A brief review on novel pyrene based fluorometric and colorimetric chemosensors for the detection of Cu2+
Zannatul
Kowser
ab,
Ummey
Rayhan
ac,
Thamina
Akther
a,
Carl
Redshaw
 d and
Takehiko
Yamato
d and
Takehiko
Yamato
 *a
*a
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Saga University, Honjo-machi 1, Saga 840-8502, Japan. E-mail: yamatot@cc.saga-u.ac.jp
bDepartment of Chemistry, Faculty of Science, Jashore University of Science and Technology, Jashore-7408, Bangladesh
cDepartment of Chemistry, Dhaka University of Engineering & Technology, Gazipur-1700, Bangladesh
dDepartment of Chemistry, The University of Hull, HU6 7RX, UK
First published on 12th January 2021
Abstract
The development of colorimetric and fluorometric chemosensors that capable of detecting Cu2+ ions by a change in colour and fluorescence intensity has been described. Herein, chemosensors having pyrene functional groups as a signaling moiety are discussed in detail as pyrene derivatives show significant photophysical properties being superior to those of other commonly used scaffolds. This review article provides a detailed overview of pyrene containing chemosensors based on fluorescence mechanisms, such as excimer/exciplex formation, photoinduced electron transfer (PET), photoinduced charge transfer (PCT), aggregation induced emission (AIE), ligand to metal charge transfer process (LMCT), chelation enhanced quenching mechanism (CHEQ), Cu2+-selective reactions for the selective and sensitive detection of Cu2+. Potential future applications are also discussed because of the fact Cu2+ ion recognition has a great significance in the biological, environmental and medical sectors.
1. Introduction
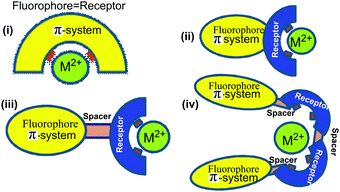
The development of fluorescent sensors has received increasing attention in recent years due to their simplicity and low detection limits.1 For the determination of copper ions, various techniques such as atomic absorption spectrometry,2 inductively coupled plasma mass spectroscopy (ICPMS),3 inductively coupled plasma atomic emission spectrometry (ICP-AES),4 and voltammetry5 and anodic stripping voltammetry (ASV)6 have been applied. However, these methods cause operational difficulties, requiring well-trained personnel and high-cost analytical instrumentation. These techniques also lead to the destruction of the cells of biological samples and cannot be used to visualize biological phenomena in situ.7 For this reason, fluorescent sensors have emerged as a useful technique for high sensitivity, simplicity and for the non-destructive imaging of intracellular distribution for the detection of cations, anions and neutral species that are of great importance in the biological and environmental sectors.8–10Chemosensors are molecules of abiotic origin, which show changes of one or more properties such as colour (colorimetric chemosensors), fluorescence (fluorescent chemosensors) or redox potentials (electrochemical sensors) upon interaction with guest species.1,11–13 These sensors are mainly organic molecules, and may be categorized into four classes: (i) as fluorescent ligands which have identical receptor (the recognition site) and fluorophore (the signal source) (ii) as fluoroionophores where the fluorophore and receptor are involved in direct electronic conjugation, (iii) fluoroionophores combined via fluorophore–spacer–receptor systems, (iv) exciplex or excimer forming probes (EPs) where, the fluorophore and receptor units can construct an intramolecular exciplex or excimer (Fig. 1). Herein, strong intramolecular geometry changes are observed after binding with the analyte by increasing or decreasing the ratio of excimer-to-monomer emission.14,15 To be an ideal fluorescent chemosensor, the receptor must have the strongest affinity for the relevant target (binding-selectivity) and the fluorescence signal should avoid any environmental interference (signal-selectivity).16
Among the heavy and transition metal ions, copper is one of the important trace elements for both plants and animals, including humans.17 When levels of Cu2+ exceeds cellular needs, it can be considered as toxic to biological systems.18 The US Environmental Protection Agency (EPA) has set the limit of 1.3 ppm (∼20 μM) for copper in drinking water.14 For this reason, improved fluorescent chemosensors for the selective and sensitive detection of Cu2+ are of great importance.19–22 It is noteworthy that Cu2+ ion detection poses some challenges when designing fluorescence turn-on (fluorescence intensity increases) sensors due to its paramagnetic nature with an unfilled d orbital. Moreover, according to the Irving–Williams rule, Cu2+ has the strongest binding ability versus any other divalent metal ion of the first transition series. The paramagnetic cations Cu2+, Ni2+ and Co2+ are usually more strongly bound than the diamagnetic ions Zn2+ or Cd2+, which is determined on the basis of the ionic radius and the second ionization potential.14,23 Therefore, strong fluorescence “turn-off” (fluorescence intensity decreases) sensors were predominantly observed due to fluorescence quenching, upon addition of Cu2+.24,25 Recently, many turn-on sensors have been studied for Cu2+ detection by using the concept of ion-induced changes in the geometry or the flexibility of the ligand as well as from the availability of certain functional groups involved in fluorescence quenching for the ligand in the unbound state.14 In this review, we discuss the sensing mechanisms of Cu2+ with pyrene based fluorescent sensors. This will help shape the design of new pyrene chemosensors for copper ion determination based on different mechanisms such as excimer/exciplex formation, photoinduced electron transfer (PET), photoinduced charge transfer (PCT), aggregation induced emission (AIE), ligand to metal charge transfer process (LMCT), chelation enhanced quenching mechanism (CHEQ) and Cu2+-selective reactions.
2. The sources, applications, effects of Cu2+
2.1. Sources of copper
Copper is the third most abundant essential transition metal ion after iron and zinc in the human body.26 It is found in both natural sources and all body tissues. In the body, the liver, brain, heart, kidneys, and skeletal muscle contain the maximum amount of copper. High doses of copper are found in a commercially available multivitamins. Moreover, naturally rich sources of copper are exhibited in oysters, sesame seeds, tahini, cocoa powder, chocolate, nuts, calamari, lobster, sunflower seeds, sun dried tomatoes, roasted pumpkin, squash seeds and dried herbs, etc. Additionally, because of copper plumbing, it is present in water. In most of the vegetarian diets, copper is present in the food items.272.2. Applications of copper
Copper is an important trace element for human metabolism and plays a vital role in the physiological processes of organisms. It contributes to the formation of red blood cells and in maintaining nerve cells and the immune system. It also helps to generate energy in the body and plays a significant role for collagen production and the absorption of iron. Copper intake also reduces the chance of cardiovascular disease and osteoporosis. Therefore, for normal development and proper working of the brain and as a cofactor of many enzymes, the presence of copper is essential.272.3. Deficiency and toxicity of copper
Copper present at low or high concentrations can increase the risk to health. Free Cu2+ ion is both acutely and chronically toxic for aquatic life and microorganisms, even at micromolar concentrations. Copper deficiency is associated with growth failure and can affect deterioration of the nervous system. However, an excessive accumulation of copper may lead to neurodegenerative disorders, abnormalities in red blood cells and heart problems. Gene mutations are responsible for the two major genetic disorders of copper metabolism in humans such as Menkes' disease and Wilson's disease, and it is found that these diseases are the result of excessive intracellular copper transport. Increased serum copper levels have been linked to a higher risk of cardiovascular disease. Different diseases, including Alzheimer's disease, Indian childhood cirrhosis (ICC) and prion disease are also associated with the toxicity of Cu2+. Besides, copper is one of the most common metal pollutants because of its huge applications in our daily lives. For adults (19 years and above), the upper limit is 10 mg a day, above this it is considered toxic. Moreover, Cu2+ ions are often essential components in biochemical reactions such as catalysis, transport or biosynthesis at trace levels (<1 μM). However, in the presence of large amounts of Cu2+, unhealthy interactions occur in biochemical redox processes and can, for instance result in the inhibition of enzyme activity or nephrotoxicity.27,28 Therefore, it is necessary to design a technique that is effective for a rapid response toward Cu2+ even at micromolar concentrations. The design and synthesis of fluorescent chemosensors for copper ion determination could help resolve these issues.3. Pyrene and pyrene derivatives as fluorophore
Pyrene and pyrene derivatives have interesting photophysical properties notably a long lifetime, high quantum yield (τM = 450 ns and ΦM = 0.60 in cyclohexane) and expanded π electron delocalization of the pyrene monomers. Pyrene derivatives generally display five well-shaped and fine absorption bands between 210 and 330 nm and the emission spectra usually consist of a broad band centered at 400 nm. Fluorescence quenching of a pyrene fluorophore can easily occur in the presence of a variety of quenchers (heavy metals, anionic species and NPs), which may be due to through-bond and through-space energy transfer between pyrene and the quenchers.29 A pyrene based fluorescent “off–on” chemosensor has been developed for detection of cyanide via ligand to Cu2+ complex as Cu2+ generally performs as a fluorescence quencher via a PET mechanism.30 Pyrene also can perform as either an electron donor or acceptor during the energy transfer depending on the substituents attached to it, and as a result blue or red shifted emission bands are easily observed from the pyrene monomer emission band. One of the most interesting features of pyrene derivatives is that a pyrene monomer (λabs = 350 nm, λem = 398 nm) can combine with another pyrene monomer to form an excimer (or excited state dimer, λem = 485 nm) because of its affinity for strong π–π interactions.31 The changes in the emission properties of the pyrene excimer from a monomer has been used for designing sensors.32–34 A pyrene-based triazole ligand was reported which exhibited self-assembly in the presence of ZnCl2. The free ligand showed monomer emission bands at 382 and 402 nm with a broad shoulder at 420 nm. After addition of ZnCl2, it can bind with ZnCl2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio due to strong excimer emission at 410 nm.35
1 ratio due to strong excimer emission at 410 nm.35
4. Mechanism of Cu2+ sensing
Fluorescent chemosensors for Cu2+ detection have been designed based on different binding mechanisms which fulfill the criteria of affinity, selectivity and sensitivity (Table 1). In general, the chemosensor is designed such that it contains oxygen or nitrogen donor atoms for co-ordination of the Cu2+.36 In most of the cases, fluorescent sensors bind Cu2+ by the fluorescence quenching process due to its paramagnetic nature which opens up the excited state de-excitation pathways by enhancing the rate of non-radioactive processes such as electronic energy transfer (EET) and/or PET process involving the metal centers. Also, the free electron in the orbital is likely to quench the fluorescence via spin–orbit coupling.37 There is also an enhancement of the fluorescence intensity in turn-on sensors, which are more desirable than turn-off sensors, with a red or blue shift of the emission after Cu2+ ion binding with ligand. Recently, some new strategies have been developed for fluorescence off–on sensors for Cu2+ detection. The classical sensing mechanisms such as PET,38,39 PCT,40,41 excimer/exciplex formation42 are mainly mentioned when investigating binding phenomenon. There are also some mechanisms which control the response of a fluorophore to substrate binding including AIE, LMCT, CHEQ and Cu2+-assisted reactions.4.1. Excimer emission mechanism
When an excited fluorophore during its lifetime in an excited state interacts with a ground state fluorophore, an excimer is formed.43 Excimer emission typically shows a red shifted broad fluorescence band in most aromatic molecules,44 by about 6000 cm−1 to lower energies than the uncomplexed (“monomer”) fluorophore emission. There are two kinds of excimers based on the origin of the pyrene dimer: a dynamic excimer and a static excimer. The dynamic or static excimer formation depends on the distance between the two pyrene units (Fig. 2).45 The conventional dynamic excimers are produced due to the interaction of the diffused ground state fluorophore with the electronic excited state fluorophore during its lifetime within van der Waals contact distances. On the other hand, in case of the static excimer, initially a pyrene dimer is formed in the ground state. After photo-excitation, the monomer units interact with each other at very close distances (3.5–3.9 Å in the case of polycyclic hydrocarbons) to form the excimer. Importantly, metal ion co-ordination with the ligand controls the separation and relative orientation of multiple fluorophore units attached to the ligands. For this reason, cation recognition is investigated by the monomer to excimer fluorescence intensity ratio.1,46–50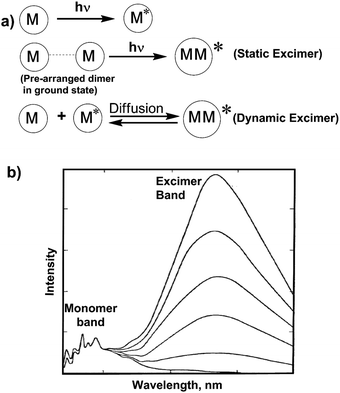 | ||
| Fig. 2 (a) Mechanism of static and dynamic excimer formation. (b) The monomer and excimer emission band of pyrene at various concentration in cyclohexane. | ||
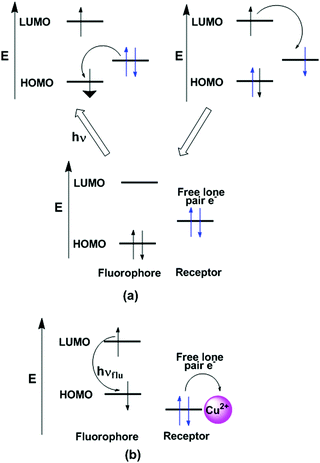
4.2. Photoinduced electron transfer (PET)
In the PET process, an excited electron is transferred from the donor to the acceptor. These ideas can also be explained with the help of molecular orbital energy diagrams when the molecular device is non-luminescent and luminescent (Fig. 3). The emission of photons from LUMO to HOMO after excitation of electrons is called fluorescence and the molecule is termed as a fluorophore. However, PET occurs when one electron from the HOMO of the electron donor is transferred efficiently to the hole in the HOMO of the fluorophore and the initially excited electron of the LUMO moves to the HOMO of the electron donor. This event happens if the HOMO of the electron donor is higher in energy than the singly occupied orbital of the fluorophore HOMO. The PET mechanism leads to nonradiative deactivation of the excited state, and as a result the emission intensity is decreased or “quenching” of fluorescence is observed.38,51–55 However, after interaction with metal ions, the donor electron containing orbital shifts its position from higher energy to lower energy and inhibits the electron-transfer process, resulting a radiative emission. In the case of metal ion binding, this effect is referred to as chelation-enhanced fluorescence (CHEF). It is observed that in detection of Cu2+ by the PET method, the pyrene containing chemosensor mostly exhibits a weak fluorescence due to fluorescence quenching from the nitrogen lone pairs onto pyrene. The binding of Cu2+ with the ligand prevents the PET mechanism, resulting in a significant enhancement in pyrene fluorescence.56,574.3. Photoinduced charge transfer (PCT)
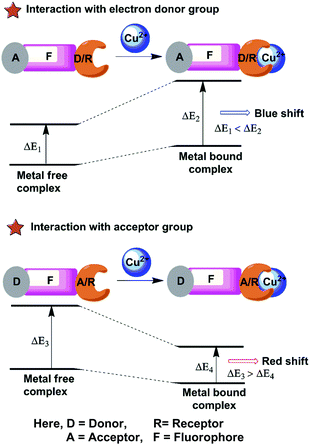
The chemosensors based on PCT or ICT are designed in which an electron withdrawing unit is conjugated with electron-donating substituents in the same molecule (Fig. 4). Therefore, the “push–pull” π electron system of the chemosensor occurs in the excited state58,59 and the charge transfer can occur over long distances through conjugation and is related with major dipole moment changes. This phenomenon is used for cation sensing, as the close interaction of cations with the donor or the acceptor moiety affects the photophysical properties of the fluorophore.60 Cation complexation of an electron donor group within a fluorophore decreases the electron-donating character of the donor group and produces a blue shift in the spectrum due to reduction of conjugation. On the other hand, a red-shifted absorption spectrum is observed for metal ion binding to the acceptor group that enhances its electron-withdrawing character.61 The fluorescence spectra should be shifted in the same direction as the absorption spectra. Receptors for the determination of Cu2+ sensing are designed from this PCT mechanism by using either the electron-donating, electron withdrawing ability or π-conjugation degree of the fluorophores.5. Chemosensors for sensing Cu2+
5.1. Chemosensors based on excimer emission
Calixarenes are an important class of phenol-based macrocycles having some unique properties, specifically a nonpolar cavity, that are able to encapsulate various guest species, and possess well-defined conformations with tunable functionalization of their lower and upper rims. Like calixarenes, thiacalix[4]arenes containing bridging sulfur atoms with their 3-dimensional structure are an ideal platform for the development of chemosensors.62 Thiacalix[4]arenes appended with a pyrene moiety have been successfully designed utilizing the intensity ratio of the monomer to excimer emission (IM/IE) of the pyrene moiety for the recognition of cations, anions or neutral molecules.63 In 2013, Kumar et al. developed a heteroditopic receptor based on a thiacalix[4]arene possessing 1,3-alternate conformation. The receptor has two urea linked pyrene moieties and a crown-ether moiety at the opposite sides of the thiacalix[4]arene cavity, and this exhibited ratiometric fluorescence for the selective recognition of F− and CN− ions in THF.64 In 2014, the Yamato group established a heterodimeric system based on a thiacalix[4]arene possessing a 1,3-alternate conformation which was capable of binding K+ ions and various anions via the crown-5-ring moiety and the two urea linked pyrene moieties in a CH2Cl2–DMSO solvent system. Moreover, a positive allosteric effect was observed for the formation of a heterogeneous dinuclear complex with Br− and K+ ions and a negative allosteric effect induces the decomplexation of the K+ ion from the crown-5 ring for the recognition of Cl−.65 However, very few ratiometric fluorescence sensors based on a thiacalix[4]arene have been designed for the recognition of Cu2+. For this purpose, in 2015 the Yamato group developed a ratiometric fluorescent chemosensor 1, in which an acetate group was introduced between the thiacalix[4]arene and triazolyl-pyrenyl groups in a 1,3-alternate conformation, which displays high selectivity toward Cu2+ ions (Fig. 5).66 The free ligand 1 exhibits strong face-to-face π–π stacking because of excimer emission at 484 nm and weaker monomer emissions at 379 and 397 nm. After gradual addition of Cu2+ ions (up to 20 equiv.), the monomer to excimer relative emission intensity ratio of 1 + Cu2+ increased 228-fold versus the monomer to excimer intensity ratio of the free ligand 1, which was 0.54. The ligand 1 at low concentrations causes excimer quenching and monomer enhancing due to the coordination bond of a Cu2+ ions between the nitrogen atoms of the triazole ring and the adjacent oxygen atoms of 1. This event forces the pyrenyl groups of 1 to move away from each other resulting in a conformational change. Notably, the monomer emission intensity of 1 was vividly reduced on addition of 5 to 45 equiv. of Cu2+ ions in EtOH solution, and this can be explained by a reverse PET from the pyrene unit to the nitrogen atoms of the triazole ring or to a heavy atom effect at high ionic solution strength. A Job's plot for the ligand to Cu2+ complexation revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with the detection limit 1.44 × 10−7 M. The IR spectra of 1 revealed that the strong absorption band for the triazole group had disappeared and the band for the –COO– group changed to a weak absorption after binding with Cu2+. Moreover, 1H NMR titration experiments for 1 were also consistent with reversibility characteristics as tested with Cu2+ and ethylenediamine. By using Density Functional Theory (DFT) computational studies, the geometry-optimized energies of 1 with Cu2+ complexes were calculated.
1 stoichiometry with the detection limit 1.44 × 10−7 M. The IR spectra of 1 revealed that the strong absorption band for the triazole group had disappeared and the band for the –COO– group changed to a weak absorption after binding with Cu2+. Moreover, 1H NMR titration experiments for 1 were also consistent with reversibility characteristics as tested with Cu2+ and ethylenediamine. By using Density Functional Theory (DFT) computational studies, the geometry-optimized energies of 1 with Cu2+ complexes were calculated.
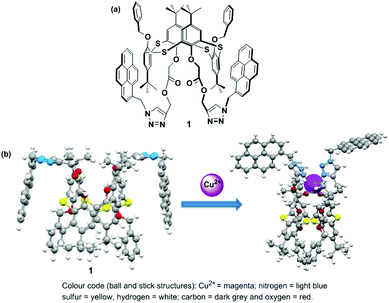 | ||
| Fig. 5 (a) The molecular structure of ligand 1; (b) geometry-optimized structures of 1 for Cu2+ in gas phase. Reprinted from ref. 66, with permission of Copyright 2015, Elsevier. | ||
Homooxacalix[3]-arenes with a basic C3-symmetric cavity are related to calixarenes and crown ethers. Recently, homooxacalix[3]-arenes appended with pyrene functionality have also been used for the development of novel fluorescence chemosensors.67 Our group reported a new type of fluorescent chemosensor based on a homooxacalix[3]arene which is connected with a pyrene moiety through a triazole group. The sensor exhibits a great sensitivity and selectivity for the recognition of Pb2+ compared with most other competitive metal ions apart from Cu2+ where quenching was observed in an aqueous organic solvent system.68 Bearing this in mind, we developed a novel ratiometric chemosensor 2 using a pyrene linked triazole modified homooxacalix[3]arene for the recognition of Hg2+ and Cu2+.69 The free ligand 2 exhibited fluorescence monomer and excimer emissions at wavelengths of 396 and 485 nm, respectively. The fluorescence spectral changes of the chemosensor 2 showed that the excimer and monomer emission of pyrene was dramatically quenched in the presence of Cu2+ and Hg2+ ions in pure acetonitrile solution. Interestingly, the monomer emission of 2 appeared to enhance with the addition of Cu2+ and Hg2+ to the organic/aqueous solutions. The time-dependent fluorescence spectral changes explained the detailed information about monomer enhancement of the 2 + Hg2+ and 2 + Cu2+ complexes at 396 nm in the presence of 5% water in acetonitrile solution. The binding phenomenon was further confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) and by 1H NMR spectroscopic titration experiments. The participation of water molecules in the complexation procedure of 2 with Hg2+ and Cu2+ inhibits the heavy atom effect and thereby the enhancement of monomer emission is observed. Similar phenomenon was observed using CH3OH, CH3CH2OH or 4,4′-bipyridine as solvent/melt for the 2 + Cu2+ and 2 + Hg2+ complexes (Fig. 6).
 | ||
| Fig. 6 (a) The heavy atom effect of 2 + M2+ complex blocked by solvent S; (b) fluorescence response of 2 + M2+ upon interaction with various solvents. | ||
Hexahomotrioxacalixarenes are defined as a class of synthetic macrocycles with phenolic units connected by CH2OCH2 bridges. By introducing different ionophores, homotrioxacalix[3]arenes have been used as potential receptors.70 The Yamato group incorporated a 2,2′-bipyridyl group attached via a carbonyl group at the upper rim and a diethylacetamide group at the lower rim of the hexahomotrioxacalix[3]arene that served as a tritopic receptor for Ag+, Li+ and Na+ ions in a cooperative fashion.71 Another representative example was reported by taking advantage of the excellent fluorescent properties of pyrene. In particular, we synthesized a pyrene-armed hexahomotrioxacalix[3]arene 3 as a ratiometric fluorescent sensor for the selective and sensitive detection of Cu2+via a Zn2+ or Cd2+ triggered synergistic effect in a CH3CN/CH2Cl2 solvent system.72 Furthermore, the ligand 3 with Cu2+ plays a vital role as an indirect sensor for F− recognition through demetallation. In this case, the fluorescence spectra of 3 in CH3CN/CH2Cl2 exhibit a comparatively strong excimer emission at 518 nm and a weak monomer emission at 415 nm, with an intensity ratio of monomer to excimer emission of 0.09. The addition of Cu2+ ions to a solution of 3 leads to a significant increase in the monomer emission and a comparative decrease in the excimer emission to reveal a ratiometric change from 0.09 to 4.36. These spectral changes of sensor 3 with Cu2+ can be ascribed to the cooperating effect of the geometrical structural changes and the reduced PET effect. The complex follows a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with a binding constant (3.57 ± 0.1) × 105 M−1. Furthermore, the 3 + Cu2+ complex displays a highly sensitive response at 415 nm to both Zn2+ and Cd2+ through a synergistic effect. In this case, the quantum yield of the 3 + Cu2+ complex is considerably greater than before (from 0.05 to 0.19) upon addition of Zn2+ or Cd2+. From the fluorescence and NMR spectroscopic titration experiments, the following can be proposed: (i) the nitrogen atom of ligand 3 interacts with both Cu2+ and Zn2+. The Cu2+ coordination with nitrogen of 3 inhibits the electron transfer from the nitrogen to the photo-excited pyrene moieties, resulting in the enhancement of monomer emission. (ii) The presence of both Zn2+ and Cu2+ further decreases the electron density at the nitrogen atom which leads to the significant increase in the monomer emission intensity of the pyrene moieties and forms a trimer complex. However, spectral changes suggest that ligand 3 can sequentially recognize Cu2+ and F− through a two-step process, namely a complexation approach between 3 and Cu2+, and then a Cu2+ displacement approach by F− to form CuF2. Therefore, sensor 3 can act as a multifunctional molecular device by constructing a fluorescence-based combinational logic gate using the Cu2+, Zn2+/Cd2+ and F− fluorescence responses (Fig. 7).
1 stoichiometry with a binding constant (3.57 ± 0.1) × 105 M−1. Furthermore, the 3 + Cu2+ complex displays a highly sensitive response at 415 nm to both Zn2+ and Cd2+ through a synergistic effect. In this case, the quantum yield of the 3 + Cu2+ complex is considerably greater than before (from 0.05 to 0.19) upon addition of Zn2+ or Cd2+. From the fluorescence and NMR spectroscopic titration experiments, the following can be proposed: (i) the nitrogen atom of ligand 3 interacts with both Cu2+ and Zn2+. The Cu2+ coordination with nitrogen of 3 inhibits the electron transfer from the nitrogen to the photo-excited pyrene moieties, resulting in the enhancement of monomer emission. (ii) The presence of both Zn2+ and Cu2+ further decreases the electron density at the nitrogen atom which leads to the significant increase in the monomer emission intensity of the pyrene moieties and forms a trimer complex. However, spectral changes suggest that ligand 3 can sequentially recognize Cu2+ and F− through a two-step process, namely a complexation approach between 3 and Cu2+, and then a Cu2+ displacement approach by F− to form CuF2. Therefore, sensor 3 can act as a multifunctional molecular device by constructing a fluorescence-based combinational logic gate using the Cu2+, Zn2+/Cd2+ and F− fluorescence responses (Fig. 7).
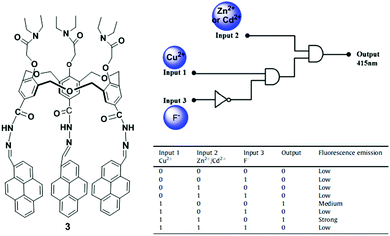 | ||
| Fig. 7 The molecular structure of receptor 3 and the combinational logic gate for 3 with truth table using Cu2+, Cd2+/Zn2+ and F− as chemical inputs. Reproduced from ref. 72 with permission from the Elsevier, copyright 2015. | ||
Li and co-workers have reported a pyrene based novel ratiometric fluorescent chemosensor for Cu2+ containing thiophene, namely compound 4 (Fig. 8).73 The monomer to excimer conversion of chemosensor 4 occurred on gradual addition of Cu2+, owing to intermolecular π–π stacked dimerization of the two pyrenes. The fluorescent emission of 4 (10 μM) in Tris–HNO3 buffer solution with high sensitivity and selectivity increases 127-fold with 2 equiv. of Cu2+ (20 μM). In addition, the detection of Cu2+ present in environmental samples such as river and pond water has been determined by using 4. Moreover, cytotoxicity tests on 4 (from 0 μM to 20 μM) using cells showed that more than 96% of the cells were viable which established the real applicability of the chemosensor 4 in biological samples. The association constant (Ka) for Cu2+ binding by chemosensor 4 was determined to be 2.18 × 104 M−1. There was a good linear relationship covering 1.0 × 10−7 to 2.0 × 10−5 M between the fluorescence ratio (I460/I375) of 4 and the concentration of Cu2+ with a detection limit of 2 × 10−8 M.
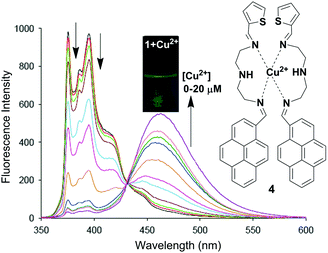 | ||
| Fig. 8 Proposed binding mechanism of chemosensor 4 with fluorescence colour changes. Fluorescence intensity changes of compound 4 (10 μM) with different concentrations of Cu2+ in Tris–HNO3 buffer solution at λexc = 342 nm. Reproduced from ref. 73 with permission from the Elsevier, copyright 2016. | ||
Two new chemosensors bearing either a single 5 or two 6 pyrene units linked by a flexible polyoxaethylene bridge have been developed that are sensitive to water and metal cations (Fig. 9).74 Compound 5 in dioxane showed a single monomer absorption and emission band, whilst compound 6 gave an absorption band in the 400–500 nm region with an emission band with maxima at ∼500 nm. In dioxane/water mixtures with xH2O = 0.29, the two probes exhibited a new band (with maxima varying from 405 to 490 nm) due to exciplex formation. Time-resolved experiments have been used to explain a two-state system for ligand 5, which involves the monomer and a charged species, which can potentially be assigned to an exciplex-like species, whereas with 6, a three-state system is involved. After addition of the metal ions (Cu2+, Zn2+ and Ag+) to probe 5 in dioxane followed by absorption and emission studies, a gradual quenching effect of the monomer emission was observed and this was significantly selective for Cu2+. In case of ligand 6, after the interaction with metal cations, the emission band decreases at approximately 550 nm and the monomer emission band increases at ∼450 nm. The binding ratio of ligand (5 and 6) to metal was proposed to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
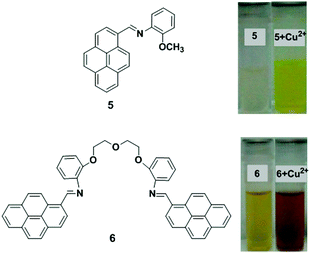 | ||
| Fig. 9 Molecular structures of receptors 5 and 6. Observed colour change 5 and 6 with Cu2+. Reproduced from ref. 74 with permission from the American Chemical Society, copyright 2013. | ||
A pentiptycene-bispyrenyl system 7 was developed from the reaction of pentiptycene hydroquinone with 1-bromomethylpyrene (Fig. 10).75 Yang et al. reported compound 7 as a selective and sensitive fluorescent chemosensor for Ca2+, Cd2+ and Cu2+. The fluorescence spectra of compound 7 exhibited typical monomer emission at 375 and 395 nm and an excimer emission at 475 nm in CH2Cl2. In particular, upon addition of Cu2+, a strong blue shifted (from 475 to 440 nm) pyrene excimer emission was observed with increased intensity. This behaviour of sensor 7 was clarified by the excitation spectra which clearly showed a significant red shift at Cu2+-induced 440 nm than 375 nm, due to the conversion of a partially overlapping static excimer from a sandwich-like dynamic excimer. Moreover, the 7 + Cu2+ complex at 440 nm has a short fluorescence lifetime (approximately 16-times) in comparison with 475 nm excimer emissions, which was explained by observation of the fluorescence decay times. Therefore, the radiative decay rate constant was larger for the static emission at 440 nm than the dynamic emission at 475 nm. In this case, Cu2+ binding with 7 brings the pyrene groups together in the ground state for the formation of static excimers and minimizes the relaxation event in the excited state leading to a blue-shifted excimer emission. The UV-vis absorption spectra of ligand 7 also exhibited a clear broadening of the spectrum with four defined isobestic points at 325, 331, 339 and 348 nm and suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with a binding constant of 4.4.
1 complex with a binding constant of 4.4.
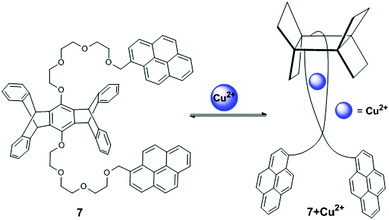 | ||
| Fig. 10 Structure of chemosensor 7 and possible binding modes for Cu2+. Reproduced from ref. 75 with permission from the American Chemical Society, copyright 2001. | ||
Kim et al. reported the mono-pyrenylalkylamine derivative 8.76 Upon addition of Cu2+, chemosensor 8 exhibited band broadening and a red-shift (from 342 to 455 nm) in a mixed solvent system of CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 11). These observations were explained by favourable intermolecular π–π stacking dimerization of the two pyrenes in the ground state. In the Job's plot measurements, the maximum point appeared at the mole fraction of 0.6, which suggested a 2
1) (Fig. 11). These observations were explained by favourable intermolecular π–π stacking dimerization of the two pyrenes in the ground state. In the Job's plot measurements, the maximum point appeared at the mole fraction of 0.6, which suggested a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal complex. Fluorescence spectral changes of 8 revealed high selectivity toward Cu2+ over other competitive species (Li+, Na+, K+, Rb+, Cs+, Mg2+, Ba2+, Ca2+, Sr2+, Ag+, Zn2+, Cd2+, Hg2+ and Pb2+). Moreover, the theoretical DFT calculations revealed that the pre-organized cavity is essential with two proximate nitrogen atoms of the sulfonamide groups to stabilize the complex of 8 with Cu2+ for static excimer emission.
1 ligand-to-metal complex. Fluorescence spectral changes of 8 revealed high selectivity toward Cu2+ over other competitive species (Li+, Na+, K+, Rb+, Cs+, Mg2+, Ba2+, Ca2+, Sr2+, Ag+, Zn2+, Cd2+, Hg2+ and Pb2+). Moreover, the theoretical DFT calculations revealed that the pre-organized cavity is essential with two proximate nitrogen atoms of the sulfonamide groups to stabilize the complex of 8 with Cu2+ for static excimer emission.
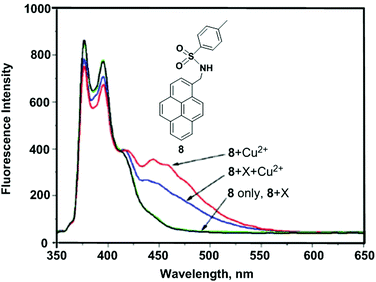 | ||
| Fig. 11 The fluorescence spectra of 8 in the presence of Cu2+ and miscellaneous cations, X (10 equiv.). Reproduced from ref. 76 with permission from the American Chemical Society, copyright 2008. | ||
The design and synthesis of new pyrene-derived fluorescent sensors containing quinolinylamide groups (9–11) was also reported by Kim and coworkers. The coordination behaviour was examined with variations in length of the methylene groups (n = 0, 1, 3) between the pyrene and quinolinylamide groups of 9–11 toward Cu2+ by fluorescence spectroscopy and theoretical DFT calculations (Fig. 12).77 Addition of Cu2+ to ligand 9 (n = 0) produced a new broad emission band which supports strong static excimer emission at 460 nm, whilst the binding constant was found to be 5.42 × 105 M−1. Moreover, compound 9 interacts with Cu2+ following a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry as deduced from the Job's plot analysis, mass spectra and fluorescence behaviour. A DFT study confirmed that the minimum intramolecular distance between the pyrene and quinoline amide is the main factor for Cu2+ ion detection. In the presence of 10 equiv. of Cu2+, the excimer emission intensity of 9 became approximately 8 times greater than that of probe 10 and 11 (n = 1, 3). Thus, it was concluded that the methylene spacers between the pyrene and carbonyl unit have a great impact on strong intermolecular Py–Py* formation in order to show an intense static excimer band.
1 stoichiometry as deduced from the Job's plot analysis, mass spectra and fluorescence behaviour. A DFT study confirmed that the minimum intramolecular distance between the pyrene and quinoline amide is the main factor for Cu2+ ion detection. In the presence of 10 equiv. of Cu2+, the excimer emission intensity of 9 became approximately 8 times greater than that of probe 10 and 11 (n = 1, 3). Thus, it was concluded that the methylene spacers between the pyrene and carbonyl unit have a great impact on strong intermolecular Py–Py* formation in order to show an intense static excimer band.
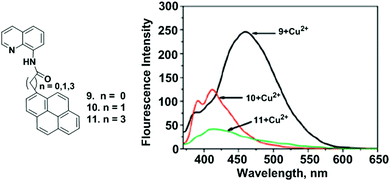 | ||
| Fig. 12 The structures of the molecular receptors 9, 10 and 11. Fluorescence spectra of 9–11 upon addition of Cu(ClO4)2 (10 equiv.). Reproduced from ref. 77 with permission from the American Chemical Society, copyright 2009. | ||
A pyrene containing Schiff base fluorosensor 12 has been developed which showed a high selectivity and sensitivity towards Cu2+ ions.78 This was due to the hindrance of the PET process upon complexation of the pyrene moiety with Cu2+ through interaction of the nitrogen lone pair electron of 12 with Cu2+ that induced the intermolecular excimer formation (Fig. 13). The free ligand 12 exhibited two weak broad emissions centred at 385 nm and 452 nm in Tris–HCl (10 mM, pH = 7) buffer containing CH3CN–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium which were assigned to the monomer and excimer of the pyrene moieties respectively. Moreover, the presence of Cu2+ triggered a noticeable enhancement (10 fold) of the excimer peak at 452 nm along with two prominent monomer peaks at 378 and 396 nm. The detection limit of the sensor 12 was found to be 4 × 10−8 M. The emission property of 12 (5 μM) was dependent on the nature of the solvent, and was enhanced on increasing the concentration of water up to 80% and there after reduced at 90% water fraction. Moreover, since some active groups (e.g. –NH2) are present in the probe which are sensitive to H+ ions, there is an effect of pH on the fluorescent response of the probe.
1) medium which were assigned to the monomer and excimer of the pyrene moieties respectively. Moreover, the presence of Cu2+ triggered a noticeable enhancement (10 fold) of the excimer peak at 452 nm along with two prominent monomer peaks at 378 and 396 nm. The detection limit of the sensor 12 was found to be 4 × 10−8 M. The emission property of 12 (5 μM) was dependent on the nature of the solvent, and was enhanced on increasing the concentration of water up to 80% and there after reduced at 90% water fraction. Moreover, since some active groups (e.g. –NH2) are present in the probe which are sensitive to H+ ions, there is an effect of pH on the fluorescent response of the probe.
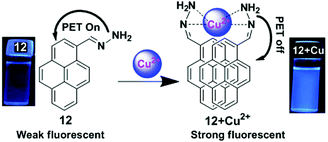 | ||
| Fig. 13 Schematic representation of Cu2+ sensing by fluorosensor 12 and emission photograph of 12 and 12 + Cu2+ under UV radiation. Reproduced from ref. 78, with permission from the Royal Society of Chemistry. | ||
Lin and co-workers have designed a simple pyrene containing derivative as a Cu2+ turn-on chemosensor 13.79 The probe 13 in CH3CN revealed a greater photoluminescence intensity enhancement at 459 nm for Cu2+ (Φ = 0.284) versus other different metal ions (Li+, Ag+, K+, Na+, Cs+, Ni2+, Fe3+, Co2+, Zn2+, Cd2+, Pb2+, Ca2+, Cr3+, Mg2+, Cu2+, Mn2+, Hg2+, Fe2+ and Ag+). The sensing mechanism involves heteroatoms (O and N) to form the excimer in which one Cu2+ binds with two N and two O (one N and one O from one molecule) (Fig. 14). The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the 13 + Cu2+ complex was calculated from Job's plots based on UV-vis absorption titrations. Moreover, the 13 + Cu2+ sensor was found to be active over a wide range of pH (1–14). After 10 minutes, the relative fluorescence intensity changes for 13 + Cu2+ reached a maximum value and so the system is effective with respect to time (0–10 minutes). The detection limit (LOD) of 13 with Cu2+ was calculated at 9.72 × 10−7 M. Similarly, based on fluorescent binding isotherms, the association constant (Ka) of the complex was estimated at 1.96 × 106 M−1.
1 stoichiometry of the 13 + Cu2+ complex was calculated from Job's plots based on UV-vis absorption titrations. Moreover, the 13 + Cu2+ sensor was found to be active over a wide range of pH (1–14). After 10 minutes, the relative fluorescence intensity changes for 13 + Cu2+ reached a maximum value and so the system is effective with respect to time (0–10 minutes). The detection limit (LOD) of 13 with Cu2+ was calculated at 9.72 × 10−7 M. Similarly, based on fluorescent binding isotherms, the association constant (Ka) of the complex was estimated at 1.96 × 106 M−1.
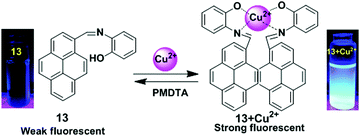 | ||
| Fig. 14 Schematic presentation for detection of Cu2+ using receptors 13. Photographs of 13 and 13 + Cu2+ visualized under UV-light irradiations. Reproduced from ref. 79, with permission from the Royal Society of Chemistry. | ||
A novel fluorescent chemosensor 14, made up of pyrene units connected by a binaphthyl-crown derivative was designed and prepared for the selective detection of Cu2+ in the presence of other metal cations (Ca2+, Cd2+, Co2+, Cs+, Cu2+, Hg2+, K+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, and Zn2+) in acetonitrile solution (Fig. 15).80 Compound 14 (1 μM) shows monomer and excimer emissions at 376 and 477 nm respectively for excitation at 342 nm. Addition of Cu2+ produced a new blue shifted excimer emission band centered at 447 nm with increasing intensity maximum up to 3-fold. The sensor 14 exhibits static excimer emission characteristics upon addition of Cu2+ that can be easily understood from excitation spectra. Here, the emission at 477 nm resulted from a dynamic excimer due to the identical excitation spectrum at both the monomer wavelength (376 nm) and the excimer wavelength (477 nm). The excitation spectrum of ligand 14 with Cu2+ monitored at 447 nm was significantly red-shifted compared with the spectrum taken at 376 nm which was attributed to the 447 nm from the static excimer. Moreover, from the fluorescence titration experiments, the association constant of 14 with Cu2+ was determined to be 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1.
600 M−1.
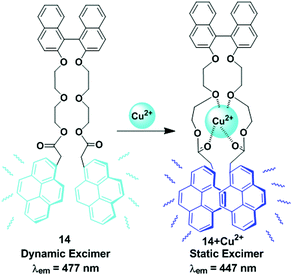 | ||
| Fig. 15 Schematic representation of Cu2+ sensing by pyrene appended-binaphthyl-crown derivative compound, 14. Reproduced from ref. 80 with permission from the Elsevier, copyright 2006. | ||
Kumar et al. have developed a new heteroditopic receptor 15, based on the 1,3-alternate conformation of thiacalix[4]arene bearing amine groups appended with pyrene moieties and a crown-4 ring (Fig. 16).81 The receptor 15 can detect Cu2+ ions selectively among other metal ions with a detection limit of 40 × 10−9 mol L−1 and follows a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry of ligand to Cu2+ complexation. It shows negative allosteric behaviour between Cu2+/Li+ ions in mixed aqueous media. The binding behaviour of compound 15 was studied by UV-vis and fluorescence spectroscopy which suggests the Cu2+ ions interact with the amino nitrogen centres and Li+ ions co-ordinate to the crown ether ring of receptor 15. The excimer emission band of receptor 15 (1.0 μM; EtOH–H2O; 8
2 stoichiometry of ligand to Cu2+ complexation. It shows negative allosteric behaviour between Cu2+/Li+ ions in mixed aqueous media. The binding behaviour of compound 15 was studied by UV-vis and fluorescence spectroscopy which suggests the Cu2+ ions interact with the amino nitrogen centres and Li+ ions co-ordinate to the crown ether ring of receptor 15. The excimer emission band of receptor 15 (1.0 μM; EtOH–H2O; 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) for the pyrene moieties was clearly observed at 466 nm due to the fully overlapped position of the two pyrene units resulting in the folded conformation of 15. Notably, after gradual addition of only Cu2+ ions, the monomer fluorescence emission at 378 nm shows a major enhancement with a remarkable decrease in the excimer emission intensity and the variation of these emissions led to the formation of an isoemissive point at 430 nm. This ratiometric behaviour of receptor 15 in the presence of Cu2+ ions was due to the decreased electron density at nitrogen leading to the conformational changes that moved the overlapped pyrene units away from each other.
2, v/v) for the pyrene moieties was clearly observed at 466 nm due to the fully overlapped position of the two pyrene units resulting in the folded conformation of 15. Notably, after gradual addition of only Cu2+ ions, the monomer fluorescence emission at 378 nm shows a major enhancement with a remarkable decrease in the excimer emission intensity and the variation of these emissions led to the formation of an isoemissive point at 430 nm. This ratiometric behaviour of receptor 15 in the presence of Cu2+ ions was due to the decreased electron density at nitrogen leading to the conformational changes that moved the overlapped pyrene units away from each other.
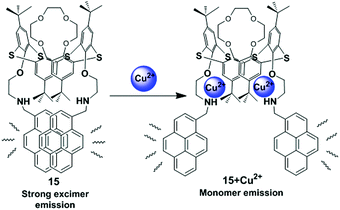 | ||
| Fig. 16 Schematic representation of possible sensing mechanism for 15 on interaction with Cu2+. Reproduced from ref. 81 with permission from the Royal Society of Chemistry. | ||
A novel pyrene containing chemosensor 16 which possesses a self-assembled 3D crystal structure via C–H⋯π, π⋯π and different types of H-bonding interactions, has been designed for the efficient and selective detection of Cu2+ and F− ions in a dual sensing mode which do not interfere with each other.82 The sensor 16 can recognize Cu2+ by fluorimetric experiments and by visual colour changes under UV-light. The emission spectra of sensor 16 (1 × 10−7 M) in a methanol–water mixture (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 7
water 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) shows, on changing the concentration, a local emission band at 466 nm which remains almost unchanged, whilst the band at 530 nm was continuously altered indicating the formation of excimers. After addition of Cu2+ to the sensor 16, a decrease of the excimer bands at 520 nm and 560 nm was initially observed as a result of interaction of the copper with the sensor and then the 16 + Cu2+ complex causes an increment of the band at 466 nm (Fig. 17). The above phenomenon can be explained by an excimer switch-off mechanism for promoting complexation with Cu2+. A Benesi–Hildebrand plot and Job's plot from UV-vis titrations demonstrated that the stoichiometry of the complex formed by 16 and Cu2+ is 1
3 v/v) shows, on changing the concentration, a local emission band at 466 nm which remains almost unchanged, whilst the band at 530 nm was continuously altered indicating the formation of excimers. After addition of Cu2+ to the sensor 16, a decrease of the excimer bands at 520 nm and 560 nm was initially observed as a result of interaction of the copper with the sensor and then the 16 + Cu2+ complex causes an increment of the band at 466 nm (Fig. 17). The above phenomenon can be explained by an excimer switch-off mechanism for promoting complexation with Cu2+. A Benesi–Hildebrand plot and Job's plot from UV-vis titrations demonstrated that the stoichiometry of the complex formed by 16 and Cu2+ is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant of 4.8 × 106 M−1. In order to confirm the binding phenomena, the structure and electronic properties of 16 and its copper complex 16 + Cu2+ have been investigated by Density Functional Theory (DFT). Moreover, the sensor 16 has potential for biological applications when detecting Cu2+ as it is transported across mammalian cell membranes (HEK 293 cells).
1 with a binding constant of 4.8 × 106 M−1. In order to confirm the binding phenomena, the structure and electronic properties of 16 and its copper complex 16 + Cu2+ have been investigated by Density Functional Theory (DFT). Moreover, the sensor 16 has potential for biological applications when detecting Cu2+ as it is transported across mammalian cell membranes (HEK 293 cells).
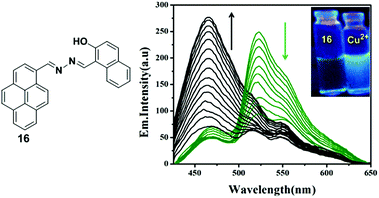 | ||
| Fig. 17 Structure of compound 16 showing fluorescence emission spectra of 16 with the addition of Cu2+. Inset: Visual fluorescence changes of 16 and upon addition of Cu2+ using a handheld UV lamp. Reproduced from ref. 82, with permission from the Royal Society of Chemistry. | ||
Mallik et al. have reported the development of four new saturated, pyrene-containing, metal-chelating lipids as fluorescent sensors (17–20).83 These lipids can interact with transition metal ions via a variety of metal-chelating head groups such as iminodiacetate (IDA), EDTA, DTPA (Fig. 18). However, all the lipids (17–20) in DMSO solution failed to show the presence of any excimers in the emission spectra. They can form liposomes (2.0 mg mL−1) under standard liposome formation conditions. The emission spectrum of 17 showed that both pyrene monomers (395 nm) and excimers (470 nm) were present in the liposomes in which the excimer to monomer intensity ratio (0.69) clearly indicated that lipid 17 was aggregated in the liposomes. Upon treatment with Cu2+, the overall fluorescence intensity of the liposomes decreased due to quenching of the excited states of the pyrene monomer by Cu2+ compared to the pyrene excimer, leading to an increase in excimer-to-monomer ratio. Similar behaviour was also observed for liposomes from 17–19 except 20 which showed very little change. Other transition metal ions (Ni2+, Hg2+ and Zn2+) did not show any response in the excimer to monomer intensity ratio.
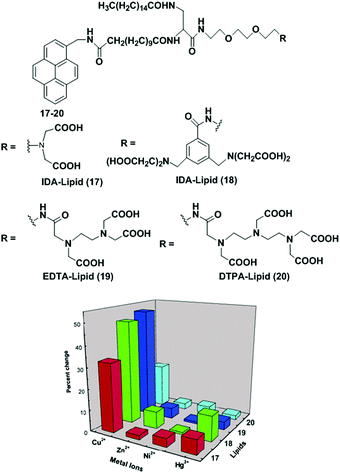 | ||
| Fig. 18 Structures of molecular receptor 17–20. Relative increase of the I470/I395 ratio with transition metal ions for the saturated lipids. Reproduced from ref. 83 with permission from the American Chemical Society, copyright 2003. | ||
Another pyrene-appended ratiometric fluorescent chemosensor 21 has been reported by Yamato and coworkers in 2015. In this case, the homooxacalix[3]arene acts as a molecular spacer incorporating three pyrene fluorophore at the upper rim and three substituted triazole arms as ionophore at the lower rim (Fig. 19).84 The sensor 21 can serve as a ratiometric chemosensor for heavy and transition metal (HTM) ions (Cu2+, Hg2+, Pb2+ and Zn2+) resulting in a conformational change for 21. The fluorescence emission spectra of free 21 show a characteristic pyrene excimer band at 482 nm in CH3CN solution. The interaction of 21 with Cu2+ and other HTM ions led to the enhancement of monomer emission accompanied by excimer quenching. Herein, the ditopic calixarene as a molecular spacer prevents the heavy atom effect of the HTM ion by insulating the fluorophore from the ionophore through a long distance. The association constant for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of 21 + Cu2+ was calculated to be 1.89 × 105 M−1. FT-IR spectra of the chemosensor 21 have been recorded, and the absorption band of C–O present on the lower rim is shifted at 1184 cm−1 whereas no absorption band change is observed for C
1 complexation of 21 + Cu2+ was calculated to be 1.89 × 105 M−1. FT-IR spectra of the chemosensor 21 have been recorded, and the absorption band of C–O present on the lower rim is shifted at 1184 cm−1 whereas no absorption band change is observed for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at the upper rim of 21 upon complexation with Cu2+. The authors stated that three substituted triazole moieties at the lower rim bind with Cu2+ and cause conformational changes that make the pyrene units move far away from one another.
O at the upper rim of 21 upon complexation with Cu2+. The authors stated that three substituted triazole moieties at the lower rim bind with Cu2+ and cause conformational changes that make the pyrene units move far away from one another.
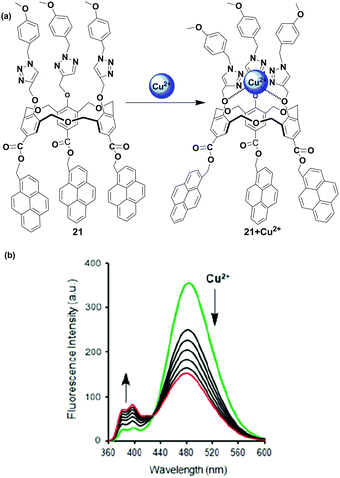 | ||
| Fig. 19 (a) The structure of ratiometric sensor 21 and it's binding mode with Cu2+; (b) showing fluorescence for the Cu2+ ion in CH3CN. Reproduced from ref. 84 with permission from the Taylor & Francis, copyright 2015. | ||
5.2. Chemosensors based on PET mechanism
Receptor molecules have been designed for the enhancement of fluorescence intensity upon binding with a metal ion through the PET mechanism.85 In 2015, based on the PET mechanism, our group developed 7-tert-butylpyrene-appended fluorescent sensors containing dipicolylamine (Dpa) linkages.86 Both the mono-chelate and bis-chelate ligand showed selectivity for Zn2+ and Cd2+ ions. Herein, we explain that the binding phenomenon of both ligands towards Zn2+ and Cd2+ varied on changing the solvent system. However, the addition of Cu2+ showed almost no fluorescence enhancement yet Cu2+ quenched the fluorescence of the bis-chelate ligand and Zn2+ complex, which can be explained by the paramagnetic nature of Cu2+. For the selective and sensitive detection of Cu2+, our group designed and synthesized three new fluorescence ‘off–on’ chemosensors 22–24 based on pyrene containing Schiff base derivatives with different chain lengths (Fig. 20).87 Compound 22 was synthesized from the condensation reaction of 1-pyrene carbaldehyde with 4-(hydrazidocarbonyl)(N,N-diethylaminocarbonylmethoxy)benzene. The other compounds 22 and 23 were easily synthesized via condensation reactions, and were compared against 22 towards Cu2+ in terms of binding ability. The probe 22 (1.0 μM) was highly sensitive for Cu2+ detection due to strong inhibition of PET. After addition of Cu2+, the emission intensity was enhanced approximately 65 times more (Φ = 0.31) than that of the free ligand 22 (Φ = 0.01), and was 57 times and 40 times greater than free 23 and 24, respectively. A Job's plot analysis was carried out at 405 nm in which the fluorescence intensity showed a maximum at the mole fraction 0.5 which corresponds to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ligand and Cu2+ complex. The resulting association constant for 22 was found to be 1.29 × 105 ± 0.32 M−1. A DFT computational study together with 1H NMR and 13C NMR spectroscopic titration experiments revealed that Cu2+ was bound at the imine nitrogen atom and the amide carbonyl oxygen of ligands 22 and 23. The diethylaminocarbonylmethoxy group of 22 provides a contribution for 22 and Cu2+ complexation through an inductive effect.
1 ratio of ligand and Cu2+ complex. The resulting association constant for 22 was found to be 1.29 × 105 ± 0.32 M−1. A DFT computational study together with 1H NMR and 13C NMR spectroscopic titration experiments revealed that Cu2+ was bound at the imine nitrogen atom and the amide carbonyl oxygen of ligands 22 and 23. The diethylaminocarbonylmethoxy group of 22 provides a contribution for 22 and Cu2+ complexation through an inductive effect.
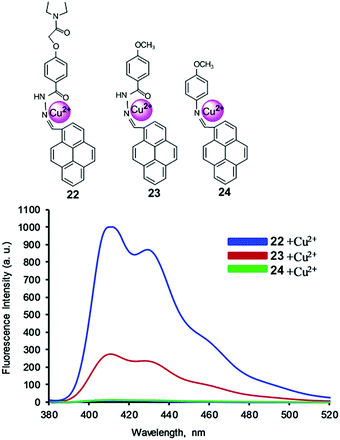 | ||
| Fig. 20 Structure of chemosensors 22, 23 and 24. Fluorescence response of ligands upon addition of Cu2+ ions in CH3CN/CH2Cl2. Reproduced from ref. 87 with permission from the Elsevier, copyright 2016. | ||
A pyrene derivative containing a benzothiazolenhydrazone receptor as a fluorescent sensor 25 was reported by Wang and Wu (Fig. 21).88 Upon addition of Cu2+ (25 μM), probe 25 (50 μM) exhibited a significant fluorescence enhancement at 468 nm in acetonitrile–water (v/v = 3/1, 5 mM HEPES, pH 7.0) from which the limit of detection of chemosensor 25 was estimated to be 2.73 μM. The coordination of Cu2+ ions inhibited the PET process resulting in a significant enhancement of fluorescence intensity. The Job's plot experiments revealed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for ligand 25 to metal complex. Density functional theory (DFT) calculations determined that two chemosensors 25 bind Cu2+ using four nitrogen atoms. Moreover, the fluorescence and bright-field images indicated that the probe 25 is useful for the detection of Cu2+ ions in living cells.
1 stoichiometry for ligand 25 to metal complex. Density functional theory (DFT) calculations determined that two chemosensors 25 bind Cu2+ using four nitrogen atoms. Moreover, the fluorescence and bright-field images indicated that the probe 25 is useful for the detection of Cu2+ ions in living cells.
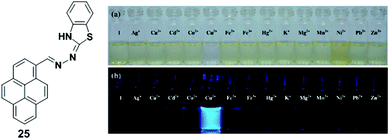 | ||
| Fig. 21 Structures of probe 25 and colour (a) and fluorescence (b) changes of chemosensor 25 (500 μM) after addition of various metal ions (500 μM). Reproduced from ref. 88 with permission from the Elsevier, copyright 2013. | ||
Molina et al. designed the chemosensor 26 in which a 2-aza-1,3-diene moiety was used as the ionophore for the recognition of Cu2+ (Fig. 22).89 The absorption spectrum of chemosensor 26 exhibited two well-defined isosbestic points at 275 and 440 nm with a gradual increase of a new red shifted band at 496 nm in the presence of Cu2+ ion. This was responsible for the change of colour from yellowish to deep orange. The fluorescence intensity of 26 (2.5 × 10−5 M) in CH3CN increased upon addition of Cu2+ (Icomplex/Ifree ligand = 22-fold), where the excimer emission shifted from 450 to 429 nm. From the emission intensity data, the association constant of the chemosensor 26 + Cu2+ system was found to be 5.71 × 105 M−1. The stoichiometry of the ligand 26 to metal complex system was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The response of the fluorescence of 26 was also studied in CH3CN/H2O (70/30). Under these conditions, titration experiments demonstrated that the Cu2+ ions yielded a 10-fold enhancement of quantum yield along with a slight red shift (10 nm) of the excimer emission band. The titration data indicated the calculated detection limit of 26 was 3.91 × 10−6 M for Cu2+.
1. The response of the fluorescence of 26 was also studied in CH3CN/H2O (70/30). Under these conditions, titration experiments demonstrated that the Cu2+ ions yielded a 10-fold enhancement of quantum yield along with a slight red shift (10 nm) of the excimer emission band. The titration data indicated the calculated detection limit of 26 was 3.91 × 10−6 M for Cu2+.
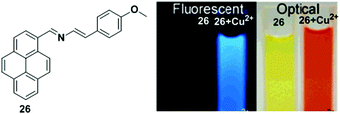 | ||
| Fig. 22 Structure of chemosensor 26 and colour and fluorescence changes of 26 with Cu2+ in CH3CN. Reproduced from ref. 89 with permission from the American Chemical Society, copyright 2006. | ||
A pyrene based fluorescent chemosensor 27 containing a picolinohydrazide receptor was developed by Wu and co-workers (Fig. 23).90 Binding with Cu2+ ions in mixed aqueous media (CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7
H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to the chemosensor 27 blocks PET and greatly enhanced the fluorescence of pyrene. After gradual addition of Cu2+ to the chemosensor 27 (25 μM), a new emission band appeared at 455 nm and the quantum yield of that emission band was 0.267, which is 20-fold that of chemosensor 27, 0.013. The Job's plot experiment indicated that the binding ratio for the chemosensor 27 + Cu2+ complex was 1
3) to the chemosensor 27 blocks PET and greatly enhanced the fluorescence of pyrene. After gradual addition of Cu2+ to the chemosensor 27 (25 μM), a new emission band appeared at 455 nm and the quantum yield of that emission band was 0.267, which is 20-fold that of chemosensor 27, 0.013. The Job's plot experiment indicated that the binding ratio for the chemosensor 27 + Cu2+ complex was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It was also confirmed from 1H NMR and IR spectroscopy that the Cu2+ was bound to one nitrogen atom from a pyridine and one nitrogen atom from an amide. Moreover, fluorescence signals determined by a fluorescence microscope indicated that chemosensor 27 possessed good cell-membrane permeability and could detect Cu2+ present in living cells.
1. It was also confirmed from 1H NMR and IR spectroscopy that the Cu2+ was bound to one nitrogen atom from a pyridine and one nitrogen atom from an amide. Moreover, fluorescence signals determined by a fluorescence microscope indicated that chemosensor 27 possessed good cell-membrane permeability and could detect Cu2+ present in living cells.
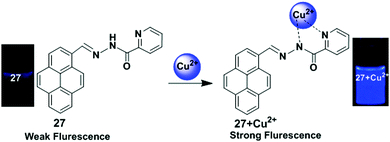 | ||
| Fig. 23 Schematic representation of the sensing mechanism of probe 27 with Cu2+. Reproduced from ref. 90 with permission from the Springer Nature, copyright 2012. | ||
A pyrene-based chemosensor 28 in which diaminomaleonitrile acted as a chelator, was synthesized and designed for Cu2+ ion detection (Fig. 24).91 It was observed from UV-visible spectroscopy that the absorbance at 420 nm was reduced and a new band appeared at 355 nm after gradual addition of Cu2+ to chemosensor 28. The colour of 28 also changed from yellow to colourless. Moreover, free ligand 28 exhibited very weak fluorescence (Φ = 0.0045) due to a PET process from the lone pair electrons of the two-nitrile groups to pyrene. However, the Cu2+-bound probe 28 inhibited the electron withdrawing ability of the two nitrile groups and showed a sharp emission peak at 417 nm (Φ = 0.59) under excitation of 350 nm in acetonitrile–water.
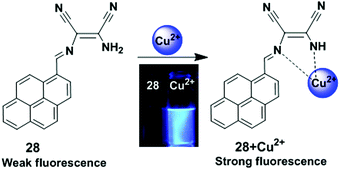 | ||
| Fig. 24 Structure of chemosensor 28 and possible binding modes for Cu2+. Reproduced from ref. 91 with permission from the Elsevier, copyright 2010. | ||
A newly designed fluorescence chemosensor 29 that contains a pyrene motif with a hydrazinylpyridine moiety has been reported.92 The sensor 29 revealed high sensitivity towards Cu2+ ions in a CH3CN/H2O solvent system over the pH range of 5.0–10. The interaction of Cu2+ inhibited the PET mechanism from the nitrogen lone pairs to the pyrene, resulting in significant enhancement in pyrene fluorescence (Fig. 25). The quantum yield of ligand 29 to Cu2+ complex at the emission band 389 nm was 0.56, which was 560 times greater than that of the free ligand 29 at 0.001. The association constant (Ka) of 29 with Cu2+ was found to be 1.0 × 104 M−1. On binding with Cu2+, the absorption spectra exhibited a 42 nm blue shift because of hindered conjugation between the double bonds of 29. The colour also changed from light yellow to colourless. Moreover, confocal fluorescence microscopy imaging for detecting Cu2+ in living cells showed that chemosensor 29 can be used as an effective fluorescent probe due to its (<30 μM) low cytotoxicity and ability to easily penetrate cell membranes.
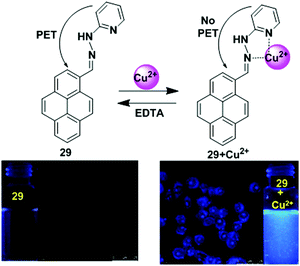 | ||
| Fig. 25 Possible binding modes and fluorescence images of macrophage (RAW 264.7) cells treated with 29 (left) and then 29 + Cu2+ (right). Inset: Fluorescence colour changes of solution 29 before and after addition of Cu2+. Reproduced from ref. 92 with permission from the Royal Society of Chemistry. | ||
A Schiff-base fluorescent compound 30 was developed by exploiting the PET process for the detection Cu2+ ions (Fig. 26).93 Ligand 30 (4 × 10−6 M) shows weak fluorescence because of PET. The fluorescence intensity was remarkably enhanced after gradual addition of Cu2+ ions (0–65 equiv.) to the chemosensor 30 in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, HEPES = 50 mM, pH = 7.4). From the emission intensity data, the detection limit of chemosensor 30 + Cu2+ system was found to be 0.26 × 10−6 M with an association constant 1.16 × 104 M−1. A Job's plot experiment showed that the 30 to Cu2+ complex followed a 1
1 v/v, HEPES = 50 mM, pH = 7.4). From the emission intensity data, the detection limit of chemosensor 30 + Cu2+ system was found to be 0.26 × 10−6 M with an association constant 1.16 × 104 M−1. A Job's plot experiment showed that the 30 to Cu2+ complex followed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. Treatment of chemosensor 30 with various metal ions such as Pb2+, Ce3+, Cd2+, La3+, Mg2+, Zn2+, Ba2+, Bi2+, Hg2+, Fe3+, Ag+, Ni2+, Cr3+, Ca2+, Mn2+, Na+, K+, Co2+, Zr2+, Li+, Sr2+, Fe2+, Al3+ and Th4+ (100 equiv.) resulted in insignificant changes in the fluorescence intensity, which indicate high selectivity of 30 towards the Cu2+ ion. Time-dependence fluorescence intensity indicated that sensor 30 completely binds with Cu2+ ion within 5 min. Moreover, the bio-imaging and spectroscopic methods confirmed its detection ability for Cu2+ ions in living cells.
1 binding model. Treatment of chemosensor 30 with various metal ions such as Pb2+, Ce3+, Cd2+, La3+, Mg2+, Zn2+, Ba2+, Bi2+, Hg2+, Fe3+, Ag+, Ni2+, Cr3+, Ca2+, Mn2+, Na+, K+, Co2+, Zr2+, Li+, Sr2+, Fe2+, Al3+ and Th4+ (100 equiv.) resulted in insignificant changes in the fluorescence intensity, which indicate high selectivity of 30 towards the Cu2+ ion. Time-dependence fluorescence intensity indicated that sensor 30 completely binds with Cu2+ ion within 5 min. Moreover, the bio-imaging and spectroscopic methods confirmed its detection ability for Cu2+ ions in living cells.
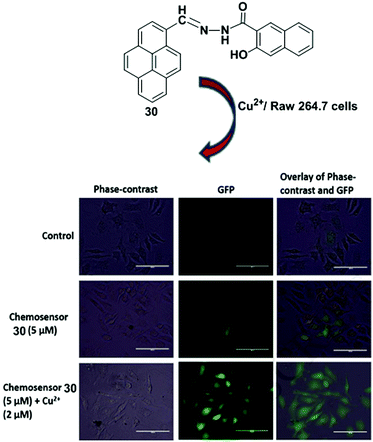 | ||
| Fig. 26 Structure of ligand 30. Fluorescence images of RAW 264.7 cells treated with chemosensor 30 and Cu2+ ions. Phase-contrast (left); GFP image (λex = 393 nm) (middle); and overlay of phase contrast and GFP (right). Reproduced from ref. 93 with permission from the Elsevier, copyright 2018. | ||
Patra and co-workers have developed a chemosensor 31 containing a benzilmonohydrazone moiety for the detection of Cu2+.94 The chemosensor 31 exhibited a significant colour change from yellow to colourless in the presence of 10 equiv. of Cu2+ ions. The binding properties of 31 were further investigated by fluorescence titration experiments in acetonitrile–water (2/1, v/v). The fluorescence quantum yield increased from 0.035 to 0.67 after gradual addition of Cu2+. In this case, Cu2+ is coordinated with the outer azino nitrogen atom of the ligand 31, which inhibits the PET mechanism (Fig. 27). The detection limit of 31 based on fluorescent-titration experiments was found to be 7.8 nM for Cu2+. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between 31 and Cu2+ was observed by 1H NMR spectroscopic data, the Job's plot and the ESI-MS spectrum. The ligand 31 was efficiently applied to real samples for the recognition of Cu2+ over the wide pH range of 4–11.
1 stoichiometric complexation between 31 and Cu2+ was observed by 1H NMR spectroscopic data, the Job's plot and the ESI-MS spectrum. The ligand 31 was efficiently applied to real samples for the recognition of Cu2+ over the wide pH range of 4–11.
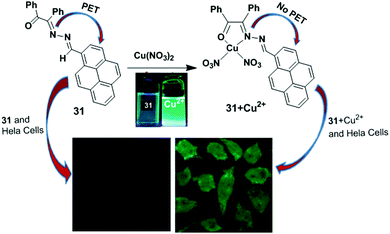 | ||
| Fig. 27 Representation of possible sensing mechanism of probe 31. Fluorescence images of HeLa cells; cells incubated with probe 31 only and cells incubated with 31 + Cu2+. Reproduced from ref. 94 with permission from the Royal Society of Chemistry. | ||
A new 2-aza-1,3-butadiene ionophore which connects two pyrene groups has been synthesized and studied as a colorimetric and fluorescent chemosensor 32 for Cu2+ ions (Fig. 28).95 The UV-vis and fluorescence measurements illustrated the binding mechanism of receptor 32 (c = 2.5 × 10−5 M) with several metal cations (Li+, Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+, Sm3+, Eu3+, Yb3+ and Lu3+) in CH3CN. The receptor 32 showed selective changes for Cu2+ and Hg2+ ions with a visible colour change by naked eye detection. Compound 32 exhibits a very weak fluorescence due to PET quenching from the lone pair electrons on the nitrogen atom in the 2-azadiene bridge to the excited state of the pyrene moiety when excited at 350 nm (Φ = 0.014). Upon addition of Cu2+ to 32, a chelation enhanced fluorescence intensity (CHEF) as well as excimer emission band was observed which was 27 times stronger in acetonitrile and 2.7 times stronger in an acetonitrile/water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent system. Moreover, the detection limit in both solvent systems was 10−6 M with a 1
3) solvent system. Moreover, the detection limit in both solvent systems was 10−6 M with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric for the 32 to Cu2+ complex.
1 stoichiometric for the 32 to Cu2+ complex.
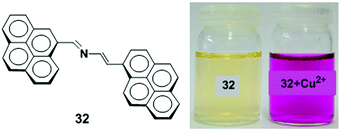 | ||
| Fig. 28 Molecular structure of receptor 32. Visual changes in the colour of 32 (left) and after addition of Cu2+ ion (right). Reproduced from ref. 95 with permission from the Elsevier, copyright 2010. | ||
A new fluorescent chemosensor 33 incorporating 1-nitronyl nitroxide pyrene has been developed as an off–on sensor for the selective recognition of Cu2+ (Fig. 29).96 As a Cu2+ selective sensor, the fluorogenic behaviour of 33 (10 mM) was investigated in CH3CN in which a weak fluorescence signal at 378 nm for the free solution of 33 remarkably increased at 444 nm after addition of 10.0 equiv. of Cu2+. This phenomenon can be explained by the inhibition of PET from nitronyl nitroxide to the pyrene moiety. An ESR experiment confirmed the inhibition caused by the coordination between Cu2+ and nitronyl nitroxide of 33. On the other hand, the fluorescence intensity of 33 showed no significant changes with various other metal ions (Li+, Na+, Ba2+, Fe3+, Fe2+, Mn2+, Mg2+, Ag+, Pb2+, Cd2+, Co2+, Ni2+, Zn2+, Hg2+). Moreover, the absorbances at 345 nm and 276 nm reduced sharply and increased at 402 and 295 nm and were accompanied with four well-defined isosbestic points, which indicated 33 + Cu2+ complexation; the colour changed from purple to bright yellow. In contrast, a reversible absorption response was observed at 345 nm by titration of 2,2′-bipyridine with the 33 + Cu2+ complex, which resulted in the recovery of the colour which is probably due to a stronger coordination between Cu2+ and 2,2′-bipyridine. Furthermore, the Job's plot calculated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio between Cu2+ and 33 with a detection limit of 3.60 × 10−7 M in CH3CN.
1 binding ratio between Cu2+ and 33 with a detection limit of 3.60 × 10−7 M in CH3CN.
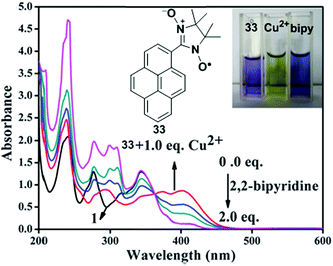 | ||
| Fig. 29 The UV-vis absorption spectra of 33 + Cu2+ and upon addition of 2,2′-bipyridine in CH3CN. Inset: Visual changes of the colour of compound 33; compound 33 + Cu2+; compound 33 + Cu2+ + 2,2′-bipyridine (from left to right). Reproduced from ref. 96 with permission from the Centre National de la Recherche Scientifique (CNRS) and the Royal Society of Chemistry. | ||
5.3. Chemosensor based on PCT mechanism
The calix[4] crown fluorescent chemosensor 34 bearing two facing pyreneamide groups has been designed based on the PCT mechanism (Fig. 30).97 After addition of Pb2+ or Cu2+, probe 34 showed fluorescence quenching in both the excimer and monomer emissions owing to reverse PET and conformational changes. The 34/Cu2+ complex gave a significant red-shifted excitation spectrum in an acetonitrile solvent system at the excimer wavelength 470 nm in comparison with monomer wavelength 380 nm, which suggests that the two-pyrene groups form the static excimer. Moreover, in both the fluorescence and absorption spectra, wavelength shifts of 34 are noted on binding of Cu2+ with the nitrogen atoms of the amide groups, resulting in a PCT mechanism.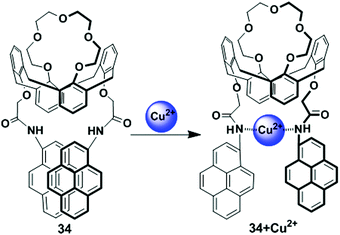 | ||
| Fig. 30 Schematic representation of proposed sensing mechanism for probe 34 in presence of Cu2+. Reproduced from ref. 97 with permission from the American Chemical Society, copyright 2006. | ||
5.4. Chemosensor based on LMCT process
The LMCT process occurs in a complex when electrons are transferred from a molecular orbital (σ, σ*, π, π* and non-bonding) of the ligand to the empty or partially filled metal d-orbitals. Therefore, a reduction of the metal occurs in LMCT transitions. In this case, this type of transfer predominantly occurs in the case of ligands having relatively high energy lone pairs atoms (example O, S or Se) or when the metal has low lying empty orbitals. The LMCT based fluorescent sensors depend on the selection of chromophore such as a pyrene unit, along with the nature and location of the receptor. The metal ion binding with a receptor will generally lead to a change in the absorption spectra or quenching of the fluorescence of the pyrene fluorophore.98In 2017, Lu and co-workers developed chemosensor 35 by combining pyrene with a pyridine unit based on LMCT which exhibited high selectivity for Cu2+ and Fe3+ over other ions (Fig. 31).99 Sensor 35 exhibited stable absorption or fluorescence intensity over a wide range of pH from 3 to 12. Addition of Cu2+ to ligand 35 partially quenched the emission of the fluorophore through electron and/or energy transfer processes due to its paramagnetic nature with an unfilled d orbital. DFT calculations of 35 + Cu2+ also fully confirmed the LUMO was distributed more over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and receptor rather than the pyrene unit after binding with Cu2+, than that of the free compound 35. The UV-vis absorption spectra of 35 in DMF–HEPES buffer (2
N bond and receptor rather than the pyrene unit after binding with Cu2+, than that of the free compound 35. The UV-vis absorption spectra of 35 in DMF–HEPES buffer (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v, pH = 7.4) showed a red shift (21 nm) from 352 nm to 373 nm after addition of 40 equiv. of Cu2+ with an optical colour change from colourless to pink. The limit of detection toward Cu2+ was 8.5 μM. The Job's plot experiment revealed a 1
8, v/v, pH = 7.4) showed a red shift (21 nm) from 352 nm to 373 nm after addition of 40 equiv. of Cu2+ with an optical colour change from colourless to pink. The limit of detection toward Cu2+ was 8.5 μM. The Job's plot experiment revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for 35 and Cu2+.
1 binding stoichiometry for 35 and Cu2+.
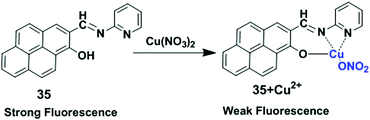 | ||
| Fig. 31 The possible binding modes of 35 with Cu2+. Reproduced from ref. 99 with permission from the Elsevier, copyright 2017. | ||
Another example of the LMCT mechanism was shown in the pyrene-based turn-off chemosensor 36 that was synthesized from the condensation reaction of 1-aminopyrene and 2,4-dihydroxy benzaldehyde (Fig. 32).100 Sensor 36 exhibited a high selectivity for recognizing Cu2+ ion in the presence of other metal ions. The UV-vis absorption spectroscopy of 36 with Cu2+ exhibited a hypsochromic shift for the absorption peaks at 368 nm and 340 nm with the formation of two isosbestic points in CH3CN/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Meanwhile, in aqueous medium, the solution colour of 36 changed from yellow to brown even at lower concentrations of Cu2+, and this was also directly observed by the naked eye. Moreover, the emission peak at 429 nm of 36 (20 μM) was gradually quenched after treatment with Cu2+ in which the quenching efficiency was 93%. This phenomenon is due to the LMCT characteristics resulting in the quenching of the fluorescence chemosensor 36 with Cu2+. The time-resolved fluorescence spectroscopy also confirmed the static quenching mechanism for 36 + Cu2+. A Job's plot experiment and ESI-mass spectroscopic analysis revealed a 2
1, v/v). Meanwhile, in aqueous medium, the solution colour of 36 changed from yellow to brown even at lower concentrations of Cu2+, and this was also directly observed by the naked eye. Moreover, the emission peak at 429 nm of 36 (20 μM) was gradually quenched after treatment with Cu2+ in which the quenching efficiency was 93%. This phenomenon is due to the LMCT characteristics resulting in the quenching of the fluorescence chemosensor 36 with Cu2+. The time-resolved fluorescence spectroscopy also confirmed the static quenching mechanism for 36 + Cu2+. A Job's plot experiment and ESI-mass spectroscopic analysis revealed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry for binding of 36 with Cu2+. The lower limit of detection of Cu2+ by 36 is 0.503 μM. The sensor 36 + Cu2+ underwent a reversibility test in the presence of histidine and regained its original colour. Therefore, the sensor 36 can be utilized in real samples to investigate trace amounts of copper.
2 stoichiometry for binding of 36 with Cu2+. The lower limit of detection of Cu2+ by 36 is 0.503 μM. The sensor 36 + Cu2+ underwent a reversibility test in the presence of histidine and regained its original colour. Therefore, the sensor 36 can be utilized in real samples to investigate trace amounts of copper.
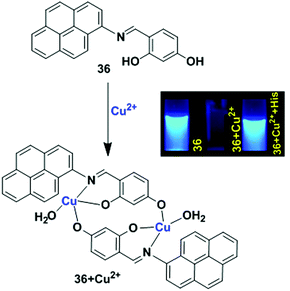 | ||
| Fig. 32 Schematic representation of possible sensing mechanism of probe 36 with Cu2+. Reproduced from ref. 100 with permission from the John Wiley and Sons, copyright 2018. | ||
5.5. Chemosensor based on ILCT mechanism
A ligand sometimes has a donor and an acceptor site simultaneously. The ILCT takes place within the ligands mediated by a metal. In most instances, these complexes exhibit long-lived excited states with low-energy absorptions. Generally, this kind of chemosensor is fluorescent in the presence of a metal ion.101Using the above technique, Mukherjee et al. reported a new simple, reversible, turn-on luminescent chemosensor, namely the hydrazone based pyrene derivative 37 for the detection of Cu2+ (Fig. 33).102 The binding of the receptor 37 with Cu2+ was studied in DMSO/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium, and the generation of new peaks at 512 nm (broad) and 345 nm through isosbestic points was evident with a colour change from yellow to reddish brown. The emission intensity of 37 was enhanced at 495 nm up to 44-fold upon addition of Cu2+ in DMSO solvent due to ILCT process. The other metal ions (Hg2+, Pb2+, Cd2+, Ni2+, Co2+, Fe2+, Fe3+, Mn2+, Zn2+, Al3+ and Cr3+) caused no significant change in the emission intensity of 37. The limit of detection (LOD) was found to be of the order of 10−8 M. DFT experiments revealed that the electron spin density was focused only on the pyrene moiety for all the frontier molecular orbitals which also supports the ILCT process. The compound 37 can extract Cu2+ with 94% extraction efficiency over the pH range of 6.5–11 from an aqueous mixture of metal ions or from real samples by a selective two-phase liquid–liquid extraction using a water–dichloromethane mixture (1
1) medium, and the generation of new peaks at 512 nm (broad) and 345 nm through isosbestic points was evident with a colour change from yellow to reddish brown. The emission intensity of 37 was enhanced at 495 nm up to 44-fold upon addition of Cu2+ in DMSO solvent due to ILCT process. The other metal ions (Hg2+, Pb2+, Cd2+, Ni2+, Co2+, Fe2+, Fe3+, Mn2+, Zn2+, Al3+ and Cr3+) caused no significant change in the emission intensity of 37. The limit of detection (LOD) was found to be of the order of 10−8 M. DFT experiments revealed that the electron spin density was focused only on the pyrene moiety for all the frontier molecular orbitals which also supports the ILCT process. The compound 37 can extract Cu2+ with 94% extraction efficiency over the pH range of 6.5–11 from an aqueous mixture of metal ions or from real samples by a selective two-phase liquid–liquid extraction using a water–dichloromethane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). During extraction, the 37 + Cu2+ complex was found to have a 2
1). During extraction, the 37 + Cu2+ complex was found to have a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ligand and Cu2+. Good recyclability and reusability were observed for chemosensor 37 as the fluorescence emission reversed to its original condition in the presence of C2O42− and the 37 + Cu2+ complex, and this characteristic was used to “INHIBIT” the logic gate application. As an analysis tool, a smartphone can also be used for detecting colour change of 37 during Cu2+ extraction.
1 ratio of ligand and Cu2+. Good recyclability and reusability were observed for chemosensor 37 as the fluorescence emission reversed to its original condition in the presence of C2O42− and the 37 + Cu2+ complex, and this characteristic was used to “INHIBIT” the logic gate application. As an analysis tool, a smartphone can also be used for detecting colour change of 37 during Cu2+ extraction.
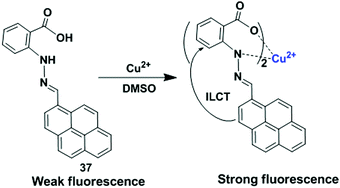 | ||
| Fig. 33 The sensing mechanism of 37 with Cu2+. Reproduced from ref. 102 with permission from the Elsevier, copyright 2018. | ||
5.6. Chemosensor based on AIE mechanism
In an AIE luminogen, the intramolecular rotations of aromatic compounds are active in dilute solutions, which serve as a relaxation channel for its excitons resulting in non-radiative decay. However, in the aggregate state, the intramolecular rotations are suppressed because of the physical constraints, which opens the radiative pathway to emit efficiently. In the crystalline state, multiple C–H–π hydrogen bonds are formed between the hydrogen atoms of the aromatic rings of one ligand and the π electrons of the aromatic rings of another ligand. These hydrogen bonds stiffen the conformations of the ligand and enhance their light emission.103For these special characteristics of light emission, Wang and co-workers have used AIE or an AEE luminogen to develop chemosensors of type 38 for Cu2+ ion detection.104 In this case, pyrene acted as the strong fluorophore and was combined with Schiff bases which were responsible for the AIE properties in semi-aqueous solution toward Cu2+ (Fig. 34). The AEE behaviour of 38 (10 μM) was explained by measurement of the fluorescence spectra in different volumes of water in mixed H2O/DMF (from 0% to 100%,) solution. The experiment suggests that with increasing percentage of water the peak continually showed a red shift because of amide–amidic acid tautomerization with water in the aggregation state of 38. Time-resolved fluorescence measurements supported the above observations. Sensor 38 became strongly emissive at 455 nm in the presence of Cu2+ (5 eq.) with a quantum yield Φ from 0.09 to 0.58 in H2O/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The UV-vis absorption spectroscopy also established that self-assembly of 38 (10 μM) prompted by Cu2+ involves a coordination interaction and changes in the aggregation form. A Job's plot experiment evaluated from the fluorescence spectra confirmed that the binding of 38 to Cu2+ followed a 2
1, v/v). The UV-vis absorption spectroscopy also established that self-assembly of 38 (10 μM) prompted by Cu2+ involves a coordination interaction and changes in the aggregation form. A Job's plot experiment evaluated from the fluorescence spectra confirmed that the binding of 38 to Cu2+ followed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
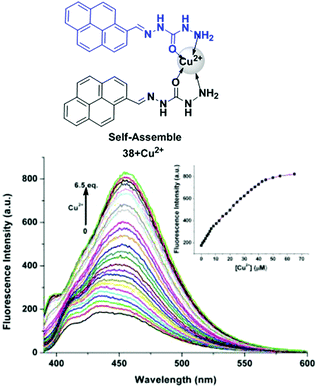 | ||
| Fig. 34 The AIE luminescence mechanism of 38 and Cu2+. The fluorescence spectra with Cu2+ ions. Inset: Emission intensity at 455 nm with Cu2+ concentration. Reproduced from ref. 104 with permission from the Elsevier, copyright 2018. | ||
Another example of aggregate formation was observed in a pyrene-based hydrophobic hydrocarbon framework in a binary water–solvent system.105 Das et al. have reported a chemosensor 39 in which the pyrene part acts as a fluorescence reporter and the antipyrene part containing the pyrazolone unit plays the role of a chelating moiety (Fig. 35). Sensor 39 exhibited a very weak fluorescence due to a PET process between N donors in the imine bond and the pyrene ring. Upon interaction with Cu2+, a fluorescence enhancement of 39 was observed at 454.5 nm along with 440 nm and 509 nm, which indicated complex formation due to prevention of the PET process by an N donor site in acetonitrile solvent. Time fluorescence titration revealed the maximum fluorescence enhancement of 39 was observed at 432 nm (blue shift) up to the addition of 150 μM of Cu2+ ion. However, with an increasing percentage of water in the acetonitrile solvent system, the fluorescence emission intensity of 39 gradually increased at 454.5 nm with a red shift with poor intensity in pure acetonitrile. At 80% of water fraction, there was 230 times increase in the emission intensity with a red shift of 16 nm. This was because of the AIE characteristics of probe 39. The critical aggregate concentration of ligand 39 in this solvent system was calculated to be 23.4 μM. Moreover, the antioxidant nature of the compound 39 was confirmed from UV studies.
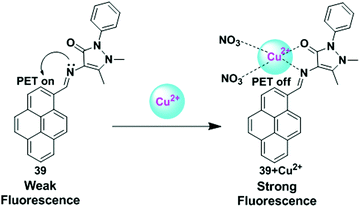 | ||
| Fig. 35 Schematic representation of Cu2+ sensing by ligand 39. Reproduced from ref. 105 with permission from the Elsevier, copyright 2018. | ||
5.7. Cu2+-Promoted reaction based chemosensors
The development of chemodosimeters that operates on the basis of analyte selective chemical reactions has attracted a great attention in the last few years. Among them, Cu2+-selective reaction-based probes show a significant fluorescence emission enhancement with high selectivity. In this case, Cu2+-assisted hydrolysis, oxidation, reduction and spirolactam ring-opening methods have been used for building Cu2+-selective reaction-based probes. Generally, fluorescent sensors of this type lead to non-emissive precursors to fluorescent products through irreversible chemical reactions. It is noticeable that Cu2+ induced-catalytic reactions to develop fluorescent sensors are the easiest way to avoid the consideration of the paramagnetic nature of Cu2+ as fluorescent products have a slight attraction to Cu2+.106Using this concept, Chang et al. have reported a chemosensor 40 in which the pyrene moiety acts as a fluorophore and a fluorescence-quenching hydrazide moiety plays the role of a signaling handle (Fig. 36).107 The pyrenecarbohydrazide probe 40 displayed a high selectivity towards Cu2+ ions via Cu2+-induced catalytic hydrolysis to pyrenecarboxylic acid and hydrazine. 1H NMR spectroscopy and mass spectrometry also confirmed the hydrolysis of probe 40. It exhibited a very weak fluorescence due to PET which was remarkably enhanced (130-fold) at 392 nm upon addition of Cu2+ in 10% aqueous DMSO solution (Tris-buffered at pH 7.0). Moreover, the Cu2+ selective fluorescence signaling behaviour of 40 was less prominent as the space between the hydrazide functionality and the pyrene fluorophore increased. Probe 40 has a wide application in environmentally related samples, especially for semiconductor waste water sample over a wide pH range. The detection limit of 40 for Cu2+ was 5.93 × 10−8 M and 6.93 × 10−8 M (0.005 ppm) in the waste water sample.
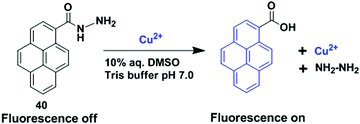 | ||
| Fig. 36 The sensing mechanism of compound 40 with Cu2+ by catalytic hydrolysis process. Reproduced from ref. 107 with permission from the Elsevier, copyright 2017. | ||
Rhodamine derivatives as chemosensors for Cu2+ detection were first utilized by Czarnik in 1997. Even now, they are used for the detection of cations and anions given their excellent photophysical properties and the ring opening process. In general, the carbonyl group in the rhodamine spirolactam form gets activated by complexation with specific metal ions using certain solvent systems and pH.108 Kim et al. introduced a novel fluorescent sensor, a rhodamine based derivative bearing a 1,8-naphthalimide group which performed as a dual-mode sensor for Cu2+ using two mechanisms, one is the rhodamine ring-opening mechanism and the other, a ratiometric displacement from Zn2+ complexation with the ligand in CH3CN–HEPES buffer solution.109
In 2009, Yoon et al. reported a rhodamine fluorophore combined with the pyrene moiety, which was utilized as a ratiometric and “off–on” fluorescent sensor 41 for the selective recognition of Cu2+.110 Herein, the spirolactam structure (non-fluorescent) of the rhodamine derivative sensor 41 was converted into a ring-opened amide form by complexation with Cu2+ and this gave rise to strong fluorescence emission and colour changes from primrose yellow to pink (Fig. 37). From the fluorescence spectra, clear ratiometric changes of ligand 41 (20 μM) were obtained upon treatment with Cu2+ in CH3CN–HEPES buffer solution. A significant decrease in the fluorescence intensity of 424 nm and a new emission band at 575 nm, with a clear isoemission point was attributed to the Cu2+ induced ring opening process of 41. The absorption spectra of 41 showed prominent changes which were reversible by reversible titration using EDTA/Cu2+. The 41 + Cu2+ complex followed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio which was calculated from the absorption spectra data of Job's plots and the nonlinear fitting of the titration curve.
1 stoichiometric ratio which was calculated from the absorption spectra data of Job's plots and the nonlinear fitting of the titration curve.
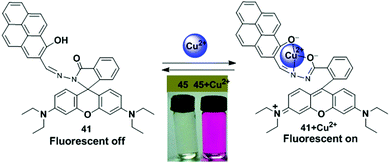 | ||
| Fig. 37 Proposed binding mechanism for 41 with Cu2+ and a photo of 41 (20 μM) as a selective naked-eye chemosensor for Cu2+. Reproduced from ref. 110 with permission from the American Chemical Society, copyright 2009. | ||
5.8. Chemosensor based on the paramagnetic nature and heavy atom effect of Cu2+
The intrinsic properties of heavy and transition metal cations usually quench the emission of organic luminophores. The emission behaviour of the metal-complexes is directly related to the periodic table and the electron configuration of the metal ions. For example, the paramagnetic Cu2+ ion has one electron in its dx2–dy2 orbital which is responsible for fluorescence quenching through an electron or energy transfer process. As the energy of this orbital places between the HOMO and LUMO of the excited fluorophore, so a non-radiative decay of the excited fluorophore can easily occur.19,106b,111 The free electron of Cu2+ also has the tendency to quench the fluorescence via spin–orbit coupling that can be explained by the heavy atom effect. The ‘heavier’ ions binding with the receptor increases the spin–orbit coupling, and hence leads to reduced fluorescence quantum yields and lifetimes.19,37bPeriasamy et al. have described the fluorescent chemosensor 42 bearing a pyrene and benzothiozole hydrazide, which shows considerably high fluorescence in the unbound state (Fig. 38).112 Upon complexation with the paramagnetic Cu2+ ions, quenching interactions probably dominate the emission characteristics of these complexes. The probe 42 displays a high sensitivity and selectivity in DMSO–H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) for Cu2+ due to chelation with 42. The ligand 42 + Cu2+ ensemble shows a high sensitivity towards S2− ions. The UV-vis titration of 42 showed that the absorption bands at 395 and 410 nm gradually reduced and the band at 455 nm increased upon addition of Cu2+ (0–100 μM). The association constant (Ka) of 42 with Cu2+ ions was found to be 2.3 × 105 M−1. The change in the appearance of solution 42 from the yellow to brown colour was observed after the addition of Cu2+ ions (100 μM) which can be seen by the naked eye. Moreover, the addition of Cu2+ to the solution of 42 gradually quenched the fluorescence emission intensity at 510 nm which was explained by the paramagnetic nature and the CHEQ effect of the Cu2+ ions. The ligand 42 was cell-permeable, and was efficiently utilized for the recognition of copper ions in living cells and in real water samples.
2 v/v) for Cu2+ due to chelation with 42. The ligand 42 + Cu2+ ensemble shows a high sensitivity towards S2− ions. The UV-vis titration of 42 showed that the absorption bands at 395 and 410 nm gradually reduced and the band at 455 nm increased upon addition of Cu2+ (0–100 μM). The association constant (Ka) of 42 with Cu2+ ions was found to be 2.3 × 105 M−1. The change in the appearance of solution 42 from the yellow to brown colour was observed after the addition of Cu2+ ions (100 μM) which can be seen by the naked eye. Moreover, the addition of Cu2+ to the solution of 42 gradually quenched the fluorescence emission intensity at 510 nm which was explained by the paramagnetic nature and the CHEQ effect of the Cu2+ ions. The ligand 42 was cell-permeable, and was efficiently utilized for the recognition of copper ions in living cells and in real water samples.
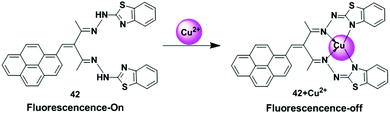 | ||
| Fig. 38 Proposed sensing mechanism of probe 42 for Cu2+. Reproduced from ref. 112 with permission from the John Wiley and Sons, copyright 2020. | ||
Similarly, quenching is promoted by paramagnetic Cu2+ ions via the heavy atom effect in a pyrene-based dipicolylamine derivative sensor 43 (Fig. 39).113 The compound 43 selectively recognized Cu2+ and Fe3+ over the other metal cations Ni2+, Mg2+, Cd2+, Hg2+, Na+, K+, Ca2+, Co2+, Cr3+, Pb2+ and Zn2+. Upon treatment of Cu2+ ions, sensor 43 exhibited well-defined absorption bands in MeOH solution in which a newly appeared band at 660 nm with red shifted peaks at 290, 333 and 358 nm were observed owing to the d–d transition of Cu(II). This phenomenon was distinguished by naked eye detection. From the results of UV-vis experiments, the stoichiometry of the Job's plot was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the binding constant 1.48 × 105 M−1 for the 43 + Cu2+ system. Moreover, 1H NMR spectroscopic titration experiments in CD3OD revealed that the seventeen aromatic protons of free receptor 43 in the 7.00–8.85 ppm region disappeared in the presence of Cu2+ ions due to the paramagnetic effect of the Cu2+ (d9 system). The fluorescence spectrum of chemosensor 43 in methanol solution showed an emission band at 392 nm (Φ ∼ 0.31) which was quenched significantly (Φ ∼ 0.05). This behaviour was observed because of the efficient non-radiative deactivation by Cu2+, which resulted in the enhanced spin–orbit coupling associated with the heavy atom effect of the complexed Cu2+.
1 with the binding constant 1.48 × 105 M−1 for the 43 + Cu2+ system. Moreover, 1H NMR spectroscopic titration experiments in CD3OD revealed that the seventeen aromatic protons of free receptor 43 in the 7.00–8.85 ppm region disappeared in the presence of Cu2+ ions due to the paramagnetic effect of the Cu2+ (d9 system). The fluorescence spectrum of chemosensor 43 in methanol solution showed an emission band at 392 nm (Φ ∼ 0.31) which was quenched significantly (Φ ∼ 0.05). This behaviour was observed because of the efficient non-radiative deactivation by Cu2+, which resulted in the enhanced spin–orbit coupling associated with the heavy atom effect of the complexed Cu2+.
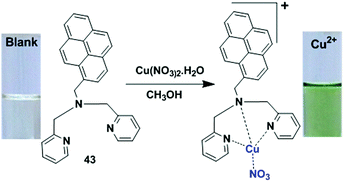 | ||
| Fig. 39 Possible binding interaction and colorimetric change of ligand 43 with Cu2+. Reproduced from ref. 113 with permission from the Elsevier, copyright 2017. | ||
Pandey et al. investigated the difference in the photophysical properties of probes 44, where coumarin–pyrene conjugates form with the non-conjugated form of probe 45 for the selective detection of Cu2+ (Fig. 40).114 Herein, it was observed that the paramagnetic nature of the Cu2+ ions have a great influence on the conjugated system of 44 rather than 45. The probe 44 has a conjugated system exhibiting C–H–π, π–π interactions, and H-bonding interactions. Successive addition of Cu2+ ions (1–10 μM) results in the gradual quenching of fluorescence at 430 nm indicating the high sensitivity of probe 44 towards Cu2+ ions. The phenomenon behind the quenching of fluorescence can be attributed to the combined effect of donation of electrons from the fluorophore fragment to the adjacent metal ion and is due to the paramagnetic nature of the Cu2+ ions. The binding constant of 44 with Cu2+ was calculated to be 2.4 × 104 M−1. Besides, sensor 45 showed no prominent change due to its non-conjugated form. Moreover, sensor 44 can be effectively applied in medical sciences for the detection of Cu2+ levels in kidney cell lining using its ability for quenching fluorescence.
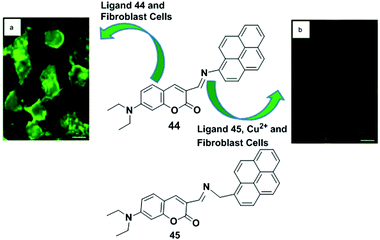 | ||
| Fig. 40 Structure of chemosensors 44 and 45. (a) Fluorescence images of 44 in a kidney cell line: cos-7 cells treated with 44 (5 mM, 20 min); (b) loss of fluorescence after addition of CuCl2 (10 mM, 30 min). Reproduced from ref. 114 with permission from the Elsevier, copyright 2016. | ||
Yamato and co-workers have described the reverse PET mechanism for the detection of paramagnetic Cu2+ ions by the heteroditopic receptor 46.115 As Cu2+ has an unfilled d orbital, it probably quenches the emission of the fluorophore via electron transfer.116 The receptor 46 has a thiacalix[4]arene moiety with two different side arms in which two pyrene-appended triazole rings are incorporated at one side of the thiacalix[4]arene cavity and the other side contains two urea moieties with various phenyl groups (Fig. 41). Herein, an effective positive allosteric effect of 46 was also observed for Ag+ with Cl−. Besides, the UV-vis absorption, fluorescence spectra and 1H NMR spectroscopic titration experiments of 46 showed changes in the presence of transition metal cations (Ag+, Cu2+ and Hg2+) and anions in the CH2Cl2–DMSO solvent system. The interaction of ligand 46 with Ag+ ion increased the monomer emission at 393 nm and decreased the excimer emission at 486 nm with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. Furthermore, upon addition of Cu2+, monomer and excimer emissions of free 46 were significantly quenched. This was because the binding with the pyrene-appended triazole groups caused a reverse PET from the pyrene moieties to the triazole groups. The association constant for the complexation of 46 + Cu2+ was found to be 330
1 binding stoichiometry. Furthermore, upon addition of Cu2+, monomer and excimer emissions of free 46 were significantly quenched. This was because the binding with the pyrene-appended triazole groups caused a reverse PET from the pyrene moieties to the triazole groups. The association constant for the complexation of 46 + Cu2+ was found to be 330![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 23
000 ± 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1.
100 M−1.
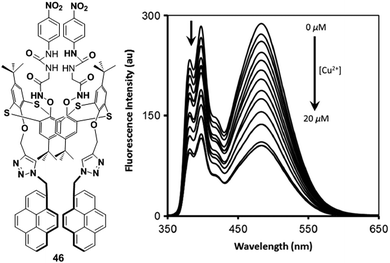 | ||
| Fig. 41 The molecular structure of fluorescence chemosensor 46, showing fluorescence quenching in the presence of Cu2+ ions. Reproduced from ref. 115 with permission from the Elsevier, copyright 2014. | ||
5.9. Miscellaneous
As discussed in the preceding part of this review, chemosensors operating using different well-known mechanisms have been reported. However, there are still other types of chemosensors which do not belong to the above classified mechanisms or they fall into the category of following several mechanisms for Cu2+ detection. Herein, we will try to briefly discuss these sensors.Two new fluorescent sensors 47, 48 based on thiacalix[4]arenes bearing two pyrene groups were developed by Kumar and co-workers for the recognition of Cu2+ and CN− ions (Fig. 42).117 The authors reported that the former receptor 47 undergoes a reverse PET process, whereas the latter 48 follows the monomer and excimer emission mechanism with a ratiometric response for Cu2+ detection in CH2Cl2/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Moreover, the UV-vis absorption spectra of both receptors upon interaction with Cu2+ showed a decrease of the blue shift band and the formation of a new red shift band. This is because of the collaboration between the Cu2+ ion with the nitrogen atoms of the amide groups which reduces the electron donating ability of the N atom and induces the red shift absorption spectra due to PCT mechanism. The strong blue fluorescence of compound 47 indicates the typical monomer emission of pyrene at 377 nm which confirms there is no π–π interaction between two pyrene units. Upon addition of Cu2+ ions (6.0–600 μM) to the solution of 47 (6.0 μM), the fluorescence emission intensity significantly decreased due to reverse PET from the pyrene units to the nitrogen atom. The association constant Ka of 47 with Cu2+ was calculated to be 8.55 × 105 M−1. On the other hand, the fluorescence properties of compound 48 were completely different compared with compound 47. The compound 48 showed a weak monomer emission at 375 nm and a strong intramolecular excimer emission at 467 nm. Upon treatment with 60 mM (10 equiv.) of Cu2+ ions, the titration profile of 48 exhibited a ratiometric response with monomer enhancement and a excimer emission quenching. The relative intensity ratio of monomer to excimer emission (IM/IE) of free ligand 48 was 0.47 and it increased by 11.7-fold to 5.52 on addition of Cu2+ ion. A selectivity test of 47 and 48 was carried out in the presence of various metal ions with Cu2+ which revealed 47 + Cu2+ and 48 + Cu2+ complexes to be selective chemosensors. This observation was in agreement with the 1
1, v/v). Moreover, the UV-vis absorption spectra of both receptors upon interaction with Cu2+ showed a decrease of the blue shift band and the formation of a new red shift band. This is because of the collaboration between the Cu2+ ion with the nitrogen atoms of the amide groups which reduces the electron donating ability of the N atom and induces the red shift absorption spectra due to PCT mechanism. The strong blue fluorescence of compound 47 indicates the typical monomer emission of pyrene at 377 nm which confirms there is no π–π interaction between two pyrene units. Upon addition of Cu2+ ions (6.0–600 μM) to the solution of 47 (6.0 μM), the fluorescence emission intensity significantly decreased due to reverse PET from the pyrene units to the nitrogen atom. The association constant Ka of 47 with Cu2+ was calculated to be 8.55 × 105 M−1. On the other hand, the fluorescence properties of compound 48 were completely different compared with compound 47. The compound 48 showed a weak monomer emission at 375 nm and a strong intramolecular excimer emission at 467 nm. Upon treatment with 60 mM (10 equiv.) of Cu2+ ions, the titration profile of 48 exhibited a ratiometric response with monomer enhancement and a excimer emission quenching. The relative intensity ratio of monomer to excimer emission (IM/IE) of free ligand 48 was 0.47 and it increased by 11.7-fold to 5.52 on addition of Cu2+ ion. A selectivity test of 47 and 48 was carried out in the presence of various metal ions with Cu2+ which revealed 47 + Cu2+ and 48 + Cu2+ complexes to be selective chemosensors. This observation was in agreement with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (H/G) binding model for 47 + Cu2+ and 48 + Cu2+ complexes respectively, which was also confirmed by the method of continuous variation (Job's plot).
2 (H/G) binding model for 47 + Cu2+ and 48 + Cu2+ complexes respectively, which was also confirmed by the method of continuous variation (Job's plot).
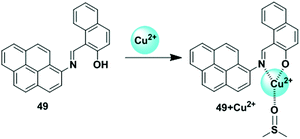 | ||
| Fig. 43 Schematic representation of possible sensing mechanism of probe 49 for Cu2+. Reproduced from ref. 118 with permission from the Elsevier, copyright 2014. | ||
A pyrene containing Schiff base colorimetric sensor 49 was synthesized via a simple one-pot reaction (Fig. 43).118 The probe 49 displayed a high sensitivity and selectivity towards Cu2+ and the sensitivity was not affected with by the presence of other relevant metal ions. In the solvent mixture DMSO/H2O (v/v = 8/2, buffered with HEPES, pH = 7.4), the absorption peaks of probe 49 at 355 and 452 nm were slowly decreased on gradual addition of Cu2+ and the peaks were blue-shifted to 326 and 410 nm with two isosbestic points at 349 and 414 nm, respectively with changes of colour from yellow to pale lemon. This phenomenon is due to the formation of the Cu2+-assisted 49-Cu2+–DMSO complex. The absorption spectrum on changing the Cu2+ concentration indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between the host 49 and the Cu2+ ions. 1H NMR spectroscopic titration experiments of 49 also found that the proton signal of the OH became broad and slightly shifted downfield with increasing Cu2+ concentration. The detection limit of 49 for Cu2+ ions was calculated to be (2.17 ± 0.02) × 10−6 M.
1 stoichiometry between the host 49 and the Cu2+ ions. 1H NMR spectroscopic titration experiments of 49 also found that the proton signal of the OH became broad and slightly shifted downfield with increasing Cu2+ concentration. The detection limit of 49 for Cu2+ ions was calculated to be (2.17 ± 0.02) × 10−6 M.
In 2010, Yen and coworkers synthesized a novel colorimetric and fluorometric receptor 50 containing a pyrene unit and a 4-methylphenylthiourea moiety for selectively sensing Cu2+ and Hg2+ (Fig. 44).119 The coordination with Cu2+ and Hg2+ ions show colour and fluorescence changes of 50 in aqueous solution (DMSO/H2O = 4/1, buffered with HEPES, pH 7.8) which allowed them to be distinguished from other metal ions. Moreover, the designed sensor 50 can recognize Cu2+ through binding with the thiourea group. For this reason, the typical pyrene absorption bands of 50 in the region of 235–350 nm gradually increased at 278, 334 nm with a shoulder peak at 388 nm and decreased at 348 nm after addition of Cu2+. The colour of the 50 + Cu2+ solution changed from pale yellow to green-yellow. Moreover, the enhancement of emission intensity (45 times more) at 396 and 439 nm was observed for 50 upon treatment with Cu2+ ions, with a colour change from pale to strong blue. The Cu2+ ion-induced complexation was also confirmed by NMR and ESI-MS spectra. The Job's plot determined the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with 1.09 × 104 M−1 binding constant for the 50 + Cu2+ complex.
1 stoichiometry with 1.09 × 104 M−1 binding constant for the 50 + Cu2+ complex.
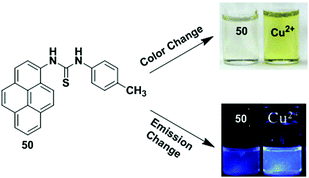 | ||
| Fig. 44 The colour and fluorescence changes of chemosensor 50 upon addition of Cu2+ ions. Reproduced from ref. 119 with permission from the Elsevier, copyright 2010. | ||
Liu et al. have developed the pyrene based diaminomaleonitrile chemosensors 51 and 52 (1 × 10−5 M) which can effectively sense Cu2+ in acetonitrile–water solution (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mM HEPES, pH = 7) (Fig. 45).120 The absorption spectra of 51 exhibits a strong red-shifted band at approximately 421 nm from that of pure pyrene at around 355 nm. On the other hand, compound 52 showed the characteristic absorption band of pyrene at 342 nm and another strong band at 380 nm, owing to the ICT mechanism. Upon gradual addition of Cu2+ to 52 (10 μM), the absorbance at 380 nm gradually decreased and the band centered at 280 nm increased, because of the Cu2+ interaction with diaminomaleonitrile moiety which prevents the charge-transfer of pyrene to the electron withdrawing diaminomaleonitrile moiety. This result is consistent with the spectral changes of receptor 51.91 The receptors 51 and 52 exhibited weak fluorescence (quantum yields in acetonitrile are 0.005 and 0.02, respectively) due to the presence of the Schiff base moiety and the overlap of the emission and absorption spectra. However, only ligand 52 gave a red-shifted band at around 590 nm in addition to the other band close to the characteristic emission band of pyrene. A significant fluorescence enhancement of 52 was observed at the emission band of pyrene on titration of Cu2+. The quantum yield of the fluorescence emission band of 52 + Cu2+ complex was 0.42, approximately 20-times more than that of free 52. The apparent association constant (Ka) of Cu2+ binding to 52 was estimated to be 5.2 × 103 M−1. However, the authors observed that on changing the solvent system from CH3CN/H2O to PBS/DMF, the receptor cannot bind with Cu2+ as DMF trapped the Cu2+ and hindered the interaction between the ligand 52 and Cu2+.
1, 10 mM HEPES, pH = 7) (Fig. 45).120 The absorption spectra of 51 exhibits a strong red-shifted band at approximately 421 nm from that of pure pyrene at around 355 nm. On the other hand, compound 52 showed the characteristic absorption band of pyrene at 342 nm and another strong band at 380 nm, owing to the ICT mechanism. Upon gradual addition of Cu2+ to 52 (10 μM), the absorbance at 380 nm gradually decreased and the band centered at 280 nm increased, because of the Cu2+ interaction with diaminomaleonitrile moiety which prevents the charge-transfer of pyrene to the electron withdrawing diaminomaleonitrile moiety. This result is consistent with the spectral changes of receptor 51.91 The receptors 51 and 52 exhibited weak fluorescence (quantum yields in acetonitrile are 0.005 and 0.02, respectively) due to the presence of the Schiff base moiety and the overlap of the emission and absorption spectra. However, only ligand 52 gave a red-shifted band at around 590 nm in addition to the other band close to the characteristic emission band of pyrene. A significant fluorescence enhancement of 52 was observed at the emission band of pyrene on titration of Cu2+. The quantum yield of the fluorescence emission band of 52 + Cu2+ complex was 0.42, approximately 20-times more than that of free 52. The apparent association constant (Ka) of Cu2+ binding to 52 was estimated to be 5.2 × 103 M−1. However, the authors observed that on changing the solvent system from CH3CN/H2O to PBS/DMF, the receptor cannot bind with Cu2+ as DMF trapped the Cu2+ and hindered the interaction between the ligand 52 and Cu2+.
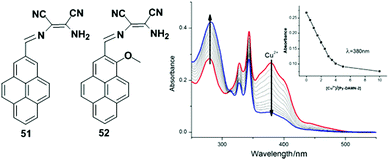 | ||
| Fig. 45 The structure of probes 51 and 52. Absorption spectra of 52 in presence of various amounts of Cu2+ in acetonitrile–water solution. Reproduced from ref. 120 with permission from the Elsevier, copyright 2015. | ||
In 2014, Goswami and co-workers designed and synthesized a new pyrene-based fluorescence probe 53 which was capable of working in both prokaryotic and eukaryotic living cells for the fluorogenic detection of Cu2+. The complexation of receptor 53 with Cu2+ occurs through a chelation-enhanced fluorescence (CHEF) mechanism and several other reasons (Fig. 46).121 In a CH3CN–HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, at pH 7.5) protic solvent system, the free receptor 53 shows a stronger fluorescence compared with aprotic CH3CN solvent. This happens because of the hydrogen bonding interaction of the solvent with the N and O lone electron pairs of 53 which weakens the intramolecular radiationless transition leading to the red shifted emission maxima (λem) on increasing protonation by the solvent. Moreover, the fluorescence behaviour of 53 upon addition of Cu2+ was enriched owing to the reduced energy gap between the ground state and the excited state of the metal bound species by possible metal–ligand charge transfer (ICT) and chelation. In addition, ESI LC-MS spectral analysis and the Job's plot confirmed the formation of a mononuclear complex of 53 with Cu2+. The fluorescence titration experiments confirmed the minimum detection limit of copper was 1.21 μM using 10 μM of the ligand 53. The selectivity of the fluorescence enhancement of 53 with Cu2+ (2.0 equivalents) was investigated in the presence of other metal ions (8.0 equivalents). With the exception of Cd2+ and Co2+, no other competing metal ions inhibited the detection of Cu2+ by 53. The NMR spectroscopic data in DMSO-d6 also demonstrated that the adduct formation between Cu2+ and 53 results in the disappearance of the phenolic protons and downfield shift of the protons of aromatic ring. The receptor 53 is cell membrane permeable and would enable detection of intracellular copper present in a biological system being incubated with copper perchlorate salt (1 mg mL−1) for 45 minutes.
3, v/v, at pH 7.5) protic solvent system, the free receptor 53 shows a stronger fluorescence compared with aprotic CH3CN solvent. This happens because of the hydrogen bonding interaction of the solvent with the N and O lone electron pairs of 53 which weakens the intramolecular radiationless transition leading to the red shifted emission maxima (λem) on increasing protonation by the solvent. Moreover, the fluorescence behaviour of 53 upon addition of Cu2+ was enriched owing to the reduced energy gap between the ground state and the excited state of the metal bound species by possible metal–ligand charge transfer (ICT) and chelation. In addition, ESI LC-MS spectral analysis and the Job's plot confirmed the formation of a mononuclear complex of 53 with Cu2+. The fluorescence titration experiments confirmed the minimum detection limit of copper was 1.21 μM using 10 μM of the ligand 53. The selectivity of the fluorescence enhancement of 53 with Cu2+ (2.0 equivalents) was investigated in the presence of other metal ions (8.0 equivalents). With the exception of Cd2+ and Co2+, no other competing metal ions inhibited the detection of Cu2+ by 53. The NMR spectroscopic data in DMSO-d6 also demonstrated that the adduct formation between Cu2+ and 53 results in the disappearance of the phenolic protons and downfield shift of the protons of aromatic ring. The receptor 53 is cell membrane permeable and would enable detection of intracellular copper present in a biological system being incubated with copper perchlorate salt (1 mg mL−1) for 45 minutes.
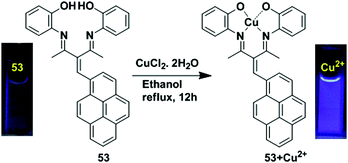 | ||
| Fig. 46 The binding modes of 53 + Cu2+ and visual colour change of 53 with the addition of 2 equiv. of CuCl2·2H2O under UV light. Reproduced from ref. 121 with permission from the Royal Society of Chemistry. | ||
| Probe no. | Solvent medium | Mechanism | Association constant | Limit of detection | λ ex/λem, (nm) | Stoichiometry (sensor/Cu2+) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | EtOH | Monomer and excimer emission | 3.5 × 105 | 1.44 × 10−7 | 344/379, 397, 484 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 57 |
| 2 | CH3CN/H2O | Monomer and excimer emission | NA | 1.87 × 10−8 | 343/396, 485 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 58 |
| 3 | CH3CN/CH2Cl2 | Monomer and excimer emission | 3.57 × 105 | NA | 367/415, 518 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 59 |
| 4 | Tris–HNO3 buffer solution | Monomer and excimer emission | 2.18 × 104 | 2 × 10−8 | 342/375, 460 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Environmental samples, live cells (HeLa cells) | 60 |
| 5 | Dioxane | Monomer and excimer emission | NA | NA | 360/420 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 61 |
| 6 | Dioxane | Monomer and excimer emission | 3.10 × 10−2 | NA | 360/420, 500 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 61 |
| 7 | CH2Cl2 | Dynamic excimer to static excimer | 4.4 | NA | 335/440 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 62 |
| 8 | CH3CN/H2O | Static excimer emission | 2.8 × 104 | NA | 342/375, 455 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 63 |
| 9 | CH3CN | Static excimer emission | 5.42 × 105 | NA | 360/388, 460 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 64 |
| 10 | CH3CN | NA | NA | NA | NA | NA | NA | 64 |
| 11 | CH3CN | NA | NA | NA | NA | NA | NA | 64 |
| 12 | Tris–HCl buffer containing CH3CN/H2O | Static excimer emission | 4.583 × 103 | 4 × 10−8 | 350/452 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Drinking water | 65 |
| 13 | CH3CN | Excimer emission | 1.96 × 106 | 9.72 × 10−7 | 395/455 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 66 |
| 14 | CH3CN | Dynamic excimer to static excimer | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
NA | 342/447 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 67 |
| 15 | EtOH/H2O | Excimer to monomer emission | 11.53 | 40 × 10−9 | 340/378, 466 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
NA | 68 |
| 16 | CH3OH/H2O | Excimer switch-off | 4.8 × 106 | NA | 410/466, 520–560 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (HEK 293 cells) | 69 |
| 17 | Mixed liposomes | Monomer and excimer emission | NA | NA | 342/395, 470 | NA | NA | 70 |
| 18 | Mixed liposomes | Monomer and excimer emission | NA | NA | 342/395, 471 | NA | NA | 70 |
| 19 | Mixed liposomes | Monomer and excimer emission | NA | NA | 342/395, 472 | NA | NA | 70 |
| 20 | Mixed liposomes | Monomer and excimer emission | NA | NA | 342/395, 473 | NA | NA | 70 |
| 21 | CH3CN | Monomer and excimer emission | 1.89 × 105 | NA | 343/482 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 71 |
| 22 | CH3CN/CH2Cl2 | PET | 1.29 × 105 | 8.80 × 10−8 | 367/405 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 72 |
| 23 | CH3CN/CH2Cl2 | PET | 1.55 × 104 | 4.94 × 10−7 | 367/405 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 72 |
| 24 | CH3CN/CH2Cl2 | PET | NA | NA | 367/405 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 72 |
| 25 | CH3CN/H2O | PET | 5 × 108 | 2.73 × 10−6 | 385/468 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (RAW 264.7 cells) | 73 |
| 26 | CH3CN | PET | 5.71 × 105 | 3.91 × 10−6 | 350/429 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 74 |
| 27 | CH3OH/H2O | PET | 2.75 × 103 | NA | 360/455 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell | 75 |
| 28 | CH3OH/H2O | PET | 5.55 × 103 | NA | 350/417 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 76 |
| 29 | CH3CN/H2O | PET | 1.0 × 104 | 0.04 × 10−6 | 346/389 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (RAW 264.7 cells) | 77 |
| 30 | DMSO–H2O | PET | 1.16 × 104 | 0.26 × 10−6 | 393/463 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (RAW 264.7 cells) | 78 |
| 31 | CH3CN/H2O | PET | NA | 7.8 × 10−9 | 305/444 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (HeLa cells), real samples | 79 |
| 32 | CH3CN | PET | 7.74 × 106 | 4.5 × 10−6 | 350/388, 409, 473 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 80 |
| 33 | CH3CN | PET | NA | 3.60 × 10−7 | 340/444 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 81 |
| 34 | CH3CN | PCT | NA | NA | 343/470 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 82 |
| 35 | DMF/HEPES buffer | LMCT | NA | 8.5 × 10−6 | 305/370 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 84 |
| 36 | CH3CN/H2O | LMCT | 5.65 × 105 | 0.503 × 10−6 | 384/429 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Real samples | 85 |
| 37 | DMSO/H2O | ILCT | 6.5789 × 104 | 6.865 × 10−8 | 370/390, 412 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Real samples | 86 |
| 38 | H2O/DMF | AEE | 1.89 × 109 | 35 × 10−9 | 370/455 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (HeLa cells) | 89 |
| 39 | CH3CN, CH3CN/H2O | PET, AIE | NA | 2.5 × 10−6 | 393/440, 454.5, 509 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Antioxidant property | 90 |
| 40 | 10% aqueous Tris-buffered DMSO solution | Cu2+ induced catalytic hydrolysis | NA | 5.93 × 10−8 | 340/392 | NA | Simulated semiconductor waste water | 92 |
| 41 | CH3CN/HEPES buffer solution | Cu2+ induced ring opening process | 2.5 × 104 | NA | 520/575 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 94 |
| 42 | DMSO/H2O | Paramagnetic nature, CHEQ process | 2.3 × 105 | 0.73 × 10−9 | 440/510 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell (A549 cells) and real water samples | 96 |
| 43 | CH3OH solution | Heavy atom effect | 1.48 × 105 | 10−6 M | 340/392 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 97 |
| 44 | Aqueous HEPES buffer (H2O/CH3CN) | Paramagnetic nature | 2.4 × 104 | NA | 353/435 | NA | Live cell (COS-7 kidney cells), biological samples | 98 |
| 45 | Aqueous HEPES buffer (H2O/CH3CN) | NA | NA | NA | NA | NA | NA | 98 |
| 46 | CH2Cl2/DMSO | Paramagnetic nature, reverse PET | 3.3 × 105 | NA | 343/393, 486 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 99 |
| 47 | CH2Cl2/CH3CN | Reverse PET | 4.939 | NA | 342/377 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 101 |
| 48 | CH2Cl2/CH3CN | Monomer and excimer emission | 10.3086 | NA | 342/375, 467 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
NA | 101 |
| 49 | DMSO/H2O | Cu2+-Assisted-complexation | NA | 2.17 × 10−6 | NA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Environmental systems | 102 |
| 50 | DMSO/H2O | Cu2+ induced complexation | 1.09 × 104 | 1.0 × 10−4 | 348/396, 439 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | 103 |
| 51 | CH3CN/H2O | Inhibition of ICT process | NA | NA | NA | NA | NA | 104 |
| 52 | CH3CN/H2O | Inhibition of ICT process | 5.2 × 103 | NA | 340/420, 590 | NA | NA | 104 |
| 53 | CH3CN/HEPES buffer | CHEF and ICT process | NA | 1.21 × 10−6 | 330/414 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Live cell | 105 |
6. Conclusion and future perspectives
This review article focuses on a particular type of fluorescent probe that contains a pyrene functional group and that have become interesting tools in modern biology and in environmental work. We have focused on the design of fluorescent sensors based on different mechanisms including monomer and excimer emission, PET, PCT, AIE, LMCT, CHEQ, Cu2+-assisted reaction and others for the detection of Cu2+. Interestingly, there have been a large number of fluorescent sensors which show fluorescence enhancement following monomer and excimer emission and inhibition of PET for sensing Cu2+. In the monomer and excimer emission mechanism, the fluorescence response can experience three main spectroscopic signals as monomer, static or dynamic excimer emission processes depending on the binding nature of the ligand to Cu2+. Based on the monomer and excimer emission mechanism, Yamato and co-workers as well as Kumar et al. have developed several ratiometric chemosensors which can easily diagnose Cu2+ ions. Moreover, a Cu2+-promoted reaction based chemosensor in protic solvent systems was reported by Chang et al. and Yoon et al. and such systems may exhibit unique advantages and find special applications in environmental systems. Some probes based on the pyrene moiety show fluorescence quenching due to the paramagnetic nature of Cu2+ ions and LMCT processes or reverse PET mechanisms with prominent selective and sensitive responses for Cu2+ ions. It is noteworthy to mention that N- and/or O- and/or S-containing ligands hold more promise for the detection of Cu2+ ions. However, there are very few chemosensors described with reversible properties, which is a weakness in this type of host–guest chemistry. Therefore, it is necessary to consider the need for recycle of these resources to avoid waste generation. Besides, the design and synthesis of fully water soluble efficient pyrene chemosensor remains a challenging task for the detection of Cu2+. Most of the pyrene sensors in this article are found to be only partially soluble in water, on mixing with organic solvents like CH3CN, CH3OH, CH2Cl2. The introduction of polar functional groups or polar moieties to the chemosensor may increase the water solubility and make it more efficient for sensing Cu2+ in biological and environmental arenas. This review article could help to explore new concepts for designing and synthesizing chemosensors based on pyrene-derivatives and more compelling advances are anticipated in the near future.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
CR thanks the EPSRC (EP/R023816/1) for an overseas travel grant.Notes and references
- (a) B. Valeur, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed; (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Signaling recognition events with fluorescent sensors and switches, Chem. Rev., 1997, 97, 1515 CrossRef CAS.
- A. P. S. Gonzales, M. A. Firmino, C. S. Nomura, F. R. P. Rocha, P. V. Oliveira and I. Gaubeur, Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry, Anal. Chim. Acta, 2009, 636, 198–204 CrossRef CAS.
- J. S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska and M. V. Zoriy, Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (slugs–genus arion) measured by laser ablation inductively coupled plasma mass spectrometry, Anal. Chem., 2007, 79, 6074–6080 CrossRef CAS.
- Y. Liu, P. Liang and L. Guo, Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry, Talanta, 2005, 68, 25–30 CrossRef CAS.
- A. A. Ensafi, T. Khayamian, A. Benvidi and E. Mirmomtaz, Simultaneous determination of copper, lead and cadmium by cathodic adsorptive stripping voltammetry using artificial neural network, Anal. Chim. Acta, 2006, 561, 225–232 CrossRef CAS.
- J. S. Becker, M. V. Zoriy, C. Pickhardt, N. Palomero-Gallagher and K. Zilles, Imaging of copper, zinc, and other elements in thin section of human brain samples (hippocampus) by laser ablation inductively coupled plasma mass spectrometry, Anal. Chem., 2005, 77, 3208–3216 CrossRef CAS.
- N. Mekjinda, S. Phunnarungsi, V. Ruangpornvisuti, R. J. Ritchie, I. Hamachi, A. Ojida and J. Wongkongkatep, Masking phosphate with rare earth elements enables selective detection of arsenate by dipycolylamine-ZnII chemosensor, Sci. Rep., 2020, 10, 2656 CrossRef CAS.
- (a) I. Takashima, M. Kinoshita, R. Kawagoe, S. Nakagawa, M. Sugimoto, I. Hamachi and A. Ojida, Design of ratiometric fluorescent probes based on arene-metal-ion interactions and their application to CdII and hydrogen sulfide imaging in living cells, Chem. – Eur. J., 2014, 20, 2184–2192 CrossRef CAS; (b) J. Guan, P. Zhang, T.-B. Wei, Q. Lin, H. Yao and Y.-M. Zhang, A highly selective PET-based chemosensor for instant detecting of Zn2+, RSC Adv., 2014, 4, 35797–35802 RSC; (c) S. Uchinomiya, N. Matsunaga, K. Kamoda, R. Kawagoe, A. Tsuruta, S. Ohdo and A. Ojida, Fluorescence detection of metabolic activity of the fatty acid beta oxidation pathway in living cells, Chem. Commun., 2020, 56, 3023–3026 RSC; (d) S. I. Reja, N. Sharma, M. Gupta, P. Bajaj, V. Bhalla, R. D. Parihar, P. Ohri, G. Kaur and M. Kumar, A highly selective fluorescent probe for detection of hydrogen sulfide in living systems: in vitro and in vivo applications, Chem. – Eur. J., 2017, 23, 9872–9878 CrossRef CAS; (e) A. Kathiravan, A. Gowri, T. Khamrang, M. D. Kumar, N. Dhenadhayalan, K.-C. Lin, M. Velusamy and M. Jaccob, Pyrene-based chemosensor for picric acid—fundamentals to smartphone device design, Anal. Chem., 2019, 91, 13244–13250 CrossRef CAS.
-
(a) S. Lee, K. K. Y. Yuen, K. A. Jolliffe and J. Yoon, Fluorescent and colorimetric chemosensors for pyrophosphate, Chem. Soc. Rev., 2015, 44, 1749–1762 RSC;
(b) H. N. Lee, Z. Xu, S. K. Kim, K. M. K. Swamy, Y. Kim, S.-J. Kim and J. Yoon, Pyrophosphate-selective fluorescent chemosensor
at physiological pH:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formation of a unique excimer upon addition of pyrophosphate, J. Am. Chem. Soc., 2007, 129, 3828–3829 CrossRef CAS.
formation of a unique excimer upon addition of pyrophosphate, J. Am. Chem. Soc., 2007, 129, 3828–3829 CrossRef CAS. - (a) W. Shi and H. Ma, Spectroscopic probes with changeable π-conjugated systems, Chem. Commun., 2012, 48, 8732–8744 RSC; (b) Bioinorganic Chemistry, ed. A. X. Trautwein, Wiley-VCH, Weinheim, 1997 Search PubMed; (c) Metals and Their Compounds in the Environment, ed. E. Merian, VCH, Weinheim, 1991 Search PubMed; (d) Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Macro-/micro-environment-sensitive chemosensing and biological imaging, Chem. Soc. Rev., 2014, 43, 4563 RSC.
- (a) N. Kaur and S. Kumar, Colorimetric metal ion sensors, Tetrahedron, 2011, 67, 9233–9264 CrossRef CAS; (b) E. V. Anslyn, Supramolecular Analytical Chemistry, J. Org. Chem., 2007, 72, 687–699 CrossRef CAS; (c) P. D. Beer, Transition-metal receptor systems for the selective recognition and sensing of anionic guest species, Acc. Chem. Res., 1998, 31, 71–80 CrossRef CAS; (d) C. Bargossi, M. C. Fiorini, M. Montalti, L. Prodi and N. Zaccheroni, Recent developments in transition metal ion detection by luminescent chemosensors, Coord. Chem. Rev., 2000, 208, 17–32 CrossRef CAS.
- A. B. Ellis and D. R. Walt, Guest editorial, Chem. Rev., 2000, 100, 2477–2478 CrossRef CAS.
- L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Luminescent chemosensors for transition metal ions, Coord. Chem. Rev., 2000, 205, 59–83 CrossRef CAS.
- K. Rurack, Flipping the light switch 'on'-the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions, Spectrochim. Acta, Part A, 2001, 57, 2161–2195 CrossRef CAS.
- (a) A. T. Afaneh and G. Schreckenbach, Fluorescence enhancement/quenching based on metal orbital control: computational studies of a 6-thienyllumazine-Based mercury sensor, J. Phys. Chem. A, 2015, 119, 8106–8116 CrossRef CAS; (b) J.-P. Desvergne, F. Fages, H. Bouas-Laurent and P. Marsau, tunable photoresponsive supramolecular systems, Pure Appl. Chem., 1992, 64, 1231 CAS.
- Z. Xu, J. Yoon and D. R. Spring, Fluorescent chemosensors for Zn2+, Chem. Soc. Rev., 2010, 39, 1996–2006 RSC.
- D. G. Barceloux and D. D. Barceloux, Copper, Clin. Toxicol., 1999, 37, 217–230 CAS.
- (a) Z. L. Harris and J. D. Gitlin, Genetic and molecular basis for copper toxicity, Am. J. Clin. Nutr., 1996, 63, 836S–841S CrossRef CAS; (b) I. H. Scheinberg and I. Sternlieb, Wilson disease and idiopathic copper toxicosis, Am. J. Clin. Nutr., 1996, 63, 842S–845S CrossRef CAS.
- (a) Y. Xiang, A. Tong, P. Jin and Y. Ju, New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity, Org. Lett., 2006, 8, 2863–2866 CrossRef CAS; (b) X. Zhang, Y. Shiraishi and T. Hirai, Cu(II)-selective green fluorescence of a rhodamine–diacetic acid conjugate, Org. Lett., 2007, 9, 5039–5042 CrossRef CAS.
- G. Li, Z. Xu, C. Chen and Z. Huang, A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim, Chem. Commun., 2008, 1774–1776 RSC.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in Living Cells, J. Am. Chem. Soc., 2009, 131, 2008–2012 CrossRef CAS.
- S.-P. Wu and S. R. Liu, A new water-soluble fluorescent Cu(II) chemosensor based on tetrapeptide histidyl-glycyl-glycyl-glycine (HGGG), Sens. Actuators, B, 2009, 141, 187–191 CrossRef CAS.
- H. Irving and R. J. P. Williams, Order of Stability of Metal Complexes, Nature, 1948, 162, 746–747 CrossRef CAS.
- T. L. Banfield and D. Husain, Electronic energy transfer from triplet state acridine to paramagnetic ions, Trans. Faraday Soc., 1969, 65, 1985–1991 RSC.
- A. W. Varnes, R. B. Dodson and E. L. Wehry, Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies, J. Am. Chem. Soc., 1972, 94, 946–950 CrossRef CAS.
- P. C. Bull, G. R. Thomas, J. M. Rommens, J. R. Forbes and D. W. Cox, The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene, Nat. Genet., 1993, 5, 327–337 CrossRef CAS.
- (a) D. Udhayakumari, S. Naha and S. Velmathi, Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–15, Anal. Methods, 2017, 9, 552–578 RSC; (b) M. Ware, Health benefits and risks of copper, Medical News Today, October 23, 2017; (c) B. Sarkar, Treatment of Wilson and Menkes diseases, Chem. Rev., 1999, 99, 2535–2544 CrossRef CAS; (d) J. A. Cowan, Inorganic Biochemistry: An Introduction, Wiley-VCH, New York, NY, USA, 2nd edn, 1997, pp. 133–134 Search PubMed; (e) M. DiDonato and B. Sarkar, Copper transport and its alterations in Menkes and Wilson diseases, Biochim. Biophys. Acta, 1997, 1360, 3–16 CrossRef CAS.
- (a) K. J. Barnham, C. L. Masters and A. I. Bush, Neurodegenerative diseases and oxidative stress, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS; (b) S. H. Hahn, M. S. Tanner, D. M. Danke and W. A. Gahl, Normal metallothionein synthesis in fibroblasts obtained from children with Indian childhood cirrhosis or copper-associated childhood cirrhosis, Biochem. Mol. Med., 1995, 54, 142–145 CrossRef CAS; (c) D. R. Brown, Copper and prion disease, Brain Res. Bull., 2001, 55, 165–173 CrossRef CAS; (d) D. Beyersmann, The significance of interactions in metal essentiality and toxicity, in Metals and Their Compounds in the Environment, ed. E. Merian, VCH, Weinheim, 1991, p. 491 Search PubMed.
- (a) M. Kaur, P. Kaur, V. Dhuna, S. Singh and K. Singh, A ferrocene–pyrene based ‘turn-on’ chemodosimeter for Cr3+–application in bioimaging, Dalton Trans., 2014, 43, 5707–5712 RSC; (b) I. Berlman, Handbook of Fluorescence Spectra of Aromatic Molecules, Academic Press, New York, 2nd edn, 1971 Search PubMed.
- M. Wang, J. Xu, X. Liu and H. Wang, A highly selective pyrene based “off–on” fluorescent chemosensor for cyanide, New J. Chem., 2013, 37, 3869–3872 RSC.
- (a) D. Udhayakumari, Chromogenic and fluorogenic chemosensors for lethal cyanide ion. A comprehensive review of the year 2016, Sens. Actuators, B, 2018, 259, 1022–1057 CrossRef CAS; (b) M.-H. Yang, P. Thirupathi and K.-H. Lee, Selective and sensitive ratiometric detection of Hg(II) Ions using a simple amino acid based sensor, Org. Lett., 2011, 13, 5028–5031 CrossRef CAS.
- (a) X.-L. Ni, S. Wang, X. Zeng, Z. Tao and T. Yamato, Pyrene-linked triazole-modified homooxacalix[3]arene: A unique C3 symmetry ratiometric fluorescent chemosensor for Pb2+, Org. Lett., 2011, 13, 552–555 CrossRef CAS; (b) Q. Dai, W. Liu, X. Zhuang, J. Wu, H. Zhang and P. Wang, Ratiometric fluorescence sensor based on a pyrene derivative and quantification detection of heparin in aqueous solution and serum, Anal. Chem., 2011, 83, 6559–6564 CrossRef CAS; (c) G. Sivaraman, T. Anand and D. Chellappa, Development of a pyrene based “turn on” fluorescent chemosensor for Hg2+, RSC Adv., 2012, 2, 10605–10609 RSC.
- F. Wang, R. Nandhakumar, J. H. Moon, K. M. Kim, J. Y. Lee and J. Yoon, Ratiometric fluorescent chemosensor for silver ion at physiological pH, Inorg. Chem., 2011, 50, 2240–2245 CrossRef CAS.
- L. Wang, M. Yu, Z. Liu, W. Zhao, Z. Li, Z. Ni, C. Li and L. Wei, A visible light excitable “on–off” and “green–red” fluorescent chemodosimeter for Ni2+/Pb2+, New J. Chem., 2012, 36, 2176–2179 RSC.
- E. Manandhar, J. H. Broome, J. Myrick, W. Lagrone, P. J. Cragg and K. J. Wallace, A pyrene-based fluorescent sensor for Zn2+ ions: a molecular ‘butterfly’, Chem. Commun., 2011, 47, 8796–8798 RSC.
- H. Irving and R. J. P. Williams, The stability of transition-metal complexes, J. Chem. Soc., 1953, 3192–3210 RSC.
- (a) A. W. Varnes, R. B. Dodson and E. L. Whery, Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies, J. Am. Chem. Soc., 1972, 94, 946–950 CrossRef CAS; (b) G. Sivaraman, M. Iniya, T. Anand, N. G. Kotla, O. Sunnapu, S. Singaravadivel, A. Gulyani and D. Chellappa, Chemically diverse small molecule fluorescent chemosensors for copper ion, Coord. Chem. Rev., 2018, 357, 50–104 CrossRef CAS; (c) K. Rurack, U. Resch, M. Sensoer and S. Daehne, A new fluorescence probe for trace metal ions: Cation-dependent spectroscopic properties, J. Fluoresc., 1993, 3, 141–143 CrossRef CAS; (d) K. Li, N. Li, X. Chen and A. Tong, A ratiometric fluorescent chemodosimeter for Cu(II) in water with high selectivity and sensitivity, Anal. Chim. Acta, 2012, 712, 115–119 CrossRef CAS; (e) X. Lou, D. Ou, Q. Li and Z. Li, An indirect approach for anion detection: the displacement strategy and its application, Chem. Commun., 2012, 48, 8462–8477 RSC.
- (a) I. Aoki, H. Kawabata, K. Nakashima and S. Shinkai, Fluorescent calix[4]arene which responds to solvent polarity and metal ions, J. Chem. Soc., Chem. Commun., 1991, 1771–1773 RSC; (b) I. Aoki, T. Sakaki and S. Shinkai, A new metal sensory system based on intramolecular fluorescence quenching on the ionophoric calix[4]arene ring, J. Chem. Soc., Chem. Commun., 1992, 730–732 RSC.
- (a) H.-F. Ji, R. Dabestani, G. M. Brown and R. A. Sachleben, A new highly selective calix[4]crown-6 fluorescent caesium probe, Chem. Commun., 2000, 833–834 RSC; (b) H.-F. Ji, R. Dabestani, G. M. Brown and R. L. Hettich, Synthesis and sensing behavior of cyanoanthracene modified 1,3-alternate calix[4]benzocrown-6: a new class of Cs+ selective optical sensors, J. Chem. Soc., Perkin Trans. 2, 2001, 585–591 RSC.
- (a) C. D. Gutsche, in Calixarenes in Monographs in Supramolecular Chemistry, ed. J. F. Stoddart, Royal Society of Chemistry, Cambridge, UK, 1989, vol. 1 Search PubMed; (b) J. D. V. Loon, W. Verboom and D. N. Reinhoudt, Selective functionalization and conformational properties of calix[4]arenes: A review, Org. Prep. Proced. Int., 1992, 24, 437–462 CrossRef.
- (a) R. Ungaro and A. Pochini, in Frontiers in Supramolecular Organic Chemistry and Photochemistry, ed. H.-J. Schneider, VCH, Weinheim, Germany, 1991, pp. 57–81 Search PubMed; (b) T. Jin, K. Ichikawa and T. Koyama, A fluorescent calix[4]arene as an intramolecular excimer-forming Na+ sensor in nonaqueous solution, J. Chem. Soc., Chem. Commun., 1992, 499–501 RSC.
- S. Nishizawa, H. Kaneda, T. Uchida and N. Teramae, Anion sensing by a donor–spacer–acceptor system: an intra-molecular exciplex emission enhanced by hydrogen bond-mediated complexation, J. Chem. Soc., Perkin Trans. 2, 1998, 2325–2328 RSC.
- (a) S. K. Kim, S. H. Lee, J. Y. Lee, J. Y. Lee, R. A. Bartsch and J. S. Kim, An excimer-based, binuclear, on–off switchable calix[4]crown chemosensor, J. Am. Chem. Soc., 2004, 126, 16499–16506 CrossRef CAS; (b) F. M. Winnik, Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media, Chem. Rev., 1993, 93, 587–614 CrossRef CAS; (c) S. H. Lee, S. H. Kim, S. K. Kim, J. H. Jung and J. S. Kim, Fluorescence ratiometry of monomer/excimer emissions in a space-through PET system, J. Org. Chem., 2005, 70, 9288–9295 CrossRef CAS; (d) J. Y. Lee, S. K. Kim, J. H. Jung and J. S. Kim, Bifunctional fluorescent calix[4]arene chemosensor for both a cation and an anion, J. Org. Chem., 2005, 70, 1463–1466 CrossRef CAS.
- J. B. Briks, Excimers, Rep. Prog. Phys., 1975, 38, 903–974 CrossRef.
- (a) J. K. Choi, S. H. Kim, J. Yoon, K.-H. Lee, R. A. Bartsch and J. S. Kim, A PCT-based, pyrene-armed calix[4]crown fluoroionophore, J. Org. Chem., 2006, 71, 8011–8015 CrossRef CAS; (b) J.-S. Yang, C.-S. Lin and C.-Y. Hwang, Cu2+-induced blue shift of the pyrene excimer emission: A new signal transduction mode of pyrene probes, Org. Lett., 2001, 3, 889–892 CrossRef CAS; (c) H. J. Kim, S. K. Kim, J. Y. Lee and J. S. Kim, Fluoride-sensing calix-luminophores based on regioselective binding, J. Org. Chem., 2006, 71, 6611–6614 CrossRef CAS.
- (a) J. B. Birks, Organic Molecular Photophysics, J. Wiley, London, New York, 1973–1975 Search PubMed; (b) J. B. Birks, Photophysics of Aromatic Molecules, Wiley-Interscience, London, 1970 Search PubMed.
- S. Sarkar, S. Roy, A. Sikdar, R. N. Saha and S. S. Panja, A pyrene-based simple but highly selective fluorescence sensor for Cu2+ ions via a static excimer mechanism, Analyst, 2013, 138, 7119–7126 RSC.
- (a) B. Valeur and I. Leray, Ion-responsive supramolecular fluorescent systems based on multichromophoric calixarenes: A review, Inorg. Chim. Acta, 2007, 360, 765–774 CrossRef CAS; (b) S. H. Kim, H. J. Kim, J. Yoon and J. S. Kim, Fluorescent chemosensors, in Calixarenes in the nanoworld, ed. J. Vicens and J. Harrowfield, Springer, Dordrecht, The Netherlands, 2007, pp. 311–334 Search PubMed.
- (a) F. M. Winnik, Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media, Chem. Rev., 1993, 93, 587–614 CrossRef CAS; (b) A. Okamoto, T. Ichiba and I. Saito, Pyrene-labeled oligodeoxynucleotide probe for detecting base insertion by excimer fluorescence emission, J. Am. Chem. Soc., 2004, 126, 8364–8365 CrossRef CAS.
- H. Nohta, H. Satozono, K. Koiso, H. Yoshida, J. Ishida and M. Yamaguchi, Highly selective fluorometric determination of polyamines based on intramolecular excimer-forming derivatization with a pyrene-labeling reagent, Anal. Chem., 2000, 72, 4199–4204 CrossRef CAS.
- A. P. de Silva, T. S. Moody and G. D. Wright, Fluorescent PET (Photoinduced Electron Transfer) sensors as potent analytical tools, Analyst, 2009, 134, 2385–2395 RSC.
- M. Sauer, Single-Molecule-Sensitive Fluorescent sensors Based on photoinduced intramolecular charge transfer, Angew. Chem., Int. Ed., 2003, 42, 1790–1793 CrossRef CAS.
- Y. Bao, B. Liu, F. Du, J. Tian, H. Wang and R. Bai, A new strategy for highly selective fluorescent sensing of F− and Zn2+ with dual output modes, J. Mater. Chem., 2012, 22, 5291–5294 RSC.
- H.-F. Ji, G. M. Brown and R. Dabestani, Optical sensing of cesium using 1,3-Alternate calix[4]-mono- and di(anthrylmethyl)aza-crown-6, Chem. Commun., 1999, 609–610 RSC.
- I. Leray, F. O’Reilly, J.-L. Habib Jiwan, J.-P. Soumillion and B. Valeur, A new calix[4]arene-based fluorescent sensor for sodium ion, Chem. Commun., 1999, 795–796 RSC.
- J. S. Kim, K. H. Noh, S. H. Lee, S. K. Kim, S. K. Kim and J. Yoon, Molecular taekwondo. 2. A new calix[4]azacrown bearing two different binding sites as a new fluorescent ionophore, J. Org. Chem., 2003, 68, 597–600 CrossRef CAS.
- J. S. Kim, O. J. Shon, J. A. Rim, S. K. Kim and J. Yoon, Pyrene-armed calix[4]azacrowns as new fluorescent ionophores: “molecular taekowndo” process via fluorescence change, J. Org. Chem., 2002, 67, 2348–2351 CrossRef CAS.
- Z. R. Grabowski and J. Dobkowski, Twisted intramolecular charge transfer (TICT) excited states: energy and molecular structure, Pure Appl. Chem., 1983, 55, 245–252 CAS.
- M. Liu, X. Yu, M. Li, N. Liao, A. Bi, Y. Jiang, S. Liu, Z. Gong and W. Zeng, Fluorescent probes for the detection of magnesium ions (Mg2+): from design to application, RSC Adv., 2018, 8, 12573–12587 RSC.
- W. Rettig and R. Lapouyade, Probe design and chemical sensing, in Topics in Fluorescence Spectroscopy, ed. J. R. Lakowicz, Plenum Press, New York, 1994, vol. 4, pp. 109–149 Search PubMed.
- H.-G. Löhr and F. Vögtle, Chromo- and fluoroionophores. A new class of dye reagents, Acc. Chem. Res., 1985, 18, 65–72 CrossRef.
- (a) P. Neri, J. L. Sessler and M.-X. Wang, Calixarenes and Beyond, Springer, 2016 CrossRef; (b) R. Kumar, A. Sharma, H. Singh, P. Suating, H. S. Kim, K. Sunwoo, I. Shim, B. C. Gibb and J. S. Kim, Revisiting fluorescent calixarenes: from molecular sensors to smart materials, Chem. Rev., 2019, 16, 9657–9721 CrossRef; (c) J. S. Kim and D. T. Quang, Calixarene-derived fluorescent probes, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS; (d) K. Sharma and P. Cragg, Calixarene based chemical sensors, Chem. Senses, 2011, 1, 1–18 Search PubMed; (e) R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Recent developments of thiacalixarene based molecular motifs, Chem. Soc. Rev., 2014, 43, 4824–4870 RSC; (f) J.-Y. Fu, L. Mu, X. Zeng, J.-L. Zhao, C. Redshaw, X.-L. Ni and T. Yamato, An NBD-armed thiacalix[4]arene-derived colorimetric and fluorometric chemosensor for Ag+: a metal–ligand receptor of anions, Dalton Trans., 2013, 42, 3552–3560 RSC.
- (a) J.-L. Zhao, H. Tomiyasu, X.-L. Ni, X. Zeng, M. R. J. Elsegood, C. Redshaw, S. Rahman, P. E. Georghiou and T. Yamato, Synthesis and evaluation of a novel ionophore based on a thiacalix[4]arene derivative bearing imidazole units, New J. Chem., 2014, 38, 6041–6049 RSC; (b) J.-L. Zhao, C. Wu, X. Zeng, S. Rahman, P. E. Georghiou, M. R. J. Elsegood, T. G. Warwick, C. Redshaw, S. J. Teat and T. Yamato, Thiacalix[4]arene derivatives bearing imidazole units: A ditopic hard/soft receptor for Na+ and K+/Ag+ with an allosteric effect and a reusable extractant for dichromate anions, ChemistrySelect, 2016, 1, 1541–1547 CrossRef CAS; (c) J.-L. Zhao, C. Wu, H. Tomiyasu, X. Zeng, M. R. J. Elsegood, C. Redshaw and T. Yamato, A rare and exclusive endoperoxide photoproduct derived from a thiacalix[4]arene crown-shaped derivative bearing a 9, 10-substituted anthracene moiety, Chem. – Asian J., 2016, 11, 1606–1612 CrossRef CAS; (d) S. Rahman, H. Tomiyasu, H. Kawazoe, J.-L. Zhao, H. Cong, X.-L. Ni, X. Zeng, M. R. J. Elsegood, T. G. Warwick, S. J. Teat, C. Redshaw, P. E. Georghiou and T. Yamato, A study of anion binding behaviour of 1,3-alternate thiacalix[4]arene-based receptors bearing urea moieties, New J. Chem., 2016, 40, 9245–9251 RSC; (e) M. Kumar, A. Dhir and V. Bhalla, On–Off switchable binuclear chemosensor based on thiacalix[4]crown armed with pyrene moieties, Eur. J. Org. Chem., 2009, 4534–4540 CrossRef CAS.
- M. Kumar, R. Kumar and V. Bhalla, Differential fluorogenic sensing of F− versus CN− based on thiacalix[4]arene derivatives, Tetrahedron Lett., 2013, 54, 1524–1527 CrossRef CAS.
- H. Tomiyasu, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, A study of allosteric binding behaviour of a 1,3-alternate thiacalix[4]arene-based receptor using fluorescence signal, Org. Biomol. Chem., 2014, 12, 4917–4923 RSC.
- J.-L. Zhao, H. Tomiyasu, C. Wu, H. Cong, X. Zeng, S. Rahman, P. E. Georghiou, D. L. Hughes, C. Redshaw and T. Yamato, Synthesis, crystal structure and complexation behaviour study of an efficient Cu2+ ratiometric fluorescent chemosensor based on thiacalix[4]arene, Tetrahedron, 2015, 71, 8521–8527 CrossRef CAS.
- (a) X.-L. Ni, X. Zeng and T. Yamato, Ratiometric fluorescent receptors for both Zn2+ and H2PO4− ions based on a pyrenyl-linked triazole-modified homooxacalix[3]arene: A potential molecular traffic signal with an R-S latch logic circuit, J. Org. Chem., 2011, 76, 5696–5702 CrossRef CAS; (b) X.-L. Ni, S. Rahman, S. Wang, C.-C. Jin, X. Zeng, D. Hughes, C. Redshaw and T. Yamato, hexahomotrioxacalix[3]arene derivatives as ionophores for molecular recognition of dopamine, serotonin and phenylethylamine, Org. Biomol. Chem., 2012, 10, 4618–4626 RSC.
- X.-L. Ni, S. Wang, X. Zeng and T. Yamato, Pyrene-linked triazole-modified homooxacalix[3]arene: A unique C3 symmetry ratiometric fluorescent chemosensor for Pb2+, Org. Lett., 2011, 13, 552–555 CrossRef CAS.
- X.-L. Ni, Y. Wu, C. Redshaw and T. Yamato, Direct evidence of a blocking heavy atom effect on the water-assisted fluorescence enhancement detection of Hg2+ based on ratiometric chemosensor, Dalton Trans., 2014, 43, 12633–12638 RSC.
- (a) X.-L. Ni, C.-C. Jin, X.-K. Jiang, M. Takimoto, S. Rahman, X. Zeng, D. L. Hughes, C. Redshaw and T. Yamato, Tri-substituted hexahomotrioxacalix[3]arene derivatives bearing imidazole units: synthesis and extraction properties for cations and chromate anions, Org. Biomol. Chem., 2013, 11, 5435–5442 RSC; (b) C.-C. Jin, T. Kinoshita, H. Cong, X.-L. Ni, X. Zeng, D. L. Hughes, C. Redshaw and T. Yamato, Synthesis and inclusion properties of C3-symmetric triazole derivatives based on hexahomotrioxacalix[3]arene, New J. Chem., 2012, 36, 2580–2586 RSC; (c) X.-L. Ni, X. Zeng, D. L. Hughes, C. Redshaw and T. Yamato, Novel ion-pair receptors based on hexahomotrioxacalix[3]arene derivatives, Org. Biomol. Chem., 2011, 9, 6535–6541 RSC.
- C.-C. Jin, H. Cong, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, Synthesis and inclusion behavior of a heterotritopic receptor based on hexahomotrioxacalix[3]arene, RSC Adv., 2014, 4, 31469–31475 RSC.
- C.-C. Jin, M. Fukuda, C. Wu, X. Jiang, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, A pyrene-armed hexahomotrioxacalix[3]arene as a multi-sensor via synergistic and demetallation effects, Tetrahedron, 2015, 71, 9593–9597 CrossRef CAS.
- Y.-S. Wu, C.-Y. Li, Y.-F. Li, D. Li and Z. Li, Development of a simple pyrene-based ratiometric fluorescent chemosensor for copper ion in living cells, Sens. Actuators, B, 2016, 222, 1226–1232 CrossRef CAS.
- J. Fernández-Lodeiro, C. Núñez, J. S. S. de Melo, J. S. Capelo and L. Carlos, Steady-state and time-resolved investigations on pyrene-based chemosensors, Inorg. Chem., 2013, 52, 121–129 CrossRef.
- J.-S. Yang, C.-S. Lin and C.-Y. Hwang, Cu2+-induced blue shift of the pyrene excimer emission:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A new signal transduction mode of pyrene probes, Org. Lett., 2001, 3, 889–892 CrossRef CAS.
A new signal transduction mode of pyrene probes, Org. Lett., 2001, 3, 889–892 CrossRef CAS. - H. J. Kim, J. Hong, A. Hong, S. Ham, J. H. Lee and J. S. Kim, Cu2+-induced intermolecular static excimer formation of pyrenealkylamine, Org. Lett., 2008, 10, 1963–1966 CrossRef CAS.
- H. S. Jung, M. Park, D. Y. Han, E. Kim, C. Lee, S. Ham and J. S. Kim, Cu2+ ion-induced self-assembly of pyrenylquinoline with a pyrenyle Excimer formation, Org. Lett., 2009, 11, 3378–3381 CrossRef CAS.
- S. Sarkar, S. Roy, A. Sikdar, R. N. Saha and S. S. Panja, A pyrene-based simple but highly selective fluorescence sensor for Cu2+ ions via a static excimer mechanism, Analyst, 2013, 138, 7119–7126 RSC.
- M. Shellaiah, Y.-H. Wu, A. Singh, M. V. R. Raju and H.-C. Lin, Novel pyrene-and anthracene-based Schiff base derivatives as Cu2+ and Fe3+ fluorescence turn-on sensors and for aggregation induced emissions, J. Mater. Chem. A, 2013, 1, 1310–1318 RSC.
- E. J. Jun, H. N. Won, J. S. Kim, K.-H. Lee and J. Yoon, Unique blue shift due to the formation of static pyrene excimer: highly selective fluorescent chemosensor for Cu2+, Tetrahedron Lett., 2006, 47, 4577–4580 CrossRef CAS.
- M. Kumar, N. Kumar and V. Bhalla, Ratiometric nanomolar detection of Cu2+ ions in mixed aqueous media: a Cu2+/Li+ ions switchable allosteric system based on thiacalix[4] crown, Dalton Trans., 2012, 41, 10189–10193 RSC.
- S. Ghosh, A. Ganguly, M. R. Uddin, S. Mandal, M. A. Alam and N. Guchhait, Dual mode selective chemosensor for copper and fluoride ions: a fluorometric, colorimetric and theoretical investigation, Dalton Trans., 2016, 45, 11042–11051 RSC.
- B. C. Roy, B. Chandra, D. Hromas and S. Mallik, Synthesis of new, pyrene-containing, metal-chelating lipids and sensing of cupric ions, Org. Lett., 2003, 5, 11–14 CrossRef CAS.
- Y. Wu, X.-L. Ni, L. Mou, C.-C. Jin, C. Redshaw and T. Yamato, Synthesis of a ditopic homooxacalix[3]arene for fluorescence enhanced detection of heavy and transition metal ions, Supramol. Chem., 2015, 27, 501–507 CrossRef CAS.
- (a) S. Sumiya, Y. Shiraishi and T. Hirai, Mechanism for different fluorescence response of a coumarin–amide–dipicolylamine linkage to Zn(II) and Cd(II) in water, J. Phys. Chem. A, 2013, 117, 1474–1482 CrossRef CAS; (b) D. T. Shi, B. Zhang, Y. X. Yang, C. C. Guan, X. P. He, Y. C. Li, G. R. Chen and K. X. Chen, Bis-triazolyl indoleamines as unique “off–approach–on” chemosensors for copper and fluorine, Analyst, 2013, 138, 2808–2811 RSC; (c) C. Gao, H. Zhu, M. Zhang, T. Tan, J. Chen and H. Qiu, A new highly Zn2+-selective and “off–on” fluorescent chemosensor based on the pyrene group, Anal. Methods, 2015, 7, 8172–8176 RSC; (d) X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, A water-soluble boronate-based fluorescent probe for the selective detection of peroxynitrite and imaging in living cells, Chem. Sci., 2014, 5, 3368–3373 RSC.
- Z. Kowser, H. Tomiyasu, X. Jiang, U. Rayhan, C. Redshaw and T. Yamato, Solvent effect and fluorescence response of the 7-tert-butylpyrene-dipicolylamine linkage for the selective and sensitive response toward Zn(II) and Cd(II) ions, New J. Chem., 2015, 39, 4055–4062 RSC.
- Z. Kowser, C.-C. Jin, X. Jiang, S. Rahman, P. E. Georghiou, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, Fluorescent turn-on sensors based on pyrene-containing Schiff base derivatives for Cu2+ recognition: spectroscopic and DFT computational studies, Tetrahedron, 2016, 72, 4575–4581 CrossRef CAS.
- H.-F. Wang and S.-P. Wu, A pyrene-based highly selective turn-on fluorescent sensor for copper(II) ions and its application in living cell imaging, Sens. Actuators, B, 2013, 181, 743–748 CrossRef CAS.
- R. Martínez, F. Zapata, A. Caballero, A. Espinosa, A. Tárraga and P. Molina, 2-Aza-1,3-butadiene Derivatives Featuring an Anthracene or Pyrene Unit:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Highly Selective Colorimetric and Fluorescent Signaling of Cu2+ Cation, Org. Lett., 2006, 8, 3235–3238 CrossRef.
Highly Selective Colorimetric and Fluorescent Signaling of Cu2+ Cation, Org. Lett., 2006, 8, 3235–3238 CrossRef. - S.-P. Wu, Z.-M. Huang, S.-R. Liu and P. K. Chung, A pyrene-based highly selective turn-on fluorescent sensor for copper(II) ion and its application in live cell imaging, J. Fluoresc., 2012, 22, 253–259 CrossRef CAS.
- S.-P. Wu, T.-H. Wang and S.-R. Liu, A highly selective turn-on fluorescent chemosensor for copper(II) ion, Tetrahedron, 2010, 66, 9655–9658 CrossRef CAS.
- P. Venkatesan and S.-P. Wu, A turn-on fluorescent pyrene-based chemosensor for Cu(II) with live cell application, RSC Adv., 2015, 5, 42591–42596 RSC.
- A. Saravanan, G. Subashini, S. Shyamsivappan, T. Suresh, K. Kadirvelu, N. Bhuvanesh, R. Nandhakumar and P. S. Mohan, A selective fluorescence chemosensor: pyrene motif Schiff base derivative for detection of Cu2+ ions in living cells, J. Photochem. Photobiol., A, 2018, 364, 424–432 CrossRef.
- A. Ghorai, J. Mondal, A. K. Manna, S. Chowdhury and G. K. Patra, A novel pyrene based highly selective reversible fluorescent-colorimetric sensor for the rapid detection of Cu2+ ions: application in bio-imaging, Anal. Methods, 2018, 10, 1063–1073 RSC.
- R. Martínez, A. Espinosa, A. Tárraga and P. Molina, A new bis (pyrenyl) azadiene-based probe for the colorimetric and fluorescent sensing of Cu(II) and Hg(II), Tetrahedron, 2010, 66, 3662–3667 CrossRef.
- H. Han, M. Wang and H. Wang, 1-Nitronyl nitroxide pyrene as a new off–on fluorescent chemosensor for Cu2+, New J. Chem., 2014, 38, 914–917 RSC.
- J. K. Choi, S. H. Kim, J. Yoon, K.-H. Lee, R. A. Bartsch and J. S. Kim, A PCT-based, pyrene-armed calix[4]crown fluoroionophore, J. Org. Chem., 2006, 71, 8011–8015 CrossRef CAS.
- L. Mohapatra and K. Parida, A review of solar and visible light active oxo-bridged materials for energy and environment, Catal. Sci. Technol., 2017, 7, 2153–2164 RSC.
- Y. Guo, L. Wang, J. Zhuo, B. Xu, X. Li, J. Zhang, Z. Zhang, H. Chi, Y. Dong and G. Lu, A pyrene-based dual chemosensor for colorimetric detection of Cu2+ and fluorescent detection of Fe3+, Tetrahedron Lett., 2017, 58, 3951–3956 CrossRef CAS.
- S. Dalbera, S. Kulovi and S. Dalai, Pyrene-based Schiff base as selective chemosensor for copper(II) and sulfide Ions, ChemistrySelect, 2018, 3, 6561–6569 CrossRef CAS.
- A. Vogler and H. Kunkely, Ligand-to-ligand and intraligand charge transfer and their relation to charge transfer interactions in organic zwitterions, Coord. Chem. Rev., 2007, 251, 577–583 CrossRef CAS.
- S. Mukherjee and S. Betal, Sensing phenomena, extraction and recovery of Cu2+ followed by smart phone application using a luminescent pyrene based chemosensor, J. Lumin., 2018, 204, 145–153 CrossRef CAS.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC; (b) T. Han, Y. Hong, N. Xie, S. Chen, N. Zhao, E. Zhao, J. W. Y. Lam, H. H. Y. Sung, Y. Dong, B. Tong and B. Z. Tang, Defect-sensitive crystals based on diaminomaleonitrile-functionalized Schiff base with aggregation-enhanced emission, J. Mater. Chem. C, 2013, 1, 7314–7320 RSC; (c) T. Han, X. Gu, J. W. Y. Lam, A. C. S. Leung, R. T. K. Kwok, T. Han, B. Tong, J. Shi, Y. Dong and B. Z. Tang, Diaminomaleonitrile-based Schiff bases: aggregation-enhanced emission, red fluorescence, mechanochromism and bioimaging applications, J. Mater. Chem. C, 2016, 4, 10430–10434 RSC.
- W.-N. Wu, P.-D. Mao, Y. Wang, X.-J. Mao, Z.-Q. Xu, Z.-H. Xu, X.-L. Zhao, Y.-C. Fan and X.-F. Hou, AEE active Schiff base-bearing pyrene unit and further Cu2+-induced self-assembly process, Sens. Actuators, B, 2018, 258, 393–401 CrossRef CAS.
- N. Chakraborty, A. Chakraborty and S. Das, A pyrene based fluorescent turn on chemosensor for detection of Cu2+ ions with antioxidant nature, J. Lumin., 2018, 199, 302–309 CrossRef CAS.
- (a) N. Li, Y. Xiang and A. Tong, Highly sensitive and selective “turn-on” fluorescent chemodosimeter for Cu2+ in water via Cu2+-promoted hydrolysis of lactone moiety in coumarin, Chem. Commun., 2010, 46, 3363–3365 RSC; (b) S. Liu, Y.-M. Wang and J. Han, Fluorescent chemosensors for copper(II) ion: Structure, mechanism and application, J. Photochem. Photobiol., C, 2017, 32, 78–103 CrossRef CAS.
- H. Ryu, J. H. Baek, M. G. Choi, J. C. Lee and S.-K. Chang, Cu2+-selective turn-on fluorescence signaling based on metal-induced hydrolysis of pyrenecarbohydrazide, Tetrahedron Lett., 2017, 58, 2927–2930 CrossRef CAS.
- (a) V. Dujols, F. Ford and A. W. Czarnik, A long-wavelength, fluorescent chemodosimeter selective for Cu(II) ion in water, J. Am. Chem. Soc., 1997, 119, 7386–7387 CrossRef CAS; (b) A. Mokhir and R. Kramer, Double discrimination by binding and reactivity in fluorescent metal ion detection, Chem. Commun., 2005, 2244–2246 RSC; (c) J. Kovacs, T. Rödler and A. Mokhir, Chemodosimeter for CuII detection based on cyclic peptide nucleic acids, Angew. Chem., Int. Ed., 2006, 45, 7815–7817 CrossRef CAS; (d) J. Kovacs and A. Mokhir, Catalytic hydrolysis of esters of 2-hydroxypyridine derivatives for Cu2+ detection, Inorg. Chem., 2008, 47, 1880–1882 CrossRef CAS; (e) R. M. Kierat and R. Kraemer, A fluorogenic and chromogenic probe that detects the esterase activity of trace copper(II), Bioorg. Med. Chem. Lett., 2005, 15, 4824–4827 CrossRef CAS; (f) L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, A selective turn-on fluorescent sensor for imaging copper in living cells, J. Am. Chem. Soc., 2006, 128, 10–11 CrossRef CAS; (g) X. Qi, E. J. Jun, L. Xu, S.-J. Kim, J. S. J. Hong, Y. J. Yoon and J. Yoon, New BODIPY derivatives as OFF-ON fluorescent chemosensor and fluorescent chemodosimeter for Cu2+: cooperative selectivity enhancement toward Cu2+, J. Org. Chem., 2006, 71, 2881–2884 CrossRef CAS; (h) M. H. Kim, H. H. Jang, S. Yi, S.-K. Chang and M. S. Han, Coumarin-derivative-based off–on catalytic chemodosimeter for Cu2+ ions, Chem. Commun., 2009, 4838–4840 RSC; (i) E. J. Corey and S. Knapp, Facile conversion of N,N-dimethylhydrazones to carbonyl compounds by cupric ioncatalyzed hydrolysis, Tetrahedron Lett., 1976, 17, 3667–3668 CrossRef; (j) Q. Wu and E. V. Anslyn, Catalytic signal amplification using a Heck reaction. An example in the fluorescence sensing of Cu(II), J. Am. Chem. Soc., 2004, 126, 14682–14683 CrossRef CAS; (k) Y. Xiang and A. Tong, Ratiometric and selective fluorescent chemodosimeter for Cu(II) by Cu(II)-induced oxidation, Luminescence, 2008, 23, 28–31 CrossRef CAS; (l) L. Mei, Y. Xiang, N. Li and A. Tong, A new fluorescent probe of rhodamine B derivative for the detection of copper ion, Talanta, 2007, 72, 1717–1722 CrossRef CAS.
- J. F. Zhang, Y. Zhou, J. Yoon, Y. Kim, S. J. Kim and J. S. Kim, Naphthalimide modified rhodamine derivative: ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches, Org. Lett., 2010, 12, 3852–3855 CrossRef CAS.
- Y. Zhou, F. Wang, Y. Kim, S.-J. Kim and J. Yoon, Cu2+-selective ratiometric and “Off-On” sensor based on the rhodamine derivative bearing pyrene group, Org. Lett., 2009, 11, 4442–4445 CrossRef CAS.
- (a) T. L. Banfield and D. Husain, Electronic energy transfer from triplet state acridine to paramagnetic ions, Trans. Faraday Soc., 1969, 65, 1985 RSC; (b) A. W. Varnes, R. B. Dodson and E. L. Wehry, Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies, J. Am. Chem. Soc., 1972, 94, 946 CrossRef CAS.
- D. Rajasekaran, K. Venkatachalam and V. Periasamy, “On–off–on” pyrene-based fluorescent chemosensor for the selective recognition of Cu2+ and S2− ions and its utilization in live cell imaging, Appl. Organomet. Chem., 2020, 34, 1–9 CrossRef.
- D. Phapale, A. Gaikwad and D. Das, Selective recognition of Cu(II) and Fe(III) using a pyrene based chemosensor, Spectrochim. Acta, Part A, 2017, 178, 160–165 CrossRef CAS.
- M. A. Wani, P. K. Singh, R. Pandey and M. D. Pandey, Coumarin–pyrene conjugate: synthesis, structure and Cu-selective fluorescent sensing in mammalian kidney cells, J. Lumin., 2016, 171, 159–165 CrossRef CAS.
- H. Tomiyasu, N. Shigyo, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, Positive allosteric binding behavior of pyrene-appended triazole-modified thiacalix[4]arene-based fluorescent receptors, Tetrahedron, 2014, 70, 7893–7899 CrossRef CAS.
- G. Huang, C. Li, X. Han, S. O. Aderinto, K. Shen, S. Mao and H. Wu, Sensitive and selective detection of Cu(II) ion: A new effective 1,8-naphthalimide-based fluorescence ‘turn off’ sensor, Luminescence, 2018, 1–10 Search PubMed.
- R. Kumar, V. Bhalla and M. Kumar, Cu2+ and CN-selective fluorogenic sensors based on pyrene-appended thiacalix[4]arenes, Tetrahedron, 2008, 64, 8095–8101 CrossRef CAS.
- Y. R. Bhorge, H.-T. Tsai, K.-F. Huang, A. J. Pape, S. N. Janaki and Y.-P. Yen, A new pyrene-based Schiff-base: a selective colorimetric and fluorescent chemosensor for detection of Cu(II) and Fe(III), Spectrochim. Acta, Part A, 2014, 130, 7–12 CrossRef CAS.
- W.-C. Lin, C.-Y. Wu, Z.-H. Liu, C.-Y. Lin and Y.-P. Yen, A new selective colorimetric and fluorescent sensor for Hg2+ and Cu2+ based on a thiourea featuring a pyrene unit, Talanta, 2010, 81, 1209–1215 CrossRef CAS.
- Y. Chen, Q. Lv, Z. Liu and Q. Fang, Diaminomaleonitrile substituted pyrene as a solvent-dependent chemosensor for copper(II) ion and hypochlorite, Inorg. Chem. Commun., 2015, 52, 38–40 CrossRef CAS.
- S. Goswami, S. Chakraborty, S. Paul, S. Halder, S. Panja and S. K. Mukhopadhyay, A new pyrene based highly sensitive fluorescence probe for copper(II) and fluoride with living cell application, Org. Biomol. Chem., 2014, 12, 3037–3044 RSC.
| This journal is © the Partner Organisations 2021 |