Open Access Article
Open Access ArticleFungal remediation of Cd(II) from wastewater using immobilization techniques
Eman Abdullah M. Ali *a,
Mohsen A. Sayed
*a,
Mohsen A. Sayed a,
Tahany M. A. Abdel-Rahman
a,
Tahany M. A. Abdel-Rahman a and
Rabab Hussein
a and
Rabab Hussein b
b
aBotany and Microbiology Department, Faculty of Science, Cairo University, 12613, Giza, Egypt. E-mail: emanali@sci.cu.edu.eg
bBasic Science Department, Faculty of Engineering, Misr University for Science and Technology, Egypt
First published on 25th January 2021
Abstract
The pollution of wastewater by heavy metal ions is hazardous to the environment and human health. Cd(II) has been recognized as one of the heavy metals that causes severe toxic effects. The present study is aimed at removing Cd(II) from wastewater using fungal biomass either immobilized on loofa sponges or in Ca-alginate beads. Two fungal species were isolated from pools of Cd(II)-polluted wastewater obtained from some Egyptian industrial plants, and using internal transcribed spacer (ITS) primers, they were molecularly identified as Penicillium chrysogenum and Cephalotheca foveolata with accession numbers MT664773 and MT664745, respectively. The sorbents used in this study were heat-inactivated mycelia of P. chrysogenum (PEN), heat-inactivated mycelia of C. foveolata (CEP), P. chrysogenum immobilized on loofa sponge (PEN-ILS), C. foveolata immobilized on loofa sponge (CEP-ILS), P. chrysogenum immobilized in Ca-alginate beads (PEN-IA), and C. foveolata immobilized in Ca-alginate beads (CEP-IA). The effects of pH, contact time, initial Cd(II) concentration, and interfering ions on Cd(II) removal from aqueous solution were tested. Maximum Cd(II) sorption capacity was obtained at pH 7.0, with thirty minutes contact time and 0.5 mol l−1 initial Cd(II) concentration for all sorbents used. However, Ca2+ displayed synergistic interference with Cd(II) that was greater than that from Na+ and K+, with decreasing sorption capacity for all sorbents. Optimum conditions were applied to real wastewater samples collected from two Egyptian industrial plants. All sorbents had the ability to remove Cd(II) from wastewater samples, and enhanced removal occurred when fungal cells were immobilized as compared to free cells.
Introduction
Heavy metals are defined as elements with an atomic density of more than 6.0 g cm−3, and they are pollutants that persist in wastewater. They are mentioned as metallic elements or trace elements.1 The leakage of excessive quantities of heavy metals into water bodies can cause severe problems for health and the environment and may increase the cost of wastewater treatment.2 Heavy metals are assumed to be the most important inorganic pollutants due to their inability to be degraded and their subsequent movement in aquatic ecosystems that causes toxicity to higher life forms, even at low concentrations.3The heavy metals present in wastewater are from both natural sources and human activities. The natural sources comprise leaching of rocks, airborne dust, soil erosion, and volcanic activities, while the human sources include rapid industrialization, urbanization, anthropogenic sources, metal finishing and electroplating processes, mining extraction, nuclear power plants, and textile industries. The presence of heavy metals in water bodies affects the quality of water and threatens soil as well as plants growing in these soils. Ingestion of those plants by humans and animals results in the movement of heavy metals into the food chain via bioaccumulation and biomagnification processes. Therefore, removal of heavy metals from aquatic ecosystems is required.4–7
The heavy metals that are dangerous to human health are Pb, Hg, Cd, As, Cu, Zn, and Cr, while Pb, Cd, and Hg are referred to as “the big three” because they have such a negative impact on the environment.8 Cadmium is documented as one of the most lethal metals and has been categorized as a human carcinogen and teratogen.9 Cadmium poses a great danger due to its high environmental movement.10 It causes lung insufficiency, renal dysfunction, liver damage, bone degeneration, and hypertension in humans.11
Water quality improvement is one of the main environmental tasks that must be effectively carried out because water is important to life, as well as domestic, agricultural, and industrial activities.12 Suitable treatments of effluents before their release to water bodies are needed to prevent the toxicity of heavy metals in the environment. The procedures used to remove metal ions from aqueous solution primarily involve chemical, physical, and biological methods. The conventional techniques used to remove heavy metals from aqueous solutions are mostly dependent on the implementation of chemical precipitation, reverse osmosis, ion exchange, ultra-filtration, lime coagulation, membrane filtration, solvent extraction, and adsorption. Every method has its own limitations and merits in application. The disadvantages of these traditional techniques include energy requirements; incomplete metal removal; generation of toxic sludge and other waste products; high cost, particularly at low metal concentrations; inefficiency; and non-selectivity.13,14 Therefore, biological methods have been encouraged in recent years to overcome problems that are present due to the use of chemical treatment methods. Biological methods are divided into two wide categories: phytoremediation and microbial remediation.15 Microbial biosorption methods using algae, fungi, yeast, and bacteria for efficient heavy metal uptake from many environments are environmentally friendly and have become an alternative methodology to chemical techniques.16
In microbial remediation, the pollutants may be used as a carbon source required for microbial growth before the microorganism can sufficiently aid in the degradation. Microbial remediation can occur with the use of genetically engineered microorganisms to increase the degradation process, which occurs naturally. The microbial remediation processes for heavy metal removal from wastewater involve biosparing, bioaugmentation, and biosorption.17 Biosorption techniques include the sequestration of positively charged heavy metal ions to negatively charged microbial cell membranes and secreted polysaccharides.18 Biosorption using non-living microbial biomass has been found to be more effective and is favored for heavy metal elimination and recovery from aqueous media.19,20 Moreover, non-living microbial cell immobilization on either synthetic polymeric or natural matrices through entrapment has been found to increase the performance, biosorptive capacity, and biosorbent lifespan for recycling and regeneration by numerous orders of magnitude. Furthermore, immobilized cells are generally easier to handle because they require less complex separation systems.16,21–24 Calcium alginate and other support materials (such as alumina, organic chelate resin, silica gel, polyvinyl alcohol, and activated carbon) have been successfully used to immobilize microorganisms.25 Loofa sponge is also used for microbial immobilization because it is easy to manipulate, inexpensive, and possesses an eco-friendly matrix.26 The major advantages of microbial remediation are reduction in cost without hazardous end products.27 The major microbial groups that have been involved in the remediation of heavy metal are bacteria and fungi. In addition to bacteria and fungi, it has been observed that some protozoa and algae possess metal-reducing capabilities.28–30
The main objectives of this study were to isolate Cd(II)-resistant fungal species and to characterize the isolated species using molecular techniques. Moreover, the optimum factors related to their dried biomass, either free or immobilized in loofa sponge or Ca-alginate beads, were determined so that the maximum Cd(II) sorption capacity from aqueous solutions could be attained. The optimum conditions were applied in experiments to remove Cd(II) from two real wastewater samples collected from Egyptian manufacturing plants.
Materials and methods
Isolation of fungi from wastewater samples
Wastewater samples were collected from some industrial plants in Giza and Cairo, Egypt. Fungi were isolated from wastewater samples using pour plate method and were maintained on glucose peptone medium composed of (g l−1): 10.0 glucose; 0.5 MgSO4·7H2O; 1.0 KH2PO4; 5.0 peptone; 20.0 agar, with a pH of 6.7 at 30.0 °C.31Molecular identification of isolated fungi
Total genomic DNA was extracted from fungal mycelium using a DNA purification kit (Zymo Research). DNA crude extracts were used directly for PCR amplification analysis of internal transcribed spacer (ITS) regions. The amplification reaction was carried out using a MyTaq™ Red Mix kit (Bioline) with the universal primers ITS1 and ITS4. The thermal cycling parameters used were an initial denaturation at 94.0 °C for 6.0 min, followed by 35.0 cycles of denaturation at 94.0 °C for 45 s, annealing at 56.0 °C for 45.0 s, and extension at 72.0 °C for 1.0 min followed by a final extension at 72.0 °C for 5.0 min. Then, the DNA sequencing of PCR products was performed at the German company GATC Biotech using an ABI 3730xl DNA sequencer with forward and reverse primers. Genomes were sequenced and analyzed by combining the traditional Sanger technology with the new 454 technology with reduction in the usual project time, and in the number of coatings and gaps. The resulting sequences were then compared with those available in the public online databases of the NCBI (National Center for Biotechnology Information) using the BLAST (Basic Local Alignment Search Tool) search program (http://www.ncbi.nlm.nih.gov/blast/).32 Accession numbers of identified fungi were obtained.Immobilization of fungal biomass on loofa sponge
The loofa sponge was obtained by removing the hard pericarp tissue of the ripened dried fruit of Luffa cylindrica. The fibrous sponge was cut into discs of approximately 2.5 cm diameter, 2.0–3.0 mm thick, and were then soaked in boiling water for 30.0 min. After washing the discs under tap water, they were submerged for 24.0 h in distilled water that was changed 3.0–4.0 times, and then oven-dried at 70.0 °C and maintained in desiccators.Mycelial suspensions were prepared from 7 day old cultures grown at 30.0 °C on slants of glucose peptone medium. Sterile distilled water was poured on the slants, and then, gentle scratching was performed with a sterile glass rod. The obtained suspensions were then transferred with sterile pipettes to sterile tubes. The mycelial suspensions (0.5 ml) of the tested fungi were inoculated into 100.0 ml of growth medium containing (g l−1): D-glucose, 10.0; KH2PO4, 2.0; MgSO4·7H2O, 0.5; NH4Cl, 0.1; CaCl2·H2O, 0.1; and thiamine, 0.001. Four sterilized pre-weighed loofa sponge discs were added. The inoculated flasks were incubated at 30.0 °C with shaking at 100.0 rpm for 7 days. The free and immobilized fungal biomasses were on the loofa sponges was collected from the medium, washed twice with distilled water, and stored at 4.0 °C until use. The dry weight of the immobilized fungal biomass on the loofa sponge was determined by drying the discs for 24.0 h at 70.0 °C and weighing them before and after fungal growth.33
Immobilization of fungal biomass in Ca-alginate beads
Fungal inocula prepared from 7 day-old cultures were used to inoculate growth medium, which was then incubated with shaking. The biomass was collected by filtration, washed several times with distilled water, and homogenized with a blender to remove cell aggregates. Two grams of Na-alginate was dissolved in distilled water and then mixed with the fungal mycelium (50.0 ml, containing 0.5 g fungal biomass in sterilized distilled water). The mixture was added to CaCl2 solution (0.1 M) with a burette and stirred to avoid aggregation of beads. The fungal immobilized beads were preserved in this solution for 1.0 h, washed twice with 200.0 ml sterile distilled water, and heated in 5.0 mM CaCl2 solution for 10.0 min. The immobilized preparations were then maintained in 5.0 mM CaCl2 solution at 4.0 °C until use.34Aqueous solution factors affecting the biosorptive capacity of Cd(II)
All sorption experiments were performed at room temperature (25.0 ± 1.0 °C).Effect of pH
One ml of Cd(II) solution (0.1 mol l−1) was mixed with each sorbent (17 ± 2 mg sorbent for loofa experiments and 45 ± 2 mg sorbent for alginate experiments) in a 50.0 ml volumetric flask. Buffers were added (9.0 ml) to prepare solutions with different pH values of 3.0, 5.0, 7.0, and 9.0 (acetate buffer for pH 3.0 and 5.0, phosphate buffer for pH 7.0, and Tris–HCl buffer for pH 9.0). These mixtures were shaken at 250.0 rpm for 30.0 min and filtered.16 Residual Cd(II) in the filtrate was determined by complexometric titration against 0.01 M EDTA, and each experiment was carried out three times. The sorption capacity was calculated from the following equation:| q = (Co − C)V × 103/m | (1) |
Effect of contact time
The formerly stated batch experimental procedure was performed at different exposure times of 10.0, 20.0, 30.0, and 40.0 min with shaking at 250.0 rpm and pH 7.0. After the sorption process, the concentration of residual Cd(II) was also detected by EDTA titration, and Cd(II) sorption capacities were calculated using the previous equation.Effect of the initial Cd(II) concentration
The effect of the initial Cd(II) concentration on the sorption capacity of Cd(II) was studied using different concentrations of Cd(II): 0.025, 0.1, 0.5, and 1 mol l−1 at pH 7.0 for 30.0 min with shaking at 250.0 rpm. The residual Cd(II) concentration after the sorption process was then determined by EDTA titration as previously described.Effect of metal ion interference
The Cd(II) sorption capacity was determined in the presence of other competing metal ions. The examined solutions were prepared by mixing 0.1 mol l−l Cd(II) solution (1.0 ml) and 1.0 ml of 0.1 mol l−1 of an interfering metal ion solution (NaCl, KCl, and CaCl2). These were mixed with each sorbent and buffer solution of pH 7.0 for 30.0 min with shaking at 250.0 rpm.Removal of Cd(II) from real wastewater samples
Two samples of wastewater collected from General Motors Factory (S1) (Giza, Egypt) and Egyptian Plastic and Electricity Factory (S2) (Cairo, Egypt) were analyzed by atomic absorption spectrophotometer (JASCO V-630, Japan) to determine the initial Cd(II) concentration. The sorption experimental procedure was performed at the optimum pH and contact time that was determined from the previous experiments. The percentage of Cd(II) removal after the sorption experiment was detected by EDTA titration.Statistical analysis
All experiments were performed in triplicate. The data were analyzed by one-way analysis of variance (ANOVA) using Statistical Package of Social Science (SPSS) program version 20 and computer program Microsoft Office Excel (2010). The results are expressed as the mean ± standard deviation (mean ± SD).Results
Molecular identification of the isolated fungi
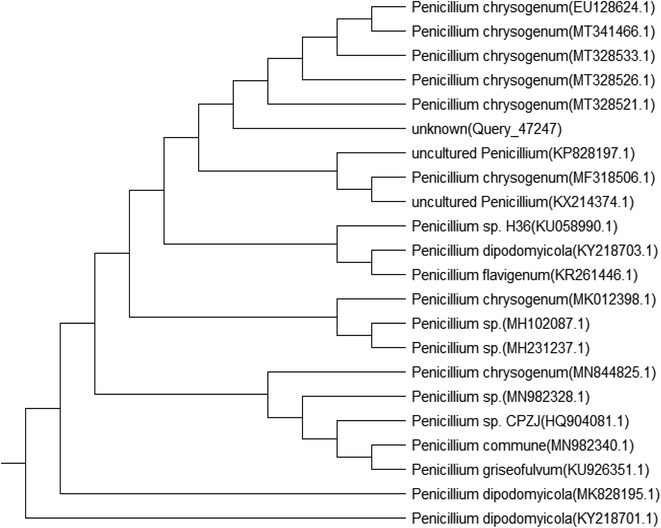
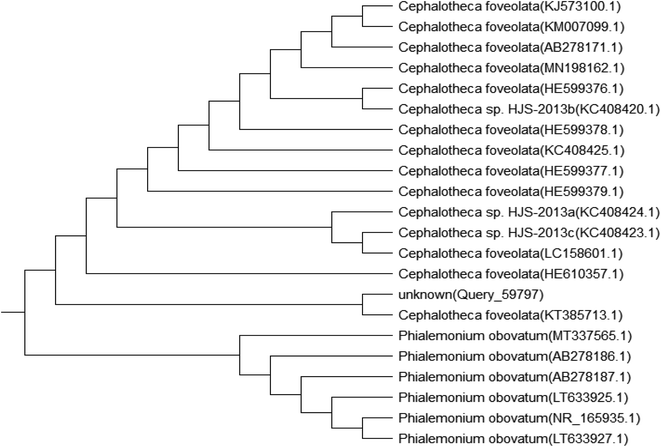
Genomic DNA was extracted from the isolated fungal species, and then, the PCR products were sequenced. The sequences were compared with those available in the public gene bank online databases of the NCBI using the BLAST alignment search program. Accession numbers of the identified fungi were obtained as MT664773 for Penicillium chrysogenum and MT664745 for Cephalotheca foveolata. Their relation to other fungal species is displayed in the following phylogenetic trees (Fig. 1 and 2).Factors affecting biosorption of Cd(II) by the immobilized biomass of P. chrysogenum and C. foveolata
In the present experiments, loofa sponge was covered with P. chrysogenum and C. foveolata mats after an incubation period of 7.0 days (Fig. 3), while heat-inactivated P. chrysogenum and C. foveolata were immobilized in Ca-alginate beads, as shown in Fig. 4.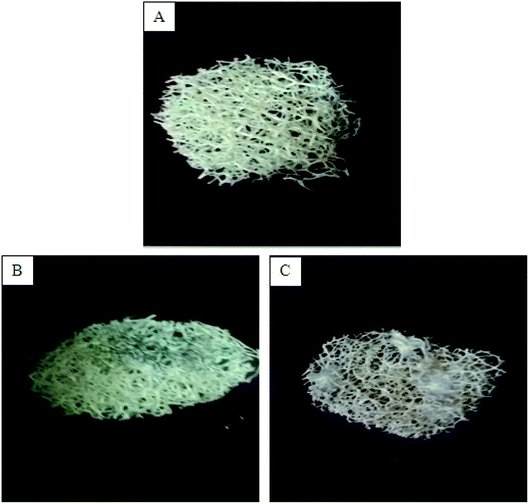 | ||
| Fig. 3 (A) Loofa sponge disc, (B) Penicillium chrysogenum immobilized on loofa sponge, (C) Cephalotheca foveolata immobilized on loofa sponge. | ||
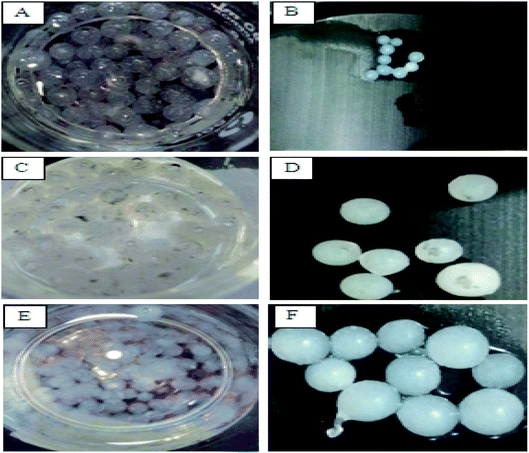 | ||
| Fig. 4 Alginate beads. (A and B) Alginate beads, (C and D) Penicillium chrysogenum immobilized in alginate beads, (E and F) Cephalotheca foveolata immobilized in alginate beads. | ||
Effect of pH
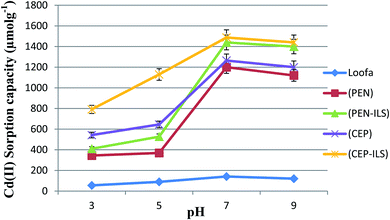
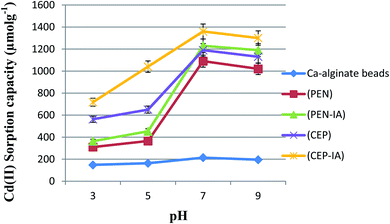
The data in Fig. 5 show that the maximum Cd(II) sorption capacity among the five sorbents was observed at pH 7.0. The sorption capacity gradually increased as the pH value was increased from 3.0–7.0. At pH 7.0, immobilized C. foveolata on loofa sponge possessed a higher capacity to adsorb Cd(II) ions from aqueous solution than that of immobilized P. chrysogenum on loofa sponge, with sorption capacities of 1487.6 and 1440.0 μmol g−1, respectively. A reduction in biosorption capacity occurred when the pH increased to 9.0.As shown in Fig. 6, the Cd(II) sorption capacity of all sorbents gradually increased as the pH values increased from 3.0 until they reached the maximum capacity at pH 7.0, and then decreased with increasing pH to 9.0. At pH 7.0, Ca-alginate-immobilized C. foveolata (CEP-IA) possessed a greater ability to adsorb Cd(II) ions from aqueous solution than that of immobilized P. chrysogenum (PEN-IA), with a sorption capacity of 1360.0 and 1230.0 μmol g−1, respectively.
Effect of contact time
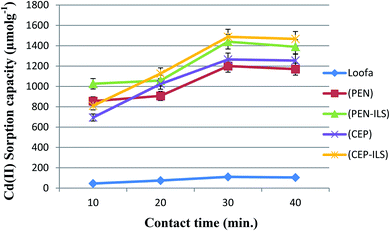
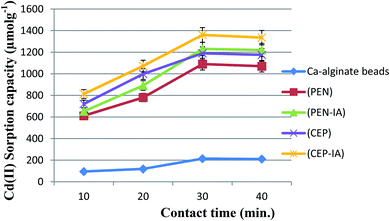
The biosorption of Cd(II) by all sorbents accelerated with increasing exposure time until the optimum was reached at 30.0 min, and maximum values of 1487.6 and 1440.0 μmol g−1 were obtained for C. foveolata and P. chrysogenum immobilized in loofa sponge, respectively (Fig. 7). When the contact time was greater than 30.0 min, there was a decrease in the sorption capacity. The fungal species immobilized on loofa sponge activated Cd(II) biosorption more than free ones at all exposure periods.As shown in Fig. 8, the biosorption of Cd(II) by all sorbents was found to be accelerated when the exposure time was increased to 30.0 min, and then slightly decreased when the time was increased to 40.0 min. The highest Cd(II) sorption capacity was recorded for C. foveolata immobilized in Ca-alginate beads followed by P. chrysogenum immobilized in Ca-alginate beads at 30.0 min exposure time.
Effect of initial Cd(II) concentration
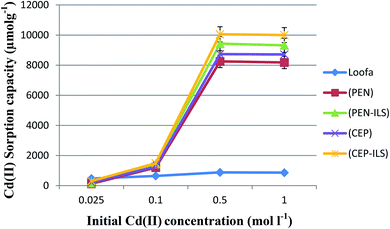
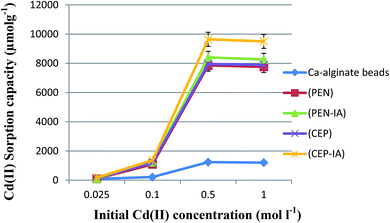
The sorption capacity of Cd(II) by free and immobilized sorbents on loofa increased as the initial concentration of Cd(II) increased until the optimum value was reached at 0.5 mol l−1. The sorption capacity increased approximately eight times at the second concentration as compared to the first, and then was enhanced approximately six times at the third concentration as compared to the second one in the case of PEN-ILS. Similarly, in the case of CEP-ILS, the sorption capacity increased approximately five times at the second concentration compared to the first one and increased seven times at the third concentration as compared to the second one (Fig. 9).The optimum initial Cd(II) concentration for the maximum sorption capacity was 0.5 mol l−1 (Fig. 10). The sorption capacity increased approximately eleven times at the second concentration compared to the first one, and then, was enhanced approximately seven times at the third concentration as compared to the second one in the case of PEN-IA. Similarly, in the case of CEP-IA, the sorption capacity increased approximately eight times at the second concentration compared to the first one, and increased seven times at the third concentration compared to the second one.
Effect of interference between ions
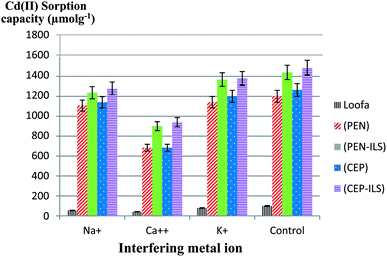
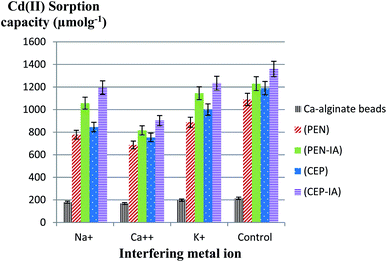
In this test, the Cd(II) sorption capacity in the presence of other interfering ions (Na+, K+, and Ca2+) was investigated. The results illustrated in Fig. 11 revealed a lower sorption capacity for Cd(II) in aqueous solution when in the presence of Ca2+ as compared to Na+ and K+ for all sorbents. For example, when using PEN-ILS, the Cd(II) sorption capacity in the presence of Ca2+, Na+, and K+ was 900.0, 1236.0, and 1368.0 μmol g−1 respectively, as compared to the control, which was recorded as 1440.0 μmol g−1 with no existence of interfering ions. In all cases, the immobilized fungal cells were more efficient in Cd(II) biosorption than free cells.The results shown in Fig. 12 indicate that Ca2+ displayed higher antagonistic interference in Cd(II) sorption capacity as compared to Na+ and K+. The sorption capacity of Cd(II) using PEN-IA was recorded as 1056.9 μmol g−1 and 1145.0 μmol g−1 in the presence of Na+ and K+, respectively, while the Cd(II) sorption capacity reached 815.8 μmol g−1 in the presence of Ca2+ ion as compared to the control, which was recorded as 1230.0 μmol g−1.
Removal of Cd(II) from real wastewater samples
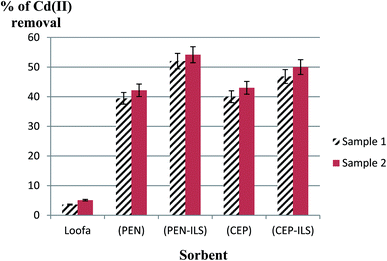
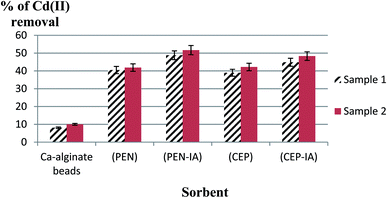
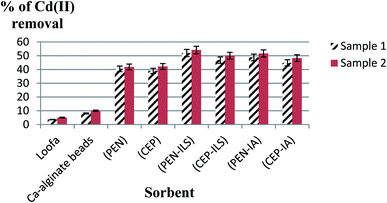
The initial Cd(II) concentrations in the wastewater samples were 0.001 mol l−1 for sample 1 and 0.002 mol l−1 for sample 2. In the first sample (Fig. 13), it was detected that usage of a loofa sponge with the immobilized biomass of P. chrysogenum provided a higher sorption capacity than usage of free P. chrysogenum, with removal of 52.0% and 39.5% of Cd(II), respectively. Similarly, loofa sponge-immobilized biomass of C. foveolata achieved higher efficiency than the usage of free C. foveolata, with 46.8% and 40.0% of Cd(II) removal from real wastewater, respectively. In the second sample, usage of loofa sponge-immobilized biomass consisting of P. chrysogenum and C. foveolata provided higher sorption capacity than the usage of the free fungal species, where they removed 54.2% and 50.0% of Cd(II) compared to 42.2% and 43.0%, respectively.In the first sample, it was detected that the usage of Ca-alginate-immobilized biomass of P. chrysogenum provided the highest performance of sorption capacity than all other sorbents, with Cd(II) removal of 48.8% followed by Ca-alginate-immobilized C. foveolata which achieved 44.9% Cd(II) removal from the real wastewater. Similarly, in the second sample, usage of Ca-alginate-immobilized biomass of P. chrysogenum exhibited the highest sorption capacity than the other sorbents by removing 51.7% of Cd(II), followed by Ca-alginate-immobilized C. foveolata with 48.3% of Cd(II) removal (Fig. 14).
Comparing all the results, it was obvious that the maximum removal of Cd(II) from wastewater was achieved by the use of PEN-ILS followed by PEN-IA. Also, CEP-ILS removed a higher percentage of Cd(II) from wastewater than CEP-IA (Fig. 15).
Discussion
Biosorption is an environmentally friendly process in which heavy metals from wastewater bind to microbial biomass surfaces by physicochemical pathways.35 Recently, there has been greater interest in using fungi as a biosorbent because their cell walls contain a varied range of functional groups that aid in the removal of metal.36Loofa sponge is derived from the plant Luffa cylindrica, which is an easily available and inexpensive material that can be used as an immobilization matrix. This plant material is an open interwoven network of fibers that are composed of cellulose, and it allows rapid growth of fungal mycelium within its fiber matrix.37 Hyphal immobilization along the surface of loofa fibers prevents clumping, in addition to the open network of the loofa sponge that increases the surface area and enhances free metal ion binding to the sorption sites.38
Alginate beads are preferred as a support material due to several advantages such as hydrophilicity, biodegradability, natural origin, and presence of carboxylic groups. The presence of carboxylic groups in the alginate structure increases the heavy metal ion sorption capacity of the system in combination with microbial cells.39,40 Also, the beads can be recycled and reused in subsequent cycles.41,42 Other advantages are their mechanical stability and low density that results in a substrate that is very suitable for several biotechnological applications. In water treatment, alginate has an essential role in eliminating heavy metal ions because of its advantages such as inexpensive, biocompatible, environmentally friendly, and non-toxic. During adsorption, the heavy metal ions appear to exchange with sodium or calcium ions via an ion exchange process.43
The removal of ions from aqueous solution is strongly dependent on the pH of the solution.44,45 It affects both the ionization state of functional groups (amino, carboxylic, and phosphate groups) on fungal cell walls and the solubility of metal ions.46 Moreover, it influences the ionization degree and Cd(II) distribution in a solution.47 Leyva-Ramos et al.48 stated that at pH < 6.0, Cd(II) is the only form in the solution, while at pH < 8.0, the dominant forms are Cd(II) and Cd(OH)+. At pH > 8.0, Cd(OH)2 dominates. By increasing the pH, the biosorption capacity increases until the maximum is reached at the optimum pH. By further increasing the pH, metals begin to precipitate due to the formation of metal hydroxides or hydroxide anionic complexes that decrease the efficiency of the metal removal.49,50 Alyasi et al.51 showed that cadmium adsorption from water increases with increase in pH, but only up to a certain point (approximately pH 5.0). This may occur due to the positive surface charge at lower pH values that results in H+ ions competing with Cd(II) for sorption sites, which leads to a decrease in Cd(II) adsorption. This is called protonation of active sites, and it causes competition between cations and metal ions for sorption sites.52,53 However, as the pH of the solution increases, the number of protons detached from the functional groups on the fungal cell wall increases so that additional negative charge groups are provided for the complexation to enhance metal uptake.54 These observations agree with those reported in earlier investigations for the removal of different metals by various biosorption materials.55,56
Similarly, in this respect, Jalija and Uzairu57 reported that Cd(II) biosorption from aqueous solution using Penicillium sp. immobilized in calcium alginate increased with an increase in solution pH. Alameen and Majeed58 demonstrated that as the solution pH increases from 5.0 to 7.0, the cadmium removal efficacy increased, while for a pH greater than 7.0, the cadmium removal efficacy decreases. Ad et al.59 showed that the optimum pH for cadmium biosorption by L. cylindrica in aqueous solution was 6.0. Yan and Viraraghavan60 recorded that the removal of lead increases with increasing pH. Iqbal and Edyvean33 reported the same results for the removal of lead, zinc, and copper. Renu et al.61 found that the optimum pH value for cadmium removal was in the range from 4.0 to 7.0. From the previous studies, it was obvious that the pH of a solution is a critical parameter because it controls the adsorption of metal ions.
The Cd(II) removal percentage by Penicillium sp. increased from 69.7% to 88.8% at 10.0 and 150.0 minutes contact time, respectively, with 150 minutes being the optimum contact time.56 Renu et al.61 stated that the optimum contact time for maximum removal of cadmium is in the range of 5.0–120.0 minutes. Kocaobaa and Arısoy62 showed that the optimum parameters for Cd(II) biosorption from aqueous solutions using Pleurotus ostreatus immobilized on bentonite were 30.0 min contact time and pH 5.0. As time increased, saturation occurred, and then the adsorbate tended to desorb back into the solution. At equilibrium, the rates of adsorption and desorption will be the same.56
Jalija and Uzairu57 showed that the Cd(II) sorption capacity increased with the increase in initial Cd(II) concentrations. Sun et al.63 indicated that cadmium biosorption using Aspergillus terreus immobilized on loofa sponge increased with the increase in the initial concentration of metal ions. The increase in specific uptake with the increase in the initial Cd(II) concentration most likely occurred because with a fixed adsorbent weight, the competition of metal ions on the biosorbent surface gradually increases until saturation of active sites occurs.64 The probability of Cd(II) biosorption on the active sites of the yeast-alginate system increased as Cd(II) became more concentrated in the aqueous solution due to an increase in the driving force that overcame the mass transfer resistance of Cd(II) between the liquid and solid phases.65
Mahmoud et al.16 studied the effect of some interfering ions on Cd(II) sorption capacity. The tested ions were Na+, K+, Ca2+, Co2+, Ni2+, and Cu2+. Similar to our results, they found that Na+ and K+ exhibited the lowest interference with Cd(II) as compared to other tested ions, while Cu2+ exhibited the highest interference. The different affinities of interfering ions may be due to their different ionic radii and ionic charges, and additionally, the nature of the functional groups existing on the fungal cell walls.19,60
Herein, the maximum Cd(II) removal from wastewater was obtained by the immobilization of fungal biomass on loofa sponge followed by immobilization in alginate beads followed by the use of free fungal cells. Barquilha et al.66 stated that the use of immobilized cells is more efficient for removing metals than free cells. Sun et al.63 studied the cadmium uptake using free and immobilized Aspergillus terreus on loofa sponge. The maximum cadmium uptake capacity of immobilized fungus was superior to that of free fungus, which may be due to an increase in accessible binding sites in the case of immobilized fungus. Velkova et al.67 stated that free microbial biosorbents are small in size and have low density.
Immobilization of microbial biomass on the appropriate carriers removes the drawbacks of free biosorbents and provides more opportunities for practical use of biosorption. The decrease in the uptake of metals when using polyacrylamide gel may be due to changes in the microbial cell structure during the immobilization process. Furthermore, a portion of the cell surface was shielded by gel and would not be available for binding metal ions with the microbial surface, whereas no diffusional limitation was detected in the case of the loofa-immobilized biomass systems.68,69
Conclusion
In the current study, it was apparent that all sorbents used had the ability to remove Cd(II) from aqueous and wastewater samples. Optimum parameters for Cd(II) removal from aqueous solutions were achieved at pH 7.0, with thirty minutes of contact time, and 0.5 mol l−1 initial Cd(II) concentration for all sorbents used. Moreover, Ca2+ was the ion that caused the greatest interference with Cd(II) as compared to Na+ or K+, leading to decreased sorption capacity for all sorbents. Optimum factors were applied for the removal of Cd(II) from two real wastewater samples collected from Egyptian plants, and using heat-inactivated fungi, the removal of Cd(II) from wastewater was a successful process. Maximum Cd(II) removal from wastewater was achieved with fungal biomass immobilized on loofa sponge followed by immobilization in alginate beads, followed by free fungal cells. The immobilization process enhanced the biosorption capacity.Author contributions
Tahany M. A. Abdel-Rahman and Mohsen A. Sayed contributed to the study conception. Material preparation, data collection, and analysis were performed by Rabab Hussein and Eman Abdullah M. Ali. Formal analysis and software were carried out by Eman Abdullah M. Ali. The first draft of the manuscript was written by Rabab Hussein and Eman Abdullah M. Ali. Reviewing and editing were performed by Eman Abdullah M. Ali and Tahany M. A. Abdel-Rahman. All authors read and approved the final manuscript.Funding
No funding was received for conducting this study.Ethical statement
The authors declared that this work has not been published elsewhere, either completely, in part, or in another form. The manuscript has not been submitted to another journal and will not be published elsewhere.Conflicts of interest
The authors declare that there are no conflicts of interest.References
- H. M. Salem, E. A. Eweida and A. Farag, International Conference on Economics, Humanity and Management, Cairo University, Egypt, 2000, p. 542–556 Search PubMed.
- A. J. M. Barros, S. Prasad, V. D. Leite and A. G. Souza, Bioresour. Technol., 2007, 98, 1418–1425 CrossRef CAS.
- B. Preetha and T. Viruthagiri, J. Hazard. Mater., 2007, 143, 506–510 CrossRef CAS.
- M. Saidi, Int. J. Environ. Sci., 2010, 1(4), 666–676 CAS.
- D. O. Ogoyi, C. J. Mwita, E. K. Nguu and P. M. Shiundu, Open Environ. Eng. J., 2011, 4, 156–161 CrossRef CAS.
- S. P. Mishra, Curr. Sci., 2014, 107, 601–616 CAS.
- A. Elahi, I. Arooj, D. A. Bukhari and A. Rehman, Appl. Microbiol. Biotechnol., 2020, 104(2), 3729–3743 CrossRef CAS.
- B. Volesky, FEMS Microbiol. Rev., 1994, 14, 291–302 CrossRef CAS.
- K. Pyrzynska, J. Environ. Chem. Eng., 2019, 7(1), 102795 CrossRef CAS.
- T. Lebeau, D. Bagot, K. Jézéquel and B. Fabre, Sci. Total Environ., 2002, 291, 73–83 CrossRef CAS.
- M. Iqbal, A. Saeed and S. I. Zafar, J. Hazard. Mater., 2007, 148, 47–55 CrossRef CAS.
- D. C. S. Alves, J. O. Gonçalves, B. B. Coseglio, T. A. L. Burgo, G. L. Dotto, L. A. A. Pinto and T. R. S. Cadaval Jr, J. Environ. Chem. Eng., 2019, 7, 103460 CrossRef CAS.
- M. E. Mahmoud, O. F. Hafez, A. Alrefaay and M. M. Osman, Desalination, 2010, 253, 9–15 CrossRef CAS.
- M. E. Mahmoud, Desalination, 2011, 266, 119–127 CrossRef CAS.
- S. Sharma, Asian J. Pharm. Life Sci., 2012, 2(2), 202–212 Search PubMed.
- M. E. Mahmoud, A. A. Yakout, H. Abdel-Aal and M. M. Osman, Desalination, 2011, 279, 291–297 CrossRef CAS.
- J. C. Igwe and A. A. Abia, Afr. J. Biotechnol., 2006, 5(12), 1167–1179 CAS.
- R. K. Sinha, D. Valani, S. Sinha, S. Singh and S. Herat, Solid Waste Management and Environmental Remediation, Nova Science Publishers, 2009, pp. 1–72 Search PubMed.
- M. Amini, H. Younesi and N. Baramifar, Colloids Surf., A, 2009, 337, 67–73 CrossRef CAS.
- Y. Wang, H. Gao, J. Sun, J. Li, Y. Su, Y. Ji and C. Gong, Desalination, 2011, 270, 258–263 CrossRef CAS.
- W. Y. Baik, J. H. Bae, K. M. Cho and W. Hartmeier, Bioresour. Technol., 2002, 81, 167–170 CrossRef CAS.
- A. I. Anastasios, K. A. Zouboulis, M. Loukidou and F. Sebaesta, Colloids Surf., A, 2003, 212, 185–195 CrossRef.
- F. Beolchini, F. Pagnaneli, A. Esposito, L. Toro and F. Veglio, Hydrometallurgy, 2003, 70, 101–112 CrossRef CAS.
- A. K. Pandey, S. D. Pandey, V. Misra and S. Devi, J. Hazard. Mater., 2003, 98, 177–181 CrossRef CAS.
- K. Tsekova, D. Todorova, V. Dencheva and S. Ganeva, Bioresour. Technol., 2010, 101(6), 1727–1731 CrossRef CAS.
- A. Saeed and M. Iqbal, Biotechnol. Prog., 2013, 29(3), 573–600 CrossRef CAS.
- M. Ahmedna, W. F. Marshall, A. A. Husseiny, R. M. Rao and I. Goktepe, Water Res., 2004, 38(4), 1064–1068 CrossRef.
- M. A. Dias, I. C. A. Lacerda, P. F. Pimentel, H. F. Castro and C. A. Rosa, Lett. Appl. Microbiol., 2002, 34, 46–50 CrossRef CAS.
- R. Gupta, P. Ahuja, S. Khan, R. K. Saxena and H. Mohapatra, Curr. Sci., 2000, 78(8), 967–973 CAS.
- K. Ramasamy and S. P. B. Kamaludeen, Environmental Bioremediation Technologies, 2006, pp. 173–187 Search PubMed.
- H. Jung, F. Xu and K. Li, Enzyme Microb. Technol., 2002, 30, 161–168 CrossRef CAS.
- S. F. Altschul, T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller and J. D. Lipman, Nucleic Acids Res., 1997, 25, 3389–3402 CrossRef CAS.
- M. Iqbal and R. G. J. Edyvean, Miner. Eng., 2004, 17, 217–223 CrossRef CAS.
- M. Y. Arica, Y. Kacar and O. Genc, Bioresour. Technol., 2001, 80(2), 121–129 CrossRef CAS.
- A.-H. M. Rasmey, A. A. Aboseidah and A. K. Youssef, Egypt. J. Microbiol., 2018, 53, 37–48 Search PubMed.
- J. R. M. Benila Smily and P. A. Sumithra, HAYATI J. Biosci., 2017, 24(2), 65–71 CrossRef.
- D. K. Verma, S. H. Hasan, D. Ranjan and R. M. Banik, Int. J. Environ. Sci. Technol., 2014, 11, 1927–1938 CrossRef CAS.
- N. Akhtar, J. Iqbal and M. Iqbal, J. Hazard. Mater., 2004, 108, 85–94 CrossRef CAS.
- L. E. De-Bashan and Y. Bashan, Bioresour. Technol., 2010, 101, 1611–1627 CrossRef CAS.
- L. Singh, A. R. Pavankumar, R. Lakshmanan and G. K. Rajara, Ecol. Eng., 2012, 38(1), 119–124 CrossRef.
- A. Tiwari and P. Kathane, Int. Res. J. Environ. Sci., 2013, 2(7), 44–53 Search PubMed.
- A. Ahmad, A. H. Bhat and A. Buang, J. Cleaner Prod., 2017, 171, 1361–1375 CrossRef.
- N. A. Negm and H. E. Ali, Eng. Life Sci., 2010, 10, 218–224 CrossRef CAS.
- D. X. Ding, X. Tan, N. Hu and G. Y. Li, Bioprocess Biosyst. Eng., 2012, 35, 1567–1576 CrossRef CAS.
- H. B. Alhaj, S. H. Al-Mabrouk and M. M. El-ajaily, Global Sci. J., 2019, 7(2), 75–80 Search PubMed.
- A. Kapoor, T. Viraraghavan and D. R. Cullimore, Bioresour. Technol., 1999, 70, 95–104 CrossRef CAS.
- C. M. Park, J. H. Han, K. H. Chu, Y. A. J. Al-Hamadani, N. Her, J. Y. Heo and Y. M. Yoon, J. Ind. Eng. Chem., 2017, 48, 186–193 CrossRef CAS.
- R. Leyva-Ramos, J. R. Rangel-Mendez, J. Mendoza-Barron, L. Fuentes-Rubio and R. M. Guerrero-Coronado, Water Sci. Technol., 1997, 35, 205–211 CrossRef CAS.
- O. Abdi and M. Kazemi, J. Mater. Environ. Sci., 2015, 6(5), 1386–1399 Search PubMed.
- M. Bilal, T. Rasheed, J. Sosa-Hernandez, A. Raza, F. Nabeel and H. Iqbal, Mar. Drugs, 2018, 16(2), 1–16 CrossRef.
- H. Alyasi, H. R. Mackey and G. McKay, Energy Environ., 2020, 31(3), 517–534 CrossRef CAS.
- A. P. A. Salvado, L. B. Campanholi, J. M. Fonseca, C. R. T. Tarley, J. Caetano and D. C. Dragunski, Desalin. Water Treat., 2012, 48, 335–343 CrossRef CAS.
- J. M. Silva, B. S. Farias, D. D. R. Grundmann, T. R. S. Cadaval Jr, J. M. Moura, G. L. Dotto and L. A. A. Pinto, J. Appl. Polym. Sci., 2017, 134(11), 44580 CrossRef.
- J. Chang, R. Law and C. Chang, Water Res., 1997, 31, 1651–1658 CrossRef CAS.
- C. V. R. Murthy, K. K. Kumar, K. Jayaraju and S. Silas, Asian J. Chem., 2007, 19, 3502–3510 CAS.
- A. Sari and M. Tuzen, J. Hazard. Mater., 2008, 152, 302–308 CrossRef CAS.
- D. O. Jalija and A. Uzairu, Int. Res. J. Pure Appl. Chem., 2020, 21(2), 1–9 Search PubMed.
- M. Alameen and N. Majeed, J. Eng., 2020, 26(1), 24–34 Search PubMed.
- C. Ad, M. Djedid, M. Benalia, N. N. Saeed, H. Steli, S. Zaoui and H. Elmsellem, Environ. Eng. Sci., 2018, 2(1), 1–8 Search PubMed.
- G. Yan and T. Viraraghavan, Water Res., 2003, 37, 4486–4496 CrossRef CAS.
- Renu, M. Agarwal and K. Singh, J. Water Reuse Desalin., 2017, 7(4), 387–419 CrossRef.
- S. Kocaobaa and M. Arısoy, Sep. Sci. Technol., 2018, 53(11), 1703–1710 CrossRef.
- Y.-M. Sun, C.-Y. Horng, F.-L. Chang, L.-C. Cheng and W.-X. Tian, Pol. J. Microbiol., 2010, 59(1), 37–44 CAS.
- S. Ilhan, M. N. Nourbakhsh, S. Kilicarslan and H. Ozdag, Pol. J. Microbiol., 2004, 2, 50–57 Search PubMed.
- S. Mustapha, D. T. Shuaib, M. M. Ndamitso, M. B. Etsuyankpa, A. Sumaila, U. M. Mohammed and M. B. Nasirudeen, Appl. Water Sci., 2019, 9, 1–11 CrossRef CAS.
- C. E. R. Barquilha, E. S. Cossich, C. R. G. Tavares and E. A. Silva, J. Cleaner Prod., 2017, 150, 58–64 CrossRef CAS.
- Z. Velkova, G. Kirova, M. Stoytcheva, S. Kostadinova, K. Todorova and V. Gochev, Eng. Life Sci., 2018, 18, 871–881 CrossRef CAS.
- P. K. Wong, K. C. Lam and C. M. So, Appl. Microbiol. Biotechnol., 1993, 39, 127–131 CrossRef CAS.
- T. S. Prakasham, J. S. Merrie, R. Sheela, N. Saswathi and S. V. Ramakrishna, Environ. Pollut., 1999, 104, 421–427 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |