Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical characterization, cytotoxic, antioxidant, antimicrobial, and enzyme inhibitory effects of different extracts from one sage (Salvia ceratophylla L.) from Turkey: open a new window on industrial purposes
Sengul Uysal *ab,
Gokhan Zengin
*ab,
Gokhan Zengin c,
Kouadio Ibrahime Sinanc,
Gunes Akc,
Ramazan Ceylanc,
Mohamad Fawzi Mahomoodally
c,
Kouadio Ibrahime Sinanc,
Gunes Akc,
Ramazan Ceylanc,
Mohamad Fawzi Mahomoodally d,
Ahmet Uysale,
Nabeelah Bibi Sadeerd,
József Jekőf,
Zoltán Cziákyf,
Maria João Rodriguesg,
Evren Yıldıztugay
d,
Ahmet Uysale,
Nabeelah Bibi Sadeerd,
József Jekőf,
Zoltán Cziákyf,
Maria João Rodriguesg,
Evren Yıldıztugay h,
Fevzi Elbasanh and
Luisa Custodiog
h,
Fevzi Elbasanh and
Luisa Custodiog
aErciyes University Halil Bayraktar Health Services Vocational College, Kayseri, Turkey. E-mail: senguluysal@erciyes.edu.tr
bDrug Application and Research Center, Erciyes University, Kayseri, Turkey
cPhysiology and Biochemistry Research Laboratory, Department of Biology, Science Faculty, Selcuk University, Campus, Konya, Turkey
dDepartment of Health Sciences, Faculty of Medicine and Health Sciences, University of Mauritius, Réduit, Mauritius
eDepartment of Medicinal Laboratory, Vocational School of Health Services, Selcuk University, Konya, Turkey
fAgricultural and Molecular Research and Service Institute, University of Nyíregyháza, Nyíregyháza, Hungary
gCentre of Marine Sciences, University of Algarve, Faculty of Sciences and Technology, Ed. 7, Campus of Gambelas, 8005-139 Faro, Portugal
hDepartment of Biotechnology, Science Faculty, Selcuk University, Campus, Konya, Turkey
First published on 28th January 2021
Abstract
In the present study, the methanolic, hydro-methanolic, dichloromethane, hexane and aqueous extracts of Salvia ceratophylla L. (Family: Lamiaceae), a lemon-scented herb, were tested for total phenolic (TPC) and flavonoid content (TFC) and antioxidant activities were evaluated using a battery of assays (2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity, total antioxidant capacity (TAC) (phosphomolybdenum) and metal chelating). Enzyme inhibitory effects were investigated using acetyl- (AChE), butyryl-cholinesterase (BChE), tyrosinase, α-amylase and α-glucosidase as target enzymes. Regarding the cytotoxic abilities, HepG2, B164A5 and S17 cell lines were used. The phytochemical profile was conducted using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). Our data showed that the methanolic aerial extracts possessed the highest phenolic (72.50 ± 0.63 mg gallic acid equivalent per g) and flavonoid (43.77 ± 1.09 mg rutin equivalent per g) contents. The hydro-methanolic aerial extract showed significant DPPH radical scavenging activity (193.40 ± 0.27 mg TE per g) and the highest reducing potential against CUPRAC (377.93 ± 2.38 mg TE per g). The best tyrosinase activity was observed with dichloromethane root extract (125.45 ± 1.41 mg kojic acid equivalent per g). Among the tested extracts, hexane root extract exerted the highest antimicrobial potential with a minimum inhibitory concentration value of 0.048 mg mL−1. Methanolic root extract showed the lowest cytotoxicity (28%) against HepG2 cells. Phytochemical analysis revealed the presence of important polyphenolic compounds including luteolin, gallic acid, rosmarinic acid, to name a few. This research can be used as one methodological starting point for further investigations on this lemon-scented herb.
1. Introduction
Salvia ceratophylla L. (S. ceratophylla) is a biennial lemon-scented herb belonging to one of the largest genera of the Lamiaceae comprising of about 900 species distributed worldwide.1 The herb is native to numerous places such as Afghanistan, Iran, Iraq, Lebanon-Syria, Palestine, the Transcaucasus, Turkey, and Turkmenistan.2 Published literature reported that a number of different Salvia species and their respective essential oils have showed promising pharmacological propensities namely antioxidant, cytotoxicity,3 antibacterial, anti-neurodegenerative,4 anti-enzymatic (anticholinesterase, anti-urease, anti-tyrosinase, anti-elastase),5 anti-tumour6 and antidiabetic activities7 to name a few. The herb, S. ceratophylla, in particular, is aromatic and in a recent analysis its essential oil (EO) was reported to possess anti-trypanosomal effects resulting with an inhibitory concentration (IC) 50 of 2.65 μg mL−1. The hexane extract demonstrated cytotoxicity activity against mouse erythroleukemia (MEL), KB (containing human papillomavirus 18 (HPV-18)), BT-549 (human breast cancer cell line), SK-OV-3 (human ovarian cancer cell line), LLC-PK1 (renal epithelial cell line) and VERO (kidney epithelial cell line) cell lines with IC50 values ranging from 60 to 100 μg mL−1. There are further data concerning antioxidant and chemical composition of the EO.8–11 The review of Ulubelen12 stated that the terpenoids present in S. ceratophylla exhibited interesting antibacterial activity. The work of Goren et al.13 also showed that the diterpenoids identified from the root of the herb exhibited strong antibacterial activity against Staphylococcus epidermidis and Proteus mirabilis. Furthermore, two seco-4,5-abietane diterpenoids showed cytotoxic effects against MOLT-4 (human acute T lymphoblastic leukaemia cells) and MCF-7 (human breast cancer cell line) cell lines.14 In another study, the chloroform extract of S. ceratophylla significantly depressed anti-butyrylcholinesterase activity with a percentage inhibition of 91.3%.15 Based on ethnobotanical information, S. ceratophylla were used to treat cancers, infections, urinary complications,8 inflammation, and even nociceptive disorders.8,16,17 The World Health Organisation outlines that cancer is the second leading cause of death across the globe with 9.6 million deaths recorded in the year 2018.2 Despite cancer is one of the most studied disease and the clinical care and technology have advanced greatly, yet cancer remains still incurable.18 Natural products have been the only storehouse of pharmaceuticals for decades and have contributed enormously in human health through effective and unique bioactive compounds. Oxidative stress involving free radicals is the onset of several chronic diseases including cancers, neurological disorders, and cardiovascular diseases.19 Medicinal plants act as a major reserve of pharmaceuticals since the early days of mankind. Today, more than 80% of medicines are directly or indirectly linked to medicinal plants due to their strong pharmacological properties, low toxicity and low cost.20 On many occasions, natural enzyme inhibitors isolated from medicinal plants have been acknowledged as useful therapeutic tools for the management of numerous human pathologies.Therefore, the quest for novel and efficient drugs from medicinal plants should be an ongoing process and a continuing need. For this reason, we evaluated the aerial part and root extracts of S. ceratophylla prepared from polar and non-polar solvents for their antioxidant, anti-enzymatic [acetylcholinesterase (AChE), butyrylcholinesterase (BChE), amylase, glucosidase, tyrosinase], anti-microbial and cytotoxicity activities. To the best of our knowledge, this is the first time the polar and non-polar extracts of this plant will be evaluated for the aforementioned studies and compiled in one single research work. The total phenolic and flavonoid content were quantified and the prepared extracts were screened for phytochemicals using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) in order to correlate the observed biological activities with the biomolecules present. We believe that this study will add information on S. ceratophylla that can be used for further investigations.
2. Materials and methods
2.1. Plant material and preparation of extracts
Salvia ceratophylla samples were collected from natural population (Akyer village, Bozdağ national park, 1020 m, and steppes areas) in the summer period of 2019. Botanical identification was performed by one of the co-authors (Dr Evren Yıldıztugay) and a voucher specimen was kept at the herbarium of Selcuk University (EY-3005). Aerial parts and roots were carefully separated, dried in a shade for ten days, and then grinded by using a laboratory mill.Different extracts were used in this study. To this end, powdered aerials parts and roots (5 g) were extracted in n-hexane, dichloromethane (DCM), methanol, methanol–water (80%) (100 mL) under stirring for 24 h at 25 °C. After that, the solvents were removed by a rotary evaporator and the extracts stored at 4 °C until analysis. Regarding aqueous extracts, we used traditional infusion technique and the plant material (5 g) were kept with 100 mL of boiled water. The extracts were filtered and then lyophilized. All extracts were stored at 4 °C in a refrigerator. The extraction yields (%) are given in Table 1.
| Parts | Solvents | Yield (%) | TPC (mg GAE per g) | TFC (mg RE per g) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Values are reported as mean ± SD. DCM: dichloromethane; MeOH: methanol; TPC: total phenolic content; TFC: total flavonoid content; GAE: gallic acid equivalent; RE: rutin equivalent. Different letters indicate significant differences in the extracts (p < 0.05). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aerial parts | Hexane | 4.0 | 17.33 ± 0.10h | 5.25 ± 0.12f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 4.96 | 21.72 ± 0.20f | 28.79 ± 1.34b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 12.11 | 72.50 ± 0.63a | 43.77 ± 1.09a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 14.26 | 72.26 ± 0.39a | 23.69 ± 0.19c | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | 16.70 | 69.16 ± 0.56b | 18.04 ± 0.25d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Roots | Hexane | 3.81 | 19.58 ± 0.04g | 2.13 ± 0.10g | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 1.75 | 39.17 ± 0.58f | 8.70 ± 0.60e | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 10.26 | 44.27 ± 0.11e | 8.75 ± 0.48e | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 12.04 | 50.61 ± 0.40c | 3.33 ± 0.06g | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | 11.45 | 45.50 ± 0.24d | 2.52 ± 0.02g | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2.2. Profile of bioactive compounds
The total phenolic and flavonoid contents were determined using the Folin–Ciocalteu and aluminium chloride (AlCl3) assays, respectively.21,22 Results were expressed as gallic acid (mg GAEs per g extract) and rutin equivalents (mg REs per g extract) for respective assays.Chromatographic separation was accomplished with a Dionex Ultimate 3000RS UHPLC instrument, equipped with Thermo Accucore C18 (100 mm × 2.1 mm i. d., 2.6 μm) analytical column for separation of compounds. Water (A) and methanol (B) containing 0.1% formic acid were employed as mobile phases, respectively. The total run time was 70 minutes, the elution profile and all exact analytical conditions have been published.23
2.3. Determination of antioxidant and enzyme inhibitory effects
The metal chelating, phosphomolybdenum, ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) activities of the extracts (0.5–5 mg mL−1) were assessed following the methods described by Grochowski et al.24 The antioxidant activities were reported as trolox equivalents, whereas ethylenediaminetetraacetic acid (EDTA) was used for metal chelating assay. The possible inhibitory effects of the extracts (0.5–5 mg mL−1) against cholinesterases (by Ellman's method), tyrosinase, α-amylase and α-glucosidase were evaluated using standard in vitro bio-assays.24 To provide comparison with standard antioxidants and inhibitors, IC50 values were also given (this is extract concentration required for scavenging 50% of radicals, ferrous ion-ferrozine and enzyme inhibitory assays; this is effective concentration at which the absorbance was 0.5 for CUPRAC, FRAP and PBD assays).2.4. Antimicrobial evaluation
In this study totally twelve microorganisms (eleven bacteria and one yeast) were used to elucidate of antimicrobial potential of S. ceratophylla extracts. Standard microorganisms were obtained from Microbiology Research Laboratory of Vocational School of Health Services, Selcuk University. Broth micro dilution method was conducted for antimicrobial activity of extracts according to Balouiri et al.25Briefly, 96-well plates were loaded with 100 μL Mueller Hinton Broth medium. Then 100 μL S. ceratophylla extracts were transferred to first well of the plate and serial dilution was done by transferring of 100 μL volume mixture via multichannel pipette. When the extract-medium mixture was ready then fresh microorganism inoculum prepared from 0.5 Mc Farland turbidity and final concentration 5 × 105 were added to each well. Plates were sealed and incubated in an incubator at 37 °C for 18–24 hours. Gentamicin was used as positive control. After incubation period 20 μL of 2,3,5 tri phenyl tetrazolium chloride solution (0.5%) loaded to each well for detecting of minimum inhibitory concentration (MIC) of S. ceratophylla extracts. The MIC is the lowest concentration of antimicrobial agent that completely inhibits growth of the organism in tubes or microdilution wells as detected by the unaided eye.26
2.5. Cell culture
The human hepatocarcinoma HepG2 cells and murine bone marrow stromal S17 cells were kindly provided by the Centre for Molecular and Structural Biomedicine of Biomedical and Molecular BME, University of Algarve, Portugal), while mouse melanoma B16 4A5 cells was purchased from Sigma-Aldrich (Germany). All cell lines were cultured in Dulbecco's Modified Eagle medium (DMEM) supplemented with foetal bovine serum (10%), L-glutamine (2 mM, 1%), and penicillin (50 U mL−1)/streptomycin (50 μg mL−1) (1%), and kept under a humidified atmosphere at 37 °C and 5% CO2.2.6. Determination of cellular viability and selectivity
Cells were plated in 96-well plates at 5 × 103 cells per well (HepG2 and S17) and 2 × 103 cells per well (B16 4A5). After a 24 h incubation, cells were treated with the samples at the concentration of 100 μg mL−1 for 72 h. Cells incubated with DMSO at 0.5% (the highest DMSO concentration used in the test wells) were used as control. The cellular viability was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test, as described formerly.27 The percentage of viable cells was calculated relative to the control (DMSO, 0.5%). Selectivity index (SI) was calculated by the formula SI = CT/CNT, where CT and CNT stands for the cytotoxicity of the extract towards tumoral and non-tumoral cell lines, respectively.282.7. Data analysis
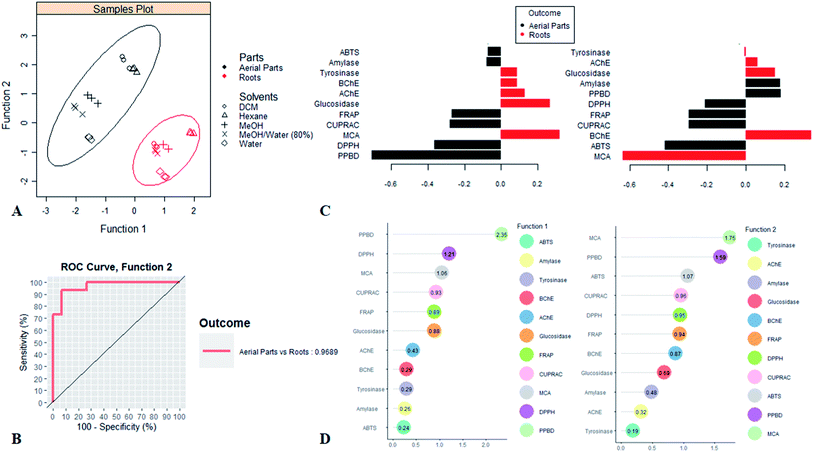
Statistical calculations were done using Xlstat 2018 and R v 3.5.1 softwares. Firstly, the one-way ANOVA with Tukey post-hoc test was performed for comparisons among samples. Pearson correlation coefficients were calculated among total bioactive compounds and biological activities. Afterwards, the biological activities dataset was analysed by supervised Partial Least Square Discriminant Analysis PLS-DA. The accuracy of model was recorded by calculating the AUC average. Finally, line plot was used following one-way ANOVA to investigate the effect of extraction solvents on the biological activities of each studied parts respectively.3. Results and discussion
3.1. Total bioactive compounds and phytochemical composition
Plants and herbs are known to be abounded with scads of phytochemicals possessing medicinal properties such as anti-inflammatory, anticancer, and antioxidant, to name a few.29 The prepared aqueous, hexane, DCM, hydro-methanolic (80%) and methanolic root and aerial part extracts were evaluated for their total phenolic and flavonoid content using colorimetric methods. Results obtained are summarized in Table 1. Upon comparison between the different extracts, hexane root and aerial extracts were found to yield the least amount of phenolic and flavonoids. The same outcomes were reported in previous studies whereby hexane solvent extracted the least amount of phenolic and flavonoid content.30–32 The methanolic aerial extract possessed the highest phenolic (72.50 ± 0.63 mg GAE per g) and flavonoid content (43.77 ± 1.09 mg RE per g). In terms of roots, phenolic content was higher in the hydro-methanolic extract (50.61 ± 0.40 mg GAE per g) in contrast to the methanolic extract (44.27 ± 0.11 mg GAE per g). It can be said that phenolic and flavonoid compounds were better extracted in hydro-methanol and methanol solvents compared to the other extraction solvents.The LC-MS/MS analysis allowed the characterization of the chemical composition of all the studied extracts obtained from S. ceratophylla. In total, 54 major compounds occurring in the aerial methanolic extract were detected, 47 in methanolic root, 48 in aqueous aerial and 37 in aqueous root extracts. The detailed chromatographic results are given Tables 2–5. Twenty-nine compounds were found in common between the aqueous root and aerial extracts (Fig. 1a) while 38 were common between methanolic root and aerial extracts (Fig. 1b). Fig. 2 shows that a total of 29 phytochemicals were found in common in all four analysed extracts (methanolic root and aerial, aqueous root and aerial).
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Confirmed by standard. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1a | Gallic acid (3,4,5-trihydroxybenzoic acid) | C7H6O5 | 2.64 | 169.01370 | 125.0230 | 97.0281 | 69.0331 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Dihydroxybenzoic acid | C7H6O4 | 5.50 | 153.01879 | 123.0437 | 109.0281 | 108.0202 | 81.0331 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Pantothenic acid | C9H17NO5 | 6.06 | 220.11850 | 202.1079 | 184.0973 | 174.1133 | 116.0346 | 90.0556 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.50 | 311.04031 | 179.0340 | 149.0080 | 135.0440 | 87.0072 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Dihydroxycoumarin-O-hexoside | C15H16O9 | 12.85 | 331.15455 | 179.0342 | 151.0390 | 133.0284 | 123.0444 | 85.0291 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Kynurenic acid | C10H7NO3 | 13.80 | 190.05042 | 162.0552 | 144.0444 | 116.0500 | 89.0392 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Caffeic acid | C9H8O4 | 15.12 | 179.03444 | 135.0439 | 107.0489 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Unidentified alkaloid | C10H11NO3 | 16.17 | 194.08172 | 166.0865 | 136.0760 | 108.0449 | 87.0447 | 80.0502 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Naringenin-6,8-di-C-glucoside | C27H32O15 | 17.31 | 595.16630 | 505.1357 | 475.1238 | 415.1028 | 385.0929 | 355.0821 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.62 | 295.04540 | 179.0340 | 135.0439 | 133.0130 | 115.0022 | 71.0122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | 4-O-Feruloylquinic acid | C17H20O9 | 18.93 | 367.10291 | 193.0499 | 173.0444 | 134.0360 | 93.0330 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Loliolide | C11H16O3 | 19.99 | 197.11777 | 179.1070 | 161.0963 | 135.1171 | 133.1015 | 107.0860 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Rosmarinic acid-di-O-hexoside | C30H36O18 | 22.30 | 683.18234 | 521.1315 | 359.0995 | 323.0777 | 197.0449 | 179.0340 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Luteolin-O-glucuronide isomer 1 | C21H18O12 | 22.49 | 461.07201 | 285.0407 | 217.0501 | 199.0396 | 151.0024 | 133.0280 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Luteolin-O-hexoside isomer 1 | C21H20O11 | 22.61 | 447.09274 | 327.0501 | 285.0407 | 284.0329 | 256.0376 | 151.0025 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Luteolin-O-glucuronide isomer 2 | C21H18O12 | 22.71 | 461.07201 | 285.0406 | 217.0500 | 199.0393 | 151.0024 | 133.0279 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Luteolin-7-O-glucoside (cynaroside) | C21H20O11 | 22.86 | 447.09274 | 327.0507 | 285.0407 | 284.0330 | 256.0381 | 151.0026 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Rosmarinic acid-O-hexoside | C24H26O13 | 23.38 | 521.12952 | 359.0730 | 323.0772 | 197.0448 | 179.0340 | 161.0232 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Methoxy-tetrahydroxy(iso)flavone-O-glucuronide | C22H20O13 | 23.40 | 491.08257 | 315.0513 | 300.0277 | 272.0327 | 151.0024 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Apigenin-O-glucuronide | C21H18O11 | 24.36 | 445.07709 | 269.0456 | 225.0554 | 175.0237 | 117.0332 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21a | Cosmosiin (Apigenin-7-O-glucoside) | C21H20O10 | 24.44 | 433.11347 | 271.0603 | 153.0183 | 119.0501 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22 | Rosmarinic acid (labiatenic acid) | C18H16O8 | 24.65 | 359.07670 | 197.0449 | 179.0340 | 161.0232 | 135.0439 | 133.0283 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23 | Methyl caffeate | C10H10O4 | 24.67 | 195.06574 | 163.0392 | 145.0287 | 135.0444 | 117.0339 | 89.0392 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 | Chrysoeriol-7-O-glucuronide | C22H20O12 | 24.82 | 475.08766 | 299.0562 | 284.0329 | 256.0376 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 | Apigenin-O-hexoside | C21H20O10 | 24.89 | 431.09782 | 311.0562 | 269.0456 | 268.0377 | 151.0021 | 117.0336 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26 | Luteolin-O-hexoside isomer 2 | C21H20O11 | 25.10 | 447.0974 | 285.0407 | 284.0330 | 255.0297 | 151.0024 | 133.0279 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27 | N-trans-feruloyltyramine | C18H19NO4 | 25.12 | 314.13924 | 194.0816 | 177.0548 | 149.0600 | 145.0286 | 121.0651 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28 | Abscisic acid | C15H20O4 | 25.75 | 263.12834 | 219.1385 | 204.1151 | 201.1281 | 152.0831 | 151.0752 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 | Martynoside or isomer | C31H40O15 | 26.20 | 651.22890 | 475.1822 | 193.0500 | 175.0390 | 160.0154 | 134.0361 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30 | Pentahydroxy(iso)flavone | C15H10O7 | 26.26 | 301.03483 | 273.0401 | 257.0444 | 151.0023 | 107.0121 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.57 | 373.09235 | 197.0449 | 179.0340 | 175.0390 | 160.0154 | 135.0439 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | Dihydroactinidiolide | C11H16O2 | 27.07 | 181.12286 | 163.1119 | 145.1015 | 135.1171 | 121.1016 | 107.0860 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33 | Methoxy-trihydroxy(iso)flavone isomer 1 | C16H12O6 | 28.06 | 299.05556 | 284.0328 | 283.0252 | 256.0378 | 228.0422 | 227,0345 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34a | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.37 | 285.03991 | 217.0495 | 199.0393 | 175.0387 | 151.0024 | 133.0282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | N1,N5,N10-Tricoumaroylspermidine | C34H37N3O6 | 29.46 | 582.26042 | 462.2038 | 436.2245 | 342.1458 | 145.0283 | 119.0488 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.22 | 269.04500 | 225.0547 | 201.0557 | 151.0024 | 149.0232 | 117.0330 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 | Chrysoeriol (3′-methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 30.44 | 299.05556 | 284.0329 | 283.0251 | 256.0376 | 227.0344 | 151.0018 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38 | Dihydrololiolide | C11H18O3 | 30.50 | 199.13342 | 181.1226 | 163.1119 | 135.1172 | 111.0445 | 107.0860 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39 | Methoxy-tetrahydroxy(iso)flavone | C16H12O7 | 30.54 | 315.05048 | 300.0277 | 272.0326 | 227.0335 | 151.0026 | 149.0233 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40 | Undecanedioic acid | C11H20O4 | 31.32 | 215.12834 | 197.1176 | 153.1272 | 125.0959 | 57.0332 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41 | Dihydroxy-trimethoxy(iso)flavone | C18H16O7 | 31.83 | 345.09743 | 330.0735 | 329.0663 | 315.0495 | 312.0631 | 284.0682 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 32.42 | 315.08686 | 300.0632 | 272.0678 | 257.0447 | 229.0487 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43 | Methoxy-trihydroxy(iso)flavone isomer 2 | C16H12O6 | 33.02 | 299.05556 | 284.0328 | 283.0237 | 256.0375 | 227.0346 | 151.0030 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44 | Hydroxy-tetramethoxy(iso)flavone | C19H18O7 | 33.31 | 359.11308 | 344.0891 | 343.0810 | 326.0790 | 315.0862 | 298.0838 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | Dodecanedioic acid | C12H22O4 | 33.75 | 229.14399 | 211.1334 | 185.1539 | 167.1431 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46a | Genkwanin (4′,5-dihydroxy-7-methoxyflavone) | C16H12O5 | 35.05 | 285.07630 | 270.0525 | 242.0574 | 213.0543 | 167.0341 | 119.0493 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47 | Hydroxy-trimethoxy(iso)flavone | C18H16O6 | 35.34 | 329.10252 | 314.0788 | 313.0701 | 299.0547 | 296.0683 | 268.0731 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 | Apigenin-4′,7-dimethyl ether (4′,7-dimethoxy-5-hydroxyflavone) | C17H14O5 | 38.71 | 299.09195 | 284.0682 | 256.0731 | 167.0338 | 133.0649 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 49 | Stearidonic acid | C18H28O2 | 40.13 | 275.20111 | 231.2107 | 177.1633 | 59.0124 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.21 | 293.21167 | 275.2020 | 235.1700 | 231.2117 | 171.1018 | 121.1008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 51 | Unidentified terpene 1 | C20H30O2 | 41.92 | 303.23241 | 285.2215 | 267.2123 | 257.2264 | 247.1695 | 201.1644 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 52 | Unidentified terpene 2 | C30H48O4 | 43.42 | 473.36309 | 455.3521 | 437.3416 | 419.3310 | 401.3207 | 359.2582 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 53 | Unidentified terpene 3 | C30H48O4 | 43.59 | 473.36309 | 455.3523 | 437.3418 | 419.3314 | 401.3216 | 359.2582 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 54 | Unidentified terpene 4 | C30H48O4 | 44.26 | 473.36309 | 455.3520 | 437.3418 | 419.3313 | 401.3202 | 109.1016 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Confirmed by standard. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Dihydroxybenzoic acid | C7H6O4 | 5.47 | 153.01879 | 123.0439 | 109.0281 | 108.0203 | 81.0331 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Pantothenic acid | C9H17NO5 | 6.03 | 220.11850 | 202.1088 | 184.0973 | 174.1128 | 116.0347 | 90.0555 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.48 | 311.04031 | 179.0340 | 149.0079 | 135.0439 | 87.0072 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Kynurenic acid | C10H7NO3 | 13.77 | 190.05042 | 162.0552 | 144.0448 | 116.0497 | 89.0394 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Caffeic acid | C9H8O4 | 15.10 | 179.03444 | 135.0439 | 107.0489 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Unidentified alkaloid | C10H11NO3 | 16.15 | 194.08172 | 166.0865 | 136.0760 | 108.0449 | 87.0447 | 80.0502 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Naringenin-6,8-di-C-glucoside | C27H32O15 | 17.28 | 595.16630 | 505.1334 | 475.1242 | 415.1036 | 385.0932 | 355.0826 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.60 | 295.04540 | 179.0340 | 135.0440 | 133.0130 | 115.0022 | 71.0122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Loliolide | C11H16O3 | 19.97 | 197.11777 | 179.1070 | 161.0963 | 135.1172 | 133.1016 | 107.0861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Rosmarinic acid-di-O-hexoside | C30H36O18 | 22.28 | 683.18234 | 521.1299 | 359.0994 | 323.0775 | 197.0449 | 179.0340 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Rosmarinic acid-O-hexoside isomer 1 | C24H26O13 | 22.37 | 521.12952 | 359.0753 | 323.0766 | 197.0449 | 179.0340 | 161.0232 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Luteolin-O-glucuronide isomer 2 | C21H18O12 | 22.65 | 461.07201 | 285.0407 | 217.0501 | 199.0389 | 151.0024 | 133.0280 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Luteolin-7-O-glucoside (cynaroside) | C21H20O11 | 22.84 | 447.09274 | 327.0524 | 285.0407 | 284.0329 | 256.0371 | 151.0023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Rosmarinic acid-O-hexoside isomer 2 | C24H26O13 | 23.36 | 521.12952 | 359.0772 | 323.0775 | 197.0448 | 179.0340 | 161.0232 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Methoxy-tetrahydroxy(iso)flavone-O-glucuronide | C22H20O13 | 23.39 | 491.08257 | 315.0514 | 300.0278 | 272.0326 | 151.0024 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16a | Cosmosiin (apigenin-7-O-glucoside) | C21H20O10 | 24.45 | 433.11347 | 271.0604 | 153.0186 | 119.0491 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Apigenin-O-glucuronide | C21H18O11 | 24.49 | 445.07709 | 269.0457 | 225.0549 | 175.0235 | 117.0332 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Methyl caffeate | C10H10O4 | 24.63 | 195.06574 | 163.0392 | 145.0287 | 135.0444 | 117.0339 | 89.0392 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Rosmarinic acid (labiatenic acid) | C18H16O8 | 24.66 | 359.07670 | 197.0449 | 179.0340 | 161.0232 | 135.0439 | 133.0282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Chrysoeriol-7-O-glucuronide | C22H20O12 | 24.82 | 475.08766 | 299.0562 | 284.0328 | 256.0385 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21 | N-trans-Feruloyltyramine | C18H19NO4 | 25.12 | 314.13924 | 194.0816 | 177.0548 | 149.0600 | 145.0286 | 121.0651 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22 | Luteolin-O-hexoside isomer 2 | C21H20O11 | 25.13 | 447.09274 | 285.0407 | 284.0328 | 255.0298 | 151.0025 | 133.0280 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23 | Abscisic acid | C15H20O4 | 25.77 | 263.12834 | 219.1385 | 204.1150 | 201.1279 | 152.0830 | 151.0752 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 | Martynoside or isomer | C31H40O15 | 26.22 | 651.22890 | 475.1835 | 193.0499 | 175.0390 | 160.0154 | 134.0362 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 | Pentahydroxy(iso)flavone | C15H10O7 | 26.28 | 301.03483 | 273.0401 | 257.0452 | 151.0025 | 107.0126 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.57 | 373.09235 | 197.0449 | 179.0340 | 175.0389 | 160.0153 | 135.0439 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27 | Dihydroactinidiolide | C11H16O2 | 27.08 | 181.12286 | 163.1120 | 145.1014 | 135.1172 | 121.1016 | 107.0860 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28 | Martynoside or isomer | C31H40O15 | 27.56 | 651.22890 | 475.1806 | 193.0501 | 175.0389 | 160.0152 | 134.0358 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 | Methoxy-trihydroxy(iso)flavone isomer 1 | C16H12O6 | 28.09 | 299.05556 | 284.0329 | 283.0256 | 256.0375 | 228.0427 | 227.0342 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30a | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.38 | 285.03991 | 217.0494 | 199.0392 | 175.0392 | 151.0024 | 133.0282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31 | N1,N5,N10-Tricoumaroylspermidine | C34H37N3O6 | 29.48 | 582.26042 | 462.2035 | 436.2205 | 342.1466 | 145.0282 | 119.0488 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.24 | 269.04500 | 225.0550 | 201.0555 | 151.0024 | 149.0233 | 117.0331 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33 | Chrysoeriol (3′-methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 30.44 | 299.05556 | 284.0329 | 283.0245 | 256.0378 | 227.0351 | 151.0027 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34 | Dihydrololiolide | C11H18O3 | 30.49 | 199.13342 | 181.1226 | 163.1119 | 135.1171 | 111.0445 | 107.0861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | Undecanedioic acid | C11H20O4 | 31.32 | 215.12834 | 197.1177 | 153.1273 | 125.0961 | 57.0333 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 | Dihydroxy-trimethoxy(iso)flavone | C18H16O7 | 31.82 | 345.09743 | 330.0737 | 329.0654 | 315.0501 | 312.0631 | 284.0682 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 32.41 | 315.08686 | 300.0631 | 272.0682 | 257.0448 | 229.0487 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38 | Methoxy-trihydroxy(iso)flavone isomer 2 | C16H12O6 | 33.03 | 299.05556 | 284.0329 | 283.0239 | 256.0371 | 227.0346 | 151.0031 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39 | Hydroxy-tetramethoxy(iso)flavone | C19H18O7 | 33.31 | 359.11308 | 344.0887 | 343.0818 | 326.0790 | 315.0881 | 298.0839 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40 | Dodecanedioic acid | C12H22O4 | 33.76 | 229.14399 | 211.1334 | 185.1530 | 167.1430 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41a | Genkwanin (4′,5-dihydroxy-7-methoxyflavone) | C16H12O5 | 35.04 | 285.07630 | 270.0526 | 242.0577 | 213.0543 | 167.0342 | 119.0494 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42 | Hydroxy-trimethoxy(iso)flavone | C18H16O6 | 35.33 | 329.10252 | 314.0786 | 313.0719 | 299.0546 | 296.0682 | 268.0732 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43 | Apigenin-4′,7-dimethyl ether (4′,7-dimethoxy-5-hydroxyflavone) | C17H14O5 | 38.71 | 299.09195 | 284.0683 | 256.0732 | 167.0344 | 133.0654 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44 | Stearidonic acid | C18H28O2 | 40.15 | 275.20111 | 231.2120 | 177.1633 | 59.0126 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.22 | 293.21167 | 275.2019 | 235.1700 | 231.2110 | 171.1016 | 121.1008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46 | Unidentified terpene 1 | C20H30O2 | 41.94 | 303.23241 | 285.2216 | 267.2104 | 257.2267 | 247.1689 | 201.1644 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47 | Unidentified terpene 2 | C30H48O4 | 43.42 | 473.36309 | 455.3527 | 437.3422 | 419.3322 | 401.3216 | 359.2585 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 48 | Unidentified terpene 4 | C30H48O4 | 44.30 | 473.36309 | 455.3526 | 437.3422 | 419.3319 | 401.3214 | 109.1017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Confirmed by standard. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1a | Gallic acid (3,4,5-trihydroxybenzoic acid) | C7H6O5 | 2.69 | 169.01370 | 125.0230 | 97.0279 | 69.0331 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Dihydroxybenzoic acid | C7H6O4 | 5.55 | 153.01879 | 123.0438 | 109.0281 | 108.0203 | 81.0331 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Pantothenic acid | C9H17NO5 | 6.17 | 220.11850 | 202.1077 | 184.0973 | 174.1124 | 116.0346 | 90.0555 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.56 | 311.04031 | 179.0341 | 149.0080 | 135.0439 | 87.0070 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Salicylic acid-2-O-glucoside | C13H16O8 | 13.50 | 299.07670 | 137.0232 | 113.0230 | 93.0330 | 85.0280 | 71.0123 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Kynurenic acid | C10H7NO3 | 13.82 | 190.05042 | 162.0552 | 144.0447 | 116.0498 | 89.0392 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Caffeoylhexose | C15H18O9 | 14.88 | 341.08726 | 179.0340 | 135.0440 | 107.0486 | 89.0229 | 71.0124 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Caffeic acid | C9H8O4 | 15.13 | 179.03444 | 135.0439 | 107.0489 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.61 | 295.04540 | 179.0341 | 135.0440 | 133.0130 | 115.0022 | 71.0122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | 4-O-Feruloylquinic acid | C17H20O9 | 18.92 | 367.10291 | 193.0498 | 173.0445 | 134.0361 | 93.0330 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Loliolide | C11H16O3 | 19.98 | 197.11777 | 179.1070 | 161.0963 | 135.1172 | 133.1015 | 107.0861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Rosmarinic acid-di-O-hexoside | C30H36O18 | 22.28 | 683.18234 | 521.1306 | 359.1000 | 323.0775 | 197.0449 | 179.0341 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Luteolin-O-glucuronide isomer 2 | C21H18O12 | 22.74 | 461.07201 | 285.0407 | 217.0495 | 199.0393 | 151.0025 | 133.0281 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 | Luteolin-7-O-glucoside (cynaroside) | C21H20O11 | 22.83 | 447.09274 | 327.0510 | 285.0408 | 284.0330 | 256.0377 | 151.0023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Rosmarinic acid-O-hexoside | C24H26O13 | 23.38 | 521.12952 | 359.0770 | 323.0774 | 197.0450 | 179.0341 | 161.0233 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16a | Cosmosiin (apigenin-7-O-glucoside) | C21H20O10 | 24.46 | 433.11347 | 271.0603 | 153.0184 | 119.0495 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Apigenin-O-glucuronide | C21H18O11 | 24.49 | 445.07709 | 269.0457 | 225.0544 | 175.0235 | 117.0330 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | Rosmarinic acid (labiatenic acid) | C18H16O8 | 24.64 | 359.07670 | 197.0450 | 179.0341 | 161.0233 | 135.0440 | 133.0283 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Methyl caffeate | C10H10O4 | 24.65 | 195.06574 | 163.0392 | 145.0287 | 135.0444 | 117.0339 | 89.0391 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | Luteolin-O-hexoside isomer 2 | C21H20O11 | 25.10 | 447.09274 | 285.0408 | 284.0336 | 255.0304 | 151.0025 | 133.0283 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21 | N-trans-Feruloyltyramine | C18H19NO4 | 25.12 | 314.13924 | 194.0820 | 177.0549 | 149.0602 | 145.0287 | 121.0652 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22 | Martynoside or isomer | C31H40O15 | 26.21 | 651.22890 | 475.1812 | 193.0500 | 175.0390 | 160.0154 | 134.0361 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.57 | 373.09235 | 197.0449 | 179.0340 | 175.0390 | 160.0154 | 135.0439 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 | Dihydroactinidiolide | C11H16O2 | 27.08 | 181.12286 | 163.1120 | 145.1016 | 135.1172 | 121.1016 | 107.0861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 | Methoxy-trihydroxy(iso)flavone isomer 1 | C16H12O6 | 28.07 | 299.05556 | 284.0330 | 283.0243 | 256.0378 | 228.0424 | 227.0351 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26a | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.36 | 285.03991 | 217.0499 | 199.0395 | 175.0390 | 151.0025 | 133.0282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.22 | 269.04500 | 225.0553 | 201.0557 | 151.0026 | 149.0233 | 117.0331 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28 | Chrysoeriol (3′-methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 30.43 | 299.05556 | 284.0329 | 283.0255 | 256.0377 | 227.0352 | 151.0023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 | Undecanedioic acid | C11H20O4 | 31.30 | 215.12834 | 197.1178 | 153.1273 | 125.0959 | 57.0332 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30 | Dihydroxy-trimethoxy(iso)flavone | C18H16O7 | 31.81 | 345.09743 | 330.0736 | 329.0659 | 315.0503 | 312.0631 | 284.0682 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 32.42 | 315.08686 | 300.0633 | 272.0681 | 257.0439 | 229.0487 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | Methoxy-trihydroxy(iso)flavone isomer 2 | C16H12O6 | 32.99 | 299.05556 | 284.0329 | 283.0252 | 256.0375 | 227.0344 | 151.0029 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33 | Dodecanedioic acid | C12H22O4 | 33.75 | 229.14399 | 211.1334 | 185.1556 | 167.1431 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34a | Genkwanin (4′,5-dihydroxy-7-methoxyflavone) | C16H12O5 | 35.04 | 285.07630 | 270.0526 | 242.0576 | 213.0552 | 167.0342 | 119.0495 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | Hydroxy-trimethoxy(iso)flavone | C18H16O6 | 35.32 | 329.10252 | 314.0788 | 313.0718 | 299.0540 | 296.0683 | 268.0732 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 | Unidentified terpene 5 | C20H30O3 | 36.08 | 319.22732 | 301.2169 | 291.2325 | 289.2166 | 277.1802 | 165.0914 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 | Unidentified terpene 6 | C20H26O4 | 38.49 | 331.19094 | 313.1800 | 295.1698 | 267.1746 | 229.1226 | 211.1121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 38 | Apigenin-4′,7-dimethyl ether (4′,7-dimethoxy-5-hydroxyflavone) | C17H14O5 | 38.69 | 299.09195 | 284.0682 | 256.0732 | 167.0340 | 133.0650 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 39 | Unidentified terpene 7 | C21H28O4 | 39.90 | 345.20658 | 327.1961 | 313.1799 | 295.1696 | 267.1746 | 229.1226 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 40 | Unidentified terpene 8 | C20H26O4 | 40.00 | 331.19094 | 313.1802 | 295.1700 | 267.1744 | 229.1226 | 211.1121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 41 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.21 | 293.21167 | 275.2020 | 235.1692 | 231.2117 | 171.1012 | 121.1012 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 42 | Unidentified terpene 9 | C21H28O4 | 41.96 | 345.20658 | 327.1966 | 313.1802 | 295.1700 | 267.1746 | 229.1226 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 43 | Viridoquinone | C20H24O2 | 42.23 | 297.18546 | 279.1748 | 269.1896 | 239.1433 | 237.1277 | 197.0966 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 44 | Unidentified terpene 2 | C30H48O4 | 43.39 | 473.36309 | 455.3525 | 437.3420 | 419.3312 | 401.3196 | 359.2586 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 45 | Unidentified terpene 3 | C30H48O4 | 43.56 | 473.36309 | 455.3528 | 437.3425 | 419.3318 | 401.3213 | 359.2586 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 46 | Unidentified terpene 4 | C30H48O4 | 44.25 | 473.36309 | 455.3527 | 437.3424 | 419.3318 | 401.3228 | 109.1017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 47 | Unidentified terpene 10 | C30H50O2 | 46.23 | 443.38891 | 425.3799 | 407.3697 | 217.1951 | 203.1799 | 191.1799 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Confirmed by standard. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Dihydroxybenzoic acid | C7H6O4 | 5.51 | 153.01879 | 123.0438 | 109.0280 | 108.0203 | 81.0332 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Pantothenic acid | C9H17NO5 | 6.15 | 220.11850 | 202.1077 | 184.0973 | 174.1128 | 116.0347 | 90.0556 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.53 | 311.04031 | 179.0340 | 149.0079 | 135.0439 | 87.0071 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Salicylic acid-2-O-glucoside | C13H16O8 | 13.49 | 299.07670 | 137.0232 | 113.0230 | 93.0330 | 85.0279 | 71.0123 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Kynurenic acid | C10H7NO3 | 13.80 | 190.05042 | 162.0552 | 144.0446 | 116.0499 | 89.0393 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Caffeoylhexose | C15H18O9 | 14.88 | 341.08726 | 179.0340 | 135.0439 | 107.0486 | 89.0228 | 71.0123 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Caffeic acid | C9H8O4 | 15.13 | 179.03444 | 135.0439 | 107.0489 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.61 | 295.04540 | 179.0340 | 135.0439 | 133.0130 | 115.0022 | 71.0122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Loliolide | C11H16O3 | 19.99 | 197.11777 | 179.1070 | 161.0963 | 135.1172 | 133.1016 | 107.0861 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Rosmarinic acid-di-O-hexoside | C30H36O18 | 22.30 | 683.18234 | 521.1307 | 359.1003 | 323.0774 | 197.0449 | 179.0340 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Luteolin-O-glucuronide isomer 2 | C21H18O12 | 22.73 | 461.07201 | 285.0406 | 217.0501 | 199.0387 | 151.0025 | 133.0280 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 | Luteolin-7-O-glucoside (cynaroside) | C21H20O11 | 22.82 | 447.09274 | 327.0513 | 285.0407 | 284.0329 | 256.0371 | 151.0023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 | Rosmarinic acid-O-hexoside | C24H26O13 | 23.38 | 521.12952 | 359.0762 | 323.0773 | 197.0449 | 179.0340 | 161.0232 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14a | Cosmosiin (Apigenin-7-O-glucoside) | C21H20O10 | 24.45 | 433.11347 | 271.0603 | 153.0183 | 119.0496 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | Apigenin-O-glucuronide | C21H18O11 | 24.48 | 445.07709 | 269.0457 | 225.0553 | 175.0238 | 117.0327 | 113.0230 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | Rosmarinic acid (labiatenic acid) | C18H16O8 | 24.67 | 359.07670 | 197.0449 | 179.0340 | 161.0232 | 135.0439 | 133.0283 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | Methyl caffeate | C10H10O4 | 24.68 | 195.06574 | 163.0392 | 145.0287 | 135.0444 | 117.0339 | 89.0391 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | N-trans-Feruloyltyramine | C18H19NO4 | 25.11 | 314.13924 | 194.0822 | 177.0547 | 149.0598 | 145.0286 | 121.0653 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | Martynoside or isomer | C31H40O15 | 26.21 | 651.22890 | 475.1839 | 193.0501 | 175.0390 | 160.0154 | 134.0361 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.57 | 373.09235 | 197.0449 | 179.0340 | 175.0390 | 160.0154 | 135.0439 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 21 | Dihydroactinidiolide | C11H16O2 | 27.07 | 181.12286 | 163.1119 | 145.1014 | 135.1172 | 121.1015 | 107.0860 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 22 | Martynoside or isomer | C31H40O15 | 27.56 | 651.22890 | 475.1825 | 193.0500 | 175.0390 | 160.0154 | 134.0361 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 23a | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.38 | 285.03991 | 217.0509 | 199.0388 | 175.0390 | 151.0023 | 133.0282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 24 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.23 | 269.04500 | 225.0549 | 201.0553 | 151.0024 | 149.0229 | 117.0332 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25 | Undecanedioic acid | C11H20O4 | 31.31 | 215.12834 | 197.1177 | 153.1273 | 125.0959 | 57.0332 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 26 | Dihydroxy-dimethoxy(iso)flavone | C17H14O6 | 32.43 | 315.08686 | 300.0630 | 272.0682 | 257.0434 | 229.0487 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 27 | Dodecanedioic acid | C12H22O4 | 33.76 | 229.14399 | 211.1334 | 185.1533 | 167.1430 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 28a | Genkwanin (4′,5-dihydroxy-7-methoxyflavone) | C16H12O5 | 35.05 | 285.07630 | 270.0528 | 242.0575 | 213.0552 | 167.0342 | 119.0497 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 29 | Hydroxy-trimethoxy(iso)flavone | C18H16O6 | 35.34 | 329.10252 | 314.0787 | 313.0709 | 299.0543 | 296.0683 | 268.0732 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 30 | Unidentified terpene 5 | C20H30O3 | 36.07 | 319.22732 | 301.2164 | 291.2324 | 289.2161 | 277.1803 | 165.0913 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 31 | Unidentified terpene 6 | C20H26O4 | 38.51 | 331.19094 | 313.1800 | 295.1696 | 267.1747 | 229.1226 | 211.1121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 32 | Apigenin-4′,7-dimethyl ether (4′,7-dimethoxy-5-hydroxyflavone) | C17H14O5 | 38.72 | 299.09195 | 284.0682 | 256.0732 | 167.0346 | 133.0650 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 33 | Unidentified terpene 8 | C20H26O4 | 40.00 | 331.19094 | 313.1799 | 295.1694 | 267.1748 | 229.1227 | 211.1121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 34 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.22 | 293.21167 | 275.2019 | 235.1702 | 231.2116 | 171.1014 | 121.1009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 35 | Viridoquinone | C20H24O2 | 42.24 | 297.18546 | 279.1748 | 269.1897 | 239.1433 | 237.1276 | 197.0965 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 36 | Unidentified terpene 2 | C30H48O4 | 43.42 | 473.36309 | 455.3525 | 437.3422 | 419.3309 | 401.3203 | 359.2583 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 37 | Unidentified terpene 4 | C30H48O4 | 44.28 | 473.36309 | 455.3522 | 437.3423 | 419.3311 | 401.3228 | 109.1017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
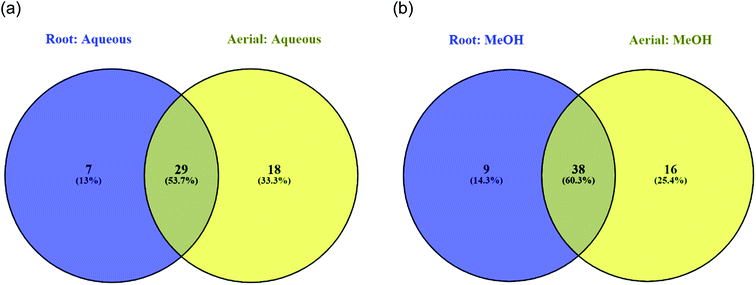 | ||
| Fig. 1 Venn diagrams displaying common compounds between different (a) aqueous (b) methanolic extracts. | ||
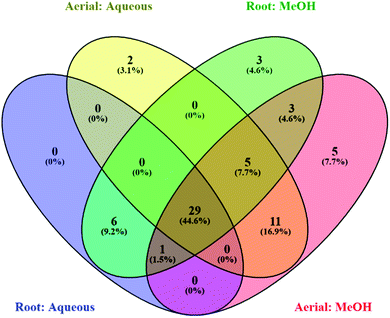 | ||
| Fig. 2 Venn diagram showing number of common compounds found in all four analysed extracts (methanolic root and aerial, aqueous root and aerial). | ||
3.2. Antioxidant activities
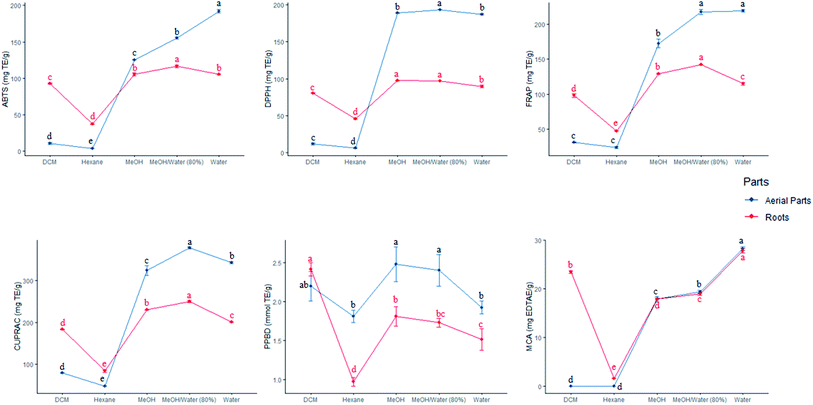
Six methods namely DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum and metal chelating were used to assess the antioxidant activities of the prepared extracts. Table 6 details the data gathered in this work. The remarkably high antioxidant activity was found to be distributed among the hydro-methanolic, methanolic and aqueous extracts while the hexane extracts exhibited the lowest antioxidant activity with all methods irrespective of the plant part used. For instance, the hydro-methanolic aerial extract showed the maximum DPPH radical scavenging activity (193.40 ± 0.27 mg TE per g) and the highest reducing potential towards copper(II) (377.93 ± 2.38 mg TE per g). Among the different root extracts analysed, the hydro-methanolic sample revealed to be the most potent ABTS radical scavenger (116.50 ± 1.65 mg TE per g) and displayed the highest reducing potential with both CUPRAC (250.03 ± 2.65 mg TE per g) and FRAP (142.00 ± 0.14 mg TE per g) assays. Similar findings were recorded in previous work showing that hydro-alcoholic extracts possessed substantially higher antioxidant activity compared to other extracts derived from low polarity solvents.33,34 In a study conducted by Orhan et al.,15 the methanolic extract displayed a high percentage inhibition of 84.8 ± 1.11 against DPPH radicals, corroborating our results. The total antioxidant capacity of the aerial and root extracts ranged from 1.81–2.48 and 0.97–2.41 mmol TE per g, respectively. The metal chelating ability was higher with aqueous aerial (28.25 ± 0.34 mg EDTAE per g) followed by root (27.83 ± 0.49 mg EDTAE per g) extracts. Mounting evidence showed that natural products play a vital role in hindering β-amyloid fibril aggregation due to their ability to bind metal ions with high affinities.35| Parts | Solvents | DPPH | ABTS | CUPRAC | FRAP | MCA | PBD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg TE per g) | IC50 (mg mL−1) | (mg TE per g) | IC50 (mg mL−1) | (mg TE per g) | IC50 (mg mL−1) | (mg TE per g) | IC50 (mg mL−1) | (mg TE per g) | IC50 (mg mL−1) | (mmol TE per g) | IC50 (mg mL−1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Values are reported as mean ± SD. DCM: dichloromethane; MeOH: methanol; TE: trolox equivalent; EDTAE: EDTA equivalent; MCA: metal chelating ability; PBD: phosphomolybdenum.; nt: no tested. Different letters indicate significant differences in the extracts (p < 0.05, the letter “a” indicates strong ability). IC50 (mg mL−1), effective concentration at which the absorbance was 0.5 for CUPRAC, FRAP and PBD assays and at which 50% of the DPPH and ABTS radicals were scavenged and the ferrous ion-ferrozine complex were inhibited. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aerial parts | Hexane | 6.47 ± 0.80h | >5 | 3.88 ± 0.34i | >5 | 48.30 ± 0.57i | 2.68 ± 0.03k | 23.91 ± 1.41h | 1.97 ± 0.12i | na | na | 1.81 ± 0.08bc | 1.44 ± 0.06cd | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 11.83 ± 1.37g | 4.66 ± 0.54h | 10.40 ± 1.38h | >5 | 79.73 ± 1.13h | 1.62 ± 0.02i | 31.27 ± 0.51h | 1.50 ± 0.02i | na | na | 2.20 ± 0.19ab | 1.19 ± 0.11bc | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 188.81 ± 0.68b | 0.29 ± 0.01c | 125.36 ± 0.43c | 0.60 ± 0.01d | 324.13 ± 11.42c | 0.40 ± 0.01d | 172.49 ± 6.32b | 0.27 ± 0.01c | 17.89 ± 0.59e | 1.15 ± 0.04f | 2.48 ± 0.22a | 1.06 ± 0.10b | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 193.40 ± 0.27a | 0.28 ± 0.01b | 155.43 ± 1.38b | 0.48 ± 0.01c | 377.93 ± 2.38a | 0.34 ± 0.01b | 217.46 ± 3.46a | 0.22 ± 0.01b | 19.38 ± 0.29c | 1.06 ± 0.02d | 2.40 ± 0.21a | 1.09 ± 0.10b | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | 187.33 ± 0.86b | 0.29 ± 0.01c | 191.93 ± 2.42a | 0.39 ± 0.01b | 342.83 ± 2.43b | 0.38 ± 0.01c | 219.20 ± 1.72a | 0.21 ± 0.01b | 28.25 ± 0.34a | 0.73 ± 0.02b | 1.93 ± 0.09bc | 1.36 ± 0.06cd | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Roots | Hexane | 46.13 ± 0.73f | 1.18 ± 0.02g | 37.03 ± 0.51g | 2.03 ± 0.03h | 84.29 ± 2.93h | 1.54 ± 0.05i | 47.72 ± 0.10g | 0.99 ± 0.01h | 1.63 ± 0.07f | >5 | 0.97 ± 0.05d | 2.68 ± 0.15e | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 80.61 ± 0.46e | 0.68 ± 0.01f | 92.76 ± 1.00f | 0.81 ± 0.01g | 183.12 ± 0.85g | 0.71 ± 0.01h | 98.33 ± 2.67f | 0.48 ± 0.01g | 23.43 ± 0.31b | 0.87 ± 0.01c | 2.41 ± 0.08a | 1.08 ± 0.04b | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 97.60 ± 0.32c | 0.56 ± 0.01d | 105.25 ± 1.97e | 0.71 ± 0.01f | 229.95 ± 0.63e | 0.56 ± 0.01f | 128.91 ± 0.83d | 0.36 ± 0.01e | 17.91 ± 0.24d | 1.14 ± 0.02e | 1.81 ± 0.12bcd | 1.45 ± 0.10cde | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 96.95 ± 0.04c | 0.56 ± 0.01d | 116.50 ± 1.65d | 0.65 ± 0.01e | 250.03 ± 2.65d | 0.52 ± 0.01e | 142.00 ± 0.14c | 0.33 ± 0.01d | 18.96 ± 0.31c | 1.08 ± 0.02d | 1.73 ± 0.05cd | 1.51 ± 0.05de | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | 89.70 ± 1.51d | 0.61 ± 0.01e | 105.46 ± 0.64e | 0.71 ± 0.01 | 200.52 ± 1.28f | 0.64 ± 0.01 | 115.01 ± 1.97e | 0.41 ± 0.01f | 27.83 ± 0.49a | 0.74 ± 0.01b | 1.51 ± 0.14d | 1.73 ± 0.15e | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standards | Trolox | — | 0.05 ± 0.01a | 0.07 ± 0.01a | — | 0.13 ± 0.01a | — | 0.05 ± 0.01a | — | nt | — | 0.65 ± 0.01a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EDTA | — | nt | nt | — | nt | — | nt | — | 0.02 ± 0.01a | — | nt | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.3. Enzyme inhibitory effects
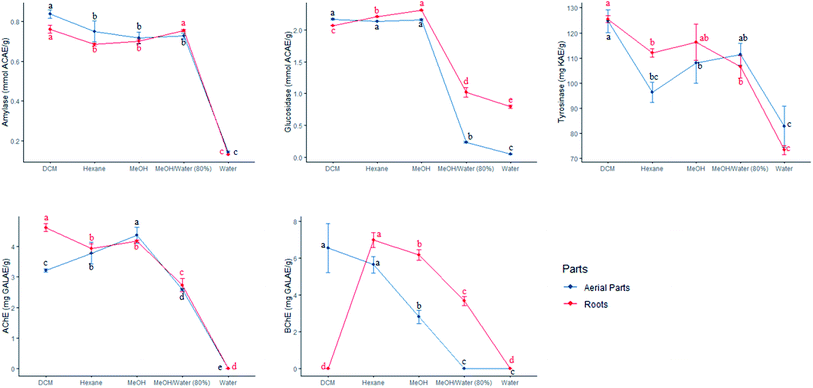
In this research work, the extracts of S. ceratophylla were screened for possible enzyme inhibitory effects against several non-communicable diseases including diabetes mellitus type II (α-amylase and α-glucosidase), Alzheimer's disease (AChE and BChE) and skin hyperpigmentation (tyrosinase). These aforementioned diseases were targeted since no cure has been found yet to combat such pathological disorders and the statistics presented by the World Health Organisation (WHO) is alarming. For instance, more than 420 million people have been diagnosed with diabetes2 and about 50 million people have dementia.2 Hence, searching for treatment and novel drugs should be an ongoing process. The WHO has approved drugs derived from plants to combat diabetes for various reasons, such as: (i) non-toxicity, (ii) negligible adverse effects compared to synthetic antidiabetic drugs, (iii) economically viable and, (iv) their safety has been confirmed through traditional medicine.36Results obtained from the enzyme inhibitory effects of S. ceratophylla are shown in Table 7. All samples exhibited inhibitory activities against tyrosinase, amylase and glucosidase. Both aqueous root and aerial extracts were ineffective against cholinesterase enzymes. The petroleum ether and ethyl acetate extracts were also found inactive against BChE according to the study of Orhan et al.15 The DCM root and aerial extracts showed the highest tyrosinase (125.45 ± 1.41 and 124.68 ± 4.47 mg KAE per g, respectively) and amylase (0.76 ± 0.02 and 0.84 ± 0.02 mmol ACAE per g, respectively) activities. To the best of our knowledge, it is the first time S. ceratophylla was screened for tyrosinase, amylase and glucosidase activities. Therefore, comparison of our data with other work was not possible.
| Parts | Solvents | AChE inhibition | BChE inhibition | Tyrosinase inhibition | Amylase inhibition | Glucosidase inhibition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg GALAE per g) | IC50 (mg mL−1) | (mg GALAE per g) | IC50 (mg mL−1) | (mg KAE per g) | IC50 (mg mL−1) | (mmol ACAE per g) | IC50 (mg mL−1) | (mmol ACAE per g) | IC50 (mg mL−1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Values are reported as mean ± SD. DCM: dichloromethane; MeOH: methanol; GALAE: galantamine equivalent; KAE: kojic acid equivalent; ACAE: acarbose equivalent; na: not active.; nt: not tested. Different letters indicate significant differences in the extracts (p < 0.05, the letter “a” indicates strong ability). IC50 (mg mL−1), inhibition concentration at which 50% of the enzyme activities were inhibited. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aerial parts | Hexane | 3.78 ± 0.36c | 0.71 ± 0.07d | 5.65 ± 0.45a | 1.06 ± 0.09b | 96.32 ± 4.09cd | 0.90 ± 0.04de | 0.75 ± 0.05b | 1.78 ± 0.12c | 2.13 ± 0.01cd | 0.55 ± 0.01de | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 3.22 ± 0.04d | 0.84 ± 0.01e | 6.55 ± 1.33a | 0.94 ± 0.19b | 124.68 ± 4.47a | 0.69 ± 0.02b | 0.84 ± 0.02a | 1.59 ± 0.03b | 2.17 ± 0.01c | 0.54 ± 0.01d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 4.37 ± 0.27ab | 0.62 ± 0.04bc | 2.81 ± 0.36b | 2.14 ± 0.25c | 107.99 ± 8.04bc | 0.80 ± 0.06cd | 0.72 ± 0.03bc | 1.85 ± 0.07cd | 2.16 ± 0.02c | 0.55 ± 0.01d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 2.58 ± 0.03e | 1.04 ± 0.01f | na | na | 111.50 ± 4.42abc | 0.78 ± 0.03bcd | 0.73 ± 0.01bc | 1.83 ± 0.03cd | 0.24 ± 0.01g | 4.99 ± 0.30h | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | na | na | na | na | 82.68 ± 8.12de | 1.05 ± 0.11ef | 0.14 ± 0.01d | >5 | 0.05 ± 0.01h | >5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Roots | Hexane | 3.93 ± 0.15bc | 0.68 ± 0.03cd | 6.99 ± 0.42a | 0.85 ± 0.05b | 112.10 ± 1.73ab | 0.77 ± 0.01bc | 0.68 ± 0.01c | 1.95 ± 0.01d | 2.21 ± 0.01bc | 0.53 ± 0.01cd | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DCM | 4.62 ± 0.13a | 0.58 ± 0.02b | na | na | 125.45 ± 1.41a | 0.69 ± 0.01b | 0.76 ± 0.02b | 1.76 ± 0.04c | 2.07 ± 0.01c | 0.57 ± 0.01d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH | 4.17 ± 0.03abc | 0.64 ± 0.01bcd | 6.19 ± 0.29a | 0.96 ± 0.04b | 116.23 ± 7.23ab | 0.74 ± 0.05bc | 0.70 ± 0.01bc | 1.90 ± 0.03cd | 2.31 ± 0.01a | 0.51 ± 0.01b | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MeOH/water (80%) | 2.73 ± 0.22de | 0.99 ± 0.08ef | 3.67 ± 0.25b | 1.63 ± 0.11c | 106.56 ± 4.50bc | 0.81 ± 0.04cd | 0.75 ± 0.01b | 1.77 ± 0.02c | 1.02 ± 0.07d | 1.16 ± 0.08e | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aqueous | na | na | na | na | 73.36 ± 1.85e | 1.18 ± 0.03f | 0.13 ± 0.01d | >5 | 0.79 ± 0.03e | 1.49 ± 0.07f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standards | Galantamine | — | 0.0027 ± 0.001a | — | 0.006 ± 0.001a | — | nt | — | nt | — | nt | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kojic acid | — | nt | — | nt | — | 0.09 ± 0.01a | — | nt | — | nt | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acarbose | — | nt | — | nt | — | nt | — | 0.86 ± 0.01a | — | 0.76 ± 0.01a | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.4. Antimicrobial evaluation
The broth microdilution assay results were given in Table 8. According to the results obtained from test, hexane extracts of aerial parts of S. ceratophylla revealed MIC values ranging between 3.12–0.019 mg mL−1 doses. It was seen that Sarcina lutea was the most sensitive bacteria against to aerial hexane extract with a dose of 0.097 mg mL−1 MIC and followed by the Bacillus cereus with 0.19 MIC value. For Citrobacter MIC was found as 1.56 mg mL−1. Aerial part hexane extract had antifungal capacity at dose of 3.12 mg mL−1 against Candida albicans. While Pseudomonas aeruginosa was resistant to aerial part hexane extract it affected from root extracts at a concentration of 1.56 mg mL−1. Same as Pseudomonas, root extract was more effective than aerial part extract against Staphylococcus aureus with 0.39 mg mL−1 MIC value. The root hexane extract of S. ceratophylla manifested very significant antibacterial activity against S. lutea and B. cereus at a dose of 0.048 mg mL−1. It was effective against Proteus at 1.56 mg mL−1 MIC and Candida was more sensitive against root extract than aerial part hexane extract with 0.78 mg mL−1 MIC. When the dichloromethane extracts were evaluated it was determined that S. lutea affected from DCM aerial part extract at a dose of 0.097 mg mL−1 and affected from root extract at a concentration of 0.048 mg mL−1. MIC values were determined as 0.097 mg mL−1 both for two extract against B. cereus. Root extracts was more effective against S. aureus than aerial part extract with 0.097 mg mL−1. Two extracts of DCM affected P. aeruginosa at 1.56 mg mL−1 dose. Antifungal activity was observed at 3.12 mg mL−1 dose for two DCM extracts. The lowest MIC value was determined for methanol aerial part extract against B. cereus and S. lutea at a dose of 0.78 mg m−1. For root methanol extract B. cereus was the sensitive bacterium with 0.19 mg mL−1 MIC value. Salmonella enteritidis, which was resistant to hexane and DCM extracts, affected from aerial part methanol extract at 1.56 mg mL−1 concentration. Similarly, Klebsiella pneumoniae was sensitive to root methanol extract at a dose of 1.56 mg mL−1 while this bacterium resistant to hexane and DCM extracts. Except for Yersinia enterocolitica and Salmonella typhimurium, most of the bacteria showed MIC value ranging between 3.12-1.56 mg mL−1 concentrations against methanol extracts.| Strains | MIC values of Salvia ceratophylla extracts (mg mL−1) | Gentamicin (μg mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | DCM | Methanol | Methanol/water | Aqueous | |||||||
| Aerial | Root | Aerial | Root | Aerial | Root | Aerial | Root | Aerial | Root | ||
| Escherichia coli ATCC 25922 | — | — | — | — | — | — | 1.56 | — | — | 6.25 | 1.95 |
| Pseudomonas aeruginosa ATCC 27853 | — | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | — | — | <0.97 |
| Klebsiella pneumoniae ATCC 70603 | — | — | — | — | — | 1.56 | 1.56 | 1.56 | — | — | 7.81 |
| Staphylococcus aureus ATCC 43300 | 3.12 | 0.39 | 3.12 | 0.097 | 1.56 | 1.56 | 1.56 | 0.78 | 1.56 | 3.12 | 1.95 |
| Salmonella enteritidis ATTC 13076 | — | — | — | — | 1.56 | — | 1.56 | 1.56 | — | 1.56 | 1.95 |
| Sarcina lutea ATCC 9341 | 0.097 | 0.048 | 0.097 | 0.048 | 0.78 | 1.56 | 0.39 | 0.19 | — | 1.95 | |
| Salmonella typhimurium NRRLE 4463 | — | — | — | — | — | — | 1.56 | 1.56 | — | — | 1.95 |
| Yersinia enterocolitica ATCC 1501 | — | — | — | — | — | — | — | — | 6.25 | 6.25 | 1.95 |
| Proteus mirabilis ATCC 25933 | 3.12 | 1.56 | 3.12 | 3.12 | 3.12 | 1.56 | 1.56 | 1.56 | 3.12 | 3.12 | 1.95 |
| Bacillus cereus ATTC 11778 | 0.19 | 0.048 | 0.097 | 0.097 | 0.78 | 0.19 | 0.39 | 0.097 | — | — | 1.95 |
| Citrobacter freundii ATCC 8090 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 1.56 | 6.25 | 6.25 | 1.95 |
| Candida albicans ATCC 26555 | 3.12 | 0.78 | 3.12 | 3.12 | 3.12 | 3.12 | 1.56 | 3.12 | 3.12 | — | 7.81 |
Methanol and water mixture aerial part extract of S. ceratophylla revealed MIC values between 3.12 to 1.56 mg mL−1 doses. Although MIC values for S. lutea and B. cereus were determined as 0.39 mg mL−1, this extract was effective against S. typhimurium at a dose of 1.56 mg mL−1 when compared previous three extracts. Escherichia coli only affected from aerial part extract at 1.56 mg mL−1 dose. The lowest MIC reported for root extract was 0.097 mg mL−1 for B. cereus. Infusion aerial part extract manifested antibacterial activity against S. aureus at a dose of 1.56 mg mL−1. Similarly, infusion root extract had antibacterial capacity against S. enteritidis (1.56 mg mL−1) only infusion extracts were effective against Y. enterocolitica with 6.25 mg mL−1 MIC value. The results showed that S. ceratophylla extracts had significant antibacterial activities against Gram positive bacteria (B. cereus, S. lutea and S. aureus) than Gram negative bacteria. Especially hexane and DCM root extracts revealed very good antibacterial activity against Gram positive bacteria at 0.048 mg mL−1 dose. The lowest MIC values were determined against S. lutea and B. cereus. The study showed that Y. enterocolitica and E. coli were the most resistant bacteria. K. pneumoniae affected from methanol-based extracts. Also extracts had antifungal capacity against Candida albicans. Hexane root extract showed the lowest antifungal activity at a dose of 0.78 mg mL−1.
Several Salvia species reported for their antimicrobial activity and pharmacological properties37,38 revealed that Salvia species contain caffeic acids, major group of phenolic acids, and derivatives. Caffeic acid plays a central role in the biochemistry of Lamiaceae and occurs predominantly in the dimer form as rosmarinic acid.39 The trimers and tetramers are also interesting from a therapeutic point of view as they have demonstrated various biological activities such as anti-oxidant, antimicrobial and anticancer.40 Chemical composition analyses showed that S. ceratophylla extracts tested in this assay included phenolic compounds such as rosmarinic acid and caffeic acid. In a study conducted by Matejczyk et al.,41 it was determined that caffeic acid revealed significant antimicrobial action against tested pathogens. Also, Li and Na salts of caffeic acid had an important activity, too. In that study also rosmarinic acid and its Li, Na and K salts were tested and better results were observed. Świsłocka42 reported that rosmarinic acid had bactericidal activity against Staphylococcus epidermidis, Stenotrophomonas maltophilia, and Enterococcus faecalis. Antimicrobial mechanisms of rosmarinic acid has not been explained clearly yet. But there were several studies about antibacterial mechanism of phenolic acids. The possible explanation for this situation could be as follows: the phenolic acids have pro-oxidative properties and they can alter the hydrophobicity and after the charging of the cell surface cellular cracking and formation can occur. The main mechanism of action of rosmarinic acid is its ability to damage the cell membrane.43 Significant antimicrobial activities of extracts determined in this study can be attributed to presence of rosmarinic and caffeic acid in S. ceratophylla.
3.5. Cytotoxicity effects
Plant-derived natural products have been considered as promising and potent chemotherapeutic agents for more than 40 years.44 In this study, the extracts of S. ceratophylla were evaluated against HepG2 (a human liver cancer cell line) and B164A5 (a skin melanoma cell line). The effects of the extracts on the viability of S17 cells, from non-tumoral origin, were also determined. Results are shown in Table 9.| Cell line | DMSO 0.5% | Aerial parts-MeOH | Aerial parts-aqueous | Roots-MeOH | Roots- aqueous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Values represent the mean ± standard error of the mean (SEM) of six replicates (n = 6). HepG2 – human hepatocellular carcinoma cells; B16 4A5 – murine melanoma cells; S17 – murine bone marrow cells (normal cells); SI – selectivity index. In the same line, values marked by different letters are significantly different according to the Tukey HSD test (P < 0.05). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HepG2 | 101 ± 7a | 75.3 ± 2.6b | 89.4 ± 6.3ab | 30.9 ± 2.5c | 34.5 ± 1.7c | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| B16 4A5 | 88.2 ± 2.1a | 90.4 ± 2.8a | 57.3 ± 1.5b | 95.1 ± 2.8a | 91.1 ± 3.7a | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S17 | 79.3 ± 4.9b | 33.8 ± 2.7c | 98.4 ± 1.0a | 42.0 ± 1.2c | 39.3 ± 3.4c | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SI – HepG2 | 0.79 | 0.45 | 1.10 | 1.36 | 1.14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SI – B16 4A5 | 0.90 | 0.37 | 1.72 | 0.44 | 0.43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Root-MeOH and root-aqueous were the most toxic towards HepG2 cells (30.9 and 34.5% of cell viability), while extract aerial part-water was more active against B16 4A5 cells (57.3% of cell viability). Regarding the non-tumoral S17 cells, all samples showed significant toxicity, except extract aerial part-water that showed higher cell viability than the control (P < 0.05). Therefore, aerial part-aqueous although displaying moderate cytotoxic activity on B16 4A5 melanoma cells, exhibited the highest selectivity index for (SI = 1.72).
The observed results could be attributed to the presence phytochemicals present in the latter extract. For instance, this finding may be linked to the presence of gallic acid, which has been claimed to inhibit carcinogenesis and induces apoptosis in previous studies.45–47 Besides, the methanolic root extract contained luteolin, a flavonoid, also known to possess anti-cancer effect.48–50 However, as a future work, further assays should be conducted with the aim to isolate and identify the phytochemicals responsible for the observed cytotoxic properties and ensure if the toxicity towards cancerous cell lines is related to specific bioactive compounds.
3.6. PLS-DA based methods to discriminate between studied parts
The present study was focused upon two parts from S. ceratophylla including aerial part and roots and it was undertaken to assess the total antioxidant and selected five enzyme inhibitory activities of diverse extracts derived from said parts. For the purpose of evaluating the variation of antioxidant and enzyme inhibitory activities between the different studied parts, the supervised partial least squares discriminant analysis (PLS-DA) was applied to the data. PLS-DA is a multivariate regression analysis aiming at find the optimal linear combinations of variables being able accurately to discriminate the sample groups. In particular, latent function emanating from the linear combinations of variables summarize as much as possible the information and reduce the dimension of the original data. Thus, to perform the model, the factor “Parts” as used as class membership criteria and the results were reported in Fig. 3. By viewing Fig. 3A, we noted a clear discrimination between the two parts. The majority of aerial parts extracts were grouped on the left side of the first function while the roots extracts were aggregated on the positive and negative side of the first two function respectively. The model, had a great performance; in particular, incorporating the first two function, it was able to discriminate the both parts with an accuracy of 96.89% (Fig. 3B).The loadings plot displayed the contribution of the biological activities on the first two function. Function 1 was positively related to MCA, glucosidase, AChE, BChE and tyrosinase and negatively bound to the other activities (PPBD, DPPH, FRAP, CUPRAC, Amylase and ABTS). While function 2 was positively determined by BChE, PPBD, amylase, glucosidase and AChE and negatively associated to MCA, ABTS, CUPRAC, FRAP, DPPH, and tyrosinase. On the other hand, this figure allowed to determine the biological activities characterizing each part. In general, antioxidant activities and anti-amylase recorded the highest value in aerial parts in contrast to roots that exhibited the best anti-cholinesterase, anti-glucosidase and anti-tyrosinase as well as metal chelating ability.
Afterwards, the biological activities which mostly varied from one part to another were observed. In this regard, the VIP score of each bioactivity was calculated and reported in figure AC. On the basis of the value above 1, it emerged that four activities including PPBD, MCA, DPPH and ABTS, differed considerably across parts. Thus, aerial parts were characterized by an excellent total antioxidant capacity and ability to scavenging ABTS and DPPH radicals while roots were distinguished by a high ability to chelate Fe2+ ion (Fig. 4).
The results of the present study indicated high levels of bioactivities variability between the areal parts and roots of S. ceratophylla. The reason is that the concentration and type of secondary metabolites involve in the evaluated bioactivities, vary according to the plants parts. This outcome are in agreement with our previous work on the topic, which has reported that different parts of the same plant are characterized by different content of secondary metabolites.51,52 Further, this variability may be due to ordered expression of the genome such that specific enzymes or group of enzymes are activated for the biosynthesis of certain molecules at particular tissue or organ of plant, and not in another. For instance, Yosr et al.53 reported that the amount in leaves of phenolic compounds compared to the other plant organs may be due to the interaction between organs and multiple processes of synthesis or degradation and transport implied in the distribution of these phenolic compounds at the plant level.
3.7. Effect of extraction solvents on the antioxidant and enzyme inhibition activities of each parts
Multiple solvents extraction condition was used with the purpose of achieving the best method to obtain a higher antioxidant and enzyme inhibitory activities of aerial parts and roots of S. ceratophylla (Fig. 3A and B). In general, a significant difference was observed between the extracts of each parts, for all biological activities. In aerial part, the extraction procedure using MeOH/water (80%) was highly efficient to scavenge DPPH radical and reduce Cu2+ ion. Similarly, as regards the roots, the same extracts exhibited highest ABTS scavenging capacity and Fe3+ and Cu2+ reducing power. Both methanol and MeOH/water (80%) extracts of roots and aerial parts scavenged DPPH radicals more effectively and presented highest total antioxidant capacity respectively. The extracts of aerial parts obtained using water possessed excellent ABTS and MCA activities while the water and MeOH/water (80%) showed a better reducing Fe3+ activity. Total antioxidant capacity of roots was ranged in order of DCM > MeOH > MeOH/water (80%) > water MeOH/water (80%) > hexane, whereas metal chelating activity increased as follows: water > DCM, MeOH/water (80%) > methanol > hexane. When it comes to enzyme inhibitory activities, hexane extract of aerial parts and roots had the highest anti-tyrosinase activity. In addition, the same extract exhibited strongest anti-BChE activity. However, in aerial parts, the activity of hexane extract was similar to that of DCM. For the second enzyme involved in the management of neurodegenerative disease, methanol (aerial parts) and DCM (roots) extractions showed the best anti-AChE activity. Regarding the anti-amylase assay, the strongest activity was shown by DCM for aerial parts and DCM and MeOH/water (80%) for roots. Furthermore, three extracts derived from aerial parts i.e., DCM, hexane and MeOH showed the highest anti-glucosidase activity while regarding the roots the best activity was presented by MeOH (Fig. 5).Historically, it is well known that extraction of secondary metabolites from plant matrix is impacted by multiple factors such as their chemical nature, the presence of interfering substances without forgetting the extraction solvent and technique used. In fact, the polarities of secondary metabolites in plants greatly vary and therefore, it is necessary to select an adequate solvent for efficient extraction in quantity and quality of the molecules of interest. As it is well known that secondary metabolites have diverse nature, concentration ranges and physicochemical properties. Accordingly, no single solvent able to recovery efficiently all of the classes of secondary metabolites from a plant matrix, simultaneously. This lends support our observations that the different solvent used, had showed each at least good result on all the evaluated biological activities. Moreover, outside the conventional extraction solvents, several researchers have employed combination of organic solvent-water for the extraction of secondary metabolites from plant. According to Cheng et al.,54 solvent mixtures allow to extract different molecules values, thanks to their differing efficacies in the penetration of plant matrixes and solubilization of the secondary metabolites. Much more, the presence of water enhance the permeability of cell membrane and therefore enables efficiently mass transfer by molecular diffusion as well as the extraction of the water soluble compounds.54
4. Conclusion
In the current work, all extracts of S. ceratophylla exhibited activity against amylase and glucosidase which are the key clinical enzymes related to diabetes, a disease affecting millions of people across the globe. A particular interest is the tyrosinase inhibitory activity displayed by the DCM root extract which can be qualified as a potent and promising activity. Thus, extract can further be examined for potential epidermal hyperpigmentation processes. Additionally, data amassed herein demonstrated that the hydro-methanolic aerial extract may act as a good antioxidant. From the antimicrobial analysis, it can be concluded that S. ceratophylla can be a potential source of bioactive compounds to combat Bacillus cereus infections. Methanolic root extract demonstrated a relatively low cytotoxicity. However, further toxicological studies should be conducted to ascertain its safety. The present study provides rationale for further in vivo/ex vivo pharmacological investigations.Author contributions
Sengul Uysal: conceptualization, data curation, formal analysis, writing – original draft. Gokhan Zengin: formal analysis, writing – original draft, supervision. Kouadio Ibrahime Sinan: methodology. Gunes Ak: methodology. Ramazan Ceylan: methodology. Mohamad Fawzi Mahomoodally: data curation, investigation, writing – original draft. Ahmet Uysal: methodology. Nabeelah Bibi Sadeer: data curation, investigation, writing – original draft. József Jekő: data curation, investigation. Zoltán Cziáky: data curation, investigation. Maria João Rodrigues: data curation, investigation. Evren Yıldıztugay: methodology. Fevzi Elbasan: methodology. Luisa Custodio: data curation, investigation.Conflicts of interest
There are no conflicts to declare.References
- Z. Aghaei Jeshvaghani, M. Rahimmalek, M. Talebi and S. A. H. Goli, Comparison of total phenolic content and antioxidant activity in different Salvia species using three model systems, Ind. Crops Prod., 2015, 77, 409–414 CrossRef CAS.
- WHO, Cancer, Available on: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed 21 November 2020).
- G. Zengin, E. J. Llorent-Martínez, M. L. F.-d. Córdova, M. B. Bahadori, A. Mocan, M. Locatelli and A. Aktumsek, Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca, Ind. Crops Prod., 2018, 111, 11–21 CrossRef CAS.
- A. Alimpić, A. Knežević, M. Milutinović, T. Stević, K. Šavikin, M. Stajić, S. Marković, P. D. Marin, V. Matevski and S. Duletić-Laušević, Biological activities and chemical composition of Salvia amplexicaulis Lam. extracts, Ind. Crops Prod., 2017, 105, 1–9 CrossRef.
- I. Yener, Determination of antioxidant, cytotoxic, anticholinesterase, antiurease, antityrosinase, and antielastase activities and aroma, essential oil, fatty acid, phenolic, and terpenoid-phytosterol contents of Salvia poculata, Ind. Crops Prod., 2020, 155, 112712 CrossRef CAS.
- R. Tundis, D. Iacopetta, M. S. Sinicropi, M. Bonesi, M. Leporini, N. G. Passalacqua, J. Ceramella, F. Menichini and M. R. Loizzo, Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae), Food Chem. Toxicol., 2017, 106, 155–164 CrossRef CAS PubMed.
- V. Adımcılar, Z. Kalaycıoğlu, N. Aydoğdu, T. Dirmenci, A. Kahraman and F. B. Erim, Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 Salvia species from Anatolia, J. Pharm. Biomed. Anal., 2019, 175, 112763 CrossRef PubMed.
- M. S. Abu-Darwish, C. Cabral, Z. Ali, M. Wang, S. I. Khan, M. R. Jacob, S. K. Jain, B. L. Tekwani, F. Zulfiqar, I. A. Khan, H. Taifour, L. Salgueiro and T. Efferth, Salvia ceratophylla L. from South of Jordan: new insights on chemical composition and biological activities, Nat. Prod. Bioprospect., 2020, 10, 307–316 CrossRef CAS.
- H. Al Jaber, Salvia ceratophylla from Jordan: Volatile Organic Compounds, Essential oil composition and antioxidant activity, Jordan J. Chem., 2016, 11, 110–121 Search PubMed.
- A. C. Gören, T. Kiliç, T. Dirmenci and G. Bilsel, Chemotaxonomic evaluation of Turkish species of Salvia: Fatty acid compositions of seed oils, Biochem. Syst. Ecol., 2006, 34, 160–164 CrossRef.
- M. Mohammadi, M. Yousefi, Z. Habibi, S. Rahmati and G. Imanzadeh, Volatile Constituents of Salvia ceratophylla L. and Salvia indica L. from Iran, J. Essent. Oil-Bear. Plants, 2010, 13, 774–780 CrossRef CAS.
- A. Ulubelen, Cardioactive and antibacterial terpenoids from some Salvia species, Phytochemistry, 2003, 64, 395–399 CrossRef CAS.
- A. C. Goren, G. Topcu, S. Oksuz, G. Kokdil, W. Voelter and A. Ulubelen, Diterpenoids from Salvia ceratophylla, Nat. Prod. Lett., 2002, 16, 47–52 CrossRef CAS PubMed.
- H. Hadavand Mirzaei, O. Firuzi, J. N. Chandran, B. Schneider and A. R. Jassbi, Two antiproliferative seco-4,5-abietane diterpenoids from roots of Salvia ceratophylla L, Phytochem. Lett., 2019, 29, 129–133 CrossRef CAS.
- I. Orhan, M. Kartal, Q. Naz, A. Ejaz, G. Yilmaz, Y. Kan, B. Konuklugil, B. Şener and M. Iqbal Choudhary, Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species, Food Chem., 2007, 103, 1247–1254 CrossRef CAS.
- A. G. Al-Bakri, G. Othman and F. U. Afifi, Determination of the antibiofilm, antiadhesive, and anti-MRSA activities of seven Salvia species, Pharmacogn. Mag., 2010, 6, 264 CrossRef CAS PubMed.
- V. Kasabri, F. U. Afifi, R. Abu-Dahab, N. Mhaidat, Y. K. Bustanji, I. Abaza and S. Mashallah, In vitro modulation of metabolic syndrome enzymes and proliferation of obesity related-colorectal cancer cell line panel by Salvia species from Jordan, Rev. Roum. Chim., 2014, 59, 693–705 Search PubMed.
- L. Scotti and M. T. Scotti, Editorial; Natural Product Inhibitors of Enzymatic Targets in Anticancer Drug Discovery - Part II, Curr. Protein Pept. Sci., 2018, 19, 342 CrossRef CAS PubMed.
- M. R. Loizzo, M. Abouali, P. Salehi, A. Sonboli, M. Kanani, F. Menichini and R. Tundis, In vitro antioxidant and antiproliferative activities of nine Salvia species, Nat. Prod. Res., 2014, 28, 2278–2285 CrossRef CAS PubMed.
- P. K. Sardar, S. Dev, M. A. Al Bari, S. Paul, M. S. Yeasmin, A. K. Das and N. N. Biswas, Antiallergic, anthelmintic and cytotoxic potentials of dried aerial parts of Acanthus ilicifolius L, Clin. Phytosci., 2018, 4, 34 CrossRef CAS.
- K. Slinkard and V. L. Singleton, Total phenol analysis: automation and comparison with manual methods, Am. J. Enol. Vitic., 1977, 28, 49–55 CAS.
- G. Zengin and A. Aktumsek, Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey, Afr. J. Tradit., Complementary Altern. Med., 2014, 11, 481–488 CrossRef PubMed.
- J. S. Katanić Stanković, R. Ceylan, G. Zengin, S. Matić, T. Jurić, A. Diuzheva, J. Jeko, Z. Cziáky and A. Aktumsek, Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition, Ind. Crops Prod., 2020, 144, 112053 CrossRef.
- D. M. Grochowski, S. Uysal, A. Aktumsek, S. Granica, G. Zengin, R. Ceylan, M. Locatelli and M. Tomczyk, In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca, Phytochem. Lett., 2017, 20, 365–372 CrossRef CAS.
- M. Balouiri, M. Sadiki and S. K. Ibnsouda, Methods for in vitro evaluating antimicrobial activity: A review, J. Pharm. Anal., 2016, 6, 71–79 CrossRef.
- Z. E. Koc and A. Uysal, Investigation of novel monopodal and dipodal oxy-Schiff base triazine from cyanuric chloride: Structural and antimicrobial studies, J. Macromol. Sci., Part A: Pure Appl.Chem., 2016, 53, 111–115 CrossRef CAS.
- M. J. Rodrigues, V. Neves, A. Martins, A. P. Rauter, N. R. Neng, J. M. Nogueira, J. Varela, L. Barreira and L. Custódio, In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers' infusions and decoctions: A comparison with green tea (Camellia sinensis), Food Chem., 2016, 200, 322–329 CrossRef CAS PubMed.
- S.-H. Oh, J. Ahn, D.-H. Kang and H.-Y. Lee, The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity, Mar. Biotechnol., 2011, 13, 205–214 CrossRef CAS.
- S. Uysal, G. Zengin, M. F. Mahomoodally, M. A. Yilmaz and A. Aktumsek, Chemical profile, antioxidant properties and enzyme inhibitory effects of the root extracts of selected Potentilla species, S. Afr. J. Bot., 2019, 120, 124–128 CrossRef CAS.
- S. Suliman, S. Yagi, A. A. Elbashir, I. Mohammed, A. Mohammed, G. Ak, G. Zengin, G. Orlando and C. Ferrante, Phenolic profile, enzyme inhibition and antioxidant activities and bioinformatics analysis of leaf and stem bark of Ficus sycomorus L, Process Biochem., 2021, 101, 169–178 CrossRef CAS.
- K. I. Sinan, M. F. Mahomoodally, O. E. Eyupoglu, O. K. Etienne, N. B. Sadeer, G. Ak, T. Behl and G. Zengin, HPLC-FRAP methodology and biological activities of different stem bark extracts of Cajanus cajan (L.) Millsp, J. Pharm. Biomed. Anal., 2021, 192, 113678 CrossRef CAS PubMed.
- D. Dhawan and J. Gupta, Comparison of Different Solvents for Phytochemical Extraction Potential from Datura metel Plant Leaves, Int. J. Biol. Chem., 2016, 11, 17–22 CrossRef.
- A. Herold, L. Cremer, A. Calugăru, V. Tamaş, F. Ionescu, S. Manea and G. Szegli, Antioxidant properties of some hydroalcoholic plant extracts with antiinflammatory activity, Roum. Arch. Microbiol. Immunol., 2003, 62, 217–227 Search PubMed.
- M. Kaneria, B. Kanani and S. Chanda, Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants, Asian Pac. J. Trop. Biomed., 2012, 2, 195–202 CrossRef CAS PubMed.
- M. Hofmann, G. Retamal-Morales and D. Tischler, Metal binding ability of microbial natural metal chelators and potential applications, Nat. Prod. Rep., 2020, 37, 1262–1283 RSC.
- J. Naveen and V. Baskaran, Antidiabetic plant-derived nutraceuticals: a critical review, Eur. J. Nutr., 2018, 57, 1275–1299 CrossRef CAS.
- M. Sharifi-Rad, B. Ozcelik, G. Altin, C. Daskaya-Dikmen, M. Martorell, K. Ramirez-Alarcon, P. Alarcon-Zapata, M. F. B. Morais-Braga, J. N. P. Carneiro, A. L. A. B. Leal, H. D. M. Coutinho, R. Gyawali, R. Tahergorabi, S. A. Ibrahim, R. Sahrifi-Rad, F. Sharopov, B. Salehi, M. D. Contreras, A. Segura-Carretero, S. Sen, K. Acharya and J. Sharifi-Rad, Salvia spp. plants-from farm to food applications and phytopharmacotherapy, Trends Food Sci. Technol., 2018, 80, 242–263 CrossRef CAS.
- G. P. P. Kamatou, N. P. Makunga, W. P. N. Ramogola and A. M. Viljoen, South African Salvia species: A review of biological activities and phytochemistry, J. Ethnopharmacol., 2008, 119, 664–672 CrossRef CAS.
- U. Gerhardt and A. Schroeter, Rosmarinic acid—a naturally occurring antioxidant in spices, Fleischwirtschaft, 1983, 63, 1628–1630 CAS.
- Y. R. Lu and L. Y. Foo, Polyphenolics of Salvia - a review, Phytochemistry, 2002, 59, 117–140 CrossRef CAS.
- M. Matejczyk, R. Swislocka, A. Golonko, W. Lewandowski and E. Hawrylik, Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds, Adv. Med. Sci., 2018, 63, 14–21 CrossRef.
- R. Świsłocka, Spectroscopic (FT-IR, FT-Raman, UV absorption, 1H and 13C NMR) and theoretical (in B3LYP/6-311++ G** level) studies on alkali metal salts of caffeic acid, Spectrochim. Acta, Part A, 2013, 100, 21–30 CrossRef.
- A. Sánchez-Maldonado, A. Schieber and M. Gänzle, Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria, J. Appl. Microbiol., 2011, 111, 1176–1184 CrossRef.
- A. L. Demain and P. Vaishnav, Natural products for cancer chemotherapy, Microb. Biotechnol., 2011, 4, 687–699 CrossRef.
- K. Khorsandi, Z. Kianmehr, Z. hosseinmardi and R. Hosseinzadeh, Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis, Cancer Cell Int., 2020, 20, 18 CrossRef PubMed.
- S. Verma, A. Singh and A. Mishra, Gallic acid: Molecular rival of cancer, Environ. Toxicol. Pharmacol., 2013, 35, 473–485 CrossRef CAS PubMed.
- Z. M. Sourani, B. P. Pourgheysari, P. M. Beshkar, H. P. Shirzad and M. M. Shirzad, Gallic Acid Inhibits Proliferation and Induces Apoptosis in Lymphoblastic Leukemia Cell Line (C121), Iran. J. Med. Sci., 2016, 41, 525–530 Search PubMed.
- M. Imran, A. Rauf, T. Abu-Izneid, M. Nadeem, M. A. Shariati, I. A. Khan, A. Imran, I. E. Orhan, M. Rizwan, M. Atif, T. A. Gondal and M. S. Mubarak, Luteolin, a flavonoid, as an anticancer agent: A review, Biomed. Pharmacother., 2019, 112, 108612 CrossRef CAS.
- Y. Lin, R. Shi, X. Wang and H.-M. Shen, Luteolin, a flavonoid with potential for cancer prevention and therapy, Curr. Cancer Drug Targets, 2008, 8, 634–646 CrossRef CAS.
- G. Seelinger, I. Merfort, U. Wölfle and C. M. Schempp, Anti-carcinogenic effects of the flavonoid luteolin, Molecules, 2008, 13, 2628–2651 CrossRef CAS PubMed.
- G. Orlando, C. Ferrante, G. Zengin, K. I. Sinan, K. Bene, A. Diuzheva, J. Jekő, Z. Cziáky, S. D. Simone, L. Recinella, A. Chiavaroli, S. Leone, L. Brunetti, C. M. N. Picot-Allain, M. F. Mahomoodally and L. Menghini, Qualitative Chemical Characterization and Multidirectional Biological Investigation of Leaves and Bark Extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. (Combretaceae), Antioxidants, 2019, 8, 343 CrossRef CAS.
- K. I. Sinan, L. Saftić, Ž. Peršurić, S. K. Pavelić, O. K. Etienne, M. C. N. Picot-Allain, M. F. Mahomoodally and G. Zengin, A comparative study of the chemical composition, biological and multivariate analysis of Crotalaria retusa L. stem barks, fruits, and flowers obtained via different extraction protocols, S. Afr. J. Bot., 2020, 128, 101–108 CrossRef CAS.
- Z. Yosr, H. Chograni, T. Rim and B. Mohamed, Changes in essential oil composition and phenolic fraction in Rosmarinus officinalis L. var. typicus Batt. organs during growth and incidence on the antioxidant activity, Ind. Crops Prod., 2013, 43, 412–419 CrossRef CAS.
- V. J. Cheng, A. E.-D. A. Bekhit, M. McConnell, S. Mros and J. Zhao, Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate, Food Chem., 2012, 134, 474–482 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |