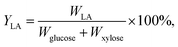Open Access Article
Open Access ArticleOpen simultaneous saccharification and fermentation of L-lactic acid by complete utilization of sweet sorghum stalk: a water-saving process
Yong Wang a,
Kai Huoa,
Lijuan Gaoa,
Guoqun Zhaoa,
Bin Wangb and
Jinlong Liu*a
a,
Kai Huoa,
Lijuan Gaoa,
Guoqun Zhaoa,
Bin Wangb and
Jinlong Liu*a
aFermentation Technology Innovation Center of Hebei Province, College of Food Science and Biology, Hebei University of Science and Technology, No. 26 Yuxiang Road, Yuhua District, Shijiazhuang 050018, PR China. E-mail: jlliu18@126.com; wangyong0520@foxmail.com
bQinhuangdao Bohai Biological Research Institute of Beijing University of Chemical Technology, Qinhuangdao 066000, PR China
First published on 27th January 2021
Abstract
A complete and efficient utilization of sweet sorghum stalk including sweet sorghum juice (SSJ) and sweet sorghum bagasse (SSB) was achieved via the open simultaneous saccharification and fermentation (SSF) of L-lactic acid. To simplify the pretreatment process and reduce water consumption, a combined hydrolysis approach was applied and the NaOH-pretreated liquor (SL) was utilized as a partial neutralizing agent. In order to further enhance the product titer, the acid hydrolysate of SSJ (SSJAH) was fed, and MgO was used as a neutralizing agent. A product titer of 94 g L−1 was obtained with a productivity of 1.55 g L−1 h−1, and the yield reached 98.31%. Totally, 274.79 g L-lactic acid was produced from 1 kg sweet sorghum stalk, and 83.22% water was saved compared with the previous study based on alkali pretreatment of SSB. This study provides an effective process for L-lactic acid biosynthesis from lignocellulosic substrates.
1 Introduction
The biosynthesis of lignocellulosic L-lactic acid has been intensively studied because of its substrate advantages including renewable nature, wide-distribution, abundance, and low cost.1 An alkali or acid pretreatment is generally required to disrupt the stable structure of lignocellulose, which further enhances the efficiency of enzymatic hydrolysis.2 Although various lignocellulosic substrates have been used for L-lactic acid biosynthesis,3–6 the compounds derived from pretreatment, which are released during the degradation of lignocellulose, have negative effects on both saccharification and the following fermentation. To remove the inhibitors from lignocellulose degradation and residual alkali or acid, thorough washing during the pretreatment process is usually conducted.7 However, a significant amount of water is consumed causing difficulties in L-lactic acid commercialization, and a substantial volume of wastewater should be strictly limited because of the high cost of treatment.8 Therefore, efficient pretreatment of a substrate without washing is the key to lignocellulosic L-lactic acid production.For bio-based bulk chemical production, sweet sorghum (Sorghum bicolor (L.) Moench) has attracted extensive attention.9 In addition, a considerable amount of soluble (43.6–58.2%) and insoluble (22.6–47.8%) sugars have been reported.10–12 Ou et al. proved that all the sugars present in sweet sorghum stalk can be utilized by Bacillus coagulans strains.13 Particularly, SSJ was adopted for L-lactic acid production, and a product titer of 142.49 g L−1 with a yield of 90% was obtained using Lactobacillus salivarius CGMCC 7.75.14 In our previous studies, SSB and SSJ have been separately applied for the efficient biosynthesis of L-lactic acid under unsterilized conditions.15,16 However, the complete utilization of sweet sorghum stalk for L-lactic acid biosynthesis has not been reported as far as we know. The water-saving process based on sweet sorghum stalk introduces a potential way for L-lactic acid industrialization, and the effects of the complex substrate system on L-lactic acid fermentation should be investigated for the utilization of lignocellulosic substrates.
In this study, sweet sorghum stalk (SSB and SSJ) was completely utilized for L-lactic acid biosynthesis, and a new thermophilic strain reported in the previous study was used.15 A combined hydrolysis approach was adopted, and the SSB slurry obtained from dilute acid pretreatment was neutralized with alkali-pretreated SSB to avoid neutralization and washing. The NaOH-pretreated liquor (SL) was used for substituting a partial neutralizing agent to increase the product yield. To further enhance the product titer, SSJ including abundant fermentable sugars was supplemented during the open SSF.17,18 Due to the promoting effect on relevant biosynthesis, which was revealed in the previous work, MgO was adopted as the neutralizing agent for the improvement of L-lactic acid biosynthesis.19 Significant reduction of water consumption was achieved in this study, and the concentration increase of inorganic salts derived from the pretreatment process did not obviously change the specific enzyme activity of cellulase and xylanase. In addition, the L-lactic acid titer, productivity, and product yield achieved were all maintained at relatively high levels in this work.
2 Methods
2.1 Materials and culture media
Sweet sorghum, YE and CSLP were obtained from the same source as previously reported.20 The specific activities of cellulase (Tianfeng Bioengineering Corporation, China) and xylanase (Imperial Jade Bio-technology CO., Ltd, China) were 78 FPU ml−1 and 100000 U g−1, respectively. The optimum temperature of both cellulase and xylanase are 50 °C, and the maximum activities of cellulase and xylanase were obtained at pH 4.8 and 5.0, respectively. All other chemicals adopted were of reagent grade and available in the market.The compositions of agar slant and inoculum were the same as that reported by Wang et al.21 To harvest mature cells of B. coagulans LA1507, an aerobic growth medium containing 10 g L−1 glucose and 15 g L−1 CSLP was adopted. The fermentation medium contained pretreated SSB slurry and 15 g L−1 CSLP. The NaOH-pretreated liquor (SL) was used for substituting the partial neutralizing agent. In batch fermentations, the initial concentration of stover was kept at 20% (w/v), and the acid hydrolysate of SSJ (SSJAH) was added as a nutritional supplement during fed-batch fermentation. Xylanase and cellulase were used for saccharification at 100 U g−1 and 25 FPU g−1 crude stover, respectively.
2.2 Pretreatment of raw materials
The squeezing of SSJ from the fresh stalks and the following acid hydrolysis were performed as mentioned by Wang et al.16 The clarified SSJAH containing 105.5 g L−1 glucose and 97.3 g L−1 fructose was stored at −20 °C for further utilization.The residuals after SSJ squeezing were chopped and dried at 80 °C. Then, the stalk was milled to a particle size of about 850 μm using a laboratory grinder. Finally, dry SSB containing 39.44% glucan, 20.16% xylan, 2.25% arabinian and 18.46% lignin was obtained. A stover loading of 10% (w/v) was adopted for acid/alkali pretreatments, and the concentration of H2SO4 or NaOH was 2% (w/w). During alkali pretreatment, SSB was pretreated at 118 °C for 80 min, and a condition of 130 °C for 60 min was maintained in dilute acid pretreatments.15,22
2.3 Influence of the pretreated liquor on enzyme activity and fermentation
Experiments were performed to study the activity changes of cellulase and xylanase in the pretreated liquor. After pretreatment, the H2SO4 pretreated liquor (HL) and SL were obtained by centrifugation, and the HL was neutralized by SL to the pH of 6.0. Cellulase at 3.21 mL and xylanase at 10 mg were added to the pretreated liquor (100 mL), respectively. Sodium acetate buffer (50 mM) was adopted in the control experiment which was conducted simultaneously. A temperature of 50 °C was maintained for a time period of 60 h, and the rotation rate was kept at 150 rpm. In addition, the composition of the pretreated liquor was analysed. To determine the influence of the pretreated liquor on fermentation, inhibitors including furfural, 5-hydroxymethylfurfural, formate, acetate, vanillin, sulfate and water-soluble lignin were separately added to the experimental flasks at the same concentration levels in pretreated liquor, and the percent OD620 of the experimental flasks relative to the control flasks (no inhibitor addition) were determined. Both the experimental flasks and control flasks contained the inoculum medium, as mentioned in Section 2.1.2.4 Enzymatic hydrolysis of the combined slurry of acid- and alkali-pretreated SSB
The pH changes were determined as different amounts of NaOH-pretreated SSB wet residue were added to the H2SO4-pretreated SSB slurry at 100 mL. Then, the mixed slurry was directly subjected to enzymatic hydrolysis. Both glucose and xylose concentration and sugar yield were determined when cellulase (25 FPU g−1 SSB) and xylanase (100 U g−1 SSB) were added. Different pH levels ranging from 5.0 to 7.0 were adopted, and a temperature of 50 °C was maintained for 60 h. The rotation rate was kept at 150 rpm.2.5 Microorganism and culture conditions
The thermophilic B. coagulans LA1507 (ref. 15) was used, and the strain was maintained at Fermentation Technology Innovation Center of Hebei Province. The preparation of inoculum was conducted as reported by Wang et al.,15 and an incubation rate of 10% (v/v) was adopted.The active cells of B. coagulans LA1507 were firstly obtained by aerobic cultivation at 50 °C, during which pH 6.0 was maintained using 33% (w/w) Ca(OH)2 as the neutralizing agent, and 2 vvm aeration was adopted with an agitation speed of 400 rpm. The initial cell density was maintained at 10 g L−1 in the following anaerobic fermentation. An open SSF at 50 °C was performed at an agitation speed of 260 rpm. A 5 L fermenter (GS-F2005AG, Good-Shine Biotechnology Limited., Shanghai, China) was used for batch and fed-batch fermentation keeping the initial volume at 2.0 L, and the SL was used for substituting partial 40% (w/w) NaOH as a neutralizing agent in batch fermentation. To further improve the L-lactic acid titer, the SSJAH was fed as a nutritional supplement in fed-batch fermentation, and the combination of SL and dry MgO was used as a neutralizing agent.
2.6 Analytical methods
L-Lactic acid and the cell density of B. coagulans LA1507 were determined by a SBA-40C biosensor analyzer and a spectrophotometer, as reported by Wang et al.23 and Ma et al.24 The calculation of L-lactic acid yield was on the basis of the equation:where YLA represents the L-lactic acid yield. The product amount accumulated is marked as WLA, and the amounts of glucose and xylose consumption are Wglucose and Wxylose, respectively. The consumption of glucose and xylose were obtained by using the method reported in the previous study.23 The quantitative determination of compositions of pretreated liquor and monosaccharides were conducted as reported by Zhang et al.25 The activities of cellulase and xylanase were determined according to methods in NREL26 and the State Standard of China (GB/T 23874-2009), respectively. In addition, the methods involved in the calculation of carbohydrates and lignin amounts from lignocellulosic substrate were according to the protocols of NREL.27
3 Results and discussion
3.1 Influence of the pretreated liquor on enzyme activity and fermentation
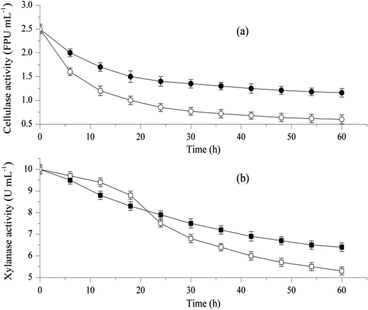
To reduce water consumption, the feasibility of the combined hydrolysis without washing was investigated, and the activity changes of cellulase and xylanase in the pretreated liquor were determined. Particularly, the pretreated liquor contained HL, which was neutralized by SL, and the control experiment was conducted as described in Section 2.3. As shown in Fig. 1a, the cellulase activity in the control condition decreased by 76% (from 2.50 FPU mL−1 to 0.60 FPU mL−1), and the cellulase activity in the pretreated liquor decreased by 53.6% (from 2.5 FPU mL−1 to 1.16 FPU mL−1) within 60 h of incubation. Similarly, a declining trend was obtained in xylanase activity (Fig. 1b), and the xylanase activities of 64% and 53% remained in the pretreated liquor and control group after 60 h, respectively. More significant enzyme activity loss for both cellulase and xylanase was observed under control conditions, which was also reported by Yu et al.26 For enzymatic activities in pretreated liquor, the decrease of 46.0% cellulase activity and 25.0% xylanase activity were observed at 30 h, and the further decline of 7.60% cellulase activity and 11% xylanase activity were obtained from 30 h to 60 h. It indicated that both cellulase and xylanase fit the slow downtrend in activity decline. The inorganic salt introduced by the neutralization scheme in combined hydrolysis contributes to the enhancement of the ionic strength, and partial enzyme inactivation under high ionic strength would result in the adverse performance of enzymatic hydrolysis, which has been reported by Palmqvist and Hahn-Hägerdal.28 However, water-soluble lignin (WSL) in pretreated liquor (Table 1) facilitates the saccharification of lignocellulose, and the interaction between the adsorption domain of hydrolysis enzymes and low-molecular-weight WSL was considered to be the potential reason.29 The results obtained indicated that pretreated liquor improved enzymatic saccharification by maintaining enzyme activities and overcame any negative effect from the increase of ionic strength. As the basic structure of the lignocellulosic substrate,30 a high association exists among macromolecular components; the deconstruction of SSB is essential for the accessibility to enzymatic hydrolysis.31 The high activities of cellulase and xylanase remaining in the pretreated liquor facilitates the enzymatic saccharification conversion of the pretreated SSB.| Composition | Concentration (g L−1) | RODc (%) |
|---|---|---|
| a HMF: 5-hydroxymethylfurfural.b Sulfate: calculated as sodium sulfate.c ROD: the percent OD620 of the experimental flask relative to the control flask (no inhibitor addition).d WSL: water-soluble lignin. | ||
| Furfural | 0.35 | 95.51 ± 2.21 |
| HMFa | 0.85 | 97.72 ± 3.32 |
| Formate | 0.31 | 96.31 ± 1.16 |
| Acetate | 3.25 | 100.10 ± 1.65 |
| Vanillin | 0.92 | 99.35 ± 2.25 |
| Sulfateb | 28.50 | 100.02 ± 0.52 |
| WSLd | 10.51 | 94.42 ± 1.42 |
The composition of pretreated liquor is shown in Table 1, and the effects of fractions on the growth of B. coagulans LA1507 were determined. Slight growth inhibitions were observed when furfural, HMF, formate and WSL, whose concentrations were equivalent to the pretreated liquor, were added in test experiments, and the ROD reached 95.51%, 97.72%, 96.31% and 94.42%, respectively. In addition, acetate, vanillin and sulfate showed little inhibitory effect on strain growth (Table 1). Thus, the pretreated SSB was expected to be utilized by B. coagulans LA1507 for L-lactic acid fermentation without washing and detoxification steps.
3.2 Enzymatic hydrolysis of pretreated SSB for fermentable sugar production
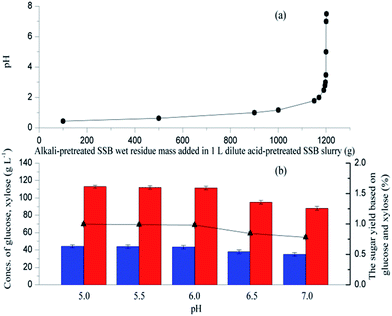
Different amounts of alkali-pretreated SSB were added in 1 L dilute acid-pretreated SSB slurry, and changes in pH were determined (Fig. 2a). The pH could be maintained at 5.0 to 7.0 when the wet residue mass added in 1 L dilute acid-pretreated SSB slurry reached about 1200 g. Furthermore, the pH level influenced the activities of cellulase and xylanase, which consequently affected the concentration and yield of sugar. In this study, different amounts of NaOH-pretreated SSB wet residue were added to H2SO4-pretreated SSB slurry at 100 mL to provide different pH levels (from 5.0 to 7.0), and the mixed slurry was directly subjected to enzymatic hydrolysis at 50 °C. As shown in Fig. 2b, the sugar yield and concentration significantly decreased when the pH was above 6.0, and the maximum sugar yield (99.7%) was obtained at pH 5.0, which provided 113 g L−1 glucose and 44.3 g L−1 xylose. The results indicated that the pH of 6.0 should be adopted with a high sugar yield and concentration while keeping a suitable pH value for bacterial growth and L-lactic acid biosynthesis.193.3 L-Lactic acid production by open SSF based on the combined hydrolysis of SSB
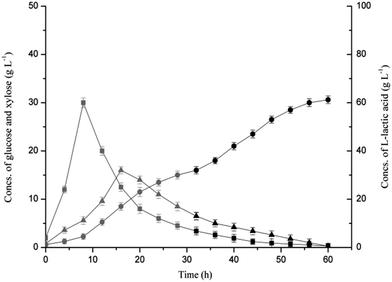
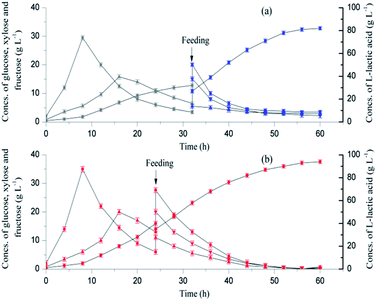
Open SSF based on the combined hydrolysis of SSB was conducted using Bacillus coagulans LA1507 in a 5 L fermenter, and SL was used to both substitute partial neutralizing agent and recover the residual sugars (Fig. 3). When SL was used as the neutralizer before 32 h, the titer of L-lactic acid reached 32 g L−1, and a productivity of 0.96 g L−1 h−1 was obtained. In the batch SSF process, the maximum concentrations of glucose and xylose reached 30 g L−1 at 8 h and 16 g L−1 at 16 h, respectively. The mixed sugars were sequentially consumed as B. coagulans LA1507 belongs to carbon catabolite repression positive strain,15 which was also observed by Zhang et al.32 At 32 h, the neutralizing agent was converted to 40% NaOH when the SL was totally consumed. A second productivity increment (1.04 g L−1 h−1), which may be caused by the reduction of the dilution effect of the neutralizing agent was observed. In addition, the concentrations of glucose and xylose decreased from 3.4 g L−1 and 6.5 g L−1 to 0.3 g L−1 and 0.25 g L−1, respectively, indicating the efficient utilization of glucose and xylose. Totally, 61.2 g L−1 L-lactic acid with an average productivity of 1.0 g L−1 h−1 was obtained, and the product yield reached 97.31%.Compared with the previous study,15 the dilution effect caused the decline of both titer and productivity when SL was used as the partial neutralizer. However, the product yield was maintained at a relatively high level (97.31%), which indicated the feasibility of efficient L-lactic acid biosynthesis based on combined hydrolysis, and the titer and productivity could be further improved by the supplementation of SSJAH and the optimization of neutralizing agents in the fed-batch fermentation. In addition, the washing process for detoxification in the substrate pretreatment was avoided, which would lead to a reduction in both water consumption and wastewater production.
3.4 The improvement of L-lactic acid biosynthesis by complete utilization of sweet sorghum stalk
To further improve L-lactic acid fermentation, MgO was used as a supplementary neutralizer due to the potential promoting effect of Mg2+ on L-lactic acid biosynthesis. Mg2+ is important for the folding and stability of the nucleic acid systems;33 it is also an activator or cofactor of the major enzymes in the glycolytic pathway.34 As a part of sweet sorghum stalk, SSJAH, which is rich in glucose and fructose, was supplemented in fed-batch fermentation.As shown in Fig. 4a, an L-lactic acid concentration of 32 g L−1 with a productivity of 0.96 g L−1 h−1 was obtained before 32 h when the SL was used as the neutralizing agent. With the SSF process, the maximum concentrations of glucose and xylose (29.5 g L−1 and 15.8 g L−1) were obtained at 8 h and 16 h, respectively. The residual glucose and xylose concentrations decreased to 3.4 g L−1 and 6.5 g L−1 at 32 h, respectively. The neutralizing agent was changed to dry MgO powder, and the SSJAH was fed as the substrate at 32 h. A second L-lactic acid productivity increment was observed, which may have been caused by the reduction of the dilution effect when the dry MgO powder was used instead of SL. However, the partial microenvironment with excessive acid or alkali caused by the incomplete mixing of the dry MgO powder limited the L-lactic acid biosynthesis, and residual glucose, xylose and fructose concentrations reached 3.5 g L−1, 2.95 g L−1, and 2.2 g L−1, respectively. Totally, a final product titer of 82 g L−1 with a product yield of 90.36% was reached, and the average productivity was 1.35 g L−1 h−1.
To avoid the incomplete mixing of dry MgO powder during fermentation, the dry MgO powder was suspended in SL with a loading of 2.5% (w/v), and the slurry obtained was used as a neutralizing agent. An L-lactic acid concentration of 94 g L−1 with a product yield of 98.31% was finally obtained, and the average productivity reached 1.55 g L−1 h−1 (Fig. 4b). The maximum concentrations of glucose and xylose were observed at 8 h (35 g L−1) and 16 h (20 g L−1), and the SSJAH was fed as the substrate at 24 h. The residual glucose, xylose and fructose concentrations were 0.1 g L−1, 0.72 g L−1 and 0.45 g L−1, respectively, indicating the efficient utilization of the carbon source.
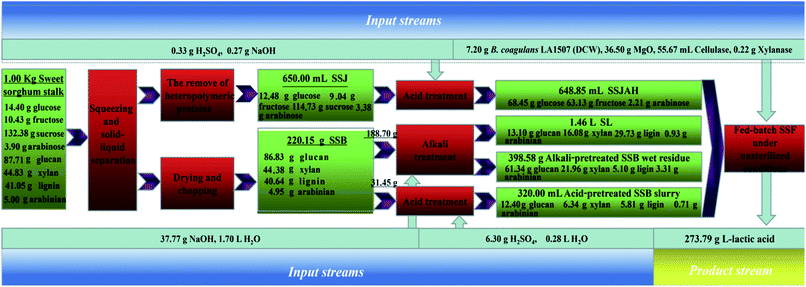
A comparison between this work and recent research on L-lactic acid biosynthesis based on lignocellulosic substrates was conducted as shown in Table 2. Different lignocellulosic substrates, including sugarcane bagasse, waste wood chips, bagasse sulfite pulp, rice straw, sweet sorghum bagasse, wheat straw and corn stover, have been intensively studied for L-lactic acid biosynthesis (Table 2). To improve the product yield and reduce water requirement and the sterilization cost, a substrate pretreatment process avoiding detoxification and washing followed by open SSF using thermophilic L-lactic acid producer such as Bacillus coagulans has been on the research front. Different from the previous studies (Table 2), the complete utilization of sweet sorghum stalk (SSB and SSJ) was achieved in L-lactic acid fermentation, and a combined hydrolysis approach excluding detoxification and washing was conducted followed by open SSF using Bacillus coagulans LA1507. In addition, balanced results were obtained in this work keeping the productivity, yield and titer at relatively high levels. A mass balance is briefly shown in Fig. 5, and a product yield of 0.274 g g−1 sweet sorghum stalk was obtained from open fed-batch SSF. The combined hydrolysis process of SSB and complete utilization of the carbon source in sweet sorghum stalk increased the product yield by 79.08% (from 0.153 g g−1 sweet sorghum stalk to 0.274 g g−1 sweet sorghum stalk), and decreased the water consumption by 83.22% (from 43.1 to 7.23 L kg−1 product) in L-lactic acid production compared with the previous study based on the alkali pretreatment of SSB.15
| Substrate | Microorganism | Fermentation type | Pretreatment mode | Washing process | Product titer (g L−1) | Yield (g g−1) | Productivity (g L−1 h−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| a g g−1 raw material.b g g−1 released sugar.c g g−1 pretreated material. | ||||||||
| Sugarcane bagasse | Lactobacillus pentosus | SSF | Acid | Inclusion | 72.75 | 0.61c | 1.01 | Unrean et al.35 |
| Waste wood chips | Lactobacillus paracasei 7BL | Fed-batch SHF | Acid and steam | None | 99 | 0.96b | 3.23 | Kuo et al.36 |
| Bagasse sulfite pulp | Bacillus coagulans CC17 | SSF | None | Inclusion | 110 | 0.72a | 0.57 | Zhou et al.37 |
| Rice straw | Bacillus coagulans LA-15-2 | Open SSF | Ethylenediamine | Inclusion | 92.5 | 0.58c | 2.01 | Chen et al.5 |
| Sweet sorghum bagasse | Bacillus coagulans LA1507 | Open SSF | Alkali | Inclusion | 111 | 0.43a | 1.59 | Wang et al.15 |
| Wheat straw | Bacillus coagulans IPE22 | Two-step SSF process![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Acid | None | 38.42 | 0.46a | 0.43 | Zhang et al.3 |
| Corn stover | Pediococcus acidilactici TY112 | Open SSF | Acid combined with biodetoxification | None | 97.3 | 0.69c | 1.5 | Qureshi et al.38 |
| Bacillus coagulans AD | Continuous![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Wet explosion | None | 35.2 | 0.95b | 3.69 | Ahring![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) et al.39 et al.39 |
|
| Pediococcus acidilactici ZY271 | SSF | Acid and biodetoxification | None | 130.3 | 0.85b | 1.81 | Wei et al.40 | |
| Lactobacillus pentosus FL0421 | Fed-batch SSF | Alkali | Inclusion | 92.3 | 0.66c | 1.92 | Hu et al.6 | |
| Sweet sorghum stalk | Bacillus coagulans LA1507 | Open SSF | Combined hydrolysis (acid and alkali) | None | 94 | 0.98b | 1.55 | This work |
Based on mass balance in this work (Fig. 5), inorganic salt (Na2SO4) introduced by the combined hydrolysis (acid and alkali) approach was about 0.024 mol L−1, which was only 2.31% of the lactate salts (1.04 mol L−1) produced. The increase in desalination pressure or the difference in wastewater production in the separation process was negligible. In addition, the purification of L-lactic acid from complex fermentation media has been achieved, and various combinations based on ultrafiltration and nanofiltration, electrodialysis, ion-exchange/adsorption, reactive distillation and hybrid short path evaporation have been reported.41,42 Thus, the further desalination for L-lactic acid purification based on the process in this study is feasible, and the product separation could sufficiently refer to the ways above.
4 Conclusions
To decrease the water consumed during L-lactic acid biosynthesis and improve the product yield, a combined hydrolysis process of SSB was conducted, and the complete utilization of sweet sorghum stalk based on open SSF was achieved. MgO was used as a supplementary neutralizer, and the L-lactic acid titer, average productivity and product yield reached 94 g L−1, 1.55 g L−1 h−1 and 98.31%, respectively, in the open fed-batch SSF. In total, 274.79 g L-lactic acid was produced from 1 kg sweet sorghum stalk, and 83.22% water was saved compared with the previous study based on alkali pretreatment. The results indicated the feasibility of efficient L-lactic acid production from lignocellulosic substrates. To further shorten the production cycle and reduce water consumption, future experiments may focus on innovation in the fermentation process, including open repeated-batch SSF and the investigation of a new biosynthesis system with higher solid loading.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the establishment of Fermentation Technology Innovation Center of Hebei Province. This work was supported by the Science and Technology Research Fund for Youth of Colleges and Universities in Hebei Province (grant no. QN2019115), National Training Program of Innovation and Entrepreneurship for Undergraduates (grant no. S202010082004), Shijiazhuang Science and Technology Research and Development Plan Project (grant no. 201490382A), the National Nature Science Foundation of China (grant no. 21908042), the Outstanding Youth Science Foundation of Hebei Province (grant no. B2020208007), and the Key Research and Development Project of Hebei Province (grant no. 20322906D).References
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286–301 CrossRef CAS.
- Z. Ruan, M. Zanotti, S. Archer, W. Liao and Y. Liu, Bioresour. Technol., 2014, 163, 12–17 CrossRef CAS.
- Y. Zhang, X. Chen, J. Luo, B. Qi and Y. Wan, Bioresour. Technol., 2014, 158, 396–399 CrossRef CAS.
- P. Unrean, Ind. Crops Prod., 2018, 111, 660–666 CrossRef CAS.
- H. Chen, W. Huo, B. Wang, Y. Wang, H. Wen, D. Cai, C. Zhang, Y. Wu and P. Qin, Ind. Crops Prod., 2019, 141, 111749 CrossRef CAS.
- J. Hu, Y. Lin, Z. Zhang, T. Xiang, Y. Mei, S. Zhao, Y. Liang and N. Peng, Bioresour. Technol., 2016, 214, 74–80 CrossRef CAS.
- Z. J. Wang, T. Q. Lan and J. Y. Zhu, Biotechnol. Biofuels, 2013, 6, 9 CrossRef CAS.
- G. Liu, J. Sun, J. Zhang, Y. Tu and J. Bao, Bioresour. Technol., 2015, 198, 803–810 CrossRef CAS.
- E. M. Rubin, Nature, 2008, 454, 841–845 CrossRef CAS.
- S. Amaducci, A. Monti and G. Venturi, Ind. Crops Prod., 2004, 20, 111–118 CrossRef CAS.
- G. Antonopoulou, H. N. Gavala, I. V. Skiadas, K. Angelopoulos and G. Lyberatos, Bioresour. Technol., 2008, 99, 110–119 CrossRef CAS.
- B. Z. Li, V. Balan, Y. J. Yuan and B. E. Dale, Bioresour. Technol., 2010, 101, 1285–1292 CrossRef CAS.
- M. S. Ou, D. Awasthi, I. Nieves, L. Wang, J. Erickson, W. Vermerris, L. O. Ingram and K. T. Shanmugam, BioEnergy Res., 2016, 9, 123–131 CrossRef CAS.
- Q. Liu, S. Wang, J. Zhi, H. Ming and D. Teng, Indian J. Microbiol., 2013, 53, 332–336 CrossRef CAS.
- Y. Wang, M. Wang, D. Cai, B. Wang, Z. Wang, P. Qin and T. Tan, RSC Adv., 2016, 6, 35771–35777 RSC.
- Y. Wang, J. Chang, D. Cai, Z. Wang and T. Tan, J. Chem. Technol. Biotechnol., 2017, 92, 1848–1854 CrossRef CAS.
- L. Laopaiboon, S. Nuanpeng, P. Srinophakun, P. Klanrit and P. Laopaiboon, Bioresour. Technol., 2009, 100, 4176–4182 CrossRef CAS.
- C. V. Ratnavathi, S. Kalyana Chakravarhy, V. V. Komala, U. D. Chavan and J. V. Patil, Sugar Tech., 2011, 13, 399–407 CrossRef CAS.
- Y. Wang, D. Cai, C. Chen, Z. Wang, P. Qin and T. Tan, Bioresour. Technol., 2015, 198, 658–663 CrossRef CAS.
- Y. Wang, S. Chen, J. Liu, P. Lv, D. Cai and G. Zhao, RSC Adv., 2019, 9, 22336 RSC.
- Y. Wang, Z. Yang, P. Qin and T. Tan, RSC Adv., 2014, 4, 8907–8913 RSC.
- Z. H. Ruan, M. Zanotti, Y. Zhong, W. Liao, C. Ducey and Y. Liu, Biotechnol. Bioeng., 2013, 110, 1039–1049 CrossRef CAS.
- Y. Wang, J. Liu, D. Cai and G. Zhao, Biotechnol. Biofuels, 2018, 11, 331 CrossRef CAS.
- K. Ma, T. Maeda, H. You and Y. Shirai, Bioresour. Technol., 2014, 151, 28–35 CrossRef CAS.
- J. Zhang, X. Ma, J. Yu, X. Zhang and T. Tan, Bioresour. Technol., 2011, 102, 4585–4589 CrossRef CAS.
- M. Yu, J. Li, S. Chang, L. Zhang, Y. Mao, T. Cui, Z. Yan, C. Luo and S. Li, Fuel, 2016, 175, 20–25 CrossRef CAS.
- R. Sindhu, M. Kuttiraja, P. Binod, R. K. Sukumaran and A. Pandey, Renewable Energy, 2014, 62, 362–368 CrossRef CAS.
- E. Palmqvist and B. Hahn-Hägerdal, Bioresour. Technol., 2000, 74, 25–33 CrossRef CAS.
- B. Jiang, J. Yu, X. Luo, Y. Zhu and Y. Jin, Process Biochem., 2018, 71, 147–151 CrossRef CAS.
- M. C. Mccann and N. C. Carpita, Curr. Opin. Plant Biol., 2008, 11, 314–320 CrossRef CAS.
- M. H. L. Silveira, A. R. C. Morais, A. M. C. Lopes, D. N. Olekszyszen, R. Bogel-Lulasik, J. Andreaus and L. P. Ramos, ChemSusChem, 2015, 8, 3366–3390 CrossRef CAS.
- Y. Zhang, X. Chen, J. Luo, B. Qi and Y. Wan, Bioresour. Technol., 2014, 158, 396–399 CrossRef CAS.
- Z. Zuo and J. Liu, J. Chem. Inf. Model., 2019, 59, 399–408 CrossRef CAS.
- T. H. Hohle and M. R. O'brian, Mol. Microbiol., 2014, 93, 736–747 CrossRef CAS.
- P. Unrean, Ind. Crops Prod., 2018, 111, 660–666 CrossRef CAS.
- Y. C. Kuo, S. F. Yuan, C. A. Wang, Y. J. Huang, G. L. Guo and W. S. Hwang, Bioresour. Technol., 2015, 198, 651–657 CrossRef CAS.
- J. Zhou, J. Ouyang, Q. Xu and Z. Zheng, Bioresour. Technol., 2016, 222, 431–438 CrossRef CAS.
- A. S. Qureshi, J. Zhang, L. da Costa Sousa and J. Bao, ACS Sustainable Chem. Eng., 2017, 5, 9254–9262 CrossRef CAS.
- B. K. Ahring, J. Traverso, N. Murali and K. Srinivas, Biochem. Eng. J., 2016, 109, 162–169 CrossRef CAS.
- C. Wei, G. Liu, J. Zhang and J. Bao, Ind. Crops Prod., 2018, 126, 415–420 CrossRef CAS.
- R. A. de Oliveira, A. Komesu, C. E. V. Rossell and R. M. Filho, Biochem. Eng. J., 2018, 133, 219–239 CrossRef.
- J. Sasiradee, M. Kienberger, M. Nuttakul and M. Siebenhofer, J. Chem. Technol. Biotechnol., 2017, 92, 2885–2893 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |