Open Access Article
Open Access ArticleSynthesis and antimicrobial activity of some novel 1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidines bearing amino acid moiety†
Mounir A. A. Mohamed a,
Adnan A. Bekhit
a,
Adnan A. Bekhit *bcd,
Omyma A. Abd Allaha,
Asmaa M. Kadrya,
Tamer M. Ibrahim
*bcd,
Omyma A. Abd Allaha,
Asmaa M. Kadrya,
Tamer M. Ibrahim e,
Salma A. Bekhitf,
Kikuko Amagaseg and
Ahmed M. M. El-Saghier*a
e,
Salma A. Bekhitf,
Kikuko Amagaseg and
Ahmed M. M. El-Saghier*a
aDepartment of Chemistry, Faculty of Science, Sohag University, Sohag, Egypt. E-mail: adnbekhit@hotmail.com; adnbekhit@pharmacy.alexu.edu.eg
bPharmaceutical Chemistry Department, Faculty of Pharmacy, Alexandria University, Alexandria, Egypt. E-mail: el_saghier@yahoo.com
cCancer Nanotechnology Research Laboratory (CNRL), Faculty of Pharmacy, Alexandria University, Alexandria 21521, Egypt
dPharmacy Program, Allied Health Department, College of Health and Sport Sciences, University of Bahrain, Zallaq, Kingdom of Bahrain
eDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh 33516, Egypt
fHigh Institute of Public Health, Alexandria University, Alexandria 21568, Egypt
gLaboratory of Pharmacology & Pharmacotherapeutics, College of Pharmaceutical Sciences, Ritsumeikan University, Kusatsu, Shiga, Japan
First published on 13th January 2021
Abstract
A new series of [1,2,4]-triazole bearing amino acid derivatives 2a–d–9a–d were synthesized under green chemistry conditions via multicomponent reaction using lemon juice as an acidic catalyst. The obtained compounds were characterized by different spectral and elemental analyses. The obtained candidates showed promising antibacterial activity against some standard bacteria and multidrug resistant (MDR) clinical isolates. In contrast to the reference drugs cephalothin and chloramphenicol, the tested compounds showed substantial better MIC values towards the tested MDR strains. The most active compounds 3c, 8a and 9d against MDR bacteria were tested for MBC and MIC index, the results indicted the bacteriostatic activity of these compounds. The most active compounds 2c, 2d, 3c, 8a, 8b, 9a, 9b, 9c and 9d showed a high selectivity index towards antimicrobial activity against K. pneumoniae and MRSA1 compared to mammalian cells, suggesting a good safety profile.
1. Introduction
Facing emerging bacterial infections has become more challenging worldwide due to the increasing number of multidrug-resistant (MDR) microbes.1–7 This indicates the crucial need to develop new efficient anti-bacterial agents. Many factors contribute to mutations in microbial genomes leading to resistance to known antibiotics. For instance, it is broadly confirmed that the abuse of antibiotics can significantly increase the development of resistant-genotypes.8–10 As the number of infectious diseases and multidrug-resistant bacterial strains continues to increase, researchers are prompted to develop novel anti-microbial molecules.11From a medicinal chemistry prospective, creating new generation of therapeutic molecules with improved pharmacological properties and drug-tolerance profile, as well as fewer side effects, is an ultimate goal.12 Hence, libraries with privileged heterocyclic scaffolds are frequently utilized in the development of new potent drugs.13 For instance, hybrids from 1,2,4-triazole derived compounds usually hold a series of pharmacological properties such as anticancer,14,15 antiviral,16 antitubercular,17,18 antifungal,19 antileishmanial20 and antibacterial21 activities. However, only few reports about fused systems of 1,2,4-triazolo[1,5-a]pyrimidines were reported in literature with pronounced antibacterial activities.22 In addition, coupling with simple amino acids, e.g., glycine and others, has been frequently attracted the interst of medicinal chemists due to its improving ability for the physicochemical and drug-likeness properties.20,23 In addition, glycine and its derivatives appear to be promising safe antimicrobial agents.24,25
Being analogues of DNA purine bases, 1,2,4-triazolo[1,5-a]pyrimidines can be regarded as plausible substrates for enzymatic biochemical processes.26 In particular, derivatives of [1,2,4]triazolo-[4,3-a]pyrimidines have recently been reported as potential antibacterials.27–30 It was reported that series of 1,2,4-triazolo[1,5-a]pyrimidines carboxamide derivatives attributed good narrow-spectrum antibacterial activity against E. faecium and possessed metabolic stability with low intrinsic clearance. Macromolecular synthesis assays revealed cell-wall biosynthesis as the target of these compounds.22 It is worth mentioning that recently, several 1,2,4-triazolo[1,5-a]pyrimidines were synthesized and screened for their antibacterial derivatives as DNA gyrase inhibitors. Some compounds possessed high activity against Gram-positive and Gram-negative bacteria with MIC values ranging from 0.25–2.0 μg mL−1. In addition they showed good toxicity profile against human kidney and red blood cell.31
Accordingly and as a continuation of our efforts to discover diverse chemotypes for potential antibacterial agents,32 we aim at preparing new triazolo[1,5-a]pyrimidine derivatives coupled with amino acids. The synthetic process comprises green conditions, e.g., using lemon juice as green catalyst and aqueous medium as green solvent.
2. Results and discussion
Chemistry
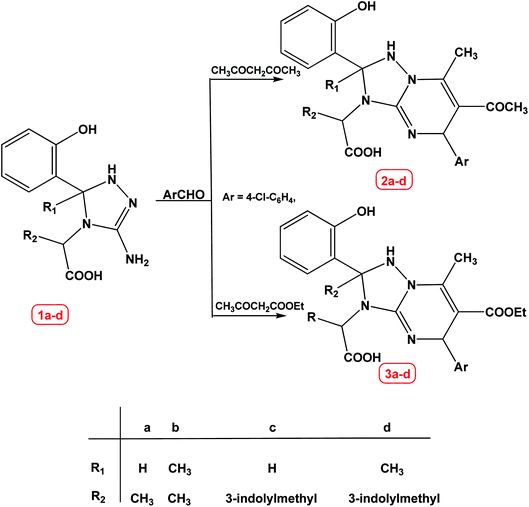
In continuation of our research program on the utility of hetrocylic moieties to find out novel antibacterial agents,20,31,32 we are going here to report an efficient and facile synthesis of some [1,2,4]triazolo[1,5-a]pyrimidine derivatives starting from 2-(3-amino-5-(2-hydroxyphenyl)-1H-1,2,4-triazol-4(5H)-yl)propanoic acid derivatives 1a–d. The tree component reaction of compound 1a–d with aromatic aldehyde (4-chlorobenzaldehyde) and acetylacetone or ethyl acetoacetate in a one pot reaction under green conditions [lemon juice, water–ethanol (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] afforded the corresponding 2-(1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(5H)-yl)propanoic acid derivatives 2a–d and 3a–d respectively, in good to excellent yield, Scheme 1.
2)] afforded the corresponding 2-(1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(5H)-yl)propanoic acid derivatives 2a–d and 3a–d respectively, in good to excellent yield, Scheme 1.
It worth mentioning that some [1,2,4]triazolo[1,5-a]pyrimidine derivatives synthesized through one-pot multicomponent reactions using a low viscous and acid-functionalized ionic liquid. The results showed that new ionic liquid can act as a green solvent and acid catalyst due to low viscosity and acid functionality.33
Structures of the newly obtained compounds were confirmed based upon their IR, 1H-NMR, 13C-MR, MS spectral data, and elemental analyses. The IR spectra of compound 2a exhibited the presence of broad band at 3455 cm−1 corresponding to two OH groups, another characteristic band at 1685 cm−1 corresponding to the α,β-unsaturated carbonyl group. The 1H-NMR spectrum of compound 2a revealed the presence of a broad band at δ 12.24 ppm characterized to the OH of the carboxyl group, a singlet at δ 9.22 ppm corresponding to the phenolic OH group, another singlet at δ 8.12 ppm for NH group, a multiplet between δ 6.92–7.60 ppm attributed to the aromatic protons, a singlet at δ 5.24 ppm corresponding to CH (triazole), a singlet at δ 4.50 ppm for CH (pyrimidine), a quartet at 2.92 ppm corresponding to 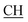 –COOH, a singlet at 2.33 ppm for δ CH3 group, a doublet at δ 2.23 ppm attributed to (CH3 alanine) and a singlet at δ 2.12 ppm characteristic for
–COOH, a singlet at 2.33 ppm for δ CH3 group, a doublet at δ 2.23 ppm attributed to (CH3 alanine) and a singlet at δ 2.12 ppm characteristic for 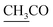 group. 13CMR spectrum of compound 2a showed the following signals: 9.88 (
group. 13CMR spectrum of compound 2a showed the following signals: 9.88 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3–CH), 20.12 (CH3), 22.02 (
H3–CH), 20.12 (CH3), 22.02 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3CO), 41.80 (
H3CO), 41.80 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H–COOH), 53.12 (CHpyrimidine), 57.80 (CHtriazole), 118.12, 119.21, 122.20, 123.41, 124.50, 126.54, 127.32, 128.62, 134.55, 136.01, 138.12, 143.21 (Ar
H–COOH), 53.12 (CHpyrimidine), 57.80 (CHtriazole), 118.12, 119.21, 122.20, 123.41, 124.50, 126.54, 127.32, 128.62, 134.55, 136.01, 138.12, 143.21 (Ar![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) ), 152.1 (C
), 152.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 178.10 (C=O), 192.54 (C=O).
N), 178.10 (C=O), 192.54 (C=O).
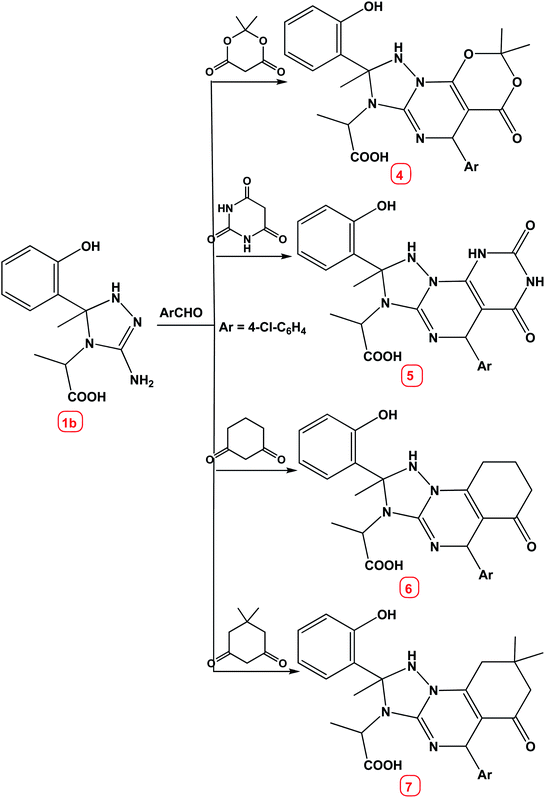
Similarly, the reaction of compound 1b with cyclic 1,3-dicarbonyl compounds viz. meldrum's acid, barbituric acid, cyclohexane-1,3-dione and/or dimedone under the same experimental conditions [lemon juice, water–ethanol (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] afforded the corresponding [1,2,4]triazolo[1,5-a]pyrimidine derivatives 4–7 respectively, Scheme 2.
2)] afforded the corresponding [1,2,4]triazolo[1,5-a]pyrimidine derivatives 4–7 respectively, Scheme 2.
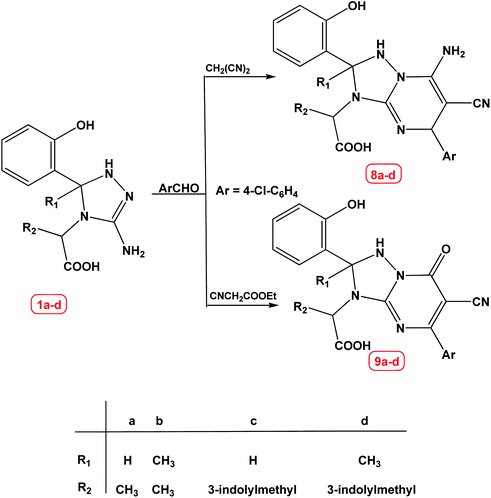
On continuation of our work, 2-(3-amino-5-(2-hydroxyphenyl)-1H-1,2,4-triazol-4(5H)-yl)propanoic acid derivatives 1a–d were allowed to react with 4-chlorobenzaldehyde and malononitrile or ethyl cyanoacetate under the same experimental conditions, where the corresponding [1,2,4]triazolo[1,5-a]pyrimidine derivatives 8a–d and 9a–d were obtained in excellent yields, Scheme 3.
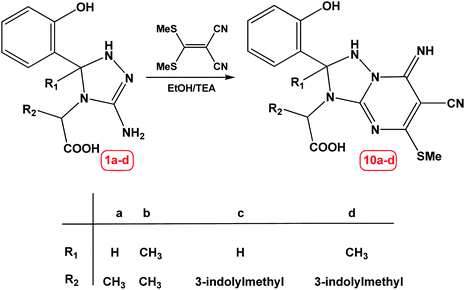
Finally, the reaction of compound 1a–d with α-cyanoketene-S,S-dithioacetal namely: 2-(bis(methylthio)methylene)malononitrile under green condition gave a product which was precipitated during the course of reaction and was identified as 2-(6-cyano-2-(2-hydroxyphenyl)-7-imino-5-(methylthio)-1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(7H)-yl)propanoic acid 10a–d, as shown in Scheme 4.
The analytical and spectral data of all compounds were found to be accordance with the structures assigned to these compounds.
Antimicrobial screening
| Comp. no. | Diameter of zone inhibition in mm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | Fungi | ||||||||
| S. aureus (ATCC 25923) | S. pyogenes (ATCC 19615) | P. phaseolicola (GSPB 2828) | P. fluorescens (S 97) | C. albicans | ||||||
| 10 μg mL−1 | 15 μg mL−1 | 10 μg mL−1 | 15 μg mL−1 | 10 μg mL−1 | 15 μg mL−1 | 10 μg mL−1 | 15 μg mL−1 | 10 μg mL−1 | 15 μg mL−1 | |
| a Less active: 6–12 mm; moderately active: 13–19 mm; highly active: 20–30 mm; no inhibition or inhibition less than 5 mm; NT – not tested. Each result represents the average of triplicate readings. S. aureus (ATCC 25923) = Staphylococcus aureus (ATCC 25923); S. pyogenes (ATCC 19615) = Streptococcus pyogenes (ATCC 19615); P. phaseolicola (GSPB 2828) = Pseudomonas phaseolicola (GSPB 2828); P. fluorescens (S 97) = Pseudomonas fluorescens (S 97); C. albicans = Candida albicans. | ||||||||||
| 2a | 10 | 16 | 12 | 17 | 18 | 28 | 16 | 25 | 8 | 9 |
| 2b | 12 | 17 | 10 | 18 | 16 | 32 | 15 | 29 | 6 | 8 |
| 2c | 19 | 32 | 18 | 30 | 11 | 23 | 12 | 22 | 6 | 9 |
| 2d | 20 | 33 | 20 | 32 | 12 | 22 | 14 | 21 | 9 | 12 |
| 3a | 11 | 20 | 12 | 22 | 18 | 32 | 16 | 30 | 6 | 8 |
| 3b | 12 | 25 | 14 | 24 | 19 | 33 | 18 | 30 | 10 | 12 |
| 3c | 19 | 33 | 18 | 32 | 10 | 21 | 8 | 23 | 6 | 7 |
| 3d | 18 | 30 | 17 | 31 | 11 | 23 | 10 | 19 | 9 | 10 |
| 4 | 10 | 24 | 8 | 22 | 7 | 18 | 12 | 25 | 9 | 12 |
| 5 | 14 | 26 | 11 | 25 | 17 | 30 | 15 | 28 | 7 | 9 |
| 6 | 15 | 25 | 10 | 24 | 18 | 29 | 19 | 33 | 7 | 8 |
| 7 | 15 | 23 | 12 | 20 | 19 | 33 | 18 | 32 | 10 | 11 |
| 8a | 13 | 20 | 12 | 21 | 18 | 30 | 17 | 29 | 10 | 13 |
| 8b | 12 | 22 | 16 | 26 | 19 | 34 | 18 | 32 | 8 | 10 |
| 8c | 18 | 30 | 19 | 33 | 14 | 24 | 13 | 23 | 8 | 11 |
| 8d | 16 | 29 | 17 | 31 | 14 | 24 | 12 | 22 | 6 | 7 |
| 9a | 14 | 26 | 12 | 27 | 18 | 30 | 17 | 30 | 6 | 9 |
| 9b | 15 | 26 | 16 | 25 | 19 | 31 | 16 | 28 | 6 | 8 |
| 9c | 18 | 32 | 19 | 34 | 10 | 22 | 11 | 20 | 9 | 11 |
| 9d | 20 | 35 | 19 | 32 | 10 | 18 | 12 | 23 | 7 | 8 |
| Cephalothin | 28 | 30 | NT | NT | ||||||
| Chloramphenicol | NT | NT | 25 | 30 | ||||||
| Clotrimazole | NT | NT | NT | NT | 36 | 44 | ||||
| Comp. no. | K. pneumoniae | MRSA1 | ||
|---|---|---|---|---|
| Diameter of zone inhibition in mm (15 μg mL−1) | MIC (μg mL−1) | Diameter of zone inhibition in mm (15 μg mL−1) | MIC (μg mL−1) | |
| 2b | 25 | 50 | 8 | — |
| 2c | 8 | — | 26 | 25 |
| 2d | 6 | — | 28 | 25 |
| 3a | 24 | 50 | 10 | — |
| 3b | 27 | 50 | 12 | — |
| 3c | 9 | — | 24 | 12.5 |
| 3d | 6 | — | 6 | — |
| 5 | 23 | 50 | 8 | — |
| 6 | 22 | 50 | 10 | — |
| 7 | 20 | 50 | 8 | — |
| 8a | 28 | 12.5 | 6 | — |
| 8b | 27 | 25 | 8 | — |
| 8c | 12 | — | 28 | 50 |
| 8d | 10 | — | 25 | 50 |
| 9a | 27 | 25 | 5 | — |
| 9b | 29 | 25 | 7 | — |
| 9c | 13 | — | 27 | 25 |
| 9d | 11 | — | 29 | 12.5 |
| Cephalothin | — | — | — | — |
| Chloramphenicol | — | — | — | — |
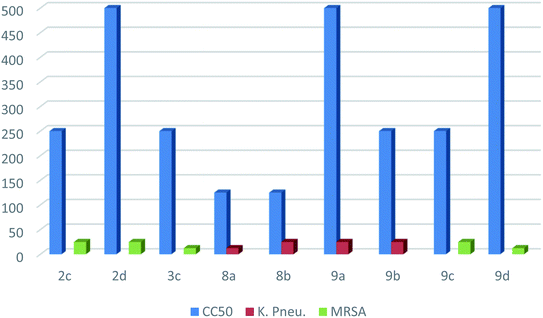
| Comp. no. | CC50a | K. pneumoniae (μg mL−1) | MRSA1 (μg mL−1) | ||
|---|---|---|---|---|---|
| MICa | SIb | MICa | SIb | ||
| a CC50 is the concentration at which 50% of the cells survive and MIC is the minimum concentration that inhibits bacterial growth reported in μg ml−1.b SI is the selectivity index regarding antimicrobial activity against K. pneumoniae and MRSA1; SI = CC50/MIC. | |||||
| 2c | 250 | — | — | 25 | 10 |
| 2d | 500 | — | — | 25 | 20 |
| 3c | 250 | — | — | 12.5 | 20 |
| 8a | 125 | 12.5 | 10 | — | — |
| 8b | 125 | 25 | 5 | — | — |
| 9a | 500 | 25 | 20 | — | — |
| 9b | 250 | 25 | 10 | — | — |
| 9c | 250 | — | — | 25 | 10 |
| 9d | 500 | — | — | 12.5 | 40 |
3. Methods
Chemistry
All melting points were determined on a Koffler melting point apparatus and are uncorrected. 1H-NMR and 13C NMR spectra were recorded on a Bruker avance 400 MHz spectrometer using TMS as internal reference (chemical shifts in δ, ppm), and IR spectra were obtained on a Nicolet 710 FT-IR spectrometer (KBr, νmax in cm−1). Mass spectra were recorded on a GC-MSQP 1000EX Schimadzu at the Microanalytical laboratory, Cairo University, Cairo, Egypt. Elemental analyses were recorded on Vario El Fab-Nr elemental analyzer (Cairo University).General procedure for the synthesis of [1,2,4]triazolo[1,5-a]pyrimidine derivatives 2a–d and 3a–d
An equimolar mixture of 2-(3-amino-5-(2-hydroxyphenyl)-1H-1,2,4-triazol-4(5H)-yl)propanoic acid derivative 1a–d (0.001 mol), 4-chlorobenzaldehyde (0.14 g, 0.001 mol) and acetylacetone or ethyl acetoacetate (0.001 mol) was mixed in water (8 mL)–ethanol (2 mL) then was treated with 1 mL of fresh lemon juice. The reaction mixture was heated under reflux for 4–6 h, then left to cool. The formed precipitates were collected by filtration, washed thoroughly with water and then recrystallized from ethanol to give the corresponding 2-(1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(5H)-yl)propanoic acid derivatives 2a–d and 3a–d respectively.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1622 (COOasy), 1582 (COOsy), 810 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.48 (d, 3H, J = 7.20 Hz, CH3), 2.10 (s, 3H,
O), 1622 (COOasy), 1582 (COOsy), 810 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.48 (d, 3H, J = 7.20 Hz, CH3), 2.10 (s, 3H, 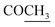 ), 2.32 (s, 3H, CH3), 3.72 (q, 1H, J = 7.14 Hz,
), 2.32 (s, 3H, CH3), 3.72 (q, 1H, J = 7.14 Hz, 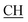 –COOH), 5.24 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.88–7.58 (m, 8H, 2ArH), 8.02 (s, 1H, NH, exchangeable by D2O), 9.06 (s, 1H, OH exchangeable by D2O), 12.25 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 17.06, 20.12, 22.02, 41.82, 57.80, 118.12, 119.21, 122.23, 123.46, 124.55, 126.57, 127.39, 128.60, 134.56, 136.08, 138.12, 143.20, 152.11, 178.16, 192.47. MS (m/z, I%): 453 (M+ − 1, 0.25%).
–COOH), 5.24 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.88–7.58 (m, 8H, 2ArH), 8.02 (s, 1H, NH, exchangeable by D2O), 9.06 (s, 1H, OH exchangeable by D2O), 12.25 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 17.06, 20.12, 22.02, 41.82, 57.80, 118.12, 119.21, 122.23, 123.46, 124.55, 126.57, 127.39, 128.60, 134.56, 136.08, 138.12, 143.20, 152.11, 178.16, 192.47. MS (m/z, I%): 453 (M+ − 1, 0.25%).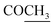 ), 2.22 (s, 3H, CH3), 2.32 (s, 3H, CH3), 3.76 (q, 1H, J = 7.14 Hz,
), 2.22 (s, 3H, CH3), 2.32 (s, 3H, CH3), 3.76 (q, 1H, J = 7.14 Hz, 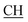 –COOH), 5.35 (s, 1H, CHpyrimidine), 6.90–7.59 (m, 8H, 2ArH), 8.01 (s, 1H, NH, exchangeable by D2O), 9.05 (s, 1H, OH exchangeable by D2O), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 17.22, 20.50, 22.37, 28.43, 41.88, 54.10, 57.67, 117.21, 118.65, 122.11, 123.42, 124.67, 127.23, 128.25, 133.16, 134.29, 136.27, 138.19, 141.16, 151.20, 178.14, 191.89. MS (m/z, I%): 468 (M+ − 1, 0.30%).
–COOH), 5.35 (s, 1H, CHpyrimidine), 6.90–7.59 (m, 8H, 2ArH), 8.01 (s, 1H, NH, exchangeable by D2O), 9.05 (s, 1H, OH exchangeable by D2O), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 17.22, 20.50, 22.37, 28.43, 41.88, 54.10, 57.67, 117.21, 118.65, 122.11, 123.42, 124.67, 127.23, 128.25, 133.16, 134.29, 136.27, 138.19, 141.16, 151.20, 178.14, 191.89. MS (m/z, I%): 468 (M+ − 1, 0.30%).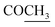 ), 2.31 (s, 3H, CH3), 3.12 (d, 2H, J = 7.6 Hz, CH2), 3.76 (q, 1H, J = 7.14 Hz,
), 2.31 (s, 3H, CH3), 3.12 (d, 2H, J = 7.6 Hz, CH2), 3.76 (q, 1H, J = 7.14 Hz, 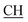 –COOH), 5.23 (s, 1H, CHtriazole), 5.38 (s, 1H, CHpyrimidine), 6.76 (s, 1H, CHindole), 6.90–7.66 (m, 12H, 3ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.05 (s, 1H, OH exchangeable by D2O), 10.24 (s, 1H, NHindole), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 20.50, 22.36, 28.40, 36.44, 41.80, 54.12, 57.60, 107.88, 117.44, 118.59, 122.15, 122.20, 123.06, 123.42, 124.67, 125.44, 127.23, 128.25, 133.16, 134.29, 135.75, 136.27, 138.19, 141.16, 144.33, 151.20, 178.14, 191.89. MS (m/z, I%): 570 (M+ − 1, 0.2%).
–COOH), 5.23 (s, 1H, CHtriazole), 5.38 (s, 1H, CHpyrimidine), 6.76 (s, 1H, CHindole), 6.90–7.66 (m, 12H, 3ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.05 (s, 1H, OH exchangeable by D2O), 10.24 (s, 1H, NHindole), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 20.50, 22.36, 28.40, 36.44, 41.80, 54.12, 57.60, 107.88, 117.44, 118.59, 122.15, 122.20, 123.06, 123.42, 124.67, 125.44, 127.23, 128.25, 133.16, 134.29, 135.75, 136.27, 138.19, 141.16, 144.33, 151.20, 178.14, 191.89. MS (m/z, I%): 570 (M+ − 1, 0.2%).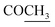 ), 2.22 (s, 3H, CH3), 2.30 (s, 3H, CH3), 3.12 (d, 2H, J = 7.6 Hz, CH2), 3.76 (q, 1H, J = 7.14 Hz,
), 2.22 (s, 3H, CH3), 2.30 (s, 3H, CH3), 3.12 (d, 2H, J = 7.6 Hz, CH2), 3.76 (q, 1H, J = 7.14 Hz, 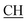 –COOH), 5.36 (s, 1H, CHpyrimidine), 6.75 (s, 1H, CHindole), 6.87–7.65 (m, 12H, 3ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 10.22 (s, 1H, NHindole, exchangeable by D2O), 12.20 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 20.31, 22.01, 34.52, 41.06, 44.22, 53.58, 57.06, 110.57, 117.26, 118.43, 121.80, 122.36, 123.12, 123.85, 124.6, 127.11, 127.94, 128.48, 133.25, 134.37, 135.41, 136.50, 138.45, 138.94, 143.10, 151.22, 178.66, 192.73. MS (m/z, I%): 583 (M+ − 1, 0.35%).
–COOH), 5.36 (s, 1H, CHpyrimidine), 6.75 (s, 1H, CHindole), 6.87–7.65 (m, 12H, 3ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 10.22 (s, 1H, NHindole, exchangeable by D2O), 12.20 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 20.31, 22.01, 34.52, 41.06, 44.22, 53.58, 57.06, 110.57, 117.26, 118.43, 121.80, 122.36, 123.12, 123.85, 124.6, 127.11, 127.94, 128.48, 133.25, 134.37, 135.41, 136.50, 138.45, 138.94, 143.10, 151.22, 178.66, 192.73. MS (m/z, I%): 583 (M+ − 1, 0.35%).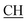 –COOH), 4.02 (q, 2H, J = 7.2 Hz, CH2), 5.24 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.94–7.52 (m, 8H, 2ArH), 8.02 (s, 1H, NH, exchangeable by D2O), 9.12 (s, 1H, OH exchangeable by D2O), 12.24 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.27, 18.25, 28.55, 44.10, 53.78, 57.88, 117.36, 118.47, 121.50, 122.30, 123.55, 124.19, 127.28, 128.72, 134.32, 136.55, 138.83, 143.01, 151.26, 178.06, 193.05.
–COOH), 4.02 (q, 2H, J = 7.2 Hz, CH2), 5.24 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.94–7.52 (m, 8H, 2ArH), 8.02 (s, 1H, NH, exchangeable by D2O), 9.12 (s, 1H, OH exchangeable by D2O), 12.24 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.27, 18.25, 28.55, 44.10, 53.78, 57.88, 117.36, 118.47, 121.50, 122.30, 123.55, 124.19, 127.28, 128.72, 134.32, 136.55, 138.83, 143.01, 151.26, 178.06, 193.05.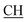 –COOH), 4.01 (q, 2H, J = 7.2 Hz, CH2), 5.38 (s, 1H, CHpyrimidine), 6.90–7.50 (m, 8H, 2ArH), 8.01 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 12.20 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.20, 18.23, 22.56, 28.50, 44.08, 53.75, 57.80, 117.30, 118.41, 121.52, 122.19, 123.55, 124.20, 127.25, 128.70, 134.33, 136.50, 138.81, 143.02, 151.25, 178.10, 193.18.
–COOH), 4.01 (q, 2H, J = 7.2 Hz, CH2), 5.38 (s, 1H, CHpyrimidine), 6.90–7.50 (m, 8H, 2ArH), 8.01 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 12.20 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.20, 18.23, 22.56, 28.50, 44.08, 53.75, 57.80, 117.30, 118.41, 121.52, 122.19, 123.55, 124.20, 127.25, 128.70, 134.33, 136.50, 138.81, 143.02, 151.25, 178.10, 193.18.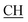 –COOH), 4.03 (q, 2H, J = 7.2 Hz, CH2), 5.23 (s, 1H, CHtriazole), 5.38 (s, 1H, CHpyrimidine), 6.90–7.64 (m, 12H, 2ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 10.18 (s, 1H, CHindole, exchangeable by D2O), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.21, 28.46, 36.48, 41.81, 53.12, 57.66, 107.86, 117.40, 118.62, 121.67, 122.15, 122.24, 123.02, 123.40, 124.61, 125.41, 127.25, 128.20, 133.32, 134.25, 135.70, 136.21, 138.22, 141.18, 144.30, 151.12, 178.25, 191.80.
–COOH), 4.03 (q, 2H, J = 7.2 Hz, CH2), 5.23 (s, 1H, CHtriazole), 5.38 (s, 1H, CHpyrimidine), 6.90–7.64 (m, 12H, 2ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.10 (s, 1H, OH exchangeable by D2O), 10.18 (s, 1H, CHindole, exchangeable by D2O), 12.23 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.21, 28.46, 36.48, 41.81, 53.12, 57.66, 107.86, 117.40, 118.62, 121.67, 122.15, 122.24, 123.02, 123.40, 124.61, 125.41, 127.25, 128.20, 133.32, 134.25, 135.70, 136.21, 138.22, 141.18, 144.30, 151.12, 178.25, 191.80.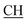 –COOH), 4.02 (q, 2H, J = 7.2 Hz, CH2), 5.38 (s, 1H, CHpyrimidine), 6.93–7.65 (m, 12H, 2ArH), 8.03 (s, 1H, NH, exchangeable by D2O), 9.12 (s, 1H, OH exchangeable by D2O), 10.23 (s, 1H, CHindole, exchangeable by D2O), 12.25 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.21, 22.58, 28.46, 36.48, 41.81, 53.12, 57.66, 107.86, 117.40, 118.62, 121.64, 122.15, 122.24, 123.02, 123.40, 124.61, 125.41, 127.25, 128.20, 133.32, 134.25, 135.70, 136.21, 138.22, 141.18, 144.30, 151.12, 178.25, 191.80.
–COOH), 4.02 (q, 2H, J = 7.2 Hz, CH2), 5.38 (s, 1H, CHpyrimidine), 6.93–7.65 (m, 12H, 2ArH), 8.03 (s, 1H, NH, exchangeable by D2O), 9.12 (s, 1H, OH exchangeable by D2O), 10.23 (s, 1H, CHindole, exchangeable by D2O), 12.25 (br, 1H, OH, exchangeable by D2O). 13CMR (DMSO-d6), δ ppm: 14.21, 22.58, 28.46, 36.48, 41.81, 53.12, 57.66, 107.86, 117.40, 118.62, 121.64, 122.15, 122.24, 123.02, 123.40, 124.61, 125.41, 127.25, 128.20, 133.32, 134.25, 135.70, 136.21, 138.22, 141.18, 144.30, 151.12, 178.25, 191.80.Reaction of compounds 1a–d with cyclic-1,3-dicarbonyl compounds: synthesis of 2-([1,2,4]triazolo[1,5-a]pyrimidin-3(2H,5H,6H)-yl)propanoic acid derivatives 4–7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] was mixed in water (8 mL)–ethanol (2 mL) then was treated with 1 mL of fresh lemon juice. The reaction mixture was heated under reflux for 5–8 h, then left to cool. The formed precipitates were collected by filtration, washed thoroughly with water and then recrystallized from ethanol to give the corresponding 4–7 derivatives.
2)] was mixed in water (8 mL)–ethanol (2 mL) then was treated with 1 mL of fresh lemon juice. The reaction mixture was heated under reflux for 5–8 h, then left to cool. The formed precipitates were collected by filtration, washed thoroughly with water and then recrystallized from ethanol to give the corresponding 4–7 derivatives.
2-(5-(4-Chlorophenyl)-2-(2-hydroxyphenyl)-2,8,8-trimethyl-6-oxo-1H-[1,3]dioxino[5,4-e][1,2,4]triazolo[1,5-a]pyrimidin-3(2H,5H,6H)-yl)propanoic acid 4. Yield (57%), brown crystals, mp 208–210 °C, anal. calcd for (C25H25ClN4O6, 512.94): C, 58.54; H, 4.91; N, 10.92; Cl, 4.91. Found: C, 58.24; H, 5.12; N, 10.97; Cl, 6.98. IR (νmax, cm−1): 3392 (2OH), 3199 (NH), 3067 (CHaromatic), 2978 (CHaliphatic), 1675 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Omeldurium), 1616 (COOasy), 1590 (COOsy), 816 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.22 (d, 3H, CH3), 2.32 (s, 3H, CH3), 2.63 (s, 3H, C–CH3), 2.65 (s, 3H, C–CH3), 3.07 (m, 1H,
Omeldurium), 1616 (COOasy), 1590 (COOsy), 816 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.22 (d, 3H, CH3), 2.32 (s, 3H, CH3), 2.63 (s, 3H, C–CH3), 2.65 (s, 3H, C–CH3), 3.07 (m, 1H, 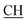 –COOH), 5.53 (s, 1H, CHpyrimidine), 6.53–8.25 (m, 8H, 2ArH), 10.66 (s, 2H, 2OH, exchangeable by D2O), 11.50 (s, 1H, NH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 14.51 (CH3), 15.57 (CH3), 18.87 (CH3), 24.61 (CH3), 46.23 (CH–COOH), 50.11 (CHpyrimidine), 56.54 (Ctriazole), 104.90, 117.38, 119.08, 120.60, 129.36, 131.32, 133.63, 135.17, 141.38, 142.89, 149.31 (m, 2ArC), 153.61 (C
–COOH), 5.53 (s, 1H, CHpyrimidine), 6.53–8.25 (m, 8H, 2ArH), 10.66 (s, 2H, 2OH, exchangeable by D2O), 11.50 (s, 1H, NH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 14.51 (CH3), 15.57 (CH3), 18.87 (CH3), 24.61 (CH3), 46.23 (CH–COOH), 50.11 (CHpyrimidine), 56.54 (Ctriazole), 104.90, 117.38, 119.08, 120.60, 129.36, 131.32, 133.63, 135.17, 141.38, 142.89, 149.31 (m, 2ArC), 153.61 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.74 (C–Cl), 167.95 (C–OH), 178.57 (C
N), 157.74 (C–Cl), 167.95 (C–OH), 178.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 181.09 (C
O), 181.09 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2-(5-(4-Chlorophenyl)-2-(2-hydroxyphenyl)-2-methyl-6,8-dioxo-1,2,6,7,8,9-hexahydropyrimido[5,4-e][1,2,4]triazolo[1,5-a]pyrimidin-3(5H)-yl)propanoic acid 5. Yield (62%), yellow crystals, mp 218–220 °C, anal. calcd for (C23H21ClN6O5, 496.91): C, 55.59; H, 4.26; Cl, 7.13; N, 16.91. Found: C, 55.70; H, 4.10; Cl, 7.33; N, 16.75. IR (νmax, cm−1): 3396 (2OH), 3294 (NH), 3238 (NHbarbaturic), 3206 (NHbarbaturic), 3024 (CHaromatic), 2996 (C–Haliphatic), 1707 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Obarbaturic), 1643 (C
Obarbaturic), 1643 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Obarbaturic), 1621 (COOasy), 1591 (COOsy), 753 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.33 (s, 3H, CH3), 3.02 (m, 1H,
Obarbaturic), 1621 (COOasy), 1591 (COOsy), 753 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.33 (s, 3H, CH3), 3.02 (m, 1H, 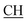 –COOH), 5.87 (s, 1H, CHpyrimidine), 6.86 (s, 1H, NH, exchangeable by D2O), 6.98–7.77 (m, 8H, 2ArH), 7.35 (s, 1H, OH, exchangeable by D2O), 10.76 (s, 1H, NHbarbaturic, exchangeable by D2O), 12.10 (s, 1H, NHbarbaturic, exchangeable by D2O), 12.32 (s, 1H, OH exchangeable by D2O).
–COOH), 5.87 (s, 1H, CHpyrimidine), 6.86 (s, 1H, NH, exchangeable by D2O), 6.98–7.77 (m, 8H, 2ArH), 7.35 (s, 1H, OH, exchangeable by D2O), 10.76 (s, 1H, NHbarbaturic, exchangeable by D2O), 12.10 (s, 1H, NHbarbaturic, exchangeable by D2O), 12.32 (s, 1H, OH exchangeable by D2O).
2-(5-(4-Chlorophenyl)-2-(2-hydroxyphenyl)-2-methyl-6-oxo-1,2,6,7,8,9-hexahydro-[1,2,4]triazolo[1,5-a]quinazolin-3(5H)-yl)propanoic acid 6. Yield (54%), yellow crystals, mp 273–275 °C, anal. calcd for (C25H25ClN4O4, 480.94): C, 62.43; H, 5.24; Cl, 7.37; N, 11.65. Found: C, 62.11; H, 5.44; Cl, 7.25; N, 16.87. IR (νmax, cm−1): 3414 (2OH), 3148 (NH), 3048 (CHaromatic), 2926 (CHaliphatic), 1663 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ocyclohexanedione), 1605 (COOasy), 1592 (COOsy), 750 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.95 (m, 2H, CH2(a)), 2.28 (t, 2H, CH2(b)), 2.34 (s, 3H, CH3), 2.53 (d, 3H, CH3), 2.64 (t, 2H, CH2(c)), 3.12 (m, 1H,
Ocyclohexanedione), 1605 (COOasy), 1592 (COOsy), 750 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.95 (m, 2H, CH2(a)), 2.28 (t, 2H, CH2(b)), 2.34 (s, 3H, CH3), 2.53 (d, 3H, CH3), 2.64 (t, 2H, CH2(c)), 3.12 (m, 1H, 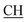 –COOH), 5.58 (s, 1H, CHpyrimidine), 6.97–7.93 (m, 8H, 2ArH), 11.92 (s, 1H, OH exchangeable by D2O), 12.48 (s, 1H, NH, exchangeable by D2O), 13.17 (s, 1H, OH exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.38 (CH3), 20.31 (CH2), 21.26 (CH3), 26.94 (CH2), 31.12 (CH–COOH), 36.86 (CH2), 48.61 (CHpyrimidine), 62.46 (Ctriazole), 115.61, 117.69, 118.02, 119.92, 128.31, 129.52, 130.34, 133.12, 136.65, 144.00, 149.91 (m, 2ArC), 160.33 (C
–COOH), 5.58 (s, 1H, CHpyrimidine), 6.97–7.93 (m, 8H, 2ArH), 11.92 (s, 1H, OH exchangeable by D2O), 12.48 (s, 1H, NH, exchangeable by D2O), 13.17 (s, 1H, OH exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.38 (CH3), 20.31 (CH2), 21.26 (CH3), 26.94 (CH2), 31.12 (CH–COOH), 36.86 (CH2), 48.61 (CHpyrimidine), 62.46 (Ctriazole), 115.61, 117.69, 118.02, 119.92, 128.31, 129.52, 130.34, 133.12, 136.65, 144.00, 149.91 (m, 2ArC), 160.33 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 165.36 (C–Cl), 168.33 (C–OH), 171.32 (C
N), 165.36 (C–Cl), 168.33 (C–OH), 171.32 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 196.64 (C
O), 196.64 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2-(5-(4-Chlorophenyl)-2-(2-hydroxyphenyl)-2,8,8-trimethyl-6-oxo-1,2,6,7,8,9-hexahydro-[1,2,4]triazolo[1,5-a]quinazolin-3(5H)-yl)propanoic acid 7. Yield (58%), pale yellow crystals, mp 257–259 °C, anal. calcd for (C27H29ClN4O4, 509): C, 63.71; H, 5.74; Cl, 6.97; N, 11.01. Found: C, 63.55; H, 5.91; Cl, 6.95; N, 11.11. IR (νmax, cm−1): 3316 (2OH), 3177 (NH), 3038 (CHaromatic), 2956 (CHaliphatic), 1705 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Odimedone), 1627 (COOasy), 1598 (COOsy), 751 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 0.91–1.07 (m, 6H, 2CH3), 2.11 (s, 2H, CH2), 2.38 (d, 3H, CH3), 2.47 (s, 3H, CH3), 2.66 (s, 2H, CH2), 3.47 (m, 1H,
Odimedone), 1627 (COOasy), 1598 (COOsy), 751 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 0.91–1.07 (m, 6H, 2CH3), 2.11 (s, 2H, CH2), 2.38 (d, 3H, CH3), 2.47 (s, 3H, CH3), 2.66 (s, 2H, CH2), 3.47 (m, 1H, 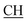 –COOH), 5.61 (s, 1H, CHpyrimidine), 6.95–7.93 (m, 8H, 2ArH), 11.93 (s, 1H, OH exchangeable by D2O), 12.86 (s, 2H, NH, OH exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.36 (CH3), 18.92 (CH3), 27.03 (CH2), 28.19 (CH3), 29.44 (CH3), 32.24 (CH2), 47.55 (
–COOH), 5.61 (s, 1H, CHpyrimidine), 6.95–7.93 (m, 8H, 2ArH), 11.93 (s, 1H, OH exchangeable by D2O), 12.86 (s, 2H, NH, OH exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.36 (CH3), 18.92 (CH3), 27.03 (CH2), 28.19 (CH3), 29.44 (CH3), 32.24 (CH2), 47.55 (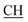 –COOH), 50.75 (CHpyrimidine), 53.69 (
–COOH), 50.75 (CHpyrimidine), 53.69 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –(CH3)2), 56.53 (Ctriazole), 117.68, 119.48, 119.93, 128.24, 130.04, 131.79, 133.12, 136.66, 149.97 (m, 2ArC), 159.98 (C
–(CH3)2), 56.53 (Ctriazole), 117.68, 119.48, 119.93, 128.24, 130.04, 131.79, 133.12, 136.66, 149.97 (m, 2ArC), 159.98 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 163.54 (C–Cl), 168.30 (C–OH), 181.36 (C
N), 163.54 (C–Cl), 168.30 (C–OH), 181.36 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 196.47 (C
O), 196.47 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Synthesis of 2-(5-(4-chlorophenyl)-6-cyano-2-(2-hydroxyphenyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(7H)-yl)propanoic acid derivatives 8a–d and 9a–d
An equimolar mixture of 2-(3-amino-5-(2-hydroxyphenyl)-1H-1,2,4-triazol-4(5H)-yl)propanoic acid derivative 1a–d (0.001 mol), 4-chlorobenzaldehyde (0.14 g, 0.001 mol) and malononitrile or ethyl cyanoacetate (0.001 mol) was mixed in water (8 mL)-ethanol (2 mL) then was treated with 1 mL of fresh lemon juice. The reaction mixture was heated under reflux for 3–6 h, then left to cool. The formed precipitates were collected by filtration, washed thoroughly with water and then recrystallized from ethanol to give the corresponding 2-(5-(4-chlorophenyl)-6-cyano-2-(2-hydroxyphenyl)-1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(7H)-yl)propanoic acid derivatives 8a–d and 9a–d respectively.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1617 (COOasy), 1539 (COOsy), 752 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.59 (d, 3H, CH3), 3.48 (q, 1H,
N), 1617 (COOasy), 1539 (COOsy), 752 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.59 (d, 3H, CH3), 3.48 (q, 1H, 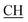 –COOH), 4.44 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.75 (s, 2H, NH2, exchangeable by D2O), 6.89–7.92 (m, 8H, 2ArH), 8.05 (s, 1H, OH, exchangeable by D2O), 8.40 (s, 1H, NH, exchangeable by D2O), 10.02 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.91 (CH3), 44.55 (
–COOH), 4.44 (s, 1H, CHtriazole), 5.36 (s, 1H, CHpyrimidine), 6.75 (s, 2H, NH2, exchangeable by D2O), 6.89–7.92 (m, 8H, 2ArH), 8.05 (s, 1H, OH, exchangeable by D2O), 8.40 (s, 1H, NH, exchangeable by D2O), 10.02 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.91 (CH3), 44.55 (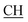 –COOH), 53.53 (CHtriazole), 80.99 (
–COOH), 53.53 (CHtriazole), 80.99 (![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) –CN), 111.44 (C
–CN), 111.44 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 116.86, 119.00, 120.84, 121.87, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C
N), 116.86, 119.00, 120.84, 121.87, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.92 (C–Cl), 167.14 (C–OH), 177.96 (C
N), 157.92 (C–Cl), 167.14 (C–OH), 177.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 178.20 (C
NH), 178.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1618 (COOasy), 1589 (COOsy), 766 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.54 (d, 3H, CH3), 2.65 (s, 3H, CH3), 3.35 (q, 1H,
N), 1618 (COOasy), 1589 (COOsy), 766 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.54 (d, 3H, CH3), 2.65 (s, 3H, CH3), 3.35 (q, 1H, 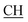 –COOH), 6.86 (s, 2H, NH2, exchangeable by D2O), 6.95–8.24 (m, 8H, 2ArH), 8.08 (s, 1H, OH, exchangeable by D2O), 11.49 (s, 1H, NH, exchangeable by D2O), 11.98 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 20.61 (CH3), 28.03 (CH3), 46.48 (
–COOH), 6.86 (s, 2H, NH2, exchangeable by D2O), 6.95–8.24 (m, 8H, 2ArH), 8.08 (s, 1H, OH, exchangeable by D2O), 11.49 (s, 1H, NH, exchangeable by D2O), 11.98 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 20.61 (CH3), 28.03 (CH3), 46.48 (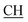 –COOH), 63.12 (Ctriazole), 78.17 (C–CN), 118.02 (C
–COOH), 63.12 (Ctriazole), 78.17 (C–CN), 118.02 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 119.63, 127.76, 129.16, 129.41, 130.48, 131.19, 131.89, 133.67, 134.69, (m, ArC), 136.74 (C
N), 119.63, 127.76, 129.16, 129.41, 130.48, 131.19, 131.89, 133.67, 134.69, (m, ArC), 136.74 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 141.30 (C–Cl), 159.45 (C–OH), 161.28 (C
N), 141.30 (C–Cl), 159.45 (C–OH), 161.28 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 178.5 (C
NH), 178.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). MS (m/z, I%): 452 (M + 2, 0.15%); 450 (0.05), 405 (0.27), 375 (0.22), 363 (0.53), 347 (0.42), 317 (0.89), 259 (4.13), 189 (6.36), 113 (24.84), 87 (19.42), 59 (100).
O). MS (m/z, I%): 452 (M + 2, 0.15%); 450 (0.05), 405 (0.27), 375 (0.22), 363 (0.53), 347 (0.42), 317 (0.89), 259 (4.13), 189 (6.36), 113 (24.84), 87 (19.42), 59 (100).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1621 (COOasy), 1578 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 3.12 (t, 1H,
N), 1621 (COOasy), 1578 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 3.12 (t, 1H, 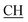 –COOH), 3.35, 3.58 (dd, 2H, CH2), 5.25 (s, 1H, CHtriazole), 6.72 (s, 2H, NH2, exchangeable by D2O), 6.80–8.12 (m, 13H, 3ArH), 8.40 (s, 1H, OH, exchangeable by D2O), 10.22 (s, 1H, NH, exchangeable by D2O), 10.48 (s, 1H, NHpyrimidine, exchangeable by D2O), 12.80 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 34.81 (CH2), 53.87 (
–COOH), 3.35, 3.58 (dd, 2H, CH2), 5.25 (s, 1H, CHtriazole), 6.72 (s, 2H, NH2, exchangeable by D2O), 6.80–8.12 (m, 13H, 3ArH), 8.40 (s, 1H, OH, exchangeable by D2O), 10.22 (s, 1H, NH, exchangeable by D2O), 10.48 (s, 1H, NHpyrimidine, exchangeable by D2O), 12.80 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 34.81 (CH2), 53.87 (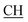 –COOH), 66.75 (CHtriazole), 77.55 (C–CN), 107.77 (C
–COOH), 66.75 (CHtriazole), 77.55 (C–CN), 107.77 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 117.30 (CHindole), 119.32, 119.50, 119.84, 121.60, 123.60, 123.96, 124.84, 128.31, 128.95, 129.12, 129.43, 130.05, 131.38, 131.50, 133.71 (ArC), 141.20 (C
N), 117.30 (CHindole), 119.32, 119.50, 119.84, 121.60, 123.60, 123.96, 124.84, 128.31, 128.95, 129.12, 129.43, 130.05, 131.38, 131.50, 133.71 (ArC), 141.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.76 (C–NH), 157.18 (C–Cl), 165.89 (C–OH), 168.50 (C
N), 153.76 (C–NH), 157.18 (C–Cl), 165.89 (C–OH), 168.50 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 175.45 (C
NH), 175.45 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1616 (COOasy), 1579 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.33 (s, 3H, CH3), 3.10 (t, 1H,
N), 1616 (COOasy), 1579 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.33 (s, 3H, CH3), 3.10 (t, 1H, 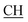 –COOH), 3.35, 3.58 (dd, 2H, CH2), 6.76 (s, 2H, NH2, exchangeable by D2O), 6.84–8.15 (m, 13H, 3ArH), 8.41 (s, 1H, OH, exchangeable by D2O), 10.20 (s, 1H, NH, exchangeable by D2O), 10.54 (s, 1H, NHpyrimidine, exchangeable by D2O), 12.86 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 20.68 (CH3), 34.80 (CH2), 53.84 (
–COOH), 3.35, 3.58 (dd, 2H, CH2), 6.76 (s, 2H, NH2, exchangeable by D2O), 6.84–8.15 (m, 13H, 3ArH), 8.41 (s, 1H, OH, exchangeable by D2O), 10.20 (s, 1H, NH, exchangeable by D2O), 10.54 (s, 1H, NHpyrimidine, exchangeable by D2O), 12.86 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 20.68 (CH3), 34.80 (CH2), 53.84 (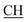 –COOH), 66.76 (CHtriazole), 77.52 (C–CN), 114.70 (C
–COOH), 66.76 (CHtriazole), 77.52 (C–CN), 114.70 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 117.32 (CHindole), 119.28, 119.54, 119.81, 121.63, 123.63, 123.96, 124.86, 128.30, 128.96, 129.10, 129.23, 130.03, 131.32, 131.55, 133.73 (m, ArC), 141.20 (C
N), 117.32 (CHindole), 119.28, 119.54, 119.81, 121.63, 123.63, 123.96, 124.86, 128.30, 128.96, 129.10, 129.23, 130.03, 131.32, 131.55, 133.73 (m, ArC), 141.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.74 (C–NH), 157.11 (C–Cl), 165.14 (C–OH), 168.32 (C
N), 153.74 (C–NH), 157.11 (C–Cl), 165.14 (C–OH), 168.32 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 175.65 (C
NH), 175.65 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). MS (m/z, I%): 568 (M + 2, 0.13), 566 (0.51), 553 (0.44), 405 (0.38), 370 (0.48), 326 (0.43), 298 (0.25), 204.20 (1.86), 194 (0.61), 158.20 (5.11), 130.15 (100), 103.10 (9.99), 77.05 (13.13).
O). MS (m/z, I%): 568 (M + 2, 0.13), 566 (0.51), 553 (0.44), 405 (0.38), 370 (0.48), 326 (0.43), 298 (0.25), 204.20 (1.86), 194 (0.61), 158.20 (5.11), 130.15 (100), 103.10 (9.99), 77.05 (13.13).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1676 (CO), 1622 (COOasy), 1548 (COOsy), 750 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.57 (d, 3H, CH3), 3.52 (q, 1H,
N), 1676 (CO), 1622 (COOasy), 1548 (COOsy), 750 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.57 (d, 3H, CH3), 3.52 (q, 1H, 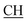 –COOH), 5.23 (s, 1H, CHtriazole), 6.94–7.59 (m, 8H, 2ArH), 8.03 (s, 1H, NH, exchangeable by D2O), 10.02 (s, 1H, OH, exchangeable by D2O), 12.22 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.91 (CH3), 44.55 (
–COOH), 5.23 (s, 1H, CHtriazole), 6.94–7.59 (m, 8H, 2ArH), 8.03 (s, 1H, NH, exchangeable by D2O), 10.02 (s, 1H, OH, exchangeable by D2O), 12.22 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.91 (CH3), 44.55 (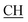 –COOH), 53.53 (CHtriazole), 107.44 (C
–COOH), 53.53 (CHtriazole), 107.44 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 116.86, 119.00, 120.84, 121.87, 123.56, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C
N), 116.86, 119.00, 120.84, 121.87, 123.56, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 157.92 (C–Cl), 167.14 (C–OH), 177.96 (C
N), 157.92 (C–Cl), 167.14 (C–OH), 177.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 178.20 (C
NH), 178.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1675 (CO), 1620 (COOasy), 1553 (COOsy), 757 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.54 (d, 3H, CH3), 2.23 (s, 3H, CH3), 3.54 (q, 1H,
N), 1675 (CO), 1620 (COOasy), 1553 (COOsy), 757 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 1.54 (d, 3H, CH3), 2.23 (s, 3H, CH3), 3.54 (q, 1H, 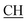 –COOH), 5.21 (s, 1H, CHtriazole), 6.92–7.56 (m, 8H, 2ArH), 8.02 (s, 1H, OH, exchangeable by D2O), 9.24 (s, 1H, OH, exchangeable by D2O), 12.18 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.87 (CH3), 18.12, 44.23 (
–COOH), 5.21 (s, 1H, CHtriazole), 6.92–7.56 (m, 8H, 2ArH), 8.02 (s, 1H, OH, exchangeable by D2O), 9.24 (s, 1H, OH, exchangeable by D2O), 12.18 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 19.87 (CH3), 18.12, 44.23 (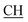 –COOH), 80.65 (Ctriazole), 107.44 (C
–COOH), 80.65 (Ctriazole), 107.44 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 116.86, 119.00, 120.84, 121.87, 123.56, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C
N), 116.86, 119.00, 120.84, 121.87, 123.56, 127.20, 129.14, 131.46, 131.94, 133.98, (m, ArC), 140.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.96 (C
N), 153.96 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH), 157.92 (C–Cl), 167.14 (C–OH), 178.15 (C
NH), 157.92 (C–Cl), 167.14 (C–OH), 178.15 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1623 (COOasy), 1578 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 3.12 (t, 1H,
N), 1623 (COOasy), 1578 (COOsy), 765 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 3.12 (t, 1H, 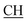 –COOH), 3.56 (d, 2H, CH2), 5.25 (s, 1H, CHtriazole), 6.74 (s, 1H, NH, exchangeable by D2O), 6.80–8.12 (m, 13H, 3ArH), 8.40 (s, 1H, OH, exchangeable by D2O), 10.25 (s, 1H, NH, exchangeable by D2O), 12.23 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 34.80, 53.83, 66.75, 107.77, 117.30, 119.32, 119.84, 121.60, 123.60, 123.96, 124.84, 128.31, 128.95, 129.12, 129.43, 130.05, 131.38, 131.50, 133.71, 141.20, 153.76, 157.16, 165.81, 168.33, 178.41.
–COOH), 3.56 (d, 2H, CH2), 5.25 (s, 1H, CHtriazole), 6.74 (s, 1H, NH, exchangeable by D2O), 6.80–8.12 (m, 13H, 3ArH), 8.40 (s, 1H, OH, exchangeable by D2O), 10.25 (s, 1H, NH, exchangeable by D2O), 12.23 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 34.80, 53.83, 66.75, 107.77, 117.30, 119.32, 119.84, 121.60, 123.60, 123.96, 124.84, 128.31, 128.95, 129.12, 129.43, 130.05, 131.38, 131.50, 133.71, 141.20, 153.76, 157.16, 165.81, 168.33, 178.41.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1625 (COOasy), 1575 (COOsy), 771 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.23 (s, 3H, CH3), 3.13 (t, 1H,
N), 1625 (COOasy), 1575 (COOsy), 771 (C–Cl). 1H-NMR (DMSO-d6), δ ppm: 2.23 (s, 3H, CH3), 3.13 (t, 1H, 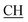 –COOH), 3.58 (d, 2H, CH2), 6.75 (s, 1H, NH, exchangeable by D2O), 6.83–8.12 (m, 13H, 3ArH), 8.38 (s, 1H, OH, exchangeable by D2O), 10.23 (s, 1H, NH, exchangeable by D2O), 12.25 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 24.13, 34.80, 53.83, 66.75, 107.76, 117.30, 119.30, 119.97, 121.60, 123.54, 123.96, 124.84, 128.31, 128.90, 129.13, 129.45, 130.05, 131.38, 131.50, 133.72, 141.25, 153.16, 157.12, 165.07, 168.27, 178.48.
–COOH), 3.58 (d, 2H, CH2), 6.75 (s, 1H, NH, exchangeable by D2O), 6.83–8.12 (m, 13H, 3ArH), 8.38 (s, 1H, OH, exchangeable by D2O), 10.23 (s, 1H, NH, exchangeable by D2O), 12.25 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 24.13, 34.80, 53.83, 66.75, 107.76, 117.30, 119.30, 119.97, 121.60, 123.54, 123.96, 124.84, 128.31, 128.90, 129.13, 129.45, 130.05, 131.38, 131.50, 133.72, 141.25, 153.16, 157.12, 165.07, 168.27, 178.48.Reaction of compound 1a–d with 2-(bis(methylthio)methylene)malononitrile
An equimolar mixture of 2-(3-amino-5-(2-hydroxyphenyl)-1H-1,2,4-triazol-4(5H)-yl)propanoic acid derivative 1a–d (0.001 mol) and 2-(bis(methylthio)methylene)malononitrile (0.17 g, 0.001 mol) was mixed in water (8 mL)–ethanol (2 mL) then was treated with 1 mL of fresh lemon juice. The reaction mixture was heated under reflux until evolution of methyl mercaptan was ceased (lead acetate, 10–12 h), then left to cool. The formed precipitates were collected by filtration, washed thoroughly with water and then recrystallized from ethanol to give the corresponding 2-(6-cyano-2-(2-hydroxyphenyl)-7-imino-5-(methylthio)-1,2-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-3(7H)-yl)propanoic acid derivatives 10a–d respectively.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1625 (COOasy), 1581 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.58 (s, 3H, S–CH3), 3.45 (q, 1H,
N), 1625 (COOasy), 1581 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.58 (s, 3H, S–CH3), 3.45 (q, 1H, 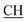 –COOH), 5.25 (s, 1H, CHtriazole), 6.78 (s, 1H, NH exchangeable by D2O), 7.34–7.56 (m, 4H, ArH), 7.96 (s, 1H, NH, exchangeable by D2O), 8.04 (s, 1H, OH, exchangeable by D2O), 12.22 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.35, 18.90, 46.76, 57.70, 66.95, 117.45, 119.11, 119.43, 128.25, 128.76, 130.13, 130.99, 131.48, 152.55, 157.02, 168.43, 181.05.
–COOH), 5.25 (s, 1H, CHtriazole), 6.78 (s, 1H, NH exchangeable by D2O), 7.34–7.56 (m, 4H, ArH), 7.96 (s, 1H, NH, exchangeable by D2O), 8.04 (s, 1H, OH, exchangeable by D2O), 12.22 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.35, 18.90, 46.76, 57.70, 66.95, 117.45, 119.11, 119.43, 128.25, 128.76, 130.13, 130.99, 131.48, 152.55, 157.02, 168.43, 181.05.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1605 (COOasy), 1587 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.33 (s, 3H, CH3), 2.53 (s, 3H, S–CH3), 3.47 (m, 1H,
N), 1605 (COOasy), 1587 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.23 (d, 3H, CH3), 2.33 (s, 3H, CH3), 2.53 (s, 3H, S–CH3), 3.47 (m, 1H, 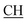 –COOH), 6.84 (s, 1H, NH exchangeable by D2O), 7.32–7.54 (m, 4H, ArH), 7.88 (s, 1H, NH, exchangeable by D2O), 8.06 (s, 1H, OH, exchangeable by D2O), 12.23 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.38, 18.96, 24.53, 46.80, 57.72, 66.99, 117.40, 119.08, 119.47, 128.33, 128.95, 130.04, 130.98, 131.48, 152.93, 157.72, 168.29, 180.98.
–COOH), 6.84 (s, 1H, NH exchangeable by D2O), 7.32–7.54 (m, 4H, ArH), 7.88 (s, 1H, NH, exchangeable by D2O), 8.06 (s, 1H, OH, exchangeable by D2O), 12.23 (s, 1H, OH, exchangeable by D2O). 13C NMR (DMSO-d6), δ ppm: 15.38, 18.96, 24.53, 46.80, 57.72, 66.99, 117.40, 119.08, 119.47, 128.33, 128.95, 130.04, 130.98, 131.48, 152.93, 157.72, 168.29, 180.98.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1625 (COOasy), 1587 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.53 (s, 3H, S–CH3), 3.47 (m, 1H,
N), 1625 (COOasy), 1587 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.53 (s, 3H, S–CH3), 3.47 (m, 1H, 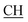 –COOH), 3.57 (d, 3H, CH2), 5.25 (s, 1H, CHtriazole), 6.85 (s, 1H, NH exchangeable by D2O), 7.32–7.54 (m, 8H, ArH), 8.04 (s, 1H, NH, exchangeable by D2O), 9.23 (s, 1H, OH, exchangeable by D2O), 12.21 (s, 1H, OH, exchangeable by D2O).
–COOH), 3.57 (d, 3H, CH2), 5.25 (s, 1H, CHtriazole), 6.85 (s, 1H, NH exchangeable by D2O), 7.32–7.54 (m, 8H, ArH), 8.04 (s, 1H, NH, exchangeable by D2O), 9.23 (s, 1H, OH, exchangeable by D2O), 12.21 (s, 1H, OH, exchangeable by D2O).![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1623 (COOasy), 1588 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.58 (s, 3H, S–CH3), 3.48 (m, 1H,
N), 1623 (COOasy), 1588 (COOsy). 1H-NMR (DMSO-d6), δ ppm: 2.58 (s, 3H, S–CH3), 3.48 (m, 1H, 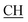 –COOH), 3.56 (d, 3H, CH2), 6.81 (s, 1H, NH exchangeable by D2O), 7.30–7.62 (m, 8H, ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.20 (s, 1H, OH, exchangeable by D2O), 12.24 (s, 1H, OH, exchangeable by D2O).
–COOH), 3.56 (d, 3H, CH2), 6.81 (s, 1H, NH exchangeable by D2O), 7.30–7.62 (m, 8H, ArH), 8.05 (s, 1H, NH, exchangeable by D2O), 9.20 (s, 1H, OH, exchangeable by D2O), 12.24 (s, 1H, OH, exchangeable by D2O).Antimicrobial screening
The antimicrobial screening was performed according to the procedure of “Agar Diffusion Method”.35,37 One mg of each of the newly synthesized compounds was dissolved in dimethyl sulphoxide (DMSO, 1 mL) then made up to 10 mL with sterile water to give a concentration of 100 μg mL−1. A solution of the tested compounds was placed separately in the agar medium. The inhibition zones were measured after 24 h incubation.
MIC index (MBC/MIC) was calculated for the antibacterial agent to determine whether the agent was bactericidal (MBC/MIC < 4) or bacteriostatic (MBC/MIC > 4) against the growth of the tested bacteria. The range of MIC index values greater than 4 and less than 32 were considered to be bacteriostatic.39
| Cell viability (%) = mean optical density/control optical density × 100% |
4. Conclusion
A new series of [1,2,4]-triazole derivatives were obtained under green reaction conditions. The obtained candidates showed promising antibacterial screening namely, 2b–d, 3a–d, 5, 6, 7, 8a–d and 9a–d. They were further subjected to a screening against MRD (multi drug resistant) clinical isolates and showed promising antibacterial activity. The most active compounds 2c, 2d, 3c, 8a, 8b, 9a, 9b, 9c and 9d showed a high selectivity index towards antimicrobial activity against K. pneumoniae and MRSA1 compared to mammalian cells, revealed a good safety profile. Moreover, in contrast to the reference drugs cephalothin and chloramphenicol, compounds 3c, 8a and 9d exhibited significant better MIC values towards the tested MDR strains. The MIC index of these compound suggesting bacteriostatic mechanism of action.Conflicts of interest
The authors confirm that the content of this article contains no conflict of interest.Acknowledgements
Authors are grateful to Department of Chemistry, Faculty of Science, Sohag University, Egypt, for unlimited support of this work.References
- B. Beović, Int. J. Food Microbiol., 2006, 112, 280–287 CrossRef
.
- D. T. W. Chu, J. J. Plattner and L. Katz, J. Med. Chem., 1996, 39, 3853–3874 CrossRef CAS
.
- R. Finch and P. A. Hunter, J. Antimicrob. Chemother., 2006, 58(Suppl 1), i3–i22 CrossRef
.
- T. D. Gootz, Crit. Rev. Immunol., 2010, 30, 79–93 CrossRef CAS
.
- D. Niccolai, L. Tarsi and R. J. Thomas, Chem. Commun., 1997, 2333–2342, 10.1039/a704497f
.
- K. M. Overbye and J. F. Barrett, Drug Discovery Today, 2005, 10, 45–52 CrossRef
.
- N. Suree, M. E. Jung and R. T. Clubb, Mini-Rev. Med. Chem., 2007, 7, 991–1000 CrossRef CAS
.
- C. Walsh, Nature, 2000, 406, 775–781 CrossRef CAS
.
- M. M. Cowan, Clin. Microbiol. Rev., 1999, 12, 564–582 CrossRef CAS
.
- V. Cattoir and B. Felden, J. Infect. Dis., 2019, 220, 350–360 CrossRef
.
- E. D. Brown and G. D. Wright, Nature, 2016, 529, 336–343 CrossRef CAS
.
- A. Travis, O. Chernova, V. Chernov and R. Aminov, Expert Opin. Drug Discovery, 2018, 13, 983–985 CrossRef CAS
.
- F. Gao, T. Wang, J. Xiao and G. Huang, Eur. J. Med. Chem., 2019, 173, 274–281 CrossRef CAS
.
- S. A. Shahzad, M. Yar, Z. A. Khan, L. Shahzadi, S. A. R. Naqvi, A. Mahmood, S. Ullah, A. J. Shaikh, T. A. Sherazi, A. T. Bale, J. Kukułowicz and M. Bajda, Bioorg. Chem., 2019, 85, 209–220 CrossRef CAS
.
- H. A. M. El-Sherief, B. G. M. Youssif, S. N. Abbas Bukhari, A. H. Abdelazeem, M. Abdel-Aziz and H. M. Abdel-Rahman, Eur. J. Med. Chem., 2018, 156, 774–789 CrossRef CAS
.
- İ. Küçükgüzel, E. Tatar, Ş. G. Küçükgüzel, S. Rollas and E. De Clercq, Eur. J. Med. Chem., 2008, 43, 381–392 CrossRef
.
- S. Zhang, Z. Xu, C. Gao, Q.-C. Ren, L. Chang, Z.-S. Lv and L.-S. Feng, Eur. J. Med. Chem., 2017, 138, 501–513 CrossRef CAS
.
- Z. Xu, S. Zhang, C. Gao, J. Fan, F. Zhao, Z.-S. Lv and L.-S. Feng, Chin. Chem. Lett., 2017, 28, 159–167 CrossRef CAS
.
- J. Xu, Y. Cao, J. Zhang, S. Yu, Y. Zou, X. Chai, Q. Wu, D. Zhang, Y. Jiang and Q. Sun, Eur. J. Med. Chem., 2011, 46, 3142–3148 CrossRef CAS
.
- A. M. El-Saghier, M. A. Mohamed, O. A. Abd-Allah, A. M. Kadry, T. M. Ibrahim and A. A. Bekhit, Med. Chem. Res., 2019, 28, 169–181 CrossRef CAS
.
- S. Eswaran, A. V. Adhikari and N. S. Shetty, Eur. J. Med. Chem., 2009, 44, 4637–4647 CrossRef CAS
.
- H. Wang, M. Lee, Z. Peng, B. Blázquez, E. Lastochkin, M. Kumarasiri, R. Bouley, M. Chang and S. Mobashery, J. Med. Chem., 2015, 58, 4194–4203 CrossRef CAS
.
- M. A. T. Blaskovich, J. Med. Chem., 2016, 59, 10807–10836 CAS
.
- A. Cosquer, M. Ficamos, M. Jebbar, J.-C. Corbel, G. Choquet, C. Fontenelle, P. Uriac and T. Bernard, Bioorg. Med. Chem. Lett., 2004, 14, 2061–2065 CrossRef CAS
.
- S. Mishra, U. Narain, R. Mishra and K. Misra, Bioorg. Med. Chem., 2005, 13, 1477–1486 CrossRef CAS
.
- E. J. Corey, L. Kürti and B. Czakó, Molecules and Medicine, Wiley & Sons, 2008, p. 272, ISBN: 978-0-470-26096-8 Search PubMed
.
- M. K. Khera, I. A. Cliffe, T. Mathur and O. Prakash, Bioorg. Med. Chem. Lett., 2011, 21, 2887–2889 CrossRef CAS
.
- T. Krishnaraj and S. Muthusubramanian, J. Heterocycl. Chem., 2014, 51, E68–E70 CrossRef CAS
.
- R. Kumar, R. R. Nair, S. S. Dhiman, J. Sharma and O. Prakash, Eur. J. Med. Chem., 2009, 44, 2260–2264 CrossRef CAS
.
- O. Prakash, V. Bhardwaj, R. Kumar, P. Tyagi and K. R. Aneja, Eur. J. Med. Chem., 2004, 39, 1073–1077 CrossRef CAS
.
- R. H. Abd El-Aleam, R. F. George, G. S. Hassan and H. M. Abdel-Rahman, Bioorg. Chem., 2020, 94, 103411 CrossRef CAS
.
- A. A. Bekhit, A. M. Farghaly, R. M. Shafik, M. M. A. Elsemary, A. E.-D. A. Bekhit, A. A. Guemei, M. S. El-Shoukrofy and T. M. Ibrahim, Bioorg. Chem., 2018, 77, 38–46 CrossRef CAS
.
- L. Zaharani, N. G. Khaligh, T. Mihankhah, Z. Shahnavaz and M. R. Johan, Synth. Commun., 2020, 50, 1633–1640 CrossRef CAS
.
- L. P. Garrod and P. M. Waterworth, J. Clin. Pathol., 1971, 24, 779–789 CrossRef CAS
.
- A. O. Abdelhamid, I. E. El Sayed, M. Z. Hussein and M. M. Mangoud, Molecules, 2016, 21 Search PubMed
.
- N. Nina, C. Quispe, F. Jiménez-Aspee, C. Theoduloz, G. E. Feresín, B. Lima, E. Leiva and G. Schmeda-Hirschmann, Molecules, 2015, 20, 18144–18167 CAS
.
- D. R. Ramadan, A. A. Elbardan, A. A. Bekhit, A. El-Faham and S. N. Khattab, New J. Chem., 2018, 42, 10676–10688 RSC
.
- G. Chamandi, Z. Olama and H. Holail, Int. J. Curr. Microbiol. Appl. Sci., 2015, 4, 328–342 CAS
.
- A. R. L. L. A. Tukappa, Am. J. Drug Discovery Dev., 2013, 3, 72–83 CrossRef
.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08189b |
| This journal is © The Royal Society of Chemistry 2021 |