Open Access Article
Open Access ArticleThe effects of long and bulky aromatic pendent groups with flexible linkages on the thermal, mechanical and electrical properties of the polyimides and their nanocomposites with functionalized silica†
Simi Annie Tharakan * and
Sarojadevi Muthusamy
* and
Sarojadevi Muthusamy
Department of Chemistry, CEG, Anna University, Chennai, India 600025. E-mail: msrde2000@yahoo.com; tharakansat@gmail.com
First published on 6th May 2021
Abstract
A novel diamine bis(4-aminophenyl)bis{3,4[(4-(8-quinolyloxymethyl carbonyl)]}methane, containing two long/bulky aromatic pendent chains was synthesized by incorporating aromatic and hetero aromatic groups with flexible linkages. Flexible, stretchable, thermally stable and processable polyimides were prepared by reacting this newly synthesized diamine with commercial tetracarboxylic acid dianhydrides like 3,3′,4,4′-benzophenone tetra carboxylic acid dianhydride (BTDA) and 4,4′-(4,4′-isopropylidenediphenoxy)diphthalic Anhydride (BPADA). Nanocomposites of polyimides were prepared using aromatic amine functionalized silica as a filler by solution casting method. The current work investigates the effects of incorporating long/bulky aromatic side chains and flexible linkages on the thermal, mechanical, electrical and optical properties of the polyimides and nanocomposites. The polyimides showed good thermal stability (T10% = 364 & 388), high flame resistance, low glass transition temperatures (Tg = 130 °C & 156 °C), very low dielectric constants (2.5 & 2.8 at 1 MHz) and good optical transparency. The neat polyimides displayed good elongation at break (133–155%) but possessed low tensile strength. The chemically imidized polyimides showed good solubility in low and high boiling solvents. Nanocomposites of polyimides based on aromatic amine functionalized silica exhibited enhanced properties with T10% values varying between 409–482 °C, Tg between 165–280 °C and higher dielectric constants (3–5.7 at 1 MHz).
Introduction
Aromatic polyimides are well recognized as high performance materials due to their excellent thermal mechanical and dielectric properties. Conventional polyimides are used in various fields like automobile, aerospace, electrical, electronics and packaging industries in different forms like coatings, composites, insulating films, adhesives, membranes, and sealants.1–3 But their applications are limited as they meet difficulties in processing due to their poor solubility in organic solvents and high melt/glass transition temperatures in the fully imidized form.4 Another major problem is the deep color and poor optical transmittance of the polyimides, making them non-suitable candidates for optoelectronics.5 All these are due to the rigidity and high crystallinity of the polymer chain backbone which is the result of the inter chain interaction and cohesive forces causing the formation of charge transfer complex formation, in their highly conjugated structures. There are many approaches to make structural modifications and to tailor desired properties. Those are introducing flexibilizing linkages in the chain backbone and attaching long and bulky groups, making coplanar and unsymmetrical structures or copolymerization.4The factors or different approaches leading to better solubility and lower transition temperatures often conflict with some requirements for high performance applications of the polymers, such as high thermo-mechanical properties and chemical resistances. Ether linkages will increase the localized segmental rotation and improve the solubility, without sacrificing much of the good thermal stabilities. The methylene or isopropylidene linkages will decrease the glass transition temperature and thermo-oxidative stability will be compromised. Amutha et al. reported that the bulky poly cyclic aryl group like anthracene in the side chain can impart elevated Tg and good thermal and mechanical properties with better solubility in the organic solvents.6,7 Wang et al.8 reported that the inclusion of long and bulky multiple alkyl side chains impart excellent solubility, toughness, flexibility and good elongation even above 200% i.e. stretchability or elastomeric properties can be imparted in the polyimides but with inferior thermal and mechanical properties. But, it has been established that the bulky aryl groups in the main and side chains impart good thermal stability and mechanical strength.9–11 Most of the measures mentioned above modified the conventional polyimides to an easily processable material with totally different and large varieties of applications like OLED,5 thin film transistors, thin film solar cell or photo voltaics,12 FPCB with new colourless polyimides, electro chromic materials,13,14 LB films, polymeric memory,15 photoresists, sensors, capacitors and solid poly electrolytes for fuel cells or batteries.16–20 They are used as membranes for gas separations21 in power plants and oil refineries.
At the same time elastomers are excellent and relatively cheap materials for various applications in many sectors including automotive, industrial, packaging and healthcare.22 Always there are requirements for elastomers with high Tg and thermal stability. There is a need of the new varieties of insulator for the aircraft and missile combustor case related to the aerodynamic heating. Silicone, with a Tg of −127 °C the material used for this purpose, has reached performance limitations. KALREZ, from DuPont is the most thermally stable commercially available fluoroelastomer, with a continuous service temperature above 260 °C.23 Recently many kinds of elastomers were reported. Ramdas et al. reported shape memory polytriazole elastomers from aromatic monomers which exhibited Tg of 35 °C, an elongation of about 80%, a shore A hardness of 55 and a T5 above 250 °C.24 Chen et al. reported a highly stretchable, self-healing, and 3D printing hydrogels with tensile strength, elongation at break, and Young's modulus of 61.0–103.4 kPa, 1150–1560%, and 42.7–125.7 kPa, respectively.25 Parmeggiani et al. reported a novel kind of PDMS/polyimide composite as elastomeric substrate for multifunctional laser-induced graphene electrodes for flexible electronic applications.26 Until now, not much research works were carried out to prepare thermally stable, stretchable polyimides from aromatic monomers. Thereby researches are required to develop a new material which incorporates the noble properties of polyimide and elastomers. Otherwise, by synthesizing new kinds of elastomers from the polyimides which are tailor made or with desired properties i.e. materials with good solubility, optimum Tg, good thermal stability, tensile strength and elongation, depending on their various service requirements. In one of our recently published work, the polyimide having single pendent groups exhibits high elongation at break of 260%.27 The effect of side groups in the polyimide chain backbone on thermal, mechanical and electrical properties were investigated in detail by Meise et al.28 and Deng et al.29
Organic/inorganic hybrid composites which combine advantages of organic polymers with the benefits of inorganic materials became an area of extensive research in the recent years.30,31 Incorporation of SiO2 nanoparticles, in the form of intercalation or exfoliation was found to improve thermal stabilities as well as mechanical strength (showed improved hardness and modulus), gas barrier properties, flame resistance and electrical properties.32 These materials act as a heat sink and protect polymer matrix from undergoing decomposition in the normal pattern. Enhancement in the properties is based on the strong interaction of nanoparticles and polyimide matrix along with the well-defined and uniform dispersion of particles in the matrix.33,34 Silica nano particle has high surface energy along with the hydrophilic hydroxyl groups on the surface which leading to self-assembly of them resulting in aggregation and low compatibility with the hydrophobic polymer matrix. In order to improve the compatibility of the nano materials with polymer, surface modification is widely used using a silane coupling agent which has amino group such as 3-amino propyl triethoxy silane (APTES), phenyl trimethoxy silane(PTMS), 3-trimethoxy-silylpropyl) diethylenetriamine (DETAS).35–37 But aliphatic groups in these coupling agents reduce the thermal stability. Taeho Kang et al. in 2016 (ref. 38) reported the surface modification of silica with PTMS and it shows increased hydrodynamic volume. Taeho Kang et al. in 2017 (ref. 39) and Ghorpade et al. in 2015 (ref. 40) reported the preparation of nanocomposites based on phenyl amino functionalized silica in which nanoparticles were dispersed without agglomeration. Modification of the silica nanoparticles with p-aminophenyl trimethoxysilane (APhTMS) was observed to maintain and enhance thermal, and mechanical properties of the polyamide-imide matrix.39 The weight percentage of nanosilica and its mode of dispersion in the polyimide matrix has tremendous effect on thermal and electrical properties of polyimide nanocomposites.41
The objective of this study is to investigate the effects of two long/bulky side chains containing aromatic/hetero aromatic groups and flexible linkages, on the thermal, mechanical and electrical properties, along with the processability of the polyimides. It has been observed that the incorporation of long and/or bulky aromatic sidechains and flexible linkages may generate highly amorphous polyimide matrix with improved free volume resulting in good flexibility/stretchability while retaining good thermal and mechanical properties. To achieve the same, a diamine having two long/bulky side chains with aromatic and heteroaromatic groups along with ether, keto and methylene linkages, was prepared. Polyimides were prepared with selected aromatic dianhydrides like 3,3′,4,4′-benzophenone tetra carboxylic acid dianhydride (BTDA) and 4,4′-(4,4′-isopropylidenediphenoxy) diphthalic anhydride (BPADA) to incorporate isopropylidene and keto groups in the polymer main chain. The nanocomposites were prepared by direct blending of the sonicated suspension of aromatic amine functionalized silica nano powder in NMP to the poly (amic acid) solution followed by the thermal imidization method. The obtained polyimides were subjected for detailed investigation on thermal/mechanical/electrical/optical properties and solubility. The detailed study of the nanocomposites on their morphology, and thermo-electrical properties is discussed.
Experimental
Materials
3,4-Dihydroxy benzaldehyde, p-hydoxy benzaldehyde and diamino diphenyl methane were purchased from Spectrochem Pvt. Ltd, India. Aniline and acetic anhydride (Fischer, India) were used as received. 3,3′,4,4′-Benzophenone tetra carboxylic acid dianhydride (BTDA, Acros, India) and bisphenol-A dianhydride (BPADA, Sigma Aldrich, USA) were recrystallized from acetic anhydride. All the reaction solvents such as dimethyl formamide (DMF), N,N-dimethyl acetamide (DMAc), N-methyl pyrollidone (NMP), dimethyl sulphoxide (DMSO), toluene, acetone, chloroform, and tetrahydrofuran (THF), were purified by appropriate method. Ethanol was purified by stirring with calcium carbonate for 12 hours followed by distillation under atmospheric pressure. 8-Hydroxy quinoline, acetanilide, silicon dioxide nano powder (15 nm) were purchased from Sisco Research Laboratories Pvt. Ltd (SRL). Concentrated hydrochloric acid, methanol and isopropyl alcohol were received from Spectrum private limited (Cochin, Kerala). 2,4′-Dibromo acetophenone (Alfa Aser India Private Limited), potassium iodide (MERCK, India), sodium nitrite (Spectrochem, India), potassium carbonate, pyridine (MERCK, India) was used as received.Characterization methods
FT-IR spectra were recorded on a Perkin Elmer RX1 spectrometer with KBr pellet. 1H-NMR and 13C-NMR spectra were recorded using a JEOL Ex-400 spectrometer with CDCl3 or DMSO as solvent and tetra methyl silane (TMS) as reference. Thermograms were obtained using TA instruments Q50V20 series thermogravimetric analyzer at a heating rate of 10 °C min−1 in with a nitrogen flow of 60 ml min−1. Differential scanning calorimetry was used to determine the Tg of the polymers using a NETZSCH DSC 204 instrument at a heating rate of 10 °C min−1 from 40 to 400 °C with a nitrogen flow of 60 ml min−1. Solubility of the polymers was tested in various solvents by mixing 2–3 mg of the polymer with 8 mL of the solvent and kept aside for 24 hours with occasional shaking. If the mixture was insoluble under cold conditions, then it was heated and cooled. Inherent viscosity measurements were conducted at 25 °C in DMAc solvent using Cannon Ubbelohde viscometer. The weight-average molecular weights (Mw) and number average molecular weights (Mn) were obtained via gel permeation chromatography (GPC) on the basis of polystyrene calibration on a PL-GPC 220 instrument with DMF as an eluent at a flow rate of 1.0 mL min−1. Wide angle X-ray diffraction measurements were performed at room temperature (about 25 °C) on an X-pert PAN analytical X-ray diffractometer using Ni-filtered Cu Kα radiation. The scanning rate was 20° min−1 over a range of 2θ = 5°–70°. Mechanical properties of the films were measured with an Instron model 1130 tensile tester using a 5 kg load cell at a cross head speed of 5 mm min−1 on strips of approximately 1 mm thick and 5 mm width with a 15 mm gauge length by the standard ASTM D638. Dielectric properties were measured with the help of an impedance analyzer (Solatron 1260 Impedance Gain phase Analyzer, UK) at room temperature. The polymer samples were cut in to circular shape (of size 1 mm thickness and 10 mm diameter) using a platinum electrode sandwich model in the frequency range of 100 Hz to 1 MHz. The morphology of the material was examined by scanning electron microscope (SEM) (JEOL, JSM-5600) at an accelerating voltage of 20 keV. Photomicrographs were taken on the surface, which was made by breaking the specimen by impact testing machine and then coating with gold powder. Transmission electron microscopic (TEM) images were recorded using JEOL TEM-3010 electron microscope operated at an accelerating voltage of 300 keV. The samples for TEM image were prepared by dispersion of the material in ethanol under sonication and deposited on a copper grid. The absorption spectra were recorded between 200 and 800 nm on Shimadzu (1650) UV-vis spectrophotometer using dimethyl acetamide as solvent.Synthesis of bis(4-aminophenyl)bis{3,4[(4-(8-quinolyloxymethyl carbonyl)phenoxy]}methane [BAPQMCPM]
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (DMSO-d6, ppm): 2 (6H, s, Ha), 8.8 (2H, s, Hb), 7 (4H, d, Hc), 7.2–7.6 (4H, d, Hd), 5.2 (1H, s, He, 6.7 (1H, dd, Hf), 6.5 (1H, d, Hg), 6.3 (1H, d, Hh), 10 (2H, s, Hi & Hj).
O). 1H-NMR (DMSO-d6, ppm): 2 (6H, s, Ha), 8.8 (2H, s, Hb), 7 (4H, d, Hc), 7.2–7.6 (4H, d, Hd), 5.2 (1H, s, He, 6.7 (1H, dd, Hf), 6.5 (1H, d, Hg), 6.3 (1H, d, Hh), 10 (2H, s, Hi & Hj).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) 1246, 1100, 1069 (stretching vibration of C–O–C groups).
O group) 1246, 1100, 1069 (stretching vibration of C–O–C groups).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) 1720 (the stretching vibration of ketonic C
O group) 1720 (the stretching vibration of ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group), 1072, 1250 (stretching vibration of aryl C–O–C group), 1597 (aromatic ring stretching). 1H-NMR (DMSO-d6, ppm): 2 (6H, s, Ha), 9.92 (2H, s, Hb), 7.96 (4H, d, Hc), 7.52 (4H, d, Hd), 5.7 (1H, s, He), 7.04 (1H, dd, Hf), 7.29 (1H, d, Hg), 7.37 (1H, d, Hh), 7.69 (2H, d, Hi), 8.61 (2H, d, Hj), 6.7 (2H, s, Hk), 7.44 (2H, dd, Hl), 8.23 (2H, t, Hm), 7.85 (2H, dd, Hn), 8.76 (2H, dd, Ho), 7.71 (2H, t, Hp), 8.93 (2H, dd, Hq).
O group), 1072, 1250 (stretching vibration of aryl C–O–C group), 1597 (aromatic ring stretching). 1H-NMR (DMSO-d6, ppm): 2 (6H, s, Ha), 9.92 (2H, s, Hb), 7.96 (4H, d, Hc), 7.52 (4H, d, Hd), 5.7 (1H, s, He), 7.04 (1H, dd, Hf), 7.29 (1H, d, Hg), 7.37 (1H, d, Hh), 7.69 (2H, d, Hi), 8.61 (2H, d, Hj), 6.7 (2H, s, Hk), 7.44 (2H, dd, Hl), 8.23 (2H, t, Hm), 7.85 (2H, dd, Hn), 8.76 (2H, dd, Ho), 7.71 (2H, t, Hp), 8.93 (2H, dd, Hq).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. Yield 76%. M.P. 180 °C. FT-IR (KBr, cm−1): 3443, 3426 (asymmetric and symmetric stretching vibrations of amino group, (aromatic C–H stretching vibrations) 2924, 2854 (aliphatic C–H stretching of –CH2 group. 1628 (N–H in-plane bending vibrations), 1660 (symmetrical stretching vibrations of C
1) mixture. Yield 76%. M.P. 180 °C. FT-IR (KBr, cm−1): 3443, 3426 (asymmetric and symmetric stretching vibrations of amino group, (aromatic C–H stretching vibrations) 2924, 2854 (aliphatic C–H stretching of –CH2 group. 1628 (N–H in-plane bending vibrations), 1660 (symmetrical stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) 1111, 1070, 1257 (stretching vibration of Ar–O–Ar and Ar–O–R groups), 1597 (aromatic ring stretching). 1H-NMR (DMSO-d6, ppm): 2.9 (4H, s, Ha), 7.1 (2H, d, Hb), 7.2 (2H, d, Hc), 5.34 (H, s, Hd), 7.36 (1H, m, He), 7.45 (1H, m, Hf), 7.5 (1H, d, Hg), 7.6–7.7 (4H, d, Hh), 7.87 (4H, d, Hi), 6.8 (4H, s, Hj), 7.95 (2H, dd, Hk), 8.45 (2H, t, Hl), 8.1 (2H, dd, Hm), 7.99 (2H, dd, Hn), 8.6 (2H, t Ho), 8.99 (2H, dd, Hp). 13C-NMR (DMSO-d6, ppm): C1 – 148.0, C2 – 128.94, C3 – 130.1, C4 – 140.96, C5 – 72.62, C6 – 147.98, C7 – 136.47, C8 – 137.1, C9 – 151.3 166, C10 – 149.4 164, C11 – 132.6, C12 – 162.6, C13 – 132.1, C14 – 132, C15 – 136.7, C16 – 194.3, C17 – 89.7, C18 – 164.5, C19 – 133.17, C20 – 129.8, C21 – 132.6, C22 – 151.3, C23 – 128.89, C24 – 129.12, C25 – 159.9, C26 – 161.2.
O group) 1111, 1070, 1257 (stretching vibration of Ar–O–Ar and Ar–O–R groups), 1597 (aromatic ring stretching). 1H-NMR (DMSO-d6, ppm): 2.9 (4H, s, Ha), 7.1 (2H, d, Hb), 7.2 (2H, d, Hc), 5.34 (H, s, Hd), 7.36 (1H, m, He), 7.45 (1H, m, Hf), 7.5 (1H, d, Hg), 7.6–7.7 (4H, d, Hh), 7.87 (4H, d, Hi), 6.8 (4H, s, Hj), 7.95 (2H, dd, Hk), 8.45 (2H, t, Hl), 8.1 (2H, dd, Hm), 7.99 (2H, dd, Hn), 8.6 (2H, t Ho), 8.99 (2H, dd, Hp). 13C-NMR (DMSO-d6, ppm): C1 – 148.0, C2 – 128.94, C3 – 130.1, C4 – 140.96, C5 – 72.62, C6 – 147.98, C7 – 136.47, C8 – 137.1, C9 – 151.3 166, C10 – 149.4 164, C11 – 132.6, C12 – 162.6, C13 – 132.1, C14 – 132, C15 – 136.7, C16 – 194.3, C17 – 89.7, C18 – 164.5, C19 – 133.17, C20 – 129.8, C21 – 132.6, C22 – 151.3, C23 – 128.89, C24 – 129.12, C25 – 159.9, C26 – 161.2.Preparation of aromatic amino functionalized silica nanoparticles
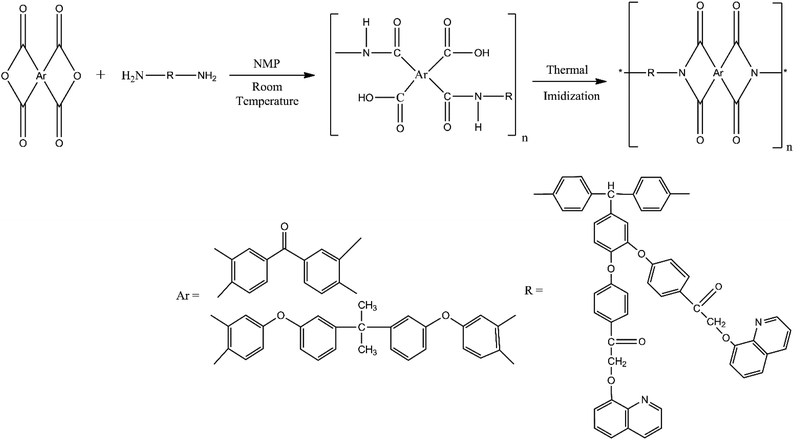
Aromatic amino functionalized nano silica particles were synthesized following a reported procedure38 (Scheme S1†).Synthesis of BAPQMCPM/BTDA-PI. BAPQMCPM (2.75 g, 0.0033 mol) was dissolved in 7 ml of NMP. After the diamine was completely dissolved, BTDA (1.1 g, 0.0033 mol) of was added to it. The reaction mixture was stirred at room temperature in nitrogen atmosphere for 16–18 hours. One portion of the obtained poly (amic acid) solution was spread on Petridish and placed in an oven at 80 °C overnight to remove the solvent. The semidried poly(amic acid) film was subjected to thermal imidization by heating from 100 °C to 300 °C in a sequential method 1 hour each at 120 °C, 150 °C, 200 °C, 250 °C, 30 minutes each at 275 °C and 300 °C to get a continuous, creasable polyimide film. Acetic anhydride (5 ml) and pyridine (2.5 ml) were added (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to the remaining poly(amic acid) solution in chemical imidization method, and the mixture was stirred well for 8 hours at a temperature of 100 °C to achieve a complete imidization. The viscous solution was poured in to methanol to get brown coloured polyimide which was filtered, washed with hot methanol and dried in vacuum oven at 60 °C for 12 h (Scheme 2). FT-IR (KBr, cm−1): 1774, 1712 (asymmetric and symmetric stretching of imide carbonyl group), 1362 (C–N–C stretching vibrations) 1155, 708 (C–N–C bending vibrations), 1160 (C–O–C stretching vibrations), 3050 (aromatic C–H stretching vibrations), 2960, 2916 (C–H stretching of methylene and methine group), 1655 (carbonyl group of BTDA and pendent group in the diamine) 1212, 1081 (Ar–O–Ar vibrations). 1H-NMR (DMSO-d6, ppm): 7–8.8 (m, 42H, aromatic protons), 6.2 (s, 2H, CH2 protons) 5.5 (s, 1H, methine CH).
1 v/v) to the remaining poly(amic acid) solution in chemical imidization method, and the mixture was stirred well for 8 hours at a temperature of 100 °C to achieve a complete imidization. The viscous solution was poured in to methanol to get brown coloured polyimide which was filtered, washed with hot methanol and dried in vacuum oven at 60 °C for 12 h (Scheme 2). FT-IR (KBr, cm−1): 1774, 1712 (asymmetric and symmetric stretching of imide carbonyl group), 1362 (C–N–C stretching vibrations) 1155, 708 (C–N–C bending vibrations), 1160 (C–O–C stretching vibrations), 3050 (aromatic C–H stretching vibrations), 2960, 2916 (C–H stretching of methylene and methine group), 1655 (carbonyl group of BTDA and pendent group in the diamine) 1212, 1081 (Ar–O–Ar vibrations). 1H-NMR (DMSO-d6, ppm): 7–8.8 (m, 42H, aromatic protons), 6.2 (s, 2H, CH2 protons) 5.5 (s, 1H, methine CH).
Synthesis of BAPQMCPM/BPADA-PI. The BAPQMCPM/BPADA-PI was prepared by adopting the same procedure used for the preparation of BAPQMCPM/BTDA-PI given above FT-IR (KBr, cm−1): 1773, 1715.35 (asymmetric and symmetric stretching of imide carbonyl group), 1364 (C–N–C stretching vibrations), 1161, 708 (C–N–C bending vibrations), 1266 (C–O–C stretching vibrations), 3051 (aromatic C–H stretching vibrations), 2962, 2921 (C–H stretching of isopropylidene, methine, and methylene groups). 1H-NMR (DMSO-d6, ppm): 7–8.5 (m, 54H, aromatic protons), 6.9 (s, 2H, OCH2 protons), 5.3 (s, 1H, methine CH), 2.8 (s, 6H, isopropylidene protons).
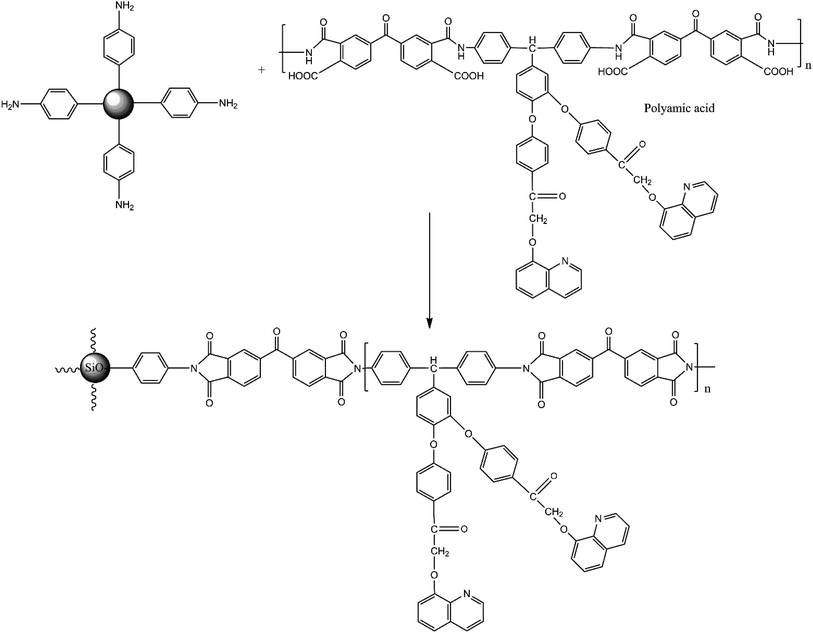
Synthesis of BAPQMCPM/BTDA polyimide/aromatic amine functionalized silica nanocomposites. 2.75 g (0.0033 mol) of BAPQMCPM was dissolved in 7 ml of NMP. After the diamine was completely dissolved, (1.1 g, 0.0033 mol) of BTDA was added to it. The reaction mixture was stirred at room temperature in nitrogen atmosphere for 16–18 hours. From the total weight of diamine and dianhydride 2% was calculated and same 2% weight of SiO2 in NMP was sonicated in an ultrasonic bath. It was then mixed with poly(amic acid) solution and sonicated again for 2 hours. The solution was poured in a Petridish, solvent was evaporated at 80 °C overnight and kept at 120 °C, 150 °C, 200 °C, 250 °C for one hour at each temperature and at 275 °C and 300 °C for half an hour each to complete the imidization. Similarly, 5% and 10% PI/SiO2 nanocomposites were also prepared using the above procedure. Continuous and creasable nanocomposite films were formed36 (Scheme 3).
BAPQMCPM/BTDA-PI/2%SiO2 nanocomposites. FT-IR (KBr, cm−1): 1777, 1716 (asymmetric and symmetric stretching of imide carbonyl groups), 1362 (C–N–C stretching vibrations) 1160, 800 (C–N–C bending vibrations) 1151, 1206 (C–O–C stretching vibrations), 3057 (aromatic C–H stretching vibrations), 2911 (C–H stretching of isopropyl and methylene group), 1658 (carbonyl group in the pendent group of diamine), 1048 (broad and strong band of Si–O–Si linkage), 710 (presence of long chain). The FT-IR spectrum of BAPQMCPM/BTDA-PI/5%SiO2 and BAPQMCPM/BTDA-PI/10%SiO2 nanocomposite also was found to have similar characteristic absorption bands like BAPQMCPM/BTDA-PI/2%SiO2 nanocomposites.
Synthesis of BAPQMCPM/BPADA poly imide/aromatic amino functionalized silica nanocomposites. The BAPQMCPM/BPADA poly imide/aromatic amino functionalized silica (2%, 5%, and 10%) nanocomposites were prepared by adopting the same procedure used for preparing BAPQMCPM/BTDA nanocomposites. FT-IR (KB(r, cm−1): 1772, 1714 (asymmetric and symmetric stretching of imide carbonyl groups), 1364 (C–N–C stretching vibrations) 1160, 749 (C–N–C bending vibrations), 1160, 1205 (C–O–C stretching vibrations), 3051 (aromatic C–H stretching vibrations), 2960, 2916 (C–H stretching of isopropyl and methylene group), 1660 (carbonyl group in the pendent group of diamine), 1070 (Si–O–Si linkage), 711 presence of long chain). The FT-IR spectrum BAPQMCPM/BPADA-PI/5%SiO2 and BAPQMCPM/BPADA-PI/10%SiO2 nanocomposite also was found to have similar characteristic absorption bands like BAPQMCPM/BPADA-PI/2%SiO2 nanocomposites.
Results and discussion
Monomer synthesis
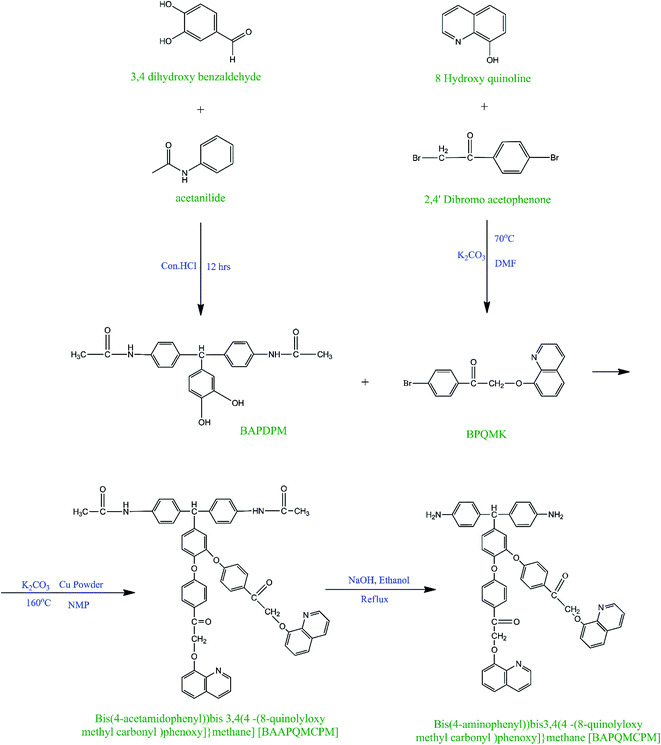
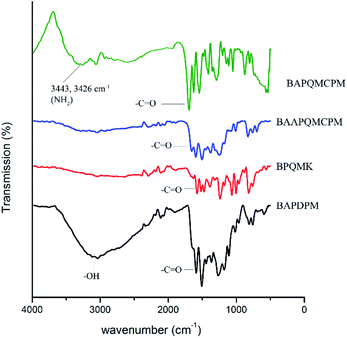
The diamine bis(4-aminophenyl)bis{3,4[4-(8-quinolyloxy methyl carbonyl)phenoxy]}methane [BAPQMCPM]was synthesized using a four-step reaction. In the first step, BAPDPM was prepared by the reaction of 3,4 dihydroxy benzaldehyde with acetanilide in the presence of concentrated hydrochloric acid in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios. Here, acetanilide is used to protect the amino group in the amide group form. The FT-IR spectrum of the BAPDPM is given in Fig. 1. The broad band at 3364 cm−1 indicates the symmetric stretching vibrations of the hydroxyl group. The small band at 3286 cm−1 is due to the symmetric –NH stretching vibrations of the secondary amide group and the strong band at 1669 cm−1 corresponds to the symmetric stretching vibrations of C
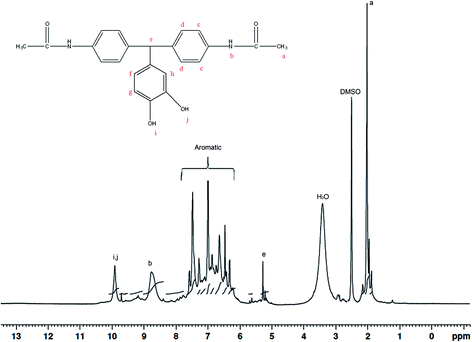
2 molar ratios. Here, acetanilide is used to protect the amino group in the amide group form. The FT-IR spectrum of the BAPDPM is given in Fig. 1. The broad band at 3364 cm−1 indicates the symmetric stretching vibrations of the hydroxyl group. The small band at 3286 cm−1 is due to the symmetric –NH stretching vibrations of the secondary amide group and the strong band at 1669 cm−1 corresponds to the symmetric stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the amide group. The band at 2793 cm−1 indicates the presence of bridging –CH group. 1H-NMR spectrum is shown in Fig. 2. The peak at 5.2 ppm corresponds to the highly shielded bridging –CH group which confirms the formation of this compound. The singlet at 8.8 ppm is due to the presence of the much shielded –NH protons and the broad peak at 10 ppm indicates the presence of both –OH protons. All the aromatic proton peaks appear between 7.2 and 9 ppm.
O group of the amide group. The band at 2793 cm−1 indicates the presence of bridging –CH group. 1H-NMR spectrum is shown in Fig. 2. The peak at 5.2 ppm corresponds to the highly shielded bridging –CH group which confirms the formation of this compound. The singlet at 8.8 ppm is due to the presence of the much shielded –NH protons and the broad peak at 10 ppm indicates the presence of both –OH protons. All the aromatic proton peaks appear between 7.2 and 9 ppm.
In the second step, the long mono bromo aryl pendent group BPQMK was prepared by the nucleophilic substitution reaction of 8-hydroxy quinoline with 2,4′-dibromo acetophenone in the presence of anhydrous potassium carbonate as base and a catalytic amount of potassium iodide in DMF. The FT-IR spectrum of BPQMK is given in Fig. 1. The strong band at 1246 cm−1 is assigned to the stretching vibration of aryl alkyl C–O–C group indicating the formation of bond. The band at 1666 cm−1 is due to the symmetric stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. The aromatic and aliphatic C–H stretching vibrations appear as strong bands at 3061 cm−1 and 2923 cm−1 respectively.
O group. The aromatic and aliphatic C–H stretching vibrations appear as strong bands at 3061 cm−1 and 2923 cm−1 respectively.
In the third step, the BAAPQMCPM which contains amide linkages and pendent groups was obtained using the classical Ullman etherification reaction, by the nucleophilic substitution reaction of BAPDPM with BPQMK in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio in the presence of anhydrous potassium carbonate and a pinch of copper powder at 160 °C in NMP as solvent. The copper powder here is used to facilitate the formation of phenoxy ion nucleophile and to assist the removal of bromine from the aromatic ring as there is no strong activation in the ortho and para positions except that of ketone group. The FT-IR spectrum of the compound is shown in Fig. 1. The secondary amide N–H group stretching vibrations appear at 3302 cm−1 and the band at 2862 cm−1 is assigned to the bridging methine and methylene groups. The strong band at 1659 cm−1 is due to the symmetric stretching vibrations of the amide C
2 mole ratio in the presence of anhydrous potassium carbonate and a pinch of copper powder at 160 °C in NMP as solvent. The copper powder here is used to facilitate the formation of phenoxy ion nucleophile and to assist the removal of bromine from the aromatic ring as there is no strong activation in the ortho and para positions except that of ketone group. The FT-IR spectrum of the compound is shown in Fig. 1. The secondary amide N–H group stretching vibrations appear at 3302 cm−1 and the band at 2862 cm−1 is assigned to the bridging methine and methylene groups. The strong band at 1659 cm−1 is due to the symmetric stretching vibrations of the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the ketonic C
O group and the ketonic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. The two strong bands at 1072, 1250 cm−1 are attributed to the stretching vibration of aryl alkyl C–O–C group. In the 1H-NMR spectrum the peak at 9.92 ppm is assigned to the highly shielded –NH proton. The methine and methylene peaks appear at 5.4 and 6.9 ppm respectively. Aromatic protons are shielded and shifted to down field between 7 ppm and 9 ppm. Highly shielded protons attached to the carbon neighbouring to the nitrogen in the quinoline ring is downshifted to 8.93 ppm.
O group. The two strong bands at 1072, 1250 cm−1 are attributed to the stretching vibration of aryl alkyl C–O–C group. In the 1H-NMR spectrum the peak at 9.92 ppm is assigned to the highly shielded –NH proton. The methine and methylene peaks appear at 5.4 and 6.9 ppm respectively. Aromatic protons are shielded and shifted to down field between 7 ppm and 9 ppm. Highly shielded protons attached to the carbon neighbouring to the nitrogen in the quinoline ring is downshifted to 8.93 ppm.
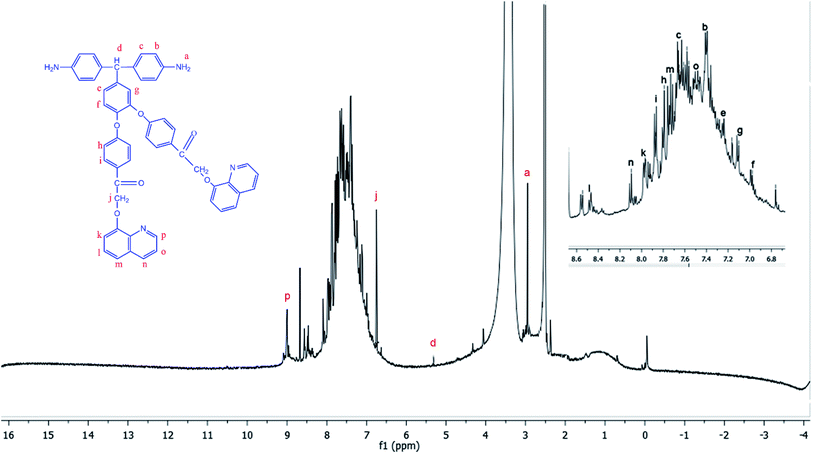
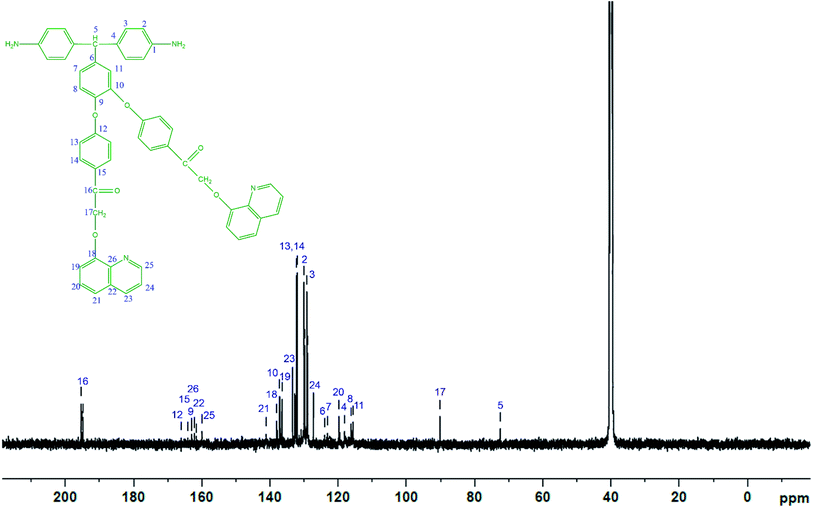
The diamine monomer BAPQMCPM was prepared by the basic hydrolysis of the diamide compound in ethanol as solvent under reflux condition followed by the careful neutralization and precipitation to prevent soluble amine salt formation. The detailed reaction scheme for the synthesis of the diamine is shown in Scheme 1. In 1H-NMR spectrum of the BAPQMCPM (Fig. 3) the peaks between 6.3 ppm and 9 ppm are assigned to the aromatic protons. Here an up-field shift is observed when the amide group is hydrolysed to amine group. The peak at 2.9 ppm corresponds to –NH2 group. The peak at 5.3 ppm represents the highly shielded –CH group. The peak at 6.7 ppm corresponds to –CH2 linkages in the pendent groups. The electron withdrawing oxygen and carbonyl linkages attached to the methylene group shift the peak to low field and so that the peaks appear at higher value. A higher resonance value (8.85 ppm) is observed for the aromatic C–H neighbouring to the nitrogen in the quinoline moiety. The 13C-NMR spectrum of the diamine BAPQMCPM (Fig. 4) is consistent with the proposed structure. The aromatic carbons appear in the range 110–170 ppm. The resonances at 72.95, and 89.3 ppm may be assigned to the methylene and the methine group respectively. The carbonyl groups in the pendent group are represented by the peak around 194 ppm.
Preparation and characterization of aromatic amino functionalized silica
Aromatic amino functionalized nano silica was prepared by a procedure reported by Ghorpade et al.40 in 2015 (ESI†). The nano silica particles were phenylated by the addition of phenyl triethoxy silane (PTEOS) to a mixture of triethylamine and nano silica suspension in toluene and subsequent reaction under reflux condition. The phenylated silica was nitrated using nitrating mixture (acetic acid + sodium nitrate) and separated by centrifugation and purified by repeated washings. The nitro phenyl functionalized silica was reduced to amino phenyl functionalized silica using stannous chloride in ethanol under reflux condition (Scheme S1, ESI†)). The FT-IR analysis of nano particles confirm modification of nano silica with aromatic amino group. Fig. S1 (ESI†) shows the FT-IR spectra of aromatic nitro functionalized silica and aromatic amino functionalized silica. The characteristic bands at 1065 cm−1 and 961 cm−1 show the stretching vibrations of Si–O–Si group.42 The band at 1651 cm−1 is due to the bending vibrations of the residual water molecules adsorbed on the surface of SiO2. The bands at 1596 cm−1 and 1342 cm−1 indicate the presence of nitro groups. The wide band around 3400 cm−1 is due to the asymmetric stretching vibrations of the amino and Si–OH groups which confirm the reduction of nitro group to amino group.43XPS analysis was performed to confirm the chemical states of elements in the aromatic amino and nitro functionalized silica. The XPS results of the aromatic amino functionalized silica are presented in Fig. S2 (ESI†). In the XPS spectra of both amino and nitro functionalized silica, O 1s, Si 2p, C 1s and N 1s peaks are found. In the XPS spectra of aromatic amino functionalized silica, the N 1s peak is at 397 eV, the C 1s is present around 287 eV, the Si 2p peak is around 104 eV, Si 2s peak is around 154 eV and the O 1s peak is around 533 eV. The small peak of N 1s which appeared at 407 eV in the aromatic nitro functionalized silica, shifts to 397 eV confirming the change in the chemical states of nitrogen due to the conversion of nitro group to amino group. The C 1s peak around 287 eV represents the phenyl group in the aromatic amino functionalized silica.40
Preparation and characterization of polyimides and their nanocomposites with aromatic amino functionalized silica
The polyimide containing long pendent chains was prepared by the conventional two step method. In the first step, which is an exothermic reaction, an equimolar amount of BAPQMCPM and BTDA (or BPADA) in a solution of 20 w% solid concentration containing in NMP were reacted. The resultant viscous solution of polyamic acid was converted into the corresponding polyimide by thermal and chemical imidization methods. In the thermal imidization method, the solvent evaporation was carried out at 80 °C for overnight and sequential heating at temperatures of 120 °C, 150 °C, 200 °C, 250 °C, 300 °C for half an hour at each temperature was carried out to convert to the fully imidized structure. Clear, continuous, creasable film was obtained. In the chemical imidization method, acetic anhydride and pyridine were added to the poly(amic acid) solution in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio and the mixture was stirred at 100 °C for 8 hours to achieve complete imidization.
1 volume ratio and the mixture was stirred at 100 °C for 8 hours to achieve complete imidization.
BAPQMCPM-PI-functionalized silica nanocomposites were synthesized by the two-step thermal imidization method. The reaction mixture of diamine and BTDA (or BPADA) was stirred in NMP at room temperature in nitrogen atmosphere for 24 hours to get a viscous polyamic acid solution. A suspension of various ratios of aromatic amino functionalized silica (2, 5, 10% by weight) taken separately in NMP were mixed with poly(amic acid) solution, sonicated and kept at 120 °C, 150 °C, 200 °C, 250 °C for one hour at each temperature and at 275 °C and 300 °C for half an hour each to complete the thermal imidization. Clear, creasable composite films were formed.
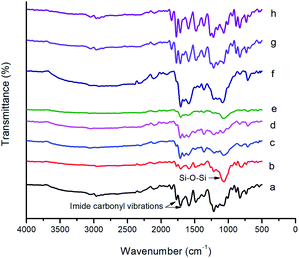
The FT-IR spectra of BAPQMCPM/BTDA-PI, BAPQMCPM/BPADA-PI and their nanocomposites are shown in Fig. 5. The bands at 1773–1779 cm−1 and 1705–1713 cm−1 indicate the presence of imide carbonyl groups. The C–N stretching of imide group was observed at 1363 cm−1 C–N bending vibrations give band at 754 cm−1. The band around 1665 cm−1 represents the BTDA carbonyl group and that around 1589 cm−1 is due to the presence of carbonyl in the diamine. In the polyimide nanocomposites the characteristic band due to Si–O–Si linkage appears at 1040–1080 cm−1. The band around 3040–3060 cm−1 is due to the stretching vibrations of the Ar–H bond. Around 2920–2970 cm−1 all kinds of C–H vibrations (methine, methylene, isopropylidene) are found.37,42,44,45
The chemical structure of the polyimides was also confirmed by 1H-NMR spectroscopy (Fig. S3a and b, ESI†). The complete imidization of the poly(amic acid) was also confirmed by the absence of residual resonance in the region 3–4.2 ppm indicating the absence of amine protons. The BPADA based polyimide shows the characteristic peak of isopropylidene group at 2.8 ppm. In BTDA based PI the peaks at 6.2 and 5.5 ppm indicate the methylene and methine groups respectively. But the peaks of methylene and methine groups are present around 6.9 and 5.3 ppm respectively in BPADA based polyimide.
Properties of polyimides and nanocomposites
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868–98
868–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246 for Mw and 42
246 for Mw and 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868–44
868–44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 for Mn with Mw/Mn values of 2.1–2.2.11,49 Polyimides show good film forming capacity and are creasable due to the formation of high molecular weight polymer.
542 for Mn with Mw/Mn values of 2.1–2.2.11,49 Polyimides show good film forming capacity and are creasable due to the formation of high molecular weight polymer.| PI & codes | Tensile strength (MPa) | Tensile elongation at break point (%) | UV λmax (nm) | Intrinsic viscosity (dL g−1) | Number average molecular weight (Mn) | Weight average molecular weight (Mw) | Mw/Mn |
|---|---|---|---|---|---|---|---|
| BAPQMCPM-BTDA | 17 | 133.5 | 325 | 1.5 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868 868 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 538 538 |
2.14 |
| BAPQMCPM-BPADA | 13 | 155.3 | 330 | 2.1 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246 246 |
2.21 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 showed that the polyetherimides with multiple alkyl side chains exhibit very good solubility in low boiling chlorinated solvents and in all high boiling solvents. Recent studies show that polyimides with aromatic pendent groups along with flexible linkages have improved solubility.55
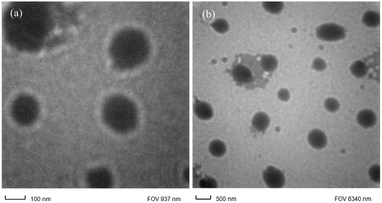
8 showed that the polyetherimides with multiple alkyl side chains exhibit very good solubility in low boiling chlorinated solvents and in all high boiling solvents. Recent studies show that polyimides with aromatic pendent groups along with flexible linkages have improved solubility.55Transmission electron microscopy (TEM) analysis was performed for nanocomposite with 2% functionalized silica, to analyze the microstructure and internal composition related to the size, shape and pattern of distribution in the polyimide matrix. Here, the light portion of the image shows the polyimide matrix part and the dark spots are the modified silica particles (Fig. 7 and S6a–i, ESI†). The modification of the silica particles plays an important role in the uniform distribution of the particles in the matrix. Here, it is observed that the aromatic amino modified silica nano particles are dispersed homogeneously in the polyimide matrix without showing any signs of aggregation. This is due to the covalent bonding between amino group of silica particle and carboxyl group of polyamic acid during the mixing and curing stage. Thus, the compatibility between polymer chain and nanoparticles is enhanced. Here the dark spherical silica particles are uniformly distributed or dispersed in nanometre range (about 50–200 nm size). There is no aggregation of the silica particles, showing uniform distribution.35
| PI & nanocomposites codes | Tg (°C) | T10% (°C) | CY (%) | LOI | Dielectric constant at 1 MHz |
|---|---|---|---|---|---|
| BAPQMCPM-BTDA | 156 | 388 | 57.0 | 40.3 | 2.8 |
| BAPQMCPM-BTDA2% nanosilica | 220 | 447 | 66.0 | 44.0 | 4.0 |
| BAPQMCPM-BTDA5% nanosilica | 263 | 454 | 69.0 | 45.1 | 4.4 |
| BAPQMCPM-BTDA10% nanosilica | 280 | 481 | 72.0 | 46.3 | 5.7 |
| BAPQMCPM-BPADA | 130 | 364 | 27.0 | 28.3 | 2.5 |
| BAPQMCPM-BPADA2% nanosilica | 165 | 409 | 38.0 | 32.7 | 3.0 |
| BAPQMCPM-BPADA5% nanosilica | 180 | 413 | 45.0 | 35.5 | 4.2 |
| BAPQMCPM-BPADA10% nanosilica | 235 | 450 | 67.0 | 44.3 | 5.3 |
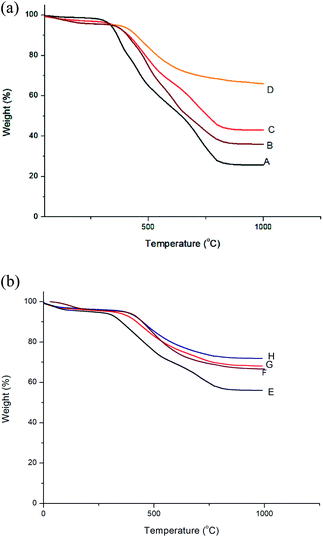
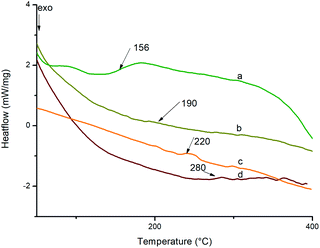
The glass transition temperature of polyimides and nanocomposites were determined using DSC at a heating rate of 10 °C min−1 in nitrogen atmosphere. The DSC curves of BTDA based polyimides are shown in Fig. 10 and those of BPADA based polyimides are given as Fig. S7 (ESI†). The values are listed in Table 2. The glass transition temperature depends upon the onset of segmental motion in a polymer molecule which in turn depends upon the molecular structure and geometry of the polymer molecule. The bulky aryl pendent groups decrease cohesive forces and inhibit the chain packing and generate greater free volume. The flexible ether and ketone linkages in the main chain improves the segmental motion so that it starts at an early stage thereby reducing Tg. The flexible linkages in the pendent groups improve the localised movements in the pendent chains. So the combined influence of both bulky aryl pendent groups and the flexible linkages in the chain backbone as well as in the pendent groups improves the available free volume so that the segmental motion starts at an early stage reducing the Tg to a greater extent.50,60,61 It is reported that, highly branched aromatic polyimides show lower Tg than the conventional PIs due to the improved free volume. The Tg values of BPADA based polyimides and nanocomposites are lesser than that of BTDA based polyimides and nanocomposites. This is due to the presence of two ether linkages in the chain backbone along with the isopropyl group, which give more segmental rotations whereas there is only a carbonyl group present in the chain backbone of BTDA based polyimides and nanocomposites. Also, the extended chain length of the BPADA based polyimides improves the free volume. It is observed that, as the percentage of silicon dioxide nano filler increases, Tg values also increases. This is consequence of the higher rigidity imparted by aromatic amino functionalized silica which occupy the available space between the chains hindering the chain mobility and reducing the total free volume, thereby the segmental motion starts at a relatively higher temperature.
Conclusions
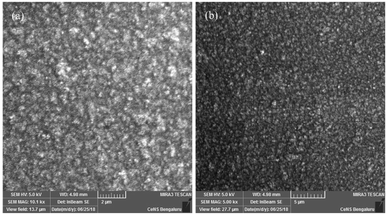
In summary, two novel flexible/stretchable polyimides were successfully synthesized by reacting a new diamine containing two long/bulky aromatic pendent chains and flexible linkages, with commercially available tetra carboxylic acid dianhydrides. Nanocomposites of polyimides were prepared with aromatic amine functionalized silica and characterized. SEM analysis shows a uniform distribution and better compatibility of the functionalized silica nanopowder in polyimide matrix. The neat polyimides show good elongation at break (133–155%) but low tensile strength. TGA shows that both the polyimides (T10% = 364 & 388) and nanocomposites (T10% = 409–482 °C) have high thermal stability. LOI values of both polyimides and nanocomposites are above 28, which indicate good self-extinguishing property. DSC analysis revealed very low Tg values for neat polyimides (130 °C and 156 °C) and for nanocomposites between 165–280 °C. Polyimides show very low dielectric constants (2.8 & 2.5) at 1 MHz showing good insulation property but the nanocomposites display higher dielectric constants (3–5.7 at 1 MHz). The prepared polyimides exhibit a better thermal stability, compared to the low thermal stability of polyimides with wholly alkyl side chains, which is attributed to the incorporation of aromatic groups in the bulky and long pendent chains. The lower Tg values for neat polyimides are ascribed to the improved segmental rotation by virtue of the flexible linkages in the enhanced free volume generated by the bulky pendent groups while nanocomposites show higher Tg due to the interrupted segmental rotation by the silica nanofiller. The enhancement in the thermal and electrical properties of the nanocomposites is ascribed to the covalent bond formation between the amino groups on the surface of silica nanoparticles with the polyimide molecules. It has been observed that incorporation of bulky aromatic side chains along with flexible linkages can improve the flexibility and elongation at break, enhance solubility, maintain optimum Tg, good thermal stability, high flame resistance and low dielectric constants. The combined effect of all these factors makes these polyimides an attractive candidate for microelectronics applications. Both the polyimides and nanocomposites can have various usages in process industries, aero-space and defence.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to the DST (FIST) and UGC (SAP) for the financial support for procuring instruments.References
- D. J. Liaw, K. L. Wang, Y. C. Huang, K. R. Lee, J. Y. Lai and C. S. Ha, Prog. Polym. Sci., 2012, 37, 907–974 CrossRef CAS.
- M. Ghosh and K. Mittal, Polyimides: Fundamental and Application, Marcel Dekker, New York, 1996 Search PubMed.
- P. Ma, C. Dai, H. Wang, Z. Li, H. Liu, W. Li and C. Yang, Compos. Commun., 2019, 16, 84–93 CrossRef.
- A. Ghosh, S. K. Sen, S. Banerjee and B. Voit, RSC Adv., 2012, 2, 5900–5926 RSC.
- W. J. Bae, M. K. Kovalev, F. Kalinina, M. Kim and C. Cho, Polymer, 2016, 105, 124–132 CrossRef CAS.
- N. Amutha, S. A. Tharakan and M. Sarojadevi, Macromol. Symp., 2016, 362, 26–38 CrossRef CAS.
- R. Hariharan, S. Bhuvana, N. Amutha and M. Sarojadevi, High Perform. Polym., 2006, 18, 893–905 CrossRef CAS.
- D. H. Wang, Z. Shen, S. Z. D. Cheng and F. W. Harris, Polymer, 2007, 48, 2572–2584 CrossRef CAS.
- M. Ghaemy and R. Alizadeh, Eur. Polym. J., 2009, 45, 1681–1688 CrossRef CAS.
- C. Wang, S. Cao, W. Chen, C. Xu, X. Zhao, J. Li and Q. Ren, RSC Adv., 2017, 7, 26420–26427 RSC.
- Y. Liu, L. Liu, B. Ren, X. Zhu, W. Zhou and W. Li, Dyes Pigm., 2019, 162, 232–242 CrossRef CAS.
- P. Ma, C. Dai and H. Liu, Polymers, 2019, 19, 555–562 CAS.
- Q. Zhang, C. Y. Tsai, L. J. Li and D. J. Liaw, Nat. Commun., 2019, 10, 2–9 CrossRef PubMed.
- S. H. Hsiao, S. C. Peng, Y. R. Kung, C. M. Leu and T. M. Lee, Eur. Polym. J., 2015, 73, 50–64 CrossRef CAS.
- Y. Tian, Y. Song, H. Yao, H. Yu, H. Tan, N. Song, K. Shi, B. Zhang, S. Zhu and S. Guan, Dyes Pigm., 2019, 163, 190–196 CrossRef CAS.
- D. Ai, H. Li, Y. Zhou, L. Ren, Z. Han, B. Yao, W. Zhou, L. Zhao, J. Xu and Q. Wang, Adv. Energy Mater., 2020, 1903881, 1–7 Search PubMed.
- C. L. Tsai, H. J. Yen and G. S. Liou, React. Funct. Polym., 2016, 108, 2–30 CrossRef CAS.
- J. C. Chen, J. A. Wu, C. Y. Lee, M. C. Tsai and K. H. Chen, J. Membr. Sci., 2015, 483, 144–154 CrossRef CAS.
- M. Hasegawa, Polymers, 2017, 9(10), 520 CrossRef PubMed.
- S. Kumar Sen and S. Banerjee, RSC Adv., 2012, 2, 6274–6289 RSC.
- L. A. Bermejo, C. Alvarez, E. M. Maya, C. García, J. G. de la Campa and A. E. Lozano, eXPRESS Polym. Lett., 2018, 12, 479–489 CrossRef CAS.
- R. A. Shanks and I. Kong, in Advances in Elastomers I. Advanced Structural Materials, ed. P. M. Visakh, S. Thomas, A. K. Chandra and A. P. Mathew, Springer, Berlin, Heidelberg, 2013, ch. 2, vol. 11, pp. 11–45 Search PubMed.
- R. A. Rhein, Thermally Stable Elastomers’ A Review, Naval Weapons Center, China Lake, California, 1983 Search PubMed.
- M. Ragin Ramdas, K. P. Vijayalakshmi, L. M. Munirathnamma, H. B. Ravikumar and K. S. Santhosh Kumar, Mater. Today Commun., 2018, 17, 180–186 CrossRef CAS.
- H. Chen, B. Hao, P. Ge and S. Chen, Polym. Chem., 2020, 11, 4741–4748 RSC.
- M. Parmeggiani, P. Zaccagnini, S. Stassi, M. Fontana, S. Bianco, C. Nicosia, C. F. Pirri and A. Lamberti, ACS Appl. Mater. Interfaces, 2019, 11, 33221–33230 CrossRef CAS PubMed.
- S. A. Tharakan, S. Raju, R. Balasubramaniam and S. Muthusamy, Polym. Compos., 2019, 40, 832–843 CrossRef CAS.
- D. Meis, A. Tena, S. Neumann, P. Georgopanos, T. Emmler, S. Shishatskiy, S. Rangou, V. Filiz and V. Abetz, Polym. Chem., 2018, 9, 3987–3999 RSC.
- B. Deng, S. Zhang, C. Liu, W. Li, X. Zhang, H. Wei and C. Gong, RSC Adv., 2018, 8, 194–205 RSC.
- S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumar, Prog. Polym. Sci., 2013, 38, 1232–1261 CrossRef CAS.
- S. K. Kumar, B. C. Benicewicz, R. A. Vaia and K. I. Winey, Macromolecules, 2017, 50, 714–731 CrossRef CAS.
- I. A. Rahman and V. Padavettan, J. Nanomater., 2012 DOI:10.1155/2012/132424.
- S. Mallakpour and M. Naghdi, Prog. Mater. Sci., 2018, 97, 409–447 CrossRef CAS.
- Z. Hong, D. Wei, F. Yong, C. Hao, Y. Yang, J. Yu and L. Jin, Mater. Sci. Eng., B, 2016, 203, 13–18 CrossRef CAS.
- Y. J. Kim, J. H. Kim, S. W. Ha, D. Kwon and J. K. Lee, RSC Adv., 2014, 4, 43371–43377 RSC.
- K. H. Moon, B. Chae, K. S. Kim, S. W. Lee and Y. M. Jung, Polymers, 2019, 11, 489–499 CrossRef PubMed.
- M. Liang, Y. Wang, S. Sun and W. Yang, J. Appl. Polym. Sci., 2020, 137, 1–12 Search PubMed.
- T. Kang, I. Jang and S. G. Oh, Colloids Surf., A, 2016, 501, 24–31 CrossRef CAS.
- T. Kang, J. H. Lee and S. G. Oh, J. Ind. Eng. Chem., 2017, 46, 289–297 CrossRef CAS.
- R. V. Ghorpade, C. R. Rajan, N. N. Chavan and S. Ponrathnam, eXPRESS Polym. Lett., 2015, 9, 469–479 CrossRef CAS.
- Y. Lin, S. Hu and G. Wu, J. Phys. Chem. C, 2019, 123, 6616–6626 CrossRef CAS.
- Y. Zhao, X. Qi, Y. Dong, J. Ma, Q. Zhang, L. Song, Y. Yang and Q. Yang, Tribol. Int., 2016, 103, 599–608 CrossRef CAS.
- C. F. Cheng, H. H. Cheng, P. W. Cheng and Y. J. Lee, Macromolecules, 2006, 39, 7583–7590 CrossRef CAS.
- X. Huang, B. Chen, M. Mei, H. Li, C. Liu and C. Wei, Polymers, 2017, 9, 484–496 CrossRef PubMed.
- H. Gao, D. Yorifuji, Z. Jiang and S. Ando, Polymer, 2014, 55, 2848–2855 CrossRef CAS.
- D. H. Wang, Z. Shen, M. Guo, S. Z. D. Cheng and F. W. Harris, Macromolecules, 2007, 40, 889–900 CrossRef CAS.
- F. Wu, X. Zhou and X. Yu, RSC Adv., 2017, 7, 35786–35794 RSC.
- S. Liu, S. Zhu, X. Wang, H. Tan and S. Guan, High Perform. Polym., 2017, 29, 46–57 CrossRef CAS.
- Y. Zhou, G. Chen, H. Zhao, L. Song and X. Fang, RSC Adv., 2015, 5, 53926–53934 RSC.
- Y. Chen, R. Huang, Q. Zhang, W. Sun and X. Liu, High Perform. Polym., 2017, 29, 68–76 CrossRef CAS.
- J. Yao, C. Wang, C. Tian, X. Zhao, H. Zhou, D. Wang and C. Chen, Des. Monomers Polym., 2017, 20, 449–457 CrossRef CAS.
- C. H. Chan, J. Joy, H. J. Maria and S. Thomas, in Natural Rubber Materials Volume 2: Composites and Nanocomposites, ed. S. Thomas, C. H. Chan, L. Pothen, J. Joy and H. J. Maria, Royal Society of Chemistry, London, 7th edn, 2013, ch. 1, vol. 2, pp. 1–33 Search PubMed.
- D. Ponnamma, R. Ramachandran, S. Hussain, R. Rajaraman, G. Amarendra, K. T. Varughese and S. Thomas, Composites, Part A, 2015, 77, 164–171 CrossRef CAS.
- Y. Li, Y. Chu, R. Fang, S. Ding, Y. Wang, Y. Shen and A. Zheng, Polymer, 2012, 53, 229–240 CrossRef CAS.
- L. Yi, W. Huang and D. Yan, SJ. Polym. Sci., Part A: Polym. Chem., 2017, 55, 533–559 CrossRef CAS.
- L. Yi, C. Li, W. Huang and D. Yan, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 976–984 CrossRef CAS.
- S. M. Amininasab, S. Esmaili, M. Taghavi and Z. Shami, J. Fluorine Chem., 2016, 192, 48–57 CrossRef CAS.
- Y. Shen and A. C. Lua, Chem. Eng. J., 2012, 188, 199–209 CrossRef CAS.
- T. Koley, P. Bandyopadhyay, A. K. Mohanty and S. Banerjee, Eur. Polym. J., 2013, 49, 4212–4223 CrossRef CAS.
- A. S. More, S. K. Pasale and P. P. Wadgaonkar, Eur. Polym. J., 2010, 46, 557–567 CrossRef CAS.
- A. S. More, A. S. Patil and P. P. Wadgaonkar, Polym. Degrad. Stab., 2010, 95, 837–844 CrossRef CAS.
- Y. Liu, Y. Zhang, Q. Lan, Z. Qin, S. Liu, C. Zhao, Z. Chi and J. Xu, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1302–1314 CrossRef CAS.
- M. Fathima Rigana, R. Balasubramanian, S. Balaji and M. Sarojadevi, Polym. Compos., 2019, 40, 600–614 CrossRef CAS.
- X. Peng, W. Xu, L. Chen, Y. Ding, T. Xiong, S. Chen and H. Hou, React. Funct. Polym., 2016, 106, 93–98 CrossRef CAS.
- K. Deshmukh, M. B. Ahamed, K. K. Sadasivuni, D. Ponnamma, M. A. A. AlMaadeed, R. R. Deshmukh, S. K. K. Pasha, A. R. Polu and K. Chidambaram, J. Appl. Polym. Sci., 2017, 134, 1–11 CrossRef.
- S. Chisca, V. E. Musteata, I. Sava and M. Bruma, Eur. Polym. J., 2011, 47, 1186–1197 CrossRef CAS.
- A. Zulkifli, in Dielectric Materials, ed. M. A. Silaghi, Intech, Croatia, 3rd edn, 2012, ch. 1, pp. 3–26 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08561h |
| This journal is © The Royal Society of Chemistry 2021 |