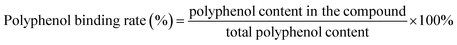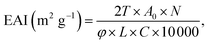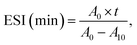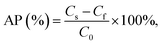Open Access Article
Open Access ArticleImprovement of protein emulsion stability through glycosylated black bean protein covalent interaction with (−)-epigallocatechin-3-gallate†
Jubing Wanga,
Huanyu Zhengbcd,
Shenyi Zhanga,
Jishu Lia,
Xiuqing Zhue,
Hua Jin*a and
Jing Xu *a
*a
aCollege of Art and Science, Northeast Agricultural University, 150030 Harbin, Heilongjiang, PR China. E-mail: 15663431982@163.com; zsylq123@163.com; ljswy123@163.com; jinhua@neau.edu.cn; xujing@neau.edu.cn
bCollege of Food Science, Northeast Agricultural University, Harbin 150030, Heilongjiang, China. E-mail: zhenghuanyu1@163.com
cHeilongjiang Green Food Science Research Institute, Harbin 150028, Heilongjiang, China
dNational Research Center of Soybean Engineering and Technology, Harbin 150028, Heilongjiang, China
eKey Laboratory of Grain Food and Comprehensive Processing of Grain Resource of Heilongjiang Province, College of Food Engineering, Harbin University of Commerce, Harbin 150076, China. E-mail: xqzhuwang@163.com
First published on 12th January 2021
Abstract
This study investigated the effects of covalent conjugates combined by glycosylated black bean protein isolate (BBPI-G) and (−)-epigallocatechin-3-gallate (EGCG) on the emulsion stability. Fourier transform infrared (FTIR) spectroscopy showed that covalent binding of EGCG with BBPI-G made the protein molecule unfolded. Besides, the emulsifying properties of BBPI-G were increased after combined with EGCG. BBPI-G–EGCG emulsion had lower mean particle size and higher content of interfacial protein adsorption (AP), which resulted in thicker and more impact oil–water interface. Therefore, the stability of emulsions was significantly improved. Furthermore, the emulsions prepared by BBPI-G–EGCG compounds exhibited considerable stability in storage, oxidation, thermal treatments, freeze–thaw and freeze-dried powders resolubility. This study demonstrated that the covalent bond of glycosylated protein and polyphenols could advance the emulsifying performance of protein, and BBPI-G–EGCG covalent complex was an effective emulsifier for preparing high stability emulsions.
1. Introduction
The content of black bean protein isolate (BBPI) is remarkable in the total weight of black bean, which is higher than for soybean and even milk.1 BBPI is rich in essential amino acids (AA), and the balance of AA is commendable.2 Moreover, it has been investigated that the solubility, emulsifying activity and emulsion stability of BBPI are excellent because of high amounts of hydrophobic amino acids.3 Thus, BBPI has great potential to stable emulsions in food industry. The oil–water emulsion is kind of delivery system to encapsulate unstable hydrophobic biological active substance (curcumin, α-tocopherol, etc.), which is able to improve stability of ingredients by reducing the exposure of hydrophobic biological active substance to external factors, such as oxygen and light.4 In the process of emulsion preparation, the emulsifier can form an interface layer to separate the oil–water phase. The densification, thickness, stability and anti-oxidation of the emulsifier layer will have an important influence on the stability of the emulsion and the encapsulated substance. However, protein is willing to aggregate itself and is susceptible to external environment brought by processing conditions in food industry. Hence, it is needed to improve the functional properties of protein via appropriate modification methods.4The interaction of protein and polyphenols is able to change the structure of protein, improve its functional characteristics, and increase the potential value of protein and polyphenols in the field of food industry. Two combination methods between protein and polyphenol are non-covalent interaction and covalent interaction. Li et al.5 reported that non-covalent complexes of (+)-catechin and rice bran protein had lower contents of α-helix and β-sheet in secondary structure, and formed more stable emulsions with smaller droplet size and greater emulsifying property at additive amount of 0.15% (w/v). Su et al.6 studied that the non-covalent interaction of β-lactoglobulin nanoparticles to (−)-epigallocatechin-3-gallate (EGCG) could increase antioxidant property of protein. Similarly, Loc et al.7 found that the thermal stability and antioxidative capacity of covalent adducts of polyphenol and flaxseed protein isolate were increased. Furthermore, Zhou et al.8 reported that non-covalent interaction and covalent interaction could both change the secondary structure and properties of soybean protein isolate (SPI), while SPI–EGCG covalent complex held more stable structure, better thermal stability and oxidation resistance than non-covalent complexes.
After glycosylation, the solubility, thermal stability, emulsifying property, foaming property and antioxidant activity of protein can be significantly improved.9–14 Zha et al.10 reported that pea protein isolate–gum arabic (PPI–GA) complex formed by glycosylating reaction had higher solubility. Laura et al.11 found that glycosylation of whey protein significantly improved the solubility and thermal stability. In recent years, the research of proteins, carbohydrates and polyphenols have all became hot topics. Perusko et al.15 investigated the binding affinity of glycosylated β-lactoglobulin with EGCG through non-covalent interaction and the antioxidant properties of β-lactoglobulin–EGCG non-covalent complex. Results showed that the binding affinity between glycosylated β-lactoglobulin and EGCG was similar to that between β-lactoglobulin and EGCG. However, antioxidant capacity of glycosylated β-lactoglobulin–EGCG non-covalent complex was higher, revealing that the combination of carbohydrate and polyphenols significantly increased the antioxidant properties of β-lactoglobulin. Meanwhile, the combination of carbohydrate and polyphenols showed synergistic and superimposed effects. Liu et al.16 researched the application of lactoferrin (LF), glucan and chlorogenic acid (CA) ternary complex through covalent interaction as emulsifier in emulsion. The results indicated that preparative ternary complex showed greater emulsification property and antioxidant activity than LF or LF–CA binary complex. The reason was that LF maintained surface activity of emulsion system as emulsifier, and the combination of carbohydrate and polyphenols enhanced spatial repulsive force and antioxidant capacity of emulsion system, respectively.
Many studies have shown that proteins, carbohydrates and polyphenols can coexist by interacting with each other in different ways, and the formation of ternary complexes can further advance protein functional properties.17 Nevertheless, to the best of our knowledge, the effects of covalent interaction between glycosylated proteins and polyphenols on the stability of emulsion has been rarely reported, and little information is available on the relevance between the changes of protein structure and emulsion stability. Therefore, a better understanding of the interaction between glycosylated proteins–polyphenol emulsifiers and emulsion properties would help to optimize protein application in food products during processing and storage. BBPI was chosen as the research subject in this study. The effects of covalent interaction between glycosylated BBPI (BBPI-G) and EGCG on the structure and properties of protein complexes were studied on the first aspect, comparing with the native BBPI as control. The emulsions were then prepared by BBPI, BBPI-G, BBPI and EGCG covalent complex (BBPI–EGCG), BBPI-G and EGCG covalent complex (BBPI-G–EGCG). The effects of glycosylation modification and polyphenols modification on the basic and stability properties of emulsions were explored. The relationship between protein structure and emulsion stability was discussed.
2. Materials and methods
2.1. Materials
BBPI were purchased from Hei Long Jiang Agriculture Company Limited (Hei Long Jiang, China). Glucose was bought from Baolingbao Biology Company (Shandong, China). (−)-Epigallocatechin-3-gallate (EGCG) was received from Yuanye Biotechnology Co. Ltd (Shanghai, China). Soya oil of gold arowana was obtained from Yihai Kerry Foodstuffs Marketing Company (Jiangsu, China). All other chemical medicines were of analytical grade.2.2. Preparation of Maillard reaction compounds
A ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (BBPI
1 (BBPI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G, w/w) of BBPI and glucose was dissolved in phosphate buffer (0.1 M, pH 7.0). Wet-Maillard reaction was executed (80 °C, 4 h) and then freeze-dried. The graft degree (DG) of 18.83% was determined by our previous study under this condition.18
G, w/w) of BBPI and glucose was dissolved in phosphate buffer (0.1 M, pH 7.0). Wet-Maillard reaction was executed (80 °C, 4 h) and then freeze-dried. The graft degree (DG) of 18.83% was determined by our previous study under this condition.18
2.3. Preparation of phenolic compounds
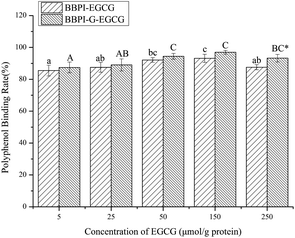
Freeze-dried powder of BBPI and BBPI-G was dissolved in phosphate buffer (0.01 M, pH 9.0). The protein solution (4 mg mL−1) was mixed with EGCG and the ultimate concentration of EGCG were (5, 25, 50, 150, 250 μmol g−1 protein), respectively. The mixture was incubated (25 °C, 12 h) for obtaining BBPI–EGCG and BBPI-G–EGCG covalent compounds.19 Then, the solution pH was regulated to 7.0. The reactive complex solutions were placed in 3500 kDa dialysis bag and dialysed in phosphate buffer (0.01 M, pH 7.0) for 48 h for removing the free polyphenols. Finally, above solutions were freeze-dried.2.4. Measurement of polyphenol binding rate
With reference to the method of Slinkard et al.,20 the Folin–Ciocalteu method was used to determine the polyphenol binding rate of protein–polyphenol complex samples. 2 mL Folin–Ciocalteu reagent (0.2 mol L−1) was added to 0.5 mL protein–polyphenol complex solution at a certain concentration, and the reaction was incubated at 25 °C for 5 min (away from light). Then, 2 mL of Na2CO3 solution (7.5%, w/v) was added to the mixture. The solution was thoroughly mixed with a vortex mixer, and reacted at 25 °C for 2 h (dark). The absorbance of the solution was determined at 760 nm. The standard solution of EGCG with different concentrations was prepared to draw the standard curve, and the polyphenol contents in the complex were calculated according to the standard curve. The polyphenol binding rate was calculated through following formula:2.5. Fluorescence spectroscopy
The measurement of fluorescence spectroscopy was slightly modified according to Wang et al.21 Sample solutions were diluted to 0.02 mg mL−1 with the phosphate buffer (0.01 M, pH 7.0). Then, the emission spectrum (slit width 5) from 300 to 550 nm was scanned with excitation wavelength 290 nm using the fluorescence spectrometer of F-4500 (Hitachi, Japan).2.6. Fourier transform infrared (FTIR) spectroscopy
FTIR of BBPI, BBPI–EGCG, BBPI-G, BBPI-G–EGCG were acquired with Bruker Vertex 70 FTIR spectrometer (Bruker Optics, Ettlingen, Germany). The wavenumber was ranging between 4000–400 cm−1. Spectroscopy data were the average results of 64 scans with a resolution of 4 cm−1. Select the range of 1600–1700 cm−1 on FTIR spectroscopy. Then, the data was fit to receive 8 groups of area under homologous wavenumbers by “Gaussian peak fitting” algorithm to obtain the percentage contents of four secondary structure by the software “Peakfit Version 4.12”.222.7. Emulsifying properties
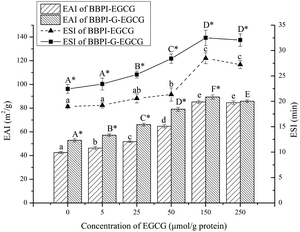
The emulsifying activity index (EAI) and emulsifying stability index (ESI) were detected by the method of Jin et al.23 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio of sample solution (1 mg mL−1) and soybean oil was taken, and homogenized the mixture (10
1 (v/v) ratio of sample solution (1 mg mL−1) and soybean oil was taken, and homogenized the mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 2 min). 40 μL liquid were at once absorbed from the emulsion bottom at 0 min and 10 min, which were then added into 5 mL of SDS solution (0.1%, w/v). Suspensions after shock blending were detected at 500 nm. Computational formula for calculating EAI and ESI were as follows:
000 rpm, 2 min). 40 μL liquid were at once absorbed from the emulsion bottom at 0 min and 10 min, which were then added into 5 mL of SDS solution (0.1%, w/v). Suspensions after shock blending were detected at 500 nm. Computational formula for calculating EAI and ESI were as follows:where T was 2.303. A0 and A10 were the absorbance of the emulsion at 0 and 10 min, respectively. N was dilution factor (125). φ was the oil volume fraction (0.25). L was path length of cuvette (1 cm). C was protein concentration (g mL−1), t was sampling interval (10 min).
2.8. Preparation of emulsions
400 mg sample powder was accurately weighed and dissolved adequately in 20 mL phosphate buffer (0.02 M, pH 7.0). Soybean oil was slowly added to the protein solution with a ratio of 3% (v/v), stirred magnetically at 25 °C for 10 min, and sodium azide (0.004%, w/v) was added for preventing microbial growth. The coarse emulsion was prepared by homogeneous dispersion machine at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 4 min. The coarse emulsion was immediately dealed with an ultrasonic processor (titanium probe with a diameter of 0.636 cm) at 500 W ultrasonic power for 20 min to form emulsion. During the whole preparation process, the temperature was kept at about 25 °C by using ice water bath.24
000 rpm for 4 min. The coarse emulsion was immediately dealed with an ultrasonic processor (titanium probe with a diameter of 0.636 cm) at 500 W ultrasonic power for 20 min to form emulsion. During the whole preparation process, the temperature was kept at about 25 °C by using ice water bath.24
2.9. Mean particle size, zeta-potential measurements
Phosphate buffer (0.01 M, pH 7.0) was used to dilute (100 times) the emulsions for ensuring uniformity and avoiding the effects of multiple scattering. Dynamic light scattering instrument (Malvern Nano-S90, Malvern Instruments, Worcestershire, UK) was used to detect the mean particle size and polydispersity index (PDI). Particle microelectrophoresis (Zetasizer Nano Z, Malvern Instruments, Worcestershire, UK) was selected to measure the zeta-potential.252.10. The percentage of interfacial protein adsorption (AP) measurements
According to Chen et al.,26 emulsion AP was determined with tiny variation. The emulsion was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 60 min). Acquired aqueous phase from the bottom was sucked with disposable syringe and filtered it to pass 0.45 μL filter membrane. The protein concentration was determined by Lowry method.27 The protein dispersion was centrifuged under the same conditions. The computational formula of AP was as follows:
000 rpm, 60 min). Acquired aqueous phase from the bottom was sucked with disposable syringe and filtered it to pass 0.45 μL filter membrane. The protein concentration was determined by Lowry method.27 The protein dispersion was centrifuged under the same conditions. The computational formula of AP was as follows:where Cf was the protein concentration of filtered subnatant from emulsion, Cs was the protein concentration of centrifugal supernatant from protein dispersion, C0 was the protein concentration of initial protein dispersion.
2.11. Storage stability of emulsions
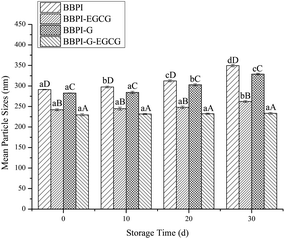
Different emulsion samples were stored at 4 °C for 30 days, and the storage stability of emulsions was analyzed by measuring the change of mean particle size of emulsions at 0, 10, 20 and 30 days.92.12. Oxidation stability of emulsions
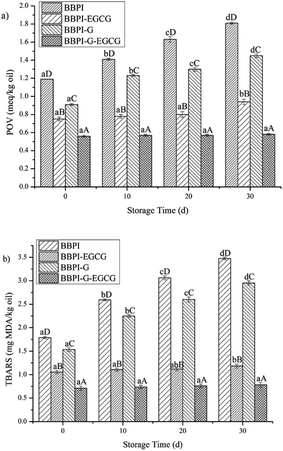
Different emulsion samples were stored at 4 °C for 30 days, and peroxide value (POV) and thiobarbituric acid reactive substances (TBARS) values of the emulsion were measured every 10 days. The oxidation stability of the emulsion was analyzed by the changes of POV and TBARS values.POV was determined according to the AOCS standard procedure with a sodium thiosulphate (Na2S2O3) titration.29 0.2 mL emulsion samples were fully mixed by vortex for 1 min with 1.5 mL mixed solution of isopropanol and isooctane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The mixtures were centrifuged (4000 rpm, 30 min). Later, mix 0.2 mL supernatant and 2.8 mL the mixture of methanol and n-butyl alcohol (2
3, v/v). The mixtures were centrifuged (4000 rpm, 30 min). Later, mix 0.2 mL supernatant and 2.8 mL the mixture of methanol and n-butyl alcohol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Then, 15 μL ammonium thiocyanate solution (3.94 M) and 15 μL bivalent iron ion solution were added, which was prepared by 1 mL of barium chloride solution (0.13 M) and 1 mL of ferrous sulfate solution (0.14 M). The mixture was centrifuged for 3 min at 3000 rpm and left for 20 minute reaction at room temperature away from light. The absorbance of the solution was determined at 510 nm. POV values of emulsion samples were calculated by using standard curve of hydrogen peroxide isopropyl benzene.
1, v/v). Then, 15 μL ammonium thiocyanate solution (3.94 M) and 15 μL bivalent iron ion solution were added, which was prepared by 1 mL of barium chloride solution (0.13 M) and 1 mL of ferrous sulfate solution (0.14 M). The mixture was centrifuged for 3 min at 3000 rpm and left for 20 minute reaction at room temperature away from light. The absorbance of the solution was determined at 510 nm. POV values of emulsion samples were calculated by using standard curve of hydrogen peroxide isopropyl benzene.
According to the method described by Mei et al.,30 the formation of TBARS was measured. TBARS value is expressed as malondialdehyde content. 2 mL mixed solution of TCA–TBA–HCl solution (0.92 M TCA, 0.02 M TBA and 1.8% (v/v) HCl) and BHT–ethanol solution (2%, v/v) (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) was added into 2 mL emulsion sample. Mixture was heated in boiling water for 15 min. After cooling to 25 °C, the supernatant was centrifuged (4000 rpm, 10 min). The absorbance of the supernatant was measured at 532 nm. The TBARS values of emulsion samples were calculated by using the standard curve of malondialdehyde.
3, v/v) was added into 2 mL emulsion sample. Mixture was heated in boiling water for 15 min. After cooling to 25 °C, the supernatant was centrifuged (4000 rpm, 10 min). The absorbance of the supernatant was measured at 532 nm. The TBARS values of emulsion samples were calculated by using the standard curve of malondialdehyde.
2.13. Thermal stability of emulsions
Emulsion samples were disposed in the boiling water for 10 min or 30 min, and then cooled to 25 °C. The changes of mean particle size were investigated to analyze thermal stability of emulsions.242.14. Freeze–thaw stability of emulsions
Different emulsion samples were frozen in a refrigerator (−20 °C, 20 h), and then thawed for 2 h to analyze the freeze–thaw stability of emulsions by measuring the changes of mean particle size of emulsion.312.15. Resolubility of freeze-dried emulsion powders
The emulsion samples were freeze-dried into powder and then dissolved back to the original volume with phosphate buffer (0.01 M, pH 7.0). The resolubility of the powder was analyzed by measuring the changes of mean particle size of the emulsions.322.16. Statistical analysis
All the tests were measured in triplicate. The results were showed as shape of mean ± standard deviation. ANOVA significance analysis (p < 0.05) and independent sample T test (T) (p < 0.05) were proceeded with statistical software (SPSS V20.0) to display significant differences.3. Results and discussion
3.1. Structure and functional properties
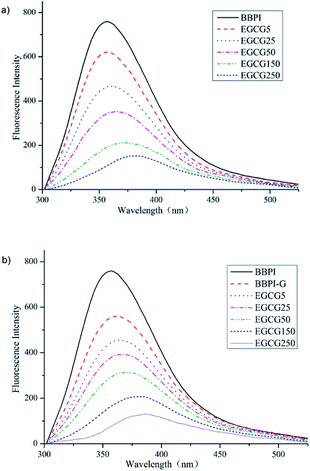 | ||
| Fig. 2 Fluorescence spectroscopy curve of BBPI–EGCG (a) and BBPI-G–EGCG (b) with EGCG amount of 5, 25, 50, 150 and 250 μmol g−1 protein. | ||
Compared Fig. 2a with Fig. 2b, it was not difficult to discover that declining degree of intensity and red-shift of BBPI-G–EGCG surpassed BBPI–EGCG. On one side, the lower fluorescence intensity was attributed to the fact that glycosylation was accompanied with the shielding effect and more hydrophobic residues were buried by the polysaccharide molecules.18 Meanwhile, the covalent binding of polyphenols led to the quenching of fluorescence intensity of glycosylated BBPI, which might be related to Forster-type fluorescence resonance energy transfer.17 On the other side, the λmax values of BBPI-G–EGCG in Fig. 2b were larger than those in Fig. 2a, showing that BBPI-G–EGCG red-shifted more obviously compared with BBPI–EGCG, which meant that BBPI-G–EGCG might have more incompact structure than BBPI–EGCG.28
| α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random coil (%) | |
|---|---|---|---|---|
| a a–e represents significant difference in the same protein with different EGCG concentrations (p < 0.05). * means significant difference between BBPI and BBPI-G (p < 0.05). | ||||
| BBPI | 16.84 ± 0.12e* | 38.29 ± 0.08d* | 28.74 ± 0.10a | 16.13 ± 0.04a* |
| BBPI–EGCG5 | 16.37 ± 0.11d* | 38.10 ± 0.08c* | 28.96 ± 0.09b | 16.57 ± 0.01b* |
| BBPI–EGCG25 | 16.03 ± 0.15c* | 38.07 ± 0.07c* | 29.09 ± 0.13b | 16.81 ± 0.07c* |
| BBPI–EGCG50 | 15.32 ± 0.16b* | 37.30 ± 0.14b* | 30.30 ± 0.06c | 17.08 ± 0.18d* |
| BBPI–EGCG150 | 14.65 ± 0.07a | 36.94 ± 0.10a* | 30.52 ± 0.09d | 17.89 ± 0.24e* |
| BBPI–EGCG250 | 14.53 ± 0.08a | 36.79 ± 0.08a* | 30.64 ± 0.10d | 18.04 ± 0.06e* |
| BBPI-G | 15.94 ± 0.08d | 35.99 ± 0.06d | 33.77 ± 0.08a* | 14.30 ± 0.10a |
| BBPI-G–EGCG5 | 15.79 ± 0.07d | 35.84 ± 0.11c | 33.84 ± 0.10a* | 14.53 ± 0.06a |
| BBPI-G–EGCG25 | 15.16 ± 0.11c | 35.66 ± 0.06b | 33.93 ± 0.07a* | 15.25 ± 0.10b |
| BBPI-G–EGCG50 | 14.93 ± 0.14b | 35.60 ± 0.07b | 34.19 ± 0.07b* | 15.28 ± 0.10bc |
| BBPI-G–EGCG150 | 14.63 ± 0.10a | 35.41 ± 0.09a | 34.44 ± 0.11c* | 15.52 ± 0.19cd |
| BBPI-G–EGCG250 | 14.48 ± 0.13a | 35.29 ± 0.05a | 34.55 ± 0.14c* | 15.68 ± 0.27d |
3.2. Emulsion properties
| Mean particle size (nm) | PDI | Zeta-potential (mV) | AP (%) | |
|---|---|---|---|---|
| a a–d represents significant difference in the same column (p < 0.05). | ||||
| BBPI | 291.30 ± 0.56d | 0.220 ± 0.007b | −24.47 ± 0.23b | 9.52 ± 0.29a |
| BBPI–EGCG | 242.17 ± 2.62b | 0.186 ± 0.011a | −30.90 ± 0.26d | 18.11 ± 0.57c |
| BBPI-G | 282.73 ± 0.35c | 0.187 ± 0.009a | −23.20 ± 0.17a | 10.53 ± 0.38b |
| BBPI-G–EGCG | 229.67 ± 2.87a | 0.172 ± 0.008a | −29.47 ± 0.61c | 20.61 ± 0.76d |
PDI value can reflect the uniformity of emulsion droplet size, and in general, small PDI value usually means narrow droplet size distribution.40 As shown in Table 2, PDI values of BBPI, BBPI–EGCG, BBPI-G, BBPI-G–EGCG emulsions were 0.220 ± 0.007b, 0.186 ± 0.011a, 0.187 ± 0.009a, 0.172 ± 0.008a, respectively. PDI values of four emulsions were all below 0.220, which indicated that uniform emulsions with narrow droplet size distribution were received. Besides, PDI values of BBPI–EGCG, BBPI-G, BBPI-G–EGCG emulsions were significantly smaller than BBPI emulsion (p < 0.05), explaining that glycosylation and polyphenol binding could not only reduce the mean particle size of emulsion, but also improve the droplet distribution to achieve the more uniform emulsion with better stability.41
For the increase of AP in BBPI-G group, Maillard cross-linking promoted molecular weight, making the thicker film. In addition, hydrophilic group of sugar offered a better hydrophilic and hydrophobic equilibrium, leading to a lifting adsorption amount on oil–water membrane.10,44 AP of BBPI–EGCG and BBPI-G–EGCG emulsions was superior to BBPI and BBPI-G emulsions, indicating that glycosylation modification had synergistic effect with EGCG covalent binding on the amount of protein adsorption on emulsion interface.
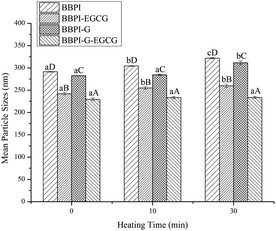
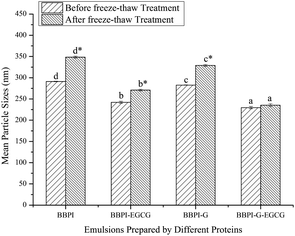
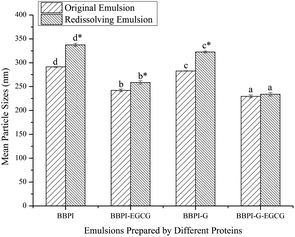
3.3. Emulsion stability measurements
4. Conclusions
In summary, this study provided the understanding of the behaviors for the potential applications of BBPI as emulsifiers in emulsions. FTIR spectroscopy showed that the structure of protein was unfolded and disordered with the treatment of glycosylated and polyphenols covalent binding. The effects of the glycosylation and polyphenols modification on the stability of emulsions were investigated. The results showed that emulsions with considerable stability in storage, oxidation, thermal treatments, freeze–thaw and freeze-dried powders resolubility were fabricated. Small mean particle size of emulsion prepared by BBPI-G–EGCG was one reason for high emulsion stability. Besides, the AP and TEM measurements found that the BBPI-G–EGCG covalent complexes had a preferably thick, continuous, compact oil–water interface in emulsions, which was another factor to enhance the stability of emulsion systems. The results of this study provided new understanding with respect to using the modified black bean proteins as natural emulsifiers.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China [No. 31901605], the Key Program of Natural Science Foundation of Heilongjiang Province [ZD2019C005] and Academic Backbone Project of Northeast Agricultural University [19XG27].References
- Y. Zhang, Y. Yin and S. Lu, Molecules, 2018, 23(9), 2127, DOI:10.3390/molecules23092127.
- L. Jiang, J. Wang and Y. Li, et al., Food Res. Int., 2014, 62, 595–601, DOI:10.1016/j.foodres.2014.04.022.
- B. Xu and S. K. C. Chang, J. Agric. Food Chem., 2008, 56(18), 8365–8373, DOI:10.1021/jf801196d.
- H. Jin, C. Liu and S. Zhang, et al., Food Funct., 2020, 11, 10205, 10.1039/D0FO01830A.
- D. Li, Y. Zhao and X. Wang, et al., Food Hydrocolloids, 2020, 98, 105306, DOI:10.1016/j.foodhyd.2019.105306.
- J. Su, Q. Guo and Y. Chen, et al., Food Hydrocolloids, 2020, 98, 105293, DOI:10.1016/j.foodhyd.2019.105293.
- B. P. Loc, B. Wang and B. Zisu, et al., Food Chem., 2019, 293, 463–471, DOI:10.1016/j.foodchem.2019.04.123.
- S.-D. Zhou, Y.-F. Lin and X. Xu, Food Chem., 2020, 309, 125718, DOI:10.1016/j.foodchem.2019.125718.
- J. Xu, D. Mukherjee and S. K. C. Chang, Food Chem., 2018, 240, 1005–1013, DOI:10.1016/j.foodchem.2017.07.077.
- F. Zha, S. Dong and J. Rao, et al., Food Chem., 2019, 285, 130–138, DOI:10.1016/j.foodchem.2019.01.151.
- J.-C. Laura, M. Villamiel and L.-F. Rosina, Food Hydrocolloids, 2007, 21(3), 433–443, DOI:10.1016/j.foodhyd.2006.05.006.
- L. Liu, J. Zheng and B. Sun, et al., Molecules, 2020, 25, 875, DOI:10.3390/molecules25040875.
- J. Xue, C. Tan and X. Zhang, et al., J. Agric. Food Chem., 2014, 62(20), 4677–4684, DOI:10.1021/jf405157x.
- Z. Yu, L. Shuang and J. Zhang, et al., LWT--Food Sci. Technol., 2020, 125, 109213, DOI:10.1016/j.lwt.2020.109213.
- M. Perusko, A. Al-Hanish and J. Mihailovic, et al., Food Chem., 2017, 232, 744–752, DOI:10.1016/j.foodchem.2017.04.074.
- F. Liu, C. Sun and W. Yang, et al., RSC Adv., 2015, 5(20), 15641–15651, 10.1039/c4ra10802g.
- W. Chen, R. Lv and A. Muhammand, et al., Ultrason. Sonochem., 2019, 58, 104678, DOI:10.1016/j.ultsonch.2019.104678.
- J. Xu, D. Han and Z. Chen, et al., Eur. Food Res. Technol., 2018, 244(6), 1111–1120, DOI:10.1007/s00217-018-3032-5.
- Z. Wei and Q. Huang, Food Hydrocolloids, 2020, 98, 105314, DOI:10.1016/j.foodhyd.2019.105314.
- K. Slinkard and V. L. Singleton, Am. J. Enol. Vitic., 1977, 28(1), 49–55, DOI:10.1002/star.19780301107.
- Y. Wang, Z. Wang and C. L. Handa, et al., Food Chem., 2017, 218, 165–172, DOI:10.1016/j.foodchem.2016.09.069.
- M. Carbonaro, P. Maselli and A. Nucara, Amino Acids, 2012, 43, 911–921, DOI:10.1007/s00726-011-1151-4.
- H. Jin, Q. Zhao and H. Feng, et al., Polymers, 2019, 11(5), 848, DOI:10.3390/polym11050848.
- H. Jin, X. Wang and Z. Chen, et al., Food Res. Int., 2018, 106, 800–808, DOI:10.1016/j.foodres.2018.01.056.
- Y. Li, H. Jin and X. Sun, et al., Nanomaterials, 2018, 9(1), 25, DOI:10.3390/nano9010025.
- M. Chen, J. Lu and F. Liu, et al., Food Hydrocolloids, 2019, 88, 247–255, DOI:10.1016/j.foodhyd.2018.09.003.
- O. H. Lowry, N. J. Rosebrough and A. L. Farr, et al., J. Biol. Chem., 1951, 193(1), 265–275, DOI:10.1515/bchm2.1951.286.1-6.270.
- H. Feng, H. Jin and Y. Gao, et al., Polymers, 2019, 11(10), 1688, DOI:10.3390/polym11101688.
- Y. Yang, S. Cui and J. Gong, et al., Food Res. Int., 2015, 69, 357–363, DOI:10.1016/j.foodres.2015.01.006.
- L. Y. Mei, D. J. McClements and J. N. Wu, et al., Food Chem., 1998, 61, 307–312, DOI:10.1016/S0308-8146(97)00058-7.
- H. Feng, H. Jin and Y. Gao, et al., Food Chem., 2020, 330, 127215, DOI:10.1016/j.foodchem.2020.127215.
- F. Liu, C. Ma and D. J. Mcclements, et al., Food Hydrocolloids, 2016, 61, 578–588, DOI:10.1016/j.foodhyd.2016.05.031.
- X. Sui, H. Sun and B. Qi, et al., Food Chem., 2018, 245, 871–878, DOI:10.1016/j.foodchem.2017.11.090.
- M. Ali, T. Homann and M. Khalil, et al., J. Agric. Food Chem., 2013, 61(28), 6911–6920, DOI:10.1021/jf402221m.
- V. Bongartz, L. Brandt and M. L. Gehrmann, et al., Molecules, 2016, 21(1), 91, DOI:10.3390/molecules21010091.
- Z. Wei, W. Yang and R. Fan, et al., Food Hydrocolloids, 2015, 45, 337–350, DOI:10.1016/j.foodhyd.2014.12.008.
- J. Wagner and J. Gueguen, J. Agric. Food Chem., 1999, 47(6), 2181–2187, DOI:10.1021/jf980977b.
- Y. Li, H. Liu and Q. Liu, et al., Food Hydrocolloids, 2019, 87, 149–157, DOI:10.1016/j.foodhyd.2018.07.052.
- C. Liu, Z. Wang and H. Jin, et al., Int. J. Biol. Macromol., 2019, 142, 658–667, DOI:10.1016/j.ijbiomac.2019.10.007.
- C. Sarah and D.-P. Gabriel, Food Chem., 2020, 338, 128083, DOI:10.1016/j.foodchem.2020.128083.
- J. Jiang and Y. L. Xiong, Meat Sci., 2015, 109, 56–65, DOI:10.1016/j.meatsci.2015.05.011.
- J. Chen, Y. Feng and B. Kong, et al., Colloids Surf., A, 2020, 604, 125332, DOI:10.1016/j.colsurfa.2020.125332.
- L. Atares, L. J. Marshall and M. Akhtar, et al., Food Chem., 2012, 134(3), 1418–1424, DOI:10.1016/j.foodchem.2012.02.221.
- J. Chen, Y. Feng and B. Kong, et al., Food Hydrocolloids, 2015, 51, 95–100, DOI:10.1016/j.foodhyd.2015.04.034.
- F. Liu, D. Wang and H. Xu, et al., Food Chem., 2016, 196, 338–346, DOI:10.1016/j.foodchem.2015.09.047.
- Y. H. Wang, Z. L. Wan and X. Q. Yang, et al., Food Hydrocolloids, 2016, 54, 40–48, DOI:10.1016/j.foodhyd.2015.09.020.
- L. Wang and Y. Xiong, J. Agric. Food Chem., 2005, 53(23), 9186–9192, DOI:10.1021/jf051213g.
- C. Liu, H. Jin and Y. Yu, et al., Nanomaterials, 2020, 10(6), 1094, DOI:10.3390/nano10061094.
- S. Dong, A. Panya and M. Zeng, et al., Food Res. Int., 2013, 51(2), 992, DOI:10.1016/j.foodres.2013.03.033.
- M. Verni, V. Verardo and C. G. Rizzello, Foods, 2019, 8(9), 362, DOI:10.3390/foods8090362.
- D. K. Sarker, P. J. Wilde and D. C. Clark, J. Agric. Food Chem., 1995, 43, 295–300, DOI:10.1021/jf00050a006.
- Z. Zhang, X. Wang and J. Yu, et al., LWT--Food Sci. Technol., 2017, 78, 241–249, DOI:10.1016/j.lwt.2016.12.051.
- Y. Chen, C. Wang and H. Liu, et al., Colloids Surf., A, 2018, 558, 330–337, DOI:10.1016/j.colsurfa.2018.08.067.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08756d |
| This journal is © The Royal Society of Chemistry 2021 |