Open Access Article
Open Access ArticleEffects of amino acid ionic liquids with different cations ([N2Py], [N2222], [P2222], and [C2mim]) on wheat seedlings
Zhonglin Chen ,
Hong Xiao,
Siqi Wu,
Jie Wang
,
Hong Xiao,
Siqi Wu,
Jie Wang *,
Jiayang Ji and
Xiao Qin
*,
Jiayang Ji and
Xiao Qin
School of Environmental Science, Liaoning University, Shenyang 110036, PR China. E-mail: 94266689@qq.com
First published on 6th January 2021
Abstract
The ecotoxicity of four ionic liquids with different cations (N-ethyl-pyridine alanine [N2Py][Ala], tetraethyl phosphine L-α-amino propionic acid salt [P2222][Ala], 1-ethyl-3-methyl-imidazolium alanine [C2mim][Ala], and tetraethyl ammonium L-α-amino propionic acid salt [N2222][Ala]) was assessed in hydroponically-grown wheat seedlings at concentrations from 200–1200 mg L−1. The results showed that type of cation has a significant influence on the growth, chlorophyll and nutrient uptake of wheat seedlings (P < 0.05). We observed decreased dry weight and shorter roots and shoots in the treated seedlings with increasing IL concentrations. The contents of Chl a and Chl b in wheat seedlings exposed to ILs showed the trend of firstly increasing followed by a decrease with increasing IL concentrations, but they peaked at different concentrations of ILs. In addition, the exposure of wheat seedling to ILs containing different cations (200–1200 mg L−1) led to first an increase and then a decrease of nitrogen content, and reduced the content of phosphorus and potassium. Moreover, the cellular structures, including nuclei, mitochondria, chloroplasts, cell membranes, and the cell walls of wheat leaf and root were affected to varying degrees by 600 mg L−1 ILs. The negative impacts of ILs on wheat seedlings ranked from high to low were: [N2Py][Ala] > [N2222][Ala] > [P2222][Ala] > [C2mim][Ala]. In this work, the relatively stronger toxicity of [N2Py][Ala] was likely contributed by ethanol, which was used to dissolve [N2Py][Ala]. Therefore, it is not recommended to use N-ethyl-pyridine alanine ([N2Py][Ala]) widely in practical applications.
1. Introduction
Ionic liquids (ILs) are organic salts composed of organic cations and organic or inorganic anions that are liquid at room temperature.1 They are considered new alternative solvents that can be widely applied, especially as an important medium, in the field of synthesis and catalysis. Different cations and anions can be combined to design a wide variety of ionic liquids. The cations of ionic liquids mainly include imidazole, pyridine, quaternary ammonium, and quaternary phosphine. The anions are mainly ionic halide salts, such as Cl− and Br−, and ionic non-halide salts, such as BF4−, PF6−, CF3SO3−, and SbF6−.2Existing studies have shown that ILs have harmful effects on fish, bacteria, algae, and higher plants.3,4 The toxicity of ILs is determined by the type of cations and anions and the length of the alkyl substituent. Tot et al. investigated the acute developmental toxicity, antioxidant activity, and membrane lipid peroxidation level of snails exposed to [Cnmim]Br (n = 6, 8, 10, 12), and found the snail death rate increased with the length of the carbon chain.5 Ma found that several imidazole-type ionic liquids with varying alkaline chain length suppressed the growth of maize seedlings, and the longer the carbon chain, the greater the toxicity.6 Other researchers found similar results in wheat, using [Cnmim][OAc] (n = 2, 4, 6) ILs, again finding that the longer the carbon chain, the greater the IL toxicity to the plants.7,8 Habibul et al. also got similar results that the toxic effects of ILs on rice growth depends on the alkyl chain length: [C8min]Br >[C4min]Br >[C2min]Br.9
There have been extensive studies on the contribution of anions to the overall toxicity of ILs.10–12 Liu et al.10 explored the effects of three imidazole-type ionic liquids containing different anions on broad bean seedlings. The results showed that the effects of toxicity on broad bean seedings were as follows: BF4− > Br− > Cl−. Imidazole-type ionic liquids containing different anions have increasing toxicity on hydrogen-producing bacteria, as the number of fluorine atoms in anions increases.11 Moreover, the toxicity of Imidazole-type ILs with different anions on wheat seedling growth was assessed in a previous study, and the toxicity of different anions followed [TfO]− > [Cl]− > [BF4]− > [Lact]− > [Ala]−.12
However, there have been few studies on the effects of cations on IL toxicity.13 Couling et al. evaluated the toxicity of 23 ILs on the aquatic organisms Vibrio cheri and Daphnia magna using the quantitative structure–property relationship. When the substitution side chain and anions were the same, the toxicity of the following five types of cationic cores on the two aquatic organisms showed similar patterns: tetrazole cations > triazole cations > imidazole cations > pyridine cations > quaternary ammonium salts cations.13 Liu found that imidazole-type ILs with different cations had greater toxicity than pyridine-type ILs on the freshwater green alga Scenedesmus obliquus.14 And the cytotoxicity of ionic liquids mainly depends on their ability to permeabilize/disrupt the cellular membrane.15 Notably, these studies of cations on IL toxicity to organisms have only involved aquatic species; the toxicity of different cations on higher plants has not been reported.
Therefore, we tested the effect of four types of ILs with different cations on wheat seedlings: [N2Py][Ala], [P2222][Ala], [C2mim][Ala], and [N2222][Ala]. The influence on the growth, chlorophyll contents, and nutrients uptake of wheat seedlings, was assessed in this study. Furthermore, we examined the ultra-microcellular structures of the root and leaf tip cells by transmission electron microscopy. This study provides data support and an important reference for assessing the ecotoxicological effects and environmental safety of ILs.
2. Materials and methods
2.1 Ionic liquids
The ionic liquids [N2Py][Ala] (25% purity, dissolved in ethanol), [P2222][Ala] (97% purity, dissolved in water), [C2mim][Ala] (97% purity, dissolved in water), and [N2222][Ala] (97% purity, dissolved in water), were attained from Lanzhou Institute of Chemical Physics, Gansu, China. In the experiment, seven different concentrations (0, 200, 400, 600, 800, 1,000, and 1200 mg L−1) of each IL as used. Furthermore, the same dose of ethanol series concentration as the solvent of [N2Py][Ala] was prepared, to analyze the interference effect of ethanol on the toxicity of [N2Py][Ala] (Fig. 1).2.2 Wheat culture
Wheat seeds were purchased from Liaoning Academy of Agricultural Sciences, Liaoning, China. Following Chen et al.,16 wheat seeds of the same size were sterilized prior to use with 0.1% HgCl2 solution for 15 min and then rinsed with deionized water until the surface mucus was washed away. After filtering and drying, 30 seeds were dispersed evenly across an 80 mm diameter Petri dish for each experimental group and then covered with three layers of gauze. Each treatment group was provided with three replicates. We added a small amount of distilled water to the control group, and the same amount of solutions of four ILs or ethanol with different concentrations (200, 400, 600, 800, 1,000, and 1200 mg L−1, prepared with distilled water) to each treatment group. The samples were incubated at 28 °C and allowed to germinate for 24 h. Additional treatment solution was added every 8 h to keep the seeds moist. Once the seeds were evenly germinated, they were transferred to a plastic beaker tied with a nylon mesh, and the beaker had been prefilled with the Hoagland nutrent soluton (Control) or Hoagland solutions containing four ILs or ethanol with the appropriate concentrations (200, 400, 600, 800, 1,000, and 1200 mg L−1).17 It should be noted that, nutrient solution was added to each beaker such that the root tips of the germinated wheat seeds were just submerged.7 The beakers were exposed to natural light with a 10 h/14 h light/dark photoperiod for 15 d with 22 °C and 17 °C day and night temperatures. The appropriate Hoagland solution was added as needed to maintain the growth of wheat plants.2.3 Determination methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) embedding medium = 1
embedding medium = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, calculated by volume) for 1 h, followed by transferring to a 1
1, calculated by volume) for 1 h, followed by transferring to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) mixture of pure acetone and Spurr's resin for 3 h at room temperature and then they were placed in pure embedding medium overnight. After that, the samples were heated at 70 °C for 12 h approximately and then the ultrathin sections were cut with a diamond knife. Later, they were stained with 50% uranyl acetate for 30 min followed by 30% lead citrate for 15 min.19 At last, the samples were scanned through TEM.
3 (v/v) mixture of pure acetone and Spurr's resin for 3 h at room temperature and then they were placed in pure embedding medium overnight. After that, the samples were heated at 70 °C for 12 h approximately and then the ultrathin sections were cut with a diamond knife. Later, they were stained with 50% uranyl acetate for 30 min followed by 30% lead citrate for 15 min.19 At last, the samples were scanned through TEM.2.4 Data analysis
A least significant difference (LSD) test of univariate analysis of variance (ANOVA) was used to examine the differences between different groups in SPSS 17.0 (IBM Corp., Armonk, NY, USA). Origin 7.0 (OriginLab Corp. Northampton, Massachusetts, USA) was used for plotting.3. Results and discussion
3.1 Effects of the ILs with different cations ([N2Py], [P2222], [C2mim] and [N2222]) on the growth of wheat seedlings
When subjected to biotic or abiotic stress, plants often show reduced dry weight, plant height, and root length, which are among the most direct growth indicators.21 Wheat growth was inhibited with increasing [N2Py][Ala], [P2222][Ala], [C2mim][Ala], [N2222][Ala], and ethanol concentrations in all five treatment groups (Fig. 2 and 3). Especially, the plants grew very short when the both treatments (N2Py [Ala] and ethanol) were given dosages greater than 800 mg L−1. Tot et al. also found that, under strong ionic liquid stress ([Im][Cl], [Bmim][Cl], [C2OC2mIm][Cl], [C1OC2mIm][Cl], [OHC3 eIm][Cl], [OHC3mIm][Cl], [OHC2mIm][Cl], 1000 mg L−1) adverse effects of ionic liquids on plant seedling growth is visible.5 The growth indices of ethanol and [N2Py][Ala] treatment groups showed the largest inhibited effects, which is consistent with the published research.16As shown in Fig. 4, different cationic ionic liquids had significant effects on the growth of wheat seedlings (P < 0.05). The aerial dry weight, underground dry weight, plant height, and root length of the treatment groups were lower than corresponding controls, except for the underground dry weight of the wheat seedlings under the 200–400 mg L−1 [C2mim][Ala] treatment, which were higher than control. Compared with control, the inhibited effects of the treatments of [N2Py]Ala, [P2222]Ala, [C2mim]Ala, [N2222]Ala and ethanol on wheat growth was the strongest at dosage of 1200 mg L−1, the corresponding inhibition rates for plant height were 83.3%, 37.0%, 43.4%, 52.7% and 87.5% respectively, the corresponding inhibition rates for root length were 96.1%, 67.6%, 50.1%, 72.9% and 78.3% respectively, the corresponding inhibition rates for aerial dry weight were 79.1%, 43.0%, 49.0%, 51.9% and 88.4% respectively, the corresponding inhibition rates for underground dry weight were 87.6%, 37.8%, 42.1%, 49.3% and 78.2% respectively. These results indicate that the growth of wheat seedlings was negatively affected by four ILs in general (Fig. 4). The increased underground dry weight under the 200–400 mg L−1 [C2mim][Ala] treatment was likely a result of the fibrous roots system of wheat plants, which had an increased number of roots despite a reduced root length under this treatment.
The treatments of ethanol-dissolved [N2Py][Ala] and pure ethanol decreased wheat growth the most among all treatments, indicating that [N2Py][Ala] and ethanol were the most toxic to wheat growth. However, the two exerted opposite effects on the underground and aboveground parts, with the aboveground part more strongly affected by ethanol than [N2Py][Ala] and the underground part more severely affected by [N2Py][Ala] than ethanol, suggesting a greater impact of [N2Py][Ala] on root length. The results are consistent with the study of Liu et al. on the effects of 1-octyl-3-methylimidazolium chloride ionic liquid on rice seedlings.21 Moreover, we found in the experiment that the roots of wheat seedlings grown in [N2Py][Ala] solution showed more serious erosion phenomenon. Habibul et al. also found the similar phenomenon in wheat root exposure to [C8mim]Ala.9 The results reveal that [N2Py][Ala] was the most toxic among the four ILs, one possible reason was that its structure was well maintained in ethanol, which exacerbated the toxicity of [N2Py][Ala], the another reason may be that cations possessing aromatic head-groups are more toxic than the non-aromatic ones.22 And the ranked degree of toxicity from high to low was: [N2Py][Ala] > [N2222][Ala] > [P2222][Ala] > [C2mim][Ala].
3.2 Effects of ILs with different cations ([N2Py], [P2222], [C2mim] and [N2222]) on the chlorophyll content of wheat seedlings
Chlorophyll is the main pigment for plant photosynthesis, the content of chlorophyll in plant leaves has a direct influence on the synthesis of organic matter in the plant body and then on the growth and development of the plant.18 The effects of four ILs on Chl a and Chl b productions in the leaves of wheat seedlings is shown in Fig. 5.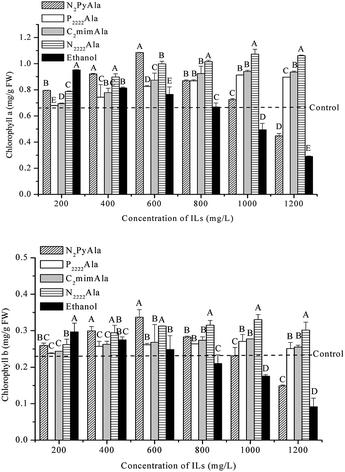 | ||
| Fig. 5 Effects of ethanol, [N2Py][Ala], [P2222][Ala], [C2mim][Ala] and [N2222][Ala] on the chlorophyll a and chlorophyll b of wheat seedlings. | ||
As shown in Fig. 5, the effect of different ILs on the chlorophyll of wheat seedlings was significantly different at the same concentration (P < 0.05). In general, the contents of Chl a and Chl b in wheat seedlings exposed to ILs ([P2222]Ala, [C2mim]Ala and [N2222]Ala) showed the same trend of slow increase followed by a slight decrease with increasing ILs concentrations. The chlorophyll (Chl a, Chl b) peaked at 600 mg L−1 ILs. At the same concentration, their contents of Chl a were 36.9%, 41.0%, and 61.2% higher than those in the control plants, respectively, whereas their contents of Chl b were 18.3%, 21.3%, and 44.5%, respectively, of those observed in control. This may be because the use of low concentration of ILs can induce the accumulation of pigment. However, with the increase of concentration of ILs, the amount of ILs entering the plant increases, and ILs could increase membrane permeability by relaxing the bilayer, cause damage to the chloroplast membrane structure and inhibit chlorophyll synthesis.23
On the other hand, the treatments of [N2Py]Ala and ethanol had the similar effects on chlorophyll in wheat seedlings. With the increase of [N2Py]Ala or ethanol dose, chlorophyll (Chl a, Chl b) contents in wheat seedlings increased at first and then decreased sharply, they were the largest at 600 mg L−1 for [N2Py]Ala and 200 mg L−1 for ethanol, respectively. At the same concentration, their contens of Chl a were 62.7% and 47.3% higher than those in control, respectively, whereas their contents of Chl b were 47.3% and 29.6% higher than those in control. However, when the concentration of [N2Py]Ala or ethanol was 1200 mg L−1, the chlorophyll content was significantly lower than that of the control group. The reason for the results was that, plants grown in a solution containing ethanol can only breathe anaerobically, which makes it difficult for plants to absorb nutrients, leading to disruption of membrane physiological functions and, consequently, to the increased toxicity.5 Zhu et al. also found that with the increasing of the concentration of IL, the number of viable cells decreased tremendously.24 Moreover, some studies suggest that, under saline–alkali stress, the content of chlorophyll b is decreased and the content of chlorophyll a is increased in order to maintain the absorption of water and nutrients.25 Whereas, Liu et al. found that, chlorophyll synthesis was severely inhibited in A. thaliana by the three ILs ([C8mim]Cl, C10mim]Cl, and C12mim]Cl).26 In our study, the contents of Chl a and Chl b in wheat seedlings showed different trends with the increase of ionic liquid, but the chlorophyll a/b values had the increasing trends except for individual treatments. This may be because different types and concentrations of ionic liquids have different effects on plants.
3.3 Effects of ILs with different cations ([N2Py], [P2222], [C2mim] and [N2222]) on the nutrient uptake of wheat seedlings
Nitrogen (N), phosphorus (P) and potassium (K) are essential macronutrients for all organisms and referred as the “three essential elements” for plant growth.27 As shown in Fig. 6, The contents of N, P and K in wheat seedlings were significantly (P < 0.05) different between treatments of ILs at the same concentration. Firstly, the contents of N in wheat seedlings exposed to [[P2222]Ala, C2mim]Ala and [N2222]Ala showed the trends of slow increase followed by a slight decrease with increasing ILs concentrations, and peaked at 1000 mg L−1 ILs. At the same concentration, their contents of N were 17.8%, 51.1%, and 64.3% higher than those in the control plants, respectively. Whereas, with the increase of [N2Py]Ala dosase, nitrogen contents in wheat seedlings increased at first and then decreased sharply, they were the largest at 600 mg L−1, and was 35.74% higher than those in control. Moreover, with the increase of ethanol concentration, the contents of N in wheat seedlings decreased gradually. Specially, the exposure to 1200 mg L−1 [N2Py]Ala and ethanol decreased N contents in plant by 24.6% and 57.3%, respectively, compared to control plants. | ||
| Fig. 6 Effects of ethanol, [N2Py][Ala], [P2222][Ala], [C2mim][Ala] and [N2222][Ala] on the nutrients' absorption of wheat seedlings. | ||
However, the contents of P showed a downward trend with increasing ILs or ethanol concentrations, which was similar to the variation trends of growth indexes. Compared with the control, the contents of P in wheat seedlings exposure to 200–1200 mg L−1 ILs ([N2Py]Ala, [P2222]Ala, [C2mim]Ala, [N2222]Ala and ethanol) declined by 23.3–85.4%, 10.1–64.9%, 9.6–61.2%, 16.3–72.2%, and 36.9–89.3%, respectively.
Meanwhile, the same variation trend of P was observed in the K contents in wheat seedlings exposure to ILs. Compared with the control, The K content in wheat seedlings exposure to the ILs with different cations ([N2Py], [P2222], [C2mim] and [N2222]) and ethanol at 200–1200 mg L−1 were reduced by 33.9–80.9%, 15.7–72.9%, 13.1–70.3%, 23.6–75.2%, and 38.0–89.9%, respectively.
Above all, the exposure of wheat seedling to ILs containing different cations (200–1200 mg L−1), led to the first increase and then decrease of nitrogen contents, and reduced the content of phosphorus and potassium. These may be because the ILs used in this experiment contains alanine ions, which could translocate into the plant and increased the nitrogen contents correspondingly. As for the declination of N contents at the dosage of being greater than 1000 mg L−1, one possible explanation was that ILs with high concentration harms plant cells and inhibits its absorption of nutrients.9 Meanwhile, the results showed that the different chemical structure and initial concentration of ILs result in the different effect on plant.9 On the other hand, different cationic ionic liquids caused different degrees of toxicity to the wheat seedlings, which resulted in the hindrance of the absorption of P and K. And the inhibited effect ranged from high to low was [N2Py][Ala] > [N2222][Ala] > [P2222][Ala] > [C2mim][Ala]. Ionic liquid is a low temperature molten salt, it may affect plants through ion toxicity, osmotic stress or the interaction of the two, and the former will lead to plant physiological drought and ion imbalance.28 Further more, the responses of different plant species, organs and growth periods to salt stress are also different.29 Song et al. have shown that, when salt content increases, leaf P content decreases, leaf N content and leaf N:P increase,29 whose results are consistent with us.
3.4 Effects of ILs with different cations ([N2Py], [P2222], [C2mim] and [N2222]) on the subcellular structure of the wheat leaves and roots
Environmental stress damages the ultrastructure of plant cells, thereby affecting plant growth and development.14 At 600 mg L−1 ILs, the morphological differences of the ultrastructure of mesophyll (photos labelled “1”) and root tip cells (photos labelled “2”) of the control group and different treatment groups are shown in Fig. 6.As shown in Fig. 7, the mesophyll cells of wheat seedlings in the control samples were morphologically intact with a clear structure, tight binding of cell wall and membrane, and smooth membrane (CK1). The nucleus was intact, the nuclear membrane was clear, and the chromatin was abundant and evenly distributed. However, upon [C2mim][Ala] exposure, the cell wall, membrane, and nuclear membrane became vague (A1), and after exposures to [P2222][Ala] (B1) and [N2222][Ala] (C1), the cell walls and cell membranes became loosely bound, the plasma membranes shrank, and local wall separation occurred. In addition, the nucleus became irregularly-shaped, the chromatin distribution became uneven, and cell contents were significantly reduced.30 The nuclear membrane even began to dissolve in [N2Py][Ala]-treated (D1) and ethanol-treated (E1) wheat seedling, suggesting that nuclei were severely damaged.31 These changes indicate that exposure to ILs affected plant cells to varying degrees.
Moreover, the chloroplasts in control samples were spindle-shaped, closely attached to the cell wall, and neatly and orderly distributed. The membrane structure of the chloroplast was intact and clear, and the thylakoids and grana lamellae in the chloroplasts were arranged neatly and parallel to the chloroplast long axis (CK1). By contrast, the thylakoids and grana lamellae were scattered and the chloroplasts were partially empty in [C2mim][Ala]-treated wheat seedlings (A1). The chloroplasts swelled even more, and the thylakoids and grana lamellae were loosely arranged and disordered in the [P2222][Ala] (B1) and [N2222][Ala] (C1) treatment groups. The thylakoids and grana lamellae became disordered and the chloroplasts were irregularly-shaped in the [N2Py][Ala] (D1) and ethanol (E1) treatment groups, suggesting damaged chloroplast structure. In addition, fewer chloroplasts were observed and some chloroplasts moved toward the center of the cell.
We observed a considerable number of mitochondria in the control (CK1) and [C2mim][Ala]-treated (A1) samples. The mitochondria were of normal configuration, contained intact membranes and clearly visible cristae, and distributed around the chloroplasts. By contrast, in the [P2222][Ala] (B1) and [N2222][Ala] (C1) treatments, the number of mitochondria decreased, and the cristae Became vague or even dissolved. Therefore, the [N2Py][Ala] and ethanol treatments had likely hindered protein synthesis in mitochondria, which caused mitochondrion swelling and intimal rupture and resulted in reduced cellular metabolism and plant growth.16
The root tip cells of the control seedlings were intact, the cell wall and membrane were tightly attached and smooth, and the nuclear membrane was intact, and the chromatin was uniformly-coloured (CK2). The mitochondria had a regular shape, the inner cristae were intact (CK2). Compared with the control, [C2mim][Ala]-treated wheat seedlings (A2) displayed swollen mitochondria but no significant change in the nuclear membrane. [P2222][Ala]- (B2) and [N2222][Ala]-treated wheat seedlings (C2) showed abnormally-stained and condensed chromosomes, unclear nuclear membrane, and fewer and scattered mitochondria with unclear crista structures. These observations indicate that the impact on mitochondria increased with increasing concentration as described above. In wheat seedlings subjected to the ethanol treatment (E2), we observed cell vacuolization, a reduced number of mitochondria, dissolution of the cristae, and scattered distribution of ribosomes. In addition, the cell walls were severely damaged and cell contents disappeared (D2).8
Compared with the control group, ILs with different cations ([N2Py][Ala], [P2222][Ala], [C2mim][Ala], and [N2222][Ala]) showed different degrees of toxicity to the cell structure of wheat seedlings, and the toxicity to root tip cells of wheat seedlings was more serious than that of mesophyll cells. This may be due to the preferential root accumulation of ILs in plant.9 Meanwhile, our results are consistent with Liu et al.21 and Xia et al.,14 who found that ILs caused damage to the ultrastructure of plants. Chen et al.16 also found that ILs with different anions mainly damage chloroplasts, mitochondria, cell walls, cell membranes, and nuclei but have minimal on the Golgi in root cells and wheat leaves, which is in good agreement with the results of this study. The ILs used in this study have different chemical structures compared with those employed in previous studies but caused similar damages to the plants on the subcellular level. Among all treatments tested in this study, ethanol and pyridine caused the greatest damage to mesophyll and apical organelles, which was in good agreement with the changes in growth indicators. Caution should be taken when interpreting the relatively stronger toxicity of pyridine compared with other ILs, which might be partly contributed by the toxicity of ethanol.
4. Conclusion
This paper revealed that ILs with different cations ([N2Py][Ala], [P2222][Ala], [C2mim][Ala], and [N2222][Ala]) at the dosage of 200–1200 mg L−1 had significant inhibition on the wheat seedling' growth (P < 0.05), led to the first increase and then decrease of nitrogen contents, and reduced the content of phosphorus and potassium significantly (P < 0.05). These may be because the ILs used in this experiment contains alanine ions, which could translocate into the plant and increased the nitrogen contents correspondingly. Above all, the inhibitory effect of ILs on the growth, chlorophyll contents, and nutrients uptake of wheat seedlings ranked from high to low was: [N2Py][Ala] > [N2222][Ala] > [P2222][Ala] > [C2mim][Ala]. Especially, the inhibitory effect of four ILs on wheat roots was significantly greater than that on shoots. Furthermore, the IL treatments exhibited toxicity to root tip and mesophyll organelle of wheat seedlings and altered their the structure to varing degrees, and the toxicity to root tip cells of wheat seedlings was more serious than that of mesophyll cells. Specially, [N2Py][Ala] is only chemically-stable and structurally-unaffected in ethanol, which we found was particularly toxic to plants. For this reason, we do not recommend the use of N-ethyl-pyridine alanine ([N2Py][Ala]) widely in practical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was supported by the National Natural Science Foundation of China (31600311), college teaching reform and research of Liaoning university (LNDXJG20183014), Peoples Republic of China and Liaoning University “College Students' Innovation and Entrepreneurship Training Program” (D201904152238479387).Notes and references
- Z. Q. Dong, L. Wei and S. W. Xu, et al., Ecotoxicol. Environ. Saf., 2018, 147, 480–486 CrossRef CAS.
- L. Y. Lu, Y. J. Zhang and J. J. Chen, et al., Chin. J. Chem. Phys., 2017, 30, 423–428 CrossRef CAS.
- S. A. S. Amiril, E. A. Rahim and S. Syahrullail, J. Cleaner Prod., 2017, 168, 1571–1589 CrossRef CAS.
- N. Meksi and A. Moussa, J. Cleaner Prod., 2017, 161, 105–126 CrossRef CAS.
- A. Tot, M. Vraneš and I. Maksimović, et al., Ecotoxicol. Environ. Saf., 2018, 147, 401–406 CrossRef CAS.
- J. G. Ma, X. Y. Dong and Q. Fang, et al., J. Biochem. Mol. Toxicol., 2014, 28, 69–75 CrossRef CAS.
- M. Yang, J. He and Y. Z. Sun, et al., J. Agro-Environ. Sci., 2017, 36, 1719–1725 Search PubMed.
- Z. L. Chen, B. Dai and W. C. Zhang, et al., RSC Adv., 2016, 6, 96908 RSC.
- N. Habibul, M. Ilmurat and Z. Habibul, et al., Chemosphere, 2020, 242, 125228 CrossRef CAS.
- T. Liu, L. S. Zhu and J. H. Wang, et al., Sci. Rep., 2015, 5, 18444 CrossRef CAS.
- H. Wang, S. V. Malhotra and A. J. Francis, Chemosphere, 2011, 82, 1597–1603 CrossRef CAS.
- Z. L. Chen, Q. Zhou and F. Leng, et al., Environmental Engineering Research, 2017, 22, 281–287 CrossRef.
- D. J. Couling, R. J. Beront and K. M. Docherty, et al., Green Chem., 2006, 8, 82–90 RSC.
- Y. L. Xia, D. D. Liu and Y. Dong, et al., Chemosphere, 2018, 195, 437–447 CrossRef CAS.
- K. Cook, K. Tarnawsky and A. J. Swinton, et al., Biomolecules, 2019, 9(6), 251 CrossRef CAS.
- Z. L. Chen, Q. Zhou and W. Guan, et al., Chemosphere, 2018, 194, 20–27 CrossRef CAS.
- D. R. Hoagland and D. I. Arnon, Calif. Agric. Exp. Stn., Circ., 1937, 347, 357–359 Search PubMed.
- X. Z. Zhang, Method of crop physiology research, Agricultural Press, Beijing, 1992, pp. 74–77 Search PubMed.
- H. J. Liu and M. Y. Xiong, Aquat. Toxicol., 2009, 93, 100–106 CrossRef CAS.
- J. B. Fang, R. L. Pang, L. L. Guo, et al., Agricultural trade standards of the People's Republic of China, NY/T 2017–2011, pp. 1–5 Search PubMed.
- H. J. Liu, S. X. Zhang and X. N. Hu, et al., Environ. Pollut., 2013, 181, 242–249 CrossRef CAS.
- N. Kaur, M. Fischer and S. Kumar, et al., J. Colloid Interface Sci., 2021, 581, 954–963 CrossRef CAS.
- R. Biczak, M. Śnioszek and A. Telesiński, et al., Ecotoxicol. Environ. Saf., 2017, 139, 463–471 CrossRef CAS.
- J. J. Zhu, Q. Kong and S. S. Zheng, et al., Int. J. Biol. Macromol., 2020, 158, 800–810 CrossRef CAS.
- Y. Y. Zhao, Z. H. Lu and L. He, et al., Appl. Biochem. Biotechnol., 2014, 173, 1680–1691 CrossRef CAS.
- H. J. Liu, Y. L. Xia and H. Y. Fan, et al., J. Hazard. Mater., 2018, 358, 327–336 CrossRef CAS.
- S. C. Yan, Y. Wu and J. L. Fan, et al., Field Crops Research, 2020, 250, 107767 CrossRef.
- L. Nielsen, M. A. Brock and K. Crossl, et al., Freshwater Biol., 2003, 48(12), 2214–2223 CrossRef.
- M. L. Song, Q. Chai and X. Z. Li, et al., Plant Soil, 2015, 387, 153–165 CrossRef CAS.
- M. A. Preston, R. K. Bhaduri and A. Klein, Phys. Today, 1976, 29, 56–57 CrossRef.
- D. Trachootham, W. Zhang and P. Huang, Cancer Cell, 2009, 7, 137–175 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |