Open Access Article
Open Access ArticleMechanically induced solvent-free esterification method at room temperature†
Lei Zhenga,
Chen Suna,
Wenhao Xuab,
Alexandr V. Dushkinc,
Nikolay Polyakov c,
Weike Su
c,
Weike Su *ab and
Jingbo Yub
*ab and
Jingbo Yub
aKey Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, College of Pharmaceutical Sciences, Zhejiang University of Technology, Hangzhou 310014, P. R. China. E-mail: pharmlab@zjut.edu.cn
bNational Engineering Research Center for Process Development of Active Pharmaceutical Ingredients, Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou 310014, P. R. China
cInstitute of Solid State Chemistry and Mechanochemistry, Novosibirsk, Russia
First published on 28th January 2021
Abstract
Herein, we describe two novel strategies for the synthesis of esters, as achieved under high-speed ball-milling (HSBM) conditions at room temperature. In the presence of I2 and KH2PO2, the reactions afford the desired esterification derivatives in 45% to 91% yields within 20 min of grinding. Meanwhile, using KI and P(OEt)3, esterification products can be obtained in 24% to 85% yields after 60 min of grinding. In addition, the I2/KH2PO2 protocol was successfully extended to the late-stage diversification of natural products showing the robustness of this useful approach. Further application of this method in the synthesis of inositol nicotinate was also discussed.
Introduction
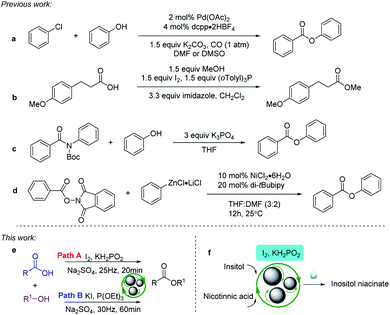
Mechanochemistry has been a popular topic in recent decades because it is a solution-free and energy-efficient approach, which complies with green chemistry. High-speed ball-milling (HSBM) is a green mechanical technique used in organic synthesis and has opened unique opportunities in synthetic organic chemistry.1 HSBM facilitates reactions with insoluble reactants, and enables solvent-free synthetic procedures to successfully avoid the use of environmentally harmful and toxic solvents, among other advantages.2 What's more, HSBM can directly break or stretch chemical bonds because the role of mechanical action is usually to provide better contact between the reagents.3 More experimental results indicate that solvent-free milling can lead to different product compositions or catalyst combinations compared to those obtained by analogous solution-based protocols. The Faraday Discussion Mechanochemistry which took place 2014 in Montreal have indicated that the importance of mechanical processing in the context of pharmaceuticals materials science.3b For these reasons, much catalysts can be used in high-speed ball-milling to optimize organic reactions.4The ester bond is found in a plethora of naturally occurring and pharmaceutically relevant molecules.5 This bond occupies an important role in pharmaceutical chemistry with esterification reactions being among the most commonly used reactions in medicinal chemistry.6 Natural products modified by esterification reactions show better biological activity than before modification, but these reactions often require large amounts of environmentally harmful solvents and catalysts.7
In recent years, many researchers have devoted themselves to exploring green and efficient esterification reactions. Common strategies of transition metal catalysis and prefunctionalization are reported for the synthesis of ester groups. Previously, Buchwald et al. described a method for the preparation of phenyl esters from aryl chlorides via palladium-catalysed carbonylation (Scheme 1a).8 In 2011, Robles et al. demonstrated a mild and regioselective method of esterification and amidation using Gregg-Samuelson-type conditions (Scheme 1b).9 In 2018, Szostak and coworkers reported an alternative transition-metal-free method for esterification of amides (Scheme 1c).10 Recently, Lee and coworkers developed a new disconnection approach for the formation of aryl esters by a nickel-catalysed radical cross-coupling reaction of a redox-active ester with an aryl zinc reagent (Scheme 1d).11 When transition metals are used in drug synthesis, the purification of the reaction becomes tedious. Prefunctionalization will reduce atom economy and increase the experimental procedures. We have currently been focusing on the development of novel, atom-economical, environmentally friendly methods for esterification under HSBM conditions.
Herein, we report two novel strategies for the synthesis of ester groups under solvent-free milling (Scheme 1e). Through modification of the reaction conditions, modes of reactivity have been observed that are different from those in solution. Compared with the preceding procedures of esterification, I2/KH2PO2 system and KI/P(OEt)3 system are solvent-free, transition-metal-free and prefunctionalization no need. Moreover, KH2PO2 and P(OEt)3 can be easily removed as effective substitutes of PPh3. In addition, we discuss the advantages of this method in the synthesis of inositol nicotinate (Scheme 1f).
Results and discussion
At the outset, reactions between benzoic acid 1a and phenol 2a were evaluated with different combinations of additives and phosphine, where anhydrous sodium sulfate was chosen as a helpful grinding auxiliary for better substrate mixing on the basis of our previous research; the selected results were summarized in Table 1. To our delight, both I2 and KI additives could afford desired product 3aa in good yields after 30 min of grinding, where I2 worked better than KI (Table 1, entries 1–7). We optimized both the I2 and KI strategies in order to discuss the different reaction efficiencies and reaction mechanisms of similar additives under the mechanochemistry process. Next, several kinds of phosphines were tested (Table 1, entries 8–16). The I2 with KH2PO2 gave the best result of 91%, while other phosphines gave lower yields under the same conditions (Table 1, entries 2–10). For KI, the reaction with P(OEt)3 as phosphine gave the most satisfactory result, while others were inferior (Table 1, entries 13–15). To further optimize the reaction conditions, we tested the amount of additives and phosphines. The best yield of 75% was given by adding 0.75 mmol KI and 0.5 mmol P(OEt)3 (Table 1, entry 17). Decreasing additive and phosphate loadings led to erosion in yield (Table 1, entries 11–13, 19 and 20). From the experimental results, the corresponding products were difficultly obtained without iodine or potassium iodide as additives (Table 1, entries 2 and 18). Furthermore, a simple comparison of the KI/P(OEt)3 induced reaction under solvent-based conditions was conducted (Table 1, entries 19 and 22), and the reaction could hardly be promoted at room temperature, which verified our hypothesis that HSBM may induce high activity when using KI/P(OEt)3. To the best of our knowledge, there was no report of inorganic iodine salts or inorganic phosphorus salts promoted esterification reactions, which inspired our interest in probing these two systems.| Entry | Additive | Phosphines | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise noted, all reactions were carried out with 1a (0.5 mmol), 2a (0.6 mmol), additive (0.5 mmol), phosphine (0.5 mmol), and anhydrous sodium sulfate (0.4 g) at 25 Hz for 30 min, using two stainless steel balls (ϕ = 1.2 cm, ΦMB = 0.036) in 50 mL stainless steel vial.b Yield based on 1a.c 0.5 equiv. I2 was used.d 0.5 equiv. KH2PO2 was used.e 1.5 equiv. KI was used.f 0.5 equiv. KI was used.g 0.5 equiv. P(OEt)3 was used.h Yield of the comparative experiment: 1a (0.5 mmol), 2a (0.6 mmol), KI (0.5 mmol), P(OEt)3 (0.5 equiv.), and CH2Cl2 (20 mL) at room temperature. | |||
| 1 | I2 | — | n.d. |
| 2 | — | PPh3 | n.d. |
| 3 | I2 | PPh3 | 84 |
| 4 | KI | PPh3 | 66 |
| 5 | NaI | PPh3 | 52 |
| 6 | CuI | PPh3 | n.d. |
| 7 | FeI2 | PPh3 | 12 |
| 8 | I2 | K3PO4 | n.d. |
| 9 | I2 | NaH2PO2 | 34 |
| 10 | I2 | KH2PO2 | 91 |
| 11c | I2 | KH2PO2 | 67 |
| 12d | I2 | KH2PO2 | 83 |
| 13 | KI | P(OEt)3 | 63 |
| 14 | KI | P(OMe)3 | 32 |
| 15 | KI | P(OBut)3 | 41 |
| 16 | I2 | P(OEt)3 | 83 |
| 17 | KI | — | n.d. |
| 18 | — | P(OEt)3 | Trace |
| 19e | KI | P(OEt)3 | 75 |
| 20f | KI | P(OEt)3 | 44 |
| 21g | KI | P(OEt)3 | 33 |
| 22h | KI | P(OEt)3 | n.d. |
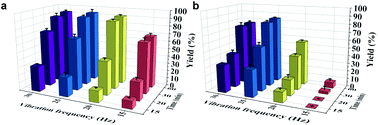
The mechanochemistry process parameters usually have a strong influence on the outcomes, as previously shown by us and others.12 Thus, a combined assessment of the grinding time and vibration frequency was carefully carried out; the results of I2/KH2PO2 system strategy are summarized in Fig. 1a. The yields increased gradually as the frequency was increased from 15 to 20 Hz. However, in contrast to the apparent influence of frequency, variation in the grinding time did not show any obvious difference from 20 to 30 Hz, where the best result could be obtained by milling at 30 Hz within 30 min (93% yield). However, the yield under optimal conditions did not change much from that conducted at 25 Hz with 20 min. For comprehensive energy consumption and environmental friendliness, we chose 25 Hz and 20 min as the subsequent experimental conditions. When we recorded the results of the KI/P(OEt)3 strategy (Fig. 1b), the best result was obtained by milling at 25 Hz within 60 min (82% yield). Of note, further increase the vibration frequency (30 Hz) led to a decrease of yield (76%). Since the reaction substrates are the same in these two systems, we speculate that distinct intermediates with inconsistent sensitivity to reaction energy would be presence.
With the optimal conditions in hand, there was a large difference in yield between the two different reaction systems, so substrate expansion was divided into two parts for elucidation. The scopes and limitations of this I2/KH2PO2 promoted methodology were investigated first by using various benzoic acids. The results are presented in Table 2. The electronic properties of the phenyl ring substituents of the carboxylic acids had some effect on the reaction. Generally, carboxylic acids possessing electron-withdrawing groups produced esters with lower yields (3ad, 3ae, and 3af). Carboxylic acids possessing electron-donating groups produced esters with higher yields (3ag, 3ah, and 3ai). The procedure tolerated a range of functional groups, such as chloro, bromo, naphthalene, and pyridine groups. The compatibility of halogen groups was synthetically useful since the products could easily be further modifiable (3ab and 3ac). Notably, 2-naphthoic acid and nicotinic acid ran smoothly under the standard conditions, producing esters in 85 and 83% yields, respectively (3aj and 3ak). Furthermore, butyric acid was explored. This reaction generated 3al in moderate yields; the reason for the lower yield might be the volatility of fatty acids. Then, the esterification of benzoic acid with several phenols and alcohols was investigated. Gratifyingly, p-cresol and 4-(tert-butyl) phenol produced p-tolyl benzoate 3ba and 4-(tert-butyl) phenyl benzoate 3bb in 87 and 76% yields, respectively. As expected, the trifluoromethyl and halogen groups on the phenyl ring of phenol derivatives were well compatible under the standard procedure (3bc–3bf). The ortho steric hindrance will reduce the yield of the product, even with a prolonged reaction time (3bj and 3bk). Phenolic compounds with electron withdrawing groups can produce corresponding esters in medium to good yields (3bg, 3bh, and 3bi). Methanol, ethanol, and butyl alcohol afforded the ester in 63%, 72% and 75% yield, respectively (3ca–3cc). Notably, the solketal could obtain the product 3cd in a medium yield.
| a Path A reaction conditions: 1 (0.5 mmol), 2 (0.6 mmol), I2 (0.5 mmol), KH2PO2 (0.5 mmol) and anhydrous sodium sulfate (0.4 g) at 25 Hz in mixer mill, using two stainless steel balls (ϕ = 1.2 cm, ΦMB = 0.036) in 50 mL stainless steel vial. Yield based on 1.b Path B reaction conditions: 1 (0.5 mmol), 2 (0.6 mmol), KI (0.75 mmol), P(OEt)3 (0.5 mmol) and anhydrous sodium sulfate (0.4 g) at 25 Hz in mixer mill, using two stainless steel balls (ϕ = 1.2 cm, ΦMB = 0.036) in 50 mL stainless steel vial.c 1 (2.0 mmol).d 2 (1.8 mmol). |
|---|
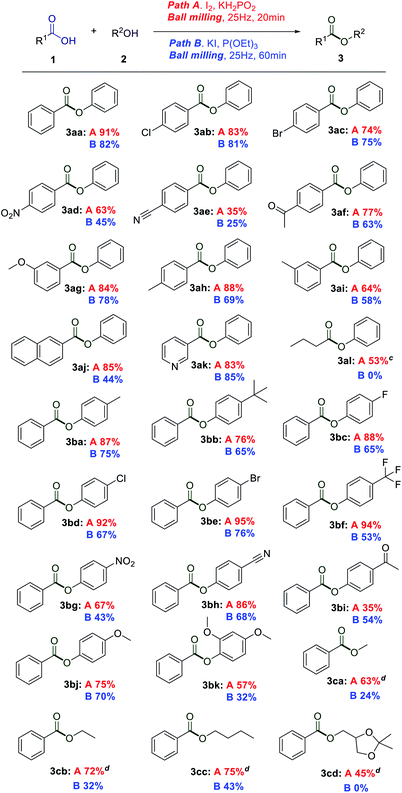 |
Next, the KI/P(OEt)3 promoted esterification reaction was examined. Unfortunately, it was observed that the KI/P(OEt)3 did not show better reaction efficiency than the I2/KH2PO2 system. As shown in Scheme 2, the varied substrate scope was evaluated. Compared with I2/KH2PO2, the yield of esterification reaction through KI/P(OEt)3 is reduced. Compared with that under I2/KH2PO2 conditions, the activity of methanol, ethanol and n-butanol in the conversion to corresponding esters was greatly weakened under KI/P(OEt)3 conditions (3ca–3cc). The solketal could not afforod product 3cd under these conditions, and butyric acid failed to deliver the desired ester. The KI/P(OEt)3 esterification reaction had serious substrate restrictions, which may be related to its possible reaction mechanism.
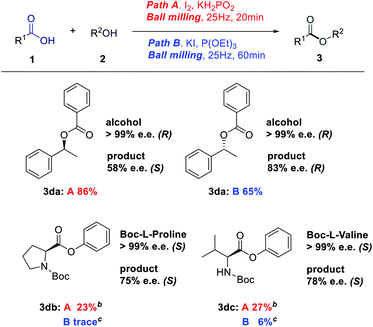
After that, the chiral molecule phenylethanol was tried. Phenylethyl benzoate could be obtained under two esterification conditions, but it was interesting that the configurations of the product were different (Table 3, 3da). Whatever, the expansion of amino acid substrates was not smooth, even if we reduced the milling frequency and prolonged the reaction time, poor yield was achieved (Table 3, 3db and 3dc).
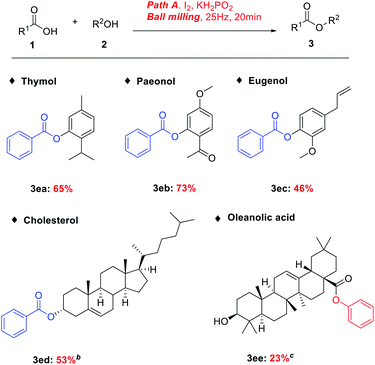
The broad synthetic utility of this process is further highlighted by the late-stage diversification of natural products. To our delight, thymol, paeonol and eugenol were tolerated, producing the resulting esters in medium yields (3da, 3db, and 3dc).13–15 We were pleased to find that the direct esterification of cholesterol (3dd),16 a cyclopentane polyhydrophenanthrene derivative necessary for the human body; and oleanolic acid (3de),17 a medicine for treating infectious acute jaundice hepatitis, furnished the esterification products in low to medium yields, demonstrating the synthetic advantage of the I2/KH2PO2 protocol (Table 4). However, the KI/P(OEt)3 protocol, delivered the esterification products only in trace amounts.
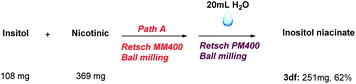
Afterwards, we explored the practicability of this method, which was exemplified through the synthesis of the antihyperlipidaemic agent inositol nicotinate (Scheme 2).18 This method can afford inositol nicotinate in moderate yield without purification by column chromatography.
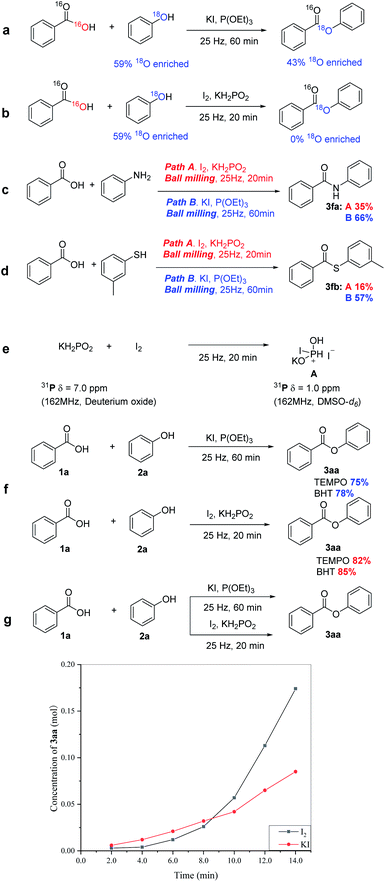
To shed light on the pathways of the two system dehydration platforms, several control experiments were performed. Firstly, we carried out mechanistic studies beginning with an isotope labelling experiment, whereby benzoic acid and 18O enriched phenol were subjected to the reaction conditions (Scheme 3a).19 In the presence of KI and P(OEt)3, the ester product was obtained with high 18O incorporation, which indicated oxygen transfer from the alcohol to the ester. In contrast, using I2 and KH2PO2, the ester product was obtained with high 16O incorporation, demonstrating oxygen transfer from the acid to the ester (Scheme 3b). After that, we verified the reaction mechanism by aniline and m-methylthiophenol. In the presence of KI and P(OET)3, the yields of N-benzamide and S-(m-tolyl) benzothioate were higher than that of I2/KH2PO2 strategy (Scheme 3c and d). Next, we examined changes to the additive under HSBM conditions. After HSBM of I2 and KH2PO2, the 31P chemical shift of KH2PO2 at 7.00 ppm was vanished, while a new peak at 1.00 ppm was formed, which was regarded as complex A, but not available in solution (Scheme 3e). We hypothesize that complex A (31P 1.00 ppm) can be produced in the HSBM environment, which is not available in solution. Both esterification methods were completely still underwent in the presence of radical scavengers, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) or 2,6-di-tert-butyl-4-methyl-phenol (BHT), to give 3aa in good yield (Scheme 3f). The difference of reaction kinetics was further studied by sampling in the first 15 minutes (the amount of 3aa was detected by HPLC). KI/P(OEt)3 reaction was almost carried out at a uniform rate. In the first 4 min, there was no obvious product formation in I2/KH2PO2 reaction, and the formation of 3aa product was accelerated after 6 min (Scheme 3g).
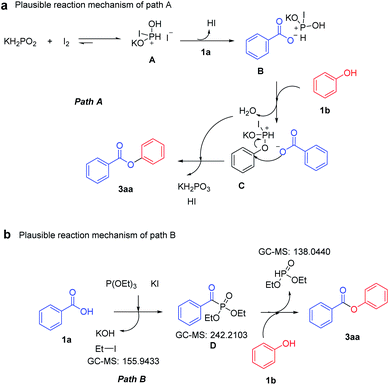
Based on the above results and Denton's work,20a a possible mechanistic pathway is proposed for the I2/KH2PO2 strategy (Path A). First, activated additive A is formed by the mechanochemical reaction of potassium hypophosphite and iodine. Then, as in the classical Mitsunobu reaction,20b,c the counter anion associated with phosphonium salt B may engage in nonproductive, reversible bonding and exchange at the phosphorus atom, but ultimately, intercepting phenol 1b to afford the conventional intermediate, the phenoxyphosphonium-nucleophile ion pair C. Subsequent nucleophilic substitution between phenoxyphosphonium salt C and intermediate B afforded the substitution product 3aa (Scheme 4a). Next, the mechanism of the KI/P(OEt)3 strategy is proposed through the results of GC-MS (Fig. S3†). The benzoic acid in the KI/P(OEt)3 system undergoes Michel–Arbuzov reaction to obtain diethyl benzoylphosphonate.21a,b According to previous reports, diethyl benzoylphosphonate can easily react with phenol to obtain the target product 3aa (Scheme 4b).21c
Conclusions
In conclusion, we have disclosed two protocols under HSBM conditions that facilitated facile esterification of carboxyl and hydroxyl groups. The I2/KH2PO2 strategy successfully replaced organic phosphorus (PPh3) with inorganic phosphorus salts (KH2PO2), while KI/P(OEt)3 strategy enabled the esterification with an easily removable P(OEt)3. Both methods are solvent-free and transition metal-free. Additionally, the feasibility of I2/KH2PO2 strategy was demonstrated by application to the esterification of natural products and the synthesis of inositol nicotinate. In addition, we discussed the different reaction mechanisms of the two systems through control experiment. Further investigation of environmentally friendly green chemistry reaction under HSBM conditions is underway in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (No. 21978270).Notes and references
- (a) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC; (b) L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC; (c) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- (a) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed; (b) S. Tabasso, D. Carnaroglio, E. C. Gaudino and G. Cravotto, Green Chem., 2015, 17, 684–693 RSC; (c) R. B. N. BaigandR and S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- (a) L. Takacs, Prog. Mater. Sci., 2002, 47, 355–414 CrossRef CAS; (b) T. Friscić, S. L. James, E. V. Boldyreva, C. Bolm, W. Jones, J. Mack, J. W. Steedh and K. S. Suslick, Chem. Commun., 2015, 51, 6248–6256 RSC.
- (a) J. B. Yu, Y. Zhang, Z. J. Jiang and W. K Su, J. Org. Chem., 2016, 81, 11514–11520 CrossRef CAS PubMed; (b) H. C Cheng, J. G. Hernández and C. Bolm, Org. Lett., 2017, 19, 6284–6287 CrossRef PubMed; (c) H. Xu, H. W Liu, K. Chen and G. W Wang, J. Org. Chem., 2018, 83, 6035–6049 CrossRef CAS.
- (a) A. Zapf and M. Beller, Top. Catal., 2002, 19, 101–109 CrossRef CAS; (b) J. Stetter and F. Lieb, Angew. Chem., Int. Ed., 2000, 39, 1724–1728 CrossRef; (c) N. Marion, S. Diez-Gonzalez and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS PubMed.
- (a) J. S. Carey, D. Laffffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC; (b) R. W. Dugger, J. A. Ragan and D. H. B. Ripin, Org. Process Res. Dev., 2005, 9, 253–258 CrossRef CAS; (c) K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef CAS PubMed; (d) T. Yuen, S. B. Sze and P. H. Toy, J. Am. Chem. Soc., 2006, 128, 9636–9637 CrossRef.
- (a) K. Ishihara, S. Ohara and H. Yamamoto, Tetrahedron, 2002, 58, 8179–8188 CrossRef CAS; (b) N. Bhatt, A. Patel, P. Selvam and K. Sidhpuria, J. Mol. Catal., 2007, 275, 14–24 CrossRef CAS; (c) N. Sanchez, M. Martinez, J. Aracil and A. Corma, J. Am. Oil Chem. Soc., 1992, 69, 1150–1153 CrossRef CAS; (d) E. Heykants, W. H. Verrelst, R. F. Parton and P. A. Jacobs, Stud. Surf. Sci. Catal., 1997, 105, 1277–1284 CrossRef.
- D. A. Watson, X. X. Fan and S. L. Buchwald, J. Org. Chem., 2008, 73, 7096–7101 CrossRef CAS PubMed.
- N. Iranpoor, H. Firouzabadi, D. Khalili and S. Motevalli, J. Org. Chem., 2011, 76, 2277–2281 CrossRef PubMed.
- G. C. Li, P. Lei and M. Szostak, Org. Lett., 2018, 20(18), 5622–5625 CrossRef CAS PubMed.
- B. H. Shih, R. S. Basha and C. F. Lee, ACS Catal., 2019, 9, 8862–8866 CrossRef CAS.
- (a) C. F. Bagamboula, M. Uyttendaele and J. Debevere, Food Microbiol., 2004, 21, 33–42 CrossRef CAS; (b) M. H. Havasia, A. J. Resslera, E. L. Parksa, A. H. Cocolasa, A. Weavera, N. P. Seeramb and G. E. Henrya, Inorg. Chim. Acta, 2020, 505, 119495–119502 CrossRef; (c) Y. Yua, Y. Liub, R. Shic, D. Zhanga, C. Lia and J. Shib, Bioorg. Chem., 2020, 98, 103644–103649 CrossRef PubMed.
- (a) K. M. Adki and Y. A. Kulkarni, Life Sci., 2020, 250, 117544–117561 CrossRef CAS PubMed; (b) Y. S. Hu, X. Han, P. J. Yu, M. M. Jiao, X. H. Liu and J. B. Shi, Bioorg. Chem., 2020, 98, 103735–103748 CrossRef CAS PubMed; (c) H. Zhang, X. Geng, Z. Li, Y. Li, K. Xu, H. Wu, J. Xie, P. Sun, S. Wei and M. Qiao, Front. Psychiatr., 2020, 11, 295–308 CrossRef; (d) Y. Peng, X. Zheng, Z. Fan, H. L. Zhou, X. X. Zhu, G. J. Wang and Z. H. Liu, Phytomedicine, 2019, 68, 1–48 Search PubMed.
- (a) C. S. Jia, D. D. Cao, S. P. Ji, X. M. Zhang and B. Muhoza, Food Hydrocolloids, 2020, 104, 105717–105769 CrossRef CAS; (b) H. G. Bilgicli, A. Kestane, P. Taslimi, O. Karabay, A. Bytyqi-Damoni, M. Zengin and I. Gulcin, Bioorg. Chem., 2019, 88, 102931–102937 CrossRef PubMed; (c) M. Govindarajan, M. Rajeswary, S. L. Hoti, A. Bhattacharyya and G. Benelli, Parasitol. Res., 2016, 115, 807–815 CrossRef.
- (a) A. Rohatgi, A. Khera, J. D. Berry, E. G. Givens, C. R. Ayers, K. E. Wedin, I. J. Neeland, I. S. Yuhanna, D. R. Rader, J. A. de Lemos and P. W. Shaul, N. Engl. J. Med., 2014, 371, 2383–2393 CrossRef CAS PubMed; (b) S. R. Pfeffer, J. Biol. Chem., 2019, 294, 1706–1709 CrossRef CAS PubMed; (c) B. M. Castellano, A. M. Thelen, O. Moldavski, M. Feltes, R. E. N. Van der Welle, L. Mydock-McGrane, X. Jiang, R. J. Van Eijkeren, O. B. Davis, S. M. Louie, R. M. Perera, D. F. Covey, D. K. Nomura, D. S. Ory and R. Zoncu, Science, 2017, 355, 1306–1311 CrossRef CAS PubMed.
- (a) T. B. Ayeleso, M. G. Matumba and E. Mukwevho, Molecules, 2017, 22, 1915–1930 CrossRef PubMed; (b) J. Pollier and A. Goossens, Phytochemistry, 2012, 77, 10–15 CrossRef CAS PubMed; (c) L. Žiberna, D. Šamec, A. Mocan, S. F. Nabavi, A. Bishayee, A. A. Farooqi, A. Sureda and S. M. Nabavi, Int. J. Mol. Sci., 2017, 18, 643–658 CrossRef PubMed.
- I. O. Poon, D. S. L. Chow and D. Liang, Am. J. Health-Syst. Pharm., 2006, 63, 2128–2134 CrossRef CAS PubMed.
- (a) S. Tanii, M. Arisawa and M. Yamaguchi, Chem. Commun., 2019, 55, 14078–14080 RSC; (b) A. N. Holding and J. B. Spencer, ChemBioChem, 2008, 9, 2209–2214 CrossRef CAS PubMed.
- (a) R. H. Beddoe, K. G. Andrews, V. Magné, J. D. Cuthbertson, J. Saska, A. L. Shannon-Little, S. E. Shanahan, H. F. Sneddon and R. M. Denton, Science, 2019, 365, 910–914 CrossRef CAS; (b) O. Mitsunobu, Synthesis, 1981, 1–28 CrossRef CAS; (c) O. Mitsunobu and M. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380–2382 CrossRef CAS; (d) J. An, R. M. Denton, T. H. Lambert and E. D. Nacsa, Org. Biomol. Chem., 2014, 12, 2993–3003 RSC.
- (a) P. J. Garegg and B. Samuelsson, J. Chem. Soc., Chem. Commun., 1979, 978–980 RSC; (b) E. D. Matveeva, T. A. Podrugina, N. G. Sandakova and N. S. Zefifirov, Russ. J. Org. Chem., 2004, 40, 1469–1472 CrossRef CAS; (c) C. A. Tolman, Chem. Rev., 1977, 77, 313–348 CrossRef CAS.
- (a) S. M. A. Kedrowski and D. A. Dougherty, Org. Lett., 2010, 12, 3990–3993 CrossRef CAS PubMed; (b) A. K. Bhattacharya and G. Thyagarajan, Chem. Rev., 1981, 81, 415–430 CrossRef CAS; (c) M. Sekine, A. Kume and T. Hata, Tetrahedron Lett., 1981, 22, 3617–3620 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, NMR spectra. See DOI: 10.1039/d0ra09437d |
| This journal is © The Royal Society of Chemistry 2021 |