Visualizing changes of molecular conformation in the solid-state by a common structural determination technique: single crystal X-ray diffraction†
Jun
Zhang‡
 abc,
Haoke
Zhang‡
abc,
Haoke
Zhang‡
 abd,
Junkai
Liu‡
ab,
Jacky Wing Yip
Lam
ab and
Ben Zhong
Tang
abd,
Junkai
Liu‡
ab,
Jacky Wing Yip
Lam
ab and
Ben Zhong
Tang
 *abdef
*abdef
aDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction and Institute for Advanced Study, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, People's Republic of China. E-mail: tangbenz@ust.hk
bHKUST-Shenzhen Research Institute, No. 9 Yuexing 1st Rd, South Area, Hi-tech Park, Nanshan, Shenzhen 518057, China
cSchool of Materials and Chemical Engineering, Anhui Jianzhu University, Hefei 230601, People's Republic of China
dDepartment of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
eCenter for Aggregation-Induced Emission, State Key Laboratory of Luminescent Materials and Devices, SCUT-HKUST Joint Research Institute, South China University of Technology, Tianhe Qu, Guangzhou 510640, China
fAIE Institute, Guangzhou Development District, Huangpu, Guangzhou 510530, China
First published on 5th October 2020
Abstract
Changes of molecular conformation in the solid state play a vital role in many advanced technologies. Single-crystal X-ray diffraction (XRD), serving as a common structural determination technique, has been widely used to perform structural analysis. In this work, variable-temperature XRD characterization has been utilized to “see” the static conformational change (SCC) and dynamic trajectory (DT) of tetraphenylethylene (TPE) in the solid state, which exhibits an aggregation-induced emission (AIE) effect. Five kinds of motions, stretching, torsion, twisting, rocking, and wagging, are observed and analyzed. In terms of the SCC, the middle double bond in TPE becomes short and twisted and the peripheral phenyl rings as a whole tend to be more twisted with increase of the testing temperature (150 to 298 K). For the DT, phenyl-ring rocking and wagging are found to be more and more vigorous along with the increase of temperature, which is closely related to the synchronously decreased luminescent intensity. This work affords direct structural evidence for the AIE mechanism of restriction of intramolecular motions and provides a new platform to investigate the photophysical processes.
Motions play essential roles in many different areas, e.g., chemistry, biology, and engineering.1,2 In the long process of human history, people have hardly ever stopped exploring the laws of motion. After long-term and persistent efforts, physicists built lots of classical and quantum mechanics to describe the motions in various scales ranging from the macroscopic universe to microscopic quarks, and also in different dimensions (e.g. 1D, 2D, and 3D). In essence, motions at the molecular level determine the properties of materials. The activity of molecular motion has a direct relationship with the existing forms of materials. For example, the synergistic molecular motion results in a non-centrosymmetric structure from a centrosymmetric one to produce phase transition, further from the novel material with strong second harmonic generation (SHG) response.3 Generally, a gaseous molecule shows a vigorous motion and the molecular motion is fairly restricted in the solid state. Undoubtedly, molecular motions, especially in the solid state, are capable of doing lots of useful studies.4–8 However, it is still a tough task to visualize and manipulate the structural change at the molecular level in the solid-state, which impedes its further applications.
Since the change of molecular conformation is so important, researchers have investigated it for a long time and developed many methods to visualize this process, i.e. spectroscopies (nuclear magnetic resonance (NMR), photoluminescence (PL), and absorption etc.),9–11 and microscopies (atomic force microscopy (AFM), scanning tunneling microscope (STM), and cryogenic electron microscopy (cryo-EM) etc.).12 However, it is still difficult, if not impossible, to realize the in situ and real-time monitoring of the conformational change at the molecular level in the solid state. For example, the obtained NMR spectra can be utilized to speculate the motional molecules with frequency, enthalpy and entropy,13 and the STM can really “see” the molecular motion, but they don’t supply the variation of the bond length/angle to build dynamic structural changes currently. Moreover, these microscopic measurements are always expensive and complicated, and the sample needs to be pretreated and fixed before the characterization, which may destroy their original structures. So, it is necessary and urgent to find a common structural determination method that can offer a platform to visualize the solid-state molecular motion.
The properties of materials should be revealed from their microscopic structures at the atomic level. Single-crystal X-ray diffraction (XRD) is a common and powerful technique to determine the molecular structure at the atomic level,14 which is also used to reveal the structural changes to investigate the properties, such as anisotropic change in the magnetic susceptibility,15 thermal hysteresis,16 organic ferroelectrics,17 phase transition,3etc. It is possible to obtain continuous dynamic trajectories of molecular motion by assembling each static configuration for the structure at different temperatures. In general, the identified atom position in XRD measurement is the average result represented as ellipsoids displaying atomic motions.18 The location distribution in ellipsoids is caused by active molecular motion which is a thorny problem for researchers. In order to obtain an accurate structure, technicians will decrease the testing temperature to slow the conformational change. Instead, can we visualize the trajectory of molecular motion by analyzing the distances, angles, and torsions? If it works for the crystalline materials, it will be a real in situ and real-time method to “see” solid-state molecular motion at the atomic level. Furthermore, the motion amplitude or even direction can be easily tuned by the ambient temperature.
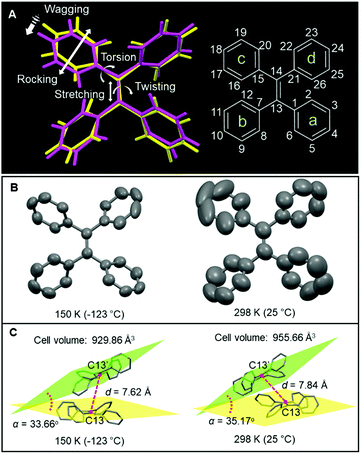
The best candidate to perform molecular motion should have a twisted structure which possesses large free volume. Tetraphenylethylene (TPE) is a typical molecular motor which shows aggregation-induced emission (AIE) effect.19 Its structure was first given in 1975 by Hoekstra et al.,20 and some excellent works about TPE were still reported recently.21–23 Previous studies reported that the molecular motion in TPE showed a direct relationship with its photoluminescence (PL) properties.20 In solution, TPE has a vigorous motion and most of the excited-state energy dissipates through a nonradiative decay pathway. Then the formation of aggregates restricts the molecular motion and strong emission is triggered. By combining experimental and computational results, restriction of intramolecular motion (RIM) is proposed as the working mechanism of AIE.24,25 The emission intensity of TPE increases with the decrease of temperature, which indicated that the molecular motions were restricted at low temperature,26 so investigating the temperature-dependent structure of TPE could provide further insight into the mechanism of AIE. Variable-temperature XRD has been used to investigate the structural variation. Based on these results, is it possible to build a direct relationship between the XRD structural analysis and photophysical performances?18 As shown in Fig. 1A, the molecular motion in TPE is divided into two categories, e.g. distance and angles. The change of distance can be caused by wagging and stretching, and the twisting/torsion/rocking are reflected in the variation of angles. Traditionally, the identification of these motions is based on speculation and they are seldom “seen” by a visual method.27 In this work, variable-temperature XRD was used to visualize the conformational change in the TPE crystal since the molecular conformation is sensitive to the surrounding temperature. As a result, with the temperature increasing from 150 to 298 K, the ellipsoid becomes bigger which suggests a more intense atomic motion at 298 K (Fig. 1B). Meanwhile, the cell volume is enlarged to 955.66 Å3 from 929.86 Å3 accompanied by the intermolecular distance (d) increasing from 7.62 to 7.84 Å. The interplanar angle (α) also exhibits a slight increase, suggesting that the molecular motion involves both inter- and intramolecular modes (Fig. 1C). This work affords direct structural evidence for the AIE mechanism of restriction of intramolecular motions and provides a new platform to investigate the photophysical processes.
A suitable crystal of TPE was picked out by microscopy to carry out XRD under various temperatures from 150 K (−123 °C) to 298 K (25 °C) with an interval of 15 K. Its crystal structures at each temperature were all perfectly determined without any disorder (Fig. S1–S11, ESI†),28,29 which indicates that the strcuctural variation of the bond length/angle is generated from the molecular motion, and not the molecular disordered packing. Firstly, the double bond-related motions were analyzed and the changes of bond length and dihedral angle with temperature were plotted (Fig. 2). Fig. 2A suggests that the double-bond length (C13–C14) decreased from 1.357 to 1.350 Å along with raising the temperature from 150 K to room temperature (RT) in accordance with the derivative of TPE.14 At the same time, the torsion angle of the double bond (C1C13C7 ∠ C15C14C21) increased from 8.9 to 9.9°, resulting in a more twisted conformation (Fig. 2B).
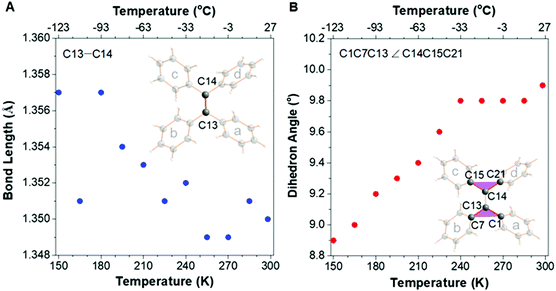 | ||
Fig. 2 Plots of the changes of (A) double-bond (C13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C14) length and (B) double-bond torsion angle (planes C1C13C7 to C15C14C21) along with the temperature. C14) length and (B) double-bond torsion angle (planes C1C13C7 to C15C14C21) along with the temperature. | ||
In addition to the double bond-related motions, the torsion angles of four peripheral phenyl rings a, b, c, and d were also analyzed (Fig. 3). Rings c and d were connected by atom C14 which showed a large steric hindrance, which is the same as the situation in rings b and a. With the temperature rising from 150 to 298 K, the torsion angle of ring c (C16C15C14C13) increased by 1.2° accompanied by the decrease of that in ring d (C22C21C14C13) (around −0.5°). Similarly, there is a 3.0° increase in torsion angle C8C7C13C14 (ring b) and the torsion angle of ring a (C2C1C13C14) simultaneously decreased from 47.8° to 45.4°.
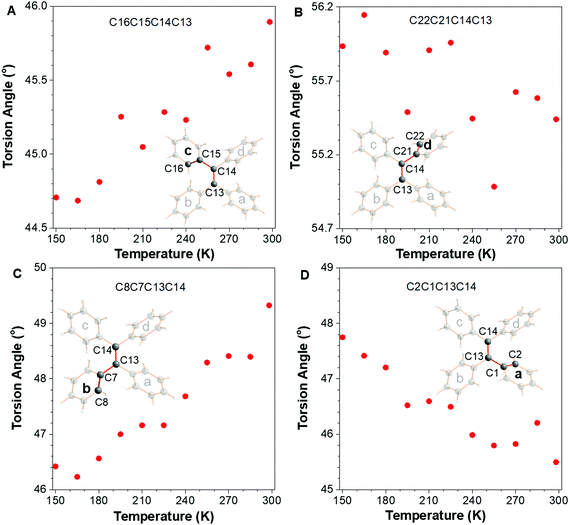 | ||
| Fig. 3 The plots of torsion angle of (A) ring c, (B) ring d, (C) ring b, and (D) ring a in TPE versus the change of temperature. | ||
The steric hindrance between rings b and c (also a and d) is smaller than that of rings c and d (a and b), which is the reason why the variation tendency of ring c is similar to ring b but opposite to ring d so as to decrease the intramolecular steric hindrance. Meanwhile, rings a and d became planar concurrently and b and c became more twisted. Overall, the peripheral phenyl rings as a whole become more twisted with the increase of temperature (Δ = 1.2°–0.5° + 3.0°–2.4° = 1.3°). (C22C21C14C13) (around −0.5°). The above mentioned results showed the “static” conformational change with the raising of temperature. In other words, the motion is represented by the conformational change under different temperatures. How about the “dynamic” molecular trajectory with variation of temperature? In order to answer this question, the sites of the molecule at different temperatures were all split and refined over two sites with site occupancy factors closed to 0.5, which represented the two extreme positions for the motion. The distance between these two positions can reflect the amplitude of the “dynamic” molecular trajectory. As shown in Fig. 4, the outermost hydrogens of H18, H24, H10, and H4 in the peripheral phenyl rings were split to generate two extreme positions A and B. The distances of H18A vs. H18B, H24A vs. H24B, H10A vs. H10B and H4A vs. H4B are 0.67, 0.44, 0.44, and 0.46 Å at 150 K, respectively. Then the rocking of the phenyl rings becomes more and more vigorous with the increase of testing temperature. At RT, the distances mentioned above increase to 0.89, 0.55, 0.59, and 0.59 Å, respectively. For most of the AIEgens, their solid-state photoluminescence is enhanced once their surrounding temperature is decreased, which is usually ascribed to the effect of restriction of intramolecular motion, indicating that the low temperature could partially freeze the molecular motions. Although the photoluminescence-related motions are in the excited state, the XRD results in this work provide compelling structural evidence for the RIM mechanism. According to the aforementioned structural changes, the temperature-dependent trajectory of molecular motion for TPE is displayed, demonstrating that the molecular motions were gradually restricted with the decrease of temperature. Combing the enhancement of PL intensity and the restricted dynamic trajectory with the decrease of temperature,26 the mechanism of RIM for AIE is further confirmed.30
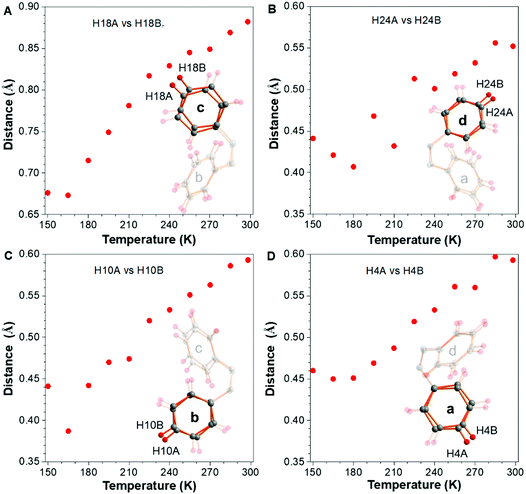 | ||
| Fig. 4 The vibration amplitude of the terminal hydrogens (A) H18, (B) H24, (C) H10, and (D) H4 under different temperatures. | ||
Conclusions
In summary, using XRD, the changes of molecular conformation in the representative AIEgen of TPE were visualized successfully. Five kinds of motions, e.g. bond stretching, double-bond torsion, phenyl-ring twisting, rocking, and wagging, have been monitored in this work. From the viewpoint of thermodynamics, these molecular motions were systematically divided into “static” configuration and “dynamic” trajectory. In terms of the static configurations, with the increase of ambient temperature, the middle double bond in TPE became short and twisted. Meanwhile, the torsion angles of the peripheral phenyl rings also showed increase or decrease but all the phenyl rings as a whole were found to be more twisted. Dynamic molecular trajectory was also observed through crystal splitting and refining, which is represented by the rocking and wagging. As expected, these kinds of dynamic trajectories tended to be more and more vigorous with the increase of temperature, which provided direct evidence for the RIM mechanism of AIE. It is noteworthy that intermolecular motions were also observed in TPE crystals in addition to these intramolecular modes. In general, the complicated molecular motions were clearly visualized so as to provide a new platform to investigate the photophysical processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (21788102 and 21671003), the Research Grants Council of Hong Kong (16308016, C6009-17G and A-HKUST 605/16), the Innovation and Technology Commission (ITC-CNERC14SC01) and the Science and Technology Plan of Shenzhen (JCYJ20160229205601482 and JCY20170818113602462).Notes and references
- M. Gao, C. Lu, H. Jean-Ruel, L. C. Liu, A. Marx, K. Onda, S.-Y. Koshihara, Y. Nakano, X. Shao, T. Hiramatsu, G. Saito, H. Yamochi, R. R. Cooney, G. Moriena, G. Sciaini and R. J. D. Miller, Mapping Molecular Motions Leading To Charge Delocalization With Ultrabright Electrons, Nature, 2013, 496, 343–346 CrossRef CAS.
- V. Garcia-Lopez, F. Chen, L. G. Nilewski, G. Duret, A. Aliyan, A. B. Kolomeisky, J. T. Robinson, G. Wang, R. Pal and J. M. Tour, Molecular Machines Open Cell Membranes, Nature, 2017, 548, 567–572 CrossRef CAS.
- J. Zhang, M. Abudoureheman, X. Ma, W. Kong, X. Xuan and S. Pan, Controllable Synthesis And Spontaneous Phase Transition Of Photonic Coordination Polymer To Produce A Strong Second-Harmonic Generation Response, Sci. China Mater., 2020, 63, 1272–1278 CrossRef CAS.
- S. Liu, Y. Li, H. Zhang, Z. Zhao, X. Lu, J. W. Y. Lam and B. Z. Tang, Molecular Motion In The Solid State, ACS Mater. Lett., 2019, 1, 425–431 CrossRef CAS.
- P. Alam, N. L. C. Leung, Y. Cheng, H. Zhang, J. Liu, W. Wu, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Spontaneous And Fast Molecular Motion At Room Temperature In The Solid State, Angew. Chem., Int. Ed., 2019, 58, 4536–4540 CrossRef CAS.
- A. Comotti, S. Bracco and P. Sozzani, Molecular Rotors Built In Porous Materials, Acc. Chem. Res., 2016, 49, 1701–1710 CrossRef CAS.
- A. Ryabchun, Q. Li, F. Lancia, I. Aprahamian and N. Katsonis, Shape-Persistent Actuators From Hydrazone Photoswitches, J. Am. Chem. Soc., 2019, 141, 1196–1200 CrossRef CAS.
- S. Liu, X. Zhou, H. Zhang, H. Ou, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, Molecular Motion In Aggregates: Manipulating Tict For Boosting Photothermal Theranostics, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS.
- J. Kaleta, J. Chen, G. Bastien, M. Dracinsky, M. Masat, C. T. Rogers, B. L. Feringa and J. Michl, Surface Inclusion Of Unidirectional Molecular Motors In Hexagonal Tris(O-Phenylene)Cyclotriphosphazene, J. Am. Chem. Soc., 2017, 139, 10486–10498 CrossRef CAS.
- L. Pejov, M. K. Panda, T. Moriwaki and P. Naumov, Probing Structural Perturbation In A Bent Molecular Crystal With Synchrotron Infrared Microspectroscopy And Periodic Density Functional Theory Calculations, J. Am. Chem. Soc., 2017, 139, 2318–2328 CrossRef CAS.
- H. Zhang, J. Z. Sun, J. Liu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Visualizing And Monitoring Interface Structures And Dynamics By Luminogens With Aggregation-Induced Emission, J. Appl. Phys., 2019, 126, 050901 CrossRef.
- T. Kudernac, N. Ruangsupapichat, M. Parschau, B. Macia, N. Katsonis, S. R. Harutyunyan, K.-H. Ernst and B. L. Feringa, Electrically Driven Directional Motion Of A Four-Wheeled Molecule On A Metal Surface, Nature, 2011, 479, 208–211 CrossRef CAS.
- S. Perez-Estrada, B. Rodriguez-Molina, E. F. Maverick, S. I. Khan and M. A. Garcia-Garibay, Throwing In A Monkey Wrench To Test And Determine Geared Motion In The Dynamics Of A Crystalline One-Dimensional (1d) Columnar Rotor Array, J. Am. Chem. Soc., 2019, 141, 2413–2420 CrossRef CAS.
- N. B. Shustova, T.-C. Ong, A. F. Cozzolino, V. K. Michaelis, R. G. Griffin and M. Dinca, Phenyl Ring Dynamics In A Tetraphenylethylene-Bridged Metal–Organic Framework: Implications For The Mechanism Of Aggregation-Induced Emission, J. Am. Chem. Soc., 2012, 134, 15061–15070 CrossRef CAS.
- Z.-S. Yao, S.-Q. Wu, Y. Kitagawa, S.-Q. Su, Y.-G. Huang, G.-L. Li, Z.-H. Ni, H. Nojiri, Y. Shiota, K. Yoshizawa, S. Kang, S. Kanegawa and O. Sato, Anisotropic Change In The Magnetic Susceptibility Of A Dynamic Single Crystal Of A Cobalt(I) Complex, Angew. Chem., Int. Ed., 2017, 56, 717–721 CrossRef CAS.
- X.-L. Wang, J.-P. Xue, X.-P. Sun, Y.-X. Zhao, S.-Q. Wu, Z.-S. Yao and J. Tao, Giant Single-Crystal Shape Transformation With Wide Thermal Hysteresis Actuated By Synergistic Motions Of Molecular Cations And Anions, Chem. – Eur. J., 2020, 26, 6778–6783 CrossRef CAS.
- Z.-S. Yao, K. Yamamoto, H.-L. Cai, K. Takahashi and O. Sato, Above Room Temperature Organic Ferroelectrics: Diprotonated 1,4-Diazabicyclo[2.2.2]Octane Shifts Between Two 2-Chlorobenzoates, J. Am. Chem. Soc., 2016, 138, 12005–12008 CrossRef CAS.
- J. D. Dunitz, E. F. Maverick and K. N. Trueblood, Atomic Motions In Molecular Crystals From Diffraction Measurements, Angew. Chem., Int. Ed. Engl., 1988, 27, 880–895 CrossRef.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS.
- A. Hoekstra and A. Vos, The Crystal and Molecular Structures Of Tetraphenylhydrazine And Related Compounds At –160 °C. Ii. The Crystal Structures of Tetraphenylethylene (Tpe) And Diphenylaminotriphenylmethane (Dtm), Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 1716–1721 CrossRef.
- J. Xiong, X. Li, C. Yuan, S. Semin, Z. Yao, J. Xu, T. Rasing and X.-H. Bu, Wavelength Dependent Nonlinear Optical Response Of Tetraphenylethene Aggregation-Induced Emission Luminogens, Mater. Chem. Front., 2018, 2, 2263–2271 RSC.
- Y.-J. Jin, H. Kim, J. J. Kim, N. H. Heo, J. W. Shin, M. Teraguchi, T. Kaneko, T. Aoki and G. Kwak, Asymmetric Restriction Of Intramolecular Rotation In Chiral Solvents, Cryst. Growth Des., 2016, 16, 2804–2809 CrossRef CAS.
- D. Li, R. Hu, D. Guo, Q. Zang, J. Li, Y. Wang, Y.-S. Zheng, B. Z. Tang and H. Zhang, Diagnostic Absolute Configuration Determination Of Tetraphenylethene Core-Based Chiral Aggregation-Induced Emission Compounds: Particular Fingerprint Bands In Comprehensive Chiroptical Spectroscopy, J. Phys. Chem. C, 2017, 121, 20947–20954 CrossRef CAS.
- N. L. C. Leung, N. Xie, W. Yuan, Y. Liu, Q. Wu, Q. Peng, Q. Miao, J. W. Y. Lam and B. Z. Tang, Restriction Of Intramolecular Motions: The General Mechanism Behind Aggregation-Induced Emission, Chem. – Eur. J., 2014, 20, 15349–15353 CrossRef CAS.
- H. Zhang, J. Liu, L. Du, C. Ma, N. L. C. Leung, Y. Niu, A. Qin, J. Sun, Q. Peng, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, J. W. Y. Lam, K. S. Wong, D. L. Phillips and B. Z. Tang, Drawing a Clear Mechanistic Picture For The Aggregation-Induced Emission Process, Mater. Chem. Front., 2019, 3, 1143–1150 RSC.
- E. P. J. Parrott, N. Y. Tan, R. Hu, J. A. Zeitler, B. Z. Tang and E. Pickwell-MacPherson, Direct Evidence To Support The Restriction Of Intramolecular Rotation Hypothesis For The Mechanism Of Aggregation-Induced Emission: Temperature Resolved Terahertz Spectra of Tetraphenylethene, Mater. Horiz., 2014, 1, 251–258 RSC.
- Y.-J. Gao, X.-P. Chang, X.-Y. Liu, Q.-S. Li, G. Cui and W. Thiel, Excited-State Decay Paths In Tetraphenylethene Derivatives, J. Phys. Chem. A, 2017, 121, 2572–2579 CrossRef CAS.
- J. Zhang, X. Ma and X. Xuan, Disordered Fragments Of Chemical Structures Based On Single-Crystal X-Ray Diffraction, Chin. J. Struct. Chem., 2020, 39, 698–708 CrossRef CAS.
- A. Hoekstra and A. Vos, The Crystal And Molecular Structures Of Tetraphenylhydrazine And Related Compounds at −160 °C. II. The Crystal Structures Of Tetraphenylethylene (TPE) And Diphenylaminotriphenylmethane (DTM), Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 1722–1729 CrossRef.
- H. Zhang, Z. Zhao, A. T. Turley, L. Wang, P. R. McGonigal, Y. Tu, Y. Li, Z. Wang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregate Science: From Structures To Properties, Adv. Mater., 2020, 32, 2001457 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm00754d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |