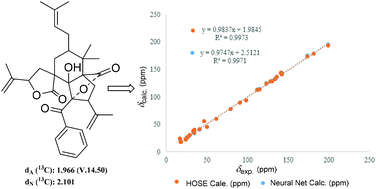 | ||
| Fig. 3 The top structure generated by Struc Eluc software, and its linear regression plots through both HOSE and Neural Net methods of 13C chemical shift prediction. | ||
The relative configuration of 1 was assigned by the ROESY experiment (Fig. 2b), in which cross-peaks observed from 5-OH to H-3, Me-13, H-7β, and H-17β, from H-20a to H-7β and H-17β, and from Me-13 to H-8 demonstrated that these groups were cofacial and randomly assigned as β-oriented. In contrast, the evident ROESY correlations of Me-12/H-22α, Me-12/H-2α, Me-21/H-18α, and H-18α/H-17α indicated α-orientation of these groups. Thus, the relative structure of 1 was elucidated as shown in Fig. 2b.
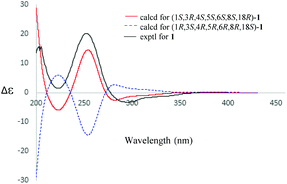
The absolute configuration of 1 was established by electric circular dichroism (ECD) analysis (Fig. 4) using the time-dependent density functional theory (TDDFT) methodology at the CAM-B3LYP/TZVP level in MeOH. The ECD spectra curves of the two opposite absolute configurations (1S,3R,4S,5S,6S,8S,18R and 1R,3S,4R,5R,6R,8R,18S) for compound 1 were calculated. Obviously, the absolute configuration of 1 was assigned as 1S,3R,4S,5S,6S,8S,18R due to the similar curves between its experimental and calculated ECD curves.
The molecular formula of compound 2 was deduced to be C34H46O6 from HRESIMS data. Analysis of its NMR data (Table S1†) revealed that 2 was similar to hyperhexanone A (3)5 except for the presence of a prenyl at C-4 instead of a geranyl, which was further verified through the HMBC correlations from Me-25 and Me-26 to C-23 and C-24, and from H2-22 to C-1, C-3, C-4, and C-5 as well as 1H–1H COSY cross-peak of H2-22/H-23 (Fig. S1†). In addition, the NOESY experiment assigned the relative configuration of 2 (Fig. S1†), in which cross-peaks of H-6/H-8 and H-6/H-22a, suggested that these groups were cofacial, and were randomly assigned as β-oriented. Thus, the prenyl group at C-4 was β-oriented. ROESY cross-peak of H-5α/H-17a suggested a cis-fusion of the cyclohexane and the tetrahydrofuran ring, implying a α-orientation of the prenyl side chain at C-2. Meanwhile, the orientation of the hydroxyl at C-1 was also determined as β-oriented and consistent with hyperhexanone A (3).5 Therefore, the relative configuration of 2 was established (Fig. S1†).
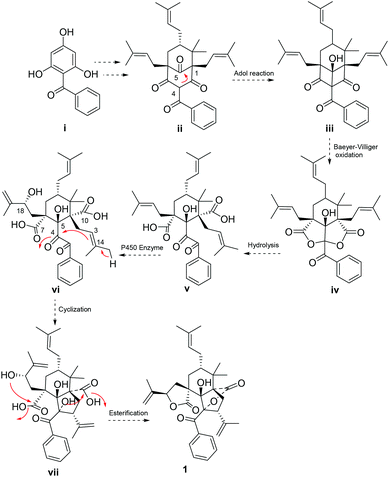
A plausible biogenetic pathway (Scheme 1) of 1 was proposed to start from an assumed benzophenone precursor (i), which underwent a series of isopentenyl transferase and cyclization to get intermediate ii.2 Intra-molecular Aldol reaction between C-4 and C-5 of ii occurred, followed by a Baeyer–Villiger oxidation to get an important intermediate iv.3 Two lactone rings of iv were opened, and then underwent a series of oxidation (P450 enzyme),16 cyclization, and intra-molecular esterification to yield compound 1.
Compounds 1–3 were screened for their multidrug resistance (MDR) reversal activity on adriamycin (ADR) resistant cancer cell lines,17,18 HepG2/ADR and MCF-7/ADR cells. Firstly, compounds 1–3 were tested for their cytotoxicity against HepG2/ADR and MCF-7/ADR cells at 10 μM, and no cytotoxicity of those compounds towards tested cell lines was observed. Two ADR resistant cancer cell lines, HepG2/ADR and MCF-7/ADR cells, revealed high ADR resistance with IC50 values of 346.40 μM and 283.54 μM. Compounds 1 and 2 showed moderate reversal activity against HepG2/ADR and MCF-7/ADR cells at the concentration of 10 μM, with reverse folds ranging from 16 to 38 (Table 2). When tested for MCF-7/ADR cells, the most active compound (2) revealed higher reversal fold than the positive control (verapamil) at the same concentration of 10 μM. Compounds 2 and 3 share the same skeleton, but their reversal activities were different. The presence of a prenyl at C-4 of 2 could be responsible for improving its reversal activity.
| Comp | HepG2/ADR | MCF-7/ADR | ||
|---|---|---|---|---|
| Sensitivity reversala | Sensitivity reversal | |||
| IC50 (μM) | RFb | IC50 (μM) | RFb | |
| a IC50 values were obtained from three independent repeats and represented as the mean ± SD. b Reversal fold (RF) is calculated as a ratio of IC50 (ADR) to IC50 (reversal agent + ADR). c Verapamil (VRP) was used as the positive control at 10 μM. | ||||
| ADR + 1 | 9.11 | 38 | 15.37 | 18 |
| ADR + 2 | 20.94 | 16 | 7.41 | 38 |
| ADR + 3 | >40 | >40 | ||
| ADR + Vc | 5.32 | 65 | 13.95 | 20 |
Conclusions
In conclusion, two rare seco-PPAPs were isolated from H. sampsonii. As far as we known, hypsampsone A (1) represents the first spirocyclic 2,3- and 3,4-seco-PPAP with fused 5/6/5/5 tetracyclic skeleton. Hyperhexanone F (2) is the second isolated novel 1,2-seco-bicyclo[3.3.1]-PPAP skeleton. Moreover, compound 2 exhibited higher reversal fold than the positive control (Verapamil) when tested for MCF-7/ADR cells at the same concentration of 10 μM. Our work may attract great attention of synthetic and biological communities for further study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by the Science and Technology Department of Guizhou Province (QKH 2020-1Z076), National Natural Science Foundation of China (U1812403 and 81502959), High-level Innovative Talents in Guizhou Province (Thousand Levels of Talent for Chunmao Yuan in 2018), and Guizhou Provincial Engineering Research Center for Natural Drugs.Notes and references
- Z. B. Zhou, Y. M. Zhang, K. Pan, J. G. Luo and L. Y. Kong, Fitoterapia, 2014, 95, 1–7 CrossRef CAS.
- X. W. Yang, R. B. Grossman and G. Xu, Chem. Rev., 2018, 118, 3508–3558 CrossRef CAS.
- R. Ciochina and R. B. Grossman, Chem. Rev., 2006, 106(9), 3963–3986 CrossRef CAS.
- H. C. Zhu, C. M. Chen, J. Yang, X. N. Li, J. J. Liu, B. Sun, S. X. Huang, D. Y. Li, G. M. Yao, Z. W. Luo, Y. Li, J. W. Zhang, Y. B. Xue and Y. H. Zhang, Org. Lett., 2014, 16(24), 6322–6325 CrossRef CAS.
- H. C. Zhu, C. M. Chen, J. Yang, D. Y. Li, J. W. Zhang, Y. Guo, J. P. Wang, Z. W. Luo, Y. B. Xue and Y. H. Zhang, Tetrahedron, 2016, 72, 4655–4659 CrossRef CAS.
- W. J. Tian, Y. Q. Qiu, X. J. Jin, H. F. Chen, X. J. Yao, Y. Dai and X. S. Yao, RSC Adv., 2016, 6, 50887–50894 RSC.
- Z. Y. Xiao, Y. H. Zeng, Q. Mu, W. K. P. Shiu and S. Gibbons, Chem. Biodiversity, 2010, 7, 953–958 CrossRef CAS.
- W. B. Xin, X. H. Man, C. J. Zheng, M. Jia, Y. P. Jiang, X. X. Zhao, G. L. Jin, Z. J. Mao, H. Q. Huang and L. P. Qin, Fitoterapia, 2012, 83, 1540–1547 CrossRef CAS.
- J. S. Zhang, Y. H. Zou, Y. Q. Guo, Z. Z. Li, G. H. Tang and S. Yin, Chem. Biodiversity, 2010, 7, 953–958 CrossRef.
- H. Y. Lou, Y. N. Li, P. Yi, J. Y. Jian, Z. X. Hu, W. Gu, L. J. Huang, Y. M. Li, C. M. Yuan and X. J. Hao, Org. Lett., 2020, 22, 6903–6909 CrossRef CAS.
- D. S. Tian, P. Yi, L. Xia, X. Xiao, Y. M. Fan, W. Gu, L. J. Huang, Y. Ben-David, Y. T. Di, C. M. Yuan and X. J. Hao, Org. Lett., 2016, 18, 5904–5907 CrossRef CAS.
- D. C. Burns, E. P. Mazzola and W. F. Reynolds, Nat. Prod. Rep., 2019, 36, 919–933 RSC.
- M. Elyashberg, A. J. Williams and K. Blinow, Nat. Prod. Rep., 2010, 27, 1296–1328 RSC.
- C. B. Naman, J. Li, A. Moser, J. M. Hendrycks, P. A. Benatrehina, H. Chai, C. H. Yuan, W. J. Keller and A. D. Kinghorn, Org. Lett., 2015, 17, 2988–2991 CrossRef CAS.
- M. Elyashberg, K. Binow, S. Molodtsov and A. J. Williams, J. Nat. Prod., 2013, 76, 113–116 CrossRef CAS.
- X. Y. Zheng, P. Li and X. Lu, J. Exp. Bot., 2019, 70, 4619–4630 CrossRef CAS.
- S. Shakera, J. Sang, X. L. Yan, R. Z. Fan, G. H. Tang, Y. K. Xu and S. Yin, Phytochemistry, 2020, 176, 112395 CrossRef.
- C. P. Liu, C. Y. Xie, J. X. Zhao, K. L. Ji, X. X. Lei, H. Sun, L. G. Lou and J. M. Yue, J. Am. Chem. Soc., 2015, 137, 10516–10519 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ob02072a |
| This journal is © The Royal Society of Chemistry 2021 |