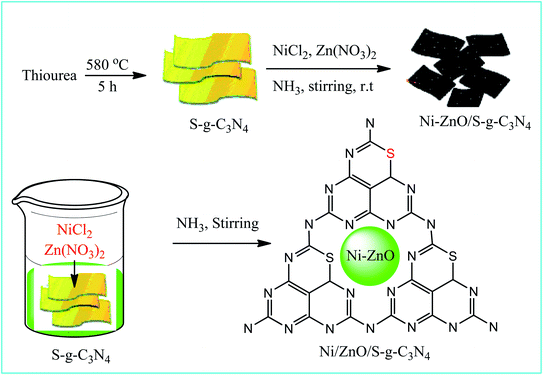
Open Access Article
Open Access ArticleDesigning a novel visible-light-driven heterostructure Ni–ZnO/S-g-C3N4 photocatalyst for coloured pollutant degradation
Ali Bahadur†
 a,
Shahid Iqbal†
a,
Shahid Iqbal† *b,
Hashem O. Alsaabc,
Nasser S. Awwadd and
Hala A. Ibrahiumde
*b,
Hashem O. Alsaabc,
Nasser S. Awwadd and
Hala A. Ibrahiumde
aDepartment of Transdisciplinary Studies, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, 08826, South Korea
bDepartment of Chemistry, School of Natural Sciences (SNS), National University of Science and Technology (NUST), H-12, Islamabad, 46000, Pakistan. E-mail: shahidiqbal.chem@sns.nust.edu.pk
cDepartment of Pharmaceutics and Pharmaceutical Technology, Taif University, P. O. Box 11099, Taif 21944, Saudi Arabia
dResearch Center for Advanced Materials Science (RCAMS), King Khalid University, P. O. Box 9004, Abha, 61413, Saudi Arabia
eDepartment of Semi Pilot Plant, Nuclear Materials Authority, P. O. Box 530, El Maadi, Egypt
First published on 12th November 2021
Abstract
In this study, photocorrosion of ZnO is inhibited by doping Ni in the ZnO nanostructure and electron–hole recombination was solved by forming a heterostructure with S-g-C3N4. Ni is doped into ZnO NPs from 0 to 10% (w/w). Among the Ni-decorated ZnO NPs, 4% Ni-doped ZnO NPs (4NZO) showed the best performance. So, 4% Ni–ZnO was used to form heterostructure NCs with S-g-C3N4. NZO NPs were formed by the wet co-precipitation route by varying the weight percentage of Ni (0–10% w/w). Methylene blue (MB) was used as a model dye for photocatalytic studies. For the preparation of the 4NZO-x-SCN nanocomposite, 4NZO NPs were formed in situ in the presence of various concentrations of S-g-C3N4 (10–50% (w/w)) by using the coprecipitation route. The electron spin resonance (ESR) and radical scavenger studies showed that O2− and OH free radicals were the main reactive species that were responsible for MB photodegradation.
1 Introduction
Water has been polluted by various organic pollutants due to rapid industrialization and uncontrolled population.1,2 Conventionally, filtration, chlorination, adsorption, and air trapping methods are used to treat polluted water but these methods are costly, hazardous to health and time-consuming, and cause secondary pollutants.3–6 Recently, photocatalytic degradation of toxic organic pollutants in water into nontoxic CO2 gas and water by using solar energy is an environment-friendly technique for water treatment.7–9Photocatalyst generates electron–hole pairs by the absorption of solar light.10–13 Electron–hole pair reacts directly with pollutant/water and generates various reactive redox species which degrade the organic pollutants and microorganisms in the wastewater.14,15 Photocatalyst faces different problems such as fast electron–hole recombination, photo-corrosion, bandgap position, and quantum efficiency.16,17 The researchers are engaged to solve these problems to make efficient photocatalysts and developed various types of photocatalysts.18,19 Among these, the heterogeneous photocatalyst is a good choice due to its efficiency under visible light.19–21 The efficiency of heterostructure photocatalysts depends upon bandgap position, bandgap energy, composition, morphology, surface area, and photostability.22,23 To construct an efficient photocatalyst that is effective under visible light is very critical and challenging.24,25 Photo-corrosion and electron–hole pair recombination are the main factors that lower the efficiency of the photocatalyst.26,27
These problems can be solved by constructing a heterostructure of a proper band gap. ZnO semiconductors make a type II heterostructure with S-g-C3N4 with better photocatalytic efficiency.21,28,29 ZnO is a low cast semiconductor with high redox potential, shows excellent optical and electrical properties.30–32 But photo-corrosion and fast electron–hole pairs recombination, have restricted its widespread applications.33,34 Photocorrosion of ZnO is prohibited by doping Ni in the ZnO nanostructure and electron–hole recombination was solved by forming heterostructure with S-g-C3N4.35–37 2D organic polymer nanosheet of eco-friendly graphitic carbon nitride (g-C3N4) showed exceptional optoelectrical properties.9,38–41 Bandgap and visible light absorption capacity were further improved by doping sulfur into g-C3N4 nanosheets.42–46
In this study, Ni is doped into ZnO NPs with different concentrations from 0 to 10%. Among the Ni-doped ZnO NPs, 4NZO NPs showed the best performance. So, 4% Ni–ZnO was used to form heterostructure NC with S-g-C3N4. Photocorrosion of ZnO was prohibited by doping Ni in the ZnO nanostructure and electron–hole recombination was solved by forming heterostructure with S-g-C3N4 with 10–50% (w/w). The 4NZO-30-SCN photocatalyst showed 97% MB dye degradation in 1 h among the other photocatalysts.
2 Experimental
2.1 Materials
For the preparation of ZnO NPs, S-g-C3N4 NS, NZO NPs, and NZO-x-SCN NC, chemicals of analytical grade were used. Nickel chloride hexahydrate (NiCl2·6H2O), zinc nitrate (Zn(NO3)2·6H2O), ammonium hydroxide (NH4OH), benzoquinone (C6H4O2), thiourea ((NH2)2CS), ammonium oxalate (C2H8N2O4), isopropanol (C3H8O), methylene blue (C16H18N3SCl), sodium lauryl sulfate, and absolute ethanol (C2H5OH), were purchased from Sigma-Aldrich.2.2 Synthesis of Ni-mixed zinc oxide (Ni/ZnO) nanocomposite
NZO NPs were formed by the co-precipitation route. For the preparation of 1% (w/w) Ni-mixed ZnO NPs (1NZO), NiCl2·6H2O (425 mg), Zn(CH3COO)2·2H2O(42.5 g), and 0.5 g of sodium lauryl sulfate were dissolved into 300 mL of deionized H2O to form homogenous solutions. After 0.5 h of stirring, NH4OH solution was added dropwise to homogenous solution under strong stirring till the pH reached 11. The precipitate of Ni decorated ZnO NPs were firmed which were further stirred for 0.5 h, washed with deionized water/ethanol, dried at 70 °C overnight and finally, calcinated (580 °C, 5 h) in a muffle furnace. The calcinated sample were stored in a desiccator for further studies. The calcinated 1% Ni-mixed ZnO NPs were designed as 1NZO (Fig. 1). Similarly, other samples were prepared just by changing the concentration of Ni from 0 to 10% (w/w) (Table 1). ZnO NPs were synthesized by the same method without using Ni precursor.| Sr. No. | Nanoparticles | Wt ratio (%) | pH | MB dye degradation (%) | |
|---|---|---|---|---|---|
| NiCl2 | Zn(NO3)2 | ||||
| 1 | Pure ZnO | 0 | 100 | 11 | 18 |
| 2 | 1NZO | 1 | 99 | 11 | 32 |
| 3 | 2NZO | 2 | 98 | 11 | 47 |
| 4 | 4NZO | 4 | 96 | 11 | 62 |
| 5 | 6NZO | 6 | 94 | 11 | 56 |
| 6 | 10NZO | 10 | 90 | 11 | 50 |
2.3 Synthesis of 4NZO/S-g-C3N4 NC
Sulfurized g-C3N4 (SCN) was synthesized by the method described in our previous work.47 For the preparation of 4NZO-x-SCN nanocomposite, 4% (w/w) Ni decorated ZnO NPs were formed in situ in the presence of various concentrations of SCN (10–50% (w/w)) by using the previous method (Fig. 1 and Table 2).| Sr. No. | Nanocomposites | Wt% ratio of NCs | MB degradation (%) | |
|---|---|---|---|---|
| S-g-C3N4 | 4NZO NPs | |||
| 1 | S-g-C3N4 | 100 | 0 | 27 |
| 2 | 4NZO-10-SCN | 90.0 | 10.0 | 45 |
| 3 | 4NZO-20-SCN | 80.0 | 20.0 | 69 |
| 4 | 4NZO-30-SCN | 70.0 | 30.0 | 97 |
| 5 | 4NZO-40-SCN | 60.0 | 40.0 | 87 |
| 6 | 4NZO-50-SCN | 50.0 | 50.0 | 79 |
2.4 Characterization
The morphological studies were performed by using a TEM (JEOL-JEM-1230) and SEM; (Quanta-200; USA). FTIR study was carried out using the Bruker V70 spectrometer. The crystal structure of nanomaterials was determined by using an X-ray diffractometer (Bruker-D8-Advance). The photocatalytic MB degradation was studied by using UV-vis spectrophotometer (JESCO, USA). Fluorolog-3 spectrometer (HORIBA) was used for the photoluminescence (PL) study. The Brunauer–Emmett–Teller was carried out by using a gas-sorption-analyzer (ASAP-2020, USA). Chemical composition was confirmed by using X-ray photoelectron spectroscopy (XPS, Kratos Axis-Ultra multifunctional X-ray spectrometer). Transient photocurrent responses were determined by CHI-602 electrochemical-workstation.2.5 Photocatalytic dye degradation study
Photodegradation of methyl blue was studied in the presence of photocatalyst under sunlight. 100 mg of tested catalyst was added in 100 mL MB solution (10 mg L−1) and stir under dark for 30 min to get adsorption equilibrium. Then the suspension was exposed to sunlight in an ambiance environment (68–73 Klux) for the initiation of degradation of MB. 5 mL suspension was withdrawn after every 10 min, centrifuge, and MB concentration was determined by UV-vis spectrometer. The degradation efficiency was determined by the following equation.where C0 and C are the initial and final concentrations at time 0 and t min, respectively.
3 Results and discussion
3.1 Physicochemical properties of undoped ZnO, 4NZO, S-g-C3N4, 0NZO-30-SCN, and 4NZO-30-SCN
The XRD was performed to explore the structural parameters and phase purity of the ZnO, 4NZO, S-g-C3N4, 0NZO-30-SCN, and 4NZO-30-SCN photocatalysts. As depicted from Fig. 2, the powdered XRD patterns of each of the constructed materials agree well with the traditional (JCPDS 01-079-2205) hexagonal wurtzite phase of ZnO. We observed that the peak locations of 4NZO are slightly shifted to higher 2θ values along with lower peak intensities and broadening, revealing the perfect inclusion of Ni2+ into pristine ZnO and a combination of 4NZO with pure S-g-C3N4. Furthermore, we noticed that decorating of ZnO with Ni did not vanish or added an XRD peak in powdered XRD measurements of 4NZO and 4NZO-30-SCN NCs that establish that Ni incorporating has not modified the wurtzite configuration. On the other hand, the XRD measurements of undoped S-g-C3N4 suggest the distinctive diffraction peak of graphitic C. The existence of diffraction peaks of 4NZO and 0NZO-30-SCN in the 0NZO-30-SCN XRD measurements indicating the presence of Ni-doped ZnO on the surface of pristine S-g-C3N4.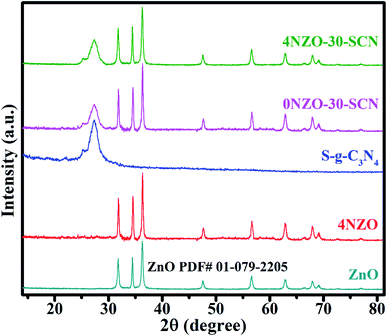 | ||
| Fig. 2 X-ray diffraction patterns of pristine ZnO, 4NZO, S-g-C3N4, 0NZO-30-SCN, and 4NZO-30-SCN nanocomposites. | ||
The surface morphologies of as constructed photocatalysts were explored by TEM and HRTEM. Fig. 3a and b demonstrate that the ZnO and 4NZO NPs have possessing identical round structures and remain in the average size scale of nearly 40–70 nm and 35–65 nm respectively. The undoped S-g-C3N4 reveals its flexible graphene-like folded NSs (Fig. 3c). We observed that the TEM images of 0NZO-30-SCN and 4NZO-30-SCN evince the heterointerface present between 4NZO NPs and S-g-C3N4 as illustrated by Fig. 3d and e. The ZnO and 4NZO NPs are uniformly distributed over the S-g-C3N4 network to construct heterojunctions as declared by TEM analysis. The 4NZO NPs are captivated by the S-g-C3N4 flexible NSs which operate as a support to protect the given NPs and aid to boost the photocatalytic performance of the NCs.
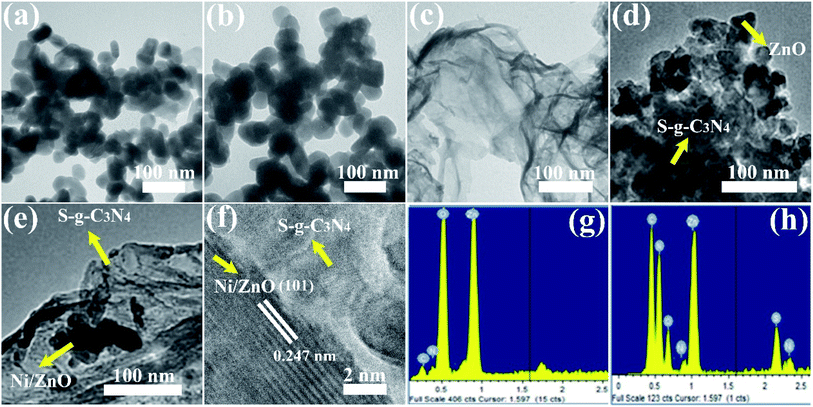 | ||
| Fig. 3 TEM images of (a) ZnO, (b) NZO, (c) S-g-C3N4 NSs, (d) 0NZO-30-SCN and (e) 4NZO-30-SCN. (f) HRTEM image of 4NZO-30-SCN NCs. (g) and (h) Show the EDX of 4NZO and 4NZO-30-SCN NCs, respectively. | ||
The appropriate integration between 4NZO NPs and S-g-C3N4 (Fig. 3e) also indicating the construction of 4NZO-30-SCN NCs. As illustrated in Fig. 3f, the lattice fringes of 4NZO disclose interplanar gaps of 0.247 nm in the NPs, which agrees perfectly with the (101) facet of the hexagonal ZnO wurtzite phase. Moreover, as can be noticed from Fig. 3f, the 4NZO NPs are well circulated in the S-g-C3N4 network with an average size of 55 nm, and the lattice pattern of 4NZO NPs can be observed evidently. The HRTEM image (Fig. 3f) revealed that the heterointerface interaction among the 4NZO and S-g-C3N4 was a well-defined heterojunction, where the lattice scale heterointerface 4NZO/S-g-C3N4 was at the nano-level. We noticed that the heterojunction between the 4NZO and S-g-C3N4 (Fig. 3f) were strongly attached, which will be liable for unusual boosting in the photocatalytic capability of constructed systems. Moreover, to evaluate the surface component metal element of 4NZO and 4NZO-30-SCN NCs the EDX elemental mapping was carried out (Fig. 3g and h). Zinc, nickel, oxygen, carbon, nitrogen, and sulfur were all observed with strong peaks, implying that each component was disseminated uniformly throughout the 4NZO-30-SCN NCs.
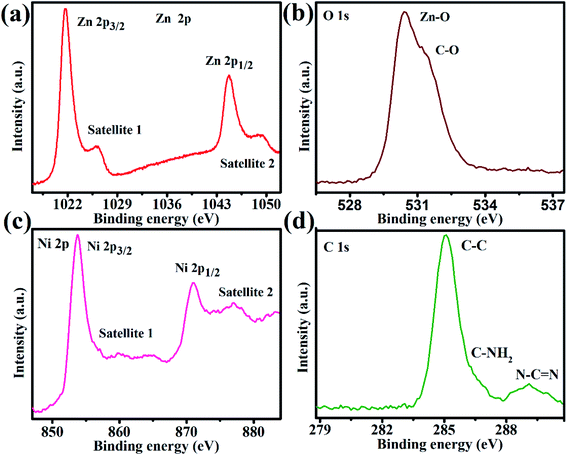
To further examine the existence of Zn, O, Ni, and C and the elemental compositional dissemination in 4NZO-30-SCN NCs, the high-resolution XPS measurement was carried out (Fig. 4). As indicated in Fig. 4a, the high deconvoluted XPS analysis of Zn 2p in 4NZO-30-SCN NCs displays distinct peaks of Zn 2p3/2 (1021.39 eV) and Zn 2p1/2 (1044.3 eV), which is matching well with the literature values for ZnO NPs.48,49 The high deconvoluted O 1s result (Fig. 4b) of the NCs reveals two distinct peaks that emerged at the requisite energies of 531.1 and 530.3 eV which can be credited to C–O and Zn–O, respectively.50 The high deconvoluted Ni 2p measurements (Fig. 4c) of the 4NZO-30-SCN NCs display two feature peaks at the binding energies of 871.04 and 853.4 eV which were allocated to the 4NZO-30-SCN of Ni2+, which matches well with already published results for Ni-doped ZnO.51,52 The XPS measurements explored that the interaction between Ni and ZnO is strong, where the nano-level interface, 4NZO-30-SCN, is established. The C 1s results have a primary peak at 285.05 eV credit to the C–C groups and the other three distinct peaks at 289.06, 287.03, and 286.1 eV related to C–(C)3 N–H and N–C–N, correspondingly.35 Thus, the XPS measurements also evidently recommended the successful manufacture of 4NZO-30-SCN and the creation of heterointerface between 4NZO and S-g-C3N4.
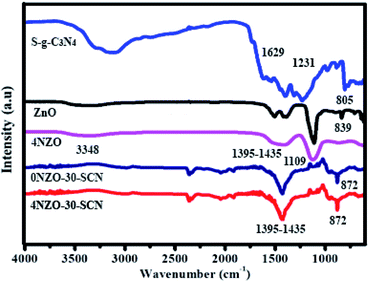
Fig. 5 demonstrates the FTIR results of ZnO, 4NZO, S-g-C3N4, 0NZO-30-SCN, and 4NZO-30-SCN. The FTIR feature peaks at 839 and 1109 cm−1 are liable for symmetrical extending pulsation types for both 4NZO and ZnO, correspondingly. The wideband at 3348 cm−1 reveals the O–H extending pulsation of H2O fragments for 4NZO and ZnO. The existence of a major group at about 1395–1435 cm−1 in 4NZO and 4NZO-30-SCN is acknowledged to the O–C–O extending pulsation type and a feature peak nearly at 872 cm−1 in 4NZO-30-SCN NCs is ascribed to the pulsation type of Zn–O.53 Furthermore, the stretching rates associated with S-g-C3N4, 4NZO, and 4NZO-30-SCN in the range of 2820 to 3430 cm−1 are accountable for N–H widening, and the distinct peak at 2344 cm−1 is responsible for adsorbed CO2 from the air. For flexible S-g-C3N4 NSs, the FTIR peak at 805 cm−1, 1231 cm−1, and 1629 cm−1 are recognized to the tri-s-triazine ring, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N widening pulsations, respectively.54 FTIR findings of 4NZO-30-SCN NCs possess all the liable feature peaks of the Ni decorated metal and metal–oxygen interaction, in addition to observable peaks of flexible S-g-C3N4 NSs confirming the effective construction of 4NZO-30-SCN NCs.
N and C–N widening pulsations, respectively.54 FTIR findings of 4NZO-30-SCN NCs possess all the liable feature peaks of the Ni decorated metal and metal–oxygen interaction, in addition to observable peaks of flexible S-g-C3N4 NSs confirming the effective construction of 4NZO-30-SCN NCs.
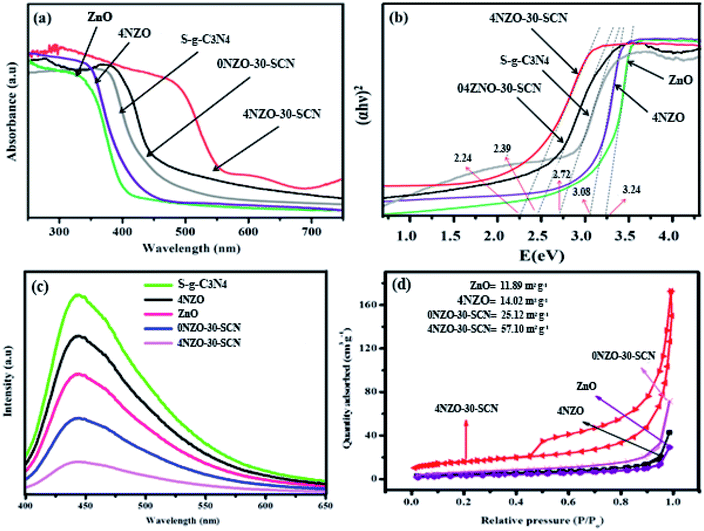
Fig. 6a and b demonstrate the light-harvesting characteristics of the constructed catalysts as evaluated by the UV-vis technique.55 The undoped ZnO demonstrated a band edge at around 389 nm, suggesting that these synthesized NPs are inactive in the visible region related to their larger band gap energy value of 3.24 eV. Moreover, a marginal enhancement in visible-light harvesting is reflected for 4NZO, which is compatible with a decrease in their bandgap value (Eg = 3.08 eV). Contrasting ZnO NPs, pristine S-g-C3N4 NSs generate an absorption band at 439 nm compatible with the conventional bandgap value of 2.72 eV and which is responsible to boost its catalytic capability in visible light. As well, the final 4NZO-30-SCN displayed outstanding visible radiation response, indicating its visible-light responsive catalytic ability. Noticeably, the 0NZO-30-SCN and 4NZO-30-SCN NCs exhibited a considerably increased harvesting (red-shift) in the visible region comparative to undoped ZnO and S-g-C3N4. As illustrated in Fig. 6b, the bandgap values of the constructed catalysts are calculated using Tauc's relation. The estimated bandgap values for 0NZO-30-SCN and 4NZO-30-SCN are 2.39 eV and 2.24 eV, correspondingly.
PL spectroscopy is a significant technique frequently utilized to explore the photo-excited (e− and h+ sets) charge separation capability in the constructed photocatalytic schemes. Fig. 6c represents the PL emission results of the constructed catalysts at the irradiance λ of 370 nm. The high-intensity emission peak at 458 nm might be apparent for the flexible S-g-C3N4 NSs and that recommends the extreme recombination percentage of photo-induced e− and h+ pair of S-g-C3N4. A noteworthy decline in PL peak intensity of 4NZO is noticed as associated with ZnO NPs, and this decrease might be attributed to the beginning of trapped channels, reduction in the inherent defects (antisite oxide and oxygen vacancies (OV)) compactness, and the mid-gap extending the time of the e− and h+ pairs. A weakening in PL peak strength of 0NZO-30-SCN is observed as compared to the undoped S-g-C3N4 peak strength, and it is attributed to the surface OV, Zn defects, and the formation of 0NZO-30-SCN heterojunction. The lowest PL peak is revealed by the 4NZO-30-SCN NCs, verifying that the e− and h+ set recombination in the 4NZO-30-SCN NCs is excellently prevented as of the effective heterointerface between the 4NZO NPs and S-g-C3N4 NSs. These findings reveal that the 4NZO-30-SCN NCs efficiently perform the photo-induced charges that are utilized in reactive oxygen species (ROS) fabrication. Subsequently, the e− and h+ recombination frequency is prevented and photocatalytic proficiency of 4NZO-30-SCN NCs is boosted.
The surface area and pore volumes of the constructed photocatalysts were assessed via the BET approach via N2 adsorption–desorption procedure. The nitrogen adsorption–desorption measurements findings are presented in Fig. 6d. Enhanced surface area and larger pore volume are noticed for the 4NZO-30-SCN NCs as contrasted to the other synthesized photocatalysts. This may be recognized by the nickel dopant inserted among the inside of pristine ZnO NPs and S-g-C3N4 flexible NSs. It is acknowledged that the higher surface areas create extra highly active places and facilitate the catalysts to adsorb extra contaminants on the upper surface. Therefore, the highest content of the contaminants may be adsorbed and reduced by the 4NZO-30-SCN NCs equated to other synthesized samples.
3.2 The photocatalytic evolutions of undoped ZnO, 4NZO, S-g-C3N4, 0NZO-30-SCN, and 4NZO-30-SCN
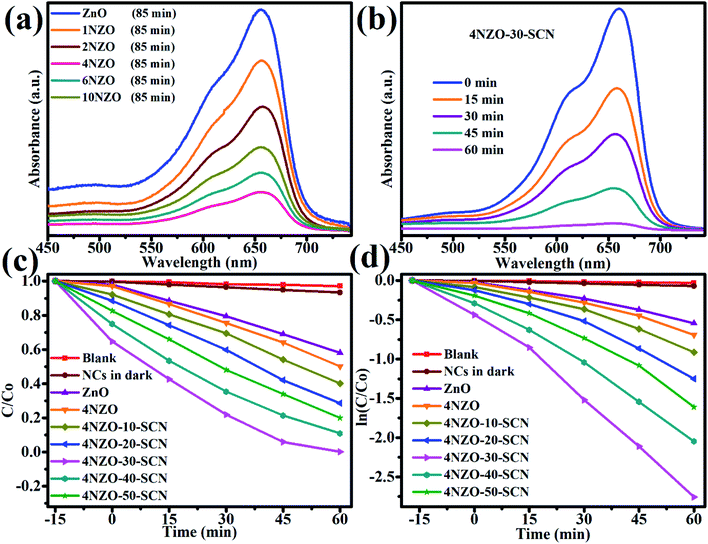
The photocatalytic removal assessment of organic pollutants in sunlight by employing the undoped ZnO and Ni/ZnO (1, 2, 4, 6, & 10 wt%) photocatalysts was explored. The dye removal investigation consequences are displayed in Fig. 7. Before the light illumination, each constructed catalyst was disconcerted with dye suspension in dark for 30 min to achieve the equilibrium point of adsorption. As exhibited in Fig. 7a, the 4NZO showed the highest adsorption (18%) as associated with other Ni decorated NPs and this may be ascribed to its small dimension and enhanced surface area. At the start of the reaction, the photocatalytic dye removal frequency is improved but then declines quickly. Fig. 7a reveals that 4NZO revealed the greatest photocatalysis equated to that of Ni/ZnO (0, 1, 2, 6, and 10 wt%) and removed 62% dye after 85 min. We noticed that the enhanced photocatalytic ability of 4NZO may be owing to the creation of additional trapping channels in the ZnO NPs owing to the nickel engineering. These extra trapping channels extend the period of e− and h+ sets and diminish their recombination rate. Afterward, the charge transporters move to the photocatalyst upper surface and suitably decrease the adsorbed MB.To further improve the photocatalytic ability of 4ZnO, these constructed NPs were integrated with changeable contents of flexible S-g-C3N4. The consequential NCs (4NZO-10-SCN, 4NZO-20-SCN, 4NZO-30-SCN, 4NZO-40-SCN, 4NZO-50-SCN, and 0NZO-30-SCN) with variable S-g-C3N4 proportions were then functional for the photocatalytic removal of dye and the consistent products are specified in Fig. 7b–d. The constructed catalysts adsorbed different contents as displayed in Fig. 7c. The highest adsorption is accomplished by 4NZO-30-SCN, and it might be owing to the superior pore size and surface area as equated to the rest of the synthesized samples.
The MB removal was initiated by irradiating the 4NZO-30-SCN NCs and dye suspension in sunlight after attaining the adsorption–desorption point of equilibrium. Furthermore, all the constructed NCs displayed substantial degradation effectiveness and the equivalent findings are demonstrated in Fig. 7c and d. The dye mineralization effectiveness of the 4NZO-30-SCN is much better than that of ZnO, 4NZO, and 0NZO-30-SCN (Fig. 7c). The optimum MB removal is revealed by the 4NZO-30-SCN NCs, and this finding is significant of its boosted e− and h+ sets segregation and the higher visible-light harvesting (Fig. 7b and c). The dye degradation abilities of the 4NZO-30-SCN are elucidated in Fig. 7c and the sequence of the photodegradation rates can be observed from Fig. 7d.
We found that all our synthesized photocatalysts obeyed the first-order kinetics in the photodegradation of MB reaction's reaction mechanism and the rate law was adopted to estimate the reaction rate constant (k) as given below:
Photocatalyst stability is very important for its practical application. So, the stability of the NiZG photocatalyst was checked up to 7 cycles. Fig. 8a showed that the photocatalytic performance of 4NZO-30-SNC has remained almost constant even after the 7th cycle of photodegradation. XRD spectra of 4NZO-30-SNC were capered before the photodegradation study and after 7th cycle (Fig. 8b). There was no modification in both XRD spectra which endorsed the cyclic stability of 4NZO-30-SNC NC.
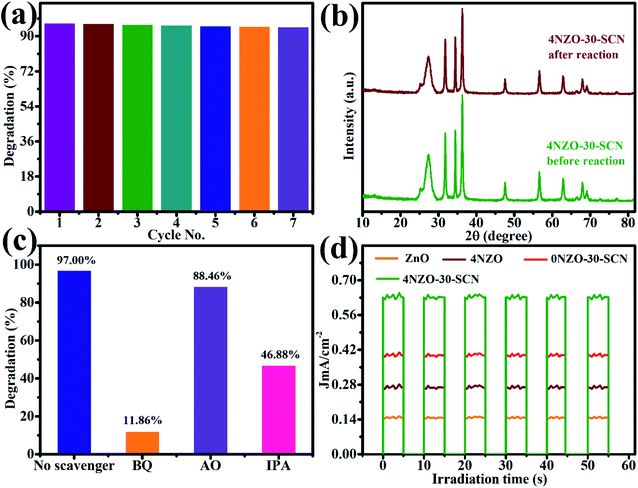 | ||
| Fig. 8 (a) Cyclic stability (b) XRD spectra after 7th recycle. (c) Scavenger's effect of the 4NZO-30-SCN NC (d) photocurrent density for MB degradation. | ||
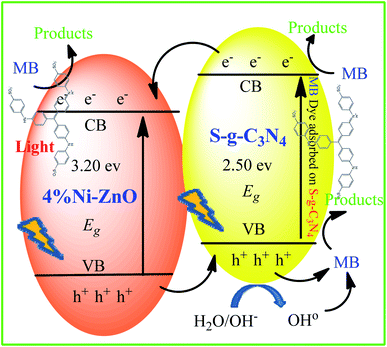
The photocatalytic MB degradation mechanism has been provided in Fig. 9. When 4NZO-30-SNC heterostructure photocatalyst is irradiated with solar light, electrons excited from valence band (VB) to the conduction band (CB), generate electron–hole pair. Due to the narrow bandgap alignment of the heterostructure, the hole is transferred from VB of NZO to VB of SCN and electron from CB of SCN to CB of NZO respectively. These excited electrons react with water and generate reactive oxygen species (ROS) which mineralize the MB. The hole generates hydroxyl radicals which oxidize the MB. To confirm which ROS is responsible for dye degradation, benzoquinone (BQ), isopropanol (IPA), and ammonium oxalate (AO) radical trapping agents were used to trap the ˙O2− radicals, ˙OH radicals, and holes, respectively. These AO, BQ, and IPA trapping agents inhibit 88.14%, 53.12%, and 11.54% MB degradation respectively. The results showed that O2− and ˙OH are the most ROS which plays a major role in MB degradation while hole has a negligible role in dye degradation (Fig. 8c).
Photoelectrochemical analysis was performed to confirm active charge transfer/separation mechanism. The photocurrent densities of the ZnO, 4NZO, 0NZO-30-SCN, and 4NZO-30-SCN were observed in 0.5 M Na2SO4 solution by irradiating chopped light at zero volt (Fig. 8d). 4NZO-30-SCN showed 4.1, 2.15, and 1.46-fold sharp increase in photocurrent density (0.6 J mA cm−2) as compare to ZnO, 4NZO, 0NZO-30-SCN respectively, in the same environment. This is due to doping effect of Ni and coupling with SCN which improve effective electron–hole pair separation and transfer. This result also confirmed the effect of heterojunction between 4NZO and SCN which result effective electron–hole pair separation and transfer to solvent useful for MB degradation.
4 Conclusion
The photocorrosion of ZnO was reduced by incorporating Ni and coupling with SCN. A novel NC was constructed by in situ forming 4NZO in the presence of different concentration of SCN (10–50% (w/w)) to construct heterostructure visible-light-driven photocatalyst (4NZO-x-SCN) for MB degradation. Among NZO NPs series, 4NZO NPs showed maximum MB degradation of 62% in 85 min. Interestingly, 4NZO-30-SCN NC showed the highest MB degradation (97%) in 1 h. Ni–ZnO/S-g-C3N4 exhibited catalytic stability consistently for 7 successive cycles. XRD measurements of 4NZO-30-SCN NCs confirmed that Ni incorporating has not modified the wurtzite configuration. TEM images demonstrate that the ZnO and 4NZO NPs have possessing identical round structures with average size scale of nearly 40–70 nm and 35–65 nm respectively. The ZnO NPs, 4NZO, SCN, 0NZO-30-SCN and 4NZO-30-SCN NCs have 3.24, 3.08, 2.72, 2.39 and 2.24 eV bandgap respectively. The highest adsorption is accomplished by 4NZO-30-SCN, and it might be owing to the superior pore size and surface area as equated to the rest of the synthesized samples. 4NZO-30-SCN has the highest k = 0.032 min−1, which is nearly 7.51 times greater than 4NZO (0.0040 min−1), and 7.74 time greater than S-g-C3N4 (0.0039 min−1). The trapping agent inhibition results showed that ˙O2− and ˙OH are the most ROS which plays a major role in MB degradation while hole has a negligible role in dye degradation.Conflicts of interest
There is no conflict of interest to be reported.Acknowledgements
Authors extend their appreciation to the Research Center for Advanced Materials Science (RCAMS) at King Khalid University for supporting this work through a research project program under grant number RCAMS/KKU/003-21. Hashem O. Alsaab would like to acknowledge Taif University Researchers Supporting Project number (TURSP-2020/67), Taif University, Taif, Saudi Arabia.References
- M. A. Qamar, S. Shahid, M. Javed, M. Sher, S. Iqbal, A. Bahadur and D. Li, Colloids Surf., A, 2021, 611, 125863 CrossRef CAS.
- A. Ajmal, I. Majeed, R. N. Malik, H. Idriss and M. A. Nadeem, RSC Adv., 2014, 4, 37003–37026 RSC.
- S. Iqbal, N. Ahmad, M. Javed, M. A. Qamar, A. Bahadur, S. Ali, Z. Ahmad, R. M. Irfan, G. Liu, M. B. Akbar and M. A. Qayyum, J. Environ. Chem. Eng., 2021, 9, 104919 CrossRef CAS.
- R. Vinayagam, R. Selvaraj, P. Arivalagan and T. Varadavenkatesan, J. Photochem. Photobiol. B, 2020, 203, 111760 CrossRef CAS PubMed.
- M. R. Abhilash, G. Akshatha and S. Srikantaswamy, RSC Adv., 2019, 9, 8557–8568 RSC.
- N. Daneshvar, D. Salari and A. Khataee, J. Photochem. Photobiol., A, 2004, 162, 317–322 CrossRef CAS.
- M. Sher, M. Javed, S. Shahid, S. Iqbal, M. A. Qamar, A. Bahadur and M. A. Qayyum, RSC Adv., 2021, 11, 2025–2039 RSC.
- S. M. T. H. Moghaddas, B. Elahi and V. Javanbakht, J. Alloys Compd., 2020, 821, 153–519 Search PubMed.
- W. Hussain, H. Malik, A. Bahadur, R. A. Hussain, M. Shoaib, S. Iqbal, H. Hussain, I. R. Green, A. Badshah and H. Li, Kinet. Catal., 2018, 59, 710–719 CrossRef CAS.
- M. Waqas, S. Iqbal, A. Bahadur, A. Saeed, M. Raheel and M. Javed, Appl. Catal., B, 2017, 219, 30–35 CrossRef CAS.
- Q. Li, T. Zhao, M. Li, W. Li, B. Yang, D. Qin, K. Lv, X. Wang, L. Wu and X. Wu, Appl. Catal., B, 2019, 249, 1–8 CrossRef CAS.
- H. Anwer, A. Mahmood, J. Lee, K.-H. Kim, J.-W. Park and A. C. Yip, Nano Res., 2019, 12, 955–972 CrossRef CAS.
- S. Iqbal, Appl. Catal., B, 2020, 274, 119097 CrossRef CAS.
- S. Iqbal, S. Nadeem, A. Bahadur, M. Javed, Z. Ahmad, M. N. Ahmad, M. Shoaib, G. Liu, A. Mohyuddin and M. Raheel, JOM, 2021, 73, 380–386 CrossRef CAS.
- M. Basith, R. Ahsan, I. Zarin and M. Jalil, Sci. Rep., 2018, 8, 1–11 CAS.
- S. Iqbal, A. Bahadur, S. Anwer, S. Ali, A. Saeed, R. Muhammad Irfan, H. Li, M. Javed, M. Raheel and M. Shoaib, Appl. Surf. Sci., 2020, 526, 146691 CrossRef CAS.
- A. T. Kuvarega, N. Khumalo, D. Dlamini and B. B. Mamba, Sep. Purif. Technol., 2018, 191, 122–133 CrossRef CAS.
- S. Iqbal, A. Bahadur, S. Anwer, S. Ali, R. M. Irfan, H. Li, M. Shoaib, M. Raheel, T. A. Anjum and M. Zulqarnain, Colloids Surf., A, 2020, 601, 124984 CrossRef CAS.
- N. M. Flores, U. Pal, R. Galeazzi and A. Sandoval, RSC Adv., 2014, 4, 41099–41110 RSC.
- S. Iqbal, A. Bahadur, A. Saeed, K. Zhou, M. Shoaib and M. Waqas, J. Colloid Interface Sci., 2017, 502, 16–23 CrossRef CAS PubMed.
- X. Chen, Z. Wu, D. Liu and Z. Gao, Nanoscale Res. Lett., 2017, 12, 1–10 CrossRef CAS PubMed.
- W. Hussain, H. Malik, R. A. Hussain, H. Hussain, I. R. Green, S. Marwat, A. Bahadur, S. Iqbal, M. U. Farooq, H. Li and A. Badshah, J. Electron. Mater., 2019, 48, 2278–2288 CrossRef CAS.
- M. N. Hussain, S. M. Shah, G. Shabir, A. S. Bhatti, A. Badshah, A. Bahadur, A. Saboor, S. Iqbal and K. Khan, Phys. E, 2019, 108, 307–316 CrossRef CAS.
- A. Bahadur, A. Saeed, M. Shoaib, S. Iqbal, M. I. Bashir, M. Waqas, M. N. Hussain and N. Abbas, Mater. Chem. Phys., 2017, 198, 229–235 CrossRef CAS.
- H. Wang, C. Xie, W. Zhang, S. Cai, Z. Yang and Y. Gui, J. Hazard. Mater., 2007, 141, 645–652 CrossRef CAS PubMed.
- S. Iqbal, M. Javed, A. Bahadur, M. A. Qamar, M. Ahmad, M. Shoaib, M. Raheel, N. Ahmad, M. B. Akbar and H. Li, J. Mater. Sci.: Mater. Electron., 2020, 31, 8423–8435 CrossRef CAS.
- L. Di, H. Yang, T. Xian and X. Chen, Micromachines, 2018, 9, 613 CrossRef PubMed.
- S. Iqbal, A. Bahadur, S. Anwer, M. Shoaib, G. Liu, H. Li, M. Raheel, M. Javed and B. Khalid, CrystEngComm, 2020, 22, 4162–4173 RSC.
- K. Byrappa, A. Subramani, S. Ananda, K. L. Rai, R. Dinesh and M. Yoshimura, Bull. Mater. Sci., 2006, 29, 433–438 CrossRef CAS.
- S. A. Abubshait, S. Iqbal, H. A. Abubshait, A. A. AlObaid, T. I. Al-Muhimeed, H. S. M. Abd-Rabboh, A. Bahadur and W. Li, Colloids Surf., A, 2021, 628, 127390 CrossRef CAS.
- R. Saravanan, E. Sacari, F. Gracia, M. M. Khan, E. Mosquera and V. K. Gupta, J. Mol. Liq., 2016, 221, 1029–1033 CrossRef CAS.
- M. Y. Guo, A. M. C. Ng, F. Liu, A. B. Djurisic, W. K. Chan, H. Su and K. S. Wong, J. Phys. Chem. C, 2011, 115, 11095–11101 CrossRef CAS.
- A. Bahadur, S. Iqbal, M. Shoaib and A. Saeed, Dalton Trans., 2018, 47, 15031–15037 RSC.
- J. Becker, K. R. Raghupathi, J. S. Pierre, D. Zhao and R. T. Koodali, J. Phys. Chem. C, 2011, 115, 13844–13850 CrossRef CAS.
- S. Iqbal, A. Bahadur, M. Javed, O. Hakami, R. M. Irfan, Z. Ahmad, A. AlObaid, M. M. Al-Anazy, H. B. Baghdadi, H. S. M. Abd-Rabboh, T. I. Al-Muhimeed, G. Liu and M. Nawaz, Mater. Sci. Eng., B, 2021, 272, 115320 CrossRef CAS.
- M. A. Qamar, S. Shahid, M. Javed, S. Iqbal, M. Sher, A. Bahadur, M. M. Al-Anazy, A. Laref and D. Li, Colloids Surf., A, 2021, 614, 126176 CrossRef CAS.
- S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, Appl. Catal., B, 2014, 144, 885–892 CrossRef CAS.
- Y. He, L. Zhang, M. Fan, X. Wang, M. L. Walbridge, Q. Nong, Y. Wu and L. Zhao, Sol. Energy Mater Sol. Cells, 2015, 137, 175–184 CrossRef CAS.
- S. Hu, X. Qu, P. Li, F. Wang, Q. Li, L. Song, Y. Zhao and X. Kang, Chem. Eng. J., 2018, 334, 410–418 CrossRef CAS.
- S. Hu, X. Chen, Q. Li, F. Li, Z. Fan, H. Wang, Y. Wang, B. Zheng and G. Wu, Appl. Catal., B, 2017, 201, 58–69 CrossRef CAS.
- S. Hu, X. Sun, Y. Zhao, W. Li, H. Wang and G. Wu, J. Taiwan Inst. Chem. Eng., 2020, 107, 129–138 CrossRef CAS.
- A. Bahadur, W. Hussain, S. Iqbal, F. Ullah, M. Shoaib, G. Liu and K. Feng, J. Mater. Chem. A, 2021, 9, 12255–12264 RSC.
- A. Bahadur, S. Iqbal, A. Saeed, M. I. Bashir, M. Shoaib, M. Waqas, G. Shabir and A. Jabbar, Chem. Pap., 2017, 71, 1445–1451 CrossRef CAS.
- M. Nemiwal, T. C. Zhang and D. Kumar, Sci. Total Environ., 2021, 144896 CrossRef CAS PubMed.
- X. Qu, S. Hu, J. Bai, P. Li, G. Lu and X. Kang, J. Mater. Sci. Technol., 2018, 34, 1932–1938 CrossRef.
- S. Hu, X. Qu, J. Bai, P. Li, Q. Li, F. Wang and L. Song, ACS Sustainable Chem. Eng., 2017, 5, 6863–6872 CrossRef CAS.
- S. Iqbal, A. Bahadur, S. Ali, Z. Ahmad, M. Javed, R. M. Irfan, N. Ahmad, M. A. Qamar, G. Liu, M. B. Akbar and M. Nawaz, J. Alloys Compd., 2021, 858, 158338 CrossRef CAS.
- Z. Ma, Y. He, X. Li, C. Zhou and L. Deng, Mater. Res. Bull., 2022, 146, 111591 CrossRef CAS.
- A. Chinnathambi, J. Alloys Compd., 2022, 890, 161742 CrossRef CAS.
- W. Ye, Y. Jiang, Q. Liu, D. Xu, E. Zhang, X. Cheng, Z. Wan and C. Liu, J. Alloys Compd., 2022, 891, 161898 CrossRef CAS.
- I. N. Reddy, C. V. Reddy, J. Shim, B. Akkinepally, M. Cho, K. Yoo and D. Kim, Catal. Today, 2020, 340, 277–285 CrossRef CAS.
- M. S. Abdel-wahab, A. Jilani, I. S. Yahia and A. A. Al-Ghamdi, Superlattices Microstruct., 2016, 94, 108–118 CrossRef CAS.
- M. Sher, M. Javed, S. Shahid, O. Hakami, M. A. Qamar, S. Iqbal, M. M. Al-Anazy and H. B. Baghdadi, J. Photochem. Photobiol., A, 2021, 418, 113393 CrossRef CAS.
- M. Š. Slušná, D. Smržová, P. Ecorchard, J. Tolasz, M. Motlochová, I. Jakubec, M. Maříková, M. Kormunda and V. Štengl, J. Phys. Chem. Solids, 2022, 160, 110340 CrossRef.
- C. Zhang, M. Jia, Z. Xu, W. Xiong, Z. Yang, J. Cao, H. Peng, H. Xu, Y. Xiang and Y. Jing, Chem. Eng. J., 2022, 430, 132652 CrossRef CAS.
Footnote |
| † The authors have equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |