Open Access Article
Open Access ArticleCharacterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu)
Xiaobai Li *a,
Jian Sunb,
Zhen Chenc,
Jingyong Jiangd and
Aaron Jacksone
*a,
Jian Sunb,
Zhen Chenc,
Jingyong Jiangd and
Aaron Jacksone
aZhejiang Academy of Agricultural Sciences, Hangzhou, 310021, China. E-mail: hufanfan1982815@outlook.com; lixiaobai@mail.zaas.ac.cn
bZhejiang Research Institute of Traditional Chinese Medicine Co., Ltd., Hangzhou, 310023, China
cCollege of Life Sciences, Taizhou University, Taizhou, 318000, China. E-mail: chenzh@tzc.edu.cn
dTaizhou Academy of Agricultural Sciences, Linhai, 317000, China. E-mail: jjy5971@163.com
eSouth Oak, Stuttgart, AR 72160, USA. E-mail: sativadna@gmail.com
First published on 15th March 2021
Abstract
Chinese raspberry (Rubus chingii Hu) is a fruit valued for it's health benefits, which is indigenous to China. It is a great source of antioxidants. However, the fruit phytochemicals are poorly understood. Phenolics and carotenoids attract much attention for their antioxidant capability, and they dramatically change during fruit ripening, leading to the difference in color, flavor and medicinal components. In this study, we investigated the change of carotenoids, phenolics and antioxidant activity using spectrophotometry during four different ripening phases i.e. mature green (MG), green yellow (GY), yellow orange (YO) and red (RE). The major components of carotenoids, anthocyanins, ellagitannins and flavonols were identified and quantified by LC-MS/MS. As a result, five carotenoids (mainly β-Citraurin and its esters), six anthocyanins (mainly anthocyanins covalently linked to another flavonoid unit), methyl (S)-flavogallonate and rourinoside were first identified in Rubus. In contrast to other known raspberries, R. chingii had a continuous decrease in total phenolics during fruit ripening, which was due to a continuous decrease in flavonoids (including anthocyanin). Total anthocyanin and flavonoid respectively declined from 19.5 to 6.9 mg/100 g FW, and 646.2 to 128.5 mg/100 g FW during fruit maturation and coloration. Accordingly, the components of anthocyanins, ellagitannins and flavonols also declined, thus resulting in a decrease in antioxidant activity (from 41.2 to 10.1 TEAC/100 g FW in ABTS and from 35.3 to 7.7 mmol TEAC/100 g FW in FRAP). In contrast, total carotenoid increased from 184.2 to 305.4 mg/100 g FW. Accordingly, the components of carotenoids also increased, with the exception of lutein. Additionally, kaempferol and quercetin were the main flavonoid aglycones, which were linked to a variety of glycosides. These kaempferol- and quercetin-glycosides mainly accumulated in epidermal hair and placentae. Notably, carotenoids (i.e. β-citraurin esters), instead of anthocyanins, gradually accumulated during fruit ripening, imparting the reddish color to ripe fruit.
1. Introduction
Rubus chingii Hu with the Chinese name “Fu-Pen-Zi” is also called Chinese raspberry, which is indigenous to China. The unripe fruit is often used in Chinese medicine. It provides health promoting and protective properties against a variety of human diseases, e.g. improving renal function,1 protecting hepatocyte function2 and relieving anxiety, pain and inflammation.3 The ripe fruit is a nutritional fruit, just like red or black raspberry.The health benefits of the Rubus genus are believed to be mainly due to their abundance of phenolics and carotenoids. In Rubus, phenolics mainly consist of anthocyanins, flavonols and ellagitannins.4,5 Red and black raspberry share the same profile of anthocyanins. Their anthocyanins are predominantly cyanidin glycosides (e.g. glucosides, sophorosides, rutinosides, sambubioside and glucosyl-rutinosides), but they only contain low to trace levels of pelargonidin glycosides.4,6–8 Black raspberry has up to five-fold greater anthocyanin content than red raspberry.9,10 Flavonols in red and black raspberry are mainly kaempferol/quercetin glycosides with glucosides, rutinoside and coumaroylglucoside.7,8 Elagitannins in red and black raspberry comprise dimeric HHDP (hexahydroxydiphenic) sanguiin H-6 and a tetrameric HHDP lambertianin C, as well as ellagic acid.7,10,11 Previous studies have focused on a few compounds in unripe fruit,1,2,12 but a comprehensive analysis of phenolics throughout the whole fruit ripening process has not been done until now.
Carotenoids benefit human health for antioxidant capability of combating the “superoxide anion radical” to reduce cancer risk. Some components are transformed into vitamin A, which is required for healthy skin and mucus membranes, and night vision. In raspberries fruit, apocarotenoids are very abundant e.g. α- and β-ionone, responsible for a large part of the characteristic raspberry aroma, but amounts of xanthophyll are relatively low.13 However, the information on carotenoids in R. chingii has been very limited until now.
In R. chingii, the unripe and ripe fruits are used differently. The different uses are attributed to the discrepancy in phytochemicals, especially for phenolics and carotenoids. They dramatically change throughout the process of fruit ripening, which has attracted considerable research attention in other Rubus species. This study was undertaken to investigate the composition of phenolics and carotenoids, and their changes during fruit ripening.
2. Experimental
2.1. Plant material
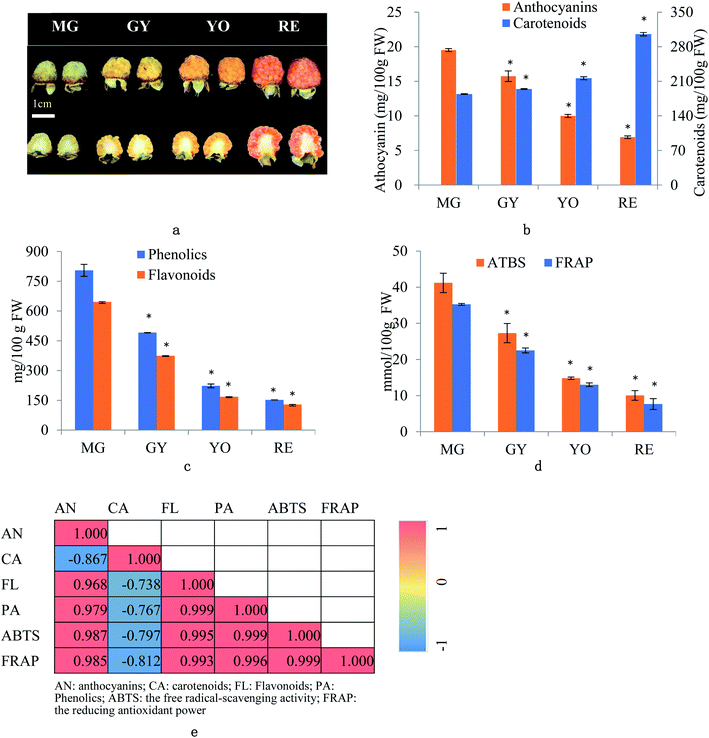
R. chingii fruits were collected from five to six plants at different ripening phases i.e. mature green (MG), green yellow (GY), yellow orange (YO) and red (RE) during the growing season (May, 2019) at Linhai, Zhejiang, China (Fig. 1a). Ten fruits were pooled as one replicate. Three biological replicates were designed for further experiments. The whole fruit were grounded with liquid nitrogen into powders, which were used for analysis of mRNA, protein and metabolites. The whole fruit tissues were “ground” in liquid nitrogen into “powder” for further analysis.2.2. Total carotenoid, anthocyanin, flavonoid, and phenolic content
Fruits were ground with liquid nitrogen and into powder, of which approximately. 0.3 g was mixed with 8 mL extraction solvent (ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone = 1
acetone = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The extract was sonicated and set aside in the dark for half-hour until the residues became colorless. The absorbance of the extract was measured at 440, 645 and 663 nm for carotenoid, chlorophyll a and b respectively.
2). The extract was sonicated and set aside in the dark for half-hour until the residues became colorless. The absorbance of the extract was measured at 440, 645 and 663 nm for carotenoid, chlorophyll a and b respectively.| Chlorophyll a (mg g−1 FW) = 0.01 × (12.7 × A663 − 2.69 × A645) × V/(M × 1000); |
| Chlorophyll b (mg g−1 FW) = 0.01 × (22.9 × A645 − 4.68 × A663) × V/(M × 1000); |
| Total chlorophyll (mg g−1 FW) = (20.21 × A645 + 8.02 × A663) × V/(M × 1000); |
| Carotenoids (mg g−1 FW) = 4.695 A 440 − 0.268 [chlorophyll (a + b)] × V/(M × 1000); |
Total anthocyanin content was determined via spectrophotometry. Approximately, 0.3 g of ground tissue with liquid nitrogen were added to 10 mL 1% (v/v) HCl methanol and sonicated for half-hour at room temperature in the dark. After centrifuging, supernatants were measured for absorbance at 530, 620 and 650 nm. The anthocyanin content was estimated using the following formulas:
| The anthocyanin content = [(A530 − A620) − 0.1 × (A650 − A620)] × V × M/(ε × m). |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), M is the molecular weight of cyanidin-3-glucoside (449 g mol−1), and M is the mass of the fruit extracted. The results were expressed as cyanidin-3-glucoside equivalents (mg CG/g FW).
600), M is the molecular weight of cyanidin-3-glucoside (449 g mol−1), and M is the mass of the fruit extracted. The results were expressed as cyanidin-3-glucoside equivalents (mg CG/g FW).
Total flavonoid content was quantified by a colorimetric assay method. Approximately 0.3 g of tissue power was mixed with 10 mL ethanol for 2 h at room temperature in the dark, and centrifuged. Of supernatant, 1 mL was mixed with 2.4 mL ethanol and 0.4 mL NaNO2. After 6 min, the mixture was added to 0.4 mL 10% Al(NO3)3 solution. After an additional 6 min, the mixture was added to 4 mL 4% NaOH and filled to 10 mL with 100% ethanol. After 15 min, the absorbance was determined at 510 nm and measured relative to a blank extraction solvent. Total flavonoid content was expressed as rutin equivalent (mg RE/g FW). Total phenolic content was determined using the Folin–Ciocalteu method following the procedure.14 Fruit tissue was finely ground in liquid nitrogen. Of tissue powders, approximately 0.3 g was mixed with 10 mL of acidified methanol (0.1% hydrochloric acid) and sonicated in ice for half-hour in the dark and centrifuged. Two mL of supernatant was transferred to another colorimetric tube, mixed with 1 mL 0.5 N Folin–Ciocalteu's phenol reagent, and set for 5 min. The reaction was neutralized with 2 mL of 5% saturated Na2CO3 and incubated for 60 min at 30 °C. The absorbance was measured at 760 nm. TPCs were expressed as gallic acid equivalent (mg GAE/g FW).
2.3. ABTS assay and FRAP assay
The free radical-scavenging activity was determined by ABTS radical cation decolorization method.15 Approximately 0.3 g was weighed and added to H2O. ABTS radical cation (ABTS˙+) was obtained by mixing 7.0 mmol L−1 ABTS solution with 2.45 mmol L−1 potassium persulfate at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) and stored in the dark for at least 16 h. The ABTS˙+ solution was diluted with 80% ethanol until its absorbance reached 0.700 ± 0.02 at 734 nm. The ABTS˙+ solution (4.0 mL, absorbance of 0.700 ± 0.02) was thoroughly mixed with 0.1 mL appropriately diluted fruit aqueous extract. The mixture was placed at room temperature for 6 min, and its absorbance was immediately measured at 734 nm. Results were expressed at Trolox equivalent (mmol TEAC/g FW).
1 (v/v) and stored in the dark for at least 16 h. The ABTS˙+ solution was diluted with 80% ethanol until its absorbance reached 0.700 ± 0.02 at 734 nm. The ABTS˙+ solution (4.0 mL, absorbance of 0.700 ± 0.02) was thoroughly mixed with 0.1 mL appropriately diluted fruit aqueous extract. The mixture was placed at room temperature for 6 min, and its absorbance was immediately measured at 734 nm. Results were expressed at Trolox equivalent (mmol TEAC/g FW).
The reducing antioxidant power of samples was determined using the FRAP method.15 Fruit tissue was completely ground with liquid nitrogen, and approximately 0.3 g of tissue powder was weighted and added to H2O. The FRAP reagent (0.3 M, pH3.6 acetate buffer, 10 M TPTZ in 40 M HCl, and 20 M FeCl3, v/v/v = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was freshly prepared. The FRAP reagent (1.0 mL) and appropriately diluted fruit aqueous extract (0.05 mL) were mixed thoroughly. The absorbance was measured at 593 nm after incubation at 37 °C for 30 min. Results were expressed as Trolox equivalent (mmol TEAC/g FW).
1) was freshly prepared. The FRAP reagent (1.0 mL) and appropriately diluted fruit aqueous extract (0.05 mL) were mixed thoroughly. The absorbance was measured at 593 nm after incubation at 37 °C for 30 min. Results were expressed as Trolox equivalent (mmol TEAC/g FW).
2.4. LC-MS/MS analysis of carotenoids, anthocyanins and flavonoids
Carotenoids were extracted by hexane/acetone/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution and sonicated in an ice bath for 30 min. The extracts was concentrated by CentriVap Refrigerated Centrifugal Concentrators at 10 °C (Labconco Models 73100 Series). The residue was dissolved with THF/acetonitrile/methanol (15
1) solution and sonicated in an ice bath for 30 min. The extracts was concentrated by CentriVap Refrigerated Centrifugal Concentrators at 10 °C (Labconco Models 73100 Series). The residue was dissolved with THF/acetonitrile/methanol (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55) solution to a final volume of 1 mL, passed through a 0.45 μm microporous membrane filter for UPLC-MS/MS analysis. Carotenoids were separated by HPLC with YMC Carotenoid C30 column (4.6 mm × 250 mm, YMC, Japan). The mobile phases were methanol (A) and acetonitrile/isopropanol (1
55) solution to a final volume of 1 mL, passed through a 0.45 μm microporous membrane filter for UPLC-MS/MS analysis. Carotenoids were separated by HPLC with YMC Carotenoid C30 column (4.6 mm × 250 mm, YMC, Japan). The mobile phases were methanol (A) and acetonitrile/isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (B). The linear gradient was: 0 min, 5%B; 60 min, 95%B; 70 min, 95%B; sample injection, 5 μL; column oven temperature, 25 °C; flow rate, 1 mL min−1.
1) (B). The linear gradient was: 0 min, 5%B; 60 min, 95%B; 70 min, 95%B; sample injection, 5 μL; column oven temperature, 25 °C; flow rate, 1 mL min−1.
Anthocyanins were extracted with 1% (v/v) HCl methanol, concentrated by CentriVap refrigerated Centrifugal Concentrators at 8 °C (Labconco Models 73100 Series) and then re-dissolved with 1 mL 1% (v/v) HCl methanol. Flavonoids was extracted with 70% methanol for 2 h at room temperature in the dark, and refrigerated Centrifugal Concentrators at 8 °C (Labconco Models 73100 Series) and then re-dissolved it with 1 mL 70% methanol. The anthocyanin and flavonoid extracts were passed through a 0.22 μm microporous membrane filter for LC-MS analysis. Anthocyanin and flavonoid were separated by UPLC with an ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 × 150 mm; Waters Corp.). For anthocyanin, the mobile phases were 1% formic acid-water (A) and acetonitrile (B). The linear gradient was as follows, 0/5, 25/35, 37/95 (min/B%); sample injection volume, 5 μL; column oven temperature, 50 °C; flow rate, 0.4 mL min−1; and the UV detector was set at 530 nm. For flavonoids and ellagitannins, the mobile phases were 0.1% formic acid-water (A) and 0.1% formic acid-acetonitrile (B). The linear gradient was as follows, 0/5, 5/10, 25/25, 37/95 (min/B%); sample injection volume, 5 μL; column oven temperature, 25 °C; flow rate, 0.3 mL min−1; and the UV detector was set at 280 and 360 nm.
The separated carotenoids and anthocyanin were analyzed by MS AB Triple TOF 5600plus System (AB SCIEX, Framingham, USA) in positive ion mode (source voltage was +5.5 kV, and the source temperature was 600 °C). The separated flavonoids were analyzed in both negative ion (source voltage at −4.5 kV, and source temperature at 550 °C) and negative ion (source voltage was +5.5 kV, and the source temperature was 600 °C). Maximum allowed error was set to ±5 ppm. Declustering potential (DP), 100 V; collision energy (CE), 10 V. For MS/MS acquisition mode, the parameters were almost the same except that the collision energy (CE) was set at 40 ± 20 V, ion release delay (IRD) at 67, ion release width (IRW) at 25. The IDA-based auto-MS2 was performed on the 8 most intense metabolite ions in a cycle of full scan (1 s). The scan range of m/z of precursor ion and product ion were set as 100–2000 Da and 50–2000 Da. The exact mass calibration was performed automatically before each analysis employing the Automated Calibration Delivery System.
The content of anthocyanin compounds was expressed as pelargonidin 3-glucoside equivalents (mg PG/g FW). The content of flavanol, ellagitannins and hydroxybenzoic acid components was determined based on their corresponding standard subtracts except methyl (S)-flavogallonate and casuarictin (galloyl-bis-HHDP-glucose), ellagic acid pentoside, rourinoside, kaempferol-3-o-rutinoside isomer, and kaempferol 3-O-hexoside isomer. The content of ellagitannins components was expressed as ellagic acid equivalents (mg g−1 FW). The content of carotenoid compounds was estimated based on the standard curve of lutein and expressed as lutein equivalents.
2.5. Frozen sections for 2-aminoethyl diphenylborate (DPBA) staining in situ
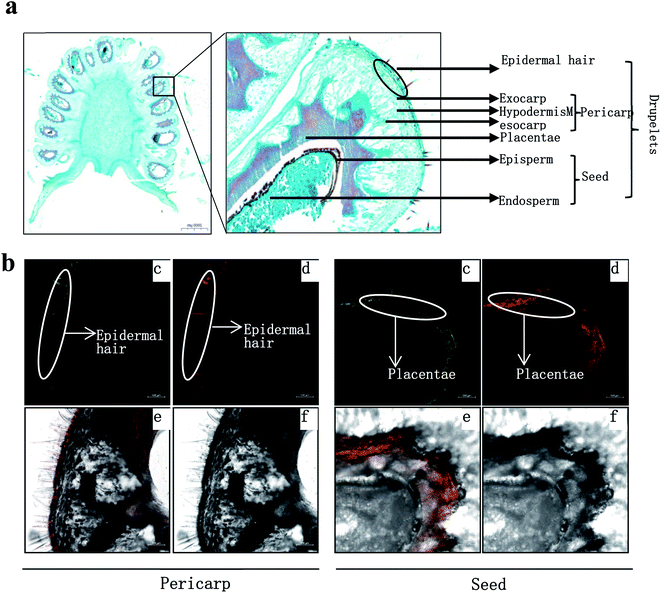
The fresh fruits were cut into several parts, and then embedded in a dedicated embedding medium (SCEM; Section-Lab). The embedded tissue was immediately frozen at −20 °C. The frozen samples in embedding medium were trimmed and then carefully sliced to produce 50–80 μm fresh-frozen sections using a Cryostat (CM1850; Leica Microsystems) set at −20 °C. The sections of fruit were stained in a freshly prepared aqueous solution of 0.25% (w/v) DPBA and 0.00375% (v/v) Triton X-100 for at least 30 min. A Zeiss LSM Zeiss LSM880 confocal laser scanning microscope was used to excite the roots with 30% maximum laser power at 458 nm, and the fluorescence was collected at 475–504 nm for kaempferol and 577 to 619 nm for quercetin.163. Results and discussion
3.1. Composition analysis of carotenoids, anthocyanins and fruit coloration
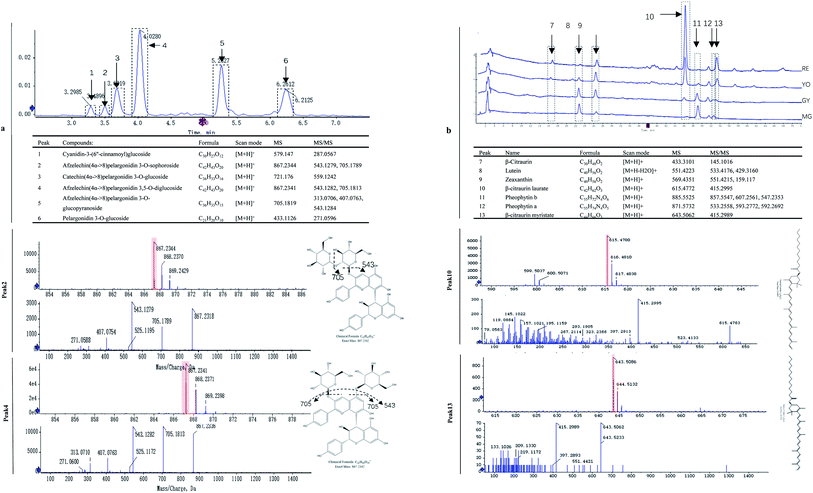
The fruit color changed during ripening (Fig. 1a) and the content of two major pigments (anthocyanin, and carotenoids) were examined (Fig. 1b). It was surprising that carotenoids were very abundant in content while anthocyanins were relatively low. More surprisingly, total anthocyanin decreased in content while total carotenoids increased. The pattern of anthocyanin change was different from previous reports in other known berries e.g. red raspberry, black raspberry, blueberry and strawberry etc. Even in other species of Rubus, i.e. red and black raspberries, concentration of fruit anthocyanins continuously increases throughout ripening.17,18 However, the pattern of carotenoid change is different among fruit crops. Tomato and loquat carotenoids progressively accumulate during fruit development and maturation.19–21 Contrarily, other fruit crops, fruit carotenoids do not accumulate but gradually decrease during ripening.22 The results suggest that the reddish color of mature fruit was probably associated with carotenoids rather than anthocyanins.These anthocyanins consisted of monomeric (i.e. pelargonidin 3-O-glucoside and cyanidin-3-(6′′-cinnamoyl)glucoside and polymeric anthocyanins covalently linked to another flavonoid unit (Fig. 2a). In agreement to total anthocyanin, these compounds also showed a continuous decrease in content during the fruit maturation process in R. chingii (Table 1). This pattern of anthocyanin change was negatively correlated with its red coloration during ripening. These polymeric anthocyanins were first identified in R. chingii, which have not been reported in Rubus. Of them, four were flavanol–anthocyanins, and pelargonidin was the main type of anthocyanin aglycones. Flavanol–anthocyanins, derived from a spontaneous condensation reaction between anthocyanins and flavanols, is usually found during storage and processing in plant-derived foods.23 Also, this type of anthocyanin (purple-colored pigments) has been reported in small amounts in a few plants. For example, 5-carboxypyrano-cyanidin/pelargonidin glycosides have been found in red onion24 and strawberries25 while 5-methylpyrano-cyanidin/delphinidin glycosides in blackcurrant seeds.26 A kind of cyanidin pigment linked to gallic acid with the C–C bonds, have been found in petals of Rosa.27 Additionally, anthocyanin linked to (epi)catechin or (epi)afzelechin moieties have been found in strawberry,25 runner beans and purple corn.28 Pelargonidin is the main type of aglycone for flavanol–anthocyanin in strawberry, while cyanidin is the main type in runner beans, and purple corn. In red raspberry, cyanidin 3-glucoside is the most prominent, followed by cyanidin 3-glucosylrutinoside and cyanidin 3-rutinoside,17 and then by pelargonidin 3-(glucosyl) rutinoside, cyanidin-3-O-rutinoside, and cyanidin-3-glucoside.18 Similarly, in black raspberry, anthocyanin increases as fruit matures,29 and cyanidin-3-O-rutinoside is the primary anthocyanin, followed by cyanidin-3-xylosylrutinoside and cyanidin-3-sambubioside.30 The results indicate that R. chingii has a special profile of anthocyanin, which is very different from other known species in Rubus.
| Peak | Compound | MG (μg g−1) | GY (μg g−1) | YO (μg g−1) | RE (μg g−1) | |
|---|---|---|---|---|---|---|
| a Carotenoid was expressed as lutein equivalents. Anthocyanin was expressed as pelargonidin 3-O-glucoside equivalents. b: was expressed as Ellagic acid equivalents. c: was expressed as kaempferol-3-o-rutinoside equivalents. *: T-test (P < 0.05). | ||||||
| Carotenoid | 1 | β-Citraurin | 42 ± 2 | 72 ± 3* | 226 ± 10* | 489 ± 20 |
| 2 | Lutein | 1450 ± 60 | 1022 ± 50* | 239 ± 10* | 111 ± 4 | |
| 3 | Zeaxanthin | 375 ± 20 | 971 ± 50* | 1136 ± 50* | 1172 ± 40 | |
| 4 | β-Citraurin laurate | 0 ± 0 | 307 ± 10* | 2961 ± 100* | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543 ± 400 543 ± 400 |
|
| 7 | β-Citraurin myristate | 405 ± 20 | 234 ± 10* | 556 ± 20* | 1494 ± 50 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Anthocyanin | 8 | Cyanidin-3-(6′′-cinnamoyl)glucoside | 18.8 ± 0.7 | 12.0 ± 0.5* | 11.6 ± 0.4* | 9.7 ± 0.4 |
| 9 | Afzelechin(4α->8)pelargonidin 3,5-O-diglucoside | 17.6 ± 0.6 | 12.0 ± 0.5* | 11.6 ± 0.5* | 10.0 ± 0.4 | |
| 10 | Catechin(4α->8)pelargonidin 3-O-β-D-glucopyranoside | 53 ± 2 | 44 ± 1* | 31 ± 1* | 16.5 ± 0.8 | |
| 11 | Afzelechin(4α->8)pelargonidin 3-O-sophoroside | 123 ± 4 | 66 ± 2* | 59 ± 3* | 21 ± 1 | |
| 12 | Afzelechin(4α->8)pelargonidin 3-O-β-D-glucopyranoside | 73 ± 3 | 57 ± 2* | 40 ± 2* | 19.6 ± 0.9 | |
| 13 | Pelargonidin 3-O-glucoside | 66 ± 2 | 45 ± 2* | 41 ± 2* | 23 ± 1 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
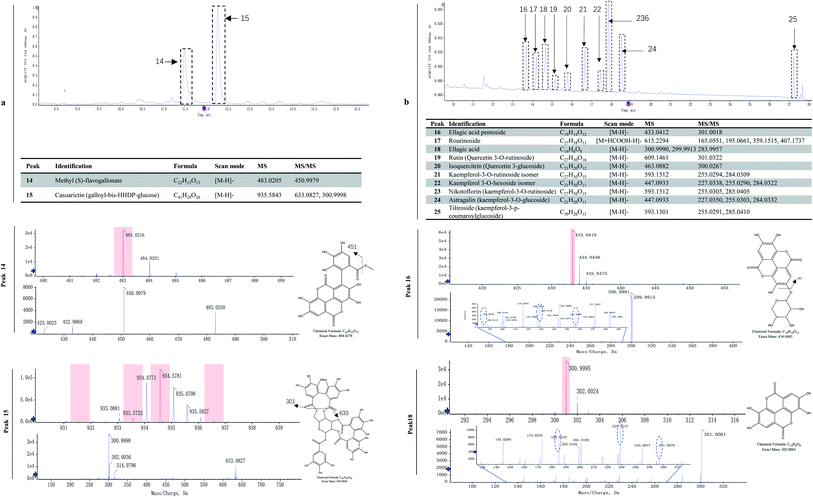
| Ellagitannins | 14 | Methyl (S)-flavogallonateb | 482 ± 20 | 279 ± 20* | 125 ± 5* | 95 ± 4 |
| 15 | Casuarictin (galloyl-bis-HHDP-glucose)b | 803 ± 40 | 490 ± 30* | 222 ± 10* | 151 ± 6 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Hydroxybenzoic derivatives, and flavonols | 16 | Ellagic acid pentosideb | 25.4 ± 0.9 | 14.6 ± 0.5* | 3.4 ± 0.1* | 4.0 ± 0.1 |
| 17 | Rourinosideb | 2.33 ± 0.08 | 1.34 ± 0.04* | 0.49 ± 0.02* | 0.32 ± 0.01 | |
| 18 | Ellagic acid | 50 ± 2 | 30 ± 1* | 14.0 ± 0.8* | 9.7 ± 0.5 | |
| 19 | Rutin (quercetin 3-O-rutinoside) | 4.7 ± 0.3 | 2.7 ± 0.1* | 1.21 ± 0.06* | 0.93 ± 0.05 | |
| 20 | Isoquercitrin (quercetin 3-glucoside) | 5.8 ± 0.3 | 3.4 ± 0.2* | 1.51 ± 0.07* | 1.16 ± 0.06 | |
| 21 | Kaempferol-3-o-rutinoside isomerc | 26.3 ± 0.8 | 15.2 ± 0.8* | 6.8 ± 0.3* | 3.1 ± 0.1 | |
| 22 | Kaempferol 3-O-hexoside isomerc | 13.3 ± 0.5 | 7.0 ± 0.3* | 3.2 ± 0.1* | 2.11 ± 0.08 | |
| 23 | Nikotoflorin (kaempferol-3-o-rutinoside) | 61 ± 3 | 31 ± 2* | 13.5 ± 0.8* | 9.7 ± 0.4 | |
| 24 | Astragalin (kaempferol-3-glucoside) | 31 ± 2 | 18 ± 1* | 8.2 ± 0.4* | 6.3 ± 0.3 | |
| 25 | Tiliroside (kaempferol-3-p-coumaroylglucoside) | 17 ± 1 | 10.4 ± 0.6* | 4.7 ± 0.2* | 3.2 ± 0.2 | |
These carotenoids primarily consisted of three apocarotenoids (β-citraurin, β-citraurin laurate, and β-citraurin myristate), and two xanthophylls (zeaxanthin, and lutein) (Fig. 2b). β-citraurin and its esters, and zeaxanthin gradually accumulated while lutein gradually decreased (Table 1). In fact, zeaxanthin was the precursor of these apocarotenoids. This is the first time these β-citraurin esters have been identified in Rubus. β-citraurin is a C30 apocarotenoid first discovered in Sicilian oranges,31 and gives rise to the peel color of citrus fruits which can range from yellow to red.31,32 However, the accumulation of β-citraurin is not common, and only observed in the flavedos of some varieties during fruit ripening.33 Raspberry is another one of the few fruits accompanied by xanthophyll degradation but by a massive apocarotenoids production during fruit ripening.13 In spite of the fact that raspberries are very rich in the apocarotenoids i.e. α- and β-ionone, responsible for a large part of the characteristic raspberry aroma, they contain relatively low amounts of carotenes.13,34 Raspberry carotenoids are diverse in composition. In yellow and red raspberry, ripe fruits contain considerable amounts of free lutein, esterified lutein (saturated fatty acids) and apocarotenoids (α- and β-ionone), but a small amount of zeaxanthin, phytoene, β-carotene and α-carotene.13,35 Lutein and β-carotene decrease in content while phytoene, esterified lutein and apocarotenoids (α- and β-ionone) increase in content during fruit ripening. In a wild raspberry (Rubus palmatus), β-cryptoxanthin accumulates during fruit ripening.36 The results indicate that β-citraurin and its esters are species-specific products in R. chingii.
Taken together, the reddish coloration of fruit is caused by β-citraurin and its esters rather than any component of anthocyanins. The profile of R. chingii carotenoids and anthocyanins is very different from that of other known Rubus species.
3.2. Phenolics and antioxidant capability
Total flavonoid content averaged 3.3 mg RE/100 g FW, and it decreased by 42.1%, 55.3%, and 23.3% from MG to GY, YO, and RE (Fig. 1b). Total phenolic content averaged 20.89 mg GAE/100 g FW, and dropped by 39.0%, 54.7%, and 31.6% from MG to GY, YO, and RE respectively (Fig. 1b). It was surprising to see that the total phenolics (including anthocyanins and other flavonoids) showed a continuous decrease during the fruit maturation process in R. chingii. The pattern of phenolic change was different from any other report in Rubus species including red and black raspberry. In red raspberry fruit, the total phenolic concentration decreases from the green to the veraison stage, and then increases until maturity.17 The increasing trend in anthocyanins and “V”-type change in phenolics are ubiquitous during fruit ripening in many berries, e.g. blueberry,14 cranberry,37 strawberry,38 and grape.39 The increase in phenolics at the later stage of maturation is mainly due to substantial increases in anthocyanin after veraison.14 In contrast to most known berries, R. chingii had the continuous decrease in anthocyanins, which contributed to the continuous decrease in flavonoids and phenolics. Antioxidant capacity of fruit was estimated by the free radical-scavenging activity (ABTS) and the reducing antioxidant power (FRAP) assay. ABTS averaged 23.3 mmol TEAC/100 g and it dropped by 33.7%, 45.8% and 32.1% from MG to GY, YO, and RE (Fig. 1d). Similarly, FRAP averaged 19.6 mmol TEAC/100 g and it dropped by 36.2%, 42.2%, and 41.0% (Fig. 1d). Anthocyanins, flavonoids, and phenolics were tightly related with antioxidant capacity (Pearson correlations ranging from 0.968 to 0.999). However, carotenoid was negatively correlated with these phenolics and antioxidant capacity (Pearson correlations ranging from −0.867 and −0.738) (Fig. 1e). The result indicates that the antioxidant capability is highly dependent on phenolic content rather than carotenoids. It may be due to that carotenoids principally scavenge singlet molecular oxygen and peroxyl radicals, and their antioxidant capability can be detected using other assays, like ORAC-L.40,41Total content of phenolics peaked at 4026.3 (mg GAE/100 g FW) at MG (Fig. 1c). It is 10-fold higher than mature fruit in red raspberry (357.8 mg GAE/100 g FW), blackberry (850.5), strawberry (621.9), blueberry (305.4) and cherry (314.5).42,43 Also, the total content of flavonoids peaked at 646.2 in MG (mg RE/100 g FW) (Fig. 1c), which was higher than that in raspberry.43 ABTS peaked at 41.2 (mmol TEAC/100 g FW) or 411.8 (μmol TEAC/g FW) in MG fruit and was over 20 folds higher than that in mature fruit of red raspberry (6.3 μmol TEAC/g FW), blackberry (13.2), strawberry (7.9), blueberry (5.9) and cherry (8.8).42 The extremely high antioxidant capacity of unripe fruit may be one of the reasons for its utilization in a traditional Chinese medicine.
3.3. Composition analysis of flavonoids and ellagitannins
Ellagitannins and other flavonoids were identified by LC-MS/MS. Two characteristic wavelengths were used to detect the compounds based on their structural properties, i.e. 280 nm for ellagitannin, and 360 nm for hydroxybenzoic acid and flavonoids. The UPLC profiles at 280 and 360 nm are shown in Fig. 3a and 4b, respectively. The major two compounds detected at 280 nm belonged to the ellagitannins family (Fig. 3a). Ellagitannins are hydrolyzable tannins, esterified with hexahydroxydiphenic acid (HHDP) and a polyol (i.e. glucose). Of ellagitannins, casuarictin was predominant in content followed by methyl (S)-flavogallonate. Both decreased in content during fruit maturation (Table 1). Of hydroxybenzoic acid and flavonoids, kaempferol-3-o-rutinoside was predominant in content, followed by ellagic acid and kaempferol-3-glucoside, while rourinoside was the least in content, followed by rutin and isoquercitrin. All of them also showed a trend of decrease in content.Plants ellagic acid is present as a free compound, in glycosylated and/or acylated form, or as ellagitannin derivatives usually esterified with glucose. In raspberries, free ellagic acid constitutes only a minor part of the total ellagic acids.44 In red raspberries, the most abundant ellagitannins are sanguiin H-6, sanguiin H-10 isomer, and lambertianin C,45,46 while less abundant ellagitannins are sanguiin H-2 and [galloyl-bis-HHDP-glucose]2-gallate.45 Both sanguiin H-2 and [galloyl-bis-HHDP-glucose]2-gallate are either present naturally or derived from degradation of lambertianin C during hot-water extraction processes.46 In black raspberries, sanguiin H-6 and its derivates, lambertianin C/D, ellagic acid and its derivates are also found.47,48 These ellagitannins can be hydrolyzed with acids or bases to release hexahydroxydiphenoyl units which spontaneously cyclizes into ellagic acid.48 The ellagic acid released after acid hydrolysis are one of important phenolic compounds in Rubus fruit, accounting for approximately 80% of the total phenolics.49 In R. chingii, the main ellagitannins (lambertianin A, sanguiin H-6 and casuarictin) and ellagic acid are found in unripe fruits.50 In this study, methyl (S)-flavogallonate and casuarictin were the main components of ellagitannins, and the former was first identified in R. chingii fruit. Ellagitannins were much higher contents than other phenolics, which contributed to a large part of antioxidant capability. These ellagitannins all decreased as the fruit matured, which was consistent to what was observed in other Rubus species.17,47 The high antioxidant capacity of ellagitannins are believed to have multiple health benefits, e.g. antiglycation activity,50 lung, oesophagus function51 and as a remedy for combating prostate cancer.52 Additionally, the ellagitannins is always correlated with oral astringency.53 The high content of ellagitannins in the unripe fruit of R. chingii also explains its use in traditional Chinese medicine, while the low content in ripe fruit makes it have a much less astringent taste than unripe fruit.
R. chingii had a varied flavonoid profile due to the occurrence of quercetin and kaempferol derivatives (Table 1; Fig. 3b). These components decreased during fruit ripening, which was consistent with what has been observed in red raspberry (S. Y. Wang et al., 2009). Of these, nikotoflorin is predominant, followed by astragalin, ellagic acid and tiliroside, which is consistent with previous reports in R. chingii.54 Ellagic acid and astragalin are prevalent in fruit, and were also reported in red raspberry7,17,45 and blackberry47 while tiliroside only exists in some varieties of Poland red raspberry8 and in leaves of Bulgarian Rubus species.55 Nikotoflorin is not found in red or black raspberry, but is found in R. chingii with high concentrations.56 Isoquercitrin and astragalin ubiquitously exist in red and black raspberries, as well as several ellagic acid pentosides, ellagic acid acetyl pentosides, hyperoside and rutin.8,47 Rourinoside was first identified in Rubus, which was also found in the fractionation of the antimalarial active CHCl3 extract of the dried stems of Rourea minor (Gaertn.).57 Isoquercitrin, nikotoflorin and tiliroside exhibit significant bioactivity, e.g. Isoquercitrin has shown bioactivity against cancer, cardiovascular disorders, diabetes and allergic reactions,58 nikotoflorin protects the liver from CCl4-induced oxidative damage,59 while tiliroside possesses anti-inflammatory, antioxidant, anticarcinogenic and hepatoprotective activities.60 The kaempferol- and quercetin-based flavonoids mainly accumulated in the fruit epidermal hair, and in the placentae and seed coats, but rarely in fruit pericarp (the exocarp, hypodermis and mesocarp) (Fig. 4). Thus, nikotoflorin and rourinoside are species-specific products, which could be applied to the taxonomic classification of Rubus species.
4. Conclusions
In R. chingii, the phenolics drastically decreased throughout fruit ripening while apocarotenoids dramatically increased, which led to discrepancy in color, flavor, and nutritional components between unripe and ripe fruits. The discrepancy determines their different uses. Unripen is extremely rich in healthy phenolic compounds, which could be useful in the development of health care products, while ripe fruit is much less astringent tasting and can be marketed as produce. Notably, R. chingii has very special profiles of phenolics and carotenoids, which is totally different form other raspberries. For example, the β-citraurin esters rather than anthocyanin components are responsible for the fruit reddish coloration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Natural Science Foundation of Zhejiang Province (LY19C160008), the Science-Technology Programs of Linqi Government, and Basic Public Welfare Projects of Zhejiang Province (2017C32082).References
- H. Y. Ding, Int. J. Mol. Sci., 2011, 12, 3941–3949 CrossRef CAS PubMed.
- T. T. Zhang, C. L. Lu, J. G. Jiang, M. Wang, D. M. Wang and W. Zhu, Carbohydr. Polym., 2015, 130, 307–315 CrossRef CAS PubMed.
- N. Sun, Y. Wang, Y. Liu, M.-L. Guo and J. Yin, Chem. Nat. Compd., 2013, 49, 49–53 CrossRef CAS.
- S. P. Mazur, A. Nes, A. B. Wold, S. F. Remberg and K. Aaby, Food Chem., 2014, 160, 233–240 CrossRef CAS PubMed.
- S. H. Nile and S. W. Park, Nutrition, 2014, 30, 134–144 CrossRef CAS PubMed.
- I. A. Ludwig, P. Mena, L. Calani, G. Borges, G. Pereira-Caro, L. Bresciani, R. D. Del, M. E. Lean and A. Crozier, Free Radical Biol. Med., 2015, 89, 758–769 CrossRef CAS PubMed.
- A. V. Rao and D. M. Snyder, J. Agric. Food Chem., 2010, 58, 3871–3883 CrossRef CAS PubMed.
- M. Kula, M. Majdan, D. Głód and M. Krauze-Baranowska, J. Food Compos. Anal., 2016, 52, 74–82 CrossRef CAS.
- L. S. Kim, S. H. Youn and J. Y. Kim, J. Korean Soc. Food Sci. Nutr., 2014, 43, 1357–1362 CrossRef CAS.
- M. Krauze-Baranowska, M. Majdan, R. Hałasa, D. Głód, M. Kula, I. Fecka and A. Orzeł, Food Funct., 2014, 5, 2536–2541 RSC.
- M. Park, H. Cho, H. Jung, H. Lee and K. T. Hwang, J. Food Biochem., 2014, 38, 259–270 CrossRef CAS.
- Q. L. Guo, J. Y. Gao and J. S. Yang, Chromatographia, 2005, 62, 145–150 CrossRef CAS.
- J. Beekwilder, I. M. van der Meer, A. Simic, J. Uitdewilligen, J. van Arkel, R. C. H. de Vos, H. Jonker, F. W. A. Verstappen, H. J. Bouwmeester, O. Sibbesen, I. Qvist, J. D. Mikkelsen and R. D. Hall, Biofactors, 2008, 34, 57–66 Search PubMed.
- X. Li, L. Jin, X. Pan, L. Yang and W. Guo, Food Chem., 2019, 290, 216–228 CrossRef CAS PubMed.
- L. Jin, X.-B. Li, D.-Q. Tian, X.-P. Fang, Y.-M. Yu, H.-Q. Zhu, Y.-Y. Ge, G.-Y. Ma, W.-Y. Wang, W.-F. Xiao and M. Li, Ind. Crops Prod., 2016, 87, 198–209 CrossRef CAS.
- D. R. Lewis, M. V. Ramirez, N. D. Miller, P. Vallabhaneni, W. K. Ray, R. F. Helm, B. S. J. Winkel and G. K. Muday, Plant Physiol., 2011, 156, 144–164 CrossRef CAS PubMed.
- S. Y. Wang, C.-T. Chen and C. Y. Wang, Food Chem., 2009, 112, 676–684 CrossRef CAS.
- J. A. Stavang, S. Freitag, A. Foito, S. Verrall, O. M. Heide, D. Stewart and A. Sønsteby, Sci. Hortic., 2015, 195, 216–225 CrossRef CAS.
- P. D. Fraser, M. R. Truesdale, C. R. Bird, W. Schuch and P. M. Bramley, Plant Physiol., 1994, 105, 405–413 CrossRef CAS PubMed.
- L. Zhang, Z. Zhang, T. Zheng, W. Wei, Y. Zhu, Y. Gao, X. Yang and S. Lin, Horticultural Plant Journal, 2016, 2, 9–15 CrossRef.
- M.-J. Rodrigo, J. F. Marcos and L. Zacarías, J. Agric. Food Chem., 2004, 52, 6724–6731 CrossRef CAS PubMed.
- K. Karppinen, L. Zoratti, M. Sarala, E. Carvalho, J. Hirsimaki, H. Mentula, S. Martens, H. Haggman and L. Jaakola, BMC Plant Biol., 2016, 16, 95 CrossRef PubMed.
- S. Remy-Tanneau, C. Le Guernevé, E. Meudec and V. Cheynier, J. Agric. Food Chem., 2003, 51, 3592–3597 CrossRef CAS PubMed.
- T. Fossen and O. M. Andersen, Phytochemistry, 2003, 62, 1217–1220 CrossRef CAS PubMed.
- T. Fossen, S. Rayyan and Ø. M. Andersen, Phytochemistry, 2004, 65, 1421–1428 CrossRef CAS PubMed.
- G. J. McDougall, S. Gordon, R. Brennan and D. Stewart, J. Agric. Food Chem., 2005, 53, 7878–7885 CrossRef CAS PubMed.
- Y. Fukui, T. Kusumi, K. Masuda, T. Iwashita and K. Nomoto, Tetrahedron Lett., 2002, 43, 2637–2639 CrossRef CAS.
- A. M. González-Paramás, F. Lopes da Silva, P. Martín-López, G. Macz-Pop, S. González-Manzano, C. Alcalde-Eon, J. J. Pérez-Alonso, M. T. Escribano-Bailón, J. C. Rivas-Gonzalo and C. Santos-Buelga, Food Chem., 2006, 94, 428–436 CrossRef.
- T. K. Hyun, S. Lee, Y. Rim, R. Kumar, X. Han, S. Y. Lee, C. H. Lee and J. Y. Kim, PLoS One, 2014, 9, e88292 CrossRef PubMed.
- M. Dossett, J. Lee and C. E. Finn, J. Funct. Foods, 2010, 2, 292–297 CrossRef CAS.
- A. L. Cual, J. Food Sci., 1965, 30, 13–18 CrossRef.
- J. N. Mi and S. Al-Babili, Mol. Plant, 2019, 12, 1173–1175 CrossRef CAS PubMed.
- G. Ma, L. Zhang, A. Matsuta, K. Matsutani, K. Yamawaki, M. Yahata, A. Wahyudi, R. Motohashi and M. Kato, Plant Physiol., 2013, 163, 682–695 CrossRef CAS PubMed.
- D. Marinova and F. Ribarova, J. Food Compos. Anal., 2007, 20, 370–374 CrossRef CAS.
- E. Carvalho, P. D. Fraser and S. Martens, Food Chem., 2013, 139, 744–752 CrossRef CAS PubMed.
- K. Mizuno, T. Tokiwano and Y. Yoshizawa, Biochem. Biophys. Res. Commun., 2017, 484, 845–849 CrossRef CAS PubMed.
- I. O. Vvedenskaya and N. Vorsa, Plant Sci., 2004, 167, 1043–1054 CrossRef CAS.
- J. Song, L. Du, L. Li, W. Kalt, L. C. Palmer, S. Fillmore, Y. Zhang, Z. Zhang and X. Li, J. Proteomics, 2015, 122, 1–10 CrossRef CAS PubMed.
- M. Giribaldi, I. Perugini, F. X. Sauvage and A. Schubert, Proteomics, 2007, 7, 3154–3170 CrossRef CAS PubMed.
- H. Liu, J. Mao, S. Yan, Y. Yu, L. Xie, J. G. Hu, T. Li, A. M. Abbasi, X. Guo and R. H. Liu, Int. J. Food Sci. Technol., 2018, 53, 381–388 CrossRef CAS.
- W. Stahl and H. Sies, Mol. Aspects Med., 2003, 24, 345–351 CrossRef CAS.
- V. R. de Souza, P. A. Pereira, T. L. da Silva, L. C. de Oliveira Lima, R. Pio and F. Queiroz, Food Chem., 2014, 156, 362–368 CrossRef PubMed.
- L. Chen, X. Xin, H. Zhang and Q. Yuan, J. Funct. Foods, 2013, 5, 508–515 CrossRef CAS.
- K. R. Määttä-Riihinen, A. Kamal-Eldin and A. R. Törrönen, J. Agric. Food Chem., 2004, 52, 6178–6187 CrossRef PubMed.
- R. Bobinaite, P. Viskelis and P. R. Venskutonis, Food Chem., 2012, 132, 1495–1501 CrossRef CAS PubMed.
- M. M. Kool, D. J. Comeskey, J. M. Cooney and T. K. McGhie, Food Chem., 2010, 119, 1535–1543 CrossRef CAS.
- L. Kaume, L. R. Howard and L. Devareddy, J. Agric. Food Chem., 2012, 60, 5716–5727 CrossRef CAS PubMed.
- U. Vrhovsek, A. Palchetti, F. Reniero, C. Guillou, D. Masuero and F. Mattivi, J. Agric. Food Chem., 2006, 54, 4469–4475 CrossRef CAS PubMed.
- H. Sh, M. Heinonen, K. So, M. Hm and R. Trrnen, Food Res. Int., 1999, 32, 345–353 CrossRef.
- C. Yue, X. Leilei, W. Yajie, C. Zhongqin and C. Haixia, LWT--Food Sci. Technol., 2019, 121, 108967 Search PubMed.
- L. A. Kresty, M. A. Morse, C. Morgan, P. S. Carlton, J. Lu, A. Gupta, M. Blackwood and G. D. Stoner, Cancer Res., 2001, 61, 6112–6119 CAS.
- N. P. Seeram, W. J. Aronson, Y. Zhang, S. M. Henning, A. Moro, R. P. Lee, M. Sartippour, D. M. Harris, M. Rettig, M. A. Suchard, A. J. Pantuck, A. Belldegrun and D. Heber, J. Agric. Food Chem., 2007, 55, 7732–7737 CrossRef CAS PubMed.
- T. Hofmann, A. Glabasnia, B. Schwarz, K. N. Wisman, K. A. Gangwer and A. E. Hagerman, J. Agric. Food Chem., 2006, 54, 9503–9509 CrossRef CAS PubMed.
- J. M. He, N. Sun, W. D. Wu, L. J. Fan and M. L. Guo, Zhongguo Zhongyao Zazhi, 2013, 38, 4351–4356 CAS.
- R. Gevrenova, I. Badjakov, M. Nikolova and I. Doichinova, Biochem. Syst. Ecol., 2013, 50, 419–427 CrossRef CAS.
- G. Yu, Z. Luo, W. Wang, Y. Li, Y. Zhou and Y. Shi, Front. Pharmacol., 2019, 10, 1323 CrossRef PubMed.
- Z.-D. He, C.-Y. Ma, G. T. Tan, K. Sydara, P. Tamez, B. Southavong, S. Bouamanivong, D. D. Soejarto, J. M. Pezzuto, H. H. S. Fong and H.-J. Zhang, Phytochemistry, 2006, 67, 1378–1384 CrossRef CAS PubMed.
- K. Valentová, J. Vrba, M. Bancířová, J. Ulrichová and V. Křen, Food Chem. Toxicol., 2014, 68, 267–282 CrossRef PubMed.
- Y. Wang, C. Tang and H. Zhang, J. Food Drug Anal., 2015, 23, 310–317 CrossRef CAS PubMed.
- T. Goto, A. Teraminami, J.-Y. Lee, K. Ohyama, K. Funakoshi, Y.-I. Kim, S. Hirai, T. Uemura, R. Yu, N. Takahashi and T. Kawada, J. Nutr. Biochem., 2012, 23, 768–776 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |