Open Access Article
Open Access ArticleMetal-free multicomponent approach for the synthesis of propargylamine: a review
Sujit Ghosh
 *a and
Kinkar Biswas
*a and
Kinkar Biswas
 *b
*b
aDepartment of Chemistry, Raiganj Surendranath Mahavidyalaya, Raiganj 733134, India. E-mail: sujit2484@gmail.com
bDepartment of Chemistry, Raiganj University, Raiganj 733134, India. E-mail: kinkar.chem@gmail.com
First published on 7th January 2021
Abstract
Propargylamines are important classes of alkyne coupled amine compounds used in heterocyclic chemistry and pharmaceuticals chemistry and have a large impact as a pharmacophore used in medicinal chemistry. One of the straightforward approaches for the synthesis of this class of compound is A3 coupling, a three-component coupling reaction among aldehyde, alkyne (terminal acetylene) and amine. However, there are many methods other than conventional three component alkyne–aldehyde–amine (A3) coupling which have also been reported for the synthesis of propargylamine. Most of these methods are based on the metal catalyzed activation of terminal alkyne. From the perspective of green and sustainable chemistry, the scientific community should necessarily focus on metal-free techniques which can access a variety of propargylamines. There are only a few reports found in the literature where propargylamines were successfully synthesized under metal-free conditions. This present review article neatly and precisely encompasses the comprehensive study of metal-free protocols in propargylamine synthesis putting forth their mechanisms and other aspects.
1. General introduction
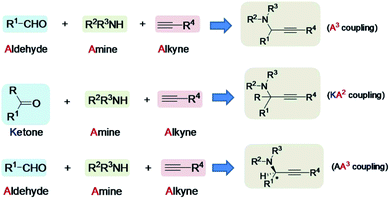
Propargylamines are important classes of organic scaffolds,1 and have significant importance as intermediates for the synthesis of multifunctional amino derivatives,2 natural products,3 as well as biologically active compounds.4 Asymmetric propargylamines are important precursors for the synthesis of many drug molecules.5 Annulation, cyclization and cascade transformation of various derivatives of propargylamine lead to the formation of miscellaneous heterocyclic compounds,6 such as pyrroles,7 pyrrolines,8 pyrrolidine,9 pyrazines,10 pyrazoles,11 thiazoles,12 thiazolidines,13 isoxazoles,14 oxazolidines,15 pyridines,16 dihydropyridines,17 etc.Among the various methods of its synthesis, metal catalytic A3 coupling reaction,18 is the major one. One-pot three-component coupling (3CC) reaction among aldehyde, amine and terminal alkyne to give propargylamine product is commonly known as the A3 coupling reaction. It is an example of multicomponent reaction (MCR). In some cases when the aldehyde is replaced by a ketone, then it is known as KA2 reaction.19 A3 coupling reaction can also produce propargylamine (chiral molecule) enantioselectively, and the reaction is called asymmetric A3 coupling or AA3 coupling reaction,20 (Fig. 1).
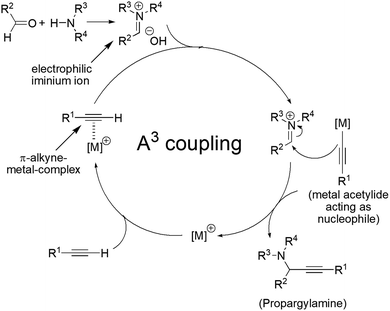
Other methods in addition of A3 coupling reaction includes alkynylation of imine,21 alkynylation of alkyl amine,22 amination of propargylic ester,23 tandem anti-Markovnikov hydroamination/alkyne addition reaction,24 which have been tacitly studied over last two decades.20,25 Almost all of these methods required transition metal based heterogeneous,26 and homogeneous metal catalysts.27 There are only a few reports published in the literature describing the A3 and other MCR (multicomponent reaction) for the metal-free propargylamine synthesis.28–42 From the green and sustainable point of view, metal-free synthesis is the best eco-friendly approach while assessing environmental safety. The object of green chemistry mainly focuses on the development of novel methodologies and alternative sustainable synthetic strategies, which must include the environmental considerations. Multicomponent reactions (MCRs),43 have been considered as benign approach in the perspective of green chemistry. Multicomponent reactions generally require at least three reactants in the same reaction vessel to generate a product containing maximum (preferably all) atoms of the substrate molecules. The dramatic shortening of the synthetic steps during MCRs reduces the chemical waste in chemical reactions. In MCRs, most of the condensation and addition steps are overlooked so that it preserves the atom economy and represents a suitable synthetic tool. Similarly, the other points of green chemistry would be satisfied rigorously by MCRs. The tentative mechanism of A3 coupling reaction is outlined in Fig. 2. The in situ generated metal acetylide from π-alkyne–metal complex reacts with imine or iminium ion, resulting in the formation of propargylamine with simultaneous regeneration of the metal catalyst.
The aim of this present review is to describe different metal-free approaches developed since last few decades for the synthesis of propargylamine. Alkynyl carboxylic acids are the important substrate,44 that can be used replacing low boiling alkyne as observed in most of the reports. Aryl boronic acid, dichloromethane (DCM) solvent, amino alcohol, amino thiol, dialkyl azo dicarboxylate have been also used as one of the coupling partners in individual metal-free approaches in addition of conventionally used aldehyde or HCHO, amine and alkyne or its precursor.
2. A3 coupling reaction using amine, HCHO & ynoic acid:
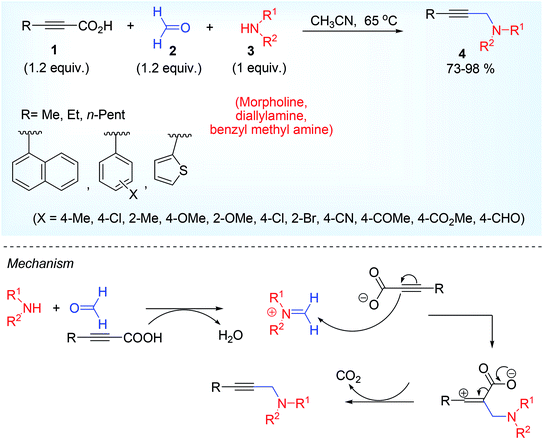
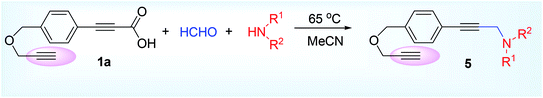
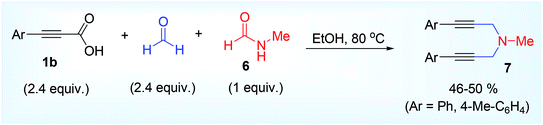
Such methodology was first reported by Lee and his groups in 2013.28 This was the metal-free decarboxylative A3 coupling reaction using paraformaldehyde 2,45 2° amine 3 and alkynyl carboxylic acid 1. Paraformaldehyde is the smallest polyoxymethylene, an oligomer or low polymer of formaldehyde with a typical degree of polymerization about 8 to 100 and generally used as a source of HCHO. The specific gravity, boiling point, melting point, flash point of paraformaldehyde is normally higher than formaldehyde, therefore the paraformaldehyde is preferentially used over formaldehyde or formalin alone (Scheme 1). The iminium ion (generated from condensation of formaldehyde and amine) gets attacked by the aryl propiolate anion to produce the vinyl cation intermediate which is very prone to decarboxylate (Scheme 1) leading to the formation of achiral propargylamine at 65 °C in acetonitrile solvent. This Eschweiler Clark,46 type decarboxylation in this protocol finds some advantages over the other existing metal catalyzed approaches in terms of moisture sensitivity, low tolerance of functional groups, unwanted Glaser homocoupled product,47 and devoid of stoichiometric metal reagents.The methodology was successful for large scale synthesis (20 g) of propargylamine. D2CO experiments showed that CH2 group in product derives from formaldehyde. Chemoselective nature of the reaction was proved by taking 1a, where triple bond attached with carboxylic acid group underwent the reaction leaving the other terminal acetylenic part intact (5, Scheme 2) which in its turn also explain the mechanistic approach given by the author that decarboxylation might act as driving force of the reaction for the regeneration of triple bond.
N-Substituted amide 6 also underwent the reaction when tested as a source of 1° amine and afforded the desired 3° amine product (7) by double propargylation reaction in good yield (Scheme 3).
However, alkynes were not used directly and only HCHO had been applied as a coupling partner. Therefore, scope and challenges remained to be explored using other aldehyde and alkynes.
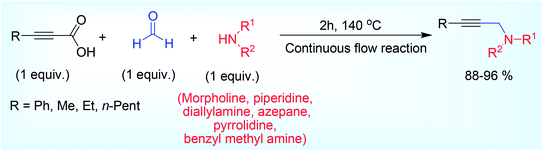
Taking exactly same coupling component set in stoichiometric ratio Lee et al.41 synthesized propargylamines in water using a continuous flow,48 reaction system (Scheme 4), which is a good alternative of lower efficiency batch reactors,49 for industrial preparation of propargylamines without the requirements for large scale reactors. The high surface area to volume ratio may be the cause of high efficiency of this protocol providing efficient heat and mass transfer, which allowed the reaction at higher temperature (140 °C) than 100 °C. Water solubility of the reactant component was the major issue in this reaction. While comparing the solubility of different components, it was observed that alkynyl carboxylic acid was insoluble in water unlike amine and paraformaldehyde, but when amine and ynoic acid were mixed they became perfectly soluble at the optimum temperature of 140 °C (but not at 100 °C) resulting a clear solution. Based on this concept two separate reservoirs were chosen before mixing the entire component. Unsymmetrical 2° amine and propiolic acid were taken in 26 mL of water and stirred at RT for 1 hour prior to send them in another reservoir. Paraformaldehyde on the other hand was taken in same amount as solvent and heated at 90 °C for some time. These two mixtures were then passed in continuous flow tube for 2 h at 140 °C (flow rate 0.0837 mL min−1). This flow technique was a good resistance of high solvent vapour pressure and found superior in terms of product yield (88–96%) than the pressure vessel reaction pot (yield: 76%) when compared under same reaction condition (140 °C, 2 h).
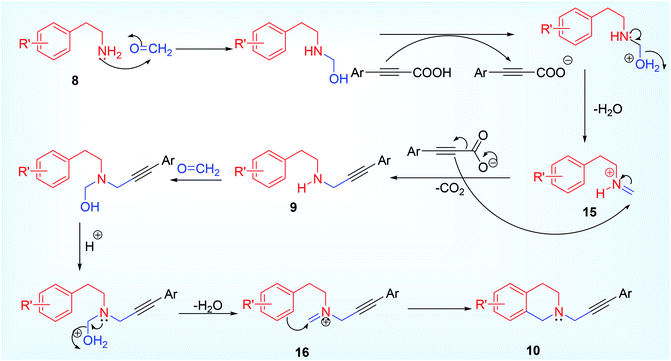
Tandem decarboxylative A3 coupling and Pictet Spengler reaction,50 is an advance methodology for the synthesis of tetrahydroisoquinoline derivatives 10,51 via propargylamine 9 under metal-free condition (Scheme 5).30 The derived functionality developed in the propargylamine after A3 coupling reaction underwent further reaction with HCHO to form an iminium ion 15 (Eschenmoscher's salt),52 via N-hydroxy methyl 2° amine derivative which underwent aromatic electrophilic substitution reaction (like Eschweiler Clark methylation) to provide the corresponding N-propargyl tetrahydroisoquinoline derivatives 10 (Scheme 6). This domino protocol was extended to heteroaromatic scaffold such as thien-3-yl 11 for the synthesis of thienotetrahydropyridine derivative 13 (Scheme 5). Though other attempted heterocyclic ring (such as pyridine-2-yl, indol-3-yl) containing ethyl amine were found un-reactive for the same reaction. Besides six membered mono N-heterocycles, the researchers also able to isolate benzodiazepine 14 (a seven membered dinitrogen heterocycle),53 using pyrimidine derivative 12 having 4,5-amino group (Scheme 5). Trace amount of product was detected while using aliphatic propiolic acid but aldehydes other than formaldehyde were not tested under the reaction condition that might yield diverse substituted tetrahydroisoquinoline.
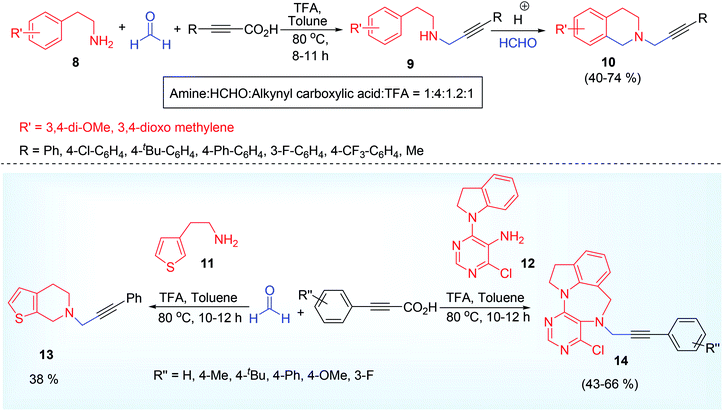 | ||
| Scheme 5 Tandem A3 coupling and Pictet Spengler reaction for the synthesis of tetrahydroisoquinoline and benzodiazepine. | ||
The tandem cyclization of A3 product (propargylamine) to tetrahydroisoquinoline derivative was assisted by the acidic additive. While finding the optimized reaction condition using various acids such as TFA, TCA, PhCOOH, MsOH, CF3SO3H, TsOH, it was observed that TFA worked better providing a maximum of 71% isolated yield, whereas highly acidic or no acidic condition gave very small amount of cyclized product (13% & 10% respectively). The author did not address the reason behind low yield of cyclized product in their paper. We therefore propose a logical explanation on this fact. In the presence of highly acidic medium, the amino group of β-arylethylamine is protonated along with formaldehyde and the –NH2 group no longer behaves as a nucleophilic site. Another interesting point is that the mostly used paraformaldehyde was not a good choice (only 20% product yield) while using alkynyl carboxylic acid as one of the A3 coupling partner, instead of 37% aqueous solution of formaldehyde worked much better (71% yield). Electronic effect in the aryl part of alkynyl carboxylic acid affects the product yield (ERG such as Me, OMe, t-Bu, Ph generated better yield than EWG such as F, Cl, CF3), but this factor was ineffective for the synthesis of benzodiazepine (43–66%). However, an attempt met failure while inspecting such cascade reaction using terminal acetylene instead of alkynyl carboxylic acid even using copper catalyst or under acidic condition, providing neither A3 coupled nor Pictet Spengler product (the tetrahydroisoquinoline).
3. MW assisted metal-free synthesis of propargylamine
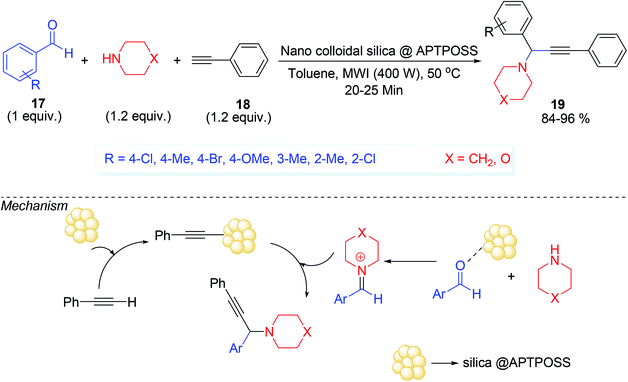
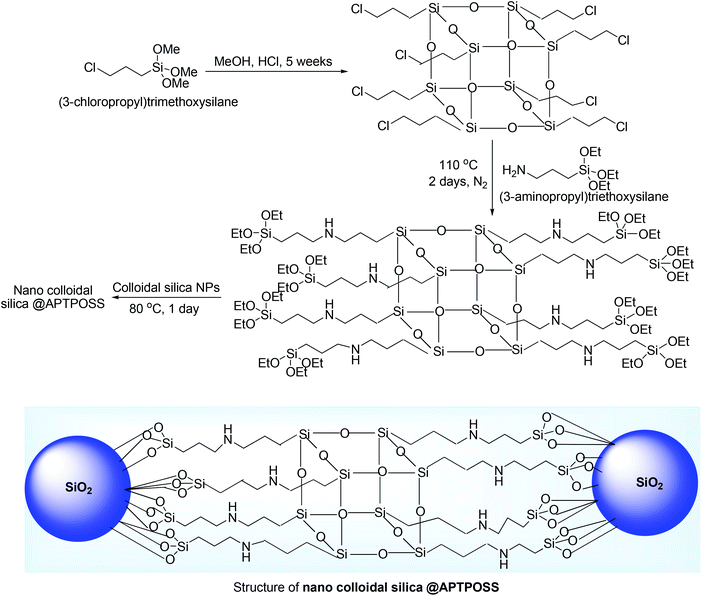
The terminal alkynes are readily available materials and can be easily synthesized whereas the alkynyl carboxylic acids are synthesized from terminal acetylenes and carbon dioxide/carbonate by transition metal catalyzed reactions.54 It is worth to use terminal alkyne rather than alkynyl carboxylic acids due to the presence of more reactive acidic terminal hydrogen in terminal acetylenes. Additionally in A3 or other multicomponent reactions, terminal alkynes are considered as good atom economic substrates than alkynyl carboxylic acids.55Nano silica in colloidal,31 form under microwave irradiation (MWI) might activate the terminal acetylene 18, which increases the alkynes nucleophilicity and thereby promote the coupling with iminium ion formed in situ from silica activated aldehyde 17 and secondary amine (Scheme 7). It was assumed that the reaction was promoted by a combination of Lewis acid and Lewis base, where catalytically active SiO2 acted as a Lewis acid and APTPOSS (–NH–) acted as a Lewis base. This A3 coupling reaction was carried out under MW irradiation and afforded the desired propargylamine 19 in good to excellent yield in very short reaction time (20–25 min) (Scheme 7).
The catalyst was prepared by stepwise manner. At first, Cl–POSS [POSS = polyhedral oligomeric silsesquioxanes (POSS),56 silsesquinoxanes are cage like colourless polymeric solid having Si–O–Si linkage] was synthesized by the hydrolysis of 3-chloropropyltrimethoxysilane under acidic conditions (MeOH, HCl, 35 days) followed by its reaction with 3-aminopropyltriethoxysilane produced APTPOSS (3-aminopropyltriethoxysilane–polyhedral oligomeric silsesquioxanes). Finally, the reaction of nano-colloidal silica with APTPOSS afforded nano-colloidal silica tethered polyhedral oligomeric silsesquioxanes named as nano-colloidal silica @APTPOSS. The catalyst was composed with eight branches of 3-aminopropyltriethoxysilane (Scheme 8). The notable point of the catalyst was that it can be reused for next several runs under the same reaction condition (washing with water followed by acetone after each reaction) which result satisfactorily yield after each run (run 1, 87%; run 2, 87%; run 3, 86%; run 4, 86%; run 5, 85%; run 6, 85%).
4. A3 coupling reaction of salicylaldehyde involving ortho-quinonoid intermediate
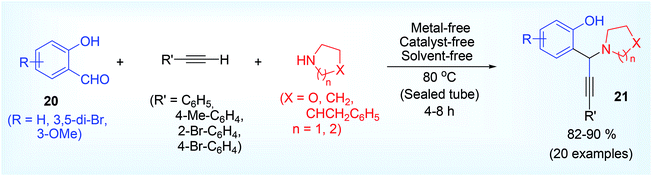
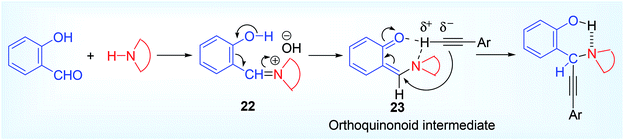
Basu et al. first reported,32 before the work of that salicylaldehyde is the only unique aldehyde that can proceed the classical A3 reaction without the requirement of metal catalyst directly using alkyne as coupling component. Striking this observation, a number of hydroxy propargylamine 21 derivative were synthesized in good to excellent yield using various derivative of salicylaldehyde 20 such as ortho-vaniline, 3,5-dibromo salicylaldehyde and 2° cyclic amine such as morpholine, piperidine, pyrrolidine etc. (Scheme 9). We suggested a tentative mechanism based on the formation of ortho-quinonoid intermediate that play the crucial role to promote the reaction without activation of terminal acetylene by metal catalyst. We certainly reported this perception based on the negative result obtained from ortho-anisaldehyde, para-hydroxy benzaldehyde and ortho-hydroxy acetophenone.While proposing the mechanism (Scheme 10), we suggested that after formation of the iminium ion 22 as in all cases ortho-hydroxyl group may undergo deprotonation forming an unstable ortho-quinonoid intermediate 23, which activates the sp carbon (C–H) of the alkyne more nucleophilic via H-bond, and finally to form the more stable A3 coupled product. The para hydroxy benzalydehyde might give rise to corresponding para-quinonoid species but it does not activate the terminal sp carbon of alkyne (probably C–H may not insert within the functionalities of para-quinonoid species). Again, in the case of ortho-hydroxy acetophenone, corresponding iminium salt might form with difficulty because of steric congestion with methyl group and the iminium carbon would be less electrophilic. Moreover, the reaction did not proceed well in a protic solvent like ethanol because of the interference with H-bond.
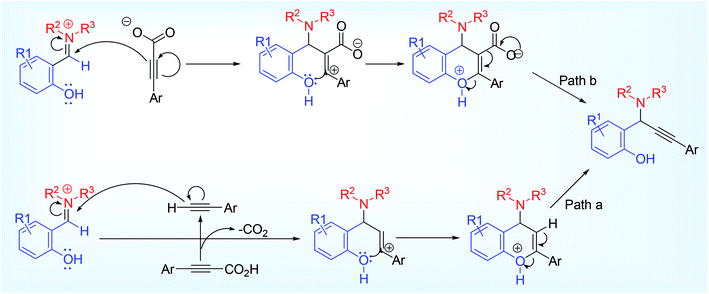
Later on, Kumar and his co-workers,33 also supported the formation of ortho-quinonoid intermediate by DFT calculation and other mechanistic study. In this reaction, alkynyl carboxylic acid was used as alkyne surrogates (Scheme 11). The ortho-quinonoid intermediate underwent a concerted Eschweiler Clarke type decarboxylation where alkynyl nucleophile generated in situ from ynoic acid and undergo nucleophilic addition (Scheme 11). The other possible mechanistic route involving vinyl cation or prior decarboxylation of acetylenic acid to terminal acetylene had been ruled out by DFT energy calculation (Scheme 12). Negative result was obtained when 1° amine was set for the reaction. When attempted for KA2 reaction taking ortho-hydroxy acetophenone similar negative result was obtained as claimed by our group. Other regioisomer of salicylaldehyde (4 or 3-hydroxy benzaldehyde) including ortho-bromo, ortho-trifluoromethyl or ortho-methoxy benzaldehyde also failed to respond the reaction. Presence of ERG or EWG in salicylaldehyde part was not comparable to focus on reaction rate and gave the corresponding hydroxy propargylamine 24 in good to excellent yield. Disappointment observed with aliphatic alkynyl carboxylic acid once again makes it more challenging to be encountered.
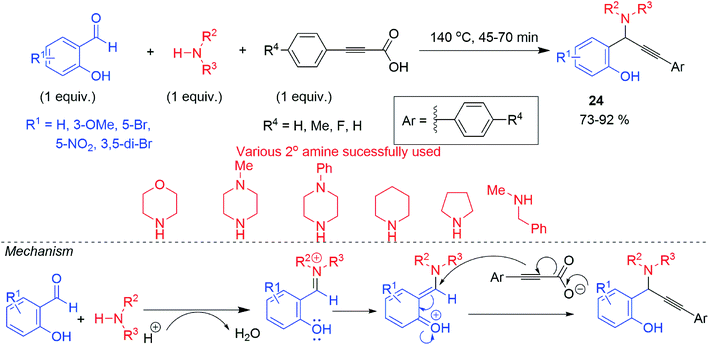 | ||
| Scheme 11 A3 coupling reaction of salicylaldehyde derivative using alkynyl carboxylic acid and its mechanism. | ||
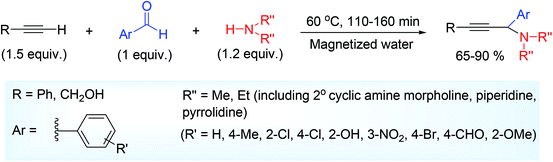
But in 2018, Gholizadeh and their groups,34 first reported the metal-free A3 coupling of other aldehydes including salicylaldehyde, terminal acetylene and 2° amine (Scheme 13) in magnetized water (MW).57 Aliphatic alkyne propargylic alcohol was made to react which afforded the corresponding hydroxylated propargylamine in good yield (68–72%). Magnetized water was prepared by exposure of magnetic field for 10 minutes, onward this magnetization property lasts up to 3 hours. Author presumed that the H-bond interaction played a crucial role in the reaction. Moreover, the molecular dynamics (MD) simulation as well as the experimental results confirmed that MW leads to strong interactions between the reagents to yield the products. The appreciable and notable importance of this protocol showed in its success with other aldehydes besides salicylaldehyde and particularly with aliphatic alkyne (propargyl alcohol).
5. Synthesis of propargylamine by MCR other than A3 coupling reaction
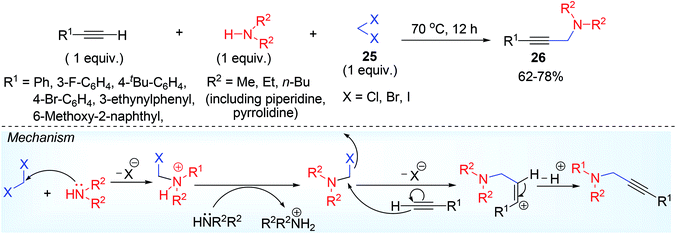
Multicomponent approach for the synthesis of propargylamine without involving aldehyde was unexplored and such report was observed by Sreedhar and his co-workers.35 They synthesized propargylamine 26 in DCM (dichloromethane) solvent 25, which acted as reactant with aromatic terminal alkyne and 2° amine (Scheme 14). The methylene group from solvent counterpart appeared in the final product adjacent to propargylic nitrogen. Conformationally restricted amines such as 2° cyclic amine, dimethylamine underwent good reaction over other amines (diethylamine, dibutylamine). Sterically crowded di-isopropyl amine, 1° aromatic amine such as aniline, butane-1-amine and aliphatic terminal acetylene failed to respond the reaction. Metal-free declaration was ascertained by ICP-AES analysis, which showed existence of possible metal in almost negligible amount (<0.1 ppm). There was a narrow difference in product yield in idealised reaction condition while compared with other dihalomethane such a CH2Br2, CH2I2.During the course of reaction, a white precipitate was found indicating piperidinium chloride salt (also confirmed by spectroscopy) might promote the reaction. A plausible mechanism in this regard has been described in Scheme 14, from which it is clear that low basicity of the aromatic amine may greatly reduce their reaction with CH2Cl2 and ultimately no product was formed. Devoid of aldehyde results no iminium ion intermediate ahead of propargylamine construction, as usually observed in conventional A3 coupling reaction.
The formation of bis-aminomethane intermediate was ruled out in acidic condition which may happen under basic condition.58 Thus, 2° amine as a nucleophile cannot preferentially compete over acetylenic carbon to attack as nucleophile to the iminium electrophile or halo-methyleneamine intermediate. It was confirmed by the author group while using bis(dimethylamino)methane separately as a starting material along with piperidine no dimethylamino derived propargylamine product was formed over reaction with alkyne.59
So far, we discussed the metal-free synthesis of propargylamine, where 2° amine has been served as a mutual and finest choice as one of the coupling partners whether it is terminal alkyne or alkynyl carboxylic acid in presence of formaldehyde, salicylaldehyde or other aldehydes even with CH2Cl2.
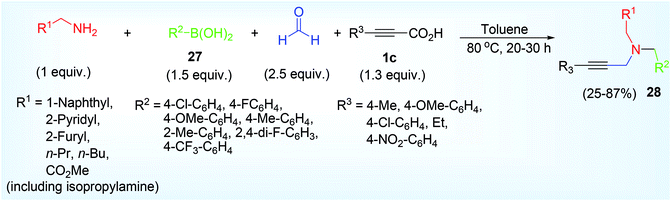
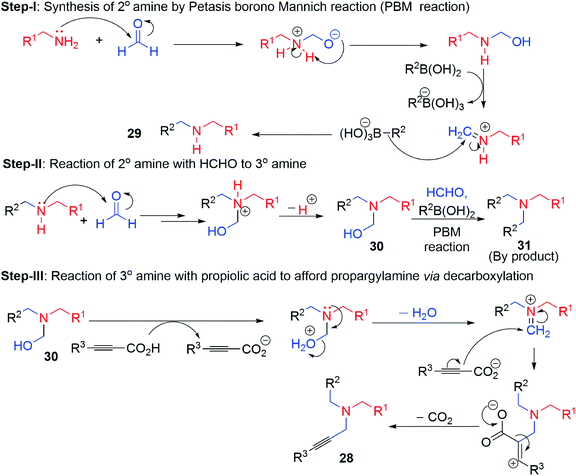
In quest of other metal-free conditions we envisioned that 1° amine can also produce various type of propargylamine under different coupling associates. Wang et al. reported,36 metal-free five-component reaction of 1° aliphatic amine, formaldehyde (2 equivalent), organoboronic acid 27, propiolic acid 1c for the direct synthesis of diverse series of propargylamine 28 by varying the both acids and amine compound in good to excellent yield via tandem PBM (Petasis Borono Mannich),60 and decarboxylation reactions (Scheme 15). Electron donating substituents (OMe, Me) on aryl part of boronic acid results a higher yield than electron withdrawing substituent (F, Cl, CF3). E-styrenyl boronic acid responded this reaction but heteroaryl boronic acid or aliphatic boronic acid remained silent for this reaction. In contrast, aliphatic propiolic acid or electron withdrawing substituent aryl propiolic acid afforded poor yield of the product.
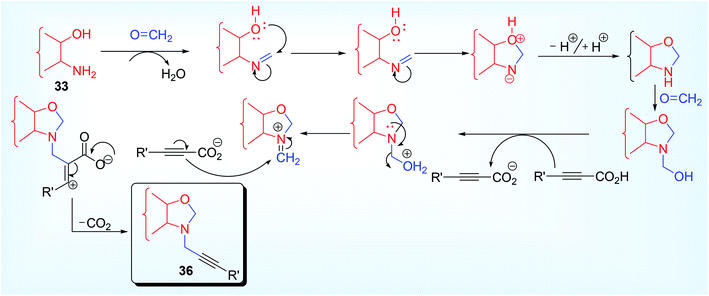
On protonation (generated by the dissociation of propiolic acid) of hemiaminal intermediate, iminium ion electrophile was formed which on further attacked by the alkynyl carbon (bonded to CO2−, carboxylate ion) to form vinylic cation which ultimately provide the desired product 28 via decarboxylation reaction (Scheme 16). Even amino alcohol compounds were applied for the synthesis of versatile propargylamine scaffold such as 1,3-oxazolidine 35, 1,3-oxazine 36 and 1,3-thiadolizine 37 (with N-propargylic group) (Scheme 17). Fenga et al.37 used 2-amino/3-amino alcohol (32, 33) and 2-amino ethane thiol 34 along with formaldehyde and alkynyl carboxylic acid. This atom economic effective protocol does not require any metal catalyst. A large variety of 5 or 6 membered heterocyclic propargylamines have been constructed by tandem annulation and decarboxylative coupling. Both exo and endo methylene groups in the cyclized propargylamine product were derived from formaldehyde molecules.
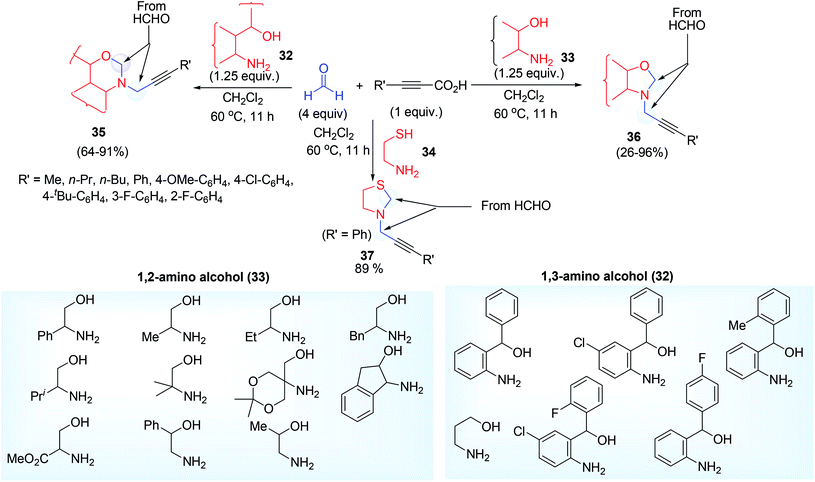 | ||
| Scheme 17 Synthesis of N-propargyl derivative of oxazolidine, oxazine and thiazolidine by metal-free multicomponent reaction. | ||
The imine formed by the reaction of 1° amine group of amino alcohol and HCHO underwent cyclization reaction to afford cyclic 2° amine which follow the expected mechanism as conveyed by the other reports using alkynyl carboxylic acid (Scheme 18). In contrast to other approaches this methodology permits aliphatic propiolic acid to afford the moderate yield of product (42% & 44% respectively from hept-2-ynoic acid and hex-2-ynoic acid).
An exceptional product was reported by Yadav et al.38 among the A3 coupling of 1° aromatic amine 38, heteroaromatic aldehyde 39 and phenylacetylene 18a using silica gel (SiO2) as catalyst under solvent-free condition.61 This methodology provided an easy access of fluorescent active indolylquinolines,62 derivatives 40 (Scheme 19) instead of propargylamine.
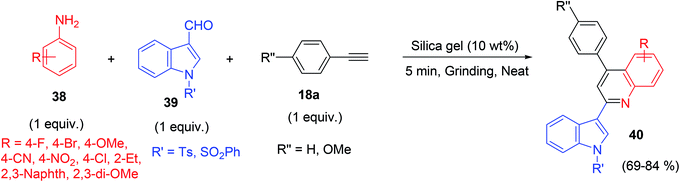 | ||
| Scheme 19 A3 coupling reaction of primary aromatic amine for the synthesis of indolylquinolines derivatives. | ||
Considering bridging amination and Seyfert Gilbert alkynylation reaction in cascade approach, Wang et al.39 reported an unusual approach for the synthesis of terminal propargylamine derivative (N-propargyl hydrazide) 44 (Scheme 20).
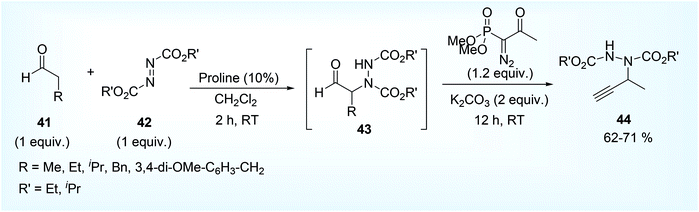 | ||
| Scheme 20 Metal-free synthesis of N-propargyl hydrazide from aldehyde, azo dicarboxylate and Ohira–Bestmann reagent. | ||
In the 1st step of this tandem reaction, carbonyl compounds having α-H atom 41 was made to react with dialkyl azo dicarboxylate 42 using proline catalyst to form an intermediate hydrazide compound 43 which on reaction with Ohira–Bestmann reagent,63 i.e. dimethyl (1-diazo-2-oxopropyl) phosphonate 46 ultimately afforded N-propargyl hydrazide 44 (Scheme 21). Reported mechanism involves an amination cycle where the intermediate compound hydrazide 45 was trapped by the carbene species 47 formed by the initial dissociation of Ohira–Bestmann reagent 46 under basic reaction condition in MeOH, giving a four membered cyclic compound 48 which on ring opening process eventually afforded the end product.
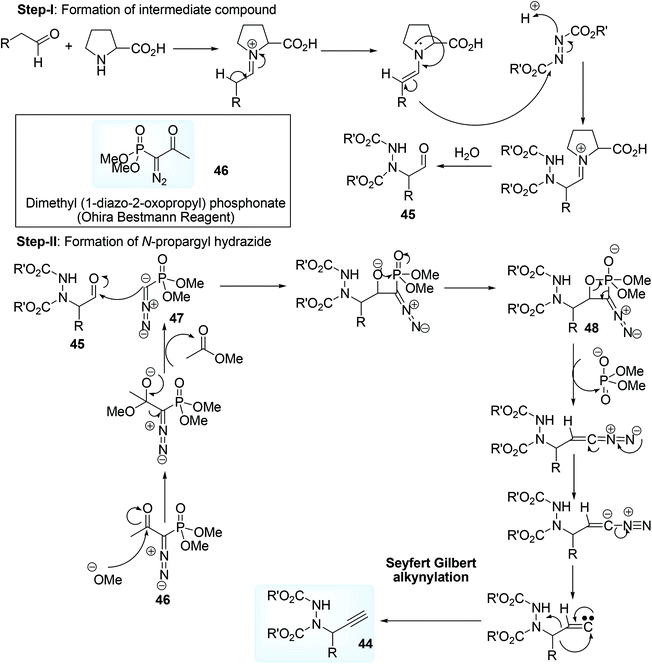 | ||
| Scheme 21 Mechanism of L-proline catalyzed synthesis of N-proparghyl hydrazide by amination and Seyfert Gilbert alkynylation reaction. | ||
Effort for chiral synthesis of propargylamine using L-proline was not much successful resulting poor enantioselectivity (14% ee) of propargylamine. However, this methodology is useful in terms of the fact that it avoids imine formation and allowing the reaction under both air and moisture environment.
6. Metal-free enantioselective synthesis of propargylamines
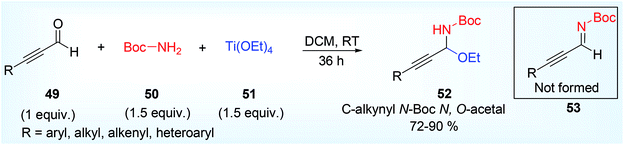
Generally asymmetric propargylamines are prepared by AA3 coupling reaction. Optically active propargylamines are stunning building blocks for the construction of highly functionalized amines.64 They are also highly important structural units for the synthesis of medicinally important and naturally occurring nitrogen-containing compounds. The alkynyl moiety present in it can be functionalized or transformed further in many ways to access new more chiral centres in the synthetically designed compounds.Wang and his group,40 prepared asymmetric syn propargylamines using Brönsted base catalyst. They synthesized unexpected C-alkynyl, N-Boc N,O-acetal 52 (Scheme 22) from alkynyl aldehyde 49, tertiary butyl carbamate (BocNH2) 50 and titanium ethoxide [Ti(OEt)4] 51 while attempted to prepare expected N-Boc protected imine 53.
It was then applied as N-protected alkynyl imine precursor 54 for the Mannich-type reaction,65 with C-nucleophile. They used ethyl-2-keto cyclopentane carboxylate 55 as an enolizable nucleophilic source for the synthesis of syn propargylamines 56 using 10 mol% chiral base as catalyst 57 (Rawal's catalyst,66 a 3° amine base having squaramide group) (Scheme 23). Use of low catalyst loading (2 mol%) also did the same result in terms of yield and stereoselectivity (both enantio and diastereoselectivity) except longer reaction time.
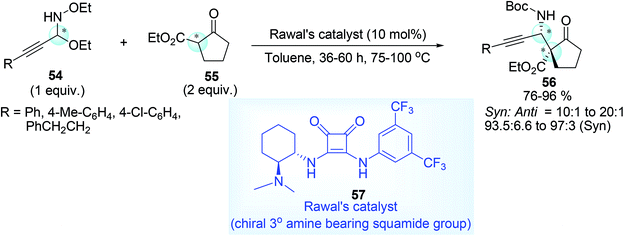 | ||
| Scheme 23 Metal-free synthesis of syn propargylamine from N-Boc N,O-acetals and five membered cyclic β-keto esters. | ||
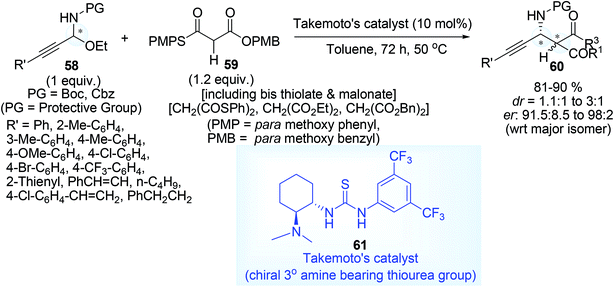
On the other hand, using various N-protected N,O-acetal 58 and thioester malonate nucleophiles 59 of the type S,O-malonate, bis-thiolate (S,S malonate), dialkyl malonate (diethyl and dibenzyl malonate) and chiral Brönsted base, a large number of substituted products 60 have been generated (Scheme 24) using Takemoto's catalyst67 (10 mol%) 61, it was hypothetically presumed by the author that both base functionality for chiral tuning and hydrogen bond factor are essential for synergistic activation of N-Boc-N,O-acetals in this enantioselective tandem process. The optically active compounds thus obtained in Scheme 24, had been applied for the synthesis of diverse functionalized compound such as β-alkynyl-β-amino thioester, β-alkynyl-β-amino acid, β-amino acid, 1,3-amino alcohol, β-amino aldehyde, δ-amino-α,β-unsaturated ester.
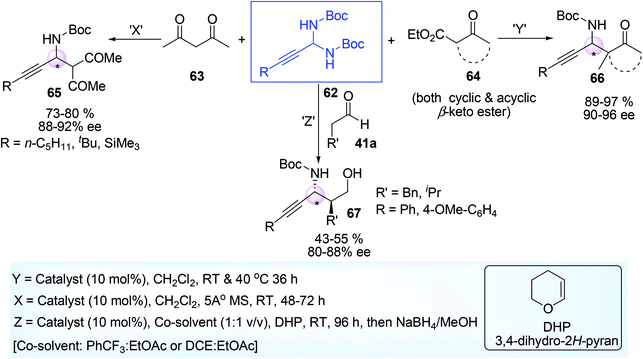
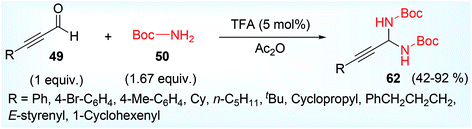
N-Boc aminal 62,68 is a good choice for the synthesis of optically active propargylamine derivatives (65, 66, 67) and this was developed by Maruoka and his co-workers.41 Less accessible N-Boc imine can be in situ obtained from more accessible N-Boc aminal under acid medium before it was made to react with various carbon nucleophile such as aldehyde 41a, β-keto ester 64, 1,3-diketones 63 having α-H atom (Scheme 25) in presence of chiral phosphoric acid catalysts (68–71, Fig. 3).
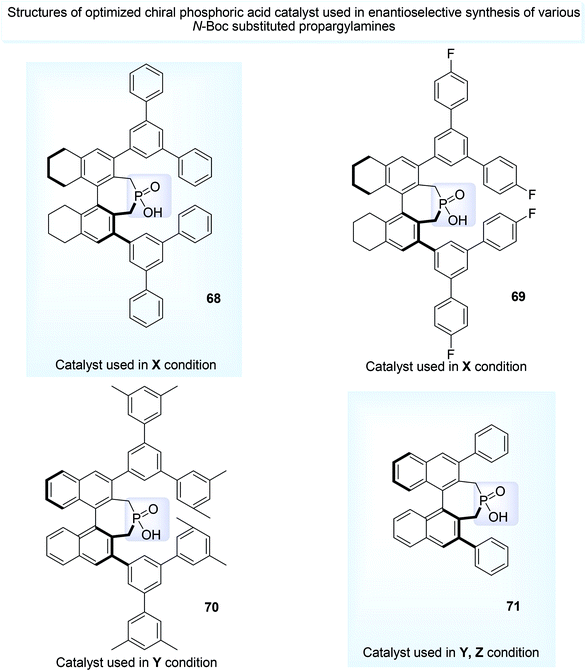 | ||
| Fig. 3 Various chiral catalysts used in the metal-free-enantioselective synthesis of propargylamines. | ||
The starting material N-Boc aminal 62 was prepared by acid catalyzed condensation reaction between tertiary butyl carbamate 50 (Boc-NH2) and aldehyde 49 (Scheme 26), some diastereomers of propargylamines were obtained with two adjacent stereocenters (one is tertiary adjacent to SP-C and another is quaternary) (Scheme 27). Such propargylamines were mainly obtained when α-substituted β-keto ester was taken as a nucleophile. It was detected that cyclic 5-membered β-keto ester was most reactive and underwent the reaction at room temperature. But other β-keto esters including acyclic and 6-membered exhibited lower reactivity under ambient temperature but afforded the expected product at higher temperature (40 °C). Moderate diastereoselectivity was found in case of acyclic compounds while cyclic compounds showed much better diastereoselectivity. Ethyl-2-keto cycloheptane carboxylate was almost inert to react (yield <5%, not shown in scheme). Acetyl acetone responded the reaction but 1,3-diester compound such as diethyl malonate was completely inactive may be due to less stabilisation of enol. Optically active amino alcohol (N-Boc propargylamine having γ-hydroxy group) was obtained by Mannich type addition followed by hydride reduction of the corresponding CHO containing propargylamine. The reaction rate was accelerated in presence of DHP (dihydropyran), act as an efficient BocNH2 scavenger.
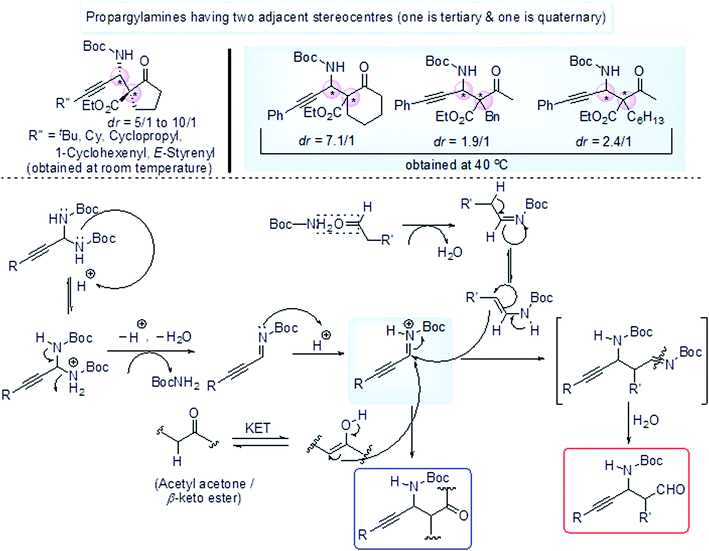 | ||
| Scheme 27 Enantioselective synthesis of various propargylamines having two stereocenters by Mannich type addition. | ||
Unlike β-diketone or β-ketoester, aldehyde required co-solvent system (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v of ethyl acetate & trifluoromethyl benzene or ethyl acetate & 1,2-dichloroethane). In the proposed mechanism (Scheme 27) of the reaction, the initially formed small amount of iminium ion electrophile (slowly generated in acid medium) consumed by the enol nucleophile (in case of β-diketone and β-keto ester), thereby shifting the equilibrium towards right direction and ended up with product. The poor enol content in the equilibrium met failure of the reaction with low iminium ion.
1 v/v of ethyl acetate & trifluoromethyl benzene or ethyl acetate & 1,2-dichloroethane). In the proposed mechanism (Scheme 27) of the reaction, the initially formed small amount of iminium ion electrophile (slowly generated in acid medium) consumed by the enol nucleophile (in case of β-diketone and β-keto ester), thereby shifting the equilibrium towards right direction and ended up with product. The poor enol content in the equilibrium met failure of the reaction with low iminium ion.
That is why DEM with low concentration of enol did not proceed the reaction. But in the case of aldehyde, the reaction was believed to take place via ene-carbamates intermediate generated in situ from BocNH2 (BocNH2 is released from the N-Boc aminal during acid catalyzed iminium ion formation). This ene-carbamate performed as a much better nucleophile than aldehyde for Mannich-type reaction. The cleavage of resultant imine product by water afforded the desired keto containing propargylamine product.
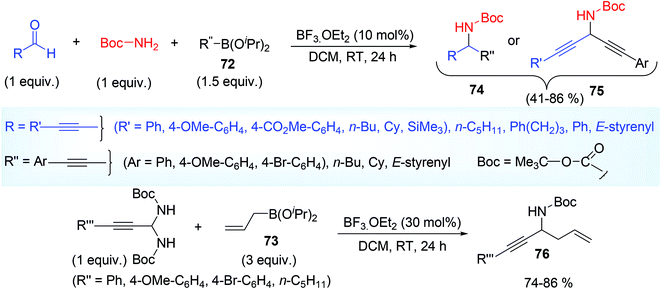
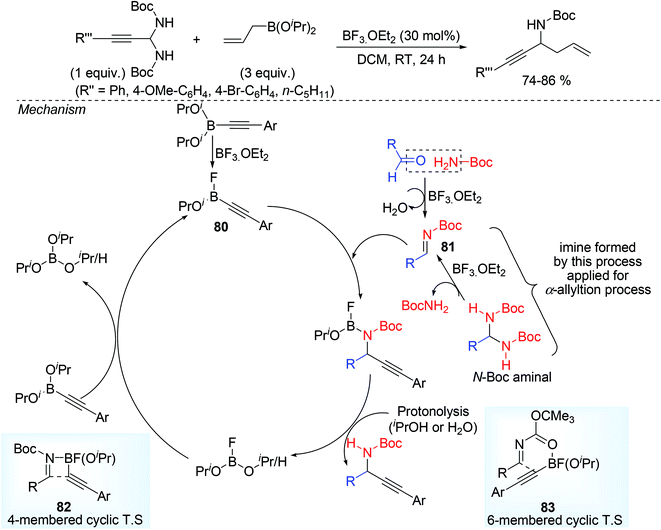
Yasumoto et al.42 applied organoborane compounds as nucleophile e.g. alkynyl or allyl boronate (72, 73) used for the synthesis of α-alkynyl and α-allyl substituted N-Boc (Boc = tertiary butyloxy carbonyl) propargylamine via alkynylation and allylation of alkynyl substituted N-Boc imines (74–76). N-Boc imines were in situ generated from same procedure but with Lewis acid catalyst BF3·OEt2 instead of protogenic acid (trifluoroacetic acid) as shown by Maruoka groups. BF3·Et2O as a catalyst played the key role during the in situ imine formation and also activates the alkyl or alkynyl boronic ester. During α-alkynylation process, aldehyde and Boc-NH2 were the optimized choice prior to imine formation whereas for α-allylation, N-Boc aminal was good precursor of N-Boc alkynyl imine. Use of phenylpropiolaldehyde and amine as a precursor of imine during α-allylation process (with allyl boronate ester) resulted propargyl alcohol (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH(OH)–CH2CH
C–CH(OH)–CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), may be attributed slower imine formation from aldehydes than the addition of nucleophiles to aldehydes. Though allylation required higher catalyst loading (30%) in comparison to that of alkynylation. A variety of propargylamines were prepared by varying alkynyl boronate and aldehyde (Scheme 28).
CH2), may be attributed slower imine formation from aldehydes than the addition of nucleophiles to aldehydes. Though allylation required higher catalyst loading (30%) in comparison to that of alkynylation. A variety of propargylamines were prepared by varying alkynyl boronate and aldehyde (Scheme 28).
Both electron rich and electron deficient aryl propiolaldehyde gave the desired product in moderate to good yield. But the use of electron rich (OMe group) alkynylboronic ester and t-Bu carbamate 77 led to moderate yield 78 (41%) may be due to the concomitant formation of cyclic carbamate 79 as side product (Scheme 29). In this mechanism, the alkynylboronic ester formed a fluoroborane intermediate by ligand exchange with BF3 thereby making it more active as nucleophile, subsequent attack by this nucleophile to electrophilic imine in the form of four or six membered cyclic T.S. centring boron atom followed by protonolysis (Scheme 30) ultimately afforded the product.
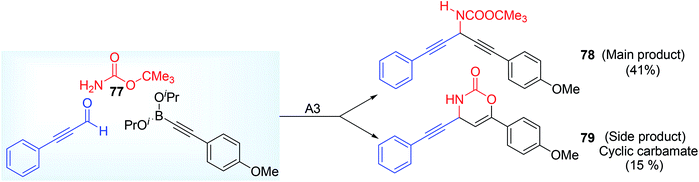 | ||
| Scheme 29 Formation of cyclic carbonate as a side product from the methoxy group containing alkynyl boronate. | ||
 | ||
| Scheme 30 Acid catalyzed mechanism for the synthesis of propargylamines from in situ obtained N-Boc imine. | ||
7. Conclusions
This review summarizes the progress of propargylamine synthesis by greener approach. The methodologies discussed under reviewing are metal-free A3 coupling and some specific multicomponent reactions. Significant efforts have been made in case of MCR to avoid metal catalyst using various types of substrate combination. Not only 2° amine, as usually observed in A3 coupling reaction but also the use of 1° aliphatic and aromatic amines have been explored. Salicylaldehyde has come out as an oversensitive aldehyde in A3 reaction circumventing alkyne activation by metal catalyst. Alkynyl carboxylic acids are the useful surrogates of low boiling alkyne compounds which undergo more facile reaction via decarboxylative coupling. Involvement of boronic acid was also found to give the propargylamine via PBM (Petasis Borono Mannich) reaction. Achievement of the reactions are observed using magnetized water, continuous flow technique, silica gel catalyst, proline, neat reaction condition and CH2Cl2 as solvent. In the case of 1° aromatic amine there is a diversion in the end step reaction to afford exceptional products other than propargylamines. Some domino A3 reaction leads to the formation of heterocyclic compound whereas a few other cascade reactions also produce the same including propargylamine. However, there are ample scopes and challenges yet to be explored such as smooth reaction with aliphatic alkyne or aliphatic ynoic acid, short reaction time and ambient reaction temperature in the perspective of environmental apprehension.Conflicts of interest
There are no conflicts to declare.Acknowledgements
KB acknowledge WBDSTBT, India for sanctioning the project (file no. ST/P/S&T/15G-16/2019). SG and KB thank to Raiganj Surendranath Mahavidyalaya and Raiganj University respectively.Notes and references
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS.
- A. G. de Viedma, V. Martínez-Barrasa, C. Burgos, M. L. Izquierdo and J. Alvarez-Builla, J. Org. Chem., 1999, 64, 1007–1010 CrossRef.
- B. M. Trost, C. K. Chung and A. B. Pinkerton, Angew. Chem., Int. Ed., 2004, 43, 4327–4329 CrossRef CAS.
- V. A. Peshkov, O. P. Pereshivko, A. A. Nechaev, A. A. Peshkov and E. V. Van der Eycken, Chem. Soc. Rev., 2018, 47, 3861–3898 RSC.
- M. Wünsch, D. Schröder, T. Fröhr, L. Teichmann, S. Hedwig, N. Janson, C. Belu, J. Simon, S. Heidemeyer, P. Holtkamp, J. Rudlof, L. Klemme, A. Hinzmann, B. Neumann, H.-G. Stammler and N. Sewald, Beilstein J. Org. Chem., 2017, 13, 2428–2441 CrossRef.
- E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 99781–99793 RSC.
- G. A. Aleku, S. P. France, H. Man, J. Mangas-Sanchez, S. L. Montgomery, M. Sharma, F. Leipold, S. Hussain, G. Grogan and N. J. Turner, Nat. Chem., 2017, 9, 961–969 CrossRef CAS.
- (a) Y. H. Shin, M. Maheswara, J. Y. Hwang and E. J. Kang, Eur. J. Org. Chem., 2014, 2305–2311 CrossRef CAS; (b) K. Jayaprakash, C. S. Venkatachalam and K. K. Balasubramanian, Tetrahedron Lett., 1999, 40, 6493–6496 CrossRef CAS.
- (a) Q. Yang, H. Alper and W.-J. Xiao, Org. Lett., 2007, 9, 769–771 CrossRef CAS; (b) B. Clique, N. Monteiro and G. Balme, Tetrahedron Lett., 1999, 40, 1301–1304 CrossRef CAS.
- (a) J. Su, H. Liu and R. Hua, Int. J. Mol. Sci., 2015, 16, 3599–3608 CrossRef CAS; (b) B. Alcaide, P. Almendros, J. M. Alonso, I. Fernández, G. GómezCampillos and M. R. Torres, Chem. Commun., 2014, 50, 4567–4570 RSC; (c) B. Roy, D. Mondal, J. Hatai and S. Bandyopadhyay, RSC Adv., 2014, 4, 56952–56956 RSC.
- J. Chen, R. Properzi, D. P. Uccello, J. A. Young, R. G. Dushin and J. T. Starr, Org. Lett., 2014, 16, 4146–4149 CrossRef CAS.
- (a) P. K. Sasmal, S. Sridhar and J. Iqbal, Tetrahedron Lett., 2006, 47, 8661–8665 CrossRef CAS; (b) V. Yarovenko, A. Polushina, I. Zavarzin, M. Krayushkin, S. Kotovskaya and V. Charushin, J. Sulfur Chem., 2009, 30, 327–337 CrossRef CAS.
- A. A. Nechaev, A. A. Peshkov, K. Van Hecke, V. A. Peshkov and E. V. Van der Eycken, Eur. J. Org. Chem., 2017, 2017, 1063–1069 CrossRef CAS.
- (a) O. Debleds, C. Dal Zotto, E. Vrancken, J.-M. Campagne and P. Retailleau, Adv. Synth. Catal., 2009, 351, 1991–1998 CrossRef CAS; (b) O. Debleds, E. Gayon, E. Ostaszuk, E. Vrancken and J.-M. Campagne, Chem.–Eur. J., 2010, 16, 12207–12213 CrossRef CAS.
- Y. Hu, R. Yi, C. Wang, X. Xin, F. Wu and B. Wan, J. Org. Chem., 2014, 79, 3052–3059 CrossRef CAS.
- (a) D. C. Mohan, N. B. Sarang and S. Adimurthy, Tetrahedron Lett., 2013, 54, 6077–6080 CrossRef; (b) P. Kaswan, K. Pericherla and A. Kumar, Synlett, 2013, 24, 2751–2757 CrossRef CAS.
- S. Tong, Q. Wang, M. X. Wang and J. Zhu, Chem.–Eur. J., 2016, 22, 8332–8338 CrossRef CAS.
- C. Wei, Z. Li and C.-J. Li, Synlett, 2004, 1472–1483 CAS.
- (a) X. Tang, J. Kuang and S. Ma, Chem. Commun., 2013, 49, 8976–8978 RSC; (b) Y. Cai, X. Tang and S. Ma, Chem.–Eur. J., 2016, 22, 2266–2269 CrossRef CAS; (c) N. V. Tzouras, S. P. Neofotistos and G. C. Vougioukalakis, ACS Omega, 2019, 4, 10279–10292 CrossRef CAS.
- B. V. Rokade, J. Barkera and P. J. Guiry, Chem. Soc. Rev., 2019, 48, 4766–4790 RSC.
- Y. Lu, T. C. Johnstone and B. A. Arndtsen, J. Am. Chem. Soc., 2009, 131, 11284–11285 CrossRef CAS.
- X. Xu and X. Li, Org. Lett., 2009, 11, 1027–1029 CrossRef CAS.
- Y. Imada, M. Yuasa, I. Nakamura and S.-I. Murahashi, J. Org. Chem., 1994, 59, 2282–2284 CrossRef CAS.
- (a) L. Zhou, D. Scott Bohle, H.-F. Jiang and C.-J. Li, Synlett, 2009, 937–940 CAS; (b) S. Takano, T. Kochi and F. Kakiuchi, Chem. Lett., 2017, 46, 1620–1623 CrossRef CAS.
- V. A. Peshkov, O. P. Pereshivko and E. V. Van der Eycken, Chem. Soc. Rev., 2012, 41, 3790–3807 RSC.
- P. Kaur, B. Kumar, V. Kumar and R. Kumar, Tetrahedron Lett., 2018, 59, 1986–1991 CrossRef CAS.
- (a) G.-J. Chen, C.-Q. Chen, X.-T. Li, H.-C. Ma and Y.-B. Dong, Chem. Commun., 2018, 54, 11550–11553 RSC; (b) M. Kassymova, A. de Mahieu, S. Chaemchuen, P. Demeyere, B. Mousavi, S. Zhuiykov, M. S. Yusubovcand and F. Verpoort, Catal. Sci. Technol., 2018, 8, 4129–4140 RSC.
- K. Park, Y. Heo and S. Lee, Org. Lett., 2013, 15, 3322–3325 CrossRef CAS.
- B. Junga, K. Parkb, K. H. Songa and S. Lee, Tetrahedron Lett., 2015, 56, 4697–4700 CrossRef.
- Y. Hu, Y. Shen, L. Huang, E. V. Van der Eycken and H. Feng, Eur. J. Org. Chem., 2020, 2020, 1695–1699 CrossRef CAS.
- J. Safaei-Ghomi, S. H. Nazemzadeh and H. Shahbazi-Alavi, Main Group Met. Chem., 2017, 40, 129–135 CAS.
- S. Ghosh, K. Biswas, S. Bhattacharya, P. Ghosh and B. Basu, Beilstein J. Org. Chem., 2017, 13, 552–557 CrossRef CAS.
- P. Kaur, B. Kumar, K. K. Gurjar, R. Kumar, V. Kumar and R. Kumar, J. Org. Chem., 2020, 85, 2231–2241 CrossRef CAS.
- F. Moosavi, R. Doosti, A. Keivanloo and M. Gholizadeh, New J. Chem., 2018, 42, 4559–4566 RSC.
- V. S. Rawat, T. Bathini, S. Govardan and B. Sreedhar, Org. Biomol. Chem., 2014, 12, 6725–6729 RSC.
- J. Wang, Q. Shen, J. Zhang and G. Song, Tetrahedron Lett., 2014, 56, 903–906 CrossRef.
- H. Feng, H. Jia and Z. Suna, Adv. Synth. Catal., 2015, 357, 2447–2452 CrossRef CAS.
- C. S. Yadav, R. Suhasini, V. Thiagarajan, D. Velmurugan and S. Kannadasan, ChemistrySelect, 2018, 3, 12576–12581 CrossRef CAS.
- Y. Wang, F. Peng, H. Zhang and Z. Shao, Synlett, 2009, 3287–3290 CAS.
- Y. Wang, M. Mo, K. Zhu, C. Zheng, H. Zhang, W. Wang and Z. Shao, Nat. Commun., 2015, 6, 8544 CrossRef.
- T. Yurino, Y. Aota, D. Asakawa, T. Kano and K. Maruoka, Tetrahedron, 2016, 72, 3687–3700 CrossRef CAS.
- K. Yasumoto, T. Kano and K. Maruoka, Org. Lett., 2019, 21, 3214–3217 CrossRef CAS.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- (a) K. Park, T. Palani, A. Pyo and S. Lee, Tetrahedron Lett., 2012, 53, 733–737 CrossRef CAS; (b) M. Ke, Q. Feng, K. Yang and Q. Song, Org. Chem. Front., 2016, 3, 150–155 RSC.
- K. G. Helander, Biotech. Histochem., 2000, 75, 19–22 CrossRef CAS.
- (a) S. H. Pine, Chem. Educ., 1968, 45, 118 CrossRef CAS; (b) J. J. Li, Eschweiler–Clarke reductive alkylation of amines, in Name Reactions, Springer, Cham, 2014, pp. 235−236 CrossRef; (c) T. Rosenau, A. Potthast, J. Röhrling, A. Hofinger, H. Sixta and P. Kosma, Synth. Commun., 2002, 32, 457–466 CrossRef CAS.
- (a) L. Su, J. Dong, L. Liu, M. Sun, R. Qiu, Y. Zhou and S.-F. Yin, J. Am. Chem. Soc., 2016, 138, 12348–12351 CrossRef CAS; (b) S. Yan, S. Pan, T. Osako and Y. Uozumi, Synlett, 2016, 27, 1232–1236 CrossRef CAS; (c) K. S. Sindhua and G. Anilkumar, RSC Adv., 2014, 4, 27867–27887 RSC.
- (a) J.-i. Yoshida, A. Nagaki and D. Yamada, Drug Discovery Today: Technol., 2013, 10, 53–59 CrossRef; (b) J. Britton and C. L. Raston, Chem. Soc. Rev., 2017, 46, 1250–1271 RSC.
- L. K. Wang and Y. Li, Sequencing Batch Reactors, in Biological Treatment Processes. Handbook of Environmental Engineering, ed. L. K. Wang, N. C. Pereira and Y. T. Hung, Humana Press, Totowa, 2009, vol. 8 Search PubMed.
- (a) E. D. Cox and J. M. Cook, Chem. Rev., 1995, 95, 1797–1842 CrossRef CAS; (b) S. W. Youn, J. Org. Chem., 2006, 71, 2521–2523 CrossRef CAS.
- (a) A. P. Gray and R. H. Shiley, J. Med. Chem., 1973, 16, 859–861 CrossRef CAS; (b) A. Sirvent, M. J. García-Muñoz, M. Yus and F. Foubelo, Eur. J. Org. Chem., 2020, 2020, 113–126 CrossRef CAS.
- (a) E. F. Kleinman, Dimethylmethyleneammonium Iodide and Chloride, in Encyclopedia of Reagents for Organic Synthesis, ed. L. Paquette, J. Wiley & Sons, New York, 2004 Search PubMed; (b) J. J. Li, Eschenmoscher's salt, in Name Reactions, Springer Cham, 2014, ISBN: 978-3-319-03979-4 CrossRef.
- (a) S. Verma, S. Kumar and S. Kumara, Arabian J. Chem., 2020, 13, 863–874 CrossRef CAS; (b) N. Kaur and D. Kishore, Synth. Commun., 2014, 44, 113–126 Search PubMed; (c) S. A. Majid, W. A. Khanday and R. Tomar, J. Biomed. Biotechnol., 2012, 510650 Search PubMed; (d) C. S. Radatz, R. B. Silva, G. Perin, E. J. Lenardão, R. G. Jacob and D. Alves, Tetrahedron Lett., 2011, 52, 4132–4136 CrossRef CAS.
- F. Manjolinho, M. Arndt, K. Gooßen and L. J. Gooßen, ACS Catal., 2012, 2, 2014–2021 CrossRef CAS.
- (a) Z. Cao, Q. Zhua, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019, 30, 2132–2138 CrossRef CAS; (b) Q.-W. Gui, X. He, W. Wang, H. Zhou, Y. Dong, N. Wang, J.-X. Tang, Z. Cao and W.-M. He, Green Chem., 2020, 22, 118–122 RSC; (c) Q. Huang, L. Zhu, D. Yi, X. Zhao and W. Wei, Chin. Chem. Lett., 2020, 31, 373–376 CrossRef CAS.
- (a) C. Calabrese, V. Campisciano, F. Siragusa, L. F. Liotta, C. Aprile, M. Gruttadauria and F. Giacalone, Adv. Synth. Catal., 2019, 361, 3758–3767 CrossRef CAS; (b) Y. Leng, J. Zhao, P. Jianga and D. Lua, Catal. Sci. Technol., 2016, 6, 875–881 RSC.
- (a) A. Simpson, Green Chem., 2020, 22, 5180 RSC; (b) M. Bakherad, Z. Moosavi-Tekyeh, A. Rezaeifard, R. Doosti, N. Mehmandoost, N. Goudarziand and S. Omara, Green Chem., 2021 10.1039/D0GC01329C; (c) H. J. Choi, M. Schaffie, M. Gholizadeh and M. Ranjbar, J. Cleaner Prod., 2017, 161, 908–921 CrossRef; (d) R. V. Martín Algarra, L. Lahuerta Zamora, G. M. Antón Fos and P. A. Alemán López, J. Chem. Educ., 2008, 85, 1416 CrossRef.
- M. Tramontini and L. Angiolini, Tetrahedron, 1990, 46, 1791–1837 CrossRef CAS.
- M. Gaudry, Y. Jasor and T. B. Khac, Org. Synth., 1979, 59, 153 CrossRef.
- (a) J.-F. Soule, A. Mathieu, S. Norsikian and J.-M. Beau, Org. Lett., 2010, 12, 5322–5325 CrossRef CAS; (b) G. Muncipinto, P. N. Moquist, S. L. Schreiber and S. E. Scahus, Angew. Chem., Int. Ed., 2011, 50, 8172–8175 CrossRef CAS; (c) W.-Y. Han, Z.-J. Wu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2012, 14, 976–979 CrossRef CAS.
- (a) P. J. Walsh, H. Li and C. A. de Parrodi, Chem. Rev., 2007, 107, 2503–2545 CrossRef CAS; (b) A. L. Garay, A. P. Stuart and L. James, Chem. Soc. Rev., 2007, 36, 846–855 RSC; (c) M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, ChemSusChem, 2014, 7, 24–44 CrossRef CAS.
- (a) Y.-C. Zhang, R. Jin, L.-Y. Li, Z. Chen and L.-M. Fu, Molecules, 2017, 22, 1380 CrossRef; (b) K. H. Chang, Y. J. Chiu, S. L. Chen, C. H. Huang, C. H. Lin, T. H. Lin, C. M. Lee, C. Ramesh, C. H. Wu, C. C. Huang, H. C. Fung, Y. C. Chen, J. Y. Lin, C. F. Yao, H. J. Huang, G. J. Lee-Chen, M. C. Lee and H. M. Hsieh-Li, Neuropharmacology, 2016, 10, 309–319 CrossRef.
- (a) M. Dhameja and J. Pandey, Asian J. Org. Chem., 2018, 7, 1502–1523 CrossRef CAS; (b) S. Mohapatra, C. Bhanja, S. Jena, S. Chakroborty and S. Nayak, Synth. Commun., 2013, 43(15), 1993–2007 CrossRef CAS.
- (a) J.-N. Mo, J. Su and J. Zhao, Molecules, 2019, 24, 1216 CrossRef; (b) T. K. Saha and R. Das, ChemistrySelect, 2018, 3, 147–169 CrossRef CAS.
- (a) A. M. Faisca Phillips, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Front. Chem., 2020, 8, 30 CrossRef; (b) H. M. Torenius, H. Tye and N. E. Leadbeater, Mol. Diversity, 2003, 7, 135–144 CrossRef; (c) P. Merino and T. Tejero, Synlett, 2011, 14, 1967–1977 Search PubMed.
- (a) A. Suresh Kumar, T. Prabhakar Reddy, R. Madhavachary and D. B. Ramachary, Org. Biomol. Chem., 2016, 14, 5494–5499 RSC; (b) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416–14417 CrossRef CAS; (c) H. Konishi, T. Y. Lam, J. P. Malerich and V. H. Rawal, Org. Lett., 2010, 12, 2028–2031 CrossRef CAS.
- A. Berkessel and B. Seelig, Synthesis, 2009, 2113–2115 CrossRef CAS.
- T. Kano, R. Kobayashi and K. Maruoka, Org. Lett., 2016, 18, 276–279 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |