Open Access Article
Open Access ArticleEffect of ion exchange resin particle size on homogeneity and leachability of Cs and Co in polymer waste form†
Jueun Kim a,
Bhupendra Kumar Singh
a,
Bhupendra Kumar Singh ac and
Wooyong Um
ac and
Wooyong Um *abc
*abc
aDivision of Advanced Nuclear Engineering (DANE), Pohang University of Science and Technology (POSTECH), 77 Cheongam-ro, Nam-Gu, Pohang, Gyeongbuk 790-784, Republic of Korea. E-mail: wooyongum@postech.ac.kr
bDivision of Environmental Sciences and Engineering (DESE), Pohang University of Science and Technology (POSTECH), 77 Chongam-ro, Nam-Gu, Pohang 790-784, Republic of Korea
cNuclear Environmental Technology Institute (NETI), Pohang University of Science and Technology (POSTECH), Pohang, Gyeongbuk 790-784, Republic of Korea
First published on 13th January 2021
Abstract
We report the size effect of ion exchange resins (IERs) on Cs and Co distribution in polymer waste forms. Ball mill ground IERs (BG) waste form resulted in relatively better homogeneous waste distribution and displayed superior Cs and Co leachability indexes compared with the same polymer waste form prepared with non-ground IERs (NG).
Introduction
Nuclear energy can produce an abundance of electric energy without generating carbon dioxide. However, as nuclear power plant (NPP) operates continuously, various radioactive wastes are also generated in large quantities.1,2 Radioactive waste must be isolated from humans and the environment to satisfy long-term disposal safety. Even after 300 years or more, the risk of radioactive waste must meet the safety requirements for disposal facilities.3 Therefore, it is important to reduce, treat, and immobilize the radioactive wastes in a suitable waste form for their final disposal in the repository.Ion exchange resins (IERs) are widely used in NPP operations to purify the reactor coolant systems, clean up the spent fuel storage tanks, and remove the radioactive contaminants in liquid radioactive waste management systems.4–6 The annual production of spent IERs could vary by NPP types. However, an average annual volume of spent IERs is observed as 15.7 m3 per year in the pressurized water reactor (PWR).7 Even though the radioactivity of spent IERs depends on the operation history of a reactor, 60Co and 137Cs are generally the main sources of their radioactivity. In the case of PWR, the average activities in spent IERs are 370 GBq m−3 and 1900 GBq m−3 for 60Co and 137Cs, respectively.4 These spent IERs are classified into wet solid waste and need to be solidified before their disposal in low- and intermediate-level radioactive waste (LILW) repository. The solidified matrix serves as a primary barrier to limit the leaking of radionuclides.8 The characteristics of solidified radioactive waste vary depending on the type of waste, the physicochemical properties of the radioactive waste, and the solidification medium. The most common matrices used to solidify spent IERs are cement, bitumen, and polymer.9 Cement waste form is widely used to immobilize spent IERs because it is economical, easy to process, and its alkaline chemistry ensures low solubility for cationic radionuclides.10 However, its high porosity leads to lower leaching resistance, and subsequently, the final waste volume is significantly increased.6,11 Also, spent IERs can swell by absorbing free water in the cement, which results in cracks, poor mechanical strength, and corrosion in drums.5,12 Bitumen has the advantages of volume reduction and a lower leaching rate compared with cement, but the disadvantage of requiring a high temperature thermal process during bituminization.5,13 In the case of polymer waste form, the properties depend on the type of polymer used to prepare the waste form (e.g., epoxy resin, polyester, polystyrene). However, in general, it has high compressive strength, thermal conductivity, and radiation stability.4 Cement can load less than 20% of IERs, however polymer waste form can incorporate up to 50–60% of IERs.6,14 In this study, an epoxy resin, which is widely used to embed IERs in France, was used for polymer waste form because polymerization process could occur in room temperature.
The final waste form must comply with the waste acceptance criteria for compressive strength, leaching, immerion, thermal cycling, and irradiation tests. Also, homogeneity of the solidifying matrix as well as the waste distribution are the most important properties for acceptance of waste forms in long-term storage.15 Various physicochemical properties including density, porosity, permeability, compressive strength, radiation damage, and thermal conductivity of immobilized waste can be significantly varied in heterogeneous and homogeneous waste matrices.
To the best of our knowledge, no study has investigated the impact of homogeneous and heterogeneous polymer waste forms on the leaching behavior of radionuclides. Herein, we used IERs contaminated with stable Cs and Co as surrogates of 137Cs and 60Co, respectively. In addition, ball mill ground IERs (BG) and non-ground IERs (NG) were used to prepare homogeneous and heterogeneous waste forms, respectively. The major objective of this work was to evaluate Cs and Co distribution in the polymer waste forms using different analytical techniques: laser-induced breakdown spectroscopy (LIBS); scanning electron microscope-energy dispersive spectrometer (SEM-EDS); and X-ray fluorescence (XRF) analyses. In addition, leaching and compressive strength tests were conducted to compare the effects of homogeneity and heterogeneity on final waste forms. The obtained data revealed a significant impact of homogeneity on the leaching behavior of Cs and Co, which displayed an efficient leaching resistivity and immobilization capacity of homogeneous waste form for both Cs and Co over its heterogeneous counterparts.
Results and discussion
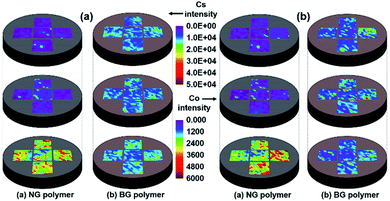
The average particle size of IERs was decreased from 527.6 to 53.4 μm after 5 h of ball mill treatment. The photographs of NG and BG polymer waste forms are shown in Fig. S1(a and b), respectively (ESI).† Both NG and BG polymer waste forms were well prepared without cracking, even at 50 wt% IERs waste loading. Fig. 1(a and b) show the distribution of Cs and Co, respectively, in NG and BG polymer waste forms, from different sections before leaching experiments. Fig. 1(a) presents the distribution of Cs in the top, middle, and bottom sections of NG (left) and BG (right) polymer waste forms; both waste forms seemed to be homogeneous in a radial direction. The concentration of Cs seemed to be negligible in both the top and middle sections of the NG polymer waste form, whereas the intensity of Cs was significantly increased in the bottom section, which confirms the heterogeneous Cs distribution in a vertical direction. LIBS analysis for Cs intensity in the different sections of BG polymer waste form displayed an approximately homogeneous distribution of Cs among all sections (top, middle, and bottom), which in turn indicates that the ball mill grinding of the IERs is necessary for the homogeneous distribution of Cs for IERs in polymer waste forms.The heterogeneity of NG polymers is likely to be caused by the mass differences between IERs and polymer, with similar trends also being observed with respect to Co distribution (Fig. 1(b)). Recently, Lin et al.16 also reported an uneven (heterogeneous) distribution of IERs in geopolymer waste form, where they worked on the solidification of IERs using metakaolin-based geopolymer binder. The authors observed that the IERs were located only in the upper half of geopolymer waste form. These results suggest that heterogeneity may occur for the IERs without grinding in other waste form matrices as well. LIBS analysis data for Cs and Co intensity in both NG and BG polymer waste forms were also plotted as a distribution chart with respect to different regions (top, middle, and bottom) (Fig. S2†). The results present similar trends as it has shown in Fig. 1 for both Cs and Co distribution in different regions of NG and BG polymer waste forms.
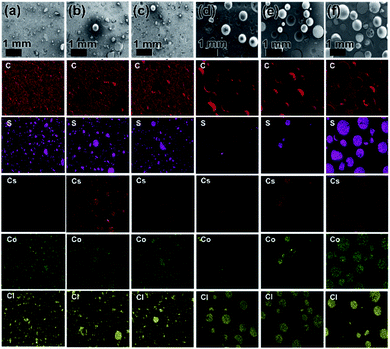
In order to evaluate the LIBS results for the distribution (hetero/homogeneity) of Cs and Co in different sections of NG and BG polymer waste forms, the samples were further characterized using SEM-EDS elemental mapping and XRF analysis (Fig. 2 and Table S1†). Both SEM-EDS and XRF provided meaningful results with respect to Cs and Co distribution in the solid waste matrices and showed almost similar trends to the LIBS results. Fig. 2 presents SEM-EDS elemental mapping results of the NG and BG polymer waste forms for C, S, Cs, Co, and Cl elements. Nearly a unique (homogeneous) distribution of both Cs and Co can be observed in all three parts of BG polymer waste forms (Fig. 2(a–c)). Whereas, an uneven (heterogeneous) distribution of Cs and Co elements can be clearly seen in top, middle, and bottom parts of NG polymer waste forms (Fig. 2(d–f)). Elemental mapping of NG polymer waste form displayed almost negligible concentrations of both Cs and Co in the top sections along with a minor presence of both elements in the middle. However, their concentrations were found to be highest in the bottom sections of the waste forms.
The XRF analysis data are given in Table S1,† which firmly corroborate the LIBS and SEM-EDS results. The concentrations of both Cs and Co were not detectable or low concentration in the top and middle parts of NG polymer waste form. However, the bottom part has displayed the highest (approximately total) concentration of Cs and Co, which indicates the heterogeneous distribution of Cs- and Co-loaded IERs in polymer waste forms. Interestingly, almost a uniform concentration of both Cs and Co was seen in all three sections with respect to BG polymer waste form. Overall, SEM-EDS and XRF data clearly support the observations made by the LIBS analysis, which in turn confirms the applicability and potential use of the LIBS technique for hetero/homogeneity determination in solid waste forms.
To investigate the effect of heterogeneity and homogeneity (waste distribution) on the immobilization capacity of NG and BG polymer waste forms, leaching experiments of Cs and Co from these waste forms were conducted up to 90 d. Fig. S1(c and d)† show the photographs of NG and BG polymer waste forms after 90 d of leaching tests, respectively. NG polymer waste form demonstrated a heterogeneous appearance after leaching tests, whereas BG polymer waste form remained intact and displayed a homogeneous property even after 90 d of leaching. In order to define their mechanical stability, compressive strength tests of both NG and BG polymer waste forms were conducted before and after leaching experiments, and the results are given in Table S2.† The uniaxial compression tests were measured three times before leaching and once after leaching. The compressive strengths of NG and BG polymers before leaching were found to be 111 and 100 MPa, respectively. BG polymer had a slightly lower compressive strength than NG polymer; however, both polymer waste forms showed remarkably elevated compressive strengths, which were ∼30 times higher than the acceptance criteria for waste form of 3.44 MPa.17 The compressive strengths of NG and BG polymer waste forms decreased to 50 and 72 MPa after 90 d of leaching tests, respectively. The mechanical strength of NG polymer waste form significantly declined about 55%, whereas BG polymer waste form showed a decrement in mechanical strength of about 28%, which clearly indicates the superiority and suitability of BG polymer waste form over NG polymer waste form.
The leachability index (LI) and the mean value of effective diffusivity (Di) of both Cs and Co are summarized in Table S3.† The LICs were calculated as 10.49 and 11.13 for NG and BG polymer waste forms, respectively, whereas the LICo were found to be 14.44 and 15.18 for NG and BG waste forms, respectively. Both NG and BG polymer waste forms strongly satisfy the requirements for waste form acceptance into the repository, which stipulates that LI must be higher than 6 for Cs- and Co-containing waste forms.18 Dermatas et al. reported that the effective diffusion coefficients (Di) generally vary from 10−5 to 10−15 cm2 s−1, where the smaller the number, the more immobile.19 In the present study, the average values of DCs for NG and BG polymer were 4.56 × 10−11 cm2 s−1 and 7.76 × 10−12 cm2 s−1, respectively, while the average values of DCo for NG and BG polymer were 4.33 × 10−13 cm2 s−1 and 4.23 × 10−15 cm2 s−1, respectively. Therefore, both Cs and Co showed low effective diffusion coefficients compared with the previous study.20
Fig. 3 gives the cumulative fraction leached (CFL) for Cs and Co leaching from the simulated NG and BG polymer waste forms over the 90 d leaching period. Cs and Co released from solidified matrices were more dominant in NG polymer waste form. Total released amount of Cs and Co in NG polymer were observed as ∼3.4 and 4.8 times higher than those in BG polymer waste form (Fig. 3(a and b)). To discuss the released mechanism of Cs and Co, the data were also presented in square rooted time versus total released amount.21 Cs released amounts in both NG and BG polymer waste forms were well fitted to the linear correlation, with correlation coefficient (R2) values of 0.982 and 0.997, respectively, which reveals that the release-controlling mechanism of Cs in polymer waste form was diffusion (Fig. 3(c and d)).21,22 Diffusion was also considered to control Co release from NG and BG polymer (R2 = 0.604 and 0.972, respectively). However, Co release from NG polymer can be attributed to the wash-off effect followed by diffusion, as Co was mainly released at the beginning of the leaching experiments.23 Generally, IERs affinity increases with an increasing charge and atomic number, therefore, IERs strongly bonded with Co2+ rather than Cs+.6 As a result, Co is expected to have less leachability than Cs during long-term waste disposal. More importantly, we can propose that the BG treatment based waste forms (which gives nearly uniform/homogenous distribution of wastes) could be better alternative for long-term disposal of nuclear waste, and hence, the immobilization of radionuclides in the underground repository.
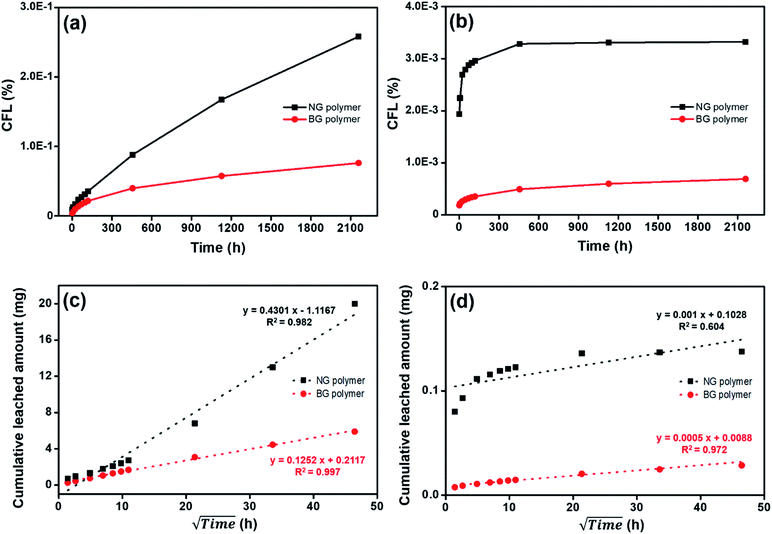 | ||
| Fig. 3 Leaching behavior of (a and c) Cs and (b and d) Co from NG and BG polymer waste forms. The data were collected up to 90 d (2160 h). | ||
Conclusions
In summary, we have developed a facile approach for the synthesis of a homogeneous epoxy polymer waste form using a ball mill treatment method. The homogeneity tests for Cs and Co in polymer waste forms were determined using LIBS analysis, which was further complemented by SEM-EDS and XRF results. Cs and Co leaching experiments from BG polymer waste forms revealed higher LIs for both Cs and Co compared with NG polymer waste form. Overall, the BG treated waste forms (which give nearly homogeneous distribution of wastes) could be better for the immobilization of radionuclides in polymer waste form for long-term disposal.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government, Ministry of Trade Industry & Energy (MOTIE) (20171520000410, Development of continuous treatment system for decommissioning radiowaste based on mechanochemistry process). We greatly appreciate to Seonggon Ryu researcher from Nuclear Engineering Services and Solutions (NESS) for supporting us to make polymer waste forms.References
- J. Saling, Radioactive waste management, CRC Press, 2001 Search PubMed.
- R. Rahman, H. Ibrahium and Y.-T. Hung, Water, 2011, 3, 551–565 CrossRef.
- K. Krauskopf, Radioactive waste disposal and geology, Springer Science & Business Media, 2013 Search PubMed.
- IAEA, Treatment of Spent Ion-exchange Resins for Storage and Disposal, IAEA, 1985 Search PubMed.
- J. Wang and Z. Wan, Prog. Nucl. Energy, 2015, 78, 47–55 CrossRef CAS.
- Application of ion exchange processes for the treatment of radioactive waste and management of spent ion exchangers, International Atomic Energy Agency (IAEA), 2002 Search PubMed.
- U. S. N. R. Commission, Final Comparative Environmental Evaluation of Alternatives for Handling Low-Level Radioactive Waste Spent Ion Exchange Resins from Commercial Nuclear Power Plants, 2013 Search PubMed.
- J. Koťátková, J. Zatloukal, P. Reiterman and K. Kolář, J. Environ. Radioact., 2017, 178, 147–155 CrossRef.
- R. O. A. Rahman, R. Z. Rakhimov, N. R. Rakhimova and M. I. Ojovan, Cementitious materials for nuclear waste immobilization, John Wiley & Sons, 2014 Search PubMed.
- M. Yousuf, A. Mollah, R. K. Vempati, T.-C. Lin and D. L. Cocke, Waste Manag., 1995, 15, 137–148 CrossRef CAS.
- Y. Zhou, G. Yun and Y. Ye, Tsinghua Sci. Technol., 2002, 7, 636–640 CAS.
- M. Matsuda, T. Nishi, K. Chino and M. Kikuchi, J. Nucl. Sci. Technol., 1992, 29, 883–889 CrossRef CAS.
- J. Sercombe, B. Gwinner, C. Tiffreau, B. Simondi-Teisseire and F. Adenot, J. Nucl. Mater., 2006, 349, 96–106 CrossRef CAS.
- C. Courtois, J. Moncouyoux and E. Revertegat, Nucl. Technol., 1996, 115, 198–207 CrossRef CAS.
- B. Rzyski and A. Suarez, Nucl. Chem. Waste Manage., 1988, 8, 211–215 CrossRef CAS.
- W. Lin, H. Chen and C. Huang, Prog. Nucl. Energy, 2020, 129, 103508 CrossRef CAS.
- J. Ahn, W.-S. Kim and W. Um, J. Nucl. Mater., 2019, 518, 247–255 CrossRef CAS.
- K. H. Kim, Y. G. Ryu and T. K. Kim, Comparison of Various Standard Test Methods for Characterization of Radioactive Waste Forms, Korea Atomic Energy Research Institute, 2008 Search PubMed.
- D. Dermatas, D. H. Moon, N. Menounou, X. Meng and R. Hires, J. Hazard. Mater., 2004, 116, 25–38 CrossRef CAS.
- I. Plecas, R. Pavlovic and S. Pavlovic, J. Nucl. Mater., 2004, 327, 171–174 CrossRef CAS.
- T. Guo, P. S. Deshpande and K. A. Rusch, Environ. Sci. Technol., 2004, 38, 603–608 CrossRef CAS.
- S. N. Lekakh, C. H. Rawlins, D. G. Robertson, V. Richards and K. D. Peaslee, Metall. Mater. Trans. B, 2008, 39, 125–134 CrossRef.
- R. Malviya and R. Chaudhary, J. Mater. Cycles Waste Manage., 2006, 8, 78–87 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09669e |
| This journal is © The Royal Society of Chemistry 2021 |