Recent progress in the piezoelectricity of molecular ferroelectrics
Qiang
Pan
,
Yu-An
Xiong
 ,
Tai-Ting
Sha
and
Yu-Meng
You
,
Tai-Ting
Sha
and
Yu-Meng
You
 *
*
Jiangsu Key Laboratory for Science and Applications of Molecular Ferroelectrics, Southeast University, Nanjing 211189, People's Republic of China. E-mail: youyumeng@seu.edu.cn
First published on 8th September 2020
Abstract
Molecular ferroelectrics (MOFEs) with excellent piezoelectricity are highly desirable for their easy and environmentally friendly processing, light weight, low processing temperature, and mechanical flexibility. However, although 100 years have passed since the discovery of the ferroelectric effect in 1920, MOFEs with a piezoelectric coefficient comparable to those of the most widely used piezoelectric ceramics, such as barium titanate (∼190 pC N−1), have rarely been found. Only in recent years has this situation changed. A series of high-performance piezoelectric MOFEs have been designed and synthesized, approaching or even exceeding some inorganic piezoelectric ceramics. Reviewing on the basis of piezoelectric MOFEs reported, we summarize several methods and strategies to synthesize performance-enhanced and application-aimed piezoelectric MOFEs, with potential as candidates for next-generation medical, micromechanical, and biomechanical devices.
1 Introduction
Piezoelectricity arises in crystalline materials without inversion symmetry, with the characteristic of developing an electrical charge under an applied mechanical pressure or vice versa.1,2 To describe the ability to generate electrical charges under the same strain, piezoelectric coefficients are defined. According to the directions of polarization and stress, piezoelectric coefficients with a third order matrix form can be categorized into two main operation modes: d31 and d33. In the d31 mode, the polarization direction “3”, i.e., the electric field, is perpendicular to the direction of the applied stress “1”. In the d33 mode, the polarization and the applied stress are in the same direction. The larger d33 or d31 is, the more desirable the piezoelectric will be. Piezoelectricity is preserved only below a certain temperature called the Curie temperature (Tc) or phase transition temperature.3In 1880, the brothers of Nobel laureates Pierre Curie and Jacques Curie first discovered the piezoelectric effect. The Curies used tourmaline, quartz, topaz, cane sugar, and Rochelle salt to demonstrate the electromechanical interactions.4 The best piezoelectrics are materials exhibiting spontaneous electric polarization, known as ferroelectrics, the direction of which can be reversed upon application of an external electric field. Although the history of ferroelectrics began with the molecular compound Rochelle salt in 1920 observed by J. Valasek,5 later inorganic ferroelectrics, such as BaTiO3 (BTO), Pb(Zr,Ti)O3 (PZT), and LiNbO3 (LNO), have long occupied the mainstream, with robust properties and great utilization potential. BTO, as a monocomposition inorganic ferroelectric material with a perovskite structure, emerged as the first practically used piezoelectric ceramic with good d33 of 90 and 190 pC N−1 along the [001] and [111] crystal orientations.6–8 Subsequently, owing to the presence of a morphotropic phase boundary (MPB), a much higher piezoelectric coefficient superior to those of BTO could be obtained in the binary system of PZT, making it extensively utilized in almost all kinds of piezoelectric applications.9,10 However, despite the prominent advantages in piezoelectricity, PZT-based ceramics generally suffer from high processing temperature, structural rigidity, energy-intensive fabrication, and especially toxic composition.
In contrast, because of the advantages of low processing temperature, mechanical flexibility, light weight, nontoxicity, biocompatibility, and easy film fabrication, piezoelectric MOFEs stand out and are expected to promote innovations in piezoelectric devices. During the past decade, significant breakthroughs have demonstrated the renaissance of MOFEs as promising alternatives or supplements to conventional inorganic ceramic ferroelectrics. For example, croconic acid was proven to have a high polarization (P) comparable to that of BTO, which reached approximately 23 μC cm−2.11 Besides, diisopropylammonium bromide (DIPAB), as a continuation of previous work,12 was found to possess a high Tc, which exceeded that of BTO at 426 K.13 In addition, new ferroelectric physics was discovered, such as charge transfer in electron donors and acceptors along the networks of π⋯π-stacked supramolecules, which caused spontaneous polarization.14
Contrary to the rapid improvement of Tc and P in MOFEs, the piezoelectric response of these molecular crystals is still orders of magnitude below those of inorganic piezoelectric ceramics, hindering the application development of MOFEs. For example, the early discovered MOFEs, such as Rochelle salt and triglycine sulfate (TGS), have d33 less than 22 pC N−1. Other organic materials have even weaker d33, 11 pC N−1 for DIPAB and 5 pC N−1 for croconic acid.15 Ferroelectric polymers (with moderate piezoelectric response: ∼20 pC N−1 for polyvinylidene fluoride (PVDF) and 2 pC N−1 for nylon) and ferroelectric liquid crystals (commonly used as display and nonlinear optical materials) are not in the scope of this review (Table 1).
| Sample | T c (K) | d 33 (pC N−1) | Ref. |
|---|---|---|---|
| Textured-KNNS | 625 | 208 | 83 |
| PbTiO3 single crystal | — | 143 | 84 |
| BaTiO3 ceramic | — | 191 | 85 |
| BaTiO3 single crystal | 393 | 85.6 | 85 |
| PMN-PZT | 489 | 1530 | 86 |
| SM-PT | 637 | 127 | 87 |
| PVDF | — | 33 | 88 |
| TGS | 322 | 22 | 15 |
| Croconic acid | — | 5 | 11 and 15 |
| DIPAB | 426 | 11 | 13 and 15 |
| ImClO4 | 373.6 | 41 | 29 |
| (ATHP)2PbBr4 | 503 | 76 | 80 |
| [C(NH2)3][ClO4] | 454 | 15 | 32 |
| [(CH3)4N][FeCl4] | 344 | 80 | 45 |
| [(CH3)4N][FeBrCl3] | 346 | 110 | 45 |
| [(CH3)4N][GaCl4] | 382 | 80 | 46 |
| [AH][ReO4] | 322 | 90 | 47 |
| [AH][IO4] | 258 | — | 47 |
| (TMCM)MnCl3 | 406 | 185 | 15 |
| (TMCM)CdCl3 | 400 | 220 | 15 |
| (TMCM)CdBr3 | 346 | 139 | 76 |
| (TMBM)MnBr3 | 415 | 112 | 77 |
| (TMFM)x(TMCM)1−xCdCl3 (x = 0.26) | 366.8 | 1540 | 78 |
| (TMFM)FeBr4 | 412.7 | — | 79 |
| MDABCO-NH4I3 | 448 | 14 | 81 |
In recent years, with the improvement of chemical synthesis and state-of-the-art characterization methods, MOFE research has become more systematic and rational. Various new MOFE materials were discovered, and several experimental design strategies were proposed.16–19 These achievements have boosted the rapid development of the piezoelectricity performance in MOFEs (Table 1). In this review, we summarize the recent progress in and approaches to piezoelectricity-related performance enhancement (d33, Tc, P), design strategies and application explorations (powder and thin film) for MOFE materials that have emerged in the last 10 years, and in the summary section, we provide conclusions regarding the potential of and challenges in MOFEs.
2 Crystalline thin films of piezoelectric MOFEs
With the vigorous development towards miniaturization, light weight and flexibility, the microelectronics and optoelectronics industries have put forward requirements for small-scale, thin-film, and on-chip integration of ferroelectric materials. In this context, the combination of ferroelectric materials and traditional semiconductor materials led to the formation of a new interdisciplinary field—integrated ferroelectrics—during the 1980s.20 Piezoelectricity was realized in practice, accompanying the boom in integrated ferroelectrics; thus, the next decades witnessed a shift in emphasis from bulk crystals/ceramics to thin films owing to their supreme advantages, aiming to overcome the initial barrier to the application of ferroelectrics, i.e., aiming to achieve a lower operating voltage.21 According to the Kay–Dunn law, for submicrometer ferroelectric thin films, a coercive field (Ec) can be achieved with just a few volts, compatible with silicon technology.22To date, a number of inorganic ferroelectric thin films have been intensively developed and broadly exploited, dominating most of the practical applications, while the processing problems are the main disadvantages in successful device design. Since lattice and thermal mismatch between a substrate and a single crystal thin film will cause strain and deteriorate the application characteristics, the careful choice of the substrate will greatly affect the growth of high-quality epitaxial or single crystal films.23 Most importantly, the properties of as-grown inorganic ferroelectric thin films rely on complicated, high-cost, energy-intensive, and time-consuming fabrication techniques involving magnetron sputtering, pulsed laser deposition, molecular beam epitaxy, and metal–organic chemical vapour deposition.24 All of these unfavourable facts have stimulated the desire to find new ferroelectric systems as viable alternatives or supplements to inorganic ferroelectric thin films.
At the same time, the emergence of MOFEs may offer more opportunities for next-generation flexible and wearable devices due to their inherent flexibility, structural tunability, environmental friendliness, light weight, low cost, low-temperature processing, low acoustic impedance, and biocompatibility. The rise of MOFE thin films apparently meets the growing demand for miniaturized multifunctional devices.
Consequently, researchers showed renewed interest in the available MOFE thin film of imidazolium perchlorate (ImClO4) processed from aqueous solution, exhibiting high spontaneous polarization, high Curie temperature, low coercivity, and good electromechanical coupling.25–27 More interestingly, the d33 of trigonal ImClO4 reaches 41 pC N−1, one of the highest values among MOFEs at that time. Researchers presented reports on the preparation of high-quality large-area MOFE films using spin-coating and in-plane liquid-phase growth.28,29 These two generally applicable approaches allow precise control of the film thickness, roughness, homogeneity, and crystal orientation by tuning the solute concentration and growth environment, thereby enabling further studies to achieve orientation-controlled polarization and customizable ferroelectric properties for memory elements and sensors.
2.1 Fabrication of ImClO4 thin film28,29
Yi Zhang et al. discovered ImClO4 (1) to be a LiNbO3-type trigonal MOFE with high spontaneous polarization and high Curie temperature and successfully processed a 1 film that maintains its ferroelectric properties and offers superior electromechanical coupling and a low coercive field. The d33 of 1 was one of the highest among MOFEs at the time, reaching 41 pC N−1.After spin-coating 2–5 layers of 1 films on amorphous Si/SiO2/Ti/Pt or quartz substrates, millimetre-level dendritic crystals grow on the substrate, with the solution having a solubility of approximately 60% at a certain temperature. Since each dendritic crystal grows from a single core, the anisotropy of the crystal and the strong low-dimensional interaction between the dendritic crystal and the substrate make the crystal grow rapidly only in favourable directions. However, at this solubility, 1 crystals grow rapidly, and the molecular diffusion length is short, causing the dendritic crystals to continue to widen and form smooth films (Fig. 1a). These are all factors that influence the growth of large dendritic crystals. The piezoelectric properties of the thin films were discovered based on piezoresponse force microscopy (PFM) by comparing the piezoresponse with that of PZT and P(VDF–TrFE) (Fig. 1b).
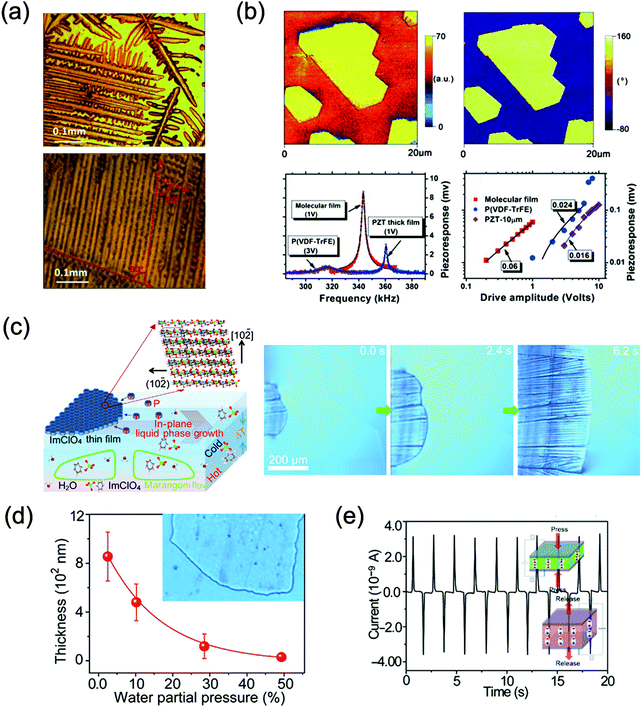 | ||
| Fig. 1 Schematic illustrations of ImClO4 thin film along with its growth, processing method and piezoelectric performance test. (a) Growth morphology and optical images of ImClO4 on a Si/SiO2/Ti/Pt or quartz substrate with dendritic crystals on the millimetre scale. (b) PFM mapping of ImClO4 thin film and comparison of PFM resonance peaks and effective piezoelectric coefficient with those of PZT and P(VDF–TrFE). (c) IP-LP growth and ImClO4 thin-film growth images for controlling the supersaturation of the air–liquid interface by adjusting the temperature gradient and external water pressure. (d) Thickness and uniformity control of ImClO4 crystal films based on the external water partial pressure. (e) Response of the piezoelectric current in the ImClO4 film with polarity reversal under the application and release of an external force of 9.8 N. The inset is a schematic illustration of the piezoelectric effect. Reproduced with permission.28,29 | ||
Zhuolei Zhang et al. reported a water-based air-processable technique to prepare large-area MOFE thin films controlled by supersaturation growth at the liquid–air interface under a temperature gradient and external water partial pressure. They used this novel “in-plane liquid-phase growth (IP-LP)” principle to fabricate a transparent thin film of 1 (Fig. 1c), consisting of a bamboo-like structure of (2,![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ,0) and (1,0,
,0) and (1,0,![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) structural variants of R3m symmetry with a reversible polarization of 6.7 μC cm−2. The resulting ferroelectric domain structure leads to a reversible electromechanical response of d33 = 38.8 pm V−1.
) structural variants of R3m symmetry with a reversible polarization of 6.7 μC cm−2. The resulting ferroelectric domain structure leads to a reversible electromechanical response of d33 = 38.8 pm V−1.
In this case, the surface tension and high spreading ability combined cause the 1 thin film to crystallize on the surface of the solution, stabilized by the hydrogen bonding between water and imidazolium molecules (Fig. 1c). The thickness of the thin film can be tuned by adjusting the evaporation rate of the water or external water partial pressure. An external water vapor flow of different saturated external water partial pressures has been used to modify the water partial pressure above the supersaturated solution to control the growth rate and uniformity of 1 crystalline films (Fig. 1d).
These researchers tested the electromechanical coupling performance of 1 by applying periodic mechanical stress to the piezoelectric film between electrodes and recording the output current and voltage signals (Fig. 1e). Based on this, the piezoelectric coefficient of the thin film was estimated to be d33 = 38.8 pm V−1, which is similar to the local value of the thin film measured by PFM. The electromechanical conversion of the 1 film is reversible and can be repeated for more than 1000 cycles, which is attractive for MOFE multifunctional materials, and the IP-LP growth method also opens up a new technical field for MOFE films.
2.2 Restrictions arising from the single polar axis in MOFE thin films
Benefiting from the easy solution-based preparation method, piezoelectric MOFE thin films have become a possible solution to the increasing demand for piezoelectric materials. Thus, improving the performance of MOFEs, especially those in piezoelectric thin film form, is urgent. However, in addition to the poor piezoelectric response, another obstacle must be overcome before a low-cost, highly efficient thin film can be realized, which is the uniaxial characteristic of MOFEs. Conventional MOFE materials, consisting of multiatomic molecules, often have relatively low symmetry in their low-temperature ferroelectric phase and a uniaxial nature with only two opposite polarization directions. For a single crystalline MOFE thin film, the crystal orientation should be well controlled to a desired direction to achieve maximum efficiency. Taking uniaxial croconic acid as an example, its single crystal was reported to have a large polarization of 20 μC cm−2,11 comparable to that of BTO; however, the polarization of the thin film reported by Jiang et al. was 0.4 μC cm−2, only 2% that of the single crystal.30 Such uniaxial limitation has been found not only in MOFEs but also in inorganic LNO thin films.31 Orientation-controlled thin-film growth requires a specific substrate and sophisticated growth techniques, which dilute the convenience of simple solution processing. Additionally, in the polycrystalline thin-film form, since each grain has an arbitrary crystal orientation, the two possible polarization directions of each grain are also randomized. Thus, only a small portion of the polarization is effective. Even after application of an electric “poling” field, only a weak macroscopic polarization can be achieved, resulting in a limited piezoelectric response, as shown in Fig. 2a and b.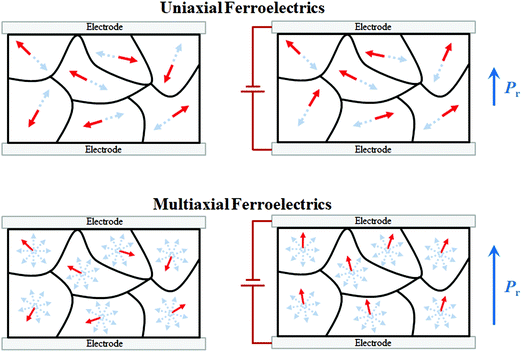 | ||
| Fig. 2 Switching of the polarization direction in uniaxial and multiaxial polycrystalline samples. (a and c) Before polarization, the polarization directions are randomly arranged. After polarization, the polarization directions of the uniaxial sample are restricted to a disordered state (b). The polarization directions of the multiaxial sample can be switched in the direction closest to the electric field to achieve a larger P (d). Reproduced with permission.32 | ||
The inadequate orientation control of MOFEs has thus far limited their practical applications because most piezoelectric materials are utilized in polycrystalline forms, such as ceramics, so a multiple polar axes characteristic is preferred to allow the polarizations of individual grains to be poled more effectively to achieve larger polarization and piezoelectric response. For the majority of inorganic piezoelectric ceramics, such as BTO, the polarization in each grain can be switched along six different directions (for the room-temperature tetragonal phase). This multiaxial property renders ceramics the most industrially favoured ferroelectric material of all time.
With increasing number of polar axes, orienting the polarization direction of grains along a specific direction throughout the entire film would become more efficient (Fig. 2c and d). For example, theoretically, for ferroelectrics with single, triple, quadruple and sextuple polar axes, the maximum effective polarization of the polycrystalline film can reach 25%, 83%, 87% and 91% that of the single crystal form, respectively.1 Thus, realization of multiple polar axes is crucial to the development of piezoelectric MOFEs.
3 Multiaxial plastic MOFEs with a large piezoelectric effect
Several attempts to obtain MOFEs with a multiaxial characteristic and achieve application of the polycrystalline form have been made since 2016, inspired by the plastic phase transition.33–35The plastic phase is a mesophase between the solid and liquid states, often found in compounds with globular molecular structures, such as adamantane and tetrachloromethane.36,37 In the plastic phase, globular molecules undergo rapid isotropic rotator motions and lose orientational order similar to in the liquid state, but the centre of each molecule retains three-dimensional long-range order as in normal solid crystals. Plastic crystals, due to the orientationally disordered globular molecules, usually crystallize into a highly symmetric cubic crystal system similar to metal crystals.38 The spherical volumes occupied by the orientationally disordered molecules are larger than those occupied by the ordered molecules, and the intermolecular interactions in plastic crystals are therefore weaker and more isotropic than those in crystals with orientational order.39 Therefore, in contrast to the characteristic rigidity and brittleness of ordinary crystals, plastic crystals exhibit mechanical deformability.
For plastic crystals, in a specific temperature range, if the crystal loses its orientational disorder and crystallizes in a polar space group, it may behave as a ferroelectric, and more interestingly, its highly disordered plastic phase may endow the plastic crystal with multiple ferroelectric polar axes. For example, recently discovered plastic MOFE crystals are attractive functional materials exhibiting multiaxial ferroelectricity.40–44 In single crystal form, their reported high-frequency performance, high Tc, and large polarization have amply demonstrated the importance and utility of these materials. Additionally, in various polycrystalline forms, these plastic MOFEs provide improved effective polarization, accompanied by potential piezoelectric applications in molecular ceramic form, compared to conventional uniaxial MOFEs, which are usually restricted to single crystals.
Herein, this section focuses on the recent developments in plastic MOFEs, especially their advances in polycrystalline forms.
3.1 [C(NH2)3][ClO4]32
Pan et al. discovered guanidinium perchlorate ([C(NH2)3][ClO4], 2) as a multiaxial ferroelectric, the powder sample of which shows a significant increase in the piezoelectric coefficient from 0 to 10 pC N−1 after poling, comparable to that of the LiNbO3 single crystal (8 pC N−1). This is the first time that such a phenomenon has been observed in MOFEs.
2 undergoes a phase transition at a record high Tc of 454 K from the paraelectric phase m![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m to the ferroelectric phase 3m. The powder X-ray diffraction (PXRD) data at 463 K refined by the Pawley method suggest that the crystal of the high-temperature paraelectric phase has a simple cubic unit cell with a = 5.4666 Å, and the most likely space group is the centrosymmetric Pm
m to the ferroelectric phase 3m. The powder X-ray diffraction (PXRD) data at 463 K refined by the Pawley method suggest that the crystal of the high-temperature paraelectric phase has a simple cubic unit cell with a = 5.4666 Å, and the most likely space group is the centrosymmetric Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (point group m
m (point group m![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). This result indicates that the molecules in the paraelectric phase are almost freely rotating and that the phase may be a plastic phase. Therefore, 2 is a BiFeO3-type MOFE, and it should have the multiaxial characteristic of four symmetry-equivalent polar axes (Fig. 3a).
m). This result indicates that the molecules in the paraelectric phase are almost freely rotating and that the phase may be a plastic phase. Therefore, 2 is a BiFeO3-type MOFE, and it should have the multiaxial characteristic of four symmetry-equivalent polar axes (Fig. 3a).
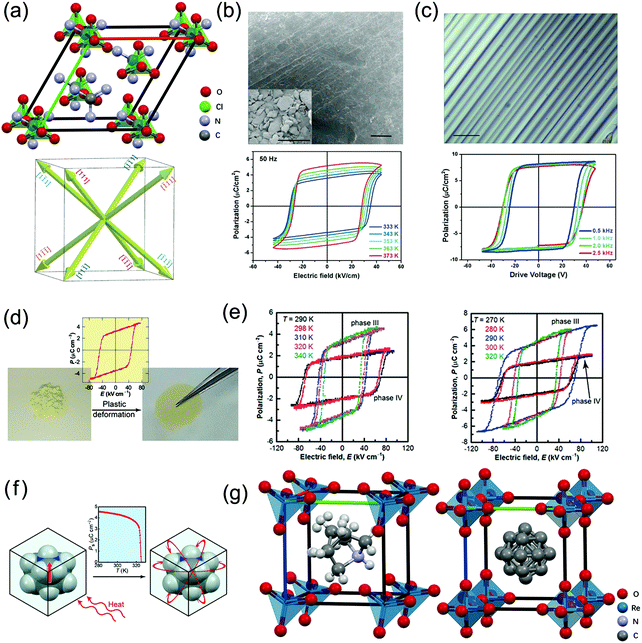 | ||
| Fig. 3 Crystal structures of three multiaxial plastic MOFEs, and performance tests of their polycrystalline thin films. (a) Schematic diagram of the crystal structure of 2, and possible equivalent polarization directions. (b) SEM images (scale bar: 50 μm) of a powder tablet of 2, and P–E hysteresis loops measured at different temperatures. (c) Optical microscope image of a thin film of 2 under crossed polarized light (scale bar: 100 μm), and hysteresis loops of GaIn/sample/ITO capacitor devices at different frequencies at room temperature. (d) Freestanding translucent films prepared by pressing powder samples of 3 exhibiting ferroelectric polarization switching at room temperature. (e) P–E hysteresis loops measured by applying an a.c. electric field (10 Hz) to polycrystalline films of 3 (left) and 4 (right) at different temperatures. (f) The P in the cation of 6 and 7 is marked with a red arrow, and the phase transition occurs with increasing temperature, which causes P to be zero due to the cation disorder. (g) Crystal structures of 6 at 300 K (ITP) (left) and 7 at 270 K (HTP) (right). The red arrow shows only one of the three equivalent polarization directions. Reproduced with permission.32,45,47 | ||
These researchers prepared compacted microcrystalline powder pellets of 2 at room temperature by the cold pressing method. This approach is different from the high-temperature sintering of inorganic ceramic ferroelectrics, realizing ferroelectricity and piezoelectricity in polycrystalline form in a low energy-intensive manner. Scanning electron microscopy (SEM) images show that the separated and dispersed microcrystals are tightly combined after pressing (Fig. 3b). By carefully dispersing the 2 deionized aqueous solution on cleaned ITO (indium tin oxide)-coated conductive glass, a thin film (≈1.5 μm thick) with uniform and high coverage of needle-like crystals was formed. The optical microscope image (Fig. 3c) shows that the film has good crystallinity and uniform crystal orientation.
Both powder and thin film samples of 2 can provide well-defined polarization–electric field (P–E) hysteresis loops with P of 5.1 and 8.1 μC cm−2, respectively (Fig. 3b and c), much higher than the value of 25% (uniaxial) the theoretical P (9.56 μC cm−2) for a single crystal. This proves that 2 does indeed have a multiaxial nature, which is one of the most valuable features of ceramic ferroelectrics.
Different from the uniaxial sample, the multiaxial MOFE has multiple polarization orientation directions, which allow each grain to be oriented in the direction closest to the external electric field. This effectively improves polarization switching and leads to maximum polarization after poling. Because the inter-grain polarizations mutually offset each other in the original powder tablet, d33 was initially approximately zero. After the P–E hysteresis loop measurements, a sufficiently large electric field that causes domain switching results in a remnant polarization and the d33 of the powder tablet being approximately 10 pC N−1, which is equivalent to the value of the single crystal sample along the [001] direction (d33 = 15 pC N−1). The powder of 2 shows moderate electromechanical activity and has the largest d33 among powder samples.
The most beneficial merit of 2 over inorganic ceramic ferroelectrics is that ferroelectricity and piezoelectricity can be realized in the polycrystalline form by cold pressing rather than energy-intensive high-temperature sintering.
This is the first case in which an MOFE exhibits piezoelectricity in polarized powder samples, demonstrating that MOFEs have great potential to be applied in the form of polycrystalline powder and are expected to be a useful supplement to traditional ceramic ferroelectrics.
3.2 [(CH3)4N][FeCl4], [(CH3)4N][FeBrCl3],45 and [(CH3)4N][GaCl4]46
Harada et al. synthesized the new plastic MOFE crystals tetramethylammonium tetrachloroferrate(III), [(CH3)4N][FeCl4] (3), and tetramethylammonium bromotrichloroferrate(III), [(CH3)4N][FeBrCl3] (4), which show plastic behaviour at high temperature and ferroelectric/piezoelectric properties in their lower-temperature phases (Fig. 3d). These researchers discovered that owing to their intrinsic multiaxial ferroelectricity and plastic deformability, the freestanding translucent films easily prepared by pressing powdered samples of these malleable compounds exhibit a d33 up to 110 pC N−1.In the plastic crystal phase, permanent deformation would occur without cracking upon application of mechanical stress on the crystal, which can be used to produce microcrystalline agglomerate films. Therefore, by applying a uniaxial stress to the microcrystalline powders of 3 and 4 in plastic phase I or applying stress multiple times at room temperature, plastic crystal freestanding translucent polycrystalline films of 3 and 4 can be easily obtained (Fig. 3d). The polycrystalline films provide well-defined hysteresis loops in phases III and IV, and their P values do not change significantly when changing the preparation conditions, such as the grain size, applied stress, and pressing temperature (Fig. 3e). PXRD spectra indicated that after high-temperature plastic deformation, the crystals maintain their crystallinity, and the polycrystals have some degree of preferred orientation.
More importantly, the plastic ferroelectric crystals of 3 and 4 show large d33 in freestanding polycrystalline film form. Applying stress to the crystals can readily alter the electrode polarization; thus, the spontaneous polarization caused by the displacement of ionic molecules contributes to the high piezoelectric response. Due to the softness of the crystal, the 3 and 4 polycrystalline films have d33 values of ∼80 pC N−1 and ∼110 pC N−1 at room temperature after being poling in phase III. These values are much larger than those of the widely used PVDF and its copolymers (∼30 pC N−1).
Li et al. reported that the MOFE tetramethylammonium tetrachlorogallate(III), [NMe4][GaCl4] (5), undergoes a plastic phase transition from Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m in the high-temperature phase (HTP) to Amm2 in the intermediate temperature phase (ITP), belonging to the multiaxial ferroelectric species of m
m in the high-temperature phase (HTP) to Amm2 in the intermediate temperature phase (ITP), belonging to the multiaxial ferroelectric species of m![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) mFmm2. The polarization–electric field (P–E) hysteresis loops of 5 were measured for its polycrystalline film in the temperature range from 280 to 380 K. The piezoelectricity of 5 was measured as d33 ≈ 80 pC N−1 in the powder form after poling, and this value is comparable to that reported for tetramethylammonium tetrachloroferrate(III).
mFmm2. The polarization–electric field (P–E) hysteresis loops of 5 were measured for its polycrystalline film in the temperature range from 280 to 380 K. The piezoelectricity of 5 was measured as d33 ≈ 80 pC N−1 in the powder form after poling, and this value is comparable to that reported for tetramethylammonium tetrachloroferrate(III).
3.3 [AH][ReO4] and [AH][IO4]47
Harada et al. synthesized the new plastic MOFE crystals 1-azabicyclo[2.2.1]heptanium perrhenate, [AH][ReO4] (6), and 1-azabicyclo[2.2.1]heptanium periodate, [AH][IO4] (7). Owing to the exceptionally small Ec (low-voltage switching <5 V), multiaxial ferroelectricity and plastic deformability (Fig. 3f), crystals of 6 can be readily processed into bulk polycrystalline forms with a variety of shapes designed for various measurements, which were employed to demonstrate various properties related to ferroelectricity, e.g., thin films for high-electric-field applications or thick and robust pellets for d33 measurements.These researchers found that the cubic crystal symmetry Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m and the fully disordered molecular orientations of 6 and 7 strongly suggest that the HTP is the plastic crystal phase and that the crystals are multiaxial plastic ferroelectric crystals.
m and the fully disordered molecular orientations of 6 and 7 strongly suggest that the HTP is the plastic crystal phase and that the crystals are multiaxial plastic ferroelectric crystals.
The plastic ferroelectric crystals of 6 have large piezoelectricity after polarization, and the d33 of polycrystalline pellets is approximately 90 pC N−1 at room temperature, which is comparable to that of tetramethylammonium plastic ferroelectric crystals. The polarization of the tetramethylammonium plastic ferroelectric crystals originates from the nonpolar symmetry arrangement of ionic molecules, and the ferroelectricity of 6 is caused by the reorientation of the polar cations (Fig. 3g). Regardless of the cause of the polarization, plastic ferroelectric crystals usually have large piezoelectricity due to the softness of the crystal lattices even in the ferroelectric phase at room temperature.
Combined with the advantageous low-temperature solution processability and ease of fabrication of polycrystalline materials with desired shapes, such plastic ferroelectric crystals with large d33 represent promising candidates for piezoelectric materials that may find a variety of applications.
4 Halogen/metal substitution-induced perovskite MOFEs with a large piezoelectric effect
In the previous work mentioned above, the largest bulk d33 among MOFEs was found in 4 (110 pC N−1), yet it is only half that of the perovskite ferroelectric BTO single crystal (d33 = 190 pC N−1 along the [111] crystal orientation). 4 has an even smaller d33 than those of binary perovskite solid solutions such as PZT, which exhibit values as high as thousands of picocoulombs per Newton with proper doping, phase, domain and strain engineering.3,48–51 Recent developments show the potential for application of organic–inorganic hybrid perovskite materials in electronics,52 light sources,53 photovoltaics,54–59 and even ferroelectric/piezoelectrics.60–67 Researchers have been trying to improve the piezoelectric response ever since the successful design of multiaxial MOFEs by adapting a hybrid perovskite structure.68,69 The inorganic framework in a hybrid perovskite not only provides high mechanical strength but also is good for strain propagation through the entire material. Such a hybrid structure also has large compositional and structural compatibility for combination with various organic cations.70–73 In this way, by introducing globular polar cations, the polarization can rotate under external strain. According to the defining equation of the piezoelectric constant as a partial derivative evaluated at constant field d = (∂D/∂T)E (where D is the dielectric displacement, T is stress, and subscript E indicates an applied constant field),3 the rotation of the polar cation under external stress can cause a change in the polarization direction, which can induce a large change in the polarization intensity and lead to a large d33, similar to in inorganics.74The BaNiO3-like perovskite [(CH3)4N]MnCl3 attracted the attention of researchers in the search for hybrid perovskite MOFEs. This compound crystallizes in the centrosymmetric space group P63/m in the room-temperature phase (RTP) and has an HTP of the centrosymmetric space group P63/mmc (Fig. 4a). This phase transition occurs because the [(CH3)4N]+ cation belonging to the molecular symmetry group Td overcomes the low potential energy barrier of the tumbling motion and rotates with a high speed in the HTP, showing a disordered isotropic state. Due to the centrosymmetric nature of [(CH3)4N]+, the RTP is not a ferroelectric polar phase, but such a hybrid compound provides a very suitable starting point for chemical design.75
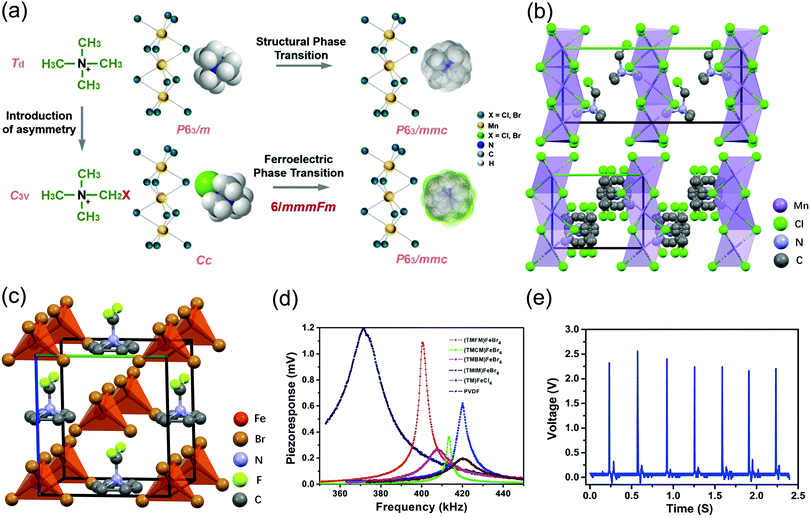 | ||
| Fig. 4 Schematic diagrams of the structural chemical design and piezoelectric/ferroelectric tests of MOFEs with halogen/metal substitution. (a) Molecular chemical design of 8 and 11, whose structural phase transitions before and after design are shown. (b) Crystal structure of 8 in the low temperature phase (LTP) (top) and HTP (bottom) with 12 different equivalent polarization directions. (c) One type of multiaxial MOFE [(CH3)3NCH2X]FeBr4 (X = F) obtained through molecular design. (d) Piezoelectric properties and (e) energy-harvesting capability of [(CH3)3NCH2X]FeBr4 (X = F, Cl, Br, I). Reproduced with permission.15,18,75,77 | ||
The “quasi-spherical theory” and the “momentum matching theory” provide us with good potential for endowing this prototypical system with ferroelectricity and piezoelectricity.18 As a result, researchers replaced a hydrogen atom on the methyl group in the [(CH3)4N]+ cation with a chlorine atom and assembled it with a [MnCl3]− inorganic framework to obtain a one-dimensional organic–inorganic hybrid perovskite material. As shown in Fig. 4a, [(CH3)3NCH2Cl]+ rotates in an isotropic spherical shape at high speed in the HTP, similar to [(CH3)4N]+, and crystallizes in the centrosymmetric space group P63/mmc. However, due to the introduction of the electronegative chlorine atom, the [(CH3)3NCH2Cl]+ organic cation has a dipole moment in the RTP, and the molecular symmetry is reduced to the C3v, resulting in a Cc polar crystal space group. With an Aizu notation of 6/mmmFm, [(CH3)3NCH2Cl]MnCl3 (8) is a multiaxial ferroelectric with 12 equivalent polarization directions (Fig. 4b).
Enlightened by the aforementioned works on [(CH3)4N]MnCl3, researchers inferred that the existence of halogen–halogen bonds in 8 is a possible factor in achieving multiaxial ferroelectricity. As a consequence of halogen/metal substitution, three isostructural perovskite structures, [(CH3)3NCH2Cl]CdCl3 (9), [(CH3)3NCH2Cl]CdBr3 (10) and [(CH3)3NCH2Br]MnBr3 (11), were designed. 9 and 11 both crystallize in the polar monoclinic space group Cc at room temperature and belong to the centrosymmetric hexagonal space group P63/mmc in the HTP (Fig. 4a). With the Aizu notation of 6/mmmFm, both 9 and 11 possess 12 crystallographically equivalent polarization directions, corresponding to 6 ferroelectric axes. Similarly, 10 also crystallizes in the hexagonal space group P63/mmc in the HTP. However, due to the size of the [CdBr3]− anion increasing in 10, the balance between the cation and the anion decreases, causing a relatively mismatched rotation movement of the cation. This makes 10 also crystallize in the hexagonal space group P63/mmc in the HTP, and after undergoing a phase transition written in Aizu notation as 6/mmmF6mm, the symmetry can only become lower to the polar hexagonal space group P63mc in the RTP.
Remarkably, in addition to the preservation of ferroelectricity, the moderate halogen bonding interactions further act as an essential driving force in the attractive piezoelectric performance of the above four compounds, whose d33 values are as large as 185, 220, 139 and 112 pC N−1. These compounds with perovskite structure have the four largest bulk d33 among monocomposition MOFE crystals.
4.1 (TMCM)MnCl3 and (TMCM)CdCl315
You et al. discovered the organic–inorganic perovskite MOFE trimethylchloromethyl ammonium trichloromanganese(II), (TMCM)MnCl3 (8), which shows an excellent piezoelectric response (d33 = 185 pC N−1) close to that of inorganic piezoelectrics of BTO and a high transition temperature Tc of 406 K.The phase transition between the P63/mmc HTP and Cc RTP is induced by the order–disorder transition of the cations, leading to a multiple polar axes characteristic (Fig. 4b). Unlike inorganic ferroelectrics normally grown in the high-temperature paraelectric phase exhibiting multidomain structures and requiring high-voltage poling processing before their application, crystals of 8 grew at room temperature and exhibited a nice monodomain structure. A maximum d33 of 185 pC N−1 was obtained in proximity to along the [102] direction of the crystal in the RTP.
Moreover, 8 is also a partially ferroelastic material based on an examination of the strain tensors in the RTP. Polarization states on the same polar axis, P+/−i (i = 1, 2,…,6), belong to the same strain state Si (i = 1, 2,…,6). Under an external stress, materials with ferroelasticity switch from one strain state to the other. In this case, when an external stress switches 8 from Si to Sj (i, j = 1, 2,…,6), the polarization state is also switched from P+/−i to P+/−j (i, j = 1, 2,…,6), respectively. Such rotation of the polarization direction prompts an anomalously large change in the polarization, leading to a large piezoresponse.
The large piezoelectricity of 8 is due to the particular organic–inorganic structural property; thus, more piezoelectrics may be formed by further engineering through element substitution and molecular design, such as replacing Mn with other metals. For example, these researchers synthesized the high-temperature piezoelectric MOFE trimethylchloromethyl ammonium trichlorocadmium(II), (TMCM)CdCl3 (9), the d33 of which was measured to be 220–240 pC N−1, even larger than that of 8.
4.2 (TMCM)CdBr376
Liao et al. showed that the perovskite MOFE trimethylchloromethylammonium tribromocadmium(II), (TMCM)CdBr3 (10), was successfully developed, where the appropriate halogen bond ensures the stabilization of a one-dimensional [CdBr3]− chain and further preservation of its ferroelectricity in this organic–inorganic hybrid perovskite system.This MOFE has a high Tc of 346 K and a large d33 of 139 pC N−1, which is 1 order of magnitude higher than those of most classical uniaxial ferroelectrics, such as LiNbO3 and Rochelle salt, and comparable to those of multiaxial ferroelectrics, such as BTO. 10 is a typical ABX3-type linear chain compound consisting of infinite chains of CdBr3 along the c-axis separated by trimethylchloromethylammonium (TMCM) cations. According to the symmetry change, 10 belongs to the ferroelectric species with Aizu notion 6/mmmF6mm. The ferroelectric mechanism can be understood as being induced by the disorder–partial-order transition of the cations with breaking of the mirror symmetry. At the tapping frequency of 110 Hz, the d33 detected for the as-grown single crystal is approximately 139 pC N−1 in proximity to along the [001] direction of the crystal in the RTP.
Before 10, the largest bulk d33 among known uniaxial MOFEs was only 41 pC N−1 in 1; thus, such a large enhancement of d33 makes 10 an ideal candidate to replace conventional piezoelectric ceramics.
4.3 (TMBM)MnBr377
Liao et al. reported the perovskite MOFE trimethylbromomethylammonium tribromomanganese(II), (TMBM)MnBr3 (11), with a large d33 of 112 pC N−1 along the polar axis, comparable to those of typical monocomposition piezoceramics such as BaTiO3 along the polar axis [001] (∼90 pC N−1) and much greater than those of most known MOFEs. More significantly, the effective local piezoelectric coefficient of (TMBM)MnBr3 films is comparable to that of its bulk crystals, which ensures the feasibility of piezo film applications when combined with the multiaxial characteristic and low coercive voltage of ∼6 V.11 crystallizes in the monoclinic space group Cc at room temperature and the hexagonal space group P63/mmc at high temperature (433 K), leading to 6 polar axes in the ferroelectric phase. The ferroelectric mechanism was attributed to the order–disorder transition of the cations activated by temperature. The maximum d33 of 11 is obtained at the tapping frequency of 110 Hz, 112 pC N−1 in the vicinity of along the [102] direction of the crystal at room temperature.
Considering the similar structures of 11 and 8, the large d33 might result from the multiaxial characteristic induced by the significant symmetry change between the paraelectric and ferroelectric phases. Additionally, by observing the polarization vector and strain tensor, these researchers found that 11 and 8 are both fully ferroelectric/partially ferroelastic compounds. Therefore, for these multiaxial ferroelectrics, some polarization states are also strain states. When an external stress switches the strain state, the corresponding polarization state is also switched, inducing a large change in the polarization and a striking piezoresponse.
4.4 (TMFM)x(TMCM)1−xCdCl378
The most effective piezoelectric materials are ceramic solid solutions in which the piezoelectric effect is optimized at what are termed morphotropic phase boundaries. This strategy for optimizing the piezoelectricity was developed in 1954 by Jaffe et al., who recognized that the electromechanical response in binary PZT ceramic solid solution was maximized at phase boundaries (Fig. 5a).9,10 However, this strategy has never been realized in molecular piezoelectrics. Molecular solid solutions are very complex because mixing occurs between molecules, which requires an understanding of the distortions and spatial orientations. Designing an MOFE with piezoelectric properties comparable to those of ceramic solid solutions would open up the application space and solve a century-old challenge.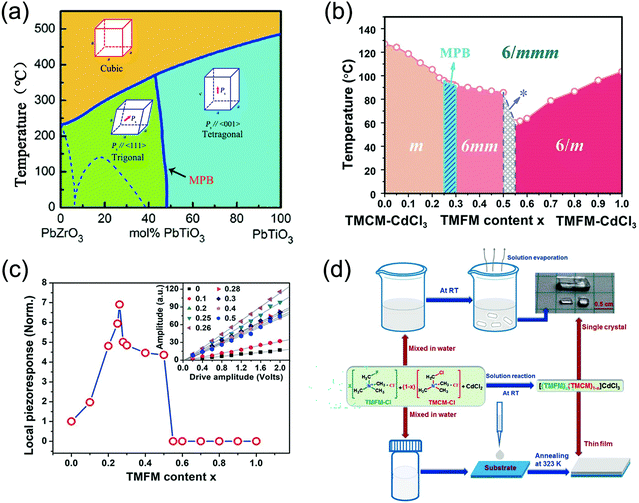 | ||
| Fig. 5 Application methods and performance tests of using the MPB in inorganic piezoelectric MOFEs. (a) PZT phase diagram, and its MPB schematic. (b) The phase diagram marked with point groups of 12 indicates that an MPB exists in the composition range of 0.25 ≤ x ≤ 0.3; for the composition of 0.5 < x <0.55, indicated by the asterisk (*), growth of a uniform crystal is difficult because severe compositional segregation occurs in this area. (c) Relationship between the piezoresponse amplitude of 12 and driving a.c. voltage under different compositions as measured by PFM, and local piezoelectric response values compared under normalization to 1 (x = 0). (d) Schematic diagram of the synthesis process and film fabrication of 12 by the room-temperature solution processing method. Reproduced with permission.76 | ||
Liao et al. synthesized piezoelectric materials from a molecular perovskite (TMFM)x(TMCM)1−xCdCl3 (12) solid solution, in which an MPB exists between monoclinic and hexagonal phases. Other binary MOFE solid-solution systems, such as the (TMFM)x(TMCM)1−xMnCl3 and (TMDFM)x(TMCM)1−xCdCl3 systems, have similar phase diagrams, including an MPB region. Both systems exhibit improvements in the piezoelectric properties near the MPB, confirming that this is a general design strategy for optimizing molecular solid solutions.
These researchers determined the phase diagram of the 12 solid solution related to the composition and observed an MPB region where the ferroelectric monoclinic m phase and ferroelectric hexagonal 6mm phase coexist within the composition range of 0.25 ≤ x ≤ 0.3 (Fig. 5b). For the composition of x = 0.26 in the MPB region, d33 is ∼1540 pC N−1 (seven times higher than that of the monocomposition piezoelectric counterpart 9, d33 ∼ 220 pC N−1) (Fig. 5c). The compositions of 0 ≤ x < 0.25 and 0.3 < x ≤ 0.5 can be expressed as 6/mmmFm and 6/mmmF6mm, respectively, in Aizu notation, which belong to 88 kinds of ferroelectric phase transition.
The 12 solid solution was synthesized as single crystals using a room-temperature solution processing method (Fig. 5d) by slow evaporation of an aqueous solution, which enables the deposition of 12 solid-solution films on various substrates, including flexible substrates, making it attractive for a variety of applications in flexible and wearable devices.
4.5 (TMXM)FeBr4 (X = F, Cl, Br, I)79
Recently, Yi Zhang et al. successfully extended the TMXM cation system to non-perovskite structures. By replacing the inorganic part, they designed and regulated four high-temperature multiaxial piezoelectric MOFEs, [(CH3)3NCH2X]FeBr4 (X = F, Cl, Br, I) (Fig. 4c). All such multiaxial ferroelectrics exhibit good piezoelectric performance. Among them, as an example, (TMFM)FeBr4 (13) (TMFM, trimethylfluoromethylammonium) has a ferroelectric phase transition with an Aizu notation of m3mFmm2, accompanied by 12 possible polarization directions and a high Tc of 412.7 K.The piezoelectric performance was examined by PFM and device measurement. The PFM probe was used to excite each film at its resonant frequency with a voltage of 10 V at room temperature. The results show that 13 has the highest peak value of the response caused by the intrinsic piezoelectricity, higher than that of the 3 thin film (Fig. 4d).
Furthermore, these researchers fabricated thin-film devices of 13 and exploited the piezoelectric response with an energy-harvesting capability. The piezoelectric voltage was recorded after repetitively applying mechanical pressure. The 13 polycrystalline sample exhibited a voltage of approximately 2.2 V (Fig. 4e).
Although the four [FeBr4]− anion compounds do not have a perovskite structure in the traditional sense, they still possess decent multiaxial ferroelectric properties and good piezoelectric performance. These results show that the organic cation design, halogen bonding strategy and “quasi-spherical” theory can be applied in a universal range and have great potential for extrapolation to other piezoelectric systems.
5 Other approaches and recent progress
5.1 Large piezoelectric voltage coefficient g3380
Chen et al. reported the two-dimensional organic–inorganic perovskite MOFE (4-aminotetrahydropyran)2PbBr4, (ATHP)2PbBr4 (14), with a high Tc of 503 K and an extremely large g33 of 660.3 × 10−3 V m N−1, much beyond that of PZT ceramics (20 to 40 × 10−3 V m N−1) and more than two times that of PVDF (approximately 286.7 × 10−3 V m N−1). The large d33 (76 pC N−1) and relatively small dielectric permittivity εr (13) contribute to the extremely large g33.Since g33 = d33/ε, to obtain a larger g33, a piezoelectric material with a larger d33 and a lower dielectric constant εr can be selected. To evaluate d33, these researchers extracted the local piezoresponse by comparing the thin film of 14 with (CHA)2PbBr4 (CHA = cyclohexylammonium, d33 = 48 pC N−1) using the PFM method under the same experimental conditions (Fig. 6a). The d33 of 14 was evaluated to be approximately 76 pC N−1. Based on the above experimental results (d33 = 76 pC N−1 and εr = 13), the g33 of 14 is extraordinarily large at approximately 660.3 × 10−3 V m N−1. Notably, 14 also possesses a high Tc of 503 K, meaning that it can maintain a stable structure and excellent performance over a broad temperature range below Tc. The excellent phase transition temperature and piezoelectric voltage coefficient indicate a large operational temperature range with ultrahigh sensitivity, endowing this MOFE with very large development potential in the field of sensing.
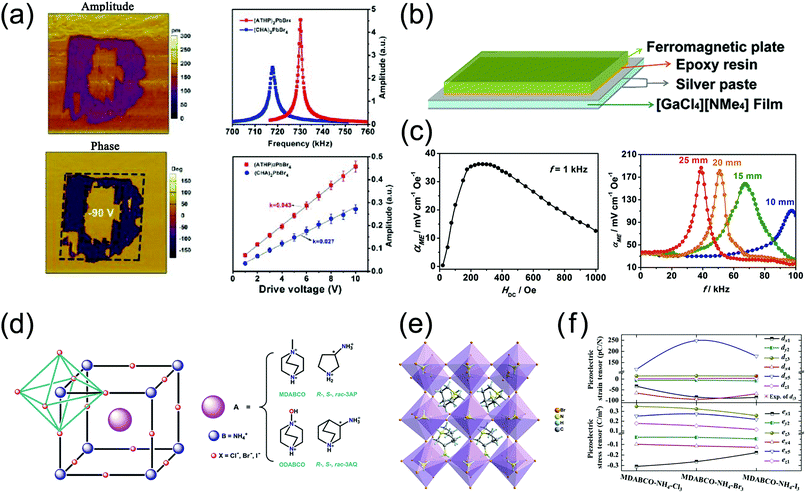 | ||
| Fig. 6 Other piezoelectric properties of MOFEs, and calculation of the piezoelectric properties of metal-free perovskite ferroelectrics. (a) PFM domain inversion image of 14, and its piezoelectric performance compared to that of (CHA)2PbBr4. (b) Layer structure of the magnetoelectric composite 5/Terfenol-D. (c) Magnetoelectric coupling coefficient tests of 5/Terfenol-D. (d) Chemical composition and structure of metal-free perovskite ferroelectrics. (e) Crystal structures of one compound in the metal-free perovskite ferroelectric family. (f) Calculated piezoelectric strain (top) and stress (bottom) tensor of MDABCO-NH4X3. Reproduced with permission.46,78–80 | ||
5.2 Magnetoelectric composites46
Cross interactions between piezoelectric and magnetic compounds at room temperature can lead to magnetoelectric materials with a large magnetoelectric response. The magnetoelectric effect in magnetoelectric composites is due to the change in the shape of the material caused by the magnetostriction under an external magnetic field, which causes the piezoelectric component to generate strain and induce electric polarization. Because the piezoelectric effect of multiaxial (plastic) MOFEs can be detected in multiple directions and polycrystalline forms, MOFEs are excellent candidates for magnetoelectric composites.Li et al. first prepared one example of a magnetoelectric composite by using the multiaxial MOFE [NMe4][GaCl4] (5) with d33 ≈ 80 pC N−1. 5 was composited due to its large piezoelectricity and easy processability, similar to the magnetoelectric composites Pb(Mg1/3Nb2/3)O3–PbTiO3/Metglas, PbZrxTi(1−x)O3/Terfenol-D, and PVDF/Metglas. Fig. 6a and b illustrates the structure of the 5/Terfenol-D composite, which can be viewed as a bilayer structure formed through connection of the polycrystalline film of 5 with silver paste coatings on both sides and the room-temperature magnetostrictive material Terfenol-D using epoxy resin. Investigation of the magnetoelectric effect of this magnetoelectric laminate composite indicates that its room-temperature magnetoelectric voltage coefficient (αME) is as high as 186 mV cm−1 Oe−1 at HDC = 275 Oe and HAC ≈ 39 kHz (Fig. 6c), providing a valid approach for the preparation of magnetoelectric materials and adding a new member to the magnetoelectric material family.
5.3 Metal-free three-dimensional perovskite MOFEs81
Different from the aforementioned one-dimensional ABX3 hybrid perovskite and Ruddlesden–Popper two-dimensional A2BX4 hybrid perovskite MOFEs, the development and design of three-dimensional ABX3 perovskites has seemed more attractive recently due to their structural similarity to BTO and inherent multiaxial nature. Motivated by their lower toxicity, wide chemical diversity, structural flexibility, and additional functionalities that are difficult to achieve with inorganic perovskites, researchers have become interested in metal-free three-dimensional ABX3 perovskites, in which an organic cation occupies the B site of the crystal.Ye et al. recently found a family of metal-free organic MOFEs with the characteristic three-dimensional perovskite structure, among which MDABCO-NH4I3 (15) (MDABCO = N-methyl-N′-diazabicyclo[2.2.2]octonium) (Fig. 6d and e) has a spontaneous polarization of 22 μC cm−2, close to that of BTO (26 μC cm−2), a high phase transition temperature of 448 K, beyond that of BTO (390 K), and the 432F3 transition with eight possible polarization directions.
Meanwhile, optically active noncentrosymmetric organic perovskites have been successfully created with chiral template organic cations, such as S-3AQ-NH4Br3 (where S-3AQ is S-3-ammonioquinuclidinium). Because of their suitable geometric sizes and exact charge balancing, molecular cationic isomers (R-3AQ)2+ and (R-3AP)2+ and (S-3AP)2+ (where S-3AP is S-3-ammoniopyrrolidinium) can be easily embedded in ammonium halide cages. To confirm the corresponding enantiomorphic relationships, these researchers performed both single crystal X-ray diffraction and vibrational circular dichroism spectroscopy, whose results clearly proved their enantiomorphic relationships.
Although the d33 of 15 is moderate at ∼14 pC N−1, according to the calculation of Wang et al., the dx4 and dx5 of the metal-free organic MOFEs are considerable: the calculated dx5 is 119 pC N−1 for MDABCO-NH4Cl3, 248 pC N−1 for MDABCO-NH4Br3 and 178 pC N−1 for MDABCO-NH4I3 (Fig. 6f).82 The success of the introduction of chirality into organic perovskite MOFEs shows the great tunability of the structure–property relationships of this family of materials. Thus, we believe that after systematic study and further optimization, this up-and-coming class of materials combining the outstanding metal-free and three-dimensional advantages certainly holds great potential for the next generation of piezoelectric MOFEs.
6 Summary and outlook
In this review, we summarized the progress in the performance enhancement and application exploration of piezoelectric MOFEs, including monoaxial thin films, multiaxial MOFEs with plastic phase transitions, and hybrid perovskite systems with giant d33, as well as some recent approaches.We believe that these work will have enlightenment significance in the rational design and wide application of piezoelectric MOFEs. The representative examples listed in the text will undoubtedly stimulate much more creative works (Table 1).
However, in such a new field of research, many unexplored areas remain, many problems are still unsolved, and some challenges must be overcome. First, the mechanisms of how the halogens, organic cations, and structures of piezoelectric MOFEs as well as the polar axes affect the properties of piezoelectric MOFEs have not been clearly studied, which will benefit the finding of new high-performance piezoelectric MOFEs. How much these factors contribute to d33 is still unknown. A theory that can be used to quantitatively predict piezoelectric properties is highly needed.
Meanwhile, the goal of piezoelectric MOFE research is still to find new piezoelectric MOFEs with larger d33, higher Tc, and more polar axes that are much more environmental friendly, lower cost, and easier to fabricate, but a sufficiently reliable approach that can guide us in designing such materials meeting the above requirements is still lacking. Though we have several experiential rules, such as those for plastic phase transition-induced and halogen/metal substitution-induced effects mentioned above, many experiments are needed to determine which compounds meet our requirements among those designed based on guiding rules. Therefore, development of a more reasonable design approach in the future will help us design piezoelectric MOFEs with higher efficiency.
In addition, many plastic MOFEs have not had their piezoelectric properties reported or been studied at all due to their time-consuming single crystal growth or other reasons. We believe that some compounds with outstanding piezoelectric performance exist, which would be beneficial for piezoelectric MOFE development.
For practical applications, many challenges also remain. In particular, some advantages of MOFEs over inorganic ceramics are practicably disadvantageous from other perspectives, such as the aqueous solubility and thermal instability. Only when the water and thermal instability problems can be solved can piezoelectric MOFEs be used for practical applications. A more universal, easier and gentler growth method needs to be proposed because various MOFEs may possess various physical properties, resulting in strict substrate and condition dependence of thin films. In addition, finding environmentally friendly piezoelectric MOFEs that can be easily degraded to replace pollution-intensive inorganic piezoelectrics is strongly preferred.
The study of piezoelectric MOFEs is clearly shifting from curiosity-driven research to problem-driven research. This trend calls for efficient collaboration among researchers in the fields of chemistry, physics, materials, microelectronics and so on. These developments will inject new vitality into the century-old piezoelectric field, attract the widespread interest of researchers from multiple subjects, and lead to piezoelectric MOFEs much closer to medical, micromechanical, and biomechanical application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21925502). The authors would like to thank Prof. Ren-Gen Xiong for his academic guidance and consultant.References
- D. Damjanovic, Ferroelectric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics, Rep. Prog. Phys., 1998, 61, 1267–1324 CrossRef CAS.
- C. Qiu, B. Wang, N. Zhang, S. Zhang, J. Liu, D. Walker, Y. Wang, H. Tian, T. R. Shrout, Z. Xu, L.-Q. Chen and F. Li, Transparent ferroelectric crystals with ultrahigh piezoelectricity, Nature, 2020, 577, 350–354 CrossRef CAS.
- B. Jaffe, Piezoelectric ceramics, Elsevier, 2012 Search PubMed.
- J. Curie and P. Curie, Développement par compression de l'électricité polaire dans les cristaux hémièdres à faces inclinées, Bull. Mineral., 1880, 3, 90–93 Search PubMed.
- J. Valasek, Piezo-electric and allied phenomena in Rochelle salt, Phys. Rev., 1921, 17, 475–481 CrossRef CAS.
- R. Bechmann, Elastic, piezoelectric, and dielectric constants of polarized barium titanate ceramics and some applications of the piezoelectric equations, J. Acoust. Soc. Am., 1956, 28, 347–350 CrossRef.
- S. Wada, K. Yako, H. Kakemoto, T. Tsurumi and T. Kiguchi, Enhanced piezoelectric properties of barium titanate single crystals with different engineered-domain sizes, J. Appl. Phys., 2005, 98, 014109 CrossRef.
- D. Berlincourt and H. Jaffe, Elastic and piezoelectric coefficients of single-crystal barium titanate, Phys. Rev., 1958, 111, 143–148 CrossRef CAS.
- G. Shirane, K. Suzuki and A. Takeda, Phase transitions in solid solutions of PbZrO3 and PbTiO3 (II) X-ray study, J. Phys. Soc. Jpn., 1952, 7, 12–18 CrossRef CAS.
- B. Jaffe, R. Roth and S. Marzullo, Piezoelectric properties of lead zirconate-lead titanate solid-solution ceramics, J. Appl. Phys., 1954, 25, 809–810 CrossRef CAS.
- S. Horiuchi, Y. Tokunaga, G. Giovannetti, S. Picozzi, H. Itoh, R. Shimano, R. Kumai and Y. Tokura, Above-room-temperature ferroelectricity in a single-component molecular crystal, Nature, 2010, 463, 789–792 CrossRef CAS.
- D. W. Fu, W. Zhang, H. L. Cai, J. Z. Ge, Y. Zhang and R. G. Xiong, Diisopropylammonium chloride: a ferroelectric organic salt with a high phase transition temperature and practical utilization level of spontaneous polarization, Adv. Mater., 2011, 23, 5658–5662 CrossRef CAS.
- D.-W. Fu, H.-L. Cai, Y. Liu, Q. Ye, W. Zhang, Y. Zhang, X.-Y. Chen, G. Giovannetti, M. Capone, J. Li and R.-G. Xiong, Diisopropylammonium Bromide Is a High-Temperature Molecular Ferroelectric Crystal, Science, 2013, 339, 425–428 CrossRef CAS.
- A. S. Tayi, A. K. Shveyd, A. C. H. Sue, J. M. Szarko, B. S. Rolczynski, D. Cao, T. J. Kennedy, A. A. Sarjeant, C. L. Stern, W. F. Paxton, W. Wu, S. K. Dey, A. C. Fahrenbach, J. R. Guest, H. Mohseni, L. X. Chen, K. L. Wang, J. F. Stoddart and S. I. Stupp, Room-temperature ferroelectricity in supramolecular networks of charge-transfer complexes, Nature, 2012, 488, 485–489 CrossRef CAS.
- Y.-M. You, W.-Q. Liao, D. Zhao, H.-Y. Ye, Y. Zhang, Q. Zhou, X. Niu, J. Wang, P.-F. Li, D.-W. Fu, Z. Wang, S. Gao, K. Yang, J.-M. Liu, J. Li, Y. Yan and R.-G. Xiong, An organic-inorganic perovskite ferroelectric with large piezoelectric response, Science, 2017, 357, 306–309 CrossRef CAS.
- W. Zhang and R.-G. Xiong, Ferroelectric Metal–Organic Frameworks, Chem. Rev., 2012, 112, 1163–1195 CrossRef CAS.
- P.-P. Shi, Y.-Y. Tang, P.-F. Li, W.-Q. Liao, Z.-X. Wang, Q. Ye and R.-G. Xiong, Symmetry breaking in molecular ferroelectrics, Chem. Soc. Rev., 2016, 45, 3811–3827 RSC.
- H.-Y. Zhang, Y.-Y. Tang, P.-P. Shi and R.-G. Xiong, Toward the Targeted Design of Molecular Ferroelectrics: Modifying Molecular Symmetries and Homochirality, Acc. Chem. Res., 2019, 52, 1928–1938 CrossRef CAS.
- Y. a. Xiong, Z. Feng, Z. Jing, Q. Pan, T. Sha, S. Miao, J. Yao, G. Du and Y. You, Recent progress in molecular ferroelectrics with perovskite structure, Chin. Sci. Bull., 2020, 65, 916–930 CrossRef.
- C. A. P. de Araujo and G. W. Taylor, Integrated ferroelectrics, Ferroelectrics, 1991, 116, 215–228 CrossRef.
- N. Setter, D. Damjanovic, L. Eng, G. Fox, S. Gevorgian, S. Hong, A. Kingon, H. Kohlstedt, N. Park and G. Stephenson, Ferroelectric thin films: Review of materials, properties, and applications, J. Appl. Phys., 2006, 100, 051606 CrossRef.
- M. Dawber, K. Rabe and J. Scott, Physics of thin-film ferroelectric oxides, Rev. Mod. Phys., 2005, 77, 1083–1130 CrossRef CAS.
- K. Hwang, T. Manabe, T. Nagahama, I. Yamaguchi, T. Kumagai and S. Mizuta, Effect of substrate material on the crystallinity and epitaxy of Pb(Zr,Ti)O3 thin films, Thin Solid Films, 1999, 347, 106–111 CrossRef CAS.
- L. B. Kong, T. Zhang, J. Ma and F. Boey, Progress in synthesis of ferroelectric ceramic materials via high-energy mechanochemical technique, Prog. Mater. Sci., 2008, 53, 207–322 CrossRef CAS.
- J. Przesławski and Z. Czapla, Calorimetric studies of phase transitions in imidazolium perchlorate crystal, J. Phys.: Condens. Matter, 2006, 18, 5517–5524 CrossRef.
- Z. Czapla, S. Dacko, B. Kosturek and A. Was’kowska, Dielectric and optical properties related to phase transitions in an imidazolium perchlorate [C3N2H5ClO4] crystal, Phys. Status Solidi B, 2005, 242, R122–R124 CrossRef CAS.
- Z. Pająk, P. Czarnecki, B. Szafrańska, H. Małuszyńska and Z. Fojud, Ferroelectric ordering in imidazolium perchlorate, J. Chem. Phys., 2006, 124, 144502 CrossRef.
- Z. Zhang, P.-F. Li, Y.-Y. Tang, A. J. Wilson, K. Willets, M. Wuttig, R.-G. Xiong and S. Ren, Tunable electroresistance and electro-optic effects of transparent molecular ferroelectrics, Sci. Adv., 2017, 3, e1701008 CrossRef.
- Y. Zhang, Y. Liu, H.-Y. Ye, D.-W. Fu, W. Gao, H. Ma, Z. Liu, Y. Liu, W. Zhang, J. Li, G.-L. Yuan and R.-G. Xiong, A Molecular Ferroelectric Thin Film of Imidazolium Perchlorate That Shows Superior Electromechanical Coupling, Angew. Chem., Int. Ed., 2014, 53, 5064–5068 CAS.
- X. Jiang, H. Lu, Y. Yin, X. Zhang, X. Wang, L. Yu, Z. Ahmadi, P. S. Costa, A. D. DiChiara, X. Cheng, A. Gruverman, A. Enders and X. Xu, Room temperature ferroelectricity in continuous croconic acid thin films, Appl. Phys. Lett., 2016, 109, 102902 CrossRef.
- V. Y. Shur, Kinetics of ferroelectric domains: Application of general approach to LiNbO3 and LiTaO3, Front. Ferroelectr., 2006, 199–210 CAS.
- Q. Pan, Z.-B. Liu, H.-Y. Zhang, W.-Y. Zhang, Y.-Y. Tang, Y.-M. You, P.-F. Li, W.-Q. Liao, P.-P. Shi, R.-W. Ma, R.-Y. Wei and R.-G. Xiong, A Molecular Polycrystalline Ferroelectric with Record-High Phase Transition Temperature, Adv. Mater., 2017, 29, 1700831 CrossRef.
- J. M. Pringle, Recent progress in the development and use of organic ionic plastic crystal electrolytes, Phys. Chem. Chem. Phys., 2013, 15, 1339–1351 RSC.
- B. Li, Y. Kawakita, S. Ohira-Kawamura, T. Sugahara, H. Wang, J. Wang, Y. Chen, S. I. Kawaguchi, S. Kawaguchi, K. Ohara, K. Li, D. Yu, R. Mole, T. Hattori, T. Kikuchi, S.-i. Yano, Z. Zhang, Z. Zhang, W. Ren, S. Lin, O. Sakata, K. Nakajima and Z. Zhang, Colossal barocaloric effects in plastic crystals, Nature, 2019, 567, 506–510 CrossRef CAS.
- J. M. Pringle, P. C. Howlett, D. R. MacFarlane and M. Forsyth, Organic ionic plastic crystals: recent advances, J. Mater. Chem., 2010, 20, 2056–2062 RSC.
- J. N. Sherwood, The Plastically crystalline state: orientationally disordered crystals, John Wiley & Sons, 1979 Search PubMed.
- J. Timmermans, Plastic crystals: a historical review, J. Phys. Chem. Solids, 1961, 18, 1–8 CrossRef CAS.
- R. Brand, P. Lunkenheimer and A. Loidl, Relaxation dynamics in plastic crystals, J. Chem. Phys., 2002, 116, 10386–10401 CrossRef CAS.
- J. Harada, T. Shimojo, H. Oyamaguchi, H. Hasegawa, Y. Takahashi, K. Satomi, Y. Suzuki, J. Kawamata and T. Inabe, Directionally tunable and mechanically deformable ferroelectric crystals from rotating polar globular ionic molecules, Nat. Chem., 2016, 8, 946–952 CrossRef CAS.
- Y.-M. You, Y.-Y. Tang, P.-F. Li, H.-Y. Zhang, W.-Y. Zhang, Y. Zhang, H.-Y. Ye, T. Nakamura and R.-G. Xiong, Quinuclidinium salt ferroelectric thin-film with duodecuple-rotational polarization-directions, Nat. Commun., 2017, 8, 1–7 CrossRef.
- Y.-Y. Tang, P.-F. Li, W.-Y. Zhang, H.-Y. Ye, Y.-M. You and R.-G. Xiong, A multiaxial molecular ferroelectric with highest Curie temperature and fastest polarization switching, J. Am. Chem. Soc., 2017, 139, 13903–13908 CrossRef CAS.
- P.-F. Li, Y.-Y. Tang, Z.-X. Wang, H.-Y. Ye, Y.-M. You and R.-G. Xiong, Anomalously rotary polarization discovered in homochiral organic ferroelectrics, Nat. Commun., 2016, 7, 1–9 Search PubMed.
- Z. Sun, T. Chen, X. Liu, M. Hong and J. Luo, Plastic transition to switch nonlinear optical properties showing the record high contrast in a single-component molecular crystal, J. Am. Chem. Soc., 2015, 137, 15660–15663 CrossRef CAS.
- H.-Y. Ye, J.-Z. Ge, Y.-Y. Tang, P.-F. Li, Y. Zhang, Y.-M. You and R.-G. Xiong, Molecular ferroelectric with most equivalent polarization directions induced by the plastic phase transition, J. Am. Chem. Soc., 2016, 138, 13175–13178 CrossRef CAS.
- J. Harada, N. Yoneyama, S. Yokokura, Y. Takahashi, A. Miura, N. Kitamura and T. Inabe, Ferroelectricity and piezoelectricity in free-standing polycrystalline films of plastic crystals, J. Am. Chem. Soc., 2018, 140, 346–354 CrossRef CAS.
- D. Li, X. M. Zhao, H. X. Zhao, X. W. Dong, L. S. Long and L. S. Zheng, Construction of Magnetoelectric Composites with a Large Room-Temperature Magnetoelectric Response through Molecular–Ionic Ferroelectrics, Adv. Mater., 2018, 30, 1803716 CrossRef.
- J. Harada, Y. Kawamura, Y. Takahashi, Y. Uemura, T. Hasegawa, H. Taniguchi and K. Maruyama, Plastic/Ferroelectric Crystals with Easily Switchable Polarization: Low-Voltage Operation, Unprecedentedly High Pyroelectric Performance, and Large Piezoelectric Effect in Polycrystalline Forms, J. Am. Chem. Soc., 2019, 141, 9349–9357 CrossRef.
- J. Hao, W. Li, J. Zhai and H. Chen, Progress in high-strain perovskite piezoelectric ceramics, Mater. Sci. Eng., R, 2019, 135, 1–57 CrossRef.
- T. Richter, S. Denneler, C. Schuh, E. Suvaci and R. Moos, Textured PMN–PT and PMN–PZT, J. Am. Ceram. Soc., 2008, 91, 929–933 CrossRef CAS.
- S. Mabud, The morphotropic phase boundary in PZT solid solutions, J. Appl. Crystallogr., 1980, 13, 211–216 CrossRef CAS.
- E. Sun and W. Cao, Relaxor-based ferroelectric single crystals: Growth, domain engineering, characterization and applications, Prog. Mater. Sci., 2014, 65, 124–210 CrossRef CAS.
- D. B. Mitzi, C. Feild, W. Harrison and A. Guloy, Conducting tin halides with a layered organic-based perovskite structure, Nature, 1994, 369, 467–469 CrossRef CAS.
- H. Cho, S.-H. Jeong, M.-H. Park, Y.-H. Kim, C. Wolf, C.-L. Lee, J. H. Heo, A. Sadhanala, N. Myoung, S. Yoo, S. H. Im, R. H. Friend and T.-W. Lee, Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes, Science, 2015, 350, 1222–1225 CrossRef CAS.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T.-Y. Yang, J. H. Noh and J. Seo, Efficient, stable and scalable perovskite solar cells using poly (3-hexylthiophene), Nature, 2019, 567, 511–515 CrossRef CAS.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Surface passivation of perovskite film for efficient solar cells, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- H. Tsai, W. Nie, J.-C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet, S. Tretiak, L. Pedesseau, J. Even, M. A. Alam, G. Gupta, J. Lou, P. M. Ajayan, M. J. Bedzyk, M. G. Kanatzidis and A. D. Mohite, High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells, Nature, 2016, 536, 312–316 CrossRef CAS.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. Chang and M. G. Kanatzidis, Lead-free solid-state organic–inorganic halide perovskite solar cells, Nat. Photonics, 2014, 8, 489–494 CrossRef CAS.
- S. Wang, X. Liu, L. Li, C. Ji, Z. Sun, Z. Wu, M. Hong and J. Luo, An Unprecedented Biaxial Trilayered Hybrid Perovskite Ferroelectric with Directionally Tunable Photovoltaic Effects, J. Am. Chem. Soc., 2019, 141, 7693–7697 CrossRef CAS.
- Y. Peng, X. Liu, Z. Sun, C. Ji, L. Li, Z. Wu, S. Wang, Y. Yao, M. Hong and J. Luo, Exploiting Bulk Photovoltaic Effect in a 2D Trilayered Hybrid Ferroelectric for Highly Sensitive Polarized Light Detection, Angew. Chem., Int. Ed., 2020, 59, 3933–3937 CrossRef CAS.
- L. Chen, W.-Q. Liao, Y. Ai, J. Li, S. Deng, Y. Hou and Y.-Y. Tang, Precise Molecular Design Toward Organic–Inorganic Zinc Chloride ABX3 Ferroelectrics, J. Am. Chem. Soc., 2020, 142(13), 6236–6243 CrossRef CAS.
- H.-Y. Zhang, X.-J. Song, X.-G. Chen, Z.-X. Zhang, Y.-M. You, Y.-Y. Tang and R.-G. Xiong, Observation of Vortex Domains in a Two-Dimensional Lead Iodide Perovskite Ferroelectric, J. Am. Chem. Soc., 2020, 142, 4925–4931 CrossRef CAS.
- J.-X. Gao, W.-Y. Zhang, Z.-G. Wu, Y.-X. Zheng and D.-W. Fu, Enantiomorphic Perovskite Ferroelectrics with Circularly Polarized Luminescence, J. Am. Chem. Soc., 2020, 142, 4756–4761 CrossRef CAS.
- H.-Y. Zhang, X.-J. Song, H. Cheng, Y.-L. Zeng, Y. Zhang, P.-F. Li, W.-Q. Liao and R.-G. Xiong, A Three-Dimensional Lead Halide Perovskite-Related Ferroelectric, J. Am. Chem. Soc., 2020, 142, 4604–4608 CrossRef CAS.
- Y. Hu, F. Florio, Z. Chen, W. A. Phelan, M. A. Siegler, Z. Zhou, Y. Guo, R. Hawks, J. Jiang, J. Feng, L. Zhang, B. Wang, Y. Wang, D. Gall, E. F. Palermo, Z. Lu, X. Sun, T.-M. Lu, H. Zhou, Y. Ren, E. Wertz, R. Sundararaman and J. Shi, A chiral switchable photovoltaic ferroelectric 1D perovskite, Sci. Adv., 2020, 6, eaay4213 CrossRef CAS.
- Q. Pan, Z.-B. Liu, Y.-Y. Tang, P.-F. Li, R.-W. Ma, R.-Y. Wei, Y. Zhang, Y.-M. You, H.-Y. Ye and R.-G. Xiong, A Three-Dimensional Molecular Perovskite Ferroelectric: (3-Ammoniopyrrolidinium)RbBr3, J. Am. Chem. Soc., 2017, 139, 3954–3957 CrossRef CAS.
- Y.-A. Xiong, T.-T. Sha, Q. Pan, X.-J. Song, S.-R. Miao, Z.-Y. Jing, Z.-J. Feng, Y.-M. You and R.-G. Xiong, A Nickel(II) Nitrite Based Molecular Perovskite Ferroelectric, Angew. Chem., Int. Ed., 2019, 58, 8857–8861 CrossRef CAS.
- T.-T. Sha, Y.-A. Xiong, Q. Pan, X.-G. Chen, X.-J. Song, J. Yao, S.-R. Miao, Z.-Y. Jing, Z.-J. Feng, Y.-M. You and R.-G. Xiong, Fluorinated 2D Lead Iodide Perovskite Ferroelectrics, Adv. Mater., 2019, 31, 1901843 CrossRef.
- C. Shi, J.-J. Ma, J.-Y. Jiang, M.-M. Hua, Q. Xu, H. Yu, Y. Zhang and H.-Y. Ye, Large Piezoelectric Response in Hybrid Rare-Earth Double Perovskite Relaxor Ferroelectrics, J. Am. Chem. Soc., 2020, 142, 9634–9641 CAS.
- Z.-X. Wang, H. Zhang, F. Wang, H. Cheng, W.-H. He, Y.-H. Liu, X.-Q. Huang and P.-F. Li, Superior Transverse Piezoelectricity in a Halide Perovskite Molecular Ferroelectric Thin Film, J. Am. Chem. Soc., 2020, 142, 12857–12864 CrossRef CAS.
- C. K. Yang, W. N. Chen, Y. T. Ding, J. Wang, Y. Rao, W. Q. Liao, Y. Y. Tang, P. F. Li, Z. X. Wang and R. G. Xiong, The First 2D Homochiral Lead Iodide Perovskite Ferroelectrics: [R-and S-1-(4-Chlorophenyl) ethylammonium] 2PbI4, Adv. Mater., 2019, 31, 1808088 CrossRef.
- Y. Ai, X.-G. Chen, P.-P. Shi, Y.-Y. Tang, P.-F. Li, W.-Q. Liao and R.-G. Xiong, Fluorine Substitution Induced High Tc of Enantiomeric Perovskite Ferroelectrics: (R)- and (S)-3-(Fluoropyrrolidinium)MnCl3, J. Am. Chem. Soc., 2019, 141, 4474–4479 CrossRef CAS.
- C. Shi, L. Ye, Z.-X. Gong, J.-J. Ma, Q.-W. Wang, J.-Y. Jiang, M.-M. Hua, C.-F. Wang, H. Yu, Y. Zhang and H.-Y. Ye, Two-Dimensional Organic–Inorganic Hybrid Rare-Earth Double Perovskite Ferroelectrics, J. Am. Chem. Soc., 2020, 142, 545–551 CrossRef CAS.
- P.-P. Shi, S.-Q. Lu, X.-J. Song, X.-G. Chen, W.-Q. Liao, P.-F. Li, Y.-Y. Tang and R.-G. Xiong, Two-Dimensional Organic–Inorganic Perovskite Ferroelectric Semiconductors with Fluorinated Aromatic Spacers, J. Am. Chem. Soc., 2019, 141, 18334–18340 CrossRef CAS.
- H. Fu and R. E. Cohen, Polarization rotation mechanism for ultrahigh electromechanical response in single-crystal piezoelectrics, Nature, 2000, 403, 281–283 CrossRef CAS.
- B. Morosin and E. Graber, Crystal structure of tetramethylammonium manganese (II) chloride, Acta Crystallogr., 1967, 23, 766–770 CrossRef CAS.
- W.-Q. Liao, Y.-Y. Tang, P.-F. Li, Y.-M. You and R.-G. Xiong, Competitive halogen bond in the molecular ferroelectric with large piezoelectric response, J. Am. Chem. Soc., 2018, 140, 3975–3980 CrossRef CAS.
- W.-Q. Liao, Y.-Y. Tang, P.-F. Li, Y.-M. You and R.-G. Xiong, Large piezoelectric effect in a lead-free molecular ferroelectric thin film, J. Am. Chem. Soc., 2017, 139, 18071–18077 CrossRef CAS.
- W.-Q. Liao, D. Zhao, Y.-Y. Tang, Y. Zhang, P.-F. Li, P.-P. Shi, X.-G. Chen, Y.-M. You and R.-G. Xiong, A molecular perovskite solid solution with piezoelectricity stronger than lead zirconate titanate, Science, 2019, 363, 1206–1210 CrossRef CAS.
- Y. Zhang, X.-J. Song, Z.-X. Zhang, D.-W. Fu and R.-G. Xiong, Piezoelectric Energy Harvesting Based on Multiaxial Ferroelectrics by Precise Molecular Design, Matter, 2020, 2, 697–710 CrossRef.
- X.-G. Chen, X.-J. Song, Z.-X. Zhang, P.-F. Li, J.-Z. Ge, Y.-Y. Tang, J.-X. Gao, W.-Y. Zhang, D.-W. Fu, Y.-M. You and R.-G. Xiong, Two-Dimensional Layered Perovskite Ferroelectric with Giant Piezoelectric Voltage Coefficient, J. Am. Chem. Soc., 2020, 142, 1077–1082 CrossRef CAS.
- H.-Y. Ye, Y.-Y. Tang, P.-F. Li, W.-Q. Liao, J.-X. Gao, X.-N. Hua, H. Cai, P.-P. Shi, Y.-M. You and R.-G. Xiong, Metal-free three-dimensional perovskite ferroelectrics, Science, 2018, 361, 151–155 CrossRef CAS.
- H. Wang, H. Liu, Z. Zhang, Z. Liu, Z. Lv, T. Li, W. Ju, H. Li, X. Cai and H. Han, Large piezoelectric response in a family of metal-free perovskite ferroelectric compounds from first-principles calculations, npj Comput. Mater., 2019, 5, 17 CrossRef.
- Y. Chang, S. F. Poterala, Z. Yang, S. Trolier-McKinstry and G. L. Messing, 〈001〉 textured (K0.5Na0.5)(Nb0.97Sb0.03)O3 piezoelectric ceramics with high electromechanical coupling over a broad temperature range, Appl. Phys. Lett., 2009, 95, 232905 CrossRef.
- T. Suwannasiri and A. Safari, Effect of Rare-Earth Additives on Electromechanical Properties of Modified Lead Titanate Ceramics, J. Am. Ceram. Soc., 1993, 76, 3155–3158 CrossRef CAS.
- R. E. Newnham, L. Bowen, K. Klicker and L. Cross, Composite piezoelectric transducers, Mater. Des., 1980, 2, 93–106 CrossRef CAS.
- S. Zhang, S.-M. Lee, D.-H. Kim, H.-Y. Lee and T. R. Shrout, Temperature dependence of the dielectric, piezoelectric, and elastic constants for Pb(Mg1/3Nb2/3)O3-PbZrO3-PbTiO3 piezocrystals, J. Appl. Phys., 2007, 102, 114103 CrossRef.
- Y. Yan, J. E. Zhou, D. Maurya, Y. U. Wang and S. Priya, Giant piezoelectric voltage coefficient in grain-oriented modified PbTiO3 material, Nat. Commun., 2016, 7, 1–10 Search PubMed.
- M. Li, H. J. Wondergem, M.-J. Spijkman, K. Asadi, I. Katsouras, P. W. Blom and D. M. De Leeuw, Revisiting the δ-phase of poly (vinylidene fluoride) for solution-processed ferroelectric thin films, Nat. Mater., 2013, 12, 433–438 CrossRef CAS.
| This journal is © the Partner Organisations 2021 |




